Abstract
Ferulic acid (FA) is a derivative of cinnamic acid. It is used in the treatment of heart head blood-vessel disease and exerts protective effects against hypoxia/ischemia-induced cell injury in the brain. This study investigated the potential neuroprotective effects of FA against ischemia/reperfusion (I/R)-induced brain injury in vivo and in vitro through hematoxylin and eosin (H&E) and Nissl staining assays, flow cytometry, Hoechst 33258 staining, quantitative PCR, western blot analysis and fluorescence microscopic analysis. In this study, models of cerebral I/R injury were established using rats and pheochromocytoma (PC-12) cells. The results revealed that treatment with FA significantly attenuated memory impairment, and reduced hippocampal neuronal apoptosis and oxidative stress in a dose-dependent manner. The results from in vitro experiments also indicated that FA protected the PC-12 cells against I/R-induced reactive oxygen species (ROS) generation and apoptosis by inhibiting apoptosis, Ca2+ influx, superoxide anion (O2−), malondialdehyde (MDA) and glutathione peroxidase (GSH-Px) production in a concentration-dependent manner. Moreover, FA inactivated the Toll-like receptor (TLR)/myeloid differentiation factor 88 (MyD88) pathway. MyD88 overexpression abolished the neuroprotective effects of FA. On the whole, we found that FA attenuated memory dysfunction and exerted protective effects against oxidative stress and apoptosis induced by I/R injury by inhibiting the TLR4/MyD88 signaling pathway. This study supports the view that FA may be a promising neuroprotective agent for use in the treatment of cerebral ischemia.
Keywords: ferulic acid, neuroprotective effect, ischemia/reperfusion, oxidation, apoptosis
Introduction
Ferulic acid (4-hydroxy-3-methoxycinnamic acid, FA), a constituent isolated from Ferula foetida, is one of many of the main active ingredients of traditional Chinese medicine (TCM) used to promote blood circulation, and is also an effective constituent of some Chinese medicinal herbs, such as Ligusticum striatum (chuanxiong) (1). Chuanxiong has been extensively used in TCM in recent years. Pharmacological studies have suggested that FA exerts antioxidant (2), free radical-scavenging (3), anti-thrombotic (4), hematic fat lowering (5), antibacterial (6), antiviral (7), anti-mutagenesis and anticancer effects (8) and has been used in the prevention and treatment of coronary heart disease (9). It has also been reported that FA exerts anti-inflammatory and antioxidant effects in some cerebral models (10).
Cerebral ischemia/reperfusion injury (IRI), is characterised by an insufficient oxygen supply and restoration of blood flow, and involves complex and multi-factorial mechanisms, and can resulted in irreversible damage to tissue (11). IRI is one of the is a threat to human health and survival and is associated with a high morbidity. IRI often contributes to the interruption of blood flow, and leads to hypoxia and hypoglycemia, subsequently promoting neuronal cell death (12,13). There is increasing evidence to suggest that cerebral IRI leads to neurological deficits, including learning and memory impairment (14–17). Cerebral IRI can induce oxidative stress, which is characterized by decreased speroxide dismutase (SOD) and glutathione peroxidase (GSH-Px) levels, and increased malondialdehyde (MDA) levels (18). Hippocampal neurogenesis is involved in memory and learning (19). Pro-inflammatory cytokines released in the periphery and neuronal apoptosis induced by cerebral IRI may exert potential damage to the hippocampal cells, leading to learning and memory impairment (20,21).
Neurons are vulnerable to hypoxia and hypoglycemia. Cerebral ischemia induces involves types of biochemical mechanisms which can damage nervous system function via the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) (22). ROS have been shown to be involved in nervous system dysfunction and brain disorders. Following brain injury, cellular functions are impaired by the increased production of free radicals, which are produced through many different cellular signal pathways (23). To investigate the mechanisms involved in neuronal cell death induced by ischemic injury and identify potential protective components, a cell model of ischemia using PC-12 cells has been used in vitro (24). Oxidative stress and mitochondrial dysfunction are known to induce the activation of the apoptotic cascade (25). Thus, the regulation of intracellular ROS and the Ca2+ influx level may be necessary for the suppression of pathological apoptosis in cerebral ischemia. Oxidative stress induced by ischemia is also involved in apoptosis. It has been shown that oxidative stress can promote the activation of caspases, subsequently inducing apoptosis (26.27). Bcl-2 anti-apoptotic family proteins, including pro-apoptotic (Bax and Bad) and anti-apoptotic (Bcl-2 and Bcl-xL) proteins, play an important role in apoptosis (28). Previous studies have suggested that apoptosis and necrosis are characterized by neuronal death during cerebral ischemia/reperfusion-induced injury (29,30). ROS attack neuronal components and finally induce apoptosis (31). The balance between anti-apoptotic Bcl-2 and pro-apoptotic Bax plays a key role in the progression of cerebral IRI (32). IRI is associated with the accumulation of ROS and apoptosis. Extended periods of ischemia can result in significant cellular dysfunction and cell death. FA has been shown to exert anti-inflammatory and antioxidant effects and can be regarded as an eliminator of free radicals (33,34).
Toll-like receptors (TLRs) also play a important role in cerebral IRI through the induction of inflammatory responses (35,36). The pathogenic role of TLRs in cerebral IRI processes was firstly implied in a mouse model of IRI, where it was found that mice lacking functional TLR4 had reduced IRI compared to wild-type mice (37). It has been reported that TLR2 can promote cellular dysfunction after ischemia, and TLR2 is also shown to aggravate ischemic damage in a myeloid differentiation factor 88 (MyD88)-dependent and/or -independent manner (38). In recent years, TLR activation has been shown to play a crucial role in IRI in the kidneys, gut and liver (39–41). TLR4 is a transmembrane receptor protein, which has a homology domain of intracellular Toll/interleukin-1 receptor (TIR). The TIR domain can interact with the adapter protein, which is named MyD88. It has been reported that MyD88-dependent signaling plays a crucial role in the pathogenesis of acute lung inflammation and injury followng ischemia and reperfusion (42). It can also promote inflammatory responses induced by IRI in mice (43). Thus, we wished to examine the association between the protective effects of FA and the TLR4/MyD88 signaling pathway.
In the past, a few studies have reported that FA attenuates IRI in the brain both in vitro and in vivo. However, the underlying molecular mechanisms remain unclear. The association between FA and IRI-induced learning and memory impairment has not been reported to date, at least to the best of our knowledge. Thus, the aim of this study was to investigate the effects of FA on IRI-induced learning and memory impairment, and to elucidate the potential mechanisms involved as regards oxidative stress and apoptosis. Our results demonstrate that FA exerts protective effects against the impairment of learning and memory induced by IRI in vivo and in vitro via antioxidant and anti-apoptotic mechanisms. Our data may provide a possible therapeutic approach with which to prevent or suppress IRI and FA may be have potential for use as a neuroprotective agent in the treatment and/or preventio of cerebral ischemia.
Materials and methods
Ethics statement
All animal experiments were approved by the Ethics Committee of Kunming Medical University [no. SYXK (dian) K2015-0002], in accordance with the animal ethical guidelines of the Chinese National Health and Medical Research Council (NHMRC).
Animals and model of cerebral ischemia/reperfusion
Male Sprague-Dawley rats (7–8 weeks old) weighing 220±20 g were purchased from the Experimental Animal Center of Kunming Medical University, Kunming, China. After anesthesia, the rats were fixed on a table, and a midline incision was made on the neck, exposing the bilateral common carotid artery (CCA) and the vagus nerve. The CCA and vagus nerve were then separated and the bilateral CCA was blocked with a vascular clamp clip for 20 min. The bilateral vascular clamp was then loosened, allowing for the recovery of blood flow. The rats were divided into 5 groups with 10 rats in each. FA was obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). The rats in 3 of these groups received various doses of FA (28, 56 and 112 mg/kg; FA-L, FA-M and FA-H groups, respectively) after ischemia for 5 consecutive days, and one group was left untreated after ischemia. The rats in the sham-operated group were treated with saline and were not subjected to any surgery. After treatment for 5 days, samples in all groups are collect for the following tests.
Passive avoidance test
The passive avoidance test (44) was performed with the Gemini Avoidance system (San Diego Instruments, San Diego, CA, USA). The test included two identical chambers (25×20×17 cm) connected by a gate (9×7 cm); 14 stainless steel rods (6 mm in diameter) were included on the floor of each chamber with an interval of 1.8 cm, connected to a shock scrambler. The passive avoidance test was performed as follows:
Habituation: 1 day prior to being subjected to cerebral IRI, the rats were placed in the bright room of this system; 10 sec later, the right chamber was illuminated, and the gate opened simultaneously. Once the rats entered the left chamber (dark room), the gate closed instantaneously. After 30 sec, the rats were shifted from the dark room to the rat cage. If the rats stayed in the bright room for >100 sec, they were excluded from the experiment.
Training: this was carried out 1 h prior to IRI. The procedure was similar to that of the habituation session. After the rats entered the dark room, the gate closed immediately, intermittent electric shocks (50 Hz, 2 sec, 0.5 mA), and the number of training sessions was recorded. After 10 sec, the rats were transferred back to the cage. The latency was then recorded if the rats did not enter the dark room. After 2 min, the aforementioned process was repeated once, and the longest latency (in seconds) that occurred first was selected. The process was repeated again after 2 min.
Retention trial: The procedure was similar to that of the training session. The time for which the rats stayed in the bright room was recorded. The retention trial was performed once a day during 3 days after reperfusion.
Morris water maze task
The standard Morris water maze task was used to evaluate the ability of hippocampal-dependent spatial navigation learning and memory in rats (45). The water maze was a 120-cm circular pool, filled 45 cm deep with 26°C water. The trials were video-recorded and scored for measures including the latency of finding the hidden platform. Rats were trained in the hidden platform of the Morris water maze system in a consistent manner. If a rat spent >20 sec to find the hidden platform, it was excluded from the experiment. The retention trial was performed once at 24 h after reperfusion and on at 7 days after reperfusion.
Hematoxylin and eosin (H&E) and Nissl staining
A total of 5 rats from each group were used for H&E and Nissl staining. After being anesthetized with choral hydrate (300 mg/kg), the rats were perfused with 0.9% NaCl and the brain tissues were removed and fixed with 4% formaldehyde. The brain tissues were dehydrated with various concentrations of xylene and were then embedded in paraffin. The hippocampal sections were observed under a microscope (Olympus, Tokyo, Japan).
Cell culture and treatment
The PC-12 cells were plated in a 6-well dish and cultured in RPMI-1640 completed medium in an incubator with 5% CO2 and 37°C. The PC-12 cells were also divided into the following groups: the control group with no treatment; the ischemic injury group stimulated with Na2S2O4 (30 nM; PeproTech, Rocky Hill, NJ, USA); the group subjected to ischemia and treated with a low concentration of FA (50 µM; termed the isch + FA-L group); the group subjected to ischemia and treated with a moderate concentration of FA (100 µM; termed the isch + FA-M group); the group subjected to ischemia and treated with a high concentration of FA (200 µM; termed the isch + FA-L group); and the group subjected to ischemia and treated with 200 µM FA and MyD88 overexpression plasmids (termed MyD88-Ov+ischemia+FA group). After 24 h, cells were harvested and processed for the following experiments.
Cell viability test
We examined PC-12 cell viability by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Beyotime, Haimen, China) assay. The cells were plated in a 96-well dish and cultured ove night. Subsequently, 10 µl MTT were added to each well followed by incubation for 4 h. After the generation of formazan crystals, the medium was aspirated and then replaced with 150 µl solubilization solution dimethylsulfoxide (DMSO) in each well and incubated for 2 h. The OD value of each well was detected using a microplate spectrophotometer (Molecular Devices Technologies, Sunnyvale, CA, USA) at 570 nm.
Flow cytometry
Apoptosis was examined using the Annexin V/propidium iodide (PI) double staining kit (Beyotime). The cells were plated in a 6-well dish and cultured overnight. After being subjected to the various treatments as described above, the cells were collected and washed 3 times with 1× phosphate-buffered saline (PBS) in 1.5 ml tube, followed by the addition of 300 µl 1× binding buffer to the tube, and then by the addition of 5 µl Annexin V. The sample tubes were then incubated at room temperature in the dark for 30 min. Subsequently, the cells from each group were incubated with 5 µl PI in the dark. Finally, the cells were transferred and analyzed in a glass flow cytometry tube with a flow cytometer (Partec GmbH, Münster, Germany).
Hoechst staining
Following treatment as described above, the cells were stained with Hoechst 33258 (Beyotime) for 30 min at room temperature in the dark. After being washed with PBS, the samples were observed under a fluorescence microscope (Olympus). Apoptotic cells are characterized by a reduced nuclear size.
Determination of SOD, GSH-Px and MDA levels
The PC-12 cells in a 96-well plate and tissue homogenate were treated as described above. The contents of SOD (xanthine oxidase assay method) and MDA (thiobarbituric acid assay method) were detected using respective kits which are purchased from Nanjing Jiancheng Bio-Engineering Institute Co., Ltd (Nanjing, China) (46,47). The GSH-Px activity was detected using a chemical assay kit following the manufacturer's instructions and as previously described (48). The changes in absorbance were determined using a microplate reader, and the results were expressed as nmol/mg for MDA and U/mg protein for SOD activity.
Measurement of ROS, superoxide anion (O2−) and Ca2+ levels
ROS generation was detected using the DCFH-DA fluorescent probe (Beyotime). Intracellular H2O2 can oxidize DCFH-DA to the highly fluorescent compound, dichlorofluorescein (DCF). After the cells were treated as described above, 10 mM DCFH-DA were added to the cells for 30 min at 37°C. Finally, after washing, the fluorescence of the cells was observed under a fluorescence microscope (Nikon TE2000U; Nikon, Tokyo, Japan).
Intracellular O2− levels were measured using the O2− fluorescent probe, dihydroethidium (DHE; Beyotime, Shanghai, China). DHE was added to the cells for 30 min, followed by washing 3 times with 1× PBS to remove the non-specific staining. The cells were observed under a fluorescence microscope.
The intracellular Ca2+ level was detected using Fluo-3/AM (Invitrogen, Carlsbad, CA, USA). Following treatment as described above, the PC-12 cells were incubated with Fluo-3/AM for 30 min in an incubator, then washed twice with 1× PBS. Finally, the cells were examined under a fluorescence microscope (Nikon TE2000U; Nikon).
Quantitative PCR (qPCR)
Total RNA from the treated cells was extracted using TRIzol reagent (Takara, Dalian, China) according to the manufacturer's instructions. cDNA was synthesized from RNA (1 µg) of each group sample using a Script RT reagent kit (Takara). The relative gene mRNA levels were then evaluated by qPCR on an ABI 7300 PCR application (Applied Biosystems, Beijing, China) with the SYBR-Green PCR kit (Takara). PCR was performed under the following conditions: 95° 5 min, (95° 30 sec, 60° 30 sec, 30 cycles). β-actin was used as the reference gene. Primers were designed using Primer Premier 5.0 software. All primers were synthesized by Invitrogen. The primers were as follows: Bax forward, 5-CTGCAGAGGATGATTGCTGA-3′ and reverse, 5′-GATCAGCTCGGGCACTTTAG-3′; Bcl-2 forward, 5′-CGACTTTGCAGAGATGTCCA-3′ and reverse, 5′-ATGCCGGTTCAGGTACTCAG-3′; caspase-3 forward, 5′-GGACCTGTGGACCTGAAAAA-3′ and reverse, 5′-GCATGCCATATCATCGTCAG-3′; TLR4 forward, 5′-TGCTCAGACATGGCAGTTTC-3′ and reverse, 5′-TCAAGGCTTTTCCATCCAAC-3′; MyD88 forward, 5′-GAGATCCGCGAGTTTGAGAC-3′ and reverse, 5′-CTGTTTCTGCTGGTTGCGTA-3′; GAPDH forward, 5′-CTCATGACCACAGTCCATGC-3′ and reverse, 5′-TTCAGCTCTGGATGACCTT-3′.
Western blot analysis
The proteins were isolated from the treated cells using lysis buffer (Beyotime), and the protein concentrations were examined using a BCA protein assay kit (Beyotime). The proteins (30 µg) were separated by denaturing 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto nitrocellulose membranes (Millipore). After the membranes were blocked with 5% milk for 2.5 h, they were incubated with the primary antibodies [caspase3 (sc-1225), Bcl-2 (sc-56015), Bax (sc-20067), TLR4 (sc-293072) and MyD88 (sc-74532); Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA] at 1:1,000 dilutions overnight. The membranes were then incubated with goat anti-mouse or rabbit HRP-conjugated secondary antibodies [goat anti-mouse (sc-2005) or rabbit (sc-2004) HRP-conjugated; Santa Cruz Biotechnology, Inc.] for 2 h at 4°C. The bands on the membranes were visualized using an ECL detection system (Thermo Fisher Scientific, Inc., Waltham, MA, USA). β-actin was used as a reference control.
Statistical analysis
All data are expressed as the means ± SD. Experiments were performed at least 3 times. Comparisons were assessed with one-way analysis of variance (ANOVA) (Tukey's post hoc test) using GraphPad Prism software version 5.0a (GraphPad, La Jolla, CA, USA). A P-value <0.05 was considered to indicate a statistically significant difference compared with the control group.
Results
FA attenuates memory impairment induced by IRI
We performed the Morris water maze test and passive avoidance tests to examine hippocampus-related learning and memory abilities. The results revealed that the escape latencies of the rats in the sham-operated group were markedly decreased and the values obtained differed significantly from those of the ischemia group (P<0.05) in 5 days. However, no statistically significant difference was observed in the ischemia group over the successive trials. However, the rats with ischemia treated with FA exhibited a significant decrease in the times for escape latency compared with the ischemia group in a concentration-dependent manner (P<0.05). Furthermore, the FA-treated ischemia rats exhibited decreased times for escape latency and these times were shorter than those of the rats in the ischemia group (Table I).
Table I.
Latency period in the water maze follwing drug treatment.
| Group | Dose (mg/kg) | Latentcy period (sec)
|
||||||
|---|---|---|---|---|---|---|---|---|
| 1 day | 2 days | 3 days | 4 days | 5 days | 6 days | 7 days | ||
| Sham | – | 11.00±0.61 | 8.80±0.62 | 7.20±1.02 | 7.60±0.42 | 5.20±0.63 | 3.80±0.16 | 2.80±0.21 |
| Ischemia | – | 37.00±0.31a | 35.80±0.17a | 39.06±0.12a | 39.80±0.10a | 40.60±0.23a | 39.40±0.02a | 40.20±0.01a |
| FA | 28 | 33..40±0.40a | 31.20±0.19a | 29.40±0.54a | 28.00±0.42a | 27.40±0.21a | 26.40±0.35a | 25.00±0.13a |
| 56 | 31.00±0.09a | 27.20±0.35a | 28.40±0.51a | 25.00±0.43a | 21.60±0.11a,b | 21.00±0.25a,b | 13.40±0.09a,b | |
| 112 | 24.60±0.17a,b | 23.60±0.26a,b | 19.80±0.13a,b | 12.00±0.19a,b | 14.20±0.23a,b | 11.00±0.17a,b | 9.00±0.09a,b | |
Sham, sham-operated;
P<0.05 vs. sham-operated group;
P<0.05 vs. ischemia group. Data are the means ± SD (n=10 rats per group).
As shown in Table II, the period of latency for the inhibitory avoidance task in the ischemia group was decreased compared to that of the sham-operated group in each session, and the values were statistically significant (P<0.05). Furthermore, with the passage of time, the rats in the ischemia group exhibited severe memory impairment and loss of memory for electrical stimulation. The period of latency in the ischemia model exhibited a steady decrease. Of note, the FA-treated rats exhibited increased escape latency times in a concentration-dependent manner compared with the ischemia group. These data indicated that FA significantly repaired the spatial cognitive and memory deficits induced by ischemia.
Table II.
Latency period in the inhibitory avoidance task following drug treatment.
| Group | Dose (mg/kg) | Latency period (sec)
|
||
|---|---|---|---|---|
| 1 day | 2 days | 3 days | ||
| Sham | – | 304.40±2.75 | 284.20±1.62 | 288.40±1.56 |
| Ischemia | – | 99.40±1.35a | 83.60±1.17a | 68.60±1.12a |
| FA | 28 | 103.00±1.36a | 108.80±1.21a | 117.00±1.54a |
| 56 | 121.40±1.21a | 121.40±1.62a | 132.20±1.51a | |
| 112 | 132.00±1.12a,b | 129.00±1.37a,b | 135.40±1.13a,b | |
Sham, sham-operated;
P<0.05 vs. sham group;
P<0.05 vs. model group. Data are the means ± SD (n=10 rats per group).
FA decreases ischemia reperfusion-induced neuronal death in the hippocampus
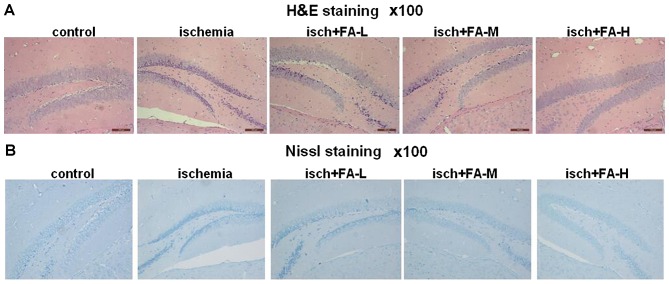
Hippocampal neurons have been reported to be closely related to learning and memory (49). We thus investigated the effects of FA on cell survival in the rat hippocampus area following IRI. We used H&E staining to observe the morphological changes in the cells in the hippocampus. As shown in Fig. 1, in the sham-operated group, the hippocampus area was found to have multiple layers, and the cells were arranged neatly, with a clear structure and normal shape, and the nucleus was round or oval, stained light blue. However, in the ischemia group, the layers of cells in the hippocampus area were decreased and exhibited a disorganized cell arrangement. Part of the hippocampus area cell exhibited nuclear condensation and deep blue staining. Of note, with the increase in the concentration of the FA (in the FA-L, FA-M, FA-H groups), the cells in the hippocampus area had more layers and were arranged more closely compared to those of the ischemia group (Fig. 1A).
Figure 1.
Ferulic acid (FA) decreases ischemia/reperfusion-induced neuronal death in the hippocampus. (A and B) Histopathology in the hippocampus region following ischemic injury shown by H&E and Nissl staining at ×100 magnification. Light microphotographs of the hippocampus in each group of H&E and Nissl staining.
In addition, we observed the changes of Nissl bodies through Nissl staining. Histological evaluation revealed that the sham-operated group had round nuclei and integral Nissl bodies, whereas the Nissl bodies in the ischemia group exhibited marked nuclear deviation, and were pathologically changed into particles. IRI induced the severe loss of Nissl bodies in the hippocampus of the rats with IRI compared with rats in the sham-operated group. Nevertheless, cell injury, as well as the abnormalities in Nissl bodies induced by IRI were attenuated in a dose-dependent manner by treatment with FA (Fig. 1B). These data indicated that FA effectively reduced IRI-induced neuronal death.
FA attenuates IRI-induced neuronal apoptosis in the hippocampus
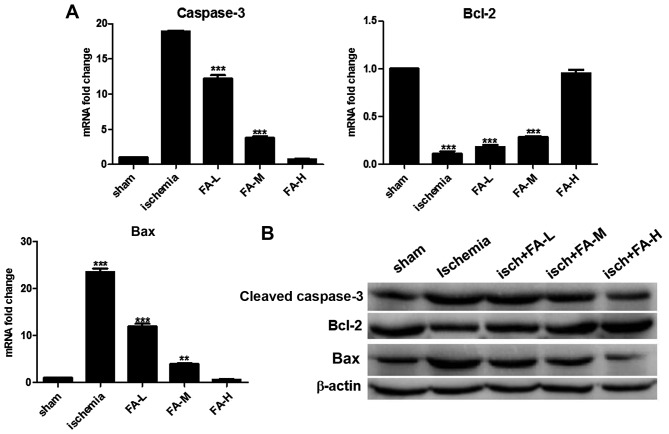
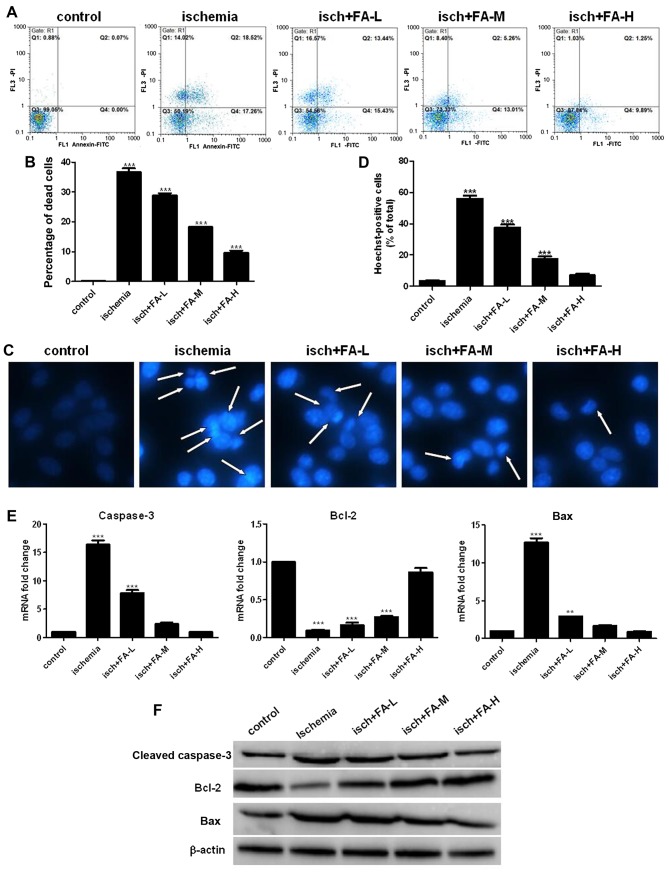
To examine the effects of FA on neuronal apoptosis, we examined the expression of cleaved caspase-3, Bcl-2 and Bax by qPCR and western blot analysis. Apoptosis was detected by measuring the levels of caspase-3, Bcl-2 and Bax in the brain tissue homogenates. We found significantly increased levels of cleaved caspase-3 and Bax, and decreased levels of Bcl-2 in the ischemia group compared with the sham-operated group. On the contrary, treatment with FA (the FA-L, FA-M, FA-H groups) effectively increased the levels of Bcl-2, and inhibited the levels of cleaved caspase-3 and Bax in a concentration-dependent manner (Fig. 2). These results suggest that FA exerts neuroprotective effects against IRI-induced brain injury through anti-apoptotic mechanisms.
Figure 2.
Ferulic acid (FA) decreases ischemia/reperfusion-induced cell apoptosis in the hippocampus. (A and B) qPCR and western blot analysis of the levels of caspase-3, Bax and Bcl-2 in each group.
FA exerts an antioxidant effect on rats with IRI
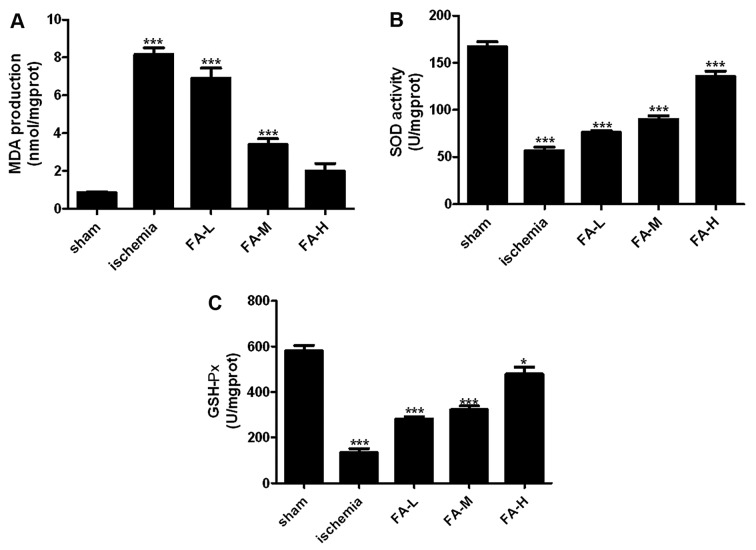
We then investigated the functions of FA as an antioxidant in rats with IRI. We wished to identify enzymatic sources of excessive ROS production following IRI, such as SOD, MDA and GSH-Px. As shown in Fig. 3, the increased contents of MDA, and the decreased activities of SOD and GSH-Px in the brain tssues were detected in the ischemia group. However, FA decreased the MDA contents and promoted the activity of SOD, GSH-Px in a dose-dependent manner (Fig. 3).
Figure 3.
Effects of ferulic acid (FA) on the content of malondialdehyde (MDA), andSOD and glutathione peroxidase (GSH-Px) activities in vivo. (A) MDA content, and (B) SOD and (C) GSH-Px activities were detected using respective kits at 532, 560 and 422 nm on a microplate reader.
Protective effects of FA on PC-12 cells following ischemic injury
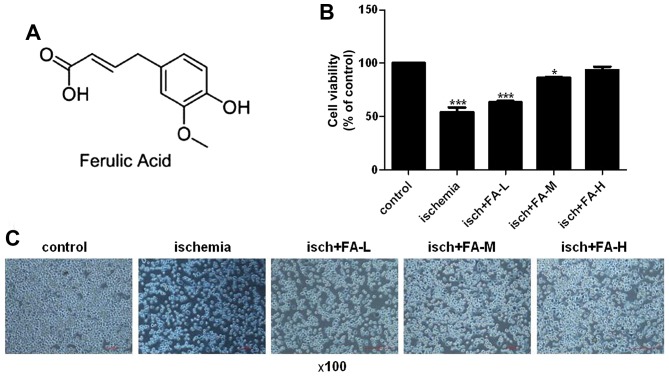
The chemical structure of FA is shown in Fig. 4A. The effect of FA on the viability of the PC-12 cells was detected by MTT assay. Compared to the control group, the viability of the cells exposed to Na2S2O4 markedly decreased (Fig. 4B and C). FA prevented the decrease in PC-12 cell viability induced by Na2S2O4 in a dose-dependent manner.
Figure 4.
Effect of ferulic acid (FA) on the viability of PC-12 cells following ischemia injury. (A) The chemical structure of FA. (B) Cell viability was detected by MTT assay. (C) Micrographs of differently treated PC-12 cells at ×100 magnification.
Anti-apoptotic effects of FA on PC-12 cells following ischemic injury
It has been reported that apoptosis plays a crucial role in ischemic injury (50). Therefore, we further examined the effects of FA on ischemia-induced apoptosis by Annexin V/PI staining with flow cytometric analysis. As shown in Fig. 5A and B, the results from Annexin V/PI staining revealed a significant increase in the apoptotic cell population in the ischemia group compared with the control group, while treatment with FA significantly decreased the apoptotic cell population induced by ischemia compared with the ischemia group in a concentration-dependent manner. The difference in nuclear morphology were also detected by Hoechst 33258 staining. The number of condensed nuclei was increased in the cells in the ischemia group compared with those in the control group. Treatment with various concentration of FA (50, 100 and 200 µM) attenauated this effect in a dose-dependent manner (Fig. 5C and D).
Figure 5.
Effect of ferulic acid (FA) on thye ischemia-induced apoptosis of PC-12 cells. (A) Annexin V/PI staining was used to determine apoptosis by flow cytometry. (B) Histograms showing the ratio of dead cells to the total nuclei. (C) Nuclearmorphology was determined by Hoechst 33258 staining; the white arrows indicate the apoptotic cells, at ×400 magnification. (D) Histograms showing the ratio of condensed nuclei to total nuclei. (E and F) The expression of apoptosis related proteins was examined by qPCR and western blot analysis.
We also examined the expression of caspase-3, Bax and Bcl-2 by qPCR and western blot analysis. As observed in Fig. 5E and F, the Bax expresion level was significantly increased in the ischemia group; however, the expression of Bcl-2 was significantly inhibited. FA decreased the Bax expression level and increased Bcl-2 expression (Fig. 5E and F). These results implied that FA inhibited the apoptosis of PC-12 cells subjected to ischemic injury.
Antioxidant effects of FA on SOD, MDA and GSH-Px
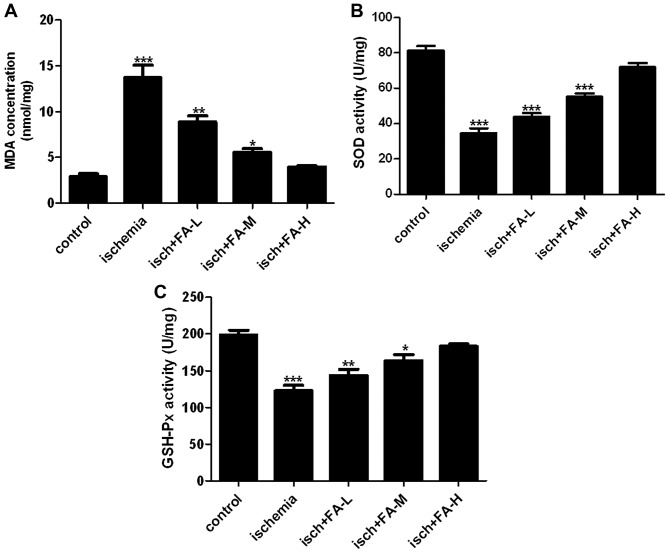
As shown in Fig. 6, after the PC-12 cells were subjected to ischemic injury, we also found that the content of MDA was elevated, and the activities of SOD and GSH-Px were decreased in the PC-12 cells. Teatment with FA significantly decreased the MDA content, and promoted the activities of SOD and GSH-Px in a dose-dependent manner.
Figure 6.
Effects of ferulic acid (FA) on the content of malondialdehyde (MDA), and activities of SOD and glutathione peroxidase (GSH-Px) in PC-12 cells subjected to ischemic injury. (A) MDA content, and (B) SOD and (C) GSH-Px activities were using respective kits at 532, 560 and 422 nm.
Antioxidant effects of FA on the ROS, O2− and Ca2+ contents
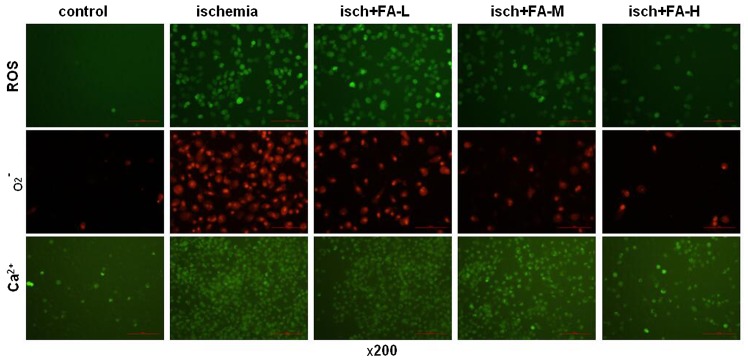
In this study, the contents of ROS, O2− and Ca2+ were measured using DCFH-DA, DHE and Flou-3/AM fluorescent probes with a fluorescence microscope. As shown in Fig. 7, the PC-12 cells subjected to ischemic injury exhibited an increase in DCF fluorescence; however, FA inhibited this increase in DCF fluorescence. A similar effect was observed for O2− generation; FA effectively reduced O2− generation in the PC-12 cells subjected to ischemic injury. The fluorescence intensity of Fluo-3/AM also was increased in the ischemia group compared to the control group. However, FA treatment decreased this fluorescence intensity. These results suggested that FA exerted the antioxidant effects by decreasing the accumulation of ROS, O2− and Ca2+.
Figure 7.
Effects of ferulic acid (FA) on the production of reactive oxygen species (ROS), O2− and Ca2+ PC-12 cells subjected to ischemic injury. ROS content, and O2− and Ca2+ production were measured using respective fluorescent probes under a fluorescence microscope, at ×400 magnification.
Effects of FA on the TLR/MyD88 signaling pathway
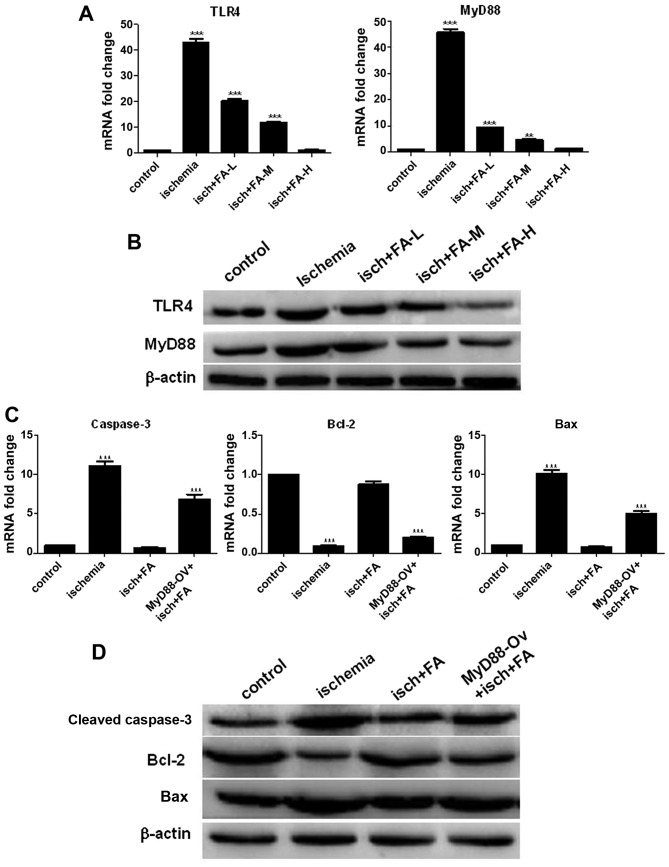
We also examined the TLR4 and MyD88 expression levels by qPCR and western blot analysis. As shown in Fig. 8A and B, ischemic injury promoted TLR4 and MyD88 expression; however, FA significantly decreased TLR4 and MyD88 expression in a concentration-dependent manner (Fig. 8A and B). These data implied that FA inhibited the activation of the TLR/MyD88 signal pathway.
Figure 8.
Ferulic acid exerts neuroprotective effects on PC-12 cells subjected to ischemic injury by inhibiting the activation of the Toll-like receptor (TLR)/MyD88 signaling pathway. (A and B) qPCR and western blot analysis of the expression of TLR4 and MyD88 in PC-12 cells subjected to ischemic injury. (C and D) The expression of apoptosis-related proteins was examined by qPCR and western blot analysis.
To investigate whether the TLR/MyD88 pathway plays an important role in ischemic injury, the effects of MyD88 overexpression on the anti-apoptotic of FA on PC-12 cells subjected to ischemic injury were determined by qPCR and western blot analysis. As shown in Fig. 8C and D, the expression levels of cleaved caspase-3 and Bax significantly increased in the ischemia group compared with the control group; however, treatment with FA decreased the expression of cleaved caspase-3 and Bax. However, these expression levels were markedly increased in the FA + MyD88-Ov group compared with the FA group. Moreover, Bcl-2 expression in the ischemia group was decreased compared to that of the control group, and the Bcl-2 level was increased following treatment with FA. However, Bcl-2 expression was markedly decreased in the FA + MyD88-Ov group compared with the FA group. Taken together, these results demonstrate that FA suppresses the apoptosis of PC-12 cells subjected to ischemic injury by inhibiting the activation of the TLR/MyD88 signaling pathway.
Discussion
Fa (4-hydroxy-3-methoxycinnamic acid) is an element isolated from Ferula foetida. FA is regarded as a free radical scavenger, and exerts antioxidant and anti-inflammatory effects in the brain. Howevere, the protective effects of FA against cerebral IRI have not been fully explored. The aim of this study was to investigate the effects of FA on learning and memory impairment induced by IRI, and to elucidate the potential underlying mechanisms responsible for the neuroprotective effects of FA against apoptosis and oxidative stress induced by IRI.
Cerebral ischemia and reperfusion can cause neuronal injury and death in the hippocampus area, subsequently inducing behavioral defects, including learning and memory impairments. Studies have reported that cognitive ability disorders are associated with the number of damaged neuronal cells in the hippocampus region, the damage of which is induced by the IRI (51–53). Importantly, the hippocampus region is the one of the most important regions for learning and memory abilities, and is vulnerable to IRI injury (54,55). Therefore, in this study, we first constructed an aninmal model of ischemic injury by surgery. We detected the spatial learning and memory deficits in rats in response to IRI using the passive avoidance tests and Morris water maze task. H&E and Nissl staining were also used to examine neuronal damage in the hippocampus region. Our results implied that FA exerted neuroprotective effects.
IRI is involved in ROS accumulation and induces apoptosis (56). Free radical production has also been widely reported in cerebral ischemic injury (57). Ischemia can induce a disadvantageous cascade reaction, including inflammatory processes, excitotoxicity and oxidative stress, and finally results in neuronal cell death (58–60). Oxidative stress is one of the primary factors in IRI (61–21). Following oxidative stress, antioxidant enzymes, such as SOD, GSH-Px (63) are included in the defensive system for protection against oxidative stress; these play a crucial role in protecting neurons against reactive oxygen species-induced cell apoptosis (64). MDA is an oxidative stress marker, which is also a product of lipid peroxidation reaction. The content of MDA can indicate the degree of lipid peroxidation in tissues (65). SOD is an important factor for the defense against the tissue damage induced by ROS, and it also catalyzes the superoxide anion transferred to hydrogen peroxide and inhibits the generation of hydroxyl radicals (66). GSH is an endogenous antioxidant, and it can react directly with ROS and/or regarded as a co-factor with the enzyme, GSH-Px to decrease hydrogen peroxide and lipid peroxide levels in tissues (67). In this study, the activities of SOD and GSH-Px were significantly decreased, while the MDA content was increased following IRI. This phenomenon indicated that oxidative stress occurs in rats suffering from IRI. Treatment with FA significantly inhibited the increased levels of MDA induced by IRI, and increased the generation of the cellular antioxidants, SOD and GSH.
We also constructed cell model of ischemic injury using sodium dithionite (Na2S2O4). In recent years, PC-12 cells are used in experiments as they constitute a widely used neuronal model system (68). The PC-12 cell line was obtained from a rat pheochromocytoma, and it is a useful cell line for studying various neuronal functions and is also widely used for the study of cellular ischemic injury (69,70). The findings of our study suggested that FA significantly decreased ischemia-induced apoptosis, as well as the contents of MDA, ROS, O2− and Ca2+ accumulation, and promoted SOD and GSH-Px activities compared to the ischemia group in vivo and in vitro. FA was suggested to exert marked neuroprotective effects. Taken together, our data suggest that FA may have potential for use in the treatment of cerebral ischemia and reperfusion injury. Our results revealed that IRI contributed to severe neuronal damage in the hippocampus, and subsequently damaged the function of spatial learning and memory in rats. However, FA reversed these effects and attenuated pathological and neurological deficits, in a dose-dependent manner.
TLRs can capture 'danger signals' coming from necrotic cells, and participate in promoting the inflammatory reaction and cell apoptosis-related processes (71,72). It has been reported that IRI, surgical injury and systemic stress can induce an inflammatory reaction in part via the TLR signaling pathway (73). In this study, we also used an in vitro model to investigate the effects and mechanisms of action of FA against Na2S2O4-induced IRI in PC-12 cells. Our findings study implied that FA exerted protective effects on the PC-12 cells subjected to ischemic injury by inhibiting the activation of the TLRs/MyD88 signaling pathway.
In conclusion, this study suggested that FA has a clearly protective function against ischemic injury in vivo and in vitro by suppressing the Ca2+ influx, the generation of O2− and ROS production, and inhibiting apoptosis. There is evidence to indicate that FA has a function of protecting neurons by confronting oxidative stress and related apoptotic processes following cerebral IRI in rats (74). In this study, the neuroprotective effects of FA were shown to be associated with the downregulated expression of TLR4 and MyD88. Above all, FA may have an anti-inflammatory effect. FA may have potential for use as a neuroprotective agent for protection against oxidative stress and apoptosis induced by ischemia. Some of the molecular mechanisms and related signaling pathways involved in the neuroprotective effects of FA were identified in this study. However, further studies are required to further elucidate and confirm the potential mechanisms.
Acknowledgments
The present study was supported by grants from Yunnan Applied Basic Research Projects-Joint Special Project (no. 2015FB010).
References
- 1.Srinivasan M, Sudheer AR, Pillai KR, Kumar PR, Sudhakaran PR, Menon VP. Influence of ferulic acid on gamma-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes. Toxicology. 2006;228:249–258. doi: 10.1016/j.tox.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan M, Sudheer AR, Menon VP. Ferulic Acid: Therapeutic potential through its antioxidant property. J Clin Biochem Nutr. 2007;40:92–100. doi: 10.3164/jcbn.40.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogiwara T, Satoh K, Kadoma Y, Murakami Y, Unten S, Atsumi T, Sakagami H, Fujisawa S. Radical scavenging activity and cytotoxicity of ferulic acid. Anticancer Res. 2002;22:2711–2717. [PubMed] [Google Scholar]
- 4.Hong Q, Ma ZC, Huang H, Wang YG, Tan HL, Xiao CR, Liang QD, Zhang HT, Gao Y. Antithrombotic activities of ferulic acid via intracellular cyclic nucleotide signaling. Eur J Pharmacol. 2016;777:1–8. doi: 10.1016/j.ejphar.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Jin Son M, W Rico C, Hyun Nam S, Young Kang M. Influence of oryzanol and ferulic acid on the lipid metabolism and antioxidative status in high fat-fed mice. J Clin Biochem Nutr. 2010;46:150–156. doi: 10.3164/jcbn.09-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo HH, Chung JG. The effects of plant phenolics, caffeic acid, chlorogenic acid and ferulic acid on arylamine N-acetyltransferase activities in human gastrointestinal microflora. Anticancer Res. 1999;19:133–139. [PubMed] [Google Scholar]
- 7.Ichimura T, Otake T, Mori H, Maruyama S. HIV-1 protease inhibition and anti-HIV effect of natural and synthetic water-soluble lignin-like substances. Biosci Biotechnol Biochem. 1999;63:2202–2204. doi: 10.1271/bbb.63.2202. [DOI] [PubMed] [Google Scholar]
- 8.Kawabata K, Yamamoto T, Hara A, Shimizu M, Yamada Y, Matsunaga K, Tanaka T, Mori H. Modifying effects of ferulic acid on azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Lett. 2000;157:15–21. doi: 10.1016/S0304-3835(00)00461-4. [DOI] [PubMed] [Google Scholar]
- 9.Yogeeta SK, Hanumantra RB, Gnanapragasam A, Senthilkumar S, Subhashini R, Devaki T. Attenuation of abnormalities in the lipid metabolism during experimental myocardial infarction induced by isoproterenol in rats: beneficial effect of ferulic acid and ascorbic acid. Basic Clin Pharmacol Toxicol. 2006;98:467–472. doi: 10.1111/j.1742-7843.2006.pto_335.x. [DOI] [PubMed] [Google Scholar]
- 10.Cheng CY, Ho TY, Lee EJ, Su SY, Tang NY, Hsieh CL. Ferulic acid reduces cerebral infarct through its antioxidative and anti-inflammatory effects following transient focal cerebral ischemia in rats. Am J Chin Med. 2008;36:1105–1119. doi: 10.1142/S0192415X08006570. [DOI] [PubMed] [Google Scholar]
- 11.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pei Z, Ho HT, Cheung RT. Pre-treatment with melatonin reduces volume of cerebral infarction in a permanent middle cerebral artery occlusion stroke model in the rat. Neurosci Lett. 2002;318:141–144. doi: 10.1016/S0304-3940(01)02503-4. [DOI] [PubMed] [Google Scholar]
- 13.Simerabet M, Robin E, Aristi I, Adamczyk S, Tavernier B, Vallet B, Bordet R, Lebuffe G. Preconditioning by an in situ administration of hydrogen peroxide: Involvement of reactive oxygen species and mitochondrial ATP-dependent potassium channel in a cerebral ischemia-reperfusion model. Brain Res. 2008;1240:177–184. doi: 10.1016/j.brainres.2008.08.070. [DOI] [PubMed] [Google Scholar]
- 14.Cao LJ, Wang J, Hao PP, Sun CL, Chen YG. Effects of ulinastatin, a urinary trypsin inhibitor, on synaptic plasticity and spatial memory in a rat model of cerebral ischemia/reperfusion injury. Chin J Physiol. 2011;54:435–442. doi: 10.4077/CJP.2011.AMM058. [DOI] [PubMed] [Google Scholar]
- 15.Feng X, Yang S, Liu J, Huang J, Peng J, Lin J, Tao J, Chen L. Electroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med Rep. 2013;7:1516–1522. doi: 10.3892/mmr.2013.1392. [DOI] [PubMed] [Google Scholar]
- 16.Wu YY, Wu WY, Gong HL, Li WZ, Yin YY. Astragalosides attenuate learning and memory impairment in rats following ischemia reperfusion injury. Mol Med Rep. 2014;9:1319–1324. doi: 10.3892/mmr.2014.1969. [DOI] [PubMed] [Google Scholar]
- 17.Kuo CT, Lin YW, Tang NY, Cheng CY, Hsieh CL. Electric stimulation of the ears ameliorated learning and memory impairment in rats with cerebral ischemia-reperfusion injury. Sci Rep. 2016;6:20381. doi: 10.1038/srep20381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Zhang L, Liang J. Activation of the Nrf2 defense pathway contributes to neuroprotective effects of phloretin on oxidative stress injury after cerebral ischemia/reperfusion in rats. J Neurol Sci. 2015;351:88–92. doi: 10.1016/j.jns.2015.02.045. [DOI] [PubMed] [Google Scholar]
- 19.Schimidt HL, Vieira A, Altermann C, Martins A, Sosa P, Santos FW, Mello-Carpes PB, Izquierdo I, Carpes FP. Memory deficits and oxidative stress in cerebral ischemia-reperfusion: neuroprotective role of physical exercise and green tea supplementation. Neurobiol Learn Mem. 2014;114:242–250. doi: 10.1016/j.nlm.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa M, Zhang JH, Nanda A, Granger DN. Inflammatory responses to ischemia and reperfusion in the cerebral microcirculation. Front Biosci. 2004;9:1339–1347. doi: 10.2741/1330. [DOI] [PubMed] [Google Scholar]
- 21.Chen B, Wu Z, Xu J, Xu Y. Calreticulin binds to Fas ligand and inhibits neuronal cell apoptosis induced by ischemia-reperfusion injury. Biomed Res Int. 2015;2015:895284. doi: 10.1155/2015/895284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Brain Res Rev. 2007;54:34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Lewén A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- 24.Tabakman R, Jiang H, Shahar I, Arien-Zakay H, Levine RA, Lazarovici P. Neuroprotection by NGF in the PC12 in vitro OGD model: Involvement of mitogen-activated protein kinases and gene expression. Ann NY Acad Sci. 2005;1053:84–96. doi: 10.1196/annals.1344.008. [DOI] [PubMed] [Google Scholar]
- 25.Nunes C, Almeida L, Laranjinha J. 3,4-Dihydroxyphenylacetic acid (DOPAC) modulates the toxicity induced by nitric oxide in PC-12 cells via mitochondrial dysfunctioning. Neurotoxicology. 2008;29:998–1007. doi: 10.1016/j.neuro.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Bhavnani BR. Glutamate-induced apoptosis in primary cortical neurons is inhibited by equine estrogens via down-regulation of caspase-3 and prevention of mitochondrial cytochrome c release. BMC Neurosci. 2005;6:13. doi: 10.1186/1471-2202-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niizuma K, Endo H, Chan PH. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J Neurochem. 2009;109(Suppl 1):133–138. doi: 10.1111/j.1471-4159.2009.05897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Susnow N, Zeng L, Margineantu D, Hockenbery DM. Bcl-2 family proteins as regulators of oxidative stress. Semin Cancer Biol. 2009;19:42–49. doi: 10.1016/j.semcancer.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu L, Wei T, Gao J, Chang X, He H, Luo F, Zhou R, Ma C, Liu Y, Yan T. The cardioprotective effect of salidroside against myocardial ischemia reperfusion injury in rats by inhibiting apoptosis and inflammation. Apoptosis. 2015;20:1433–1443. doi: 10.1007/s10495-015-1174-5. [DOI] [PubMed] [Google Scholar]
- 30.Hu J, Zhu XH, Zhang XJ, Wang PX, Zhang R, Zhang P, Zhao GN, Gao L, Zhang XF, Tian S, et al. Targeting TRAF3 signaling protects against hepatic ischemia/reperfusions injury. J Hepatol. 2016;64:146–159. doi: 10.1016/j.jhep.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Zaidi A, Fernandes D, Bean JL, Michaelis ML. Effects of paraquat-induced oxidative stress on the neuronal plasma membrane Ca(2+)-ATPase. Free Radic Biol Med. 2009;47:1507–1514. doi: 10.1016/j.freeradbiomed.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kizmazoglu C, Aydin HE, Sevin IE, Kalemci O, Yüceer N, Atasoy MA. Neuroprotective effect of resveratrol on acute brain ischemia reperfusion injury by measuring Annexin V, p53, Bcl-2 levels in rats. J Korean Neurosurg Soc. 2015;58:508–512. doi: 10.3340/jkns.2015.58.6.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanski J, Aksenova M, Stoyanova A, Butterfield DA. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: Structure-activity studies. J Nutr Biochem. 2002;13:273–281. doi: 10.1016/S0955-2863(01)00215-7. [DOI] [PubMed] [Google Scholar]
- 34.Kikuzaki H, Hisamoto M, Hirose K, Akiyama K, Taniguchi H. Antioxidant properties of ferulic acid and its related compounds. J Agric Food Chem. 2002;50:2161–2168. doi: 10.1021/jf011348w. [DOI] [PubMed] [Google Scholar]
- 35.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 36.Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- 37.Oyama J, Blais C, Jr, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in Toll-like receptor 4-deficient mice. Circulation. 2004;109:784–789. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 38.Sakata Y, Dong JW, Vallejo JG, Huang CH, Baker JS, Tracey KJ, Tacheuchi O, Akira S, Mann DL. Toll-like receptor 2 modulates left ventricular function following ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H503–H509. doi: 10.1152/ajpheart.00642.2006. [DOI] [PubMed] [Google Scholar]
- 39.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen LW, Chang WJ, Chen PH, Liu WC, Hsu CM. TLR ligand decreases mesenteric ischemia and reperfusion injury-induced gut damage through TNF-alpha signaling. Shock. 2008;30:563–570. doi: 10.1097/SHK.0b013e31816a3458. [DOI] [PubMed] [Google Scholar]
- 41.Zhai Y, Shen XD, O'Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 42.Altemeier WA, Liles WC, Villagra-Garcia A, Matute-Bello G, Glenny RW. Ischemia-reperfusion lung injury is attenuated in MyD88-deficient mice. PLoS One. 2013;8:e77123. doi: 10.1371/journal.pone.0077123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye X, Kong D, Wang J, Ishrat T, Shi H, Ding X, Cui G, Hua F. MyD88 contributes to neuroinflammatory responses induced by cerebral ischemia/reperfusion in mice. Biochem Biophys Res Commun. 2016;480:69–74. doi: 10.1016/j.bbrc.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Van Meer P, Raber J. Mouse behavioural analysis in systems biology. Biochem J. 2005;389:593–610. doi: 10.1042/BJ20042023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 46.Cao YJ, He X, Wang N, He LC. Effects of imperatorin, the active component from Radix Angelicae (Baizhi), on the blood pressure and oxidative stress in 2K,1C hypertensive rats. Phytomedicine. 2013;20:1048–1054. doi: 10.1016/j.phymed.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 47.Dong Y, Yang N, Liu Y, Li Q, Zuo P. The neuroprotective effects of phytoestrogen α-zearalanol on β-amyloid-induced toxicity in differentiated PC-12 cells. Eur J Pharmacol. 2011;670:392–398. doi: 10.1016/j.ejphar.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 48.Kazmierczak A, Strosznajder JB, Adamczyk A. Alpha-synuclein enhances secretion and toxicity of amyloid beta peptides in PC12 cells. Neurochem Int. 2008;53:263–269. doi: 10.1016/j.neuint.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Tamnes CK, Walhovd KB, Engvig A, Grydeland H, Krogsrud SK, Østby Y, Holland D, Dale AM, Fjell AM. Regional hippocampal volumes and development predict learning and memory. Dev Neurosci. 2014;36:161–174. doi: 10.1159/000362445. [DOI] [PubMed] [Google Scholar]
- 50.Dagher PC. Apoptosis in ischemic renal injury: roles of GTP depletion and p53. Kidney Int. 2004;66:506–509. doi: 10.1111/j.1523-1755.2004.761_7.x. [DOI] [PubMed] [Google Scholar]
- 51.Niu YL, Li C, Zhang GY. Blocking Daxx trafficking attenuates neuronal cell death following ischemia/reperfusion in rat hippocampus CA1 region. Arch Biochem Biophys. 2011;515:89–98. doi: 10.1016/j.abb.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 52.Bae EJ, Chen BH, Yan BC, Shin BN, Cho JH, Kim IH, Ahn JH, Lee JC, Tae HJ, Hong S, et al. Delayed hippocampal neuronal death in young gerbil following transient global cerebral ischemia is related to higher and longer-term expression of p63 in the ischemic hippocampus. Neural Regen Res. 2015;10:944–950. doi: 10.4103/1673-5374.158359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erfani S, Khaksari M, Oryan S, Shamsaei N, Aboutaleb N, Nikbakht F, Jamali-Raeufy N, Gorjipour F. Visfatin reduces hippocampal CA1 cells death and improves learning and memory deficits after transient global ischemia/reperfusion. Neuropeptides. 2015;49:63–68. doi: 10.1016/j.npep.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Leão AH, Medeiros AM, Apolinário GK, Cabral A, Ribeiro AM, Barbosa FF, Silva RH. Hippocampal-dependent memory in the plus-maze discriminative avoidance task: The role of spatial cues and CA1 activity. Behav Brain Res. 2016;304:24–33. doi: 10.1016/j.bbr.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Bird CM, Burgess N. The hippocampus and memory: Insights from spatial processing. Nat Rev Neurosci. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- 56.Arda-Pirincci P, Bolkent S. The role of epidermal growth factor in prevention of oxidative injury and apoptosis induced by intestinal ischemia/reperfusion in rats. Acta Histochem. 2014;116:167–75. doi: 10.1016/j.acthis.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Nakashima M, Niwa M, Iwai T, Uematsu T. Involvement of free radicals in cerebral vascular reperfusion injury evaluated in a transient focal cerebral ischemia model of rat. Free Radic Biol Med. 1999;26:722–729. doi: 10.1016/S0891-5849(98)00257-3. [DOI] [PubMed] [Google Scholar]
- 58.Rodrigo J, Fernández AP, Serrano J, Peinado MA, Martínez A. The role of free radicals in cerebral hypoxia and ischemia. Free Radic Biol Med. 2005;39:26–50. doi: 10.1016/j.freeradbiomed.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 59.He F, Ye B, Chen J, Sun X, Li C. Influence of hepatocyte growth factor on iNOS, NO and IL-1β in the cerebrum during cerebral ischemia/reperfusion in rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2014;39:23–9. doi: 10.11817/j.issn.1672-7347.2014.01.005. In Chinese. [DOI] [PubMed] [Google Scholar]
- 60.Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47:122–129. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Pradeep H, Diya JB, Shashikumar S, Rajanikant GK. Oxidative stress - assassin behind the ischemic stroke. Folia Neuropathol. 2012;50:219–230. doi: 10.5114/fn.2012.30522. [DOI] [PubMed] [Google Scholar]
- 62.Liu L, Sun Q, Wang R, Chen Z, Wu J, Xia F, Fan XQ. Methane attenuates retinal ischemia/reperfusion injury via anti-oxidative and anti-apoptotic pathways. Brain Res. 2016;1646:327–333. doi: 10.1016/j.brainres.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 63.Wang X, Zhou H, Yang J. GSH.Px or SOD encapsulated erythrocytes in the study of cerebral ischemia-reperfusion. Zhonghua Yi Xue Za Zhi. 1997;77:43–46. In Chinese. [PubMed] [Google Scholar]
- 64.Wang TF, Lei Z, Li YX, Wang YS, Wang J, Wang SJ, Hao YJ, Zhou R, Jin SJ, Du J, et al. Oxysophoridine protects against focal cerebral ischemic injury by inhibiting oxidative stress and apoptosis in mice. Neurochem Res. 2013;38:2408–2417. doi: 10.1007/s11064-013-1153-6. [DOI] [PubMed] [Google Scholar]
- 65.Zhang RQ, Li DY, Xu TD, Zhu SS, Pan HJ, Fang F, Wu X, Sun H. Antioxidative effect of luteolin pretreatment on simulated ischemia/reperfusion injury in cardiomyocyte and perfused rat heart. Chin J Integr Med. 2016 Mar 8; doi: 10.1007/s11655-015-2296-x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 66.Chandra Jagetia G, Rajanikant GK, Rao SK, Shrinath Baliga M. Alteration in the glutathione, glutathione peroxidase, superoxide dismutase and lipid peroxidation by ascorbic acid in the skin of mice exposed to fractionated gamma radiation. Clin Chim Acta. 2003;332:111–121. doi: 10.1016/S0009-8981(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 67.Tu Q, Wang R, Ding B, Zhong W, Cao H. Protective and antioxidant effect of Danshen polysaccharides on cerebral ischemia/reperfusion injury in rats. Int J Biol Macromol. 2013;60:268–271. doi: 10.1016/j.ijbiomac.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 68.Negis Y, Unal AY, Korulu S, Karabay A. Cell cycle markers have different expression and localization patterns in neuron-like PC12 cells and primary hippocampal neurons. Neurosci Lett. 2011;496:135–140. doi: 10.1016/j.neulet.2011.03.100. [DOI] [PubMed] [Google Scholar]
- 69.Yamazaki M, Chiba K, Mohri T. Differences in neuritogenic response to nitric oxide in PC12 and PC12h cells. Neurosci Lett. 2006;393:222–225. doi: 10.1016/j.neulet.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 70.Zhang ZG, Lü TS, Yuan HY. Neuroprotective effects of ultra-low-molecular-weight heparin in vitro and in vivo models of ischemic injury. Fundam Clin Pharmacol. 2011;25:300–303. doi: 10.1111/j.1472-8206.2010.00845.x. [DOI] [PubMed] [Google Scholar]
- 71.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. JExp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang L, Zhang X, Liu L, Cui L, Yang R, Li M, Du W. Tanshinone II A down-regulates HMGB1, RAGE, TLR4, NF-kappaB expression, ameliorates BBB permeability and endothelial cell function, and protects rat brains against focal ischemia. Brain Res. 2010;1321:143–151. doi: 10.1016/j.brainres.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 73.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 74.Cheng CY, Su SY, Tang NY, Ho TY, Chiang SY, Hsieh CL. Ferulic acid provides neuroprotection against oxidative stress-related apoptosis after cerebral ischemia/reperfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res. 2008;1209:136–150. doi: 10.1016/j.brainres.2008.02.090. [DOI] [PubMed] [Google Scholar]