Abstract
Social dysfunction is common after traumatic brain injury (TBI), contributing to reduced quality of life for survivors. Factors which influence the emergence, development or persistence of social deficits after injury remain poorly understood, particularly in the context of ongoing brain maturation during childhood. Aberrant social interactions have recently been modeled in adult and juvenile rodents after experimental TBI, providing an opportunity to gain new insights into the underlying neurobiology of these behaviors. Here, we review our current understanding of social dysfunction in both humans and rodent models of TBI, with a focus on brain injuries acquired during early development. Modulators of social outcomes are discussed, including injury-related and environmental risk and resilience factors. Disruption of social brain network connectivity and aberrant neuroendocrine function are identified as potential mechanisms of social impairments after pediatric TBI. Throughout, we highlight the overlap and disparities between outcome measures and findings from clinical and experimental approaches, and explore the translational potential of future research to prevent or ameliorate social dysfunction after childhood TBI.
Keywords: Pediatric, traumatic brain injury, social interactions, social competency, communication, children, brain, behavior, social brain network, rodents, humans
1. Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability in children and adolescents (Faul et al., 2010; Thurman, 2014). The likelihood of sustaining a TBI is particularly high among young children under five years of age, as a consequence of falls, accidents, and inflicted trauma (Crowe et al., 2015; Faul et al., 2010; Love et al., 2009). Despite this prevalence, the long-term impact of TBI during early childhood remains poorly understood. Accumulating studies, including systematic reviews, have identified cognitive, emotional and behavioral impairments in survivors of pediatric TBI, as well as an increased risk of novel psychiatric problems (Anderson et al., 2014; Beauchamp and Anderson, 2013; Karver et al., 2012; Li and Liu, 2013). Of note, a younger age at insult is predictive of poorer post-injury outcomes and a more extended recovery trajectory across multiple domains including behavior, attention, language and cognition (Garcia et al., 2015). Occurring during a time when distributed neural networks underlying these functions are undergoing rapid maturation and consolidation, a TBI during childhood not only disrupts established neural functions, but can interfere with the acquisition of new skills and developmental milestones (Anderson et al., 2011). As a result, the full extent of post-injury morbidity may not be evident until many years after the insult, when high level social functions are expected to fully manifest (Wells et al., 2009).
Historically, physical and intellectual consequences of pediatric TBI have received greater attention than psychosocial problems. Over the past decade, however, impairments in social functioning after TBI have garnered increased recognition, with psychosocial problems often reported as causing significant and persisting distress for parents, teachers and carers (Catroppa et al., 2008; Catroppa et al., 2012; Cattelani et al., 1998; Chapman et al., 2010; Li and Liu, 2013). Social dysfunction can profoundly influence psychological well-being, academic and workplace performance, and community integration (Rosema et al., 2012; Rosema et al., 2014), contributing to a decline in quality of life (QoL) for survivors of childhood TBI (Di Battista et al., 2012; Williams et al., 2014; Yeates, 2013).
Deficits in social and behavioral functioning have been documented in the acute, sub-acute and chronic phases of pediatric TBI. Importantly, there is growing awareness that psychosocial problems, including deficits in social skills, may persist among young adult survivors of pediatric TBI, often independent of injury severity (Kaldoja and Kolk, 2015; McLellan and McKinlay, 2013; Scott et al., 2015). Factors that influence the emergence, development or persistence of social deficits post- injury remain poorly understood, particularly in the context of a brain that is still maturing and consolidating network connectivity. Continued research in both clinical and experimental arenas is needed to better understand mechanisms that underlie these social impairments after injury to the developing brain. Experimental models are invaluable to complement and clarify associative findings from patient-based studies, and allow for more invasive investigation of causal mechanisms. The purpose of this review is therefore threefold: (1) to draw attention to recent findings from clinical and experimental studies regarding factors which contribute to social dysfunction after pediatric TBI; (2) to highlight the overlap and disparity between studies of social behavior in human and rodent models; and (3) to explore the potential of future translational research into underlying biological mechanisms, aiming to ultimately improve social outcomes for survivors of brain injury.
2. Social functioning influences health and post-injury recovery
Many mammalian species, including humans and rodents, are inherently social animals. For humans, social contact with others is considered a critical determinant of good QoL, psychological adjustment and general welfare (Mering and Jolkkonen, 2015). At its most primitive, social behavior in some form is necessary for reproduction, which may underlie the observation that many of the neural and hormonal processes that influence social behaviors are highly evolutionarily conserved (Feldman et al., 2015; Insel and Fernald, 2004). The interaction between brain structure and function and social behavior is a complex, bidirectional relationship. In addition to brain insults having an impact on subsequent social behavior, the quality of social support received by an individual with TBI significantly influences their recovery trajectory (Anderson et al., 2005; Vangel et al., 2011).
For children with TBI, the longevity and severity of symptoms may be influenced by the post-injury family environment, particularly parental responsiveness, adjustment and resources (Potter et al., 2011; Yeates et al., 2010). Low socioeconomic status, family stress, permissive parenting, and a lack of resources and social support, exert a negative influence on social competence after TBI (Chapman et al., 2010; Yeates et al., 2004; Yeates et al., 1997). In experimental studies, such environments can be modeled by individually housing rodents. In this context, social isolation is particularly detrimental to general health, exacerbating morbidity and mortality after a range of acute injuries including experimental TBI, stroke and myocardial infarction (Boden-Albala et al., 2005; Doulames et al., 2014; Khodaie et al., 2015; Mering and Jolkkonen, 2015). Equally, the consequences of social impairments after TBI, including social isolation and withdrawal, may also contribute to a post-injury environment that is conducive to the persistence and/or exacerbation of poor social functioning.
An environment characterized by strong family functioning and parent mental health fosters resilience and buffers against the maladaptive psychosocial consequences of TBI (Ryan et al., 2013a; Yeates et al., 1997). In young patients, supportive friendships (Heverly-Fitt et al., 2014) and positive parenting (Wade et al., 2011; Woods et al., 2014a; Yeates et al., 2010) have been identified as factors which may facilitate behavioral recovery after TBI. The influence of a supportive environment can be further explored with experimental models, for example, by introducing multisensory housing conditions including social and cognitive challenges, collectively termed environmental enrichment (Brenes et al., 2015; Doulames et al., 2014). Environmental enrichment consisting of sensorimotor, social and cognitive challenges has repeatedly demonstrated benefit in rodent models of TBI, with ongoing research aiming to optimize the intensity and timing of interventions to drive future implementation of intervention strategies in the clinic (Bondi et al., 2014). Although rarely examined in isolation, evidence suggests that socialization is an important component of environmental enrichment, and a mediator of improved neurocognitive outcomes after TBI in rats (Sozda et al., 2010). Interestingly, social group housing is reportedly sufficient to ameliorate social behavior deficits observed in a mouse model of autism (Yang et al., 2011a). Whether an enriched environment would afford similar benefit on social impairments after brain injury remains to be determined.
3. Tracing the social landscape of pediatric TBI
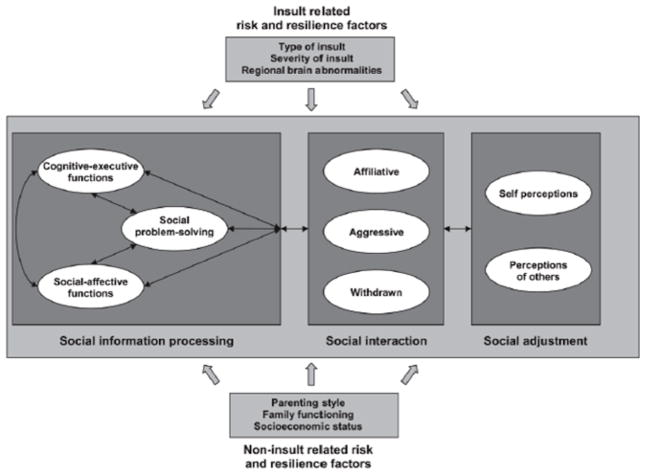
A theoretical understanding of how TBI influences social competency to manifest as aberrant social behaviors is grounded in concepts and methods from social neuroscience and developmental psychology. For example, social competence can be conceptualized as a multi-dimensional construct, partitioned into interrelated levels of social competence: individuals, interactions and relationships (Yeates et al., 2007). The Heuristic Model of Social Competence (HMSC; Figure 1) (Yeates et al., 2007) posits that at the individual level, children bring to bear individual abilities and characteristics needed to behave competently in social settings. For example, social competence has been linked to social information processing, an umbrella term that is conceived as involving a series of distinct problem solving steps that are implemented when children respond to social situations (Yeates et al., 2007). Social information processing incorporates a range of skills that include both cognitive-executive functions (e.g., inhibitory control and working memory), and social-affective processes (e.g., emotion perception, theory of mind (ToM), and empathy). These skills independently or interactively contribute to social competence firstly at the level of social interaction, which refers to children’s actual behavior and whether their responses are effective both in achieving their own goals and in maintaining positive relationships (Rubin and Bukowski, 2011). Secondly, social competence is influenced by social adjustment, which reflects the “extent to which children attain socially desirable and developmentally appropriate goals, and encompasses the quality of children’s relationships as perceived by others” (Beauchamp and Anderson, 2010). In this section, we aim to (1) critically evaluate current literature on the nature, causes, and risk factors associated with social impairment following pediatric TBI from a clinical perspective; and (2) identify and discuss the current challenges and limitations of measures used to evaluate social function in the TBI population.
Figure 1.
Heuristic model of social competence. Reproduced with Yeates et al. (2007), with permission from the American Psychological Association.
3.1. Social dysfunction post-pediatric TBI: a scoping review
As illustrated in Table 1, social adjustment is conceptualized as a multidimensional construct that encompasses parent, teacher and child perceptions of socio-emotional functioning (e.g., depression, anxiety, aggression), adaptive functioning, and social integration. Although research findings in this domain have been mixed, a large proportion of studies reveal that children and adolescents with TBI are at elevated risk for impairments in socialization, communication and adaptive behavior (Anderson et al., 2013; Chapman et al., 2010; Fletcher et al., 1990; Ganesalingam et al., 2011; Levin et al., 2009; Max et al., 2012; Poggi et al., 2005; Rosema et al., 2012; Yeates et al., 2004; Yeates et al., 2010), and are more likely to exhibit clinically significant depression, anxiety, aggression and anti-social behavior, relative to typically developing children (Andrews et al., 1998; Chapman et al., 2010; Max et al., 2012). Further, brain injury during early life is associated with poorer language competency and non-verbal communication, processes that are intricately linked with the development of social skills (Didus et al., 1999; Li and Liu, 2013; Sullivan and Riccio, 2010; Wells et al., 2009). Impairments in social communication may persist for many years after severe pediatric TBI (Cattelani et al., 1998; Hoofien et al., 2001; Ryan et al., 2013b).
Table 1.
Survey of social outcome instruments in studies of pediatric TBI.
| HMSC Construct | Definition | Measures | Author | Age range | Form |
|---|---|---|---|---|---|
| Social adjustment | “The degree to which children get along with their peers; the degree to which they engage in adaptative, competent social behaviour; and the extent to which they inhibit aversive, incompetent behaviour.” (Crick & Dodge, 1994, p. 82). | Vineland Adaptive Behavior Scale: VABS | Sparrow et al. (1984) | 0–90 years | Parent |
| Child Behaviour Checklist: CBCL | |||||
| Achenbach & Rescorla (2001) | 6–18 years | Parent | |||
| Eyberg Child Behavior Inventory: ECBI | |||||
| Eyberg and Robinson (1983) | |||||
| Preschool & Kindergarten Behavior Scales-2: PKBS-2 | Merrell (2002) | 2–16 years | Parent | ||
| Adaptive Behaviour Assessment System: ABAS | |||||
| Harrison & Oakland (2005) | 3–6 years | Parent | |||
| Children’s Loneliness Scale: CLS) Loneliness and Social Dissatisfaction Scale: LSDS) | Asher et al. (1984) | ||||
| 0–89 years | Parent | ||||
| Relational Provisions Questionnaire | Biggs et al. (2010) | ||||
| Harter Self Perception Profile for Children | 5–16 years | Parent | |||
| Community Integration Questionnaire: CIQ) | Hayden (1989) | 5–18 years | Parent | ||
| The Sydney Psychosocial Reintegration Scale for Children. | |||||
| Harter (1985) | |||||
| 5–16 years | Parent | ||||
| Willer et al., (1993) | |||||
| 8–16 years | Parent | ||||
| Soo et al., (2015) | |||||
| 5–18 years | |||||
| Parent | |||||
| 5–14 years | |||||
| Parent | |||||
| Social Interaction | “refers to the social actions and reactions between individuals and groups adapted to the situation” (Beauchamp & Anderson, 2010) | Friendship Quality Questionnaire (FQQ) | Parker & Asher (1989) | 6–18 | Child |
| Network of Relationships Inventory | Furman & Buhrmester (1985) | ||||
| 6–18 | Child | ||||
| Social information processing | “is conceived as involving a series of distinct problem solving steps that are implemented when children respond to social situations. Such steps would commonly involve interpreting cues, clarifying goals, generating alternative responses, and evaluating the outcome” (Yeates et al., 2007) | Cognitive-Executive Functions | |||
| Emotion Regulation Checklist: ERC | |||||
| 10-minute delay of gratification task: DGT | Shields and Cichetti (1998) | 5–18 years | Child | ||
| Mischel and Ebbesen (1970) | |||||
| Flanker-No-Go Task | |||||
| Bunge et al. (2001) | 5–18 years | Child | |||
| Test of Everyday Attention for Children (TeaCH) | |||||
| Manly et al. (1999) | |||||
| Social-affective functions | |||||
| 6–18 years | Child | ||||
| The Jack & Jill Task | |||||
| 6–16 years | Child | ||||
| Emotional and Emotive Faces Task | |||||
| Dennis et al. (2012) | |||||
| The Ironic Criticism and Empathic Praise Task | Dennis et al. (2013) | ||||
| The Test of Language Competence-Expanded Edition: Making Inferences | 5–16 years | Child | |||
| Dennis et al., (2001) | 5–16 years | Child | |||
| Interpersonal Negotiation Strategies Interview | |||||
| Facial Emotion Sorting | Wigg, & Secord (1989) | 5–16 years | Child | ||
| Faux Pas Test | |||||
| Florida Affect Battery: FAB | 5–18 years | Child | |||
| Mind in the Eyes Test | Schultz et al. (1988) | ||||
| Strange Stories Test | |||||
| Virtual Reality Interpersonal | Adolphs et al. (2001) | 5–18 years | Child | ||
| Negotiations Strategy | |||||
| Baron-Cohen et al. (1999) | |||||
| The Awareness of Social Inference Test: TASIT | |||||
| Bowers et al. (199) | All ages | Child | |||
| Comprehensive Assessment of Spoken Language: CALS | Baron-Cohen et al. (2001) | 6–18 years | Child | ||
| Happe (1994) | 6–18 years | Child | |||
| NEPSY-II: Theory of Mind and Affect Labeling | |||||
| Hanten et al. (2011) | 6–18 years | Child | |||
| 6–18 years | Child | ||||
| 5–18 years | Child | ||||
| Flanagan et al. (1998) | |||||
| Carrow-Woolfolk (1999) | 13–18 years | Child | |||
| Brooks et al. (2009) | 5–18 years | Child | |||
| 5–16 years | Child |
Note: Adapted from Rosema et al., 2012.
Social competence, as measured at the level of social interaction, is commonly assessed using indirect socio-metric ratings of friendship quality, such as those obtained from both parent and child ratings obtained from the Friendship Quality Questionnaire (FFQ; Table 1) (Parker and Asher, 1993). Parent report measures reveal that children with TBI tend to have fewer close friendships than typically developing children (Prigatano and Gupta, 2006; Yeates et al., 2013). In contrast, studies using child self-report ratings have found no significant differences between those children with and without TBI (Bohnert et al., 1997; Ross et al., 2011b). These discrepant findings highlight an important distinction between the perceptions of others (e.g., peers, parents, teachers) and perceptions of children with TBI, who may lack awareness of their own deficits and therefore evaluate their social interactions more positively than others do (Beauchamp and Anderson, 2010; Yeates et al., 2007).
While there exists a large body of evidence that links pediatric TBI to a range of directly observable behavioral changes at the level of social interaction and social adjustment, the mechanisms that explain this association are poorly understood. One hypothesis is that TBI affects the distributed neural networks implicated in social-affective functions, or the ability to perceive and process social cues and context (Beauchamp and Anderson, 2010). These skills emerge relatively early in development and illustrate protracted development into late childhood and mid-late adolescence, coinciding with the extended structural and functional maturation of the ‘social brain network’ (SBN), an anatomically distributed frontal temporo-limbic circuit that comprises many of the same regions most vulnerable to the effects of contusions, compression injury, and shear/strain forces (Bigler, 2013) (see Section 6.1). Consistent with the vulnerability of the SBN to early disruption from TBI, relative to typically developing controls, children with TBI exhibit significantly poorer emotion perception (Ryan et al., 2013a; Tlustos et al., 2011) and ToM (Bellerose et al., 2015; Dennis et al., 2012; Walz et al., 2010), and experience greater difficulty choosing the optimal solution to social dilemmas (Janusz et al., 2002). Despite converging evidence for deficits in social-affective functions, in the clinical context there exist a dearth of reliable and ecologically valid tools to assess these skills, and thus clinicians remain poorly equipped to identify children at risk for these deficits.
3.2. Assessment of social-affective functions in children with TBI
In contrast to behavior at the level of social adjustment and social interaction, a reliable estimate of social information processing is difficult to obtain from traditional rating scales completed by parents and teachers. As summarized in Table 1, there exist a variety of social cognitive test batteries designed to tap these specific but often highly complex social cognitive skills. Despite increasing knowledge and awareness among clinicians of the need to assess these skills in pediatric brain disorders, many of these measures lack sound theoretical grounding and are unable to isolate deficits in specific aspects of social information processing. In particular, given that social information processing is reliant on social-affective and cognitive-executive skills that are tightly linked and share overlapping neuroanatomical substrates within the developing brain (Yeates et al., 2007), the majority of measures do not distinguish between domain-specific and domain-general demands of these highly complex tasks, thus thwarting the opportunity for clinicians and researchers to identify meaningful targets for intervention and management.
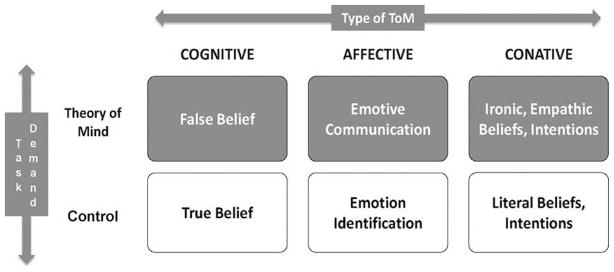
The challenges associated with social outcome measurement in pediatric TBI are perhaps best illustrated in recent approaches to the study of ToM; a multi-dimensional construct that allows individuals to ascribe a variety of psychological states, such as intentions or emotions, to understand and subsequently predict behaviors (Blakemore, 2008; Herbet et al., 2013). Grounded in evidence from lesion-deficit and neuroimaging studies, the tripartite model of ToM (Figure 2), partitions these skills into cognitive ToM, concerned with basic, belief-based inferences traditionally assessed using paradigmatic false-belief tasks (Wellman et al., 2001); and ‘complex cognitive-affective ToM’(Dennis et al., 2013b). The latter can be further parcellated into conative ToM, defined as the ability to understand how indirect speech acts involving irony and empathy are used to influence the mental or emotional state of the listener; and affective ToM, concerned with understanding that while facial expressions can convey emotions actually felt (emotion identification), they are also used for social purposes to convey emotions that we want people to think we feel (emotive communication) (Dennis et al., 2013b; Hein and Singer, 2008).
Figure 2.
Tripartite model of Theory of Mind. Reproduced from Dennis et al. (2013), with permission from Elsevier.
Previous reports show that ToM is mediated by anatomically distributed brain neural networks implicated in a range of tightly linked social-affective and cognitive-executive processes, which interact dynamically to contribute to social information processing abilities (Dennis et al., 2012; Frith and Frith, 2005; Yeates et al., 2007). Research to date has failed to clarify whether poor performance on measures of ToM reflects difficulty with the domain-general task demands, or a domain-specific ToM impairment, independent of other cognitive abilities required to complete the task. In addressing this limitation, Dennis et al. (2013) recently reported on a series of measures grounded within the tripartite model of ToM, to isolate specific inefficiencies at various levels of social information processing. These measures have been employed in a two longitudinal prospective studies of children with TBI, with results indicating that, while severe TBI was associated with significantly poorer cognitive ToM, children with TBI of all levels of severity showed poorer performance on measures of conative and affective ToM (Dennis et al., 2013a; Dennis et al., 2013b; Dennis et al., 2012). This pattern suggest that, relative to basic ToM abilities, higher-order ToM demonstrates a lower threshold for perturbation and appears vulnerable to even mild generalized injuries.
On measures of affective ToM, Dennis et al. (2013b) also report that children with TBI demonstrate comparable levels of impairment on both ToM and non-social cognitive control trials, suggesting that poorer performance at least partly reflects inefficiencies in response speed, reduced auditory attention span and poor inhibitory control. In contrast, in a second study, Ryan and colleagues employed the same ToM paradigm but found that, while children with TBI demonstrated comparable performance to typically developing controls on control trials, they were impaired on trials of cognitive, affective and conative ToM (Ryan et al., 2015c). Additional research is required to elucidate whether these difficulties may constitute a potential mechanism that underpins the empirical association between pediatric TBI and elevated risk for a range of maladaptive behaviours that are shown to persist or even worsen with time since injury.
4. Identifying social behavior impairments in brain-injured rodents
Rodent models of TBI, including fluid-percussion injury (FPI), controlled cortical impact (CCI), weight-drop and blast injury models, have been well-characterized in terms of their distinct patterns of temporal neuropathology [refer to review by (Xiong et al., 2013) for methodological details]. In both rats and mice, these models reliably reproduce many of the common cognitive, sensorimotor and neuropsychiatric consequences of human TBI (Gold et al., 2013; Malkesman et al., 2013). However, few studies to date have examined social changes in the context of experimental brain injuries. Several tests for social competence, measured primarily at the level of social interaction, are established in other neuroscience specialties and could be readily adopted by the neurotrauma field to provide insight into the mechanisms, risk factors and co-morbidities associated with social dysfunction after TBI. Paradigms to assess social interactions in rodents have been developed and extensively validated within recent years to phenotype core autistic-like symptoms in novel mouse models of autism-spectrum disorders (Crawley, 2012; Silverman et al., 2010; Wöhr and Scattoni, 2013). Many aspects of social information processing, including cognitive/executive function (e.g., working memory, inhibitory control) as well as socio-emotional functioning (e.g., anxiety, aggression and depressive-like symptoms) can be evaluated in rodents. Rodents exhibit rapid brain development, high sociability throughout life, and the capacity for genetic manipulation to explore molecular mechanisms, making them well-suited to investigate the impact of injury and interventions on social outcomes.
4.1. Key measures used to evaluate social health in rodents
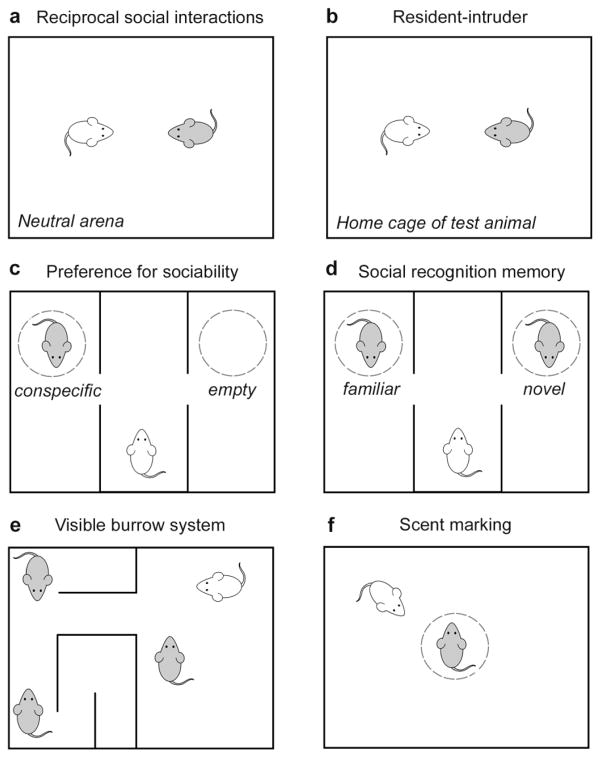
Social behavior in rodents can be investigated using various behavioral paradigms, to assess aspects of sociality, affiliation and social approach. In particular, laboratory mice Mus musculus display a broad repertoire of quantifiable social behaviors, and several etiologically-relevant assays with good face validity (i.e. resemblance to the human phenomenology) have been developed to measure different aspects of social behavior in mice (Crawley, 2007; Silverman et al., 2010; Terranova and Laviola, 2005) (see Figure 3). As in patients, a combination of complementary tasks is often the most useful to gain an overall perspective on the level of social functioning.
Figure 3.
Assays for social behavior in rodents. A range of experimental paradigms can be used to evaluate social behaviors between rodents, including the evaluation of social interactions in a neutral environment (a) or home cage of the test animal (b). The three-chamber apparatus provides a measure of sociability (c) and subsequent evaluation of social recognition memory (d). Social behaviors can alternatively be examined within a group housing context (e). Social communication may be quantified by the deposition of urinary scent marks in response to a conspecific (f). Adapted from Sandi (2015).
The most common experimental paradigm involves two unfamiliar rodents permitted free exploration for a period of time, for the quantification of social interaction or social investigation by the test subject. When conducted in a novel environment, this task is ideal for qualifying reciprocal social interactions, while the introduction of a novel stimulus rodent into the home cage of the test animal is typically referred to as a resident-intruder test (Terranova and Laviola, 2005; Wöhr and Scattoni, 2013). The latter scenario is more likely to encourage territorial and aggressive behaviors in male rodents (Duvoisin et al., 2011; Koolhaas et al., 2013). In both contexts, a wide spectrum of complex, interactive behaviors may be quantified individually (e.g., sniffing, climbing, playing), or the total duration of social investigation can be tallied for comparison between groups of subjects. Repeated testing in the same animals over time is possible, allowing for the evaluation of developmental trajectories across age (Silverman et al., 2010).
In addition to the resident-intruder paradigm, social aggression or competition can be induced between pairs of male rodents to quantify antagonistic and territorial behaviors. The tube-dominance test evaluates the dominant-submissive relationship established during a brief encounter (Molina et al., 2008; Spencer et al., 2005; Wang et al., 2011), while the visible burrow system allows for the observation of social hierarchies formed between group-housed animals (Blanchard et al., 1995).
One limitation of the above-mentioned paradigms is that social interactions may be initiated and motivated by either the test or stimulus animals. To avoid this, the three-chamber social approach task restricts the stimulus rodent to a wire-enclosed cup, such that social approach behaviors of the test animal alone can be readily quantified, and social interactions are limited to the visual, olfactory and auditory senses (Nadler et al., 2004; Silverman et al., 2010; Yang et al., 2011b). In this task, the test animal is presented with a choice between chambers containing either the stimulus animal or an empty cup, with preferential proximity and investigative sniffing of the stimulus considered to be an indicator of sociability (Moy et al., 2004; Moy et al., 2007). The subsequent addition of a second, novel stimulus animal to the previously-empty chamber next presents the test rodent with a choice of social partners, and a preference for proximity to the novel animal is quantified as an indicator of social recognition and social memory (Silverman et al., 2010). An alternative paradigm for social recognition is the habituation-dishabituation test, which involves short, sequential encounters between pairs of test and stimulus animals in an open arena, such that the test animal is repeatedly exposed to the same novel conspecific. Recognition is quantified as a decline in social investigation across repeated encounters with the same animal (i.e. habituation), which appears to be dependent upon short-term memory with a duration of between 90–180 minutes (Crawley, 2004). During a final encounter, a novel stimulus rodent is introduced, and a high degree of investigative time is expected as an indicator that the test animal exhibits a preference for social novelty, implicating intact social recognition (Gheusi et al., 1994).
As described earlier (Section 3.1), social communication problems are commonly reported after TBI. Although it remains controversial whether rodents have comparable brain regions necessary to mediate language skills and human-like vocal learning (Arriaga and Jarvis, 2013; Hammerschmidt et al., 2015), several non-verbal means of social communication are readily quantifiable in both rats and mice. Changes in social communication between rodents have recently emerged as a signature characteristic of autistic-like mouse models (Wöhr and Scattoni, 2013). Urinary scent marking is performed by male mice to deposit pheromones in a context-dependent manner, in order to mark territory, attract mates and convey information about health status (Wöhr and Scattoni, 2013), and changes in this behavior are considered an indicator of social dysfunction (Arakawa et al., 2007, 2008; Wöhr et al., 2011). Male rodents in particular also communicate via the emission of ultrasonic vocalizations as auditory signals (Bean et al., 1981). Vocalizations differ in type, pattern and frequency depending upon the social context (Wohr et al., 2015). For example, rats typically emit low frequency (22 kHz) vocalizations in response to aversive stimuli such as an aggressive encounters or exposure to a predator. In contrast, 50 kHz calls are elicited by social and sexual encounters, and are associated with activation of the dopaminergic system (Wohr and Schwarting, 2013). Ultrasonic vocalizations are readily produced by isolated mouse and rat pups, typically eliciting a maternal retrieval response. Although the precise meaning of individual vocalizations remains to be elucidated, alterations in the number and temporal patterns of ultrasonic vocalizations in a social context correlate with social deficits in rodents (Scattoni et al., 2011; Wöhr et al., 2011; Yang et al., 2013), and are typically considered to reflect social interest, recognition and motivation (Wöhr and Scattoni, 2013).
4.2. Social dysfunction after experimental TBI in adult and pediatric rodents: findings to date
The above-described paradigms to evaluate social function in rodents can be applied to neurotrauma research with several objectives; (1) to generate in-depth behavioral phenotyping and characterization of different injury models; (2) for hypothesis-driven studies designed to evaluate proposed risk factors associated with social dysfunction after TBI in patients (e.g., the effect of injury severity, location, age-at-insult); and (3) to evaluate potential therapies for the treatment of TBI-induced social deficits and co-morbidities.
Even in adult models of TBI, the number of studies that have incorporated measures of social outcomes remains limited to date (see Table 2). Across a range of injury models including FPI, CCI, impact acceleration, blast and repetitive closed skull impacts of varying severity, investigators have reported either a reduction in social investigation and social recognition after TBI (Bajwa et al., 2016; Fenn et al., 2013; Klemenhagen et al., 2013; Koliatsos et al., 2011; Pandey et al., 2009; Semple et al., 2012; Semple et al., 2014), or the lack of a difference compared to sham-operated controls (Patel et al., 2014; Shultz et al., 2012; Shultz et al., 2011). Further studies are needed to validate the incorporation of specific social tasks in this context, as the characterization of social outcomes across a range of TBI models will likely enhance our understanding of the complex interaction between neuropathology and functional outcomes.
Table 2.
Key symptoms of social dysfunction after TBI in humans, and their hypothesized analogous behavioral test in rodents.
| Human symptom | Analogous behavioral task in rodents | Evidence in experimental TBI |
|---|---|---|
| Inappropriate social interactions | Reciprocal social interactions; social approach | Reduced social investigation by male mice at adulthood after pediatric CCI (Semple et al. 2012; 2014) |
| Reduced social investigation by male mice after adult FPI (Fenn et al. 2013) | ||
| Preference for social novelty and social recognition | Reduced social recognition by male mice at adulthood after pediatric CCI (Semple et al. 2012) | |
| Reduced social recognition by male mice after adult blast injury (Koliatsos et al. 2011) | ||
| Reduced social recognition by male mice after adult repeated closed skull impacts in rats (Klemenhagen et al. 2013) | ||
| Sociosexual interactions | Reduced sexual behavior towards a stranger female at adulthood after pediatric CCI in mice (Semple et al. 2014) | |
| Reduced sexual behavior towards a stranger female after repeated adolescent closed skull impacts in rats (Greco et al. 2015) | ||
| Reduced sexual behavior towards a stranger female after adult impact acceleration injury in rats (Pandey et al. 2009) | ||
| Social play | Altered social play after mild TBI in young rats, dependent upon sex and injury status of stimulus rat (Mychasiuk et al. 2014) | |
| Social aggression | Resident-intruder paradigm | No effect on frequency or timing of fighting with a stranger male by adult male mice after pediatric CCI(Semple et al. 2012) |
| Tube dominance test | Increased likelihood of exerting dominance over a stranger male, in adult male mice after pediatric CCI (Semple et al. 2012) | |
| Visible burrow system | Not yet investigated | |
| Impairments in social communication | Behavioral responses to social olfactory cues (e.g. scent marking) | Reduced scent marking in response to a stranger female by adult male mice after pediatric CCI (Semple et al. 2014) |
| Reduced scent marking in response to a stranger female by adult male mice after adult CCI and CHI (Bajwa et al. in press) | ||
| Ultrasonic vocalizations during social encounters | Increased number of high frequency vocalizations by adult male mice after pediatric CCI, in response to a stranger female or female bedding, dependent upon prior exposure to females (Semple et al. 2014). | |
| Social cognition | Social odor recognition | Impaired social odor recognition memory at 45–90 days after mild TBI in adult rats (Zhang et al. 2015) |
| Social transmission of food preference | Not yet investigated in TBI | |
| Theory of Mind deficits | Location of buried food following observation of conspecifics | Not yet investigated in TBI |
| Social transmission of food preference | Not yet investigated in TBI |
Adapted from Crawley et al (2004). CCI = controlled cortical impact; CHI = closed head injury; FPI = fluid percussion injury.
Much of what is known regarding vulnerability of the immature brain to traumatic insult has stemmed from experimental models of TBI at different developmental ages. Unilateral CCI to the parietal lobe of mice at postnatal day 21 (p21) is an established model approximating TBI to a toddler-aged child (Semple et al., 2013; Tong et al., 2002). Injury at this age is characterized by a reproducible pattern of neuronal loss in the injured cortex and underlying sub-cortical structures, including the hippocampus and corpus callosum, leading to ongoing neurodegeneration over time (Claus et al., 2010; Pullela et al., 2006). These animals also display a phenotype of transient anxiolysis, persistent general hyperactivity, and spatial memory deficits that emerge over time (Pullela et al., 2006). Both the neuroanatomical and neurobehavioral features of this model are consistent with sequelae frequently observed in children with brain injury (Keightley et al., 2014; Konrad et al., 2000; Levin, 1998; Max et al., 2011).
During encounters with naive, unfamiliar, age-matched male mice, social behavior in this model of pediatric TBI at p21 varies according to time post-injury. Surprisingly, brain-injured mice exhibited a normal degree of social investigation at adolescence (p35); however, by adulthood (p60–70), TBI mice showed a reduction in social interactions and social recognition in the resident-intruder paradigm and three-chamber task (Semple et al., 2012). Further, at adulthood, TBI mice were more likely to exert social dominance in the tube task compared to sham-operated controls (Semple et al., 2012). Brain-injured mice also present with aberrant social communication, as evidenced by a striking reduction in scent marking behavior at adulthood after pTBI, and abnormal context-dependent ultrasonic vocalizations in response to female stimuli (Semple et al., 2014). Together, these findings reveal an emergence of aberrant social behavior over time after early-life injury, a trajectory that parallels the development of cognitive deficits in this model, as well as social dysfunction reported many years after childhood TBI in patients.
4.3. Difficulties and potential confounds in experimental studies of social behavior after TBI
There are several advantages inherent to the above-described tests for rodent social behavior, including short session lengths and the increasing automation of data collection and analysis, allowing for high-throughput screening and minimization of potential observer bias. These paradigms rely on a spontaneous, unconditioned behavioral response being elicited during an encounter with another animal, a feature which shows remarkable conservation across many mammalian species, suggesting that the underlying neural systems may also be comparable (Crawley, 2004).
The interpretation of social interactions in rodents, and translation of these findings to the clinical scenario, is not without limitations. While validated paradigms exist to evaluate aspects of cognitive and socio-emotional function in rats and mice, rodent models are lacking in their ability to assess the integrity of socio-affective processes, such as emotional perception, ToM and empathy. Several symptoms of aberrant social function, such as difficulty interpreting the subtleties of social language including sarcasm, emotive recognition and implied meanings, may be uniquely human constructs. Further, just as brain injuries show a high degree of heterogeneity (Bigler et al., 2013a), many of the psychosocial symptoms following TBI display inherent variability between individuals, manifesting in a unique manner depending upon the extent of co-morbidities, pre-existing health status and other variables (Silverman et al., 2010). As in human patients, differentiating social dysfunction from cognitive and emotional problems is challenging in rodent models, and deficits detected in rodent social tasks may be interpreted as an anxiety or depressive-like phenotype rather than social dysfunction per se (Fenn et al., 2013).
Lastly, artifacts that may confound the interpretation of social tasks require consideration. Social information processing in rodents is downstream of the main and accessory olfactory bulbs as well as the vomeronasal organ, which are thought to play a critical role in social recognition and behavioral motivation in these species (Baum and Kelliher, 2009; Kim et al., 2015). The presentation of normal social behaviors are therefore dependent upon intact olfactory function (Ryan et al., 2008), and potential deficits in olfaction should be ruled out by evaluating the animal's ability to detect and distinguish between odors alongside the assessment of general health and sensorimotor abilities (Crawley, 2004; Yang and Crawley, 2009).
A particular challenge when modeling injuries across a developmental time course is determining the appropriate age for an animal to accurately depict the human condition at the age of interest. To address this, many researchers have drawn comparisons between species in terms of key developmental milestones including brain maturation and behavioral phenotypes (Clancy et al., 2007; Prins and Hovda, 2003; Rice and Barone, 2000; Semple et al., 2013). However, differences in the time scale and rate of biological processes between species, under physiological conditions as well as in response to an insult, renders the equating of animal and human lifespan intrinsically complex (Agoston, 2015). Nevertheless, characterization of psychosocial and functional outcomes after injuries across a spectrum of brain maturation stages may reveal important insights into key developmental events which underpin age-specific vulnerabilities.
5. Risk factors for social impairments after pediatric TBI: Evidence from patients and rodent models
Though converging evidence indicates that children with TBI demonstrate impairments across a wide variety of social affective domains, outcomes for individual children are highly variable, and thus knowledge of risk factors is critical to identify and target those at elevated risk for social and behavioral impairments. Clinical research has begun to identify key risk factors which contribute to vulnerability of certain individuals to develop social dysfunction after a TBI. These include injury-specific factors (e.g., age-at-insult, severity and location) as well as pre-existing (e.g., socioeconomic status, cognitive function and family environment) and post-injury features (family environment, treatment resources, child disability). Animal-based laboratory research provides a useful platform to extend and enhance our understanding of these risk factors and how they might interact, as well as identify novel factors.
Across a range of functional domains, age at the time of a brain insult is considered to be a key predictor of long-term outcomes (Anderson et al., 2010; Anderson et al., 2014; Garcia et al., 2015). This phenomenon has recently been modeled in young mice after experimental TBI, whereby mice that received an equivalent brain injury during adolescence (p35) showed remarkable resilience to the development of social dysfunction, compared to mice injured at p21 (Semple et al., 2014). This result is consistent with findings from patient populations suggesting that the younger brain shows greater vulnerability to poor social outcomes after injury compared to older children or adults (Anderson et al., 2010; Didus et al., 1999; Wells et al., 2009). However, the relationship between injury timing and outcome is neither simple nor linear (Crowe et al., 2012; Greenham et al., 2010). Animal studies have delineated critical periods for the visual and motor systems, whereby injury during particular developmental windows results in robust neuroplasticity and structural reorganization (Marco et al., 2011). In contrast, critical developmental periods for more complex neurobehavioral processes such as social behaviors are yet to be defined (Fox et al., 2010). Mixed findings from extensive animal research over several decades, primarily employing focal surgical lesions in young rodents, cats and monkeys, have demonstrated the uncertain relationship between age and behavioral outcomes, identifying a complex interaction between developmental age, injury location, and the region-specific maturational state (Goldman et al., 1970; Kolb and Gibb, 2014; Passingham et al., 1983). These studies have shaped our current understanding, that social outcomes after TBI depend upon a balance between neural vulnerability versus plasticity during critical periods of brain development (Anderson et al., 2011).
Following a TBI during childhood, family resources are considered to be a strong predictor of social and cognitive behaviors (Anderson et al., 2012; Ewing-Cobbs et al., 2013). A low socioeconomic status exerts a negative influence on cognitive and social outcomes for children with TBI, as does family stress (Anderson et al., 2001; Schmidt et al., 2010; Yeates et al., 2004; Yeates et al., 1997; Yeates et al., 2010). On the contrary, a supportive family environment and good parent mental health may foster resilience and provide a degree of protection against the consequences of a brain injury (Holland and Schmidt, 2015; Yeates et al., 1997). Rodent studies in which the housing environment is altered (e.g., social isolation compared to enriched, complex housing) have further demonstrated how sensitive behavioral outcomes are to the environment (Kolb and Gibb, 2014), suggesting that efforts to optimize the early post-injury environment may improve social outcomes (Holland and Schmidt, 2015).
Injury severity is another proposed risk factor for social dysfunction, although the evidence from patient populations is conflicting to date. In several studies, severe TBI in young children has been associated with poorer social interactions and social communication compared to complicated-mild or moderate TBI (Catroppa et al., 2008; Catroppa et al., 2015; Ewing-Cobbs et al., 2013). Supporting this concept, experimental studies in which the brain insult was considered mild or concussive typically failed to detect social deficits (Bajwa et al., 2016; Shultz et al., 2012; Shultz et al., 2011), although such an interpretation may be confounded by differing test conditions and measures with differing sensitivities. In contrast, some have argued that even a mild TBI during early childhood can disrupt normal socio-emotional development, with significant consequences on social interactions later in life (Kaldoja and Kolk, 2015). Such findings may be explained by differing thresholds at which specific functional domains are vulnerable to perturbation after injury. Rodent models, in which a range of injury severities can be generated and compared while controlling for other potential variables, may offer further insight in this arena.
Lastly, the distribution of neuropathology resulting from a TBI during childhood or adulthood, in particular the location of focal lesions, has received considerable attention in terms of its association with functional deficits. Brain lesions resulting from pediatric TBI are very heterogeneous as in adults, displaying varying combinations of focal and diffuse regions of damage (Bigler et al., 2013a). Frontal brain regions are often affected by a TBI, likely due to their position within the skull (Wilde et al., 2012a; Wilde et al., 2005). Importantly, the frontal lobes are among the last neural structures to achieve full maturation (Giedd et al., 1999), and are crucial for higher-order social cognition and language processing (Friederici et al., 2011; Mayes et al., 2015; Wilde et al., 2012b). Indeed, several clinical studies have detected an association between frontal lobe injuries and the persistence of social deficits, including poor social competence (Bigler et al., 2013b; Eslinger et al., 2004; Levan et al., 2015; Levin et al., 2004; Spikeman et al., 2012; Wilde et al., 2012b). Such findings are inconsistent, as other investigators have failed to demonstrate a link between lesion location or laterality and either cognitive or psychosocial outcomes after a range of early life brain injuries, including focal lesions and acquired stroke (Greenham et al., 2010; Jacobs and Anderson, 2002; Jacobs et al., 2011; Pavlovic et al., 2006; Power et al., 2007). In the previously-described mouse model of pediatric TBI (Section 4.2), shifting a moderate-to-severe unilateral injury from the parietal lobe to the frontal lobe recently yielded a surprisingly phenotype of normal social behaviors after frontal injury (Chen et al., 2013). This intriguing finding raises further questions, including the possibility that social deficits are mediated at least in part by damage to hippocampal and parahippocampal structures after parietal lobe injuries (Felix-Ortiz and Tye, 2014; Rubin et al., 2014), and that the extent of damage rather than lesion location or laterality is more predictive of disruption to neural networks underlying complex social and cognitive skills (Anderson et al., 2011).
A final consideration regarding risk factors for social dysfunction after pediatric TBI is the concept that an interaction between individual factors results in poorer social outcomes compared to either risk factor alone (Holland and Schmidt, 2015). The double-hazard model describes how a combination of both severe injury and young age at insult results in the poorest outcomes (Escalona, 1982). Others have demonstrated poorer outcomes in individuals with both a more severe injury and a lower socioeconomic status compared to those with only one risk factor (Anderson et al., 2005; Taylor and Alden, 1997). Future clinical and preclinical studies in this area will provide a more comprehensive understanding of risk and resilience factors which influence how and when social dysfunction manifests after TBI, which is of importance for the appropriate timing and intensity of intervention strategies (Holland and Schmidt, 2015).
6. Moving forward: Delineating potential biological mechanisms
In this section, we consider two potential mechanisms which may underlie the manifestation of social behavior dysfunction after pediatric TBI, synthesizing recent evidence from both clinical and experimental studies.
6.1. Aberrant connectivity of the social brain network (SBN)
A complex interconnected system of functionally-specific neuroanatomical regions, distributed throughout cortical and sub-cortical structures, appear to mediate the initiation and execution of social behaviors in mammals (Figure 4). In humans, this SBN incorporates areas involved in cognitive processing (prefrontal cortex; medial, orbital and anterior cingulate), context evaluation (temporal cortex, prefrontal cortices and amygdala), social recognition (fusiform area, superior temporal gyrus and accessory olfactory bulb) and social motivation (ventral tegmental area and nucleus accumbens), with downstream information and emotional processing (thalamus, hippocampus and amygdala), and finally execution of behaviors (hypothalamus, periaqueductal gray, and brainstem motor and autonomic centers) (Beauchamp and Anderson, 2010; Blakemore, 2008; Insel and Fernald, 2004; Misra, 2014; Sandi and Haller, 2015). Laboratory studies have identified a SBN in rodents that largely overlaps with the human network, including interconnectivity between prelimbic regions, amygdala and cortical regions, as well as the hypothalamus and hippocampus (Kas et al., 2014; Siegel et al., 1999). Of note, the SBN is known to mature throughout childhood and adolescence, to gain increased refinement and functional specialization, coinciding with the protracted development of higher-order social skills. This prolonged developmental trajectory likely underlies the network's vulnerability to dysfunction when any of the many regions, neural substrates or interconnecting pathways is disrupted by injury (Misra, 2014).
Figure 4.
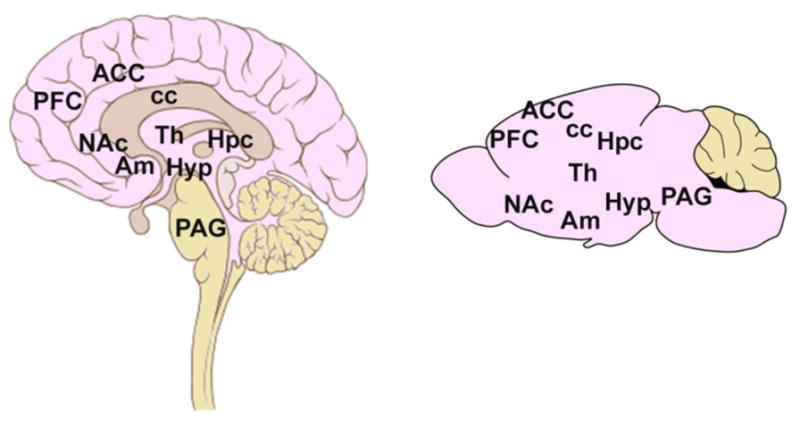
The social brain network in humans and rodents. Key brain regions involved in the social brain network in humans and rodents. PFC, prefrontal cortex; ACC, anterior cingulate cortex; cc, corpus callosum; Th, thalamus; Am, amygdala; NAc, nucleus accumbens; Hpc, hippocampus; Hyp, hypothalamus; PAG, periaqueductal gray.
Advanced neuroimaging modalities have proven particularly useful to date, identifying changes in the SBN as a result of TBI during childhood, providing a unique opportunity to quantify the impact of TBI on the developing brain, and improve prediction of social outcomes in this population. Using diffusion tensor imaging (DTI) to identify microstructural white matter integrity, several studies have demonstrated that moderate to severe TBI disrupts regions and pathways implicated in social cognitive networks (Ewing-Cobbs et al., 2008; Levin et al., 2008; Levin et al., 2011; Wilde et al., 2006). Tensor or voxel-based morphometric analyses of magnetic resonance imaging (MRI) has demonstrated widespread atrophy of the social brain network after moderate-to-severe childhood TBI (Dennis et al., 2015), including particular vulnerability of frontotemporolimbic structures implicated in poor ToM and adaptive behaviors (Bigler et al., 2013a; Bigler et al., 2013b). Further, by examining normally-developing brains and those of children diagnosed with autism spectrum disorder, specific long-range white matter tracts have been identified as crucial to complex socio-emotional processing, implicating impairments in limbic connectivity in social behavior dysfunction (Ameis and Catani, 2015).
Susceptibility weighted imaging (SWI) is a high resolution gradient-recalled echo sequence that accentuates the magnetic properties of blood and blood product in the brain, rendering it particularly sensitive to micro-hemorrhagic lesions commonly associated with traumatic axonal injury (Sehgal et al., 2005). SWI is shown to be more sensitive in detecting traumatic lesions than computed tomography (CT) or T2-weighted MRI (Beauchamp et al., 2011), and demonstrates utility for prediction of cognitive outcomes after pediatric TBI (Beauchamp et al., 2013; Ryan et al., 2014). In addition, new evidence suggests that this technique has potential to unlock early prognostic biomarkers that may add predictive value for social affective outcomes. In a recent longitudinal study that examined prospective links between social cognition and SWI, children with TBI underwent MRI including SWI sequences at 2–8 weeks post injury and subsequently completed a social cognitive test battery at 6- and 24-months post injury (Ryan et al., 2015b; Ryan et al., 2014). In line with previous evidence for heterogeneity of brain lesions in children with TBI (Bigler et al., 2013a), SWI revealed a highly variable and anatomically distributed pattern of microhemorrhagic lesions across children in the sample (Beauchamp et al., 2013).
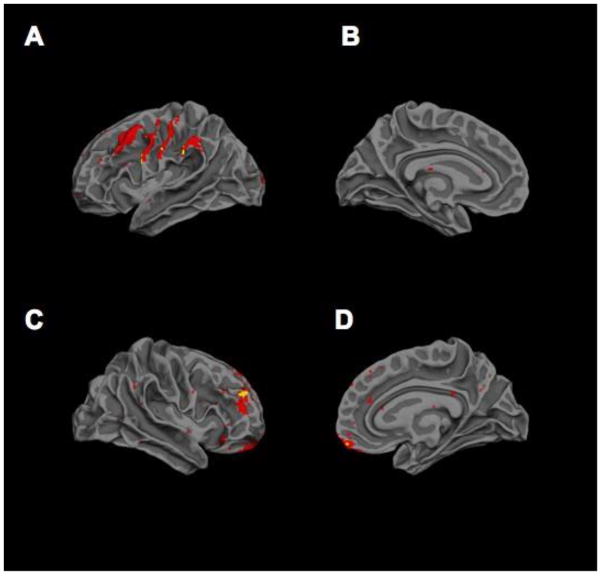
As depicted in Figure 5, microhemorrhagic lesions were most commonly identified in frontal temporo-limbic regions commonly associated with the SBN, and more diffuse SWI lesions within this network were prospectively associated with poorer cognitive ToM and pragmatic language skills at 6- and 24- months post-injury (Ryan et al., 2015a; Ryan et al., 2015c). In a subsequent sub-study that examined the relation between SWI lesions and higher level aspects of ToM, findings showed that the effect of SWI lesions on ToM may interact with the child’s developmental stage, such that more diffuse acute micro-bleeds may contribute to the time-dependent emergence of social cognitive dysfunction particularly during adolescence (Ryan et al., 2015b).
Figure 5.
Distribution of brain lesions in the social brain network after pediatric TBI. Probability distribution of brain lesions detected using SWI in the left lateral (A), left medial (B), right lateral (C) and right medial (D) hemispheres. Lesion distributions were created by aligning the individual T1 images to the Montreal Neurological Institute template using the non-linear normalization procedure in Statistical Parametric Mapping 8 (SPM8). The lesion maps were normalized using the same transformations. The aligned lesion masks were averaged to produce a single image illustrating the distribution of lesions in the study population. Hotter colors indicate the co-location of lesions in multiple subjects. Lesions were most prominent in frontal regions [frontal only = 15 patients, frontal + extrafrontal only = 6, frontal + other regions (CC (corpus callosum) = 1, deep gray + CC = 1, cerebellum = 1, cerebellum + CC = 1)], followed by extrafrontal regions only (N = 6). A small number of patients (4) had lesions in several areas (frontal + extrafrontal + cerebellum = 2, frontal + extrafrontal + deep gray = 1, frontal + extrafrontal + CC = 1). Very few patients had lesions solely in the CC (1), cerebellum (1) or deep gray (0) regions. Reproduced from Beauchamp et al. (2013).
Although such studies suggest that high resolution MRI has potential to improve prediction of acute and chronic socio-affective outcomes after TBI, knowledge of potential mechanisms to explain the robust association between pediatric TBI and broader patterns of maladaptive behavior is lacking. Based on preliminary evidence that links impairments in social cognition to diffuse structural brain abnormalities and behavior problems including aggression and rule breaking, one possibility is that injury-related alterations in SBN morphology confer risk for behavior problems via their influence on ToM. In order to test this potential mechanism, Ryan et al. (under review) examined prospective longitudinal relationships between sub-acute SBN morphology, ToM and chronic behavior problems in 98 children and adolescents with mild-severe TBI. Interestingly, and in accord with the premises of conceptual models of social outcome in childhood brain disorder, impaired ToM fully mediated the prospective association between abnormal SBN morphology and more frequent behavior problems in children with TBI.
Overall, these findings converge to suggest that impaired ToM may contribute to the association between TBI and increased risk for socially maladaptive behaviors, which persist or even worsen with time since injury. While structural change in the SBN and its putative hub regions may represent a useful imaging biomarker to identify children at risk for social cognitive impairments, these findings also have critical implications for early assessment and intervention. Since these findings suggest that social cognitive impairments contribute to the onset and maintenance of chronic behavior problems, early evaluation and assessment are critical to facilitate timely interventions and reduce risk for chronic behavior problems. Given the dearth of ecologically valid tools to capture the pre-requisite skills for real world social interactions, further research and development of this area is critical to optimize rehabilitation and outcome for children living with TBI.
6.2. A neuroendocrine basis for social dysfunction
An alternative consideration, founded in reports of aberrant growth and sex hormones after TBI, is the possibility that psychosocial outcomes after brain injury may be influenced by the neuroendocrine response. Many hormones and neuropeptides including androgens and estrogens, serotonin, oxytocin, arginine and vasopressin, modulate and influence socio-emotional and social behaviors in both rodents and humans (Meyer et al., 2012; Neumann and Landgraf, 2012). For example, oxytocin is known to promote pro-social interactions, attachment and maternal behaviours, in contrast to vasopressin which is associated with anxiogenic and aggressive behaviors (Neumann and Landgraf, 2012). The actions of these neuropeptides on social information processing and social recognition are regulated by gonadal hormones, which themselves have been implicated in social behaviors (Choleris et al., 2009). Rodent experiments using genetic or surgical manipulation have indicated that both estrogens and androgens are necessary for normal social and sociosexual behaviors in males and females (Harada et al., 2009). In addition, many neuroanatomical regions involved in social cognition are sexually dimorphic, including septal and hypothalamic nuclei, and components of the circuitry involved in behavioral motivation (Kim et al., 2015; Shah et al., 2004). The degree to which these sexual distinctions influence behavioral outcomes after TBI is uncertain, although there is evidence of differential psychosocial and communication problems in male versus female patients (Despins et al., 2015; Gerring et al., 2002; Moreno and McKerral, 2015; Scott et al., 2015), even in young children (Collins et al., 2013; Kaldoja and Kolk, 2015). Further studies are needed in this arena, as sex differences in how the developing brain responds to pTBI may have important implications for both diagnosis and treatment after pTBI.
One of the mechanisms by which TBI may influence sex hormones is via direct or indirect damage to the pituitary gland, which plays a central role in the regulation of gonadal as well as growth, diuretic and stress hormones. The narrow pituitary stalk is vulnerable to direct damage by mechanical compression, shearing or vascular injury, or indirectly compromised by brain swelling (Dusick et al., 2012; Reifschneider et al., 2015). Haemorrhage, necrosis and fibrosis of the pituitary stalk, anterior and posterior pituitary have been reported upon autopsy after fatal TBI (Klose and Feldt-Rasmussen, 2015; Kornblum and Fisher, 1969; Tanriverdi and Kelestimur, 2015), and amongst survivors of adult TBI, pituitary atrophy is evident by MRI up to at least 11 months post-injury (Maiya et al., 2008). Further, rodent studies have identified an inflammatory response in the hypothalamus, associated with impaired growth hormone levels, even in the absence of overt pituitary damage (Osterstock et al., 2014). Such pathology suggests vulnerability of this region to TBI that may underlie post-traumatic hypopituitarism seen in some patients despite the absence of obvious damage to the pituitary gland or stalk.
In recent years, numerous studies have identified hypopituitarism as a common consequence of TBI, although the reported incidence varies considerably depending upon assay methodology, diagnostic criteria, patient selection, and duration between injury and evaluation (Reifschneider et al., 2015; Richmond and Rogol, 2014). Growth hormone and gonadotropin deficiencies are the most common pituitary hormone abnormalities reported in TBI patients (Silva et al., 2015), however, any part of the hypothalamic-pituitary axis can be affected.
Endocrine abnormalities associated with pituitary or hypothalamic damage after TBI can improve or worsen with time, and in children with brain injury, such changes have the potential to significantly impact QoL, particularly in the context of ongoing growth and puberty. Although a direct link between neuroendocrine dysfunction and psychosocial abnormalities has yet to be identified, many of the symptoms commonly associated with post-TBI pituitary deficiencies are likely to influence social functioning, including fatigue, decreased memory and attention, low libido, depression and anxiety (Reifschneider et al., 2015). In a rodent model of repeated, mild TBIs at adolescence, male rats showed evidence of pituitary dysfunction including reduced levels of testosterone, growth hormone and insulin-like growth factor-1 over time post-injury (Greco et al., 2013). Concurrent observations of a reduction in sociosexual investigations at adulthood and stunted growth of sexual organs (Greco et al., 2015), implicates neuroendocrine dysfunction as a potential mechanism underlying a range of aberrant social behaviours after TBI.
7. Improving translation between experimental and human studies
At present there is considerable mismatch between study populations, testing paradigms, and outcome measures used in experimental models compared to clinical investigation of social dysfunction after TBI. Such disparities are important to define and discuss, to encourage improved translation of research findings from bench-to-bedside and back again. Intellectual advancements in this field, including the delineation of potential underlying mechanisms and therapeutic strategies, will depend upon increasing alignment between preclinical and clinical research approaches.
A difference in study populations is an obvious discrepancy between human and rodent studies of social behavior after TBI. The majority of experimental TBI studies to date have focused exclusively on male subjects (Fenn et al., 2013; Klemenhagen et al., 2013; Koliatsos et al., 2011; Pandey et al., 2009; Semple et al., 2012; Shultz et al., 2012; Shultz et al., 2011). This may be justified given the clinical presentation of a higher rate of TBI in boys compared to girls (Jager et al., 2000; Love et al., 2009), however, the potential for sex differences in both neuropathology and social outcomes after TBI (see Section 6.2) highlights the need to better understand the extent to which psychosocial functioning is affected in females as well, with implications for the treatment of persistent symptoms (Schmidt et al., 2012). Another population-related limitation is the age range of studied subjects, which is typically narrowly-defined in animal studies yet may span multiple developmental transitions in children.
As is evident from this review, there are considerable discrepancies between the relatively simple social behaviors currently assessed in experimental rodent models (e.g., social interactions), and those evaluating more complex, higher order social functions in patients (e.g. ToM constructs). Lesion studies in non-human primates provide the foundation for much of our understanding of the neuroanatomical substrates which underlie social impairments (Amaral et al., 2003; Machado and Bachevalier, 2003). However, the current use of primate and other large animal models for TBI research is limited due to restrictive costs, practical and ethical considerations. The translational gap between rodent models and the clinical context may be bridged somewhat by the inclusion of more sophisticated test paradigms in rodents, in alignment with assessment measures used in patients. For example, recent evidence provides support for social facilitation and empathy in rodents (Mogil, 2012). The affective state of one mouse (e.g., pain) can influence the response of cohabitating conspecifics to noxious stimuli (Langford et al., 2006; Panksepp and Lahvis, 2011), indicating social modulation of distress consistent with similar observations of empathy in humans (Goubert et al., 2005). This novel paradigm requires further validation across different disease and injury models, with reports of strain-specific differences in behavioral and physiological responses to distress. Another interesting and potentially useful task for the preclinical TBI setting may stem from the observation that inbred laboratory mice (e.g. C57Bl/6 strain) can distinguish some socially-atypical animals from normally-behaving controls (Shah et al., 2013). Mirroring human qualitative assessments of social aptitude, in particular, the judgment of whether another individual is interacting in a 'typical' way, this paradigm may provide an interesting parallel to questionnaire-based evaluations of social functioning after a brain insult.
Studies of social function in patients also have a unique set of challenges, with scope for improvements in study design and data collection. There remains considerable heterogeneity and a lack of consensus in the use of different outcome measures (for example, the Child Behavior Checklist versus the Strengths and Difficulties Questionnaire), which limits the ability to compare and contrast between studies (Garcia et al., 2015). Many of the existing measures cannot discriminate between specific domains of social information processing, yet recently-developed tools to better assess complex social skills have not been adequately standardized (Section 3.2). Due to age-dependent trajectories in neurocognitive and psychosocial performance, the same measure often cannot be used longitudinally, and there is a lack of consistent tools across age groups.
Discrepancies in patient and proxy perspectives can also yield considerable differences in perceived level of impairment, as noted earlier in Section 3.1. The identification of pre-existing problems in cognition and behavior, which can have a considerable influence on the post-injury recovery trajectory, is limited by our ability to measure the complexity of parenting styles and family environmental factors. Further, limited availability of sensitive assessment tools during early childhood and infancy, compared to what is available for older children and adults, restricts the ability to measure social competency particularly at a young age (Bagner et al., 2012).
Lastly, we advocate for improved incorporation and better alignment of neuroimaging modalities, including DTI and SWI, in preclinical and clinical studies. As highlighted in Section 6.1, neuroimaging techniques have considerable potential to unlock prognostic biomarkers markers of social outcomes. Clinical neuroimaging, in combination with longitudinal imaging of rodent models with region-specific experimental lesions, will continue to provide insight into key neuroanatomical regions and connections underlying the observed social changes after pediatric TBI.
8: Translating current knowledge into evidence-based practice - future directions
Empirical evidence suggests that pediatric TBI has potential to derail social functions at any stage across the course of development. The challenge remains to translate these findings into novel prognostic approaches and post-injury rehabilitation programs to optimize outcomes for survivors. Traditional clinical indicators such as Glasgow Coma Scale demonstrate poor prognostic utility for social outcomes (Dennis et al., 2012; Rosema et al., 2012). As highlighted in Section 6.1, advances in modern neuroimaging provide an opportunity to quantify the impact of TBI on the developing brain and identify early prognostic markers that may improve personalized social outcome prediction in pediatric TBI.
An enriching environment is important for optimal development for children (Belsky and de Haan, 2011), and evidence shows that the quality of the environment may play an even greater role in the context of recovery from early brain insult (Anderson et al., 2012; Giza et al., 2005; Greenough et al., 1987). The potential influence of developmental stage in treatment responses remains largely unclear, however, periods when the SBN undergoes significant structural and functional reorganization (e.g. the second decade of life in humans) may reflect a sensitive time during which this neural circuitry may be particularly amenable to environmental stimulation (Blakemore, 2008; Burnett and Blakemore, 2009). In line with evidence that there exists ‘critical periods’ during which the developing brain may be particularly amenable to enriching environmental inputs (Belsky and de Haan, 2011), rodent studies have found qualitatively different changes in the distribution of synapses in young and old animals housed in complex environments and those with tactile stimulation (Kolb et al., 2003). These morphological alterations were associated with behavioral enhancement, and benefits were significantly greater for animals injured earlier in development. Rodent models also suggest that the timing of post-injury environmental enrichment is critical, such that better recovery is associated with exposure to an enriched environment during the post-acute recovery period (Giza et al., 2005).
Interventions which directly target children’s social cognitive skills are sparse, possibly reflecting the limited availability of theoretically driven, psychometrically robust assessment tools to reliably quantify change in social cognitive function pre-and post-intervention. Further development and validation of social assessment tools in large normative samples may assist to facilitate advances in this area. Efforts by clinicians and parents to proactively target potentially modifiable, identified risk factors (e.g., socioeconomic status, family environment, access to resources) may translate into optimal recovery and outcome for children with TBI.
Context-sensitive intervention programs aim to optimize post-injury outcome by placing the family unit at the center of the rehabilitative process (Braga et al., 2005). Grounded in the developmental context that is unique to pediatric brain injury, this context-sensitive family based approach adopts a holistic, individualized focus that involves training parents and teachers to stimulate motor, cognitive, sensorial, and language development within the context of the child’s everyday routines and environment (Braga et al., 2006). This methodology involves a 2-week intensive family-focused training program that is delivered alongside an individualized family manual to reinforce learned strategies. Further, it provides tools to enable parents and clinicians to collaboratively evaluate the child’s progress, set new goals, adjust the treatment program, and modify the training manual during bi-weekly visits to the clinic over the course of one year. A randomized, controlled trial study demonstrated the effectiveness of this context-sensitive methodology, with evidence for statistically significant improvements in cognitive and motor outcomes compared to children receiving direct clinician-delivered interventions (Braga et al., 2005). Similarly, the efficacy of the family-centered model is shown to generalize to social and behavioral domains, with evidence that parent training programs for management of challenging behaviours may markedly improve child outcomes and contribute to maintenance of gains over time (Woods et al., 2014a; Woods et al., 2014b).
While hormonal processes may influence aspects of social behavior that are commonly vulnerable to the effects of pediatric TBI, the efficacy and tolerability of pharmacological treatments for post-injury social dysfunction in the TBI population is largely unknown. One potential therapeutic target is the hormone oxytocin and its receptors, which has been identified as having an important role in social cognition (Prehn et al., 2013), and is shown to enhance peer recognition and bonding behavior across numerous mammalian species (Donaldson and Young, 2008; Leknes et al., 2013). Consistent with these findings, results from a recent randomized clinical crossover trial of young children with autism revealed that compared with placebo, a 5-week oxytocin nasal spray treatment regimen led to significant improvements on the primary outcome of caregiver-rated social responsiveness (Yatawara et al., 2015). Though these preliminary findings suggest that pharmacotherapy may increase affiliative social behaviors in young children with autism, the strength of these findings is weakened by small sample size and sole reliance on a single parent-report measure of outcome. On this basis, further research is required to replicate these findings in large samples and establish whether the study findings generalize to other pediatric populations - particularly children with TBI, in whom social impairments may be associated with highly variable brain pathologies that gives rise to complex and highly heterogeneous clinical syndromes in individual children.
9. Conclusions
Social dysfunction is one of the most debilitating and persistent problems reported after pediatric TBI. Despite increasing recognition of its important influence on long-term QoL and well-being, social outcomes have been afforded little attention in experimental TBI models to date, and our understanding of how social impairments may emerge, evolve and resolve over time after early life injury remains very poor. This review has provided an overview of the psychosocial consequences that commonly result from pediatric TBI, from both clinical and experimental perspectives. An inclusive discussion of both rodent and human data identified areas requiring further translational research into underlying biological mechanisms of post-injury social impairments.
Collaboration between basic researchers and clinicians, including neuropsychologists and rehabilitation therapists, should encourage the development and incorporation of behavioral and neuroimaging assessment tools for higher order social skills, and greater alignment between experimental and clinical studies. Animal models provide an arena to test hypotheses regarding potential mechanisms underlying impaired social competency that may not be possible in the clinical setting, as well as trial interventions aimed at improving social outcomes. Further, rodent models may inform clinicians regarding novel and identified risk and resilience factors, allowing for better prediction of outcomes on an individual basis. Experimental models provide evidence that functional recovery can be modulated after early life brain injury, for example, by interactive behavioral therapies or supplementation of neurotrophic factors (Kolb et al., 2011). Clinically, although the provision of heavily-enriched social stimuli by therapists and caregivers has the potential to alleviate or prevent the emergence of social deficits over time, efficacious interventions to promote psychosocial outcomes after childhood TBI are limited to date (Ross et al., 2011a; Yeates, 2013). Understanding the neurobiological basis of social dysfunction after TBI, particularly in relation to the maturation state at the time of insult, may lead to the design of age-appropriate interventions to promote a normal developmental trajectory and enhance recovery after injury (Misra, 2014).
Highlights.
Social dysfunction is common yet understudied after traumatic brain injury (TBI)
Injury-related and environmental factors modulate social outcomes after TBI
Rodent models of TBI reveal clinically-relevant deficits in social interactions
Parallel experimental and clinical research may delineate underlying mechanisms
Acknowledgments
This work was funded through research grants and fellowships awarded by the National Health and Medical Research Council (BDS, CC and VA), The University of Melbourne (BDS), The National Institutes of Health (#R01NS077767 and R01NS050159; LJN-H), the Murdoch Childrens Research Institute and the Victorian Neurotrauma Initiative, and was supported by the Victoria Government Operational Infrastructure Scheme (#CO6E1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- CCI
controlled cortical impact
- CT
computed tomography
- DTI
diffusion tensor imaging
- FPI
fluid-percussion injury
- HMSC
Heuristic Model of Social Competence
- MRI
magnetic resonance imaging
- pTBI, p21
postnatal day 21
- QoL
Quality of Life
- SBN
social brain network
- SWI
susceptibility-weighted imaging
- TBI
traumatic brain injury
- ToM
Theory of Mind
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agoston DV. Bench-to-Bedside and Bedside Back to the Bench; Seeking a Better Understanding of the Acute Pathophysiological Process in Severe Traumatic Brain Injury. Front Neurol. 2015;6:47. doi: 10.3389/fneur.2015.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Bauman MD, Schumann CM. The amygdala and autism: implications from non-human primate studies. Genes Brain Behav. 2003;2:295–302. doi: 10.1034/j.1601-183x.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Ameis SH, Catani M. Altered white matter connectivity as a neural substrate for social impairment in Autism Spectrum Disorder. Cortex. 2015;62:158–181. doi: 10.1016/j.cortex.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Anderson V, Beauchamp MH, Yeates KO, Crossley L, Hearps SJC, Catroppa C. Social Competence at 6 Months Following Childhood Traumatic Brain Injury. J Int Neuropsychol Soc. 2013;19:539–550. doi: 10.1017/S1355617712001543. [DOI] [PubMed] [Google Scholar]
- Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Functional plasticity or vulnerability after early brain injury? Pediatrics. 2005;116:1374–1382. doi: 10.1542/peds.2004-1728. [DOI] [PubMed] [Google Scholar]
- Anderson V, Godfrey C, Rosenfeld JV, Catroppa C. Predictors of cognitive function and recovery 10 years after traumatic brain injury in young children. Pediatrics. 2012;129:e254–261. doi: 10.1542/peds.2011-0311. [DOI] [PubMed] [Google Scholar]
- Anderson V, Jacobs R, Spencer-Smith M, Coleman L, Anderson P, Williams J, Greenham M, Leventer R. Does early age at brain insult predict worse outcome? Neuropsychological implications. J Pediatr Psychol. 2010;35:716–727. doi: 10.1093/jpepsy/jsp100. [DOI] [PubMed] [Google Scholar]
- Anderson V, Spencer-Smith M, Wood A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain. 2011;134:2197–2221. doi: 10.1093/brain/awr103. [DOI] [PubMed] [Google Scholar]
- Anderson VA, Catroppa C, Haritou F, Morse S, Pentland L, Rosenfeld J, Stargatt R. Predictors of acute child and family outcome following traumatic brain injury in children. Pediatr Neurosurg. 2001;34:138–148. doi: 10.1159/000056009. [DOI] [PubMed] [Google Scholar]
- Anderson VA, Spencer-Smith MM, Coleman L, Anderson PJ, Greenham M, Jacobs R, Lee KJ, Leventer RJ. Predicting neurocognitive and behavioural outcome after early brain insult. Dev Med Child Neurol. 2014;56:329–336. doi: 10.1111/dmcn.12387. [DOI] [PubMed] [Google Scholar]
- Andrews T, Rose F, Johnson D. Social and behavioural effects of traumatic brain injury in children. Brain Injury. 1998;12:133–138. doi: 10.1080/026990598122755. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. Scent marking behavior in male C57Bl/6J mice: sexual and developmental determination. Behav Brain Res. 2007;182:73–79. doi: 10.1016/j.bbr.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. A new test paradigm for social recognition evidenced by urinary scent marking behavior in C57BL/6J mice. Behav Brain Res. 2008;190:97–104. doi: 10.1016/j.bbr.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriaga G, Jarvis ED. Mouse vocal communication system: are ultrasounds learned or innate? Brain Lang. 2013;124:96–116. doi: 10.1016/j.bandl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagner DM, Rodriguez GM, Blake CA, Linares D, Carter AS. Assessment of behavioral and emotional problems in infancy: a systematic review. Clin Child Fam Psychol Rev. 2012;15:113–128. doi: 10.1007/s10567-012-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajwa NM, Halavi S, Hamer M, Semple BD, Noble-Haeusslein LJ, Baghchechi M, Hiroto A, Hartman RE, Obenaus A. Mild concussion, but not moderate traumatic brain injury, is associated with long-term depression-like phenotype in mice. PloS ONE. 2016;11:e0146886. doi: 10.1371/journal.pone.0146886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MJ, Kelliher KR. Complementary roles of the main and accessory olfactory systems in mammalian mate recognition. Annu Rev Physiol. 2009;71:141–160. doi: 10.1146/annurev.physiol.010908.163137. [DOI] [PubMed] [Google Scholar]
- Bean NY, Nunez AA, Conner R. Effects of medial preoptic lesions on male mouse ultrasonic vocalizations and copulatory behavior. Brain Res Bull. 1981;6:109–112. doi: 10.1016/s0361-9230(81)80033-0. [DOI] [PubMed] [Google Scholar]
- Beauchamp KM, Anderson AJ. SOCIAL: an integrative framework for the development of social skills. Psychol Bull. 2010;136:39–64. doi: 10.1037/a0017768. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Anderson V. Cognitive and psychopathological sequelae of pediatric traumatic brain injury. Handbook Clin Neurol. 2013;112:913–920. doi: 10.1016/B978-0-444-52910-7.00013-1. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Beare R, Ditchfield M, Coleman L, Babl FE, Kean M, Crossley L, Catroppa C, Yeates KO, Anderson V. Susceptibility weighted imaging and its relationship to outcome after pediatric traumatic brain injury. Cortex. 2013;49:591–598. doi: 10.1016/j.cortex.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Ditchfield M, Babl FE, Kean M, Catroppa C, Yeates KO, Anderson V. Detecting traumatic brain lesions in children: CT versus MRI versus susceptibility weighted imaging (SWI) J Neurotrauma. 2011;28:915–927. doi: 10.1089/neu.2010.1712. [DOI] [PubMed] [Google Scholar]
- Bellerose J, Bernier A, Beaudoin C, Gravel J, Beauchamp MH. When Injury Clouds Understanding of Others: Theory of Mind after Mild TBI in Preschool Children. J Int Neuropsychol Soc. 2015:1–11. doi: 10.1017/S1355617715000569. [DOI] [PubMed] [Google Scholar]
- Belsky J, de Haan M. Annual Research Review: Parenting and children's brain development: the end of the beginning. J Child Psychol Psychiatry. 2011;52:409–428. doi: 10.1111/j.1469-7610.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- Bigler ED. Traumatic brain injury, neuroimaging, and neurodegeneration. Front Hum Neurosci. 2013;7:395. doi: 10.3389/fnhum.2013.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED, Abildskov TJ, Petrie J, Farrer TJ, Dennis M, Simic N, Taylor HG, Rubin KH, Vannatta K, Gerhardt CA, Stancin T, Owen Yeates K. Heterogeneity of brain lesions in pediatric traumatic brain injury. Neuropsychology. 2013a;27:438–451. doi: 10.1037/a0032837. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Yeates KO, Dennis M, Gerhardt CA, Rubin KH, Stancin T, Taylor HG, Vannatta K. Neuroimaging and social behavior in children after traumatic brain injury: Findings from the social outcomes of brain injury in kids (SOBIK) study. NeuroRehabil. 2013b;32:707–720. doi: 10.3233/NRE-130896. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Boden-Albala B, Litwak E, Elkind MS, Rundek T, Sacco RL. Social isolation and outcomes post stroke. Neurology. 2005;64:1888–1892. doi: 10.1212/01.WNL.0000163510.79351.AF. [DOI] [PubMed] [Google Scholar]
- Bohnert AM, Parker JG, Warschausky SA. Friendship and social adjustment of children following a traumatic brain injury: An exploratory investigation. Developmental Neuropsychology. 1997;13:477–486. [Google Scholar]
- Bondi CO, Klitsch KC, Leary JB, Kline AE. Environmental enrichment as a viable neurorehabilitation strategy for experimental traumatic brain injury. J Neurotrauma. 2014;31:873–888. doi: 10.1089/neu.2014.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga LW, Da Paz AC, Ylvisaker M. Direct clinician-delivered versus indirect family-supported rehabilitation of children with traumatic brain injury: a randomized controlled trial. Brain Inj. 2005;19:819–831. doi: 10.1080/02699050500110165. [DOI] [PubMed] [Google Scholar]
- Braga LW, Ylvisaker M, Rossi L, Souza LN. Cognitive development and neuropsychological disorders. In: Braga LW, Campos da Paz A, editors. The Child with Traumatic Brain Injury or Cerebral Palsy: A context-Sensitive, Family-Based Approach to Development. CRC Press; 2006. pp. 55–101. [Google Scholar]
- Brenes JC, Lackinger M, Hoglinger GU, Schratt G, Schwarting RK, Wohr M. Differential effects of social and physical environmental enrichment on brain plasticity, cognition, and ultrasonic communication in rats. J Comp Neurol. 2015 doi: 10.1002/cne.23842. [DOI] [PubMed] [Google Scholar]
- Burnett S, Blakemore SJ. The development of adolescent social cognition. Ann N Y Acad Sci. 2009;1167:51–56. doi: 10.1111/j.1749-6632.2009.04509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catroppa C, Anderson VA, Morse SA, Haritou F, Rosenfeld JV. Outcome and predictors of functional recovery 5 years following pediatric traumatic brain injury (TBI) J Pediatr Psychol. 2008;33:707–718. doi: 10.1093/jpepsy/jsn006. [DOI] [PubMed] [Google Scholar]
- Catroppa C, Crossley L, Hearps SJ, Yeates KO, Beauchamp M, Rogers K, Anderson V. Social and behavioral outcomes: pre-injury to six months following childhood traumatic brain injury. J Neurotrauma. 2015;32:109–115. doi: 10.1089/neu.2013.3276. [DOI] [PubMed] [Google Scholar]
- Catroppa C, Godfrey C, Rosenfeld JV, Hearps SS, Anderson VA. Functional recovery ten years after pediatric traumatic brain injury: outcomes and predictors. J Neurotrauma. 2012;29:2539–2547. doi: 10.1089/neu.2012.2403. [DOI] [PubMed] [Google Scholar]
- Cattelani R, Lombardi F, Brianti R, Mazzucchi A. Traumatic brain injury in childhood: intellectual, behavioural and social outcome into adulthood. Brain Inj. 1998;12:283–296. doi: 10.1080/026990598122584. [DOI] [PubMed] [Google Scholar]
- Chapman LA, Wade SL, Walz NC, Taylor HG, Stancin T, Yeates KO. Clinically significant behavior problems during the initial 18 months following early childhood traumatic brain injury. Rehabil Psychol. 2010;55:48–57. doi: 10.1037/a0018418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Noble-Haeusslein LJ, Ferriero D, Semple BD. Traumatic injury to the immature frontal lobe: A new murine model of long-term motor impairment in the absence of psychosocial or cognitive deficits. Dev Neurosci. 2013;35:474–490. doi: 10.1159/000355874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Clipperton-Allen AE, Phan A, Kavaliers M. Neuroendocrinology of social information processing in rats and mice. Front Neuroendocrinol. 2009;30:442–459. doi: 10.1016/j.yfrne.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde JR, Darlington RB, Anand KJS, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Claus CP, Tsuru-Aoyagi K, Adwanikar H, Walker B, Whetstone W, Noble-Haeusslein LJ. Age is a determinant of the inflammatory response and loss of cortical volume after traumatic brain injury. Dev Neurosci. 2010;32:454–465. doi: 10.1159/000316805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins NC, Molcho M, Carney P, McEvoy L, Geoghegan L, Phillips JP, Nicholson AJ. Are boys and girls that different? An analysis of traumatic brain injury in children. Emerg Med J. 2013;30:675–678. doi: 10.1136/emermed-2011-200496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10:248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Translational animal models of autism and neurodevelopmental disorders. Dialogues Clin Neurosci. 2012;14:293–305. doi: 10.31887/DCNS.2012.14.3/jcrawley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe LM, Catroppa C, Anderson V. Sequelae in children: developmental consequences. Handb Clin Neurol. 2015;128:661–677. doi: 10.1016/B978-0-444-63521-1.00041-8. [DOI] [PubMed] [Google Scholar]
- Crowe LM, Catroppa C, Babl FE, Rosenfeld JV, Anderson V. Timing of traumatic brain injury in childhood and intellectual outcome. J Pediatr Psychol. 2012;37:745–754. doi: 10.1093/jpepsy/jss070. [DOI] [PubMed] [Google Scholar]
- Dennis EL, Hua X, Villalon-Reina J, Moran LM, Kernan C, Babikian T, Mink R, Babbitt C, Johnson J, Giza CC, Thompson PM, Asarnow RF. Tensor-Based Morphometry Reveals Volumetric Deficits in Moderate/Severe Pediatric Traumatic Brain Injury. J Neurotrauma. 2015 Sep 22; doi: 10.1089/neu.2015.4012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Agostino A, Taylor HG, Bigler ED, Rubin K, Vannatta K, Gerhardt CA, Stancin T, Yeates KO. Emotional expression and socially modulated emotive communication in children with traumatic brain injury. J Int Neuropsychol Soc. 2013a;19:34–43. doi: 10.1017/S1355617712000884. [DOI] [PubMed] [Google Scholar]
- Dennis M, Simic N, Bigler ED, Abildskov T, Agostino A, Taylor HG, Rubin K, Vannatta K, Gerhardt CA, Stancin T, Yeates KO. Cognitive, affective, and conative theory of mind (ToM) in children with traumatic brain injury. Dev Cogn Neurosci. 2013b;5:25–39. doi: 10.1016/j.dcn.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Simic N, Gerry Taylor H, Bigler ED, Rubin K, Vannatta K, Gerhardt CA, Stancin T, Roncadin C, Yeates KO. Theory of mind in children with traumatic brain injury. J Int Neuropsychol Soc. 2012;18:908–916. doi: 10.1017/S1355617712000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despins EH, Turkstra LS, Struchen MA, Clark AN. Sex-Based Differences in Perceived Pragmatic Communication Ability of Adults With Traumatic Brain Injury. Arch Phys Med Rehabil. 2015 doi: 10.1016/j.apmr.2014.06.023. pii: S0003-9993(15)00096-9. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Di Battista A, Soo C, Catroppa C, Anderson V. Quality of life in children and adolescents post-TBI: a systematic review and meta-analysis. J Neurotrauma. 2012;29:1717–1727. doi: 10.1089/neu.2011.2157. [DOI] [PubMed] [Google Scholar]
- Didus E, Anderson VA, Catroppa C. The development of pragmatic communication skills in head injured children. Pediatr Rehabil. 1999;3:177–186. doi: 10.1080/136384999289441. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Doulames V, Lee S, Shea TB. Environmental enrichment and social interaction improve cognitive function and decrease reactive oxidative species in normal adult mice. Int J Neurosci. 2014;124:369–376. doi: 10.3109/00207454.2013.848441. [DOI] [PubMed] [Google Scholar]
- Dusick JR, Wang C, Cohan P, Swerdloff R, Kelly DF. Pathophysiology of hypopituitarism in the setting of brain injury. Pituitary. 2012;15:2–9. doi: 10.1007/s11102-008-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvoisin RM, Villasana L, Davis MJ, Winder DG, Raber J. Opposing roles of mGluR8 in measures of anxiety involving non-social and social challenges. Behav Brain Res. 2011;221:50–54. doi: 10.1016/j.bbr.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalona S. Babies at double hazard: early development of babies at biologic and social risk. Pediatrics. 1982;70:670–676. [PubMed] [Google Scholar]
- Eslinger PJ, Flaherty-Craig CV, Benton AL. Developmental outcomes after early prefrontal cortex damage. Brain Cogn. 2004;55:84–103. doi: 10.1016/S0278-2626(03)00281-1. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Mendez D, Barnes MA, Swank P. Social interaction in young children with inflicted and accidental traumatic brain injury: relations with family resources and social outcomes. J Int Neuropsychol Soc. 2013;19:497–507. doi: 10.1017/S1355617713000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Swank P, Kramer L, Cox CS, Jr, Fletcher JM, Barnes M, Zhang X, Hasan KM. Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. NeuroImage. 2008;42:1305–1315. doi: 10.1016/j.neuroimage.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States emergency departments visits, hospitalizations, and deaths. Centres for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA: 2010. [Google Scholar]
- Feldman R, Monakhov M, Pratt M, Ebstein RP. Oxytocin Pathway Genes: Evolutionary Ancient System Impacting on Human Affiliation, Sociality, and Psychopathology. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.08.008. pii: S0006-3223(15)00656-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci. 2014;34:586–595. doi: 10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Gensel JC, Huang Y, Popovich PG, Lifshitz J, Godbout JP. Immune Activation Promotes Depression 1 Month After Diffuse Brain Injury: A Role for Primed Microglia. Biol Psychiatry. 2013;25:00950–00955. doi: 10.1016/j.biopsych.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Ewing-Cobbs L, Miner ME, Levin HS, Eisenberg HM. Behavioral changes after closed head injury in children. J Consult Clin Psychol. 1990;58:93–98. doi: 10.1037//0022-006x.58.1.93. [DOI] [PubMed] [Google Scholar]
- Fox S, Levitt P, Nelson C. How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 2010;81:28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Brauer J, Lohmann G. Maturation of the language network: from inter- to intrahemispheric connectivities. PloS ONE. 2011;6:e20726. doi: 10.1371/journal.pone.0020726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C, Frith U. Theory of mind. Curr Biol. 2005;15:R644–646. doi: 10.1016/j.cub.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Ganesalingam K, Yeates KO, Taylor HG, Walz NC, Stancin T, Wade S. Executive functions and social competence in young children 6 months following traumatic brain injury. Neuropsychology. 2011;25:466–476. doi: 10.1037/a0022768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D, Hungerford GM, Bagner DM. Topical review: negative behavioral and cognitive outcomes following traumatic brain injury in early childhood. J Pediatr Psychol. 2015;40:391–397. doi: 10.1093/jpepsy/jsu093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerring JP, Slomine B, Vasa RA, Grados M, Chen A, Rising W, Christensen JR, Denckla MB, Ernst M. Clinical predictors of posttraumatic stress disorder after closed head injury in children. J Am Acad Child Adolesc Psychiatry. 2002;41:157–165. doi: 10.1097/00004583-200202000-00009. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Bluthe RM, Goodall G, Dantzer R. Social and individual recognition in rodents: Methodological aspects and neurobiological bases. Behav Processes. 1994;33:59–87. doi: 10.1016/0376-6357(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giza CC, Griesbach GS, Hovda DA. Experience-dependent behavioral plasticity is disturbed following traumatic injury to the immature brain. Behav Brain Res. 2005;157:11–22. doi: 10.1016/j.bbr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Gold EM, Su D, Lopez-Velazquez L, Haus DL, Perez H, Lacuesta GA, Anderson AJ, Cummings BJ. Functional assessment of long-term deficits in rodent models of traumatic brain injury. Regen Med. 2013;8:483–516. doi: 10.2217/rme.13.41. [DOI] [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE, Mishkin M. Evidence for behavioral impairment following prefrontal lobectomy in the infant monkey. J Comp Physiol Psychol. 1970;70:454–463. doi: 10.1037/h0028701. [DOI] [PubMed] [Google Scholar]
- Goubert L, Craig KD, Vervoort T, Morley S, Sullivan MJL, Williams ACdC, Cano A, Crombez G. Facing others in pain: the effects of empathy. Pain. 2005;118:285–288. doi: 10.1016/j.pain.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Greco T, Hovda D, Prins M. The effects of repeat traumatic brain injury on the pituitary in adolescent rats. J Neurotrauma. 2013;30:1983–1990. doi: 10.1089/neu.2013.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco T, Hovda DA, Prins ML. Adolescent TBI-induced hypopituitarism causes sexual dysfunction in adult male rats. Dev Neurobiol. 2015;75:193–202. doi: 10.1002/dneu.22218. [DOI] [PubMed] [Google Scholar]
- Greenham M, Spencer-Smith MM, Anderson PJ, Coleman L, Anderson VA. Social functioning in children with brain insult. Front Human Neurosci. 2010;4:1–10. doi: 10.3389/fnhum.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Dev. 1987;58:539–559. [PubMed] [Google Scholar]
- Hammerschmidt K, Whelan G, Eichele G, Fischer J. Mice lacking the cerebral cortex develop normal song: insights into the foundations of vocal learning. Sci Rep. 2015;5:8808. doi: 10.1038/srep08808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Wakatsuki T, Aste N, Yoshimura N, Honda SI. Functional analysis of neurosteroidal oestrogen using gene-disrupted and transgenic mice. J Neuroendocrinol. 2009;21:365–369. [Google Scholar]
- Hein G, Singer T. I feel how you feel but not always: the empathic brain and its modulation. Curr Opin Neurobiol. 2008;18:153–158. doi: 10.1016/j.conb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Herbet G, Lafargue G, Bonnetblanc F, Moritz-Gasser S, Duffau H. Is the right frontal cortex really crucial in the mentalizing network? A longitudinal study in patients with a slow-growing lesion. Cortex. 2013;49:2711–2727. doi: 10.1016/j.cortex.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Heverly-Fitt S, Wimsatt MA, Menzer MM, Rubin KH, Dennis M, Taylor HG, Stancin T, Gerhardt CA, Vannatta K, Bigler ED, Yeates KO. Friendship quality and psychosocial outcomes among children with traumatic brain injury. J Int Neuropsychol Soc. 2014;20:684–693. doi: 10.1017/S1355617714000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland JN, Schmidt AT. Static and Dynamic Factors Promoting Resilience following Traumatic Brain Injury: A Brief Review. Neural Plast. 2015;2015:902802. doi: 10.1155/2015/902802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoofien D, Gilboa A, Vakil E, Donovick PJ. Traumatic brain injury (TBI) 10–20 years later: a comprehensive outcome study of psychiatric symptomatology, cognitive abilities and psychosocial functioning. Brain Inj. 2001;15:189–209. doi: 10.1080/026990501300005659. [DOI] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Ann Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Jacobs R, Anderson V. Planning and problem solving skills following focal frontal brain lesions in childhood: analysis using the Tower of London. Child Neuropsychol. 2002;8:93–106. doi: 10.1076/chin.8.2.93.8726. [DOI] [PubMed] [Google Scholar]
- Jacobs R, Harvey AS, Anderson V. Are executive skills primarily mediated by the prefrontal cortex in childhood? Examination of focal brain lesions in childhood. Cortex. 2011;47:808–824. doi: 10.1016/j.cortex.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Jager TE, Weiss HB, Coben JH, Pepe PE. Traumatic brain injuries evaluated in U.S. emergency departments, 1992–1994. Acad Emerg Med. 2000;7:134–140. doi: 10.1111/j.1553-2712.2000.tb00515.x. [DOI] [PubMed] [Google Scholar]
- Janusz JA, Kirkwood MW, Yeates KO, Taylor HG. Social problem-solving skills in children with traumatic brain injury: long-term outcomes and prediction of social competence. Child Neuropsychol. 2002;8:179–194. doi: 10.1076/chin.8.3.179.13499. [DOI] [PubMed] [Google Scholar]
- Kaldoja ML, Kolk A. Does gender matter? Differences in social-emotional behavior among infants and toddlers before and after mild traumatic brain injury: a preliminary study. J Child Neurol. 2015;30:860–867. doi: 10.1177/0883073814544705. [DOI] [PubMed] [Google Scholar]
- Karver CL, Wade SL, Cassedy A, Taylor HG, Stancin T, Yeates KO, Walz NC. Age at injury and long-term behavior problems after traumatic brain injury in young children. Rehabil Psychol. 2012;57:256–265. doi: 10.1037/a0029522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kas MJ, Modi ME, Saxe MD, Smith DG. Advancing the discovery of medications for autism spectrum disorder using new technologies to reveal social brain circuitry in rodents. Psychopharmacol. 2014;231:1147–1165. doi: 10.1007/s00213-014-3464-y. [DOI] [PubMed] [Google Scholar]
- Keightley ML, Sinopoli KJ, Davis KD, Mikulis DJ, Wennberg R, Tartaglia MC, Chen JK, Tator CH. Is there evidence for neurodegenerative change following traumatic brain injury in children and youth? A scoping review. Front Hum Neurosci. 2014;8:139. doi: 10.3389/fnhum.2014.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodaie B, Lotfinia AA, Ahmadi M, Lotfinia M, Jafarian M, Karimzadeh F, Coulon P, Gorji A. Structural and functional effects of social isolation on the hippocampus of rats with traumatic brain injury. Behav Brain Res. 2015;278:55–65. doi: 10.1016/j.bbr.2014.09.034. [DOI] [PubMed] [Google Scholar]
- Kim Y, Venkataraju KU, Pradhan K, Mende C, Taranda J, Turaga SC, Arganda-Carreras I, Ng L, Hawrylycz MJ, Rockland KS, Seung HS, Osten P. Mapping social behavior-induced brain activation at cellular resolution in the mouse. Cell Rep. 2015;10:292–305. doi: 10.1016/j.celrep.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenhagen KC, O'Brien SP, Brody DL. Repetitive concussive traumatic brain injury interacts with post-injury foot shock stress to worsen social and depression-like behavior in mice. PloS ONE. 2013;8:e74510. doi: 10.1371/journal.pone.0074510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose M, Feldt-Rasmussen U. Hypopituitarism in traumatic brain injury - A critical note. J Clin Med. 2015;4:148–1497. doi: 10.3390/jcm4071480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gibb R. Searching for the principles of brain plasticity and behavior. Cortex. 2014;58:251–260. doi: 10.1016/j.cortex.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gibb R, Gorny G. Experience-dependent changes in dendritic arbor and spine density in neocortex vary qualitatively with age and sex. Neurobiol Learn Mem. 2003;79:1–10. doi: 10.1016/s1074-7427(02)00021-7. [DOI] [PubMed] [Google Scholar]
- Kolb B, Mychasiuk R, Williams P, Gibb R. Brain plasticity and recovery from early cortical injury. Dev Med Child Neurol. 2011;53(Suppl 4):4–8. doi: 10.1111/j.1469-8749.2011.04054.x. [DOI] [PubMed] [Google Scholar]
- Koliatsos VE, Cernak I, Xu L, Song Y, Savonenko A, Crain BJ, Eberhart CG, Frangakis CE, Melnikova T, Kim H, Lee D. A mouse model of blast injury to brain: initial pathological, neuropathological, and behavioral characterization. J Neuropathol Exp Neurol. 2011;70:399–416. doi: 10.1097/NEN.0b013e3182189f06. [DOI] [PubMed] [Google Scholar]
- Konrad K, Gauggel S, Manz A, Schöll M. Inhibitory control in children with traumatic brain injury (TBI) and children with attention deficit/hyperactivity disorder (ADHD) Brain Inj. 2000;14:859–875. doi: 10.1080/026990500445691. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Coppens CM, de Boer SF, Buwalda B, Meerlo P, Timmermans PJ. The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J Vis Exp. 2013;4 doi: 10.3791/4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblum RN, Fisher RS. Pituitary lesions in craniocerebral injuries. Arch Pathol. 1969;88:242–248. [PubMed] [Google Scholar]
- Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, Chanda ML, Levitin DJ, Mogil JS. Social modulation of pain as evidence for empathy in mice. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- Leknes S, Wessberg J, Ellingsen DM, Chelnokova O, Olausson H, Laeng B. Oxytocin enhances pupil dilation and sensitivity to 'hidden' emotional expressions. Soc Cogn Affect Neurosci. 2013;8:741–749. doi: 10.1093/scan/nss062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levan A, Baxter L, Kirwan CB, Black G, Gale SD. Right frontal pole cortical thickness and social competence in children with chronic traumatic brain injury: cognitive proficiency as a mediator. J Head Trauma Rehabil. 2015;30:E24–31. doi: 10.1097/HTR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- Levin HS. Cognitive function outcomes after traumatic brain injury. Curr Opin Neurol. 1998;11:643–646. doi: 10.1097/00019052-199812000-00006. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Li X. The relation of cognitive control to social outcome after paediatric TBI: Implications for intervention. Dev Neurorehabil. 2009;12:320–329. doi: 10.3109/17518420903087673. [DOI] [PubMed] [Google Scholar]
- Levin HS, Wilde EA, Chu Z, Yallampalli R, Hanten GR, Li X, Chia J, Vasquez AC, Hunter JV. Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. J Head Trauma Rehabil. 2008;23:197–208. doi: 10.1097/01.HTR.0000327252.54128.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Wilde EA, Hanten G, Li X, Chu ZD, Vasquez AC, Cook L, Yallampalli R, Hunter JV. Mental state attributions and diffusion tensor imaging after traumatic brain injury in children. Dev Neuropsychol. 2011;36:273–287. doi: 10.1080/87565641.2010.549885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Zhang L, Dennis M, Ewing-Cobbs L, Schachar R, Max J, Landis JA, Roberson G, Scheibel RS, Miller DL, Hunter JV. Psychosocial outcome of TBI in children with unilateral frontal lesions. J Int Neuropsychol Soc. 2004;10:305–316. doi: 10.1017/S1355617704102129. [DOI] [PubMed] [Google Scholar]
- Li L, Liu J. The effect of pediatric traumatic brain injury on behavioral outcomes: a systematic review. Dev Med Child Neurol. 2013;55:37–45. doi: 10.1111/j.1469-8749.2012.04414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love PF, Tepas JJ, 3rd, Wludyka PS, Masnita-Iusan C. Fall-related pediatric brain injuries: the role of race, age, and sex. J Trauma. 2009;67:S12–15. doi: 10.1097/TA.0b013e3181ac7f22. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Non-human primate models of childhood psychopathology: the promise and the limitations. J Child Psychol Psychiatry. 2003;44:64–87. doi: 10.1111/1469-7610.00103. [DOI] [PubMed] [Google Scholar]
- Maiya B, Newcombe V, Nortje J, Bradley P, Bernard F, Chatfield D, Outtrim J, Hutchinson P, Matta B, Antoun N, Menon DK. Magnetic resonance imaging changes in the pituitary gland following acute traumatic brain injury. Int Care Med. 2008;34:468–475. doi: 10.1007/s00134-007-0902-x. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Tucker LB, Ozl J, McCabe JT. Traumatic brain injury - modeling neuropsychiatric symptoms in rodents. Front Neurol. 2013;4:157. doi: 10.3389/fneur.2013.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EM, Macri S, Laviola G. Critical age windows for neurodevelopmental psychiatric disorders: evidence from animal models. Neurotox Res. 2011;19:286–307. doi: 10.1007/s12640-010-9205-z. [DOI] [PubMed] [Google Scholar]
- Max JE, Keatley E, Wilde EA, Bigler ED, Levin HS, Schachar RJ, Saunders A, Ewing-Cobbs L, Chapman SB, Dennis M, Yang TT. Anxiety disorders in children and adolescents in the first six months after traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2011;23:29–39. doi: 10.1176/jnp.23.1.jnp29. [DOI] [PubMed] [Google Scholar]
- Max JE, Wilde EA, Bigler ED, Thompson WK, MacLeod M, Vasquez AC, Merkley TL, Hunter JV, Chu ZD, Yallampalli R, Hotz G, Chapman SB, Yang TT, Levin HS. Neuroimaging correlates of novel psychiatric disorders after pediatric traumatic brain injury. J Am Acad Child Adolesc Psychiatry. 2012;51:1208–1217. doi: 10.1016/j.jaac.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes AK, Reilly S, Morgan AT. Neural correlates of childhood language disorder: a systematic review. Dev Med Child Neurol. 2015;57:706–717. doi: 10.1111/dmcn.12714. [DOI] [PubMed] [Google Scholar]
- McLellan T, McKinlay A. Sensitivity to emotion, empathy and theory of mind: adult performance following childhood TBI. Brain Inj. 2013;27:1032–1037. doi: 10.3109/02699052.2013.794965. [DOI] [PubMed] [Google Scholar]
- Mering S, Jolkkonen J. Proper housing conditions in experimental stroke studies-special emphasis on environmental enrichment. Front Neurosci. 2015;9:106. doi: 10.3389/fnins.2015.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DL, Davies DR, Barr JL, Manzerra P, Frster GL. Mild traumatic brain injury in the rat alters neuronal number in the limbic system and increases conditioned fear and anxiety-like behaviors. Exp Neurol. 2012;235:574–587. doi: 10.1016/j.expneurol.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Misra V. The social brain network and autism. Ann Neurosci. 2014;21:69–73. doi: 10.5214/ans.0972.7531.210208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS. The surprising empathic abilities of rodents. Trends Cogn Sci. 2012;16:143–144. doi: 10.1016/j.tics.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Molina J, Carmona-Mora P, Chrast J, Krall PM, Canales CP, Lupski JR, Reymond A, Walz K. Abnormal social behaviors and altered gene expression rates in a mouse model for Potocki-Lupski syndrome. Hum Mol Genet. 2008;17:2486–2495. doi: 10.1093/hmg/ddn148. [DOI] [PubMed] [Google Scholar]
- Moreno JA, McKerral M. Differences according to Sex in Sociosexuality and Infidelity after Traumatic Brain Injury. Behav Neurol. 2015;2015:914134. doi: 10.1155/2015/914134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression and social behaviors. Trends Neurosci. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Osterstock G, El Yandouzi T, Romano N, Carmignac D, Langlet F, Coutry N, Guillou A, Schaeffer M, Chauvet N, Vanacker C, Galibert E, Dehouck B, Robinson IC, Prevot V, Mollard P, Plesnila N, Mery PF. Sustained alterations of hypothalamic tanycytes during posttraumatic hypopituitarism in male mice. Endocrinology. 2014;155:1887–1898. doi: 10.1210/en.2013-1336. [DOI] [PubMed] [Google Scholar]
- Pandey DK, Yadav SK, Mahesh R, Rajkumar R. Depression-like and anxiety-like behavioural aftermaths of impact accelerated traumatic brain injury in rats: a model of comorbid depression and anxiety? Behav Brain Res. 2009;205:436–442. doi: 10.1016/j.bbr.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Rodent empathy and affective neuroscience. Neurosci Biobehav Rev. 2011;35:1864–1875. doi: 10.1016/j.neubiorev.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JG, Asher SR. Friendship and friendship quality in middle childhood: Links with peer group acceptance and feelings of loneliness and social dissatisfaction. Dev Psych. 1993;29:611–621. [Google Scholar]
- Passingham RE, Perry VH, Wilkinson F. The long-term effects of removal of sensorimotor cortex in infant and adult rhesus monkeys. Brain. 1983;106(Pt 3):675–705. doi: 10.1093/brain/106.3.675. [DOI] [PubMed] [Google Scholar]
- Patel TP, Gullotti DM, Hernandez P, O'Brien WT, Capehart BP, Morrison B, 3rd, Bass C, Eberwine JE, Abel T, Meaney DF. An open-source toolbox for automated phenotyping of mice in behavioral tasks. Front Behav Neurosci. 2014;8:349. doi: 10.3389/fnbeh.2014.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic J, Kaufmann F, Boltshauser E, Capone Mori A, Gubser Mercati D, Haenggeli CA, Keller E, Lutschg J, Marcoz JP, Ramelli GP, Roulet Perez E, Schmitt-Mechelke T, Weissert M, Steinlin M. Neuropsychological problems after paediatric stroke: two year follow-up of Swiss children. Neuropediatrics. 2006;37:13–19. doi: 10.1055/s-2006-923932. [DOI] [PubMed] [Google Scholar]
- Poggi G, Liscio M, Adduci A, Galbiati S, Massimino M, Sommovigo M, Zettin M, Figini E, Castelli E. Psychological and adjustment problems due to acquired brain lesions in childhood: A comparison between post-traumatic patients and brain tumour survivors. Brain Injury. 2005;19:777–785. doi: 10.1080/0269905500110132. [DOI] [PubMed] [Google Scholar]
- Potter JL, Wade SL, Walz NC, Cassedy A, Stevens MH, Yeates KO, Taylor HG. Parenting style is related to executive dysfunction after brain injury in children. Rehabil Psychol. 2011;56:351–358. doi: 10.1037/a0025445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power T, Catroppa C, Coleman L, Ditchfield M, Anderson V. Do lesion site and severity predict deficits in attentional control after preschool traumatic brain injury (TBI)? Brain Inj. 2007;21:279–292. doi: 10.1080/02699050701253095. [DOI] [PubMed] [Google Scholar]
- Prehn K, Kazzer P, Lischke A, Heinrichs M, Herpertz SC, Domes G. Effects of intranasal oxytocin on pupil dilation indicate increased salience of socioaffective stimuli. Psychophysiology. 2013;50:528–537. doi: 10.1111/psyp.12042. [DOI] [PubMed] [Google Scholar]
- Prigatano GP, Gupta S. Friends after traumatic brain injury in children. J Head Trauma Rehabil. 2006;21:505–513. doi: 10.1097/00001199-200611000-00005. [DOI] [PubMed] [Google Scholar]
- Prins ML, Hovda DA. Developing experimental models to address traumatic brain injury in children. J Neurotrauma. 2003;20:123–137. doi: 10.1089/08977150360547053. [DOI] [PubMed] [Google Scholar]
- Pullela R, Raber J, Pfankuch T, Ferriero DM, Claus CP, Koh SE, Yamauchi T, Rola R, Fike JR, Noble-Haeusslein LJ. Traumatic injury to the immature brain results in progressive neuronal loss, hyperactivity and delayed cognitive impairments. Dev Neurosci. 2006;28:396–409. doi: 10.1159/000094166. [DOI] [PubMed] [Google Scholar]
- Reifschneider K, Auble BA, Rose SR. Update of endocrine dysfunction following pediatric traumatic brain injury. J Clin Med. 2015;4:1536–1560. doi: 10.3390/jcm4081536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone JS. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond E, Rogol AD. Traumatic brain injury: endocrine consequences in children and adults. Endocrine. 2014;45:3–8. doi: 10.1007/s12020-013-0049-1. [DOI] [PubMed] [Google Scholar]
- Rosema S, Crowe L, Anderson V. Social function in children and adolescents after traumatic brain injury: a systematic review 1989–2011. J Neurotrauma. 2012;29:1277–1291. doi: 10.1089/neu.2011.2144. [DOI] [PubMed] [Google Scholar]
- Rosema S, Muscara F, Anderson V, Godfrey C, Eren S, Catroppa C. Agreement on and predictors of long term psychosocial development 16 years post childhood traumatic brain injury. J Neurotrauma. 2014;13:13. doi: 10.1089/neu.2013.3226. [DOI] [PubMed] [Google Scholar]
- Ross KA, Dorris L, McMillan T. A systematic review of psychological interventions to alleviate cognitive and psychosocial problems in children with acquired brain injury. Dev Med Child Neurol. 2011a;53:692–701. doi: 10.1111/j.1469-8749.2011.03976.x. [DOI] [PubMed] [Google Scholar]
- Ross KA, McMillan T, Kelly T, Sumpter R, Dorris L. Friendship, loneliness and psychosocial functioning in children with traumatic brain injury. Brain Injury. 2011b;25:1206–1211. doi: 10.3109/02699052.2011.609519. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Bukowski WM. Handbook of peer interactions, relationships, and groups. Guilford Press; 2011. [Google Scholar]
- Rubin RD, Watson PD, Duff MC, Cohen NJ. The role of the hippocampus in flexible cognition and social behavior. Front Hum Neurosci. 2014;8:742. doi: 10.3389/fnhum.2014.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BC, Young NB, Moy SS, Crawley JN. Olfactory cues are sufficient to elicit social approach behaviors but not social transmission of food preference in C57Bl/6J mice. Behav Brain Res. 2008;193:235–242. doi: 10.1016/j.bbr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan NP, Anderson V, Godfrey C, Beauchamp MH, Coleman L, Eren S, Rosema S, Taylor K, Catroppa C. Predictors of very long-term socio-cognitive function after pediatric traumatic brain injury: support for the vulnerability of the immature 'social brain.'. J Neurotrauma. 2013a;31:649–657. doi: 10.1089/neu.2013.3153. [DOI] [PubMed] [Google Scholar]
- Ryan NP, Anderson V, Godfrey C, Eren S, Rosema S, Taylor K, Catroppa C. Social communication mediates the relationship between emotion perception and externalizing behaviors in young adult survivors of pediatric traumatic brain injury (TBI) Int J Dev Neurosci. 2013b;31:811–819. doi: 10.1016/j.ijdevneu.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Ryan NP, Catroppa C, Beare R, Coleman L, Ditchfield M, Crossley L, Beauchamp MH, Anderson VA. Predictors of longitudinal outcome and recovery of pragmatic language and its relation to externalizing behaviour after pediatric traumatic brain injury. Brain and Language. 2015a;142:86–95. doi: 10.1016/j.bandl.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Ryan NP, Catroppa C, Cooper JM, Beare R, Ditchfield M, Coleman L, Silk T, Crossley L, Beauchamp MH, Anderson VA. The emergence of age-dependent social cognitive deficits after generalized insult to the developing brain: a longitudinal prospective analysis using susceptibility-weighted imaging. Hum Brain Mapp. 2015b;36:1677–1691. doi: 10.1002/hbm.22729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan NP, Catroppa C, Cooper JM, Beare R, Ditchfield M, Coleman L, Silk T, Crossley L, Rogers K, Beauchamp MH. Relationships between acute imaging Biomarkers and theory of mind impairment in post-acute pediatric traumatic brain injury: A prospective analysis using susceptibility weighted imaging (SWI) Neuropsychologia. 2014 doi: 10.1016/j.neuropsychologia.2014.10.040. [DOI] [PubMed] [Google Scholar]
- Ryan NP, Catroppa C, Cooper JM, Beare R, Ditchfield M, Coleman L, Silk T, Crossley L, Rogers K, Beauchamp MH, Yeates KO, Anderson VA. Relationships between acute imaging biomarkers and theory of mind impairment in post-acute pediatric traumatic brain injury: A prospective analysis using susceptibility weighted imaging (SWI) Neuropsychologia. 2015c;66:32–38. doi: 10.1016/j.neuropsychologia.2014.10.040. [DOI] [PubMed] [Google Scholar]
- Sandi C, Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci. 2015;16:290–304. doi: 10.1038/nrn3918. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AT, Hanten GR, Li X, Vasquez AC, Wilde EA, Chapman SB, Levin HS. Decision making after pediatric traumatic brain injury: trajectory of recovery and relationship to age and gender. Int J Dev Neurosci. 2012;30:225–230. doi: 10.1016/j.ijdevneu.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AT, Orsten KD, Hanten GR, Li X, Levin HS. Family environment influences emotion recognition following paediatric traumatic brain injury. Brain Inj. 2010;24:1550–1560. doi: 10.3109/02699052.2010.523047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C, McKinlay A, McLellan T, Britt E, Grace R, MacFarlane M. A comparison of adult outcomes for males compared to females following pediatric traumatic brain injury. Neuropsychology. 2015;29:501–508. doi: 10.1037/neu0000074. [DOI] [PubMed] [Google Scholar]
- Sehgal V, Delproposto Z, Haacke EM, Tong KA, Wycliffe N, Kido DK, Xu Y, Neelavalli J, Haddar D, Reichenbach JR. Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging. 2005;22:439–450. doi: 10.1002/jmri.20404. [DOI] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106–107:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Canchola SA, Noble-Haeusslein L. Deficits in social behavior emerge during development after pediatric traumatic brain injury in mice. J Neurotrauma. 2012;29:2672–2683. doi: 10.1089/neu.2012.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Noble-Haeusslein LJ, Jun Kwon Y, Sam PN, Gibson AM, Grissom S, Brown S, Adahman Z, Hollingsworth CA, Kwakye A, Gimlin K, Wilde EA, Hanten G, Levin HS, Schenk AK. Sociosexual and communication deficits after traumatic injury to the developing murine brain. PloS ONE. 2014;9:e103386. doi: 10.1371/journal.pone.0103386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah CR, Forsberg CG, Kang JQ, Veenstra-VanderWeele J. Letting a typical mouse judge whether mouse social interactions are atypical. Autism Res. 2013;6:212–220. doi: 10.1002/aur.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43:313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Shultz SR, Bao F, Omana V, Chiu C, Brown A, Cain DP. Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal model of repeated concussion. J Neurotrauma. 2012;29:281–294. doi: 10.1089/neu.2011.2123. [DOI] [PubMed] [Google Scholar]
- Shultz SR, MacFabe DF, Foley KA, Taylor R, Cain DP. A single mild fluid percussion injury induces short-term behavioral and neuropathological changes in the Long-Evans rat: support for an animal model of concussion. Behav Brain Res. 2011;224:326–335. doi: 10.1016/j.bbr.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Siegel A, Roeling TAP, Gregg TR, Kruk MR. Neuropharmacology of brain stimulation-evoked aggression. Neurosci Biobehav Rev. 1999;23:359–289. doi: 10.1016/s0149-7634(98)00040-2. [DOI] [PubMed] [Google Scholar]
- Silva PPB, Bhatnagar S, Herman SD, Zafonte R, Klibanski A, Miller KK, Tritos NA. Predictors of hypopituitarism in patients with traumatic brain injury. J Neurotrauma. 2015;32 doi: 10.1089/neu.2015.3998. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozda CN, Hoffman AN, Olsen AS, Cheng JP, Zafonte RD, Kline AE. Empirical comparison of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. J Neurotrauma. 2010;27:1047–1057. doi: 10.1089/neu.2010.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- Spikeman JM, Timmerman ME, Milders MV, Veenstra WS, van der Naalt J. Social cognition impairments in relation to general cognitive deficits, injury severity, and prefrontal lesions in traumatic brain injury patients. J Neurotrauma. 2012;29:101–111. doi: 10.1089/neu.2011.2084. [DOI] [PubMed] [Google Scholar]
- Sullivan JR, Riccio CA. Language functioning and deficits following pediatric traumatic brain injury. Appl Neuropsychol. 2010;17:93–98. doi: 10.1080/09084281003708852. [DOI] [PubMed] [Google Scholar]
- Tanriverdi F, Kelestimur F. Neuroendocrine disturbances after brain damage: an important and often undiagnosed disorder. J Clin Med. 2015;4:847–857. doi: 10.3390/jcm4050847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HG, Alden J. Age-related differences in outcomes following childhood brain insults: an introduction and overview. J Int Neuropsychol Soc. 1997;3:555–567. [PubMed] [Google Scholar]
- Terranova ML, Laviola G. Scoring of social interactions and play in mice during adolescence. Curr Prot Toxicol. 2005:13.10.1–13.10.11. doi: 10.1002/0471140856.tx1310s26. [DOI] [PubMed] [Google Scholar]
- Thurman DJ. The Epidemiology of Traumatic Brain Injury in Children and Youths: A Review of Research Since 1990. J Child Neurol. 2014 doi: 10.1177/0883073814544363. [DOI] [PubMed] [Google Scholar]
- Tlustos SJ, Chiu CY, Walz NC, Taylor HG, Yeates KO, Wade SL. Emotion labeling and socio-emotional outcomes 18 months after early childhood traumatic brain injury. J Int Neuropsychol Soc. 2011;17:1132–1142. doi: 10.1017/S1355617711001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W, Igarashi T, Ferriero DM, Noble LJ. Traumatic brain injury in the immature mouse brain: characterization of regional vulnerability. Exp Neurol. 2002;176:105–116. doi: 10.1006/exnr.2002.7941. [DOI] [PubMed] [Google Scholar]
- Vangel SJ, Jr, Rapport LJ, Hanks RA. Effects of family and caregiver psychosocial functioning on outcomes in persons with traumatic brain injury. J Head Trauma Rehabil. 2011;26:20–29. doi: 10.1097/HTR.0b013e318204a70d. [DOI] [PubMed] [Google Scholar]
- Wade SL, Cassedy A, Walz NC, Taylor HG, Stancin T, Yeates KO. The relationship of parental warm responsiveness and negativity to emerging behavior problems following traumatic brain injury in young children. Dev Psychol. 2011;47:119–133. doi: 10.1037/a0021028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz NC, Yeates KO, Taylor HG, Stancin T, Wade SL. Theory of mind skills 1 year after traumatic brain injury in 6- to 8-year-old children. J Neuropsychol. 2010;4:181–195. doi: 10.1348/174866410X488788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H. Bidirectional control of social hierachy by synaptic efficacy in medial prefrontal cortex. Science. 2011;334:693–697. doi: 10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- Wellman HM, Cross D, Watson J. Meta-analysis of theory-of-mind development: the truth about false belief. Child Dev. 2001;72:655–684. doi: 10.1111/1467-8624.00304. [DOI] [PubMed] [Google Scholar]
- Wells R, Minnes P, Phillips M. Predicting social and functional outcomes for individuals sustaining pediatric traumatic brain injury. Dev Neurorehab. 2009;12:12–23. doi: 10.1080/17518420902773109. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Chu Z, Bigler ED, Hunter JV, Fearing MA, Hanten G, Newsome MR, Scheibel RS, Li X, Levin HS. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J Neurotrauma. 2006;23:1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Hunter, Bigler ED. Pediatric traumatic brain injury: neuroimaging and neurorehabilitation outcome. NeuroRehabilitation. 2012a;31:245–260. doi: 10.3233/NRE-2012-0794. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Hunter JV, Newsome MR, Scheibel RS, Bigler ED, Johnson JL, Fearing MA, Cleavinger HB, Li X, Swank PR, Pedroza C, Roberson GS, Bachevalier J, Levin HS. Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. J Neurotrauma. 2005;22:333–344. doi: 10.1089/neu.2005.22.333. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Merkley TL, Bigler ED, Max JE, Schmidt AT, Ayoub KW, McCauley SR, Hunter JV, Hanten G, Li X, Chu ZD, Levin HS. Longitudinal changes in cortical thickness in children after traumatic brain injury and their relation to behavioral regulation and emotional control. Int J Dev Neurosci. 2012b;30:267–276. doi: 10.1016/j.ijdevneu.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MW, Rapport LJ, Millis SR, Hanks RA. Psychosocial outcomes after traumatic brain injury: life satisfaction, community integration, and distress. Rehabil Psychol. 2014;59:298–305. doi: 10.1037/a0037164. [DOI] [PubMed] [Google Scholar]
- Wohr M, Engelhardt KA, Seffer D, Sungur AO, Schwarting RK. Acoustic Communication in Rats: Effects of Social Experiences on Ultrasonic Vocalizations as Socio-affective Signals. Curr Top Behav Neurosci. 2015 doi: 10.1007/7854_2015_410. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Roullet FI, Hung AY, Sheng M, Crawley JN. Communication impairments in mice lacking Shank1: Reduced levels of ultrasonic vocalizations and scent marking behavior. PloS ONE. 2011;6:e20631. doi: 10.1371/journal.pone.0020631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, Scattoni ML. Behavioral methods used in rodent models of autism spectrum disorders: Current standards and new developments. Behav Brain Res. 2013;251:5–17. doi: 10.1016/j.bbr.2013.05.047. [DOI] [PubMed] [Google Scholar]
- Wohr M, Schwarting RK. Affective communication in rodents: ultrasonic vocalizations as a tool for research on emotion and motivation. Cell Tissue Res. 2013;354:81–97. doi: 10.1007/s00441-013-1607-9. [DOI] [PubMed] [Google Scholar]
- Woods D, Catroppa C, Godfrey C, Anderson V. Long-term maintenance of treatment effects following intervention for families with children who have acquired brain injury. Social Care Neurodis. 2014a;5:70–82. [Google Scholar]
- Woods D, Catroppa C, Godfrey C, Giallo R, Matthews J, Anderson V. Challenging behaviours following pediatric acquired brain injury (ABI): The clinical utility for a manualised behavioural intervention program. Social Care Neurodis. 2014b;5:145–159. [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14:128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr Prot Neurosci. 2009:8.21.1–8.24.12. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Loureiro D, Kalikhman D, Crawley JN. Male mice emit distinct ultrasonic vocalizations when the female leaves the social interaction arena. Front Behav Neurosci. 2013;7:159. doi: 10.3389/fnbeh.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Perry K, Weber MD, Katz AM, Crawley JN. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Res. 2011a;4:17–27. doi: 10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Curr Protoc Neurosci. 2011b;Chapter 8(Unit 8.26) doi: 10.1002/0471142301.ns0826s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.162. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO. Social outcomes in pediatric traumatic brain injury: Perspectives from social neuroscience and developmental psychology. J Int Neuropsychol Soc. 2013;19:493–496. doi: 10.1017/S1355617713000398. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Bigler ED, Dennis M, Gerhardt CA, Rubin KH, Stancin T, Taylor HG, Vannatta K. Social outcomes in childhood brain disorder: a heuristic integration of social neuroscience and developmental psychology. Psychol Bull. 2007;133:535–556. doi: 10.1037/0033-2909.133.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Gerhardt CA, Bigler ED, Abildskov T, Dennis M, Rubin KH, Stancin T, Taylor HG, Vannatta K. Peer relationships of children with traumatic brain injury. J Int Neuropsychol Soc. 2013;19:518–527. doi: 10.1017/S1355617712001531. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Swift E, Taylor HG, Wade SL, Drotar D, Stancin T, Minich N. Short- and long-term social outcomes following pediatric traumatic brain injury. J Int Neuropsychol Soc. 2004;10:412–426. doi: 10.1017/S1355617704103093. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Drotar D, Wade SL, Klein S, Stancin T, Schatschneider C. Preinjury family environment as a determinant of recovery from traumatic brain injuries in school-age children. J Int Neuropsychol Soc. 1997;3:617–630. [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Walz NC, Stancin T, Wade SL. The family environment as a moderator of psychosocial outcomes following traumatic brain injury in young children. Neuropsychology. 2010;24:345–356. doi: 10.1037/a0018387. [DOI] [PMC free article] [PubMed] [Google Scholar]