Abstract
The identification of immune correlates that are predictive of disease outcome for tuberculosis remains an ongoing challenge. To address this issue, we evaluated gene expression profiles from peripheral blood mononuclear cells following ex vivo challenge with Mycobacterium tuberculosis, among participants with active TB disease (ATBD, n = 10), latent TB infection (LTBI, n = 10), and previous active TB disease (after successful treatment; PTBD, n = 10), relative to controls (n = 10). Differential gene expression profiles were assessed by suppression-subtractive hybridization, dot blot, real-time polymerase chain reaction, and the comparative cycle threshold methods. Comparing ATBD to control samples, greater fold-increases of gene expression were observed for a number of chemotactic factors (CXCL1, CXCL3, IL8, MCP1, MIP1α). ATBD was also associated with higher IL1B gene expression, relative to controls. Among LTBI samples, gene expression of several chemotactic factors (CXCL2, CXCL3, IL8) was similarly elevated, compared to individuals with PTBD. Our results demonstrated that samples from participants with ATBD and LTBI have distinct gene expression profiles in response to ex vivo M. tuberculosis infection. These findings indicate the value in further characterizing the peripheral responses to M. tuberculosis challenge as a route to defining immune correlates of disease status or outcome.
Introduction
Globally, 10.4 million incident cases of active tuberculosis (TB) disease (ATBD) and 1.4 million TB-related deaths were reported in 2015 [1]. Over the past century, anti-TB drugs, bacille Calmette-Guérin (BCG) vaccination, and public health strategies such as directly observed treatment, have contributed to a reduction in TB-related mortality [1]. However, latent TB infection (LTBI) and recurrent ATBD remain critical issues for global control [1, 2], particularly given that previous anti-TB treatment is an established risk factor for drug resistance [3, 4].
Determining the contribution of Mycobacterium tuberculosis re-infection, as opposed to reactivation, is a challenge for numerous TB control programs, especially in endemic areas [5]. M. tuberculosis re-infection can occur at any time during ATBD and LTBI, and is independent of relapse [5]. Moreover, the inability of vaccination to protect against M. tuberculosis re-infection and reactivation represent significant gaps in research and therapeutics [6–8]. The sole approved vaccine BCG and vaccines in the development pipeline are largely protective against ATBD [9], and not LTBI or exogenous M. tuberculosis re-infection [8, 10]. Currently there is no approved and effective vaccine for individuals with LTBI [8, 10].
The assessment of vaccine efficacy hinges on identifying biomarkers predictive of disease progression and outcomes [8, 11, 12]. Given the numerous constraints for TB vaccine development, it has been hypothesized that identification of peripheral correlates of protective immunity against M. tuberculosis may be more realistic, compared to a single biomarker [13–15]. A unique transcriptional signature has been identified for ATBD; this whole-blood transcript signature was associated with disease severity and observed to resolve after treatment [16]. The extent to which this signature is predictive rather than diagnostic still needs to be determined. Previous studies have argued that differential gene expression was associated with TB disease recurrence, susceptibility, and host control [17–19]. Little is known regarding transcriptional biomarkers of post-primary M. tuberculosis re-infection or even enhanced exposure due to reactivation, despite the public health significance.
Distinguishing gene expression patterns following ex vivo challenge with M. tuberculosis among individuals with ATBD, LTBI, previous active TB disease (PTBD; after successful treatment) has significance in identifying diagnostic and predictive biomarkers, which are required for development of vaccines and therapeutics [20, 21]. Our study objective involved delineating patient responses to the ex vivo challenge of M. tuberculosis through analysis of the relative gene expression profiles between study participants with ATBD, LTBI, PTBD, compared to controls.
Materials and methods
Ethical conduct of research
The Institutional Ethics Review Committees at St. John’s Medical College and Hospital (St. John’s National Academy of Health Sciences; Bangalore, Karnataka, India) and Arogyavaram Medical Centre (Arogyavaram, Andhra Pradesh, India) approved the study protocol. Study participants provided voluntary informed consent prior to data collection.
Study population
Study participants (n = 40) were enrolled at a hospital outpatient department (St. John’s Medical College and Hospital, Bangalore, Karnataka) in India. Participants included four groups of patients with ATBD (n = 10) and LTBI (n = 10), PTBD (n = 10), and controls (n = 10). Inclusion criteria included TB status (defined below), and BCG vaccination. Exclusion criteria included: age (≤ 14 years), HIV infection (GS HIV Combo Ag/Ab EIA; Bio-Rad Laboratories, Redmond, Washington, United States).
Definitions of TB status included: ATBD, LTBI, and PTBD. ATBD status was based on acid-fast bacilli (AFB) sputum smear microscopy, which was performed by standard protocol (Ziehl-Neelsen staining), or Xpert MTB/RIF (Cepheid, Sunnyvale, California, United States). Patients with ATBD were newly diagnosed and had not initiated anti-TB treatment (or received <1 week of treatment). LTBI was diagnosed by QuantiFERON-TB Gold In-Tube (QFT-G; Cellestis Limited, Carnegie, Victoria, Australia). Blood samples were collected in QFT-G tubes, assayed according to manufacturer protocol and by enzyme-linked immunosorbent assay. Study enrollment was based on a positive QFT-G diagnostic result. PTBD was defined as having previous ATBD (pulmonary), anti-TB treatment, and post-treatment sputum conversion. PTBD patients received 6 months of anti-TB treatment for ATBD; study enrollment occurred between 2–371 days after completing treatment. Controls were considered individuals with a negative QFT-G diagnostic result.
Sample collection and peripheral blood mononuclear cell isolation
Venous blood samples (8 mL) were collected in mononuclear cell preparation tubes (CPT™; Becton Dickinson Vacutainer Systems; Franklin Lakes, New Jersey, United States), and processed per manufacturer’s instructions.
Peripheral blood mononuclear cells (PBMCs) were isolated from blood samples, based on manufacturer instructions (CPT™; Becton Dickinson Vacutainer Systems; Franklin Lakes, New Jersey, United States). PBMCs were suspended at 5 x 106 cells/mL in cryopreservation medium (45% RPMI 1640, 45% fetal bovine serum, 10% dimethyl sulfoxide), and incubated overnight at -80°C (in Mr. Frosty™ freezing container; Nalgene, Rochester, New York, United States). PBMCs were stored in liquid nitrogen prior to analyses.
Mycobacterium tuberculosis infection
Frozen PBMCs were thawed and incubated overnight (37°C in a 5% CO2 humidified incubator) in growth media (RPMI 1640 [Gibco Laboratories; Grand Island, NY] with 2mM glutamine, 10% fetal bovine serum, 10mM 2-[4-[2-hydroxyethyl]-1-piperazinyl] ethanesulfonic acid, [Antibiotic-Antimycotic solution; Gibco Laboratories; Grand Island, New York, United States]). Cells (106) were washed and re-suspended in fresh growth media containing 2 μg/ml whole cell lysate of M. tuberculosis strain H37Rv (Mycobacteria Research Laboratories at Colorado State University, Colorado, United States) for 48 hours (37°C in a 5% CO2 humidified incubator). An initial pilot study standardized the lysate concentration required for stimulation.
Ribonucleic acid isolation
Total ribonucleic acid (RNA) was extracted (RNA Easy Plus Kit; Qiagen, Hilden, Germany) and quantitated (Qubit® 2.0 fluorometer; Life Technologies, Milan, Italy) for suppression subtractive hybridization (SSH). In two patient groups (ATBD, LTBI), 300 ng total RNA from each individual were pooled and quantified. Pooled total RNA (300 ng) was used for complementary deoxyribonucleic acid (cDNA) synthesis (SMARTer™ polymerase chain reaction [PCR] cDNA Synthesis Kit; Clontech Laboratories, Inc., Palo Alto, California, United States).
Complementary deoxyribonucleic acid subtractive libraries
SSH libraries were created with the PCR-select cDNA subtraction kit (Clontech Laboratories, Inc.; Palo Alto, California, United States), per the manufacturer instructions. In forward subtraction (ATBD-LTBI), cDNA from the ATBD group was the tester and LTBI was the driver. Conversely, in reverse subtraction (LTBI-ATBD), LTBI cDNA was the tester and ATBD cDNA was the driver. Real-time PCR (Rotor Gene 6000; Qiagen Inc., Hilden, Germany) amplified the subtracted, unsubtracted, and control cDNA with M13 primers. Following PCR (DNA amplification), products were cloned in the PCR 2.1-TA vector (Invitrogen, Carlsbad, California, United States) and transformed into Escherichia coli Top10 cells. From forward subtraction, approximately 200 clones were obtained; in reverse subtraction, about 150 clones were obtained. Subtraction efficiency was evaluated by comparing glyceraldehyde-3-phosphate dehydrogenase expression in the subtracted and unsubtracted cDNA.
Dot blot hybridization
PCR product (5 μl) were transferred to two nylon membranes. The two identical membranes were hybridized with a tester and driver probe. Digoxigenin (DIG)-labeled probes (DIG-High Prime Labelling and Detection kit; Roche Diagnostics; Mannheim, Germany) visualized hybridization by a color reaction. The detected (differentially expressed) clones were sequenced (ABI 3730 DNA Analyzer; Applied Biosystems, Foster City, California, United States). Sequences were compared with the National Center for Biotechnology Information (NCBI; National Institutes of Health) reference database (Basic Local Alignment Search Tool [BLAST]).
PCR quantification of gene expression
Gene expression of the identified sequences (from hybridization) were further assessed with real-time PCR (Rotor Gene 6000; Qiagen Inc., Hilden, Germany) in replicate. Primers were designed with NCBI Primer-BLAST (S1 Table). Each PCR reaction included: 0.3 μg cDNA, 200 nM primers, 12.5 μL 2x KAPA SYBR FAST quantitative polymerase chain reaction (qPCR; KAPA Biosystems; Boston, Massachusetts, United States). PCR cycling conditions were: 1 cycle (95°C for 3 minutes); 35 cycles (95°C for 30 seconds, 60°C for 1 minute). Human acidic ribosomal protein (HUPO) was the internal control gene. Cycle thresholds (CT) were calculated through the Rotor Gene software (version 1.7.87).
Statistical analysis
Relative gene expression was reported as fold-change, based on the comparative cycle threshold (2-ΔΔCT) method [22] with HUPO as the internal control gene. Specifically:
| (1) |
where
| (2) |
[22]
For each comparison, fold-change was reported as the average of the fold-changes of the two replicates (Table 1).
Table 1. Pairwise comparison groupsa.
| ATBD | Controls |
| LTBI | Controls |
| PTBD | Controls |
| ATBD | LTBI |
| ATBD | PTBD |
| LTBI | PTBD |
aAbbreviations: active TB disease (ATBD), latent TB infection (LTBI), previous active TB disease (PTBD; after successful treatment)
For sociodemographic characteristics, the normality assumption of the age variable was assessed by the Kolmogorov-Smirnov test. Comparisons between the four study participant groups were evaluated by Kruskal-Wallis and Fisher’s exact tests. Statistical analysis was conducted with SAS (version 9.4; SAS Institute, Inc., Cary, North Carolina, United States); statistical significance was based on alpha value < 0.05 and two-tailed tests.
Results
Study population
Among the study participants, 65.0% were male (Table 2). The proportion of men differed across TB status (ATBD, LTBI, PTBD, controls) (p = 0.03). The median age was 31.5 years (interquartile range [IQR] 27.0–40.5; Table 2), and ranged between 18–65 years. Median age was similar in the four study participant groups (p = 0.32).
Table 2. Study participant characteristics (n = 40)a.
| Males, n (%) | 26 (65.0) |
| Age (years), median (IQR) | 31.5 (27.0, 40.5) |
| TB, n (%) | |
| ATBD b | 10 (25.0) |
| LTBI c | 10 (25.0) |
| PTBD d | 10 (25.0) |
| No TB (control) e | 10 (25.0) |
aAbbreviations: active TB disease (ATBD), latent TB infection (LTBI), previous active TB disease (PTBD; after successful treatment).
bAcid-fast bacilli [AFB] sputum smear microscopy
cIndividuals with a positive QuantiFERON Gold In-tube and negative AFB diagnostic
dIndividuals with previous ATBD who recently received anti-TB treatment for pulmonary TB (2–371 days prior to study enrollment), and post-treatment sputum conversion (based on AFB)
eControls were considered individuals with negative QFT-G and AFB diagnostic results.
Active TB disease associated with increased cytokine gene expression
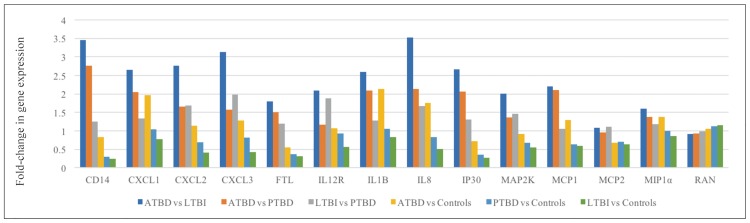
After M. tuberculosis infection, ATBD patient samples had increased gene expression of chemotactic factors (chemokine [C-X-C motif] ligand 1 [CXCL1], 3 [CXCL3], 8 [CXCL8 or interleukin [IL]-8]; chemokine [C-C motif] ligand 2 [CCL2; monocyte chemotactic protein [MCP]1; macrophage inflammatory protein [MIP] 1α] compared to controls (Fig 1, S2 Table). The fold-increases in chemokine expression were also greater among ATBD samples (2.7-fold CXCL1, 2.8-fold CXCL2, 3.1-fold CXCL3, 3.5-fold CXCL8, 2.2-fold CCL2, 1.6-fold MIP1α), compared to LTBI (Fig 1, S3 Table). In contrast, CCL8 (MCP2) gene expression was similar in comparing ATBD versus LTBI 1.1-fold (S3 Table).
Fig 1. Relative gene expression among patients with ATBD, LTBI, PTBD, and controlsa.
a Abbreviations: active TB disease (ATBD), chemokine (C-X-C motif) ligand: (CXCL), cluster of differentiation (CD), ferritin light chain (FTL), gamma-interferon-inducible protein (IP30), interleukin (IL), latent TB infection (LTBI), mitogen activated protein kinase kinase (MAP2K), macrophage inflammatory protein (MIP), monocyte chemotactic protein (MCP), previous active TB disease (PTBD; after successful treatment), Ras-related nuclear protein (RAN).
Relative to controls, ATBD was associated with higher gene expression of ILs (2.1-fold IL1B; Fig 1; S2 Table), which regulate the T helper 2 (Th2) response. In comparison to LTBI, ATBD samples had elevated IL1B (2.6-fold) and IL-12R (2.1-fold) gene expression (Fig 1).
Latent TB infection versus previous active TB disease
In comparing LTBI against PTBD groups, several chemotactic factors were elevated (1.7-fold CXCL2, 2.0-fold CXCL3, 1.7-fold CXCL8 [IL-8]) although others were similar (1.1-fold CCL2 [MCP1]; Fig 1).
Discussion
In summary, the gene expression profiles in ATBD, LTBI, PTBD, and control human study participants exhibit distinct patterns. These data provide a foundation for characterizing biomarker panels as correlates of protective immunity, which would serve as valuable surrogates in future development of TB vaccines and therapeutics.
Our findings are consistent with previous literature that observed elevated innate immune responses to M. tuberculosis, as well as M. tuberculosis evasion tactics in ATBD [23–28]. Similar to our results, other studies have established the importance of cytokines (including constitutive and stimulated chemokine gene expression such as MCP-1, IL-8, MIP1α) in host protection against M. tuberculosis [24–28]. M. tuberculosis has been shown to induce IL1B in dendritic cells, which upregulates the host Th2 response and dampens the protective Th1 response [23].
Additionally, similar to our results other data indicated that the gene expression of chemokines (including MIP1α) may differ during ATBD. Studies have identified a role for microbial lipoproteins in stimulating cytokine production (such as IL-12) in macrophages (via Toll-like receptors) [29]. MIP has also been found to be present in infectious pathogens, such as Chlamydial trachomatis [30].
Broadly, other studies have also highlighted the potential of biomarker panels (including host protein biosignatures) as indicators of the immune response against M. tuberculosis that could have diagnostic and/or predictive value [14–16, 31]. Furthermore, prior studies contend that differential gene expression was associated with increased risk of TB disease progression and relapse [17–19]. One study with whole-blood microarray analysis reported differential gene expression between patients with ATBD and LTBI, and identified gene expression profiles associated with host control of M. tuberculosis (specifically apoptosis and natural killer cell activity) [17]. Two studies indicated that differential gene expression profiles could associate with TB relapse [18, 19]. These studies show that biomarker profiles have potential to be more robust than single biomarkers of TB immunity. Interferon (IFN)-gamma, for example, is perhaps the most utilized candidate TB biomarker for a protective immune response against M. tuberculosis [32–35]; however IFN-gamma has low accuracy and predictive power, especially as a biomarker of protection or disease outcome [33].
Our study has several limitations. Firstly, PBMCs were cryopreserved prior to M. tuberculosis stimulation and RNA extraction, which could affect transcriptional processes. Secondly, the interpretation of gene expression in biological function remains preliminary [36]. Additionally, given the use of 2-ΔΔCT as a relative gene expression quantitation method [22], the findings need to be confirmed and extended with additional housekeeping controls [37]. Due to the pooling of samples in each group, there was no variance data, which restricted statistical analyses and corrections for multiple hypothesis testing. Further confirmatory studies could alternatively utilize groupwise comparisons.
Future studies are needed to elucidate the role of transcriptional signatures as immune correlates (including via single cell analysis [38, 39], prior to any claims that such profiles are predictive of clinical morbidities (ATBD, LTBI) and treatment outcomes. Studies to date have assessed cross-sectional data and therefore have not allowed for causal inference, which we could potentially address in a human cohort. Furthermore, studies need to account for other potential confounding factors, including the heterogeneity of patient immune response [40, 41]. Nonetheless, these data provide a useful platform in defining initial immunological themes that allow us to differentiate between human patients that fall into the active (ATBD), latent (LTBI), and active-treated (PTBD) disease classes.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Data Availability
Data are available in the eCommons repository at Cornell University (https://doi.org/10.7298/X4JH3JBN).
Funding Statement
Research reported in this publication was supported by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases; T32-DK007158 award); the Mario Einaudi Center for International Studies (South Asia Program), Human Ecology Alumni Association, Graduate School, Division of Nutritional Sciences at Cornell University (for E.A.Y. research and travel support). D.G.R. is supported by National Institutes of Health (National Institute of Allergy and Infectious Diseases; AI118582 award). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Allergy and Infectious Diseases, or the National Institutes of Health.
References
- 1.World Health Organization. Global tuberculosis report 2016. Geneva: World Health Organization; 2016.
- 2.Cox HS, Morrow M, Deutschmann PW. Long term efficacy of DOTS regimens for tuberculosis: systematic review. BMJ. 2008;336(7642):484–7. doi: 10.1136/bmj.39463.640787.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization and International Union Against Tuberculosis and Lung Disease (WHO/Union) Global Project on Anti-Tuberculosis Drug Resistance Surveillance 2002–2007. Anti-tuberculosis drug resistance in the world: fourth global report. Geneva: World Health Organization; 2008. [Google Scholar]
- 4.World Health Organization. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. Geneva: World Health Organization; 2010. [Google Scholar]
- 5.Lambert M-L, Hasker E, Van Deun A, Roberfroid D, Boelaert M, Van der Stuyft P. Recurrence in tuberculosis: relapse or reinfection? Lancet Infect Dis. 2003;3(5):282–7. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann SH. Fact and fiction in tuberculosis vaccine research: 10 years later. Lancet Infect Dis. 2011;11(8):633–40. doi: 10.1016/S1473-3099(11)70146-3 [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann SH, Hussey G, Lambert P-H. New vaccines for tuberculosis. Lancet. 2010;375(9731):2110–9. doi: 10.1016/S0140-6736(10)60393-5 [DOI] [PubMed] [Google Scholar]
- 8.Russell DG, Barry CE 3rd, Flynn JL. Tuberculosis: what we don't know can, and does, hurt us. Science. 2010;328(5980):852–6. doi: 10.1126/science.1184784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doherty TM, Dietrich J, Billeskov R. Tuberculosis subunit vaccines: from basic science to clinical testing. Exp Opin Biol Ther. 2007;7(10):1539–49. [DOI] [PubMed] [Google Scholar]
- 10.Lin MY, Ottenhoff TH. Not to wake a sleeping giant: new insights into host-pathogen interactions identify new targets for vaccination against latent Mycobacterium tuberculosis infection. Biol Chem. 2008;389(5):497–511. [DOI] [PubMed] [Google Scholar]
- 11.Walzl G, Ronacher K, Hanekom W, Scriba TJ, Zumla A. Immunological biomarkers of tuberculosis. Nat Rev Immunol. 2011;11(5):343–54. doi: 10.1038/nri2960 [DOI] [PubMed] [Google Scholar]
- 12.Petruccioli E, Scriba TJ, Petrone L, Hatherill M, Cirillo DM, Joosten SA, et al. Correlates of tuberculosis risk: predictive biomarkers for progression to active tuberculosis. Eur Respir J. 2016;48(6):1751–63. doi: 10.1183/13993003.01012-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobsen M, Mattow J, Repsilber D, Kaufmann SH. Novel strategies to identify biomarkers in tuberculosis. Biol Chem. 2008;389(5):487–95. [DOI] [PubMed] [Google Scholar]
- 14.Doherty TM, Wallis RS, Zumla A. Biomarkers of disease activity, cure, and relapse in tuberculosis. Clin Chest Med. 2009;30(4):783–96. doi: 10.1016/j.ccm.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 15.Doherty M, Wallis RS, Zumla A. Biomarkers for tuberculosis disease status and diagnosis. Curr Opin Pulm Med. 2009;15(3):181–7. [DOI] [PubMed] [Google Scholar]
- 16.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466(7309):973–7. doi: 10.1038/nature09247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maertzdorf J, Repsilber D, Parida SK, Stanley K, Roberts T, Black G, et al. Human gene expression profiles of susceptibility and resistance in tuberculosis. Genes Immun. 2011;12(1):15–22. doi: 10.1038/gene.2010.51 [DOI] [PubMed] [Google Scholar]
- 18.Mistry R, Cliff JM, Clayton CL, Beyers N, Mohamed YS, Wilson PA, et al. Gene-expression patterns in whole blood identify subjects at risk for recurrent tuberculosis. J Infect Dis. 2007;195(3):357–65. doi: 10.1086/510397 [DOI] [PubMed] [Google Scholar]
- 19.Cliff JM, Cho JE, Lee JS, Ronacher K, King EC, van Helden P, et al. Excessive cytolytic responses predict tuberculosis relapse after apparently successful treatment. J Infect Dis. 2016;213(3):485–95. doi: 10.1093/infdis/jiv447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parida SK, Kaufmann SH. The quest for biomarkers in tuberculosis. Drug Discov Today. 2010;15(3):148–57. [DOI] [PubMed] [Google Scholar]
- 21.Ma Z, Lienhardt C, McIlleron H, Nunn AJ, Wang X. Global tuberculosis drug development pipeline: the need and the reality. Lancet. 2010;375(9731):2100–9. doi: 10.1016/S0140-6736(10)60359-9 [DOI] [PubMed] [Google Scholar]
- 22.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3(6):1101–8. [DOI] [PubMed] [Google Scholar]
- 23.Dwivedi VP, Bhattacharya D, Chatterjee S, Prasad DV, Chattopadhyay D, Van Kaer L, et al. Mycobacterium tuberculosis directs T helper 2 cell differentiation by inducing interleukin-1beta production in dendritic cells. J Biol Chem. 2012;287(40):33656–63. doi: 10.1074/jbc.M112.375154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrero E, Biswas P, Vettoretto K, Ferrarini M, Uguccioni M, Piali L, et al. Macrophages exposed to Mycobacterium tuberculosis release chemokines able to recruit selected leucocyte subpopulations: focus on gamma delta cells. Immunology. 2003;108(3):365–74. doi: 10.1046/j.1365-2567.2003.01600.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monin L, Khader SA. Chemokines in tuberculosis: the good, the bad and the ugly. Semin Immunol. 2014;26(6):552–8. doi: 10.1016/j.smim.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhoades ER, Cooper AM, Orme IM. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect Immun. 1995;63(10):3871–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strieter RM, Belperio JA, Keane MP. Cytokines in innate host defense in the lung. J Clin Invest. 2002;109(6):699–705. doi: 10.1172/JCI15277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowdish DM, Sakamoto K, Kim MJ, Kroos M, Mukhopadhyay S, Leifer CA, et al. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 2009;5(6):e1000474 Epub 2009/06/13. doi: 10.1371/journal.ppat.1000474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285(5428):732–6. [DOI] [PubMed] [Google Scholar]
- 30.Neff L, Daher S, Muzzin P, Spenato U, Gulacar F, Gabay C, et al. Molecular characterization and subcellular localization of macrophage infectivity potentiator, a Chlamydia trachomatis lipoprotein. J Bacteriol. 2007;189(13):4739–48. doi: 10.1128/JB.01889-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Achkar JM, Cortes L, Croteau P, Yanofsky C, Mentinova M, Rajotte I, et al. Host protein biomarkers identify active tuberculosis in HIV uninfected and co-infected individuals. EBioMedicine. 2015;2(9):1160–8. doi: 10.1016/j.ebiom.2015.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178(6):2249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K, et al. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection-United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1–25. [PubMed] [Google Scholar]
- 34.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178(6):2243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goletti D, Sanduzzi A, Delogu G. Performance of the tuberculin skin test and interferon-gamma release assays: an update on the accuracy, cutoff stratification, and new potential immune-based approaches. J Rheumatol Suppl. 2014;91:24–31. doi: 10.3899/jrheum.140099 [DOI] [PubMed] [Google Scholar]
- 36.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–50. doi: 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, Hennen G, et al. Housekeeping genes as internal standards: use and limits. J Biotechnol. 1999;75(2–3):291–5. [DOI] [PubMed] [Google Scholar]
- 38.Fritzsch FS, Dusny C, Frick O, Schmid A. Single-cell analysis in biotechnology, systems biology, and biocatalysis. Annu Rev Chem Biomol Eng. 2012;3:129–55. doi: 10.1146/annurev-chembioeng-062011-081056 [DOI] [PubMed] [Google Scholar]
- 39.Rubakhin SS, Romanova EV, Nemes P, Sweedler JV. Profiling metabolites and peptides in single cells. Nat Methods. 2011;8(4s):S20–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su W-L, Perng W-C, Huang C-H, Yang C-Y, Wu C-P, Chen J-H. Association of reduced tumor necrosis factor alpha, gamma interferon, and interleukin-1β (IL-1β) but increased IL-10 expression with improved chest radiography in patients with pulmonary tuberculosis. Clin Vaccine Immunol. 2010;17(2):223–31. doi: 10.1128/CVI.00381-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barry CE, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7(12):845–55. doi: 10.1038/nrmicro2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Data are available in the eCommons repository at Cornell University (https://doi.org/10.7298/X4JH3JBN).