Abstract
We aimed to investigate the diagnostic yield of stool cultures and identify predictive factors for positive cultures in patients with diarrheal illness.
A total of 13,327 patients who underwent stool cultures due to diarrheal illness were reviewed. Stool cultures were performed for enteric pathogens, including Salmonella, Shigella, Vibrio, Klebsiella oxytoca, and Yersinia. The culture-positive group was compared with the culture-negative group who were randomly selected from culture negative patients.
A total of 196 patients (1.47%) were diagnosed with positive stool culture. In 196 culture positive patients, Salmonella spp. (75.0%) was detected most commonly, followed by Vibrio (19.4%). Univariate analyses showed fever (>37.8°C), vomiting, duration and frequency of diarrhea, and high C-reactive protein (CRP) were significantly associated with positive stool culture. Multivariate analysis showed fever (odds ratio [OR], 2.33; 95% confidence interval [CI], 1.25–4.35; P = .008), ≥5/day of diarrhea (OR, 3.52; 95% CI, 1.93–6.44; P < .001) and >50 mg/L of CRP (OR, 2.27; 95% CI, 1.18–4.36; P = .014) were independent predictors for positive stool culture. OR in patients with all 3 factors was 6.55 (95% CI, 2.56–16.75; P < .001). Vomiting (OR, 0.32; 95% CI, 0.17–0.57; P < .001) was a negative predictive factor.
Diagnostic yield of stool culture in patients with diarrheal illness is very low. Fever, frequency of diarrhea, and high CRP are predictors for positive stool cultures. These findings may lead to more discerning and cost-effective utilization of stool culture by clinicians.
Keywords: CRP, diarrhea, stool culture
1. Introduction
Acute diarrheal disease is a common condition encountered in the clinical field worldwide. Acute diarrhea is one of the major causes of morbidity and mortality around the world. There are approximately 2 billion cases of diarrheal disease worldwide every year, and 1.9 million children younger than 5 years of age die from diarrhea each year in developing countries.[1]
In the case of community-acquired diarrhea, infections are caused by environmental enteric bacteria, viruses, or parasites.[2] These are mostly transmitted by contaminated food or water sources, but can be spread via a person to person, fecal-oral route. Diarrhea is usually self-limiting and does not require the use of antibiotics.[3] Regardless of the cause of diarrhea, it is primarily treated by oral and intravenous fluid replacement therapy to restore nutritional defects and dehydration. Although fluid replacement therapy reduces the risk of dehydration and death, it is not effective in shortening the duration of diarrhea and vomiting.[4]
Infection is the most common cause of diarrheal illness; thus, the cornerstone of diagnosis is microbiologic analysis of the stool. Stool analysis can help prevent epidemics and management for severely ill and immunocompromised patients. However, stool cultures have been shown to have poor yield and high costs.[5] In addition, stool culture results take up to several days to become available. Though several guidelines have recommended indications for stool culture to maximize their positive rate, stool culture testing has still been routinely used for most patients with diarrheal illness.[3,4,6]
Previous studies showed 2.4% to 32% positive rates in stool cultures.[7–9] In a study of Pakistan, the positivity of stool culture was 42%, and Vibrio cholerae was found to be the most prevalent organism. They suggested younger age, number of unformed stools, and low serum bicarbonate level as predictors of positive stool culture.[10] However, there are not many studies of this kind, and the causative organisms and the culture positive rates may show regional differences.
We hypothesized that the positive rate of routine stool culture would be low in patients with diarrheal illness in Republic of Korea, and the finding some clinical factors that were associated with positive stool culture would help clinicians be more discerning and cost-effective when ordering stool cultures. The aims of this study were to investigate the positive rates of stool cultures and to determine predictors of positive cultures in patients with diarrheal illness.
2. Materials and methods
2.1. Study setting and population
All patients including children, adolescents, and adults who underwent stool cultures for episodes of diarrheal illness at Chung-Ang University Hospital, from December 2005 to November 2014, were included in the study and their clinical and laboratory data were reviewed. Chung-Ang University Hospital is an 840-bed university-affiliated, tertiary referral hospital in Seoul, Republic of Korea, and built electronic medical record systems from 2005 year.
Acute diarrhea was defined as 3 or more loose or watery stools in a 24-hour period and lasting less than 14 days.[6]
2.2. Stool culture
Stool specimens were collected in aseptic storage bins and strictly handled by standard laboratory procedures. Yield of any enteric pathogens from stool samples was considered to be a positive stool culture. Based on previous studies, we defined enteric pathogens as follows: Aeromonas species (spp.), Klebsiella oxytoca, Salmonella spp., Shigella spp., Vibrio spp. and Yersinia spp. Pathogenic Escherichia coli was excluded.[10–14]
Salmonella spp. and Shigella spp. were routinely examined all year round and Vibrio spp. was examined between May and October every year during the diarrhea epidemic period. MacConkey agar, Salmonella Shigella agar (BBL), and Selenite broth were used for Enterobacteriaceae. Vibrio species was incubated in Thiosulfate citrate bile salts sucrose (TCBS) agar plate. Suspected Yersinia infection was incubated using the Cefsulodin-Irgasan-Novobiocin (CIN) medium and subsequently the antibody test was performed. In the case of significant colony formation, additional serological and biochemical tests were performed and antibiotic susceptibility tests were conducted on the suspected strains.
2.3. Determining predictive factors for positive stool culture
After determining culture positive rate, the characteristics of the culture positive group were compared with those of same number of culture negative group as controls. The control group was randomly selected from patients who underwent stool culture and showed negative culture results after matching age and gender. The demographic characteristics, length of hospital stay, laboratory findings, and frequency and duration of presenting symptoms, including fever, abdominal pain, vomiting, and loose/watery stool, were compared between the 2 groups. Patients were considered to have fever if the temperature recorded was ≥37.8°C during the illness.
The study protocol was approved by the Institutional Review Board of Chung-Ang University Hospital.
2.4. Statistical analyses
To investigate the predictors for positive stool cultures, we compared the characteristics between patients with positive culture and those without. Continuous variables were analyzed by independent sample t-test and expressed as the mean ± standard deviation. The chi-square test or Fisher's exact test was used to compare categorical variables. Factors associated with positive stool culture were analyzed using multivariate logistic regression analysis. For multivariate analysis, we selected variables which were significant in univariate analysis. Odds ratios and 95% confidence intervals were calculated to estimate the relative risk of positive stool culture and their associations with various parameters. SPSS version 18.0 software (SPSS Inc., Chicago, IL) was used for all statistical analyses. A P of less than 0.05 was considered statistically significant.
3. Results
3.1. Stool culture positive rate and bacterial isolation
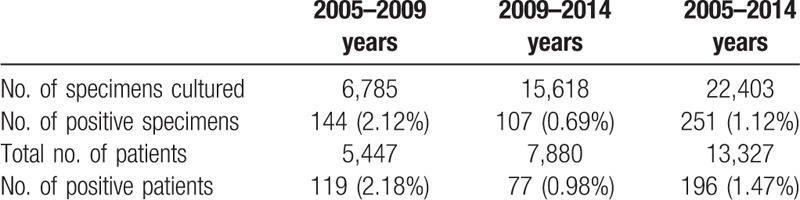
During the study period, a total of 22,403 stool cultures were performed at our hospital from consecutive 13,327 patients [6537 (49.1%) female] with diarrheal illness. Patient mean age was 37.3 ± 32.0 (range, 0–100 years). An average of 1.68 stool cultures per patient was performed. Among those, 251 positive stool cultures from 196 patients were found. The positive stool culture rate was 1.47% (196/13327) excluding duplicated samples in the patients having stool cultures (Table 1).
Table 1.
Stool culture positive rates.
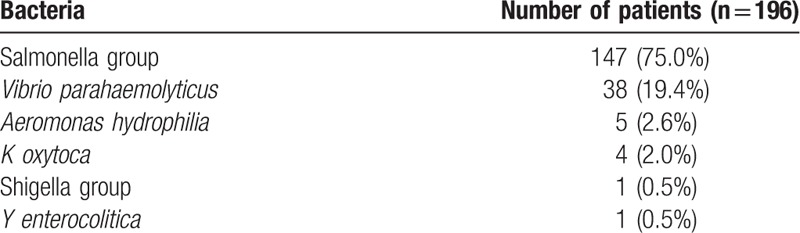
The types of bacterial species documented in 196 patients with positive stool cultures are shown in Table 2. In the 196 culture positive patients, Salmonella spp. (147/196, 75.0%) was detected most commonly followed by Vibrio spp. (38/196, 19.4%), Aeromonas hydrophilia (5/196, 2.55%), K oxytoca (4/196, 2.04%), Shigella sonnei (1/196, 0.51%), and Yersinia enterocolitica (1/196, 0.51%). The distribution of infectious etiologies was also similar in 13 patients in whom diarrhea lasted more than 10 days.
Table 2.
Stool isolates in patients with diarrheal illness.
3.2. Comparison between the stool culture positive and negative groups and the predictors for positive culture
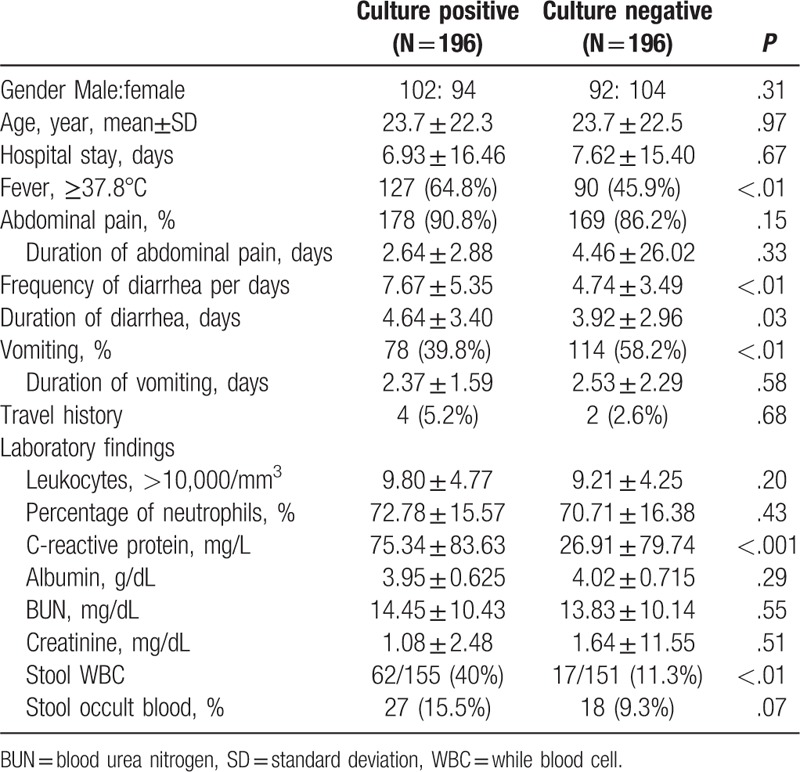
Mean age was 23.7 ± 22.3 (range, 0–87 years) in the positive group and 23.7 ± 22.5 (range, 0–87 years) in the control group. Men represented 52.0% of the positive culture group and 46.9% of the negative culture group. No significant difference in the length of hospital stay was observed between the 2 groups. Travel history was very rare in both groups.
In the univarate analysis, the following were identified as significant factors associated with the isolation of pathogens in patients with diarrheal illness: fever (≥37.8°C) (odds ratio [OR], 2.17; 95% confidence interval [CI], 1.44–3.25; P < .01), frequency of diarrhea (OR, 3.33; 95% CI, 2.20–5.04; P < .01), duration of diarrhea (OR, 1.92; 95% CI, 1.29–2.87; P = .001), vomiting (OR, 0.48; 95% CI, 0.32–0.71; P < .01), high C-reactive protein (CRP, OR, 4.16; 95% CI, 2.36–7.32; P < .01), and stool WBC (OR, 5.26; 95% CI, 2.89–9.56; P < .01) (Table 3).
Table 3.
Demographics and clinical features of stool culture positive and negative patients.
Multivariate logistic regression analysis showed that fever (OR, 2.33; 95% CI, 1.25–4.35; P = .008), ≥5/day of diarrhea (OR, 3.52; 95% CI, 1.93–6.44; P < .001), >50 mg/L of CRP (OR, 2.27; 95% CI, 1.18–4.36; P = .014), and stool WBC (OR, 3.12; 95% CI, 1.49–6.53; P = .003) were independent risk factors associated with positive stool culture (Table 4). The frequency of diarrhea showed the highest odds ratio as a single factor for positive stool culture.
Table 4.
Independent predictors of positive stool culture in multivariate regression analysis.
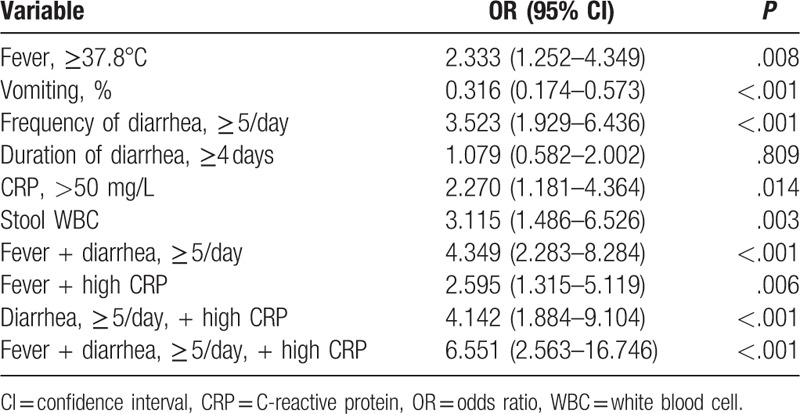
Those patients with 2 or more of the independent factors above had a significantly higher rate of positive stool culture yield. OR for patients who had all 3 factors except stool WBC was 6.55 for positive stool culture (95% CI, 2.56–16.75; P < .001). Vomiting (OR, 0.32; 95% CI, 0.17–0.57; P < .001), on the other hand, was a negative predictive factor (Table 4).
Among the 196 patients with positive stool culture, 172 patients received antibiotics. Fluoroquinolone was most commonly administered as a single agent in 68 adult patients. In pediatric patients, cephalosporine and aminoglycoside were most commonly used. In the control group, 165 patients were administered antibiotics. Likewise, fluroquinolones and cephalosporins were the most common antibiotics prescribed in adult and pediatric patients, respectively. There were no significant differences between the patients with positive culture and those with negative culture in the proportion of patients who received antibiotics (P = .309). All patients from both groups improved without changes in their initial antibiotic treatment. In the 196 patients with positive stool culture, 12 patients were severely ill status (systolic blood pressure < 80 mm Hg and/or serum creatinine>2.0 mg/dL); however, their diarrheal illnesses were also improved by the treatments of empirical antibiotics without changing antibiotics. The number of severely ill patients was 10 in the control group and the proportion was not different from that in the culture positive group.
4. Discussion
Acute diarrheal illness is commonly encountered in the clinical field, which usually has a self-limiting course. When patients develop fever, abdominal pain, and diarrhea, physicians order a stool culture test easily. Recent studies have questioned the value of routine screening culture of all stool specimens in patients with diarrheal illness because the positive yield of a bacterial pathogen is known to be very low and obtaining results takes time. Most cases require no change in treatment even when positive culture results are obtained.[7,11] Thus, stool culture has been treated like a diagnostic tool which causes expense without yielding useful results. These findings were also found in our study. The stool culture positive rate was very low and the treatments were not changed after the culture results. However, the stool culture test can still be important in some clinical situations, especially for critically ill or immunocompromised patients. Appropriate antibacterial treatment is crucial to reduce the mortality rate for those high risk patients. In addition, using empirical antibiotics widely before obtaining culture results may lead to antibiotic resistance.
In our study, the positive culture rate for diarrheagenic bacteria was 1.47%. This is much lower than the results reported in other studies. Previous studies showed 2.4% to 32% positive rates.[7–9] This very low positive rate may be influenced by some reasons: First, important pathogens of infectious diarrhea such as Campylobacter and diarrheagenic E coli were undetected because our hospital does not routinely use the selective medium for these pathogens. Second, younger children who have a high incidence of viral gastroenteritis were included in the present study. Third, some stool samples might have been taken after patients started antibiotics.
The number of specimens for stool culture increased considerably from 6,785 during 2005–2009 to 15,618 during 2010–2014, whereas the ratio of positive specimens decreased from 2.18% (119 cases) to 0.98% (77 cases) in the present study. The low positive rate of bacterial culture in the later 5 years may be explained by an increasing surveillance culture for diarrheal illness accompanied by improvements in public health and medical facilities. In addition, this may be due to the increase of viral or Clostridium difficile infection following the increased use of antibiotics in the later period.
In our study, Salmonella spp. (67.7%) was most commonly detected, followed by Vibrio spp. (25.0%), A hydrophilia (2.55%), K oxytoca (2.04%), S sonnei (0.51%), and Y enterocolitica (0.51%). These results are similar to earlier reports about the etiology of gastroenteritis. Previous studies on the etiology of infectious colitis have reported that Campylobacter spp. were found to be the most frequently cited bacteria (5–20%) in western countries and industrialized nations,[5,15] whereas Cholera continues to pose a threat in underdeveloped Asian[10] and African countries.[16] In South Korea, a multi-center study using the polymerase chain reaction method for detection of Campylobacter jejuni showed a tendency to increase to just about 1.7%.[17] Genetic variance and country-specific environmental exposure might have affected these regional differences. Further studies dealing with these issues are required.
Several guidelines for stool culture testing have been published; however, the physicians’ choice of performing the test is still varied.[6] If we can predict the positivity of stool culture, it will help physicians order stool culture tests appropriately and determine proper management for diarrheal illness. Previous studies suggested several factors, including fever, duration and frequency of loose stool, duration of abdominal pain, and intravenous fluid therapy might predict positive stool culture in adult patients with infectious diarrhea.[15] Some of these factors such as fever, duration, and frequency of diarrhea were found in our data. However, the length of hospital stay, duration of abdominal pain and travel history were not significant factors associated with positive stool culture results in the present study. In the case of vomiting, however, there was a negative relationship with positive stool culture. That may be because vomiting is more common in viral gastroenteritis (e.g., norovirus and rotavirus infection), especially in young children.[18] Of the laboratory findings, CRP and stool WBC also had statistically significant associations with positive stool culture. Previous study suggested that a simple scoring system including clinical presentation and CRP might be useful in predicting the positivity of stool culture and, therefore, could be helpful in targeting patients who require antimicrobial therapy.[12] Another study suggested that procalcitonin (PCT) offered better specificity than CRP for differentiating between viral and bacterial infection etiology with similar sensitivity. PCT also offered better sensitivity and specificity than CRP for differentiating between invasive and noninvasive infection.[19] Our study was not novel one, however, included a large number of patients over 13,000 people and investigated both symptoms and laboratory tests, including CRP as predictors for stool culture. We think that our study results can be used as basic data about the yield of stool culture and the predictors in South Korea that is a developed country in Asia.
Several studies have recommended some strategies to increase the rate of positive stool culture: (1) not performing routine cultures in patients who experience the onset of diarrhea 3 days or more after admission to the hospital,[11,20] (2) not multiple specimens but 1 appropriate specimen, (3) not culturing by smears of rectal swabs, and (4) elimination of stool specimens in patients being treated with antibiotics prior to obtaining a specimen.[21] Our study showed that culture positive rates may be increased by performing stool culture in patients who have symptoms of fever, frequent diarrhea (≥5/day), and/or elevated CRP (i.e., >50 mg/L). The OR for positive stool culture was 6.55 in patients who had all 3 of those factors. Although we could not obtain a definite cut-off value for positive stool cultures for these 3 factors, we suggested that adding these 2 or 3 factors could increase the yield of stool culture. Considering the cost-effectiveness, it would be advisable that stool culture should be performed when at least one of these factors are present.
There are several limitations to this study. First, some clinical data might have been missed in cases due to the study's retrospective design. Second, in the process of culturing bacterial pathogens, different media are required for the growth of certain bacteria. Thus, there could be some undetected pathogens including Campylobacter and diarrheagenic E coli because they are not routinely performed in our hospital unless physicians make a special request. On the other hand, since the number of samples exceeded 13,000 specimens, contamination also might be present in a small number. Third, we could not perform analysis separately by the departments where stool cultures were performed such as inpatients, outpatients, or emergency room. The culture positive rate may be different by department. In spite of these limitations, we consider our data valuable, because our study included a very large sample size, and suggested some good clinical factors that may predict a positive stool culture.
In conclusion, the positive rate for stool culture in patients with diarrheal illness was very low (1.47%). Fever, frequency of diarrhea, and high CRP were the significant and independent predictors associated with positive stool cultures. If these clinical factors are considered when ordering stool culture tests, the positive yield may be higher and laboratories can minimize unnecessary tasks. Our study findings will likely lead to more discerning and cost-effective utilization of stool culture testing by clinicians.
Footnotes
Abbreviations: BUN = blood urea nitrogen, CI = confidence interval, CRP = C-reactive protein, OR = odds ratio, PCR = polymerase chain reaction, SD = standard deviation, Spp. = species, V. cholerae = Vibrio cholerae, WBC = white blood cell.
JYL and SYC contributed equally to this work.
This study was presented as a presidential poster at the annual scientific meeting of American College of Gastroenterology, Las Vegas, October 16 to 18, 2016.
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2017R1D1A1B03031924).
The authors have no conflicts of interest to disclose.
References
- [1].Farthing M, Salam MA, Lindberg G, et al. Acute diarrhea in adults and children: a global perspective. J Clin Gastroenterol 2013;47:12–20. [DOI] [PubMed] [Google Scholar]
- [2].Elliott EJ. Acute gastroenteritis in children. BMJ 2007;35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Guerrant RL, Van Gilder T, Steiner TS, et al. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis 2001;32:331–51. [DOI] [PubMed] [Google Scholar]
- [4].Murphy M. Guidelines for managing acute gastroenteritis based on a systematic review of published research. Arch Dis Child 1998;79:279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jansen A, Stark K, Kunkel J, et al. Aetiology of community-acquired, acute gastroenteritis in hospitalised adults: a prospective cohort study. BMC Infect Dis 2008;8:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Riddle MS, DuPont HL, Connor BA. ACG Clinical Guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol 2016;111:602–22. [DOI] [PubMed] [Google Scholar]
- [7].Rohner P, Pittet D, Pepey B, et al. Etiological agents of infectious diarrhea: implications for requests for microbial culture. J Clin Microbiol 1997;35:1427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chau ML, Hartantyo SHP, Yap M, et al. Diarrheagenic pathogens in adults attending a hospital in Singapore. BMC Infect Dis 2016;16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Koplan JP, Fineberg HV, Ferraro MJ, et al. Value of stool cultures. Lancet 1980;2:413–6. [DOI] [PubMed] [Google Scholar]
- [10].Riaz MM, Patel MJ, Khan MS, et al. Clinical characteristics and predictors of positive stool culture in adult patients with acute gastroenteritis. J Pak Med Assoc 2012;62:20–4. [PubMed] [Google Scholar]
- [11].Kobayashi M, Sako A, Ogami T, et al. Validation of the 3-day rule for stool bacterial tests in Japan. Intern Med 2014;53:533–9. [DOI] [PubMed] [Google Scholar]
- [12].Cadwgan AM, Watson WA, Laing RB, et al. Presenting clinical features and C-reactive protein in the prediction of a positive stool culture in patients with diarrhoea. J Infect 2000;41:159–61. [DOI] [PubMed] [Google Scholar]
- [13].Thia KT-J, Chan ES-Y, Ling K-L, et al. Role of procalcitonin in infectious gastroenteritis and inflammatory bowel disease. Dig Dis Sci 2008;53:2960–8. [DOI] [PubMed] [Google Scholar]
- [14].Chan SSW, Ng KC, Lam PK-w, et al. Predictors of positive stool culture in adult patients with acute infectious diarrhea. J Emerg Med 2002;23:125–30. [DOI] [PubMed] [Google Scholar]
- [15].Wong H, Que T, Ho P. What have we learnt from bacterial stool culture results?: a retrospective study of hospitalised gastroenteritis cases in a regional hospital in Hong Kong. Hong Kong J Emerg Med 2010;17:27. [Google Scholar]
- [16].Sauvageot D, Njanpop-Lafourcade B-M, Akilimali L, et al. Cholera incidence and mortality in Sub-Saharan African sites during multi-country surveillance. PLoS Negl Trop Dis 2016;10:e0004679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lee JK, Kim KY, Koo MS, et al. Detection of Campylobacter jejuni by multiplex PCR and patterns of pulsed-field gel electrophoresis. Korean J Clin Microbiol 2002;5:35–41. [Google Scholar]
- [18].Payne DC, Vinjé J, Szilagyi PG, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med 2017;376:1121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lopez AF, Cubells CL, Garcia JJG, et al. Procalcitonin in pediatric emergency departments for the early diagnosis of invasive bacterial infections in febrile infants: results of a multicenter study and utility of a rapid qualitative test for this marker. Pediatr Infect Dis J 2003;22:895–904. [DOI] [PubMed] [Google Scholar]
- [20].Barbut F, Leluan P, Antoniotti G, et al. Value of routine stool cultures in hospitalized patients with diarrhea. Eur J Clin Microbiol Infect Dis 1995;14:346–9. [DOI] [PubMed] [Google Scholar]
- [21].Chitkara YK. Limited value of routine stool cultures in patients receiving antibiotic therapy. Am J Clin Pathol 2005;123:92–5. [DOI] [PubMed] [Google Scholar]


