Abstract
Honey samples (n = 126) from Castilla-La Mancha (Central Spain) were characterized based on their physicochemical properties and a melissopalynological analysis. The latter showed that Echium pollen type was the dominant palynomorph in most samples, representing at least 30% of the pollen in each sample. As anticipated, a relationship was observed between the proportion of this pollen and the properties of the honey. One goal of this study was to set a threshold that defines the percentage of pollen necessary for Viper’s bugloss honey to be considered monofloral or multifloral. This is a mandatory requirement in light of the publication of the European Directive 2014/63/EU establishing the regulations governing the labelling and control of honey to eradicate fraud (BOE n° 147, June 2015). By analyzing how the proportions of Echium pollen type affected the physicochemical and sensory parameters of the honey, the honeys analyzed could be segregated into multifloral and monofloral honeys. The data indicates that the proportion of pollen necessary to discriminate monofloral Viper’s bugloss honey lies at 70%.
Introduction
While the Borage family is native to southern Europe, the members of this family also occur in most countries worldwide. The species of the family Boraginaceae are clearly entomophilous, attracting insects with the reward of both pollen and nectar. The position of their anthers and the smallness of the grains ensures that large amounts of pollen are also released into the atmosphere, with its consequent anemophilous transport. In fact, these plants may free about 35.9 grams of pollen grains per cubic meter at atmosphere, although this varies depending on climatology [1].
The Echium spp. are wild plants that prosper in dry meadows and fields, waste ground and roadsides. They are an important nitrophile in pastures and meadows across the Iberian Peninsula [2] and their wildflowers are blue, or occasionally white or pink, appearing from late spring to mid-summer. According to Flora Ibérica [3], seven species of the genus Echium can be found in Castilla-La Mancha (Central Spain): E. boissieri Steudel, E. creticum L., E. flavum Desf., E. asperrimum Lam; E. humile Desf.; E. vulgare L., E. creticum L. and E. plantagineum L. Of these, the most abundant is without doubt E. plantagineum L., followed by Echium vulgare.
Palynologically, most of these species produce the same pollen type described by Díez [4,5], characteristically small in size (P = 13–25μm; E = 8–15μm) and heteropolar. While this facilitates their rapid identification, it does not favor palynological identification of the different species in honey. Echium species produce large amounts of pollen. In the case of E. albicans, the density of flowers reaches 14,639 (±3,720)/m2 with a production of 123,331 (±32.12) pollen grains per flower, whereas the density of flowers in the case of E. plantagineum reaches 27,845 ± 4,927/m2 and the pollen grains 97,571 ± 21,456 [6,7]. If we compare these numbers of pollen grains per flower with those of the Lamiaceae (Labiatae) family, such as Rosmarinus officcinalis L. or Thymus, the contribution of the pollen of these latter apiculture sources (nectariferous plants) is much lower: R. officcinalis, pollen contributes 8,300 grains; T. mastichyna (L.) L. 4,321; and Teucrium fruticans L., 13,999.
The Echium spp. are assiduously sought out by bees and bumblebees also due to the large amount of nectar they produce [8,9]. However, little is known about the composition of the nectar in Mediterranean communities and plants. Several aspects of pollination are influenced by climate, such as nectar quantity and concentration, flowering time and pollinator assemblages [10]. Indeed, nectary morphology, as well as the amount and concentration of nectar sugars, are related to the type of pollinator a plant is visited by [11–13]. In the case of E. plantagineum, each flower receives around 5,955 visits throughout the day, 90.2% of the time from the Apidae family. Of these, 87.8% correspond to Bombus terrestres, which visits 23.1 flowers per minute, and 2.4% to Apis mellifera, visiting approximately 15.4 flowers per minute [14].
In this study we set out to define different parameters of Echium (Viper′s bugloss) flower honey. Honey is a viscous and appreciated aromatic product prepared by bees mainly from flower nectar or honeydew [15]. In ancient Greece, it was believed that honey promoted longevity and virility, and there are written reports of its use in traditional medicine in ancient Egypt (5,000 years ago), as well as in ancient China and even Russia. The characteristics of honey, including its appearance, flavor, sweetness and texture, as well as its medicinal properties, attract thousands of consumers [15]. Although there is considerable variation in honey composition depending on the edaphic and botanical origin [16], the typical composition of honey is: moisture, 20.0%; carbohydrates, 79.7%; proteins, 0.2%; and ash, 0.1% [17]. Honey also contains a number of components that act as conservatives, such as vitamin C, flavonoids and other phenols, as well as enzymes like glucose oxidase, catalase and peroxidase [18]. Indeed, it has been suggested that the conservative properties of some of these substances is due to their antioxidant activity [19,20].
Honey has been termed a value added product ever since the initial studies confirmed that the antioxidant properties of polyphenols lie at the heart of their cosmetic [21–23], medical [24–26] and alimentary applications [27]. Indeed, antioxidant supplementation is beneficial in preventing certain diseases. Antioxidant capacity is generally assessed by two main methods, the quenching of various free radicals or the reduction of metal ions like iron, copper and chromium [28]. Hence, it is clearly important to determine the specific properties of each type of honey [17].
Significantly, E. vulgare is also a traditional medicinal herb that is used to treat kidney and respiratory diseases, to soothe irritated tissues, and to aid in wound healing. In Spain, this plant has been used in traditional medicine as an anti-catarrhal agent [29] and there are references to the properties of E. plantagineum to treat stomach ache [30]. Moreover, it is often one of the many blossoms contributing to multifloral honeys worldwide, accounting for at least 45% of the content except in monofloral honeys [31]. Accordingly, it is important to determine the precise proportions of pollen necessary to discriminate Echium monofloral honeys, and to quantify and specify the characteristics of viper’s bugloss honey in the Iberian Peninsula. Defining the features necessary to classify viper’s bugloss honey as monofloral will aid its labeling, thereby helping to control fraud [32], and it will help consumers who are looking for specific properties in the honey they consume, in line with the European Directive 2014/63/UE.
Materials and methods
Location of beehives and definition of the study area
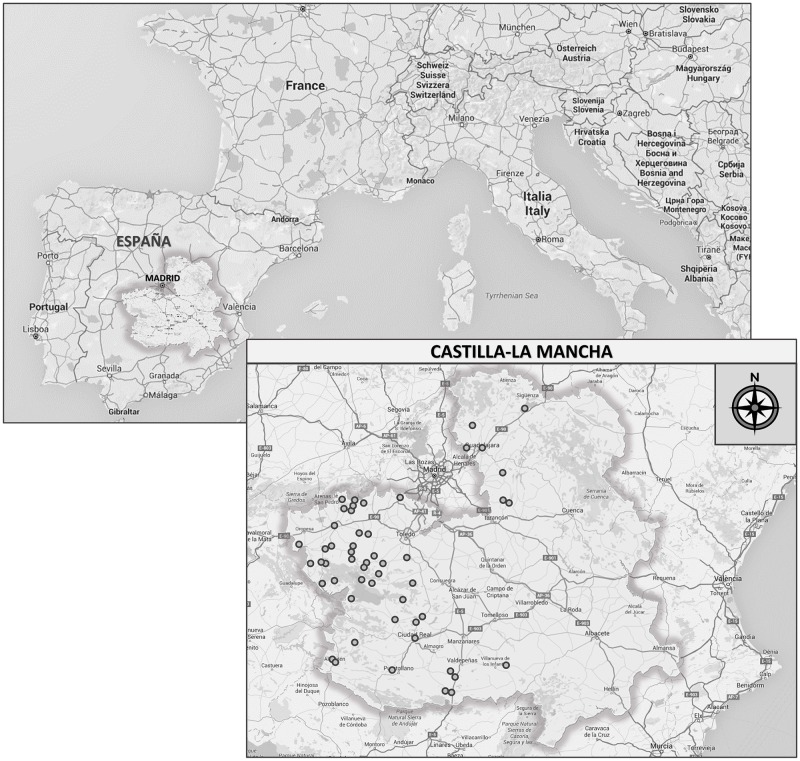
The honey samples were collected directly by beekeepers in different areas of Castilla-La Mancha, Spain (Fig 1, Table 1). There is no transhumance of the hives in this region and the honey is extracted by centrifugation of the combs. The beehives are located in open areas dominated by herbaceous (annual and biannual) and woody communities, indicating impaired land use. Hence, perennial plants that contribute to shrub substitution (Thymus, Lavandula, etc.) are represented in the honey, as well as other plants (Echium plantagineum, E. vulgare, Brassica, Diplotaxis, etc.), which indicate soil subnitrification or nitrification. Nevertheless, the most abundant plant is without any doubt E. plantagineum, a major component of the nitrophiles.
Fig 1. Map of “Castilla La Mancha” showing the sampling sites.
Table 1. Production sites and geographical coordinates.
| PRODUCTION SITES | LATITUDE | LONGITUDE | |
|---|---|---|---|
| Almaden | Ciudad Real | 38.774464 | -4.832621 |
| Almodovar Del Campo | Ciudad Real | 38.70828 | -4.175695 |
| Almuradiel | Ciudad Real | 38.513078 | -3.496555 |
| Anchuras | Ciudad Real | 39.479808 | -4.837157 |
| Chillon | Ciudad Real | 38.823685 | -4.872809 |
| Cortijos (Los) | Ciudad Real | 39.314464 | -4.051939 |
| Encomienda De Mudela | Ciudad Real | 38.641947 | -3.464775 |
| Fernan Caballero | Ciudad Real | 39.123329 | -3.901818 |
| Granatula De Calatraba | Ciudad Real | 38.798713 | -3.742239 |
| Horcajo De Los Montes | Ciudad Real | 39.327835 | -4.64891 |
| Malagon | Ciudad Real | 39.166806 | -3.853225 |
| Porzuna | Ciudad Real | 39.146809 | -4.155215 |
| Retuerta Del Bullaque | Ciudad Real | 39.463764 | -4.411779 |
| Saceruela | Ciudad Real | 38.945204 | -4.607595 |
| Santa Cruz De Mudela | Ciudad Real | 38.641078 | -3.467508 |
| Villahermosa | Ciudad Real | 38.750094 | -2.871427 |
| Viso Del Marques | Ciudad Real | 38.521597 | -3.562291 |
| Saceda-Trasierra | Cuenca | 40.154047 | -2.853658 |
| Algora | Guadalajara | 40.961388 | -2.666711 |
| Cantalojas | Guadalajara | 41.235902 | -3.245163 |
| Illana | Guadalajara | 40.183198 | -2.908477 |
| Malaguilla | Guadalajara | 40.821234 | -3.258991 |
| Pastrana | Guadalajara | 40.416124 | -2.921341 |
| Valdeavero | Guadalajara | 40.633257 | -3.33287 |
| Alcaudete De La Jara | Toledo | 39.785595 | -4.868919 |
| Aldeanueva De San Bartolome | Toledo | 39.636348 | -5.110638 |
| Almendral De La Cañada | Toledo | 40.183349 | -4.740042 |
| Belvis De La Jara | Toledo | 39.760413 | -4.948077 |
| Buenasbodas | Toledo | 39.642705 | -4.948727 |
| El Carpio De Tajo | Toledo | 39.888132 | -4.456805 |
| Garciotur | Toledo | 40.098992 | -4.646972 |
| Hinojosa De San Vicente | Toledo | 40.104194 | -4.723873 |
| Hontanar | Toledo | 39.612367 | -4.498327 |
| La Nava De Ricomalillo | Toledo | 39.63785 | -4.987049 |
| Los Navalmorales | Toledo | 39.725507 | -4.641161 |
| Los Navalucillos | Toledo | 39.664984 | -4.642452 |
| Malpica De Tajo | Toledo | 39.897551 | -4.548012 |
| Mazarambroz | Toledo | 39.694596 | -4.019743 |
| Menasalbas | Toledo | 39.641411 | -4.283342 |
| Montes De Toledo | Toledo | 39.55 | -4.333333 |
| Navahermosa | Toledo | 39.635595 | -4.472251 |
| Navas De Estena | Toledo | 39.493866 | -4.519261 |
| Nombela | Toledo | 40.156354 | -4.504445 |
| Nuño Gomez | Toledo | 40.114132 | -4.619857 |
| Pelahustan | Toledo | 40.176256 | -4.599207 |
| San Martin De Montalban | Toledo | 39.701862 | -4.388637 |
| San Martin De Pusa | Toledo | 39.782966 | -4.632208 |
| San Pablo De Los Montes | Toledo | 39.541629 | -4.332111 |
| Sevilleja De La Jara | Toledo | 39.460882 | -4.979902 |
| Talavera De La Reina | Toledo | 39.962884 | -4.830454 |
| Valdeverdeja | Toledo | 39.797424 | -5.246246 |
| Valmojado | Toledo | 40.204946 | -4.088713 |
The sampling territory is located in two biogeographic provinces: the Western Mediterranean Iberian province that includes Northwest Toledo and Guadalajara, where Quercus. suber L., Q. robur L. and Castanea sativa Mill. are found; and the Central Mediterranean Iberian Province that includes Albacete, Ciudad Real, Cuenca and Guadalajara. In both these territories, Quercus rotundifolia Lam. oak woods dominate the vegetation.
Samples
Samples of honey harvested consecutively from 2005 to 2013 were analyzed. The Echium honey was classified by melissopalynology and its sensorial characteristics were defined. Each of the honey samples (1 kg) was separated into two parts: one was stored at room temperature (18–22°C) and it was used for physicochemical and sensorial analyses; and the other was frozen at –30°C for further analysis. Except for the sensorial analyses, all the other characteristics were measured in duplicate. Of the 210 samples, 126 were selected in which Echium was present (157 samples in which Echium pollen represented at least 30% and 126 with more than 45% Echium pollen). At present, viper’s bugloss honey is considered monofloral when Echium pollen represents at least 45% of total pollen load [31].
Pollen analysis
The pollen was analyzed based on previously described methods [31]. The honey samples were treated chemically with acidified water (sulfuric acid, 10%), and a qualitative and quantitative analysis was performed (Von der Ohe et al. [32]) on the sediment recovered from 5 g aliquots. A minimum of 300 pollen grains were counted in each sample (between 300 at 1200 pollen grains), and the pollen grains from each sample were identified and classified on the basis of the identification keys available at the Centro Agrario de Marchamalo (CAR) honey laboratory [33–37]. This resource was supplemented by the manual and digital pollen collection that contains pollen slides already available in the laboratory, as well as by new pollen slides produced from the material collected during this study. The International Commission for Plant-Pollinator Relationships’ (ICPPR) recommendations were followed to classify the honey according to its floral origin [31], bearing in mind the minimum percentages of nectariferous pollen for monofloral honeys.
A quantitative analysis was performed on an aliquot (10μL) of the sediment obtained, expressing the results as the number of pollen grains per gram of honey. The number of pollen grains in the aliquot was counted under light microscopy and the magnification that is most suitable for identifying the various elements in the sediment (400 to 1000 ×). A qualitative analysis was also performed on the same sediment. The results were expressed as percentages and divided into the following frequency classes: P, pollen present, always below 1% of the pollen spectrum; R, minor pollen, representing between 1% and 3%; I, important pollen, representing between 3% and 15%; A, accompanying or secondary pollen, representing between 15% and 45%; and D, dominant pollen, equivalent to or above 45%.
Sensorial analysis
The sensory attributes were evaluated by a panel of trained honey analysts at the CAR. The 7 assessors on the sensory panel are trained according to the general guidelines (ISO 8586–1, [38], ISO 8586–2 [39] based on ISO 5492 [40], and they followed the protocol closest to that established and published in Apidologie by the research group for the sensorial description of monofloral honeys of the International Honey Commission (IHC: [33]). These analysts have 10 years of experience in describing honeys from the Alcarria and Castilla-La Mancha regions. The 7 assessors evaluated the color, odor (family odor and other olfactory perceptions, intensity and persistence), aftertaste, taste (salty, sweetness, bitterness and acidity), aroma, intensity and persistence and astringency of the honey, as well as the presence of crystallization. A descriptive score sheet was designed with a structured scale and a blank space where the assessors indicated their perceptions. The scale was designed with numerical intervals of 0–3 points and the assessors described the characteristics of each perception (especially family odor, secondary olfactory perceptions and taste attributes).
Physicochemical analysis
The parameters for honey quality were determined using the methods adopted and reviewed by the IHC [41] Moisture was determined by refractometry using a refractometer (Abbe 325; Auxilab S.L., Navarra, Spain), and the color of the honey was measured spectrophotometrically and using a Lovibond apparatus/tintometer (expressed as Pfund values). The pH of a 10% (w/v) solution of honey prepared in freshly boiled distilled water was measured potentiometrically at 20°C using an Eutech System XS PC510 pH-meter. The free acidity was obtained by plotting the neutralization curve with a titrated NaOH solution and determining the pH of the equivalence point. Electrical conductivity was measured at 20°C on a 20% (w/v) solution of honey (dry matter basis) prepared in deionized water using a Radiometer CDM-83 conductimeter and the results were expressed as mScm-1. Hydroxymethylfurfural (HMF) content was determined using the White spectrophotometric method.
Determination of the antioxidant capacity
The antioxidant activity was evaluated spectrophotometrically using the stable free radical DPPH test (1,1-diphenyl-2-picrylhydrazyl: see Vela et al., [42]).
Vitamin C
The vitamin C content was determined using the 2,6-dichloroindophenol titrimetric method (AOAC method for juices). The AOAC method involves a redox titration with 2,6-dichloroindophenol [43]. The vitamin C content was determined by RP-HPLC in isocratic mode, with a mobile phase of 0.01% (v/v) H2SO4 (Panreac) at pH 2.5, a flow rate of 0.9 mL/min, and UV detection at 245 nm and 25 (± 1)°C (the method used in this work was adapted from Vázquez-Oderiz et al., [44].and León-Ruiz et al., [45]).
Sugar profile
This approach aimed to identify parameters that discriminate between the different honey samples using a method described previously [45].The sugar content (glucose and fructose) was determined by HPLC but using refractometry detection (Merck RI-71). A mixture of acetonitrile:water (87:13% v/v) was used as the mobile phase at a flow rate of 1 mL/min. Separation was carried out at 40°C using a Lichrospher 100 NH2 (5 μm: Merck Darmstadt, Germany). Honey samples were dissolved to 5% (w/v) in water and filtered through a nylon syringe filter (0.45 μm).
Statistical analysis
Of the 210 samples collected in the sampling area containing viper’s bugloss pollen, 157 had at least 30% representation and these were the samples used in the present study. Among these samples, there are data on 23 chemical and 40 pollen variables that define the general spectrum of viper’s bugloss honey, although only some define the specific character of these honeys. After applying a second filter that removes those samples that do not have data for at least 10% of the variables, the study population was reduced to 126 samples.
To discern which variables most strongly influence the monofloral character of this type of honey, an initial correlation analysis was carried out between the percentages of Echium pollen (Echium type will appoint as from this moment) and each of the variables. This initial approach identified the variables most strongly related to a high representation of Echium type pollen, allowing the most robust parameters that present the strongest relationships to be selected, whether directly or inversely proportional. Subsequently, groups and cohorts were established with variations in the presence of pollen from the species under analysis within the ranges of 3%, 5% and 10%. A Principal Components Analysis (PCA) and a Cluster analysis of these cohorts was used to establish, with the greatest possible precision, at what percentage of pollen there is a tighter relationship between the proportion of Echium and the physicochemical characteristics studied. The Variables diagram revealed the evolution of these characteristics with the change in the percentage of Echium pollen. These statistical and graphical analyses were carried out with specific software, including Biplot 1.1 [46] and Olea-DP [47] working on Microsoft Excel and Tilia [48,49] or Conniss [50].
Results and discussion
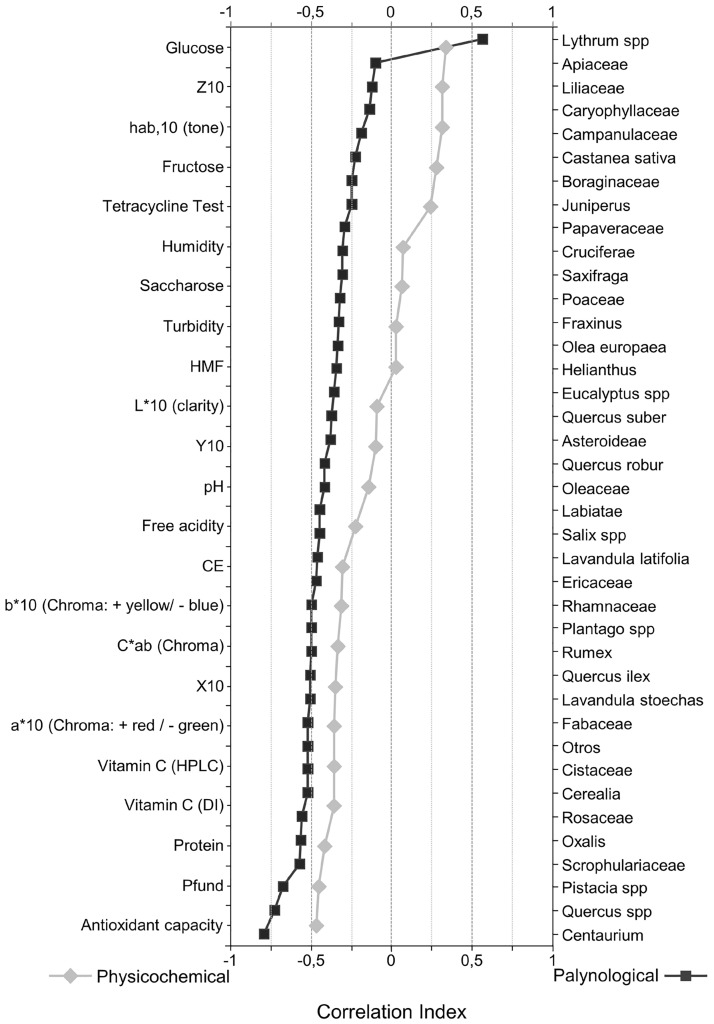
After the correlation analyses of the representation of Echium pollen type with respect to each of the variables analyzed in the samples, we obtain a first approximation as to which variables will have a stronger influence on the properties of the honey. As a result, there are clearly physicochemical variables that presented correlation indices close to 0, like HMF content and moisture, indicating that these variables have nothing to do with the changes in the proportion of Echium pollen in the honey (Fig 2, see also S1 and S2 Tables). The same can be seen with some pollen variables (Apiaceae, Liliaceae,…) that were clearly not related to the relative presence of Echium pollen and they were so weakly correlated that they were not considered further to avoid them contributing noise to the analysis. In view of these results, the number of physicochemical variables was reduced from 23 to 9, and the pollen variables from 60 to 22, selecting only those variables that were robustly related to the proportional representation of Echium pollen type. Indeed, the objective of this analysis was to summarize the information, eliminating the noise in order to ensure a correct interpretation of the results.
Fig 2. Correlation index: Variable analysis diagram.
Cohorts were generated in function of the changes in the representation of pollen of the species studied that were in the range of 3%, 5% and 10%. The PCA and Cluster analysis of these cohorts established, with the highest possible precision, the proportion of Echium pollen that is most closely related with the physicochemical characteristics studied. After this first filter, the number of samples was reduced based purely on quantitative criteria. Thus, all the samples that did not present information for at least 10% of the variables were excluded, a filter that reduced the number of samples from 157 to 126. Finally, the grouping of the samples into ranges or percentages provided a more comprehensive view of the data, leaving only the problem of expressing the data clearly and concisely.
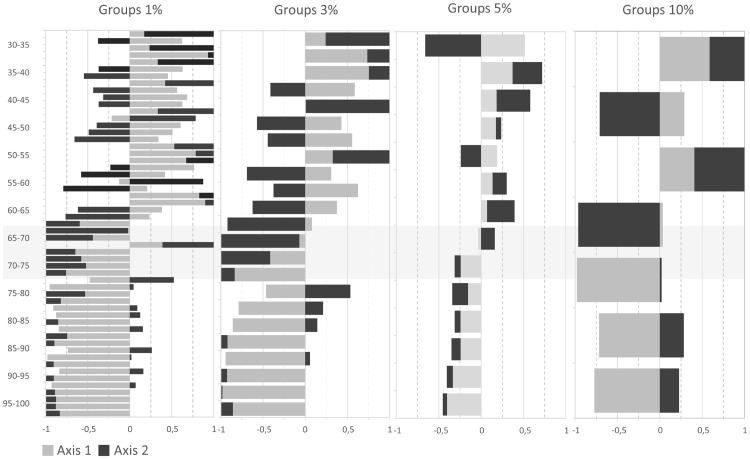
When the samples were grouped into cohorts that varied by 3%, 5% or 10%, a parallel behavior was evident when the PCA was applied. These analyses were carried out for a maximum of 4 axes and each of the models explains at least 76% of the variance. Ultimately, the cohort of 5% was chosen because it statistically presents a more homogeneous number of samples per segment. A clear distribution of variables was evident in this group, although it did not differ substantially from the distribution in the other groups (Fig 3).
Fig 3. Pollen cohorts.
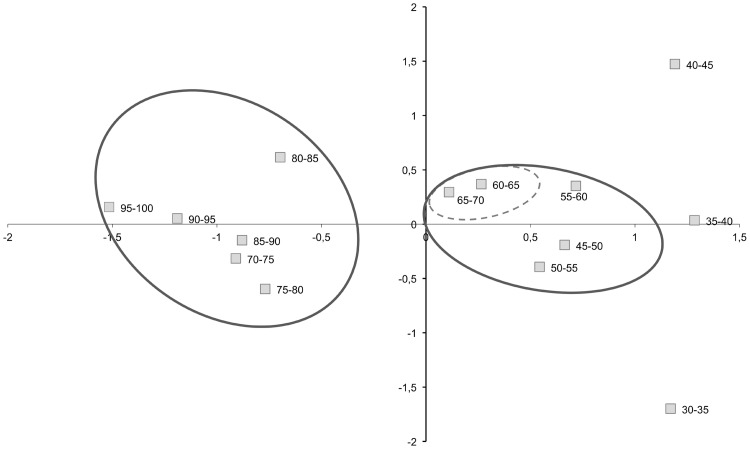
In the graphical representation of the PCA (Fig 4), the honeys with a pollen load above 65–70% displayed a distinct behavior in Axis 1. These honeys formed a group with more stable and homogeneous physicochemical characteristics, which distinguished them from the rest of the samples. These samples were situated in quadrants 2 and 3, with negative values of axis 1, while the rest of the honeys had positive values in this axis. In addition, the ranges of 60–65% and 65–70% formed a transitional subgroup, while the ranges with values of 30–35%, 35–40% and 40–45% present an anodyne dynamic.
Fig 4. Principal component analysis.
Segregation of the honeys in function of the proportion of viper’s bugloss pollen.
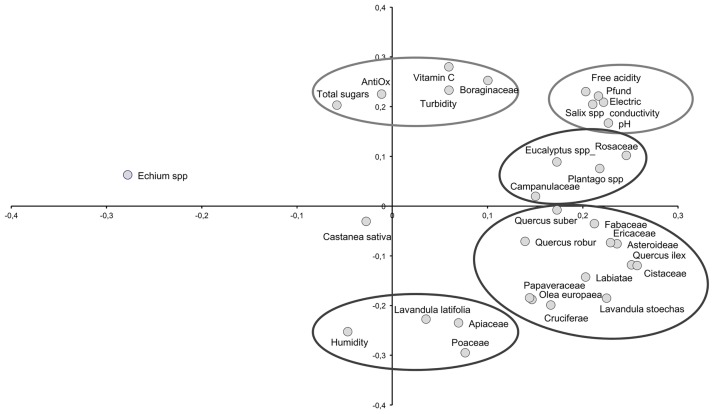
This segregation defined the evolution of the honeys with respect to the percentage of Echium pollen. We went from samples that had nothing to do with each other (30–45%), to samples with a consistency in terms of their physicochemical and pollinic characteristics (> 70%). A transition phase (60–70%) marked an evolution from multifloral honeys with a high percentages of Echium pollen to monofloral Echium honeys. As for the distribution, the physicochemical variables were situated in quadrants 1 and 2. This PCA distribution represented 88% of the variance within the first 2 axes (Fig 5).
Fig 5. Principal component analysis.
Distribution of variables.
Variables such as pH, color, conductivity and acidity were negatively correlated with the honeys that had an Echium pollen content >70%. By contrast, the turbidity, Vitamin C and sugar content, and the antioxidant capacity were strongly correlated with this type of honey. An increase in the amount of sugars in honey with a high representation of Echium has been confirmed previously, with viper's bugloss honeys reported to be richer in fructose and glucose in a study of monofloral honey [51]. This may reflect the local nectar composition, as the nectar of the Echium genus, and of the Borraginaceae family in general, is richer in sucrose than that of Rosemary, Eucalyptus, Citrus and Rosaceae, and as evident in monofloral honeys of these species and in several multifloral honeys [52–54]. Nevertheless, while this Borraginaceae family provides bees with more sucrose, glucose and fructose, the relationship between sucrose and hexoses (fructose + glucose) may be significant [12,13]. In the light of this relationship, the most attractive families are likely to be the Fabaceae, Ranunculaceae and Labiatae (28%, 14% and 4%, respectively) followed by the Borraginaceae, Asteraceae and Dipsacaceae (3%, 2% and 0.5%). The least attractive families in this sense are the Apiaceae (0.2%) and Liliaceae (0.3%: [10]). While the intense production of pollen and nectars by plants in the Echium genus does not situate them among the most attractive families, in open habitats, they are still sufficiently attractive as to produce monofloral honeys.
In terms of the antioxidant capacity of the honeys, which given the presence of polyphenols, flavonoids, etc. is not currently questioned [55,17], our data present this activity near the y-axis. All the honeys in our study are spring honeys and hence, their humidity is similar, situating this parameter in the horizontal axis to indicate that there is no relationship or variability. A negative correlation was seen between the pollen content and Pfund values. As the presence of viper’s bugloss pollen increases, honeys become more golden and transparent. This is consistent with the sensory characteristics described previously [17], reflecting a honey with a yellow gold color, and with a light clean taste, a floral bouquet and lemon characteristics.
The pollinic variables were distributed between quadrants 1 and 4, with higher values along axis 1, whereas Echium appears at negative values along this axis. Coupled with the segregation of these variables into three groups, this is indicative of a differentiation in function of ecology, plant size and the composition of the vegetation. The herbaceous species and communities rich in Echium constitute open subnitrophilous meadowlands (quadrant one), while the decrease in the representation of Echium pollen occurs in communities enriched with arboreal or woody species (quadrant four: [2,56,57]).
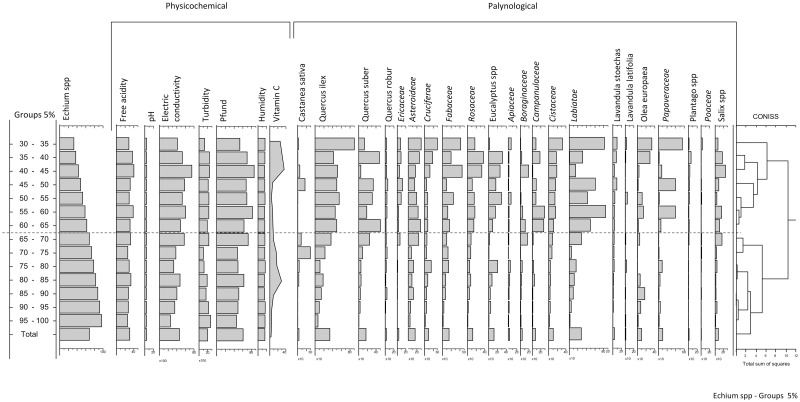
In a diagram of Variables (Fig 6) in which each are serially represented, a new Cluster analysis was carried out using the Coniss package [50]. This analysis segregated the samples into two clearly differentiated groups, where the boundary between the two groups was established at 65% of Echium pollen. This corroborates the data obtained previously in the PCA. Analyzing each parameter individually, we can see that the physicochemical parameters vary from 30 to 70% and that they follow nearly parallel Gaussian curves, such that the behavior of these parameters is almost similar to an Echium pollen load of 30% rather than that of 50%. From a representation of 70% Echium pollen onwards, the lines obtained are virtually exponential, indicating a perfect correlation that differs considerably from that seen at 45% representation, the minimum value currently established to consider viper’s bugloss honey monofloral.
Fig 6. Diagram of the variables: Cluster analysis, Coniss package.
Conclusions
In view of the analysis and results obtained, we found that a representation of Purple Viper's bugloss pollen in honey below 70% can be considered as multifloral. Therefore, we propose that to consider it as monofloral, the percentage of Echium type in the honey sediment should be at least 70%. After a statistical analyses of the physicochemical, sensory and pollen data of 126 honey samples in our study, the parameters that define the monofloral character of Purple Viper's-bugloss honey are essentially: pH, color, electrical conductivity, acidity and Echium pollen content. In addition, the monofloral honey of Purple Viper's bugloss produced in the center of the Iberian Peninsula has the following characteristics: high levels of major sugars like hexoses (glucose/fructose) and sucrose; relatively light amber golden honey; and a pollen spectrum rich in herbaceous taxa and low woody pollen taxa.
Supporting information
(PDF)
(PDF)
Acknowledgments
The authors wish to thank the professional beekeepers for supplying the samples. We also want to thank F. Besga, R. León, S. Rodrigo and A. Sanz for their support in the laboratory analysis.
Data Availability
All relevant data underlying this study come from the Database of CIAPA and can be found in the Supporting Information tables.
Funding Statement
This study was partly funded by the Junta de Comunidades de Castilla-La Mancha (05-299/IA-47, PAI09-0018-9267), INIA-FEDER (RTA2007-00072-C03 and Researcher Contract INIA-CCAA) and CONVENIO2014-001 JCCMGEACAM grants. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Muñoz Rodríguez A F, Tormo Molina R, Silva Palacioa I, Moreno Corchero A, Tavira Muñoz J. Airborne behaviour of pollen Echium pollen. Aerobiologia. 2005; 21:125–130. [Google Scholar]
- 2.Devesa JA, Ruiz T. Vegetación In: Devesa JA, editor. Flora y Vegetación de Extremadura. Universitas Editorial, Badajoz, España; 1995. pp. 81–115 [Google Scholar]
- 3.Valdés B. Echium L. In: Talavera et al., editors. Flora Ibérica vol. XI. Gentianaceae-Boraginaceae. Real Jardín Botánico, CSIC. Madrid, España; 2012. pp. 413–446
- 4.Díez MJ. Contribución al Atlas palinológico de Andalucía Occidental. I. Boraginaceae. Lagascalia. 1984; 13:147–171. [Google Scholar]
- 5.Díez MJ. Boraginaceae In: Valdés B, Díez MJ, Fernández I, editors. Atlas polínico de Andalucía Occidental. Instituto de Desarrollo Regional de Universidad de Sevilla, Utrera, Sevilla; 1987. pp. 265–281 [Google Scholar]
- 6.Hidalgo MI, Cabezudo B, Recio M. Producción floral en un matorral del Sur de España. Anales Jard. Bot. Madrid. 1996; 54:547–553. [Google Scholar]
- 7.Hidalgo MI, Recio M, Cabezudo B. Producción de polen en un matorral del Sur de España. Acta Bot. Malacitana. 1996; 21:49–55. [Google Scholar]
- 8.Corbet SA, Delfosse ES. Honeybees and the nectar of Echium plantagineum L. in the south-eastern Australia. Aust. J. Ecol. 1984; 9:125–139. [Google Scholar]
- 9.Corbet SA, Williams IH, Osborne JL. Bees and the pollination of crops and wild flowers in the European Community. Bee World. 1991; 72: 47–59. [Google Scholar]
- 10.Petanidou T. Sugars in Mediterranean floral nectars: an ecological and evolutionary approach. Journal of Chemical Ecology. 2005; 31: 065–1088. [DOI] [PubMed] [Google Scholar]
- 11.Baker HG, Baker I. Chemical constituents of nectar in relation to pollination mechanisms and phylogeny In: Nitecki HM, editors. Biochemical aspects of evolutionary biology. IL: Chicago University Press Chicago; 1982. pp. 131–171 [Google Scholar]
- 12.Baker HG, Baker I. A brief historical review of the chemistry of floral nectar In: Bentley B, Elias T, editors. The biology of nectarines. Columbia University Press; New York; 1983. pp. 126–152 [Google Scholar]
- 13.Baker HG, Baker I. Floral nectar sugar constituents in relation to pollinator type In: Little RJ, Jones CE, editors. Handbook of pollination biology. NY: Scientific and Academic Editions New York; 1983. pp. 117–141 [Google Scholar]
- 14.Guitián J, Guitián P, Navarro L. Tamaño del núcleo de población y polinización en E. plantagineum. Anales del Jardín Botánico de Madrid. 1993; 51 (1): 65–72. [Google Scholar]
- 15.Dustmann J H. Honey, Quality and its Control. American Bee Journal. 1993; 133(9): 648–651. [Google Scholar]
- 16.González-Porto AV, Martín Arroyo T, Bartolomé Esteban C. How soil type (gypsum or limestone) influences the properties and composition of thyme honey. SpringerPlus. 2016; 5(1), 1663 doi: 10.1186/s40064-016-3243-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagai T, Tanoue Y, Kai N, Suzuki N. Functional Property of Honey from Echium. Food and Nutrition Sciences. 2012; 3: 614–620. [Google Scholar]
- 18.Ferreres F, García Viguera C, Tomás Lorente F, Tomás Barberán FA. Hesperetin C a Marker of the Floral Origin of Citrus Honey, Journal of the Science of Food and Agriculture. 1993; 61: 121–123. [Google Scholar]
- 19.Çoban Ç, Ökkeş Y, Ayşe Dilek Ö, Oğuz Ayhan K. Biochemical analysis of some honey varieties of Ardahan region. Mellifera. 2014; 14 27-28:17–26. [Google Scholar]
- 20.Cerutti P. Oxy-Radicals and Cancer. Lancet. 1994; 344 n° 8926: 862–863. [DOI] [PubMed] [Google Scholar]
- 21.Jiménez MM, Fresno MJ, Selles E. The Galenic Behaviour of a Dermopharmaceutical Excipient Containing Honey. International Journal of Cosmetic Science. 1994; 16, 5: 211–226. doi: 10.1111/j.1467-2494.1994.tb00098.x [DOI] [PubMed] [Google Scholar]
- 22.Ediriweera ERHSS, Premarathna NYS. Medicinal and cosmetic uses of Bee’s Honey–A review. Ayu. 2012; 33(2): 178–182. http://doi.org/10.4103/0974-8520.105233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burlando B, Cornara L. Honey in dermatology and skin care: a review. Journal of Cosmetic Dermatology. 2013; 12: 306–313. doi: 10.1111/jocd.12058 [DOI] [PubMed] [Google Scholar]
- 24.Cooper RA, Molan PC, Harding KG. The sensitivity to Honey of Gram-Positive Cocci of Clinical Significance Isolated from Wounds. Journal of Applied Microbiology.2002; 93(5): 857–863. [DOI] [PubMed] [Google Scholar]
- 25.Khan IU, Dubey W, Gupta V. Medicinal Properties of Honey: A Review. Int. J. Pure App. Biosci. 2014; 2(5): 149–156. [Google Scholar]
- 26.Marshall S, Gu L, Schneider KR. Health Benefits and Medicinal Value of Honey. Food Science and Human Nutrition. FSHN15-04. 2015 [Google Scholar]
- 27.Nagai T, Inoue R, Kanamori N, Suzuki N, Nagashima T. Characterization of Honey from Different Floral Sources. Its Functional Properties and Effects of Honey Species on Storage of Meat. Food Chemistry. 2006; 97(2): 256–262. [Google Scholar]
- 28.Imlay JA. Pathways of oxidative damage, The Annual Review of Microbiology. 2003; 57: 395–418. doi: 10.1146/annurev.micro.57.030502.090938 [DOI] [PubMed] [Google Scholar]
- 29.Blanco Guzmán E, Ruano López A, Ugarte Libano R. Prescripción de antitusígenos por pediatras de Atención Primaria del País Vasco. Pediatría Atención Primaria. 2013; 15 (59): e85–e88. [Google Scholar]
- 30.Gürdal B, Kültür S. An ethnobotanical study of medicinal plants in Marmaris (Muğla, Turkey). J Ethnopharmacol. 2013; March 7; 146(1): 113–26. doi: 10.1016/j.jep.2012.12.012 Epub 2012 Dec 20. [DOI] [PubMed] [Google Scholar]
- 31.Louveaux J, Maurizio A, Vorwohl G. Methods of melissopalynology. Bee World.1978; 59 (4): 139–157. [Google Scholar]
- 32.Von Der Ohe W, Persano Oddo L, Piana ML, Morlot M, Martin P. Harmonized methods of melissopalynology. Apidologie. 2004; 35 (Suppl.1): S18–S25. [Google Scholar]
- 33.Oddo L, Piro R, Ivanov T, Piškulová J, Flamini C, Lheritier J, et al. Main European unifloral honeys: descriptive sheets. Apidologie. 2004; 35 (Suppl.1): S38–S81. [Google Scholar]
- 34.Valdés B, Díez, MJ. Fernández, I. Atlas polínico de Andalucía occidental. Instituto de Desarrollo Regional, Universidad de Sevilla, Excma. Diputación de Cádiz, Cádiz; 1987.
- 35.Carretero JL. Análisis polínico de la miel. Ed. Mundi-prensa, Madrid; 1989. [Google Scholar]
- 36.Moore PD, Webb JA, Collinson ME. Pollen analysis. Blackwell Scientific; London; 1991 [Google Scholar]
- 37.Saa Otero MP, Suarez Cervera M, Rodriguez-Gracia V. Atlas de polen de Galicia I. Deputación de Ourense. Ourense; 1996.
- 38.NO, ISO Standard. 8586–1. Sensory analysis–General guidance for the selection, training and monitoring of assessors–Part, vol. 1. 1993.
- 39.NO, ISO 8586–2. Sensory analysis. General guidance for the selection, training and monitoring of assessors-Part 2: experts. 1994.
- 40.Piana M L, Persano Oddo L, Bentabol A, Bruneau E, Bogdanov S, Guyot Declerck C. Sensory analysis applied to honey: state of the art. Apidologie. 2004; 35 (Suppl 1): S26–S37. [Google Scholar]
- 41.Bogdanov S, Kaspar Ruoff, Livia Oddo. Physico-chemical methods for the characterisation of unifloral honeys: a review. Apidologie. Springer Verlag. 2004; 35 (Suppl. 1): S4–S17. [Google Scholar]
- 42.Vela L, Lorenzo C, Pérez RA. Antioxidant capacity of Spanish honeys and its correlation with polyphenol content and other physicochemical properties. J. Sci. Food Agric. 2007; 87: 1069–1075. [Google Scholar]
- 43.International AOAC. Official methods of analysis of AOAC International. AOAC International; 2005. [Google Scholar]
- 44.Vázquez-Odériz ML, Vázquez-Blanco ME, López-Hernández J, Simal-Lozano J, Romero-Rodríguez MA. Simultaneous determination of organic acids and vitamin C in green beans by liquid chromatography. J AOAC Int. 1994; 77: 1056–1059. [PubMed] [Google Scholar]
- 45.León-Ruiz V, Vera S, González-Porto AV, San Andrés MP. Vitamin C and sugar levels as simple markers for discriminating Spanish honey sources. J. Food Sci. 2011; 76: 356–361. [DOI] [PubMed] [Google Scholar]
- 46.Smith EP, Lipkovich IA Biplot 1.1. Statistics Department. 1999–2002.
- 47.Martín Arroyo T, Ruíz Zapata MB, Gil García MJ. OLEA-DP: a new application used to plot pollen. In: Pollen. 2nd International APLE-APLF Congress: Pollen Biotechnology, Diversity and Function in a Changing Environment. 17–20 September 2013. Madrid, Spain. 2014; pp 139
- 48.Grimm EC. Tilia version 2. Illinois State Museum. Research and Collection Center, Springfield. IL 62703. USA. 1992.
- 49.Grimm EC. TGView. Illinois State Museum. Springfield. USA. 2004.
- 50.Grimm EC. CONISS: a FORTRAN 77 program for stratigraphicall Y constrained cluster analysis b Y the method of incremental sum of squares. Computers Geosciences. 1987; 13 (1): 13–35. [Google Scholar]
- 51.De La Fuente E, Ruiz-Matute AI, Valencia-Barrera RM, Sanz J, Castro IM. Carbohydrate composition of Spanish unifloral honeys. Food Chemistry. 2011; 129 (4): 1483–1489. [Google Scholar]
- 52.Maurizio A, Grafl I. Das Trachtpflanzenbuch Ehrenwirth Verlag. 1969. München. [Google Scholar]
- 53.Chwil M, Weryszko-Chmielewska E. Nectary structure and nectar secretion of Echium russicum J. F. Gmel. flowers. Acta Agrobot. 2007; 60 (1): 25–33. [Google Scholar]
- 54.Chwil M, Weryszko-Chmielewska E. Nectar production and pollen yield of Echium vulgare L. in the climatic conditions of Lublin. Acta Sci. Pol., Hortorum Cultus. 2011; 10 (3): 187–196. [Google Scholar]
- 55.Sanz ML, Gonzalez M, De Lorenzo C, Sanz J, Martınez-Castro I. A contribution to the differentiation between nectar honey and honeydew honey. Food Chemistry. 2005. 91 (2): 313–317. [Google Scholar]
- 56.Álvarez M L, Abreu OS, Molero Mesa J M, Guadalupe M L, Zafra ML, Calderón G M, et al. Algunas consideraciones sobre las comunidades nitrófilas de Granada (España). Anales del Jardín Botanico de Madrid. 1980; 37 (2): 737–763. [Google Scholar]
- 57.Álvarez M L, Andrés FN, Gutiérrez CJV. Comunidades nitrófilas salmantinas. Studia Botanica. 1983; 2:7–67. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All relevant data underlying this study come from the Database of CIAPA and can be found in the Supporting Information tables.