Abstract
Background:
Immune and nutritional status of patients have been reported to predict postoperative complications, recurrence, and prognosis of patients with cancer. Therefore, this retrospective study aimed to explore the prognostic value of preoperative inflammation-based prognostic scores [neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR)] and nutritional status [prognostic nutritional index (PNI), body mass index (BMI), hemoglobin, albumin, and prealbumin] for overall survival (OS) in adenocarcinoma of esophagogastric junction (AEG) patients.
Methods:
A total of 355 patients diagnosed with Siewert type II/III AEG and underwent surgery between October 2010 and December 2011 were followed up until October 2016. Receiver operating characteristic (ROC) curve analysis was used to determine the cutoff values of NLR, PLR, and PNI. Kaplan–Meier curves and Cox regression analyses were used to calculate the OS characteristics.
Results:
The ideal cutoff values for predicting OS were 3.5 for NLR, 171 for PLR, and 51.3 for PNI according to the ROC curve. The patients with hemoglobin <120 g/L (P = .001), prealbumin <180 mg/L (P = .000), PNI <51.3 (P = .010), NLR >3.5 (P = .000), PLR >171 (P = .006), and low BMI group (P = .000) had shorter OS. And multivariate survival analysis using the Cox proportional hazards model showed that the tumor-node-metastasis stage, BMI, NLR, and prealbumin levels were independent risk factors for the OS.
Conclusion:
Our study demonstrated that preoperative prealbumin, BMI, and NLR were independent prognostic factors of AEG patients.
Keywords: adenocarcinoma of esophagogastric junction, neutrophil-lymphocyte ratio, overall survival, platelet-lymphocyte ratio, prognostic nutritional index
1. Introduction
Gastric cancer (GC) is the second most prevalent malignant tumors worldwide and has a high mortality.[1] The adenocarcinoma of the esophagogastric junction (AEG), as one of special clinical disease with unique clinicopathological characteristic and biological behavior, was initially proposed in 1999 by Siewert.[2] According to the location of tumor epicenter, Siewert classified AEG into 3 subgroups, type I is esophageal cancer located 1 to 5 cm above the esophagogastric junction (EGJ), which is correlative with the occurrence of gastroesophageal reflux and Barrett esophagus; types II and III AEG are more common than type I and they are mostly treated as GC.[3,4] During recent decades, the incidence and prevalence of AEG were arising globally.[5–7] The reason was still unclear. Despite the improvements in multiple treatment strategies, the prognosis of AEG remains poor.[8]
In the clinic, electronic gastroscope, computed tomography (CT), and endoscopic ultrasonography are important methods to diagnose GC and judge the disease progression, but the value of these techniques are limited by its cost, risk, and inconvenience. Therefore the development of noninvasive and sensitive index that can accurately evaluate the prognosis of GC would be beneficial, among them, immune and nutritional status of patients have been reported to predict postoperative complications, recurrence, and prognosis.[9–12] Related studies have shown that the presence of malnutrition and systematic inflammatory response can cause shorter survival and higher possible complications in patients with malignancy.[13,14] The indexes of systemic inflammatory and nutritional scores include neutrophil-lymphocyte ratio (NLR),[15,16] platelet-lymphocyte ratio (PLR),[17,18] prognostic nutritional index (PNI),[19,20] body mass index (BMI),[21] hemoglobin,[22] albumin,[23–25] and prealbumin,[26,27] and they can get easily and conveniently before surgery. However, the value of these indexes for prediction of overall survival (OS) in AEG patients is still unclear. To our best knowledge, there are few studies that had republished to access the prediction of these indexes for OS in AEG patients.
Therefore, this retrospective study aimed to explore the prognostic value of preoperative inflammation-based prognostic scores (NLR, PLR) and nutritional status (PNI, BMI, hemoglobin, albumin, and prealbumin) for OS in AEG patients.
2. Materials and methods
2.1. Patients
Clinicopathological data of 355 patients, who were diagnosed as Siewert type II/III AEG based on postoperative pathology in the First Affiliated Hospital of Anhui Medical University between October 2010 and December 2011, were analyzed retrospectively in this study; all patients received the preoperative peripheral blood tests, including neutrophil, lymphocyte, platelet, hemoglobin, albumin, and prealbumin. In addition, complete clinicopathological characteristics including age, sex, height, weight, tumor location, differentiation grade, tumor size, invasion depth, lymph node metastasis, the surgery time, and tumor-node-metastasis (TNM) stage were also recorded in our study.
2.2. Inclusion and exclusion criteria
Before surgery, endoscopy and barium swallows were performed to help diagnose Siewert type, CT, and magnetic resonance imaging examinations were used to determine the clinical stage. The eligibility criteria included the following: the clinical information of AEG patients was complete; they had no heart disease or other important organ dysfunction; the need of surgery was definite.
Exclusion criteria from the study included the following conditions: the patient had previous malignant tumors or multiple primary cancers; the patient had received radiotherapy or chemotherapy before the operation; the patients had some disease, which can affect the counting of peripheral blood cells, such as liver cirrhosis, infection, and so on; the patient died within 30 days after surgery in the process of follow-up.
2.3. Treatment and follow-up
The resected specimens were examined by the same group of gastrointestinal pathologists in our hospital. All AEG patients were classified based on the AEG criteria recommended by Siewert (1998), the disease progression was classified using the guidelines outlined in the seventh edition of the American Joint Committee on Cancer (AJCC) about TNM staging.[28]
All Siewert type II/III of AEG patients underwent radical surgery with celiac and mediastinal lymphadenectomy. They accepted 4 to 6 cycles of first-line adjuvant combination chemotherapy after surgery with oxaliplatin plus 5-fluorouracil/leucovorin or a prodrug of 5-fluorouracil (capecitabine; CapeOX), and they were prospectively followed up until October 2016, follow-up results were obtained through reviews of the hospital records and outpatient visit. Follow-up was performed in regular intervals (every 3 months for the first 2 years after treatment, every 6 months in years 3–5, and every 12 months after 5 years). The OS was calculated from the day of surgery to AEG-related death or the end of follow-up.
2.4. Definition of prognostic factors
Peripheral blood tests were obtained within 1 week before surgery, we determine the following indexes (NLR, PLR, BMI, hemoglobin, albumin, prealbumin, and PNI). NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count, PLR was calculated by dividing the absolute palate count by the absolute lymphocyte count, PNI was calculated using the following formula: serum albumin (g/L) + 5 × total lymphocyte count (×109/L).[29] The recommended cutoff values for preoperative NLR, PLR, and PNI were determined using receiver operating characteristic (ROC) curve analysis based on the Youden index [maximum (sensitivity + specificity − 1)].[30] The BMI was calculated from preoperative height and weight, BMI was divided into 3 groups: <18.5 (low group), 18.5 to 24.9 (normal group), and ≥25 (high group). According to clinical standard, the cut-off values of albumin and prealbumin were set at 40 g/L and 180 mg/L, respectively. The surgery time was divided into 2 groups according to the median value.
The new scores model (including BMI, prealbumin, and NLR) was calculated as follows (Table 1).
Table 1.
The new scores model.

2.5. Statistical analysis
All statistical analyses were conducted using SPSS software (version 16.0). For analysis of survival data, Kaplan–Meier curves were constructed, and statistical analysis was carried out using the log-rank test. Both univariate and multivariate survival analyses were performed using the Cox proportional hazard model. For all 2-sided tests, a P value of <.05 was considered to be statistically significant.
2.6. Ethics statement
This study was approved by the Ethics Committee of The First Affiliated Hospital of Anhui Medical University. Written informed consent was obtained from each patient.
3. Results
3.1. Patients’ characteristics
A total of 467 patients were initially enrolled, and 355 cases were included in the final analysis based on the eligibility criteria. Based on the cut-off values of NLR, PLR, and PNI, the patients were divided into low- and high-level groups for further analysis. Overall, 281 (79.2%) patients were men and 74 (20.8%) patients were women. The median age of patients was 64 years (range, 34–82). The median follow-up month was 52 (range, 1–81).
3.2. The cutoff values of prognostic factors
The predictive ability of prognostic factors for OS was compared by ROC curves (Figs. 1 and 2). Using the ROC curve, we determined the recommended cutoff value of NLR, PLR, and PNI was 3.5 [area under the curve (AUC), 0.585; P = .006], 171 (AUC, 0.565; P = .034), and 51.3 (AUC, 0.573; P = .017), respectively.
Figure 1.
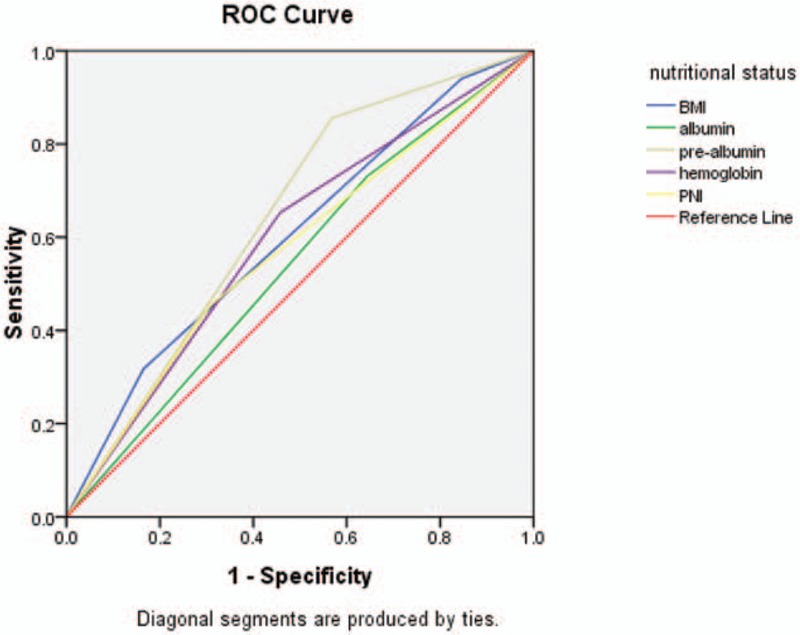
The ROC curve of nutritional status (BMI, albumin, prealbumin, hemoglobin, and PNI). BMI = body mass index, PNI = prognostic nutritional index, ROC = Receiver operating characteristic.
Figure 2.

The ROC curve of inflammation-based scores (NLR and PLR). NLR = neutrophil-lymphocyte ratio, PLR = platelet-lymphocyte ratio, ROC = Receiver operating characteristic.
3.3. The Kaplan–Meier survival curves of patients
The Kaplan–Meier survival curves for patients in high BMI group, normal BMI group, and low BMI group (Fig. 3); high NLR group and low NLR group (Fig. 4); high PLR group and low PLR group (Fig. 5); high PNI group and low PNI group (Fig. 6); high albumin group and low albumin group (Fig. 7); high prealbumin group and low prealbumin group (Fig. 8); and high hemoglobin group and low hemoglobin group (Fig. 9) are showed as follows. The patients with hemoglobin <120 g/L (P = .001), prealbumin <180 mg/L (P = .000), PNI <51.3 (P = .010), NLR >3.5 (P = .000), PLR >171 (P = .006), and low BMI group (P = .000) had shorter OS.
Figure 3.

The Kaplan–Meier survival curves of BMI. BMI = body mass index.
Figure 4.

The Kaplan–Meier survival curves of NLR. NLR = neutrophil-lymphocyte ratio.
Figure 5.
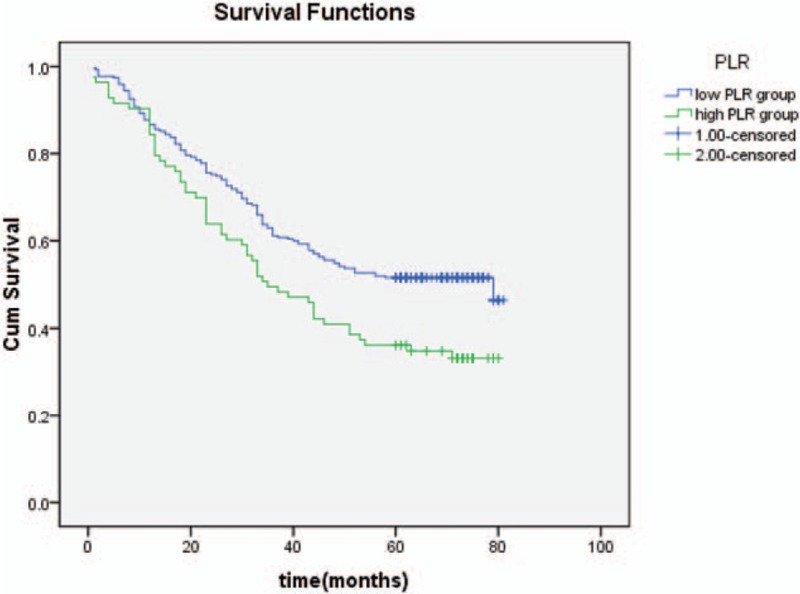
The Kaplan–Meier survival curves of PLR. PLR = platelet-lymphocyte ratio.
Figure 6.
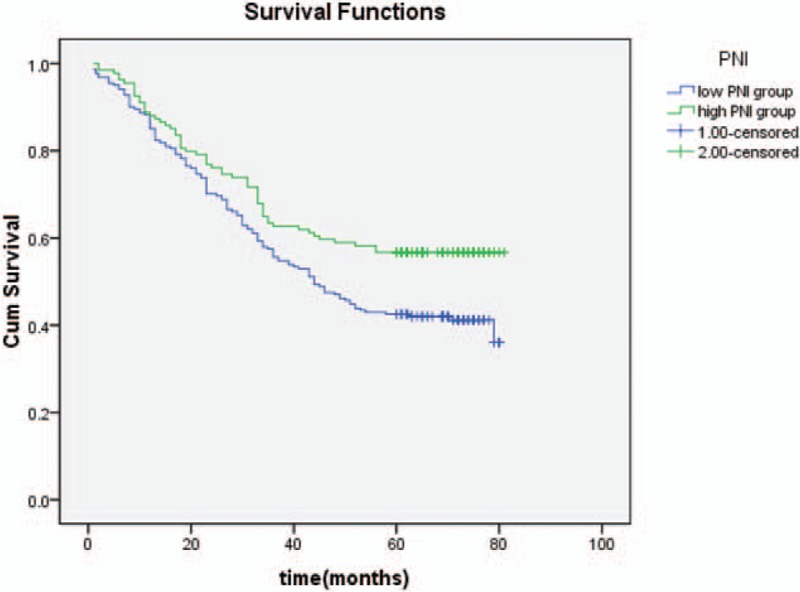
The Kaplan–Meier survival curves of PNI. PNI = prognostic nutritional index.
Figure 7.

The Kaplan–Meier survival curves of albumin.
Figure 8.

The Kaplan–Meier survival curves of prealbumin.
Figure 9.
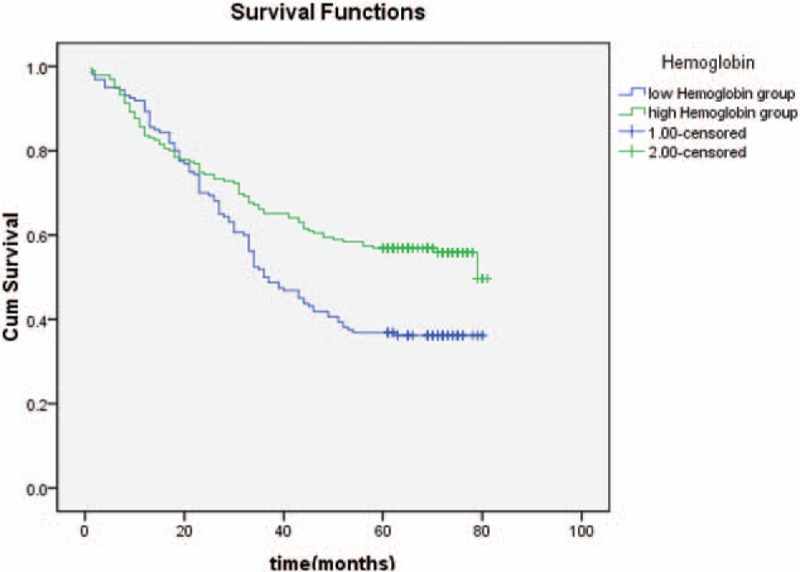
The Kaplan–Meier survival curves of hemoglobin.
3.4. The risk factors for overall survival
As indicated by a univariate analysis, hemoglobin, BMI, prealbumin, PNI, NLR, PLR, TNM, tumor size, and differentiation grade significantly impacted the OS (Table 2). And multivariate survival analysis using the Cox proportional hazards model showed that the TNM stage, BMI, NLR, and prealbumin levels were independent risk factors for the OS (Table 3).
Table 2.
The univariate analyses of prognostic factors for OS in AEG patients.

Table 3.
Multivariate analyses of prognostic factors for OS in AEG patients.

4. Relationship of baseline NLR, prealbumin, and BMI levels with clinicopathologic characteristics
The association is shown in Table 4. The serum NLR was significantly correlated with tumor size (P = .011); the BMI was significantly with surgery time (P < .05); the associations between prealbumin and tumor size (P < .001), differentiation grade (P < .05), and TNM stage (P < .05) were significant.
Table 4.
Association of the patients’ characteristics with NLR, BMI, and prealbumin.

5. Predictive value of the new scores model
To further evaluate the prognostic values of the prognostic scores, ROC analysis was performed (Figs. 10 and 11). The new scores model had a higher AUC value (0.712; P = .000) than BMI (AUC, 0.604; P = .001), prealbumin (AUC, 0.644; P = .000), NLR (AUC, 0.585; P = .006), and TNM (AUC, 0.673; P = .000).
Figure 10.

The ROC of new scores model and independent risk factors (BMI and prealbumin) of overall survival in adenocarcinoma of esophagogastric junction (AEG) patients. BMI = body mass index, ROC = receiver operating characteristic.
Figure 11.

The ROC of independent risk factors (NLR and TNM) of overall survival in adenocarcinoma of esophagogastric junction (AEG) patients. NLR = neutrophil-lymphocyte ratio, ROC = Receiver operating characteristic, TNM = tumor-node-metastasis.
6. Discussion
Adenocarcinoma of the esophagogastric junction (AEG), which located within 5 cm from EGJ, was classified into 3 subgroups. Type I AEG (adenocarcinoma of the distal esophagus) is the most prevalent type in Western countries, types II (adenocarcinoma of the cardiac) and III AEG (subcardial adenocarcinoma) are commoner than type I in Asia. In a few decades, there has been an alarming rise in the incidence of AEG, but the etiological factor is not clear. Radical resection with D2 lymph node cleaning remains the only potentially curative treatment option, the overall 5 years survival rates of locally advanced AEG was poor.[31,32] Immune and nutritional status had gradually become the focus in the field of predicting prognosis of patients with cancer nowadays.[33] The aim of our retrospective study was to compare the prognostic value of inflammation-based prognostic scores (NLR, PLR) and nutritional status (BMI, hemoglobin, PNI, albumin, and prealbumin) for OS in AEG patients.
In recent years, the study about the relationship between the tumor and inflammation has been developed, the inflammation caused by tumors can lead to DNA damage, out control of cell cycle, and micrometastasis lesions.[34] Preoperative NLR and PLR detected in peripheral blood are simple inflammatory markers, which have been identified in various malignancies, including colon cancer, esophageal cancer, ovarian cancer, lung cancer, and breast cancer.[35–39] But few studies about the relationship between the value of NLR and PLR with prognosis of AEG patients have been published. This large retrospective evidence showed that NLR was an independent prognostic factor in patients with nonmetastatic Siewert type II/III AEG, whereas PLR had limited significance. Considering the interactions of inflammation-based scores for survival outcomes, ROC curve was constructed to estimate their discrimination ability (Fig. 2). The AUC of NLR was 0.585 [95% confidence interval (CI) 0.526–0.644]; the AUC of PLR was 0.565 (95% CI 0.506–0.625); therefore, the performance abilities of NLR may be more significant than PLR for predicting OS of AEG patients. The high NLR and PLR group of AEG patients have a poor prognosis, and the NLR was significantly related with the tumor size (P < .05), so the inflammation indexes may have a connection with pathological progression of tumors. Thus we may delay the tumors’ progression through anti-inflammatory treatment, but the conclusion need more experiments to prove.
Another index for assessing nutritional status and systematic inflammatory response was BMI. Obesity is showing a rising trend in the worldwide with the improvement of living standards[40]; it had been reported that the BMI was associated with the surgical outcomes in colorectal cancer, pancreatic cancer, liver cancer, and so on.[41–45] This study suggested that BMI was not related with the pathological features of tumor; however, BMI had a significant value with surgery time (P < .05). Patients with a normal level of BMI had a longer OS than the low-level group in AEG patients, and BMI was also an independent factor for predicting OS. Related studies have shown that low BMI was associated with postoperative pulmonary infection and other complications.[46] Postoperative complications of surgery are still the most troublesome problems, which can lead to longer hospital stay and increased medical expenses; thus, if we can provide potential nutrition support before surgery, the low preoperative BMI concentrations of patients may be modified, which can reduce the risk of postoperative complications. Some studies showed that higher BMI of the patients (BMI ≥25) would bring longer operative time and fewer lymph nodes,[47] our study was consistent with this. The patients with obesity need proper exercise, which may have some help to their surgery outcome.[48] The patients with a higher level of BMI had a longer OS. Some experiments showed that low PNI combined with low BMI accurately predicted poorer outcome[49]; the main mechanism is that the higher BMI patients may have a higher nutritional level to withstand the occurrence of perioperative complications.
Studies have showed that serum albumin is related with the survival of patients[23,50,51]; however, prealbumin is a 54 kDa protein, which synthesized in the liver has a shorter half-life (nearly 2 days) than albumin (nearly 20 days), so prealbumin is affected earlier in acute protein change especially during the perioperative period. Currently, prealbumin became the research focus as a serum index for assessment of nutritional status. In this study, we explored the prognostic value of prealbumin in AEG patients, we concluded that prealbumin was an independent factor for predicting postoperative survival outcomes, and ROC curve was constructed to estimate its discrimination ability with albumin (Fig. 1). The AUC of prealbumin was 0.644 (95% CI 0.586–0.701) and the AUC of albumin was 0.543 (95% CI 0.484–0.603); therefore, prealbumin may have a more sensitive value for predicting OS of AEG patients than albumin. What's more, from the relationship of baseline prealbumin levels and clinicopathologic characteristics, we can know that the serum prealbumin was significantly correlated with tumor size (P < .001) and TNM stage (P < .05), so the prealbumin may have a significant value to determine the progression of AEG patients.
The Kaplan–Meier survival curves revealed that AEG patients with hemoglobin <120 g/L (P = .001), prealbumin <180 g/L (P = .000), low BMI group (P = .000), PNI < 51.3 (P = .010), NLR >3.5 (P = .000), PLR >171 (P = .006) had shorter OS; thus, we can make comprehensive assessment with patients before surgery, and then using different therapies. For example, we can take radio or chemotherapy or provide potential nutrition support before surgery to improve the prognosis of these patients to achieve better treatment effect, and these patients also need more attention to care and follow-up after surgery.
Our study has determined the value of NLR, BMI, and prealbumin to be comparable to TNM stage, were independent prognostic factors of AEG patients, so these indexes should be included in the routine assessment of AEG patients. These markers in combination may effectively provide individualized prognostic support for AEG patients.
In addition, we constructed a new score model to better evaluate the prognosis of AEG patients. Multivariate survival analysis showed that the TNM stage, BMI, NLR, and prealbumin levels were independent risk factors for the overall survival. Considering that the pTNM stage can only be evaluated in specimens after surgery, so the TNM stage was excluded from this score model. We can see the new scores model had a higher AUC value (0.712; P = .000) than BMI, NLR, TNM, and prealbumin, showing a reliable discrimination ability to act as a prognostic index of AEG patients. This index is also simple and inexpensive to get before surgery; it may benefit a lot to our patients.
There are some potential limitations of this study. Firstly, other potential confounding factors including specific tumor markers and inflammatory markers that are not included in this study, besides, there might be other reasonable cut-off levels for each ratio.
To our knowledge, this is the first study about the prognostic value of preoperative inflammation-based prognostic scores (NLR, PLR) and nutritional status (PNI, BMI, hemoglobin, albumin, and prealbumin) for OS in AEG patients and we found that the patients with a high level of albumin, pre-albumin, hemoglobin, PNI, BMI, and low NLR, PLR level had longer OS. Moreover, our study further demonstrated that preoperative prealbumin, BMI, and NLR were independent prognostic factors of AEG patients and the new scores model may serve as a reliable index to evaluate the prognosis of GC patients.
Footnotes
Abbreviations: AEG = adenocarcinoma of esophagogastric junction, AUC = area under the curve, BMI = body mass index, CT = computed tomography, EGJ = esophagogastric junction, GC = gastric cancer, NLR = neutrophil-lymphocyte ratio, OS = overall survival, PLR = platelet-lymphocyte ratio, PNI = prognostic nutritional index.
LZ and YS contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (No.: 81572350, to Prof. A-Man Xu).
The authors report no conflicts of interest.
References
- [1].Naghavi M. The global burden of cancer. JAMA Oncol 2015;1:505–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Siewert JR. Adenocarcinoma of the esophago-gastric junction. Gastric Cancer 1999;2:87–8. [DOI] [PubMed] [Google Scholar]
- [3].Fang WL, Wu CW, Chen JH, et al. Esophagogastric junction adenocarcinoma according to Siewert classification in Taiwan. Ann Surg Oncol 2009;16:3237–44. [DOI] [PubMed] [Google Scholar]
- [4].Huang Q, Fan X, Agoston AT, et al. Comparison of gastrooesophageal junction carcinomas in Chinese versus American patients. Histopathology 2011;59:188–97. [DOI] [PubMed] [Google Scholar]
- [5].Steevens J, Botterweck AA, Dirx MJ, et al. Trends in incidence of oesophageal and stomach cancer subtypes in Europe. Eur J Gastroenterol Hepatol 2010;22:669–78. [DOI] [PubMed] [Google Scholar]
- [6].Deans C, Yeo MS, Soe MY, et al. Cancer of the gastric cardia is rising in incidence in an Asian population and is associated with adverse outcome. World J Surg 2011;35:617–24. [DOI] [PubMed] [Google Scholar]
- [7].Kusano C, Gotoda T, Khor CJ, et al. Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan. J Gastroenterol Hepatol 2008;23:1662–5. [DOI] [PubMed] [Google Scholar]
- [8].Hosoda K, Yamashita K, Katada N, et al. Overview of multimodal therapy for adenocarcinoma of the esophagogastric junction. Gen Thorac Cardiovasc Surg 2015;63:549–56. [DOI] [PubMed] [Google Scholar]
- [9].Meguid MM, Mughal MM, Debonis D, et al. Influence of nutritional status on the resumption of adequate food intake in patients recovering from colorectal cancer operations. Surg Clin North Am 1986;66:1167–76. [DOI] [PubMed] [Google Scholar]
- [10].Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 2014;10:1–7. [PubMed] [Google Scholar]
- [11].Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984;85:1001–5. [PubMed] [Google Scholar]
- [12].Gomes de Lima KV, Maio R. Nutritional status, systemic inflammation and prognosis of patients with gastrointestinal cancer. Nutr Hosp 2012;27:707–14. [DOI] [PubMed] [Google Scholar]
- [13].Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr 2002;56:779–85. [DOI] [PubMed] [Google Scholar]
- [14].Crumley ABC, McMillan DC, McKernan M, et al. An elevated C-reactive protein concentration, prior to surgery, predicts poor cancer-specific survival in patients undergoing resection for gastro-oesophageal cancer. Br J Cancer 2006;94:1568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ying HQ, Deng QW, He BS, et al. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol 2014;31:305. [DOI] [PubMed] [Google Scholar]
- [16].Azab B, Shah N, Radbel J, et al. Pretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patients. Med Oncol 2013;30:432. [DOI] [PubMed] [Google Scholar]
- [17].Smith RA, Bosonnet L, Raraty M, et al. Preoperative platelet–lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg 2009;197:466–72. [DOI] [PubMed] [Google Scholar]
- [18].He W, Yin C, Guo G, et al. Initial neutrophil lymphocyte ratio is superior to platelet lymphocyte ratio as an adverse prognostic and predictive factor in metastatic colorectal cancer. Med Oncol 2013;30:439. [DOI] [PubMed] [Google Scholar]
- [19].Kasymjanova G, MacDonald N, Agulnik JS, et al. The predictive value of pre-treatment inflammatory markers in advanced non-small-cell lung cancer. Curr Oncol 2010;17:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow inflammation outcome study. Eur J Cancer 2011;47:2633–41. [DOI] [PubMed] [Google Scholar]
- [21].Cespedes Feliciano EM, Kwan ML, Kushi LH, et al. Body mass index, PAM50 subtype, recurrence and survival among patients with nonmetastatic breast cancer. Cancer 2017;123:2535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Caro JJ, Salas M, Ward A, et al. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer 2001;91:2214–21. [PubMed] [Google Scholar]
- [23].Bountziouka V, Panagiotakos BD. Statistical methods used for the evaluation of reliability and validity of nutrition assessment tools used in medical research. Curr Pharm Des 2010;16:3770–5. [DOI] [PubMed] [Google Scholar]
- [24].Suh B, Park S, Shin DW, et al. Low albumin-to-globulin ratio associated with cancer incidence and mortality in generally healthy adults. Ann Oncol 2014;25:2260–6. [DOI] [PubMed] [Google Scholar]
- [25].Kinoshita A, Onoda H, Imai N, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol 2015;22:803–10. [DOI] [PubMed] [Google Scholar]
- [26].Byström P, Berglund A, Nygren P, et al. Evaluation of predictive markers for patients with advanced colorectal cancer. Acta Oncologica 2012;51:849–59. [DOI] [PubMed] [Google Scholar]
- [27].Kelly P, Paulin F, Lamont D, et al. Pre-treatment plasma proteomic markers associated with survival in oesophageal cancer. Br J Cancer 2012;106:955–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 2010;17:3077–9. [DOI] [PubMed] [Google Scholar]
- [29].Nozoe T, Ninomiya M, Maeda T, et al. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today 2010;40:440–3. [DOI] [PubMed] [Google Scholar]
- [30].Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- [31].Hulscher JB, Van Sandick JW, De Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662–9. [DOI] [PubMed] [Google Scholar]
- [32].Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007;246:992–1000. [DOI] [PubMed] [Google Scholar]
- [33].Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 2014;20:6212–22. [DOI] [PubMed] [Google Scholar]
- [34].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- [35].Walsh SR, Cook EJ, Goulder F, et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 2005;91:181–4. [DOI] [PubMed] [Google Scholar]
- [36].Sharaiha RZ, Halazun KJ, Mirza F, et al. Elevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol 2011;18:3362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Thavaramara T, Phaloprakarn C, Tangjitgamol S, et al. Role of neutrophil to lymphocyte ratio as a prognostic indicator for epithelial ovarian cancer. J Med Assoc Thai 2011;94:871–7. [PubMed] [Google Scholar]
- [38].Kemal Y, Yucel I, Ekiz K, et al. Elevated serum neutrophil to lymphocyte and platelet to lymphocyte ratios could be useful in lung cancer diagnosis. Asian Pac J Cancer Prev 2014;15:2651–4. [DOI] [PubMed] [Google Scholar]
- [39].Azab B, Bhatt VR, Phookan J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol 2012;19:217–24. [DOI] [PubMed] [Google Scholar]
- [40].Yanovski SZ, Yanovski JA. Obesity prevalence in the United States—up, down, or sideways? N Engl J Med 2011;364:987–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Amri R, Bordeianou LG, Sylla P, et al. Obesity, outcomes and quality of care: body mass index increases the risk of wound-related complications in colon cancer surgery. Am J Surg 2014;207:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Balentine CJ, Wilks J, Robinson C, et al. Obesity increases wound complications in rectal cancer surgery. J Surg Res 2010;163:35–9. [DOI] [PubMed] [Google Scholar]
- [43].Santoso JT, Barton G, Riedley-Malone S, et al. Obesity and perioperative outcomes in endometrial cancer surgery. Arch Gynecol Obstet 2012;285:1139–44. [DOI] [PubMed] [Google Scholar]
- [44].Tsai S, Choti MA, Assumpcao L, et al. Impact of obesity on perioperative outcomes and survival following pancreaticoduodenectomy for pancreatic cancer: a large single-institution study. J Gastrointest Surg 2010;14:1143–50. [DOI] [PubMed] [Google Scholar]
- [45].Bhayani NH, Hyder O, Frederick W, et al. Effect of metabolic syndrome on perioperative outcomes after liver surgery: a National Surgical Quality Improvement Program (NSQIP) analysis. Surgery 2012;152:218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wightman SC, Posner MC, Patti MG, et al. Extremes of body mass index and postoperative complications after esophagectomy. Dis Esophagus 2017;30:1–6. [DOI] [PubMed] [Google Scholar]
- [47].Bickenbach KA, Denton B, Gonen M, et al. Impact of obesity on perioperative complications and long-term survival of patients with gastric cancer. Ann Surg Oncol 2013;20:780–7. [DOI] [PubMed] [Google Scholar]
- [48].Cho H, Yoshikawa T, Oba MS, et al. Matched pair analysis to examine the effects of a planned preoperative exercise program in early gastric cancer patients with metabolic syndrome to reduce operative risk: the Adjuvant Exercise for General Elective Surgery (AEGES) study group. Ann Surg Oncol 2014;21:2044–50. [DOI] [PubMed] [Google Scholar]
- [49].Ji F, Liang Y, Fu S, et al. Prognostic value of combined preoperative prognostic nutritional index and body mass index in HCC after hepatectomy. HPB (Oxford) 2017;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [50].Suh B, Park S, Shin DW, et al. Low albumin-to-globulin ratio associated with cancer incidence and mortality in generally healthy adults. Ann Oncol 2014;25:2260–6. [DOI] [PubMed] [Google Scholar]
- [51].Kinoshita A, Onoda H, Imai N, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol 2015;22:803–10. [DOI] [PubMed] [Google Scholar]


