Abstract
The aim of our study was to investigate the impact of interleukin (IL)-6 190C/T, IL-6 174G/C, IL-6 572G/C, tumor necrosis factor-alpha (TNF-α) 308G/A, and angiotensin-converting enzyme (ACE) I/D gene polymorphisms on Helicobacter pylori (H. pylori) infection in children.
A cross-sectional study was performed on 126 children (57 children with H. pylori infection and 69 children without H. pylori infection) aged between 3 and 18 years presenting to a Pediatrics Tertiary Hospital from Romania. Children were assessed clinically, endoscopically, histopathologically, and genetically.
In our study, we found that the presence of the CT and CT+TT genotypes of IL-6 190C/T (P < .002 and P = .04), allele G of IL-6 572 G/C polymorphism (P = .01), genotypes GA and AA of TNF-α 308 G/A polymorphism (P = .04, P = .01), and genotype II of ACE I/D polymorphism (P = .02) were associated with H. pylori infection, while the CC genotype of IL-6 174G/C polymorphism was scarcely encountered in children with H. pylori infection [P = .02, odds ratio (OR) = 0.06; 95% confidence interval (95% CI): 0.003–0.128]. Taking under consideration the 4 variant genotypes (IL-6 572G/C, IL-6 190C/T, TNF-α 308G/A, and ACE I/D), we noticed a 2 times higher incidence of H. pylori infection (OR = 6.34; 95% CI: 2.15–25.8).
We may consider that the IL-6 190C/T, IL-6 174G/C, IL-6 572G/C, TNF-α 308G/A, and ACE I/D gene polymorphisms may increase the children's susceptibility for acquiring H. pylori infection; therefore, they may contribute to the pathogenesis of H. pylori gastritis.
Keywords: children, genetic susceptibility, H. pylori infection
1. Introduction
Infection with Helicobacter pylori (H. pylori) is one of the most worldwide spread infections. It is acquired in the first years of life in both developed and developing countries.[1,2] The socioeconomic status plays an essential role in acquiring H. pylori infection; therefore, the lowest incidence is encountered in the developed countries from Northern and Western Europe, while people from underdeveloped and developing countries, such as those from the South and East of Europe, South America, Asia, and Africa, present a much higher incidence.[3–5]
In children, peptic ulcer disease has a lower incidence than in adults. In a multicentric study performed on 1233 symptomatic children with H. pylori infection, peptic ulcer disease was diagnosed in 10% of teenagers and in <5% of the children with the age under 12 years.[6] Most of the time, children cannot exactly locate or describe the pain characteristics.[7,8] In addition, certain disorders such as idiopathic thrombocytopenic purpura were proven to be associated with H. pylori infection only in adults, not in children, probably due to their different pathogenesis.[9,10] Gastric cancer is considered the fourth leading cause of death due to cancer in adults worldwide reaching percentages of 16% to 18% of all cancers, H. pylori being responsible for 75% of gastric cancers.[11] Long-term infection with H. pylori is associated with an increased risk of gastric adenocarcinoma or malignant lymphoma in adults, few cases being reported at pediatric age.[12,13]
Gastritis, inflammatory processes of the gastric mucosa, are commonly associated with peptic ulcer and duodenitis.[14] They have a great importance and a certain actuality in the field of pediatric pathology due to both their increased incidence and complications in children. Recent data suggest that gastritis may have an inflammatory etiology, associating a low degree of proinflammatory status.[15] Some cases of gastritis associated with H. pylori infection do not heal despite the correct application of treatment regimens due to the virulence of microorganisms in association with the hosts‘ genetic predisposition.[15] In the beginning, H. pylori determines acute gastritis, developing afterwards into a chronic process. If the inflammation persists, possibly influenced and intensified by the proinflammatory cytokines activity and through the stimulation of the immune system by H. pylori, it will lead eventually to atrophic gastritis, hypochlorhydria, and even gastric cancer in adults.[16,17] Even though H. pylori induces an immune response through both innate and acquired immunity, the host is unable to clear the organism from the mucosa, a fact that leads to a life-long infection.[18,19] The study of Hwang et al[20] proved that H. pylori stimulated the synthesis and transcription of certain proinflammatory cytokines such as interleukin IL-1β, IL-2, IL-6, IL-8 and tumor necrosis factor alpha (TNF-α), and of several anti-inflammatory ones, such as IL-4 and IL-10.
Genetic polymorphisms of several inflammatory and immunoregulatory cytokines were investigated for their possible association with the risk for specific H. pylori associated disease.[15,21] The review of Sugimoto et al[22] underlined the fact that certain polymorphisms such as IL-4 590 and IL-6 572 were associated with gastric cancer, while others such as IL-4 590, IL-6 572, and IL-8 251 were specific for peptic ulcer disease among the people from Western in comparison to Eastern Asia. IL-6 is a multifunctional cytokine produced by immune or nonimmune cells, and its functions as an inflammatory endocrine and metabolic function mediator are well documented in the literature.[18,23] A correlation has been reported between H. pylori and IL-6 due to the fact that mRNA levels of this cytokine within the gastric mucosa were found to be associated with the inflammation level.[24] In normal conditions, IL-6 is involved in host defense mechanisms functioning as a messenger between innate and adaptive systems through the stimulation of interferon gamma production in T-cells, through the promotion of immunoglobulin secretion in activated B-cells, and also by activating polymorphonuclear cells.[15,18] Sugimoto et al [22] and Hwang et al [20] also underlined that IL-1B-511, IL-10, TNF-α polymorphisms were related to gastric cancer and ulcerous disease. In addition, the studies of Ianovich et al [25] and Achyut et al [26] pointed out that the A allele of TNF-α 308G/A gene polymorphism was more frequently associated with H. pylori infection. Also, the study of Zambon et al[27] proved that H. pylori infection was associated with TNF-α 308 AG genotype. Sugimoto et al,[28] in a study on Japanese subjects, noticed that the II genotype of ACE polymorphism was associated with gastric cancer and gastric ulcer, and Goto et al[29] underlined the fact that the DD genotype was associated with gastric tumor progression and metastases.
All the above-mentioned published data underline the fact that proinflammatory and anti-inflammatory cytokines play an important role in gastric pathology. Nevertheless, only a few studies assessed these cytokines in pediatric patients, and none of them evaluated the combined effect of these 5 gene polymorphisms, some of the most frequently described to be involved in both inflammation and cancers of the gastric mucosa.
On the basis of all these facts, the objective of our study was to assess the impact of host's genetic susceptibility in acquiring H. pylori infection. Therefore, we assessed the role of 5 frequently described cytokines (IL-6 190C/T, IL-6 174C/G, IL-6 572G/C, TNF-α 308G/A, and ACE I/D) gene polymorphisms in acquiring H. pylori in children, by evaluating both the role of each polymorphism and the combined effect of all 5 in determining H. pylori induced gastritis at this age, also taking into consideration symptoms, endoscopic aspects, and histopathological features.
2. Methods
A cross-sectional prospective study was performed on 126 children aged between 3 and 18 years, with the following symptoms: abdominal pain, nausea, heart burn, and vomiting. Children were evaluated in a Pediatrics Tertiary Hospital from Romania, between January 2014 and December 2015. Patients were divided according to the presence or absence of H. pylori infection into the study group—57 children with H. pylori infection and control group—69 children without H. pylori infection. All children were clinically, endoscopically, histopathologically, and genetically assessed.
The inclusion criteria in our study were suggestive symptoms for gastritis, such as heart burn, nausea, vomiting, epigastric pain, diffuse abdominal pain, loss of appetite. The exclusion criteria were as follows: presence of any infectious disease, acute surgical abdomen or children who presented other complains than those mentioned above, and children under the age of 3 years due to the fact that our endoscope had a diameter of 9 mm and it was designed to be used only for children above the age of 3.
To increase the comparative statistical power, we chose a bigger control group than the study one.
The subjects’ parents gave their written informed consent before the inclusion in the study and the procedures were explained to all children according to their age in order to receive their assent. The study was performed in compliance with the principles of the Helsinki Declaration, and was approved by the Ethics Committee of the University of Medicine and Pharmacy of Tîrgu Mureş (No. 13/July 18, 2011, and No. 35/April 7, 2017).
2.1. Endoscopic exploration and histopathological evaluation
A single trained person performed all esophagogastroduodenoscopies with an videogastroscop Olympus Exera III GIF-HQ190 9,9 mm flexible endoscope, under local anesthesia with 2% Xylocaine and proper premedication, after a fasting period of 8 to 12 hours. During the endoscopic exploration, 2 antral biopsies and 2 from the gastric corpus were taken, which were afterwards histopathologically examined. H. pylori infection was diagnosed by processing the previously taken biopsy specimens through standard histological methods: formalin fixation, sampling, paraffin inclusion, sectioning, hematoxylin–eosin and Giemsa staining.
2.2. Genotyping description
For the investigation of gene polymorphisms, we used gDNA (genomic DNA) isolated from blood samples. The ACE I/D gene polymorphism was analyzed using the method recommended by Rigat et al [30] and by Shanmugam et al.[31] For the genotyping of IL-6 572G/C and IL-6 190T/C polymorphisms, we performed a simple method namely polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay. [32] Amplification-refractory mutation system PCR (ARMS-PCR) method described by Daneshmandi et al[33] was used for the analysis of IL-6 174G/C and TNF-α 308G/A gene polymorphisms. For the IL-6 and TNF-α investigated gene polymorphisms, the most frequent allele found in the control group was considered as dominant and it was used as a reference.
2.3. Statistical analysis
Data analysis was performed using the Statistical Package for Social Sciences (SPSS, version 20; SPSS Inc, Chicago, IL). The associations of distribution (IL-6 190C/T, IL-6 174 G/C, IL-6 572 G/C, TNF-α 308 G/A, and ACE I/D polymorphisms) with category variables were assessed using Chi-square or Fisher exact tests. Differences in continuous variables were analyzed using Student t test (data expressed as mean ± SD), and Mann–Whitney test (data expressed as median, range). We performed multivariate analysis (simple binary logistic regression and multiple binary logistic regression). We included the data in a multivariable model as independent variables that achieved the criterion of significance at P < .25 in a univariate analysis. The odds ratio (OR) was calculated in a similar way, to demonstrate the probability or susceptibility to H. pylori infection according to the given variables. The level of statistical significance was set at P < .05.
3. Results
3.1. Characteristics of the subjects
We included 126 children in our study, and we divided them according to the presence or absence of H. pylori infection into 2 groups: the study group—57 children with H. pylori infection and the control group—69 children without H. pylori infection, based on the histopathological examination from the gastric biopsy specimens.
Mean age in the study group was 9.85 ± 4.08 years, while in the control group, it was 10.72 ± 4.54 years, without significant statistical difference between the 2 groups (P = .31). Also, regarding gender distribution, we did not encounter any significant statistical difference between the 2 groups (P = .29). Therefore, we can say that the groups were age- and sex-matched.
3.2. The IL-6 174G/C, IL-6 190C/T, IL-6 572G/C, TNF-α 308G/A, and ACE I/D polymorphisms
Regarding the repartition of polymorphisms in our groups, we observed that for the IL-6 174G/C gene polymorphism, CC genotype frequency (8.7%) was higher in the control group, suggesting the fact that this genotype may present a defensive factor against the infection (P = .02, OR = 0.06; 95% CI: 0.003–0.128) (Table 1).
Table 1.
Distribution of IL-6 174 G/C, IL-6 190 C/T, IL-6 572 G/C, TNF-α 308 G/A, and ACE I/D genotypes among H. pylori infection patients and controls.

For the IL-6 190C/T gene polymorphism, the frequency of CT genotype was significantly higher in the group with H. pylori infection (68.4%) in comparison to the control group (33.4%) (P < .002, OR = 3.51, and 95% CI: 1.54–7.98). Also, the combined genotype CT+TT of IL-6 190C/T gene polymorphism was more frequently encountered in patients with H. pylori infection, 75.4% versus 58.0% in the control group (P = .04, OR = 2.22 and 95% CI: 1.03–4.81).
Distribution of the C allele of IL-6 572G/C gene polymorphism varied significantly between the 2 groups, 3.5% in the study group and 12.3% in the control group (P = .01, OR = 0.25, 95% CI: 0.08–0.74). The G allele of the same polymorphism was also more frequently encountered in children with H. pylori infection (Table 1).
For the TNF-α 308G/A gene polymorphism, the frequency of genotypes, GA (P = .04, OR = 2.26, 95% CI: 1.01–5.04), AA (P = .01, OR = 3.63, 95% CI: 1.26–10.4), or variant genotype GA+AA (P = .008, OR = 2.63, 95% CI: 1.27–5.42) were significantly higher in the study group in comparison to the control group. Similarly, allele A of the same polymorphism was significantly more frequent in the study group versus the control group (P = .002, OR = 2.31, 95% CI: 1.35–3.97) (Table 1).
Regarding the ACE I/D gene polymorphism, the II genotype presented a significantly increased frequency among children with H. pylori infection (29.8%) versus the control group (P = .02, OR = 5.1, 95% CI: 1.28–20.2). Also, I allele of this polymorphism was encountered significantly more frequent in the study group (P = .006, OR = 2.73, 95% CI: 1.31–5.69) (Table 1).
The distribution of the assessed polymorphisms in patients with H. pylori infection through the variants of combined genotypes (heterozygotes and homozygotes), stratified according to symptoms and the macroscopic aspect of the gastric mucosa is presented in Table 2. It was noticed that for IL-6 174G/C polymorphism, vomiting was significantly more frequent for the GG genotype in the control group, and GC+CC genotype, respectively, in the group with H. pylori infection (P = .03, OR = 0.12, 95% CI: 0.01–0.96), actually a reverse association.
Table 2.
H. pylori patients’ symptoms and endoscopic aspects according to IL-6 174 C/G, IL-6 190 C/T, IL-6 572 G/C, TNF-α 308 G/A, and ACE I/D genotypes.
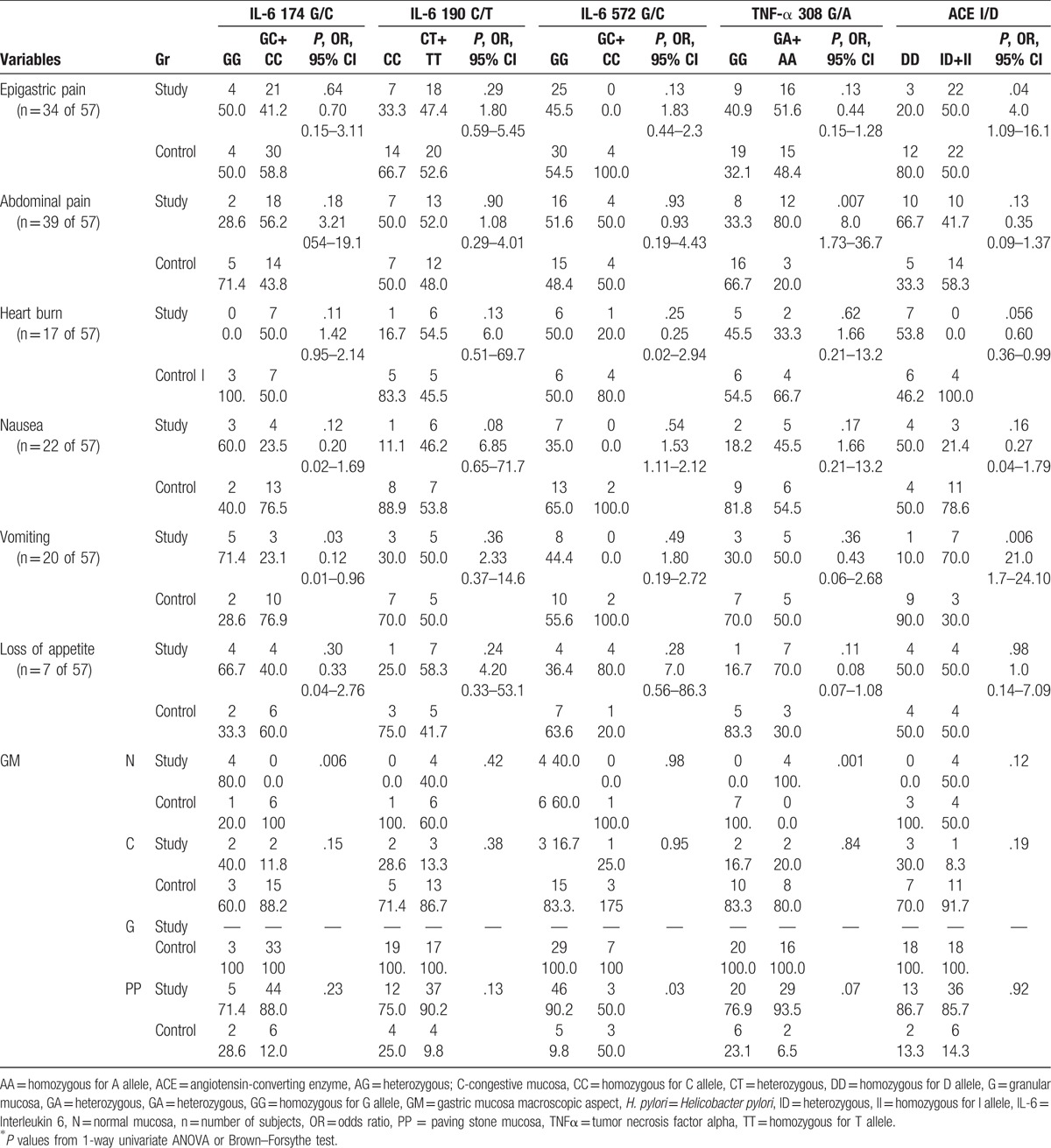
Presence of the variant genotype in the control group was associated with abdominal pain for TNF-α 308G/A gene polymorphism (P = .007, OR = 8.0, 95% CI: 1.73–36.7), with epigastric pain or vomiting in case of the ID+II genotypes of ACE I/D polymorphism (P = .04, OR = 4.0, 95% CI: 1.09–16.1, and P = .006, OR = 21.0, 95% CI: 1.7–24.1, respectively). The “paving-stone” aspect was more frequently observed in children who presented the normal genotype of IL6 572G/C polymorphism (P = .03) (Table 2).
Taking into account the association hypothesis between these polymorphisms with H. pylori infection, it was noticed that combining the variant genotypes of IL-6 190C/T and TNF-α 308G/A polymorphisms resulted in a statistically significant association (Chi-square test, χ2 = 7.128 and P = .007, OR = 3.95; 95% CI: 1.37–11.4). For the presence of 3 variant genotypes (IL-6 190 C/T, TNF-α 308 G/A, and ACE I/D), the infection risk was OR = 4.51 (95% CI: 1.10–15.9), and in case of 4 variant genotypes (IL-6 572 G/C, IL-6 190 C/T, TNF-α 308 G/A, and ACE I/D), the risk of infection was OR = 6.34 (95% CI: 2.15–25.8).
Through univariate logistic regression presented in Table 3, we followed the estimated level of statistical significance, associated with every independent variable. A statistically significant dependency was observed between H. pylori infection and the presence of variant alleles of IL-6 190 C/T (P = .041) and TNF-α 308 G/A polymorphisms (P = .009). Relations closely to the significance threshold, therefore smaller than P < .25, were observed related to age (P = .087), suggesting that small age could be a risk factor. The same fact was also noticed for the ACE I/D polymorphism, with ID+II (P = .076). These 4 independent variables were included into a model of multivariate logistic regression with the dependent variable (Table 4)—presence or absence of H. pylori infection, which was also taken in the statistical analysis in case of the univariate simple logistic regression.
Table 3.
The results from univariate logistic regression for IL-6 174 G/C, of IL-6 190 C/T, IL-6 572 G/C, TNF-α 308 G/A, and ACE I/D polymorphisms.
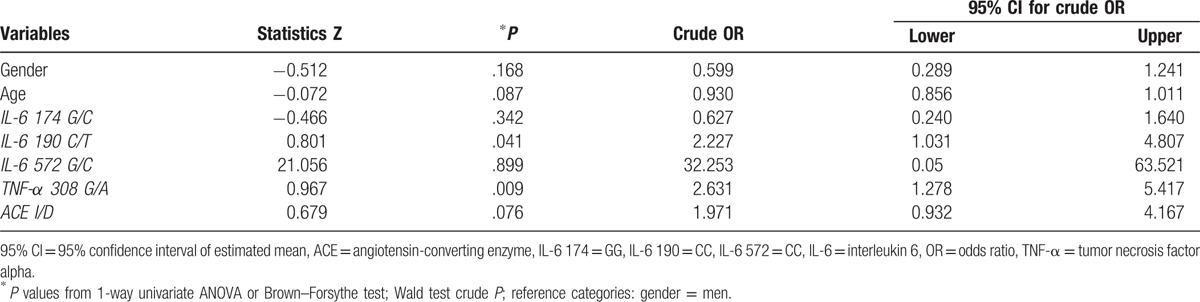
Table 4.
The results from multivariate logistic regression.
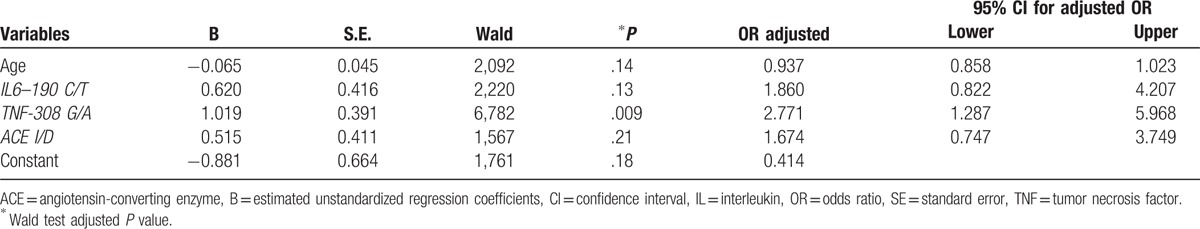
Among the 4 independent variables considered in the regression analysis, only TNF-α 308 polymorphism with variant GA+AA could be considered an independent predictor or an independent risk factor for the predisposition to H. pylori infection (P < .009), the OR risk being 2.77 times higher in comparison to the reference for this polymorphism, namely the GG genotype. IL6–190 C/T and ACE I/D had a positive effect on the risk of H. pylori infection, but without significant associations (P = .13, P = .21) (Table 4).
4. Discussion
4.1. Considerations regarding the IL-6 174 G/C, IL-6 190 C/T, and IL-6 572 G/C gene polymorphisms in children's H. pylori gastritis
IL-6 is a multifunctional cytokine produced by immune and nonimmune cells, and is an inflammatory mediator and metabolic regulator.[15,34] Previous studies reported that high IL-6 mucosal levels are associated with H. pylori infection.[35] Multiple gene polymorphisms present inflammatory and immune-regulatory role, being associated with H. pylori infection,[15,21] this itself stimulating the synthesis of several proinflammatory cytokines, including IL-6.[20] Sugimoto et al [22] in a review pointed out the fact that the IL-6 572 G/C gene polymorphism was not only associated with gastric cancer but also with ulcerous disease among Asiatic people. Kang et al [36] proved that the G/G homozygotes and G carriers allele of the Il-6 572 G/C gene polymorphisms presented smaller chances of developing duodenal ulcer associated with H. pylori infection than patients with nonulcerous dyspepsia. In our study in exchange, we observed that the G allele of IL-6 572 G/C was significantly more frequent in patients with gastritis determined by H. pylori (P = .01, OR = 0.25, 95% CI 0.08–0.74). Studies regarding the IL-6 174 G/C polymorphism are often contradictory. Therefore, Gatti et al [18] and Pohjanen et al [23] proved that G carriers developed gastric cancer more frequently than nonulcerous dyspepsia, and contrariwise, Kamangar et al [37] pointed out that GG homozygotes of the same polymorphism presented a more decreased risk of developing gastric cancer. In our study, we noticed that the CC genotype of the IL-6 174 G/C gene polymorphisms has a protecting role against H. pylori infection (P = .02, OR = 0.06, 95% CI: 0.003–0.128). The study of Gatti et al [18] from 2005 underlined the fact that G carriers of IL-6 174 G/C produced higher levels of IL-6, [15,38] but without any association with chronic gastritis and inflammatory processes, in adult patients.[15] Also, Gatti et al [18] did not find any association between the inflammation degrees (none, mild, moderate, and severe, according to Sydney system) and the Il-6 174 G/C polymorphism, nor related to gender. They only encountered concordances between the GG genotype of Il-6 174 G/C and adenocarcinoma, independent of the histopathological tumor aspect. In our study, we did not identify any relation between the previously mentioned gene polymorphism and patients’ gender. Hwang et al [20] proved that the C allele is more frequently associated with H. pylori in the Asian population than in the Columbian one, fact explained by ethnic differences. In our study, we noticed that vomiting was more frequent in case of the GG genotype in the control group and in those carrying the GC+CC genotypes in the study group (P = .03). We did not obtain significant correlations between the Il-6 polymorphisms and other symptoms or macroscopic aspects of the gastric mucosa associated with H. pylori infection.
Even though in the literature we did not find correlations or data regarding the IL-6 190 C/T gene polymorphism and H. pylori infection, in our study, the CT heterozygote genotype, and the variant genotype CT+TT, respectively, were more frequently observed in children with H. pylori infection (P = .002, P = .004, respectively).
4.2. Considerations regarding the TNF-α 308 G/A gene polymorphism in children's H. pylori gastritis
Tumor necrosis factor has an important role in the pathogenesis of gastric diseases, an abnormal inflammatory response being associated in these patients.[25]H. pylori infection can induce a gastric mucosal inflammatory response that may be influenced by serum TNF-α or the TNF-α 308 G/A polymorphism.[26] The study of Ianovich et al[25] showed a correlation between the risk of duodenal ulcer and the A allele of TNF-α 308 G/A in the assessed patients. Likewise, we also found a correlation between the A allele of TNF-α 308 G/A and H. pylori infection. In exchange, other studies such as those by Oliviera et al [39] and Santos et al [40] did not obtain any association between TNF-α 308 G/A or IL-8 251 A/T polymorphisms and the lesions from the adult's gastritis. Achyut et al [26] proved a trend toward association of lymphoid follicles and the A allele of TNF-α 308 G/A carriers with H. pylori infection compared with those without the presence of this bacteria (58.5% vs 22.2%; P = .064). In the study performed by Wilschanski et al [41] on 113 biopsies in children with the age between 2 and 18 years, among whom 23 had duodenitis and 90 gastritis, H. pylori infection was diagnosed by bacterial culture and histology and antral biopsies were used to characterize the genetic polymorphism of TNF-α 308 G/A and 238 A/G by PCR. All H. pylori strains were examined for cytotoxin-associated gene A and induced-by-contact-with-epithelium gene (iceA1).[41] The authors noticed that the combination of bacterial iceA1 and TNF-α 238 G/A polymorphism could be a risk factor for peptic ulcer disease in children infected with H. pylori. Nevertheless, larger studies were needed to confirm this association.[41] Also the study of Zambon et al [27] in which the existence of a correlation between H. pylori associated diseases and H. pylori virulence genes or IL-1B, IL-1RN, IFN-G, TNF-α, IL-10 genetic polymorphisms were assessed, showed that H. pylori infection was associated with TNF-α 308 A/G, while the IFN-γ 874 AA genotype was associated with cagA (cytotoxin-associated gene A virulence factor of H. pylori) positive infections. Yea et al[42] underlined that patients with the GA genotype of TNF-α 308 G/A presented chronic gastritis, while cagA was more frequently associated with H. pylori infection. Zabaglia et al[43] proved that H. pylori infection was associated with the G allele or the GC genotype of TNF-α 308 G/A, also observing an association between cagA and alleles s1/m1 of the vacA gene in samples with G allele or GC genotype. In addition, the authors underlined the fact that H. pylori infection induced an important increase in TNF-α expression in patients with gastritis regarding the inflammatory process, but after developing into a malignant one, the bacteria no longer influenced the expression, underlying in this manner that TNF-α owns an important role in the progression of gastritis toward cancer.[43] Regarding our findings, a statistically significant dependency was observed between the infection with H. pylori and presence of the mutant alleles of IL-6 190 C/T (P = .041) and TNF-α 308 G/A gene polymorphisms (P = .009).
4.3. Considerations regarding the ACE I/D gene polymorphism in children's H. pylori gastritis
Angiotensin-converting enzyme (ACE) owns multiple effects. It is an enzyme playing a role in the regulation of the renin-angiotensin-aldosterone (RAA) system. ACE is expressed by different types of cells [44] and generates angiotensin II that was recently proved to be involved in the regulation of cellular proliferation, angiogenesis, and inflammation through the type 1 receptors of angiotensin II,[45,46] which are expressed in tumor and endothelial cells. Several studies proved that the ACE I/D polymorphism was related to the development of gastric cancer, and also to atrophic gastritis.[28,29] The study of Kupcinskas et al[47] did not notice any association between ACE I/D and gastric cancer. Contrariwise, in our study, we noticed that the ACE II genotype was more frequently encountered in children with H. pylori infection (P = .02, OR = 5.1 95% CI: 1.28–20.2), and the I allele (P = .006, OR = 2.73, 95% CI: 1.31–5.69). In addition, ACE I/D presented a positive effect on the risk of H. pylori infection in our study.
We must underline some limitations of our study, as the small number of cases (126 children included in the study) that reduced the power of the study. Also, the fact that the group came from a single clinic, from a single geographic area of Romania represented another weakness of the study. Follow-up of children after treatment, at well settled intervals until the proved eradication of H. pylori infection, and data regarding H. pylori infection among the family members and resistance to treatment could have provided supplementary information to our study. We also must not forget food habits, environmental, and geographic factors that could have interfered with our results. It is recommended to extend the study on a higher geographic area, on a higher number of cases also taking into account H. pylori infection in the family members.
It is also very important to mention the strong points of our study, namely the very high accuracy of estimation of the statistical parameters due to adequate sample size. Another strength is represented by the fact that a single well trained person provided all endoscopic examinations, H. pylori examinations, and also a single person assessed the histopathological examinations. To our best knowledge, this is the first study that assessed the correlations between H. pylori infection and 5 of the most frequently reported gene polymorphisms involved in the etiology of gastric pathologies, evaluating not only the effect of each of them on H. pylori infection but also the combined effect of all 5 in children diagnosed with H. pylori induced gastritis. Multiple factors involved in child's gastritis were assessed in this study. Even though these strengths present a great impact in the study, we must carry on the research, assessing the children further on in life, prior, and after the treatment, on long term—longitudinally due to the well-known hypothesis that H. pylori in also incriminated in oncogenesis, therefore in the increasing incidence of gastric cancer during adulthood.
5. Conclusion
Our findings support the fact that presence of the variant genotype of IL-6 190 C/T, G allele of IL-6 572 G/C, variant genotype of TNF-α 308 G/A, and II genotype of ACE I/D polymorphism were associated with H. pylori infection. Taking into account the combined effect of these gene polymorphisms, we noticed that the risk for acquiring H. pylori infection was 2 times higher in cases where 2 variant alleles coexisted.
So, we may consider that the IL-6 190 C/T, IL-6 174 G/C, IL-6 572 G/C, TNF-α 308 G/A, and ACE I/D gene polymorphisms present a role in developing H. pylori gastritis in children. Therefore, they may explain a small amount of the individual genetic susceptibility to this infection. The clinical relevance of our findings is based on the fact that different gene polymorphisms can influence not only the susceptibility to H. pylori infection but can also influence the response to treatment. Therefore, further longitudinal studies on bigger groups and more extended geographical areas are required, with a potential role in predicting children's gastritis.
Footnotes
Abbreviations: AA = homozygous for A allele, ACE = angiotensin-converting enzyme, AG = heterozygous, ARMS-PCR = amplification-refractory mutation system PCR, AT = heterozygous, cag A = cytotoxin-associated gene A virulence factor of Helicobacter pylori, CC = homozygous for C allele, CI = Confidence interval, CT = heterozygous, DD = homozygous for D allele, DNA = deoxiribonucleic acid, GA = heterozygous, GG = homozygous for G allele, H. pylori = Helicobacter pylori, ID = heterozygous, II = homozygous for I allele, IL = interleukin, IL-10 = interleukin 10, IL-2 = interleukin 2, IL-4 = interleukin 4, IL-6 = interleukin 6, IL-8 = interleukin 8, n = absolute number, OR = odds ratio, PCR = polymerase chain reaction, PCR-RFLP = polymerase chain reaction-restriction fragment length polymorphism, SD = standard deviation, TNF-α = tumor necrosis factor alpha, TT- = homozygous for T allele, vacA = vacuolating cytotoxin of H. pylori.
None of the authors has any conflicts of interest.
References
- [1].Rowland M, Daly L, Vaughan M, et al. Age-specific incidence of Helicobacter pylori. Gastroenterology 2006;130:65–72. [DOI] [PubMed] [Google Scholar]
- [2].Goodman KJ, O’rourke K, Day RS, et al. Dynamics of Helicobacter pylori infection in a US-Mexico cohort during the first two years of life. Int J Epidemiol 2005;34:1348–55. [DOI] [PubMed] [Google Scholar]
- [3].Kawakami E, Machado RS, Ogata SK, et al. Decrease in prevalence of Helicobacter pylori infection during a 10-year period in Brazilian children. Arq Gastroenterol 2008;45:147–51. [DOI] [PubMed] [Google Scholar]
- [4].Elitsur Y, Dementieva Y, Rewalt M, et al. Helicobacter pylori infection rate decreases in symptomatic children: a retrospective analysis of 13 years (1993-2005) from a gastroenterology clinic in West Virginia. J Clin Gastroenterol 2009;43:147–51. [DOI] [PubMed] [Google Scholar]
- [5].Azevedo NF, Huntington J, Goodman KJ. The epidemiology of Helicobacter pylori and public health implications. Helicobacter 2009;14(Suppl 1):1–7. [DOI] [PubMed] [Google Scholar]
- [6].Koletzko S, Richy F, Bontems P, et al. Prospective multicentre study on antibiotic resistance of Helicobacter pylori strains obtained from children living in Europe. Gut 2006;55:1711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chitkara DK, Rawat DJ, Talley NJ. The epidemiology of childhood recurrent abdominal pain in Western countries: a systematic review. Am J Gastroenterol 2005;100:1868–75. [DOI] [PubMed] [Google Scholar]
- [8].Kalach N, Mention K, Guimber D, et al. Helicobacter pylori infection is not associated with specific symptoms in nonulcer-dyspeptic children. Pediatrics 2005;115:17–21. [DOI] [PubMed] [Google Scholar]
- [9].Khurana R, Fischbach L, Chiba N, et al. Meta-analysis: Helicobacter pylori eradication treatment efficacy in children. Aliment Pharmacol Ther 2007;25:523–36. [DOI] [PubMed] [Google Scholar]
- [10].Bourke B, Ceponis P, Chiba N, et al. Canadian Helicobacter Study Group Consensus Conference: update on the approach to Helicobacter pylori infection in children and adolescents: an evidence-based evaluation. Can J Gastroenterol 2005;19:399–408. [PubMed] [Google Scholar]
- [11].Walker MM, Talley NJ. Review article: bacteria and pathogenesis of disease in the upper gastrointestinal tract: beyond the era of Helicobacter pylori. Aliment Pharmacol Ther 2014;39:767–79. [DOI] [PubMed] [Google Scholar]
- [12].Moschovi M, Menegas D, Stefanaki K, et al. Primary gastric Burkitt lymphoma in childhood: associated with Helicobacter pylori? Med Pediatr Oncol 2003;41:444–7. [DOI] [PubMed] [Google Scholar]
- [13].Poddar U, Yachha SK. Helicobacter pylori in children: an Indian perspective. Indian Pediatr 2007;44:761–70. [PubMed] [Google Scholar]
- [14].Feldman M, Jensen P. Classification and Diagnosis of Gastritis and Gastropathy. Available at: http://www.uptodate.com/contents/classification-and-diagnosis-of-gastritis-and-gastropathy#H1. Accessed August 22, 2016. [Google Scholar]
- [15].Lobo Gatti L, Zambaldi Tunes M, de Lábio RW, et al. Interleukin-6 polymorphism and Helicobacter pylori infection in Brazilian adult patients with chronic gastritis. Clin Exp Med 2005;5:112–6. [DOI] [PubMed] [Google Scholar]
- [16].Shimoyama T, Crabtree JE. Bacterial factors and immune pathogenesis in Helicobacter pylori infection. Gut 1998;43(Suppl 1):S2–5. [PMC free article] [PubMed] [Google Scholar]
- [17].Sipponen P. Gastric cancer: a long-term consequence of Helicobacter pylori infection? Scand J Gastroenterol Suppl 1994;201:24–7. [PubMed] [Google Scholar]
- [18].Gatti LL, Burbano RR, Zambaldi-Tunes M, et al. Interleukin-6 polymorphisms, Helicobacter pylori infection in adult Brazilian patients with chronic gastritis and gastric adenocarcinoma. Arch Med Res 2007;38:551–5. [DOI] [PubMed] [Google Scholar]
- [19].Kranzer K, Eckhardt A, Aigner M, et al. Induction of maturation and cytokine release of human dendritic cells by Helicobacter pylori. Infect Immun 2004;72:4416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hwang I-R, Kodama T, Kikuchi S, et al. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology 2002;123:1793–803. [DOI] [PubMed] [Google Scholar]
- [21].Basso D, Scrigner M, Toma A, et al. Helicobacter pylori infection enhances mucosal interleukin-1 beta, interleukin-6, and the soluble receptor of interleukin-2. Int J Clin Lab Res 1996;26:207–10. [DOI] [PubMed] [Google Scholar]
- [22].Sugimoto M, Yamaoka Y, Furuta T. Influence of interleukin polymorphisms on development of gastric cancer and peptic ulcer. World J Gastroenterol 2010;16:1188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pohjanen V-M, Koivurova O-P, Mäkinen JM, et al. Interleukin 6 gene polymorphism -174 is associated with the diffuse type gastric carcinoma. Genes Chromosomes Cancer 2013;52:976–82. [DOI] [PubMed] [Google Scholar]
- [24].Harris PR, Smythies LE, Smith PD, et al. Inflammatory cytokine mRNA expression during early and persistent Helicobacter pylori infection in nonhuman primates. J Infect Dis 2000;181:783–6. [DOI] [PubMed] [Google Scholar]
- [25].Ianovich OO, Nosova ES, Titov LP. [Polymorphism of the genes IL-1RA and TNF-alpha in patients with gastritis and duodenal ulcer associated with Helicobacter pylori]. Mol Genet Mikrobiol Virusol 2013;31–4. [PubMed] [Google Scholar]
- [26].Achyut BR, Tripathi P, Ghoshal UC, et al. Interleukin-10 (-819 C/T) and tumor necrosis factor-alpha (-308 G/A) gene variants influence gastritis and lymphoid follicle development. Dig Dis Sci 2008;53:622–9. [DOI] [PubMed] [Google Scholar]
- [27].Zambon C-F, Basso D, Navaglia F, et al. Pro- and anti-inflammatory cytokines gene polymorphisms and Helicobacter pylori infection: interactions influence outcome. Cytokine 2005;29:141–52. [DOI] [PubMed] [Google Scholar]
- [28].Sugimoto M, Furuta T, Shirai N, et al. Influences of chymase and angiotensin I-converting enzyme gene polymorphisms on gastric cancer risks in Japan. Cancer Epidemiol Biomark Prev 2006;15:1929–34. [DOI] [PubMed] [Google Scholar]
- [29].Goto Y, Ando T, Nishio K, et al. The ACE gene polymorphism is associated with the incidence of gastric cancer among H. pylori seropositive subjects with atrophic gastritis. Asian Pac J Cancer Prev APJCP 2005;6:464–7. [PubMed] [Google Scholar]
- [30].Rigat B, Hubert C, Corvol P, et al. PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme gene (DCP1) (dipeptidyl carboxypeptidase 1). Nucleic Acids Res 1992;20:1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shanmugam V, Sell KW, Saha BK. Mistyping ACE heterozygotes. PCR Methods Appl 1993;3:120–1. [DOI] [PubMed] [Google Scholar]
- [32].Zhang D, Zhou Y, Wu L, et al. Association of IL-6 gene polymorphisms with cachexia susceptibility and survival time of patients with pancreatic cancer. Ann Clin Lab Sci 2008;38:113–9. [PubMed] [Google Scholar]
- [33].Daneshmandi S, Pourfathollah AA, Pourpak Z, et al. Cytokine gene polymorphism and asthma susceptibility, progress and control level. Mol Biol Rep 2012;39:1845–53. [DOI] [PubMed] [Google Scholar]
- [34].Lauta VM. Interleukin-6 and the network of several cytokines in multiple myeloma: an overview of clinical and experimental data. Cytokine 2001;16:79–86. [DOI] [PubMed] [Google Scholar]
- [35].Yamaoka Y, Kita M, Kodama T, et al. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology 1996;110:1744–52. [DOI] [PubMed] [Google Scholar]
- [36].Kang JM, Kim N, Lee DH, et al. The effects of genetic polymorphisms of IL-6, IL-8, and IL-10 on Helicobacter pylori-induced gastroduodenal diseases in Korea. J Clin Gastroenterol 2009;43:420–8. [DOI] [PubMed] [Google Scholar]
- [37].Kamangar F, Abnet CC, Hutchinson AA, et al. Polymorphisms in inflammation-related genes and risk of gastric cancer (Finland). Cancer Causes Control CCC 2006;17:117–25. [DOI] [PubMed] [Google Scholar]
- [38].Fernández-Real JM, Broch M, Vendrell J, et al. Interleukin-6 gene polymorphism and lipid abnormalities in healthy subjects. J Clin Endocrinol Metab 2000;85:1334–9. [DOI] [PubMed] [Google Scholar]
- [39].de Oliveira JG, Rossi AFT, Nizato DM, et al. Influence of functional polymorphisms in TNF-(, IL-8, and IL-10 cytokine genes on mRNA expression levels and risk of gastric cancer. Tumour Biol J Int Soc Oncodevelopmental Biol Med 2015;36:9159–70. [DOI] [PubMed] [Google Scholar]
- [40].Santos JC, Ladeira MSP, Pedrazzoli J, et al. Relationship of IL-1 and TNF-( polymorphisms with Helicobacter pylori in gastric diseases in a Brazilian population. Braz J Med Biol Res 2012;45:811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wilschanski M, Schlesinger Y, Faber J, et al. Combination of Helicobacter pylori strain and tumor necrosis factor-alpha polymorphism of the host increases the risk of peptic ulcer disease in children. J Pediatr Gastroenterol Nutr 2007;45:199–203. [DOI] [PubMed] [Google Scholar]
- [42].Yea SS, Yang YI, Jang WH, et al. Association between TNF-alpha promoter polymorphism and Helicobacter pylori cagA subtype infection. J Clin Pathol 2001;54:703–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zabaglia LM, Ferraz MA, Pereira WN, et al. Lack of association among TNF-( gene expression, -308 polymorphism (G>A) and virulence markers of Helicobacter pylori. J Venom Anim Toxins Trop Dis 2015;21:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bauvois B. Transmembrane proteases in cell growth and invasion: new contributors to angiogenesis? Oncogene 2004;23:317–29. [DOI] [PubMed] [Google Scholar]
- [45].Fujita M, Hayashi I, Yamashina S, et al. Blockade of angiotensin AT1a receptor signaling reduces tumor growth, angiogenesis, and metastasis. Biochem Biophys Res Commun 2002;294:441–7. [DOI] [PubMed] [Google Scholar]
- [46].Deshayes F, Nahmias C. Angiotensin receptors: a new role in cancer? Trends Endocrinol Metab TEM 2005;16:293–9. [DOI] [PubMed] [Google Scholar]
- [47].Kupcinskas J, Wex T, Bornschein J, et al. Lack of association between gene polymorphisms of angiotensin converting enzyme, Nod-like receptor 1, Toll-like receptor 4, FAS/FASL and the presence of Helicobacter pylori-induced premalignant gastric lesions and gastric cancer in Caucasians. BMC Med Genet 2011;12:112. [DOI] [PMC free article] [PubMed] [Google Scholar]


