Abstract
Effective immunotherapy, whether by checkpoint blockade or adoptive cell therapy, is limited in most patients by a key barrier: the immunosuppressive tumor microenvironment. Suppression of tumor-specific T cells is orchestrated by the activity of a variety of stromal myeloid and lymphoid cells. These often display inducible suppressive mechanisms that are triggered by the same anti-tumor inflammatory response that the immunotherapy intends to create. Therefore, a more comprehensive understanding of how the immunosuppressive milieu develops and persists is critical in order to harness the full power of immunotherapy of cancer.
Tumors depend on suppressive mechanisms
Malignant cells are visible to the immune system. Mutations and aberrant processing of proteins can give rise to neoantigens; chronic stress creates signals that alert the immune system; and constant cell death and turnover displays antigens and inflammatory intracellular contents in an immunogenic context. Thus, the tumor’s only option to evade immune surveillance is to evolve mechanisms to suppress the immune response. To achieve this goal, the tumor does not create a new set mechanisms from scratch; rather, it pathologically exaggerate the normal regulatory circuits that control self-tolerance, homeostasis of myeloid cells, wound-healing and response to dying cells.
Suppressive mechanisms are often inducible
Many of the regulatory mechanisms hijacked by the tumors are, in fact, counter-regulatory: i.e., mechanisms whose expression is induced by the inflammatory signals that they will suppress. These inflammatory signals may result from attempted T cell activation, or from cell death. As a consequence, not all of the inducible suppressive mechanisms that may operate in a tumor are necessarily expressed at baseline. Unlike a driver oncogene, which is present at diagnosis, some immune-escape mechanisms may only be up-regulated in response to our attempted immunotherapy: i.e., the more the tumor comes under attack, the more counter-regulatory mechanisms may be induced. This implies that patients cannot be simply pre-screened at diagnosis for expression of all the mechanisms that may be relevant. Conversely, this can also offer an opportunity to exploit potent synergy, by adding agents that blocks the inducible counter-regulatory pathways used for immune escape.
CTLA-4 and PD-1 pathways: the classic immune checkpoints
CTLA-4 and PD-1 are inhibitory molecules expressed by activated T cells. Blocking antibodies against these molecules can enhance T cell-mediated attack to tumors, particularly in those patients who already have a significant anti-tumor T cell response present prior to therapy. Anti-CTLA-4 antibody was the first checkpoint-inhibitor to be approved for clinical use [1]. When used as a single agent, durable responses have been rather limited; but when combined with PD-1 blockade the responses were better than with either agent alone [2].
With single-agent CTLA-4 blockade, clinical benefit was greatest in patients with a high mutational burden in their tumor genome, and a large number of predicted mutational neoantigens [3,4]. Patients treated with CTLA-4 blockade showed a significant increase in the frequency of T cells reacting with a set of known melanoma-associated antigens [5]. Importantly, the major change was not simply due to expanding the initial T cell clones already present at baseline (which remained unchanged), but the emergence of new clones reactive with a diverse array of additional epitopes. Of note, the epitopes tested in this study were not mutational neoantigens, but were simply known shared-self antigens associated with melanoma. Thus, blocking CTLA-4 may enhance the ability of the host immune system to respond to endogenous tumor antigens, including breaking functional tolerance to shared self antigens overexpressed by the tumor.
Exactly how this enhanced antigen response arises remains to be elucidated, because the mechanism of action of CTLA-4 blockade is still rather unclear [6]. Blocking CTLA-4 may render T cells more responsive to antigen (by lowering the activation threshold), and/or it may inhibit or deplete regulatory T cells (Tregs) [6,7]. Since Tregs can potently suppress the function of antigen-presenting cells (APCs) in the tumor microenvironment [8,9], CTLA-4 antibody might thus enhance functional cross-presentation of tumor antigens. Whatever the mechanism, one key message from these studies is that the host T cell response to endogenous tumor antigens is not fixed, and can be increased by therapy if the relevant suppressive circuits can be removed.
The PD-1/PD-ligand pathway
PD-1 is a second inhibitory molecule expressed on activated T cells. Some tumors express PD-L1 constitutively, while others may up-regulate PD-L1 in response to inflammatory signals. In addition, host APCs (DCs, MDSCs and macrophages) may also express PD-L1 or PD-L2. Blocking either PD-1 or PD-L1 can trigger some striking clinical responses, especially in melanoma and lung cancer. Like CTLA-4 blockade, response to single-agent therapy was most likely in patients who already had an anti-tumor response at baseline [10,11], and/or who had a high mutational burden [12]. In some studies, response also appeared more likely in patients with constitutive PD-L1 expression in the tumor [13]. Since PD-L1 expression is highly inducible by T cell-derived signals such as IFNγ, PD-L1 expression at baseline may in some cases be a proxy for the T cell response [14]. Intriguingly, in one recent trial, when CTLA-4 blockade was added to boost T cell responses, blocking PD-1 in this setting provided benefit even in those patients who previously lacked PD-L1 expression at diagnosis [2]. This study did not include on-treatment biopsies to ask whether the addition of anti CTLA-4 increased the T cell responses and caused inducible up-regulation of PD-L1, but this would be a reasonable hypothesis.
Thus, the early results from trials using antibody-mediated blockade of CTLA-4 or the PD-1/PD-L1 pathway are encouraging, especially when used in combination. Nevertheless, most patients with most tumor types still do not respond, and many of the responders will subsequently progress. Thus, additional strategies are needed to further enhance the anti-tumor immunity and build upon these successes.
Defective APCs in the tumor milieu
CTLA-4 and PD-1 are expressed on T cells. Blocking these pathways can enhance T cells after they are activated, but it cannot affect the underlying nature of the APC that presents the antigen initially. If these tumor-associated APCs do not effectively cross-present tumor antigens – or worse, if they are actively suppressive and tolerogenic – then T cells may never become activated in the first place, or might be silenced instead of stimulated. Unfortunately, the APCs in tumors display multiple defects. The APCs recruited by tumors are ideally suited for supporting tissue remodeling and wound-healing, but they are not effective in the cross-presentation of tissue antigens; indeed, they may actively enforce T cell tolerance. As Virchow observed, tumors appear as wounds that do not heal [15,16]. Tumors resemble healing wounds in their enhanced and often anarchic angiogenesis, in their constant tissue remodeling, and in their accumulation of fibroblasts and reparative macrophages [17,18]. Remodeling tissues must dispose of many apoptotic cells and their associated self-antigens, to which T cell tolerance must be strictly maintained. Thus, while a sterile wound may appear “inflamed”, the actual cytokines such as TGFβ and VEGF that are associated with angiogenesis and the reparative macrophage response are actively tolerogenic for T cells. These signals potently inhibit immunogenic antigen presentation to T cells [19,20]. Therefore, in the case of many of these pathways, tumors do not need to “evolve” their immunosuppressive mechanisms: as long as they simply resemble a remodeling normal tissue, these suppressive mechanisms will naturally be activated.
Tumor-associated macrophages
Tumor-associated macrophages (TAMs) are key coordinators of tumor-promoting angiogenesis, fibrous stroma deposition and metastasis formation [21]. TAMs are also inhibitory for T cell responses [20]. In part, this immunosuppressive phenotype may simply recapitulate the “reparative” phenotype of macrophages during tissue remodeling [17], but signals in the tumor such as acidosis and hypoxia may further drive their phenotype and function [22]. Molecular mechanisms of TAM-induced immune suppression are not yet well defined, but likely include production of VEGF and TGFβ, both of which inhibit T cell responses. Whatever the mechanism, destabilization of the intra-tumoral macrophage pool, e.g., by blocking the CSF-1-receptor, significantly impairs tumor growth and enhances anti-tumor immune responses [23,24].
Myeloid-derived suppressor cells
While TAMs may resemble normal “M2-like” macrophages or reparative macrophages, the tumor microenvironment is actually highly abnormal, and the myeloid lineage in tumors is profoundly disordered [21]. Bone-marrow myelopoiesis is altered by factors secreted from the tumor, such as GM-CSF and IL-6, which affects both the monocytic and granulocytic lineages [25]. These abnormal circulating cells become further altered when they are recruited into the tumor microenvironment, with its chronic low-grade inflammation, free-radical flux and constant metabolic stress [26]. The presence of IDO and activated Tregs in the tumor may enhance the recruitment of these cells, and render them even more suppressive [27]. Once in place, this heterogeneous population of “myeloid-derived suppressor cells” (MDSCs) create an immunosuppressive milieu via elaboration of nitric oxide, arginase and reactive oxygen species [21,28].
Dendritic cells in tumors
Even more damaging than the direct suppression mediated by MDSCs may be the defects created by what they are not: they completely fail to differentiate into inflammatory, immunogenic dendritic cells (DCs) that could effectively cross-present tumor antigens. Tumors are treated by the immune system like a slow, lingering wound; yet they ought to receive as much attention as a fulminant, life-threatening viral infection. Oddly, a small virus may introduce fewer “neoantigens” into its host cell than a mutated tumor genome, yet the virus generates robust immune activation and antigen cross-presentation, usually followed by CD8+ T-cell-mediated eradication. The reason for this difference in response – a difference which ultimately allows the cancer to kill the patient – is not yet entirely clear.
Ironically, the response of myeloid-lineage immune cells to viral and other infections is actually quite similar to the tumor-induced myelopoiesis of MDSCs, with the dramatic exception that MDSCs fail to differentiate into activated, immunogenic myeloid DCs and inflammatory macrophages [29]. Immunogenic CD103+ myeloid DCs have recently been identified in tumors, and they are potent cross-presenting APCs for tumor antigens [30]. However, in most tumors these cells are extremely rare, whereas their defective alternatives (the suppressive MDSCs) are present in large numbers [30]. Whatever the factors in the tumor microenvironment that block the beneficial differentiation of inflammatory myeloid DCs, the consequences to the host are dire. Thus, identifying the factors that inhibit DC differentiation and function in tumors, and developing strategies that may be able to restore such differentiation [31,32], is an important priority for the field. As one recent example, oncogenic drivers such as the WNT/β-catenin signaling pathway can inhibit priming of T cells in the tumor, by altering the access of CD103+ DCs to the tumor environment [33].
IDO and counter-regulation
IDO is a tryptophan-catabolizing enzyme that plays a regulatory and tolerogenic function in the immune system [34]. The biologic role of IDO is narrower and more focused than CTLA-4 or PD-1, but in certain settings it can be non-redundant for creating acquired peripheral tolerance. Thus, in contexts as diverse as mucosal tolerance, tissue transplantation or mammalian pregnancy, interrupting the IDO pathway can convert a normally tolerogenic antigen exposure into an immunogenic T cell response [34]. IDO is also up-regulated by exposure to apoptotic cells; and mice lacking the IDO1 gene rapidly develop lethal lupus-like autoimmunity when challenged repeatedly with self apoptotic cells [35]. In these various models, it is important to note that it was not the nature of the antigens themselves that dictated tolerance versus immunity, but rather the presence or absence of the instructive contextual signals delivered by IDO and the downstream pathways it elicits.
In tumors, IDO can be expressed by the tumor cells themselves, or by host cells such as DCs and macrophages. Often, both tumor and host cells can express IDO. Expression of IDO may have several effects: it may contribute to activation of suppressive Tregs within the tumor, as discussed below; it may create a local milieu that is deficient in tryptophan, and thus inimical to T cell activation [36]; and it may produce increased levels of kynurenine metabolites, which can themselves affect the immune system by activating the aryl hydrocarbon receptor (AhR) [37]. IDO expression may also recruit MDSCs into the tumor, thereby increasing the suppressive milieu [27].
Expression of IDO may be constitutive (e.g., in tumor cells), or it may be induced in response to local inflammatory signals [14]. In part this may explain why IDO expression in certain tumors can be paradoxically associated with increased T cell infiltration – in this case, IDO is an elicited counter-regulatory response, rather than a primary inhibitory mechanism. In the normal immune system, IDO is usually counter-regulatory, meaning that it is elicited by inflammation (colitis, autoimmunity, infection, apoptotic cells) and then acts to suppress or limit the damaging immune response. This highly inducible nature of IDO has implications for tumor immunotherapy, where the goal of treatment is specifically to create immune activation and inflammation in the tumor. If these inflammatory signals also elicit counter-regulatory IDO, then the therapy itself may unintentionally blunt its own effectiveness. Thus, combination of an IDO-inhibitor drug with other immunotherapy may be synergistic, as suggested by preclinical studies of combination with anti-CTLA-4, anti-PD-1 or CAR-T cells [38–41]. In addition, useful and potent vaccine adjuvants such as TLR-ligands and STING agonists are also potentially inducers of counter-regulatory IDO expression [42].
Activated Tregs and tolerance to dying tumor cells
Tregs are an important suppressive population in tumors [43]. Depleting Tregs [44] or inhibiting signaling pathways that they require [45] rescues immune surveillance against the tumor. However, it remains unclear how Tregs exert their suppressive function. Tregs are known to produce local IL-10 and TGFβ, but an additional important mechanism may be their inhibitory effect on tumor-associated APCs [8,9]. In the presence of Tregs, tumor-associated DCs lose co-stimulatory ligands and cannot support T cell activation [8].
One important unanswered question is why Treg activity is so excessive and dominant in tumors. In part, this may be due to extra Tregs that arise against tumor neoantigens; but this seems unlikely to be the major explanation, and many of the Tregs in tumors appear to recognize the same normal self antigens as in the tissue of origin [46]. Thus, the higher degree of suppression in tumors may reflect a higher degree of Treg functional activation, and this functional activation step is not well understood.
Several upstream pathways are known to activate Treg function in tumors, including IDO [47] and neuropilin-1[48]. Recently, it was found that when Tregs are activated by IDO they up-regulate expression of the PD-1 receptor; this PD-1 then maintains the suppressive Treg phenotype in long-term, by signaling via PTEN phosphatase [49]. Neuropilin-1 also activates PTEN in Tregs [48], and PTEN has been recently implicated in maintaining normal function and stability of Tregs [50,51]. Thus, PTEN may be an important, centrally-positioned pathway in tumor-induced Treg activation. In tumor-bearing mice, genetic ablation or pharmacologic inhibition of the PTEN pathway in Tregs prevented tumors from creating an immunosuppressive local microenvironment, and markedly enhanced immune responses to dying tumor cells after chemotherapy [49].
This effect on the immunogenicity of dying tumor cells is potentially important. When certain transplantable tumors are treated with anthracycline chemotherapy, they can undergo a form of cell death that is spontaneously immunogenic [32]. If dying tumor cells could be rendered consistently immunogenic, this could have major implications for response to chemotherapy or radiation, and for epitope-spreading after vaccination or T cell adoptive transfer. However, true “immunogenic” cell death (i.e., capable of generating an anti-tumor immune response by itself) is rare, and occurs only under certain circumstances [52]. This is probably because dying normal cells are usually tolerogenic, not immunogenic [53], and dying tumor cells may elicit suppressive pathways such as IDO, TGFβ and Treg activation, which inhibit the attempted immune response [35,49]. Thus, strategies to circumvent these inhibitory pathways, or to enhance immunogenic cross-presentation such as blocking CD47 [54], may allow a robust immune response to dying tumor cells under a much wider variety of conditions.
Conclusions
We are starting to appreciate that tumors can hijack a number of potent, non-redundant negative regulators of the immune system in order to survive (Figure 1). This review discusses certain of these immunoregulatory circuits, with a focus on how they may relate to emerging immunotherapeutic approaches in the clinic. Numerous other inhibitory pathways exist, and have been recently reviewed elsewhere [21,28,55]. But from a therapeutic standpoint, the key question is: how many of these molecules must we target at the same time in order to improve the clinical outcome in patients undergoing immune-based therapy? The answer will require empirical data from patient outcomes in the clinic, focused in particular on those patients who fail our current single-agent therapies. Data from on-treatment biopsies and similar studies will help to establish whether a hierarchy of pathways exists, with certain mechanisms being induced in response to attempted interventions. Other open questions include characterization of immunosuppressive mechanisms according to stage or tumor type, or to genetic subtypes of cancers. Here the power of bioinformatics may help pick up unexpected associations and signatures [56]. When these same techniques can also be applied to on-treatment samples from patients undergoing immunotherapy, then a clearer picture of the immune-regulatory network in the tumor microenvironment may emerge.
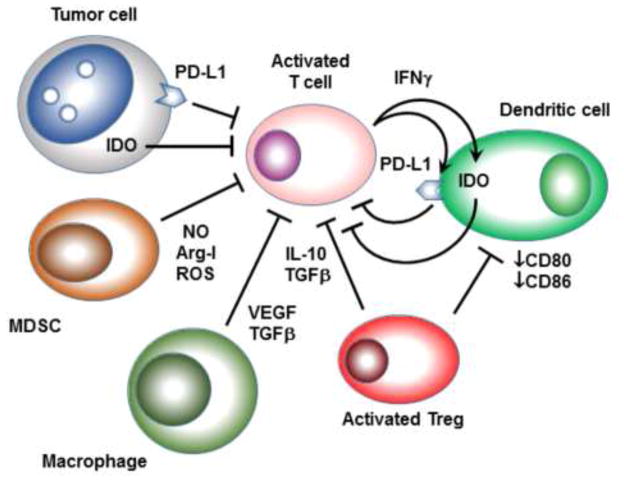
Figure 1. Examples of constitutive and inducible suppressive mechanisms in the tumor microenvironment.
T cells attempting to activate in the tumor microenvironment may face constitutive expression of PD-L1 and IDO by the tumor cells. Myeloid-derived suppressor cells (MDSCs) in the tumor may produce immunosuppressive nitric oxide (NO), arginase-I or reactive oxygen species (ROS). Tumor-associated macrophages may produce TGFβ and VEGF, which can be inhibitory for both T cells and dendritic cells. Activated Tregs can produce IL-10 and TGFβ, which may directly suppress T cells. Tregs may also inhibit expression of costimulatory ligands CD80 and CD86 on local DCs, thus rendering them ineffective and tolerizing antigen-presenting cells. As effector T cells attempt to activate, their production of IFNγ and other pro-inflammatory cytokines may actively up-regulate expression of IDO and PD-L1 by DCs, thus eliciting counter-regulatory suppression. Many tumor cells may also respond to IFNγ by up-regulating IDO and PD-L1.
Highlights.
Tumors exaggerate and exploit natural immunosuppressive and tolerogenic mechanisms
This milieu favors angiogenesis and tissue remodeling but is suppressive for T cells
T cell activation in tumors may paradoxically elicit counter-regulatory suppression
Acknowledgments
This work was supported by grants from the Italian Ministry of Health; the Italian Ministry of Education, Universities and Research (FIRB cup: B31J11000420001); the Italian Association for Cancer Research (AIRC 6599, 12182 and 14103); and the Italian Ministry of Health (FINALIZZATA 2011-2012 RF-2011-02348435 cup: E35G1400019001); and NIH grants R01CA096651 and R01CA103320.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David H. Munn, Georgia Regents University Cancer Center and the Medical College of Georgia, Augusta GA 30192, USA
Vincenzo Bronte, University Hospital and Department of Medicine, University of Verona, 37134, Italy.
References
- 1.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Geukes Foppen MH, Goldinger SM, et al. Genomic correlates of response to CTLA4 blockade in metastatic melanoma. Science. 2015;350:207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Kvistborg P, Philips D, Kelderman S, Hageman L, Ottensmeier C, Joseph-Pietras D, Welters MJ, van der Burg S, Kapiteijn E, Michielin O, et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med. 2014;6:254ra128. doi: 10.1126/scitranslmed.3008918. This study shows that CTLA-4 blockade can allow T cell responses to new tumor-associated antigens (suggesting improved cross-presentation of endogenous tumor antigens), and that these antigens are not confined only to unique mutational neoantigens, but can also be tumor-associated shared-self antigens. [DOI] [PubMed] [Google Scholar]
- 6.Walker LS, Sansom DM. Confusing signals: recent progress in CTLA-4 biology. Trends Immunol. 2015;36:63–70. doi: 10.1016/j.it.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Bauer CA, Kim EY, Marangoni F, Carrizosa E, Claudio NM, Mempel TR. Dynamic Treg interactions with intratumoral APCs promote local CTL dysfunction. J Clin Invest. 2014;124:2425–2440. doi: 10.1172/JCI66375. This study shows that one major target of Tregs in the tumor microenvironment is inhibition of APC function (antigen presentation and costimulation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, DuPage M, Tammela T, Kerper NR, Farago AF, et al. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity. 2015;43:579–590. doi: 10.1016/j.immuni.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. This study demonstrates that the attempted immune response by tumor-infiltrating CD8 T cells can paradoxically up-regulate inhibitory pathways such as IDO and PD-L1, and recruit additional Tregs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 16.Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Molec Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 17.Rybinski B, Franco-Barraza J, Cukierman E. The wound healing, chronic fibrosis, and cancer progression triad. Physiol Genomics. 2014;46:223–244. doi: 10.1152/physiolgenomics.00158.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 19.Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11:702–711. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- 20.Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, Pamer EG, Li MO. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ugel S, De Sanctis F, Mandruzzato S, Bronte V. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Invest. 2015;125:3365–3376. doi: 10.1172/JCI80006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, Belaygorod L, Carpenter D, Collins L, Piwnica-Worms D, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013;73:1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. This study demonstrates that TAMs are not only important for initial tumor growth, but also play an on-going maintenance/sustaining role that can be targeted for immunotherapy. [DOI] [PubMed] [Google Scholar]
- 25••.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. This study demonstrates that tumor-derived cytokines such as GCSF, GM-CSF and IL-6 can drive systemic MDSC production, resulting in inhibition of anti-tumor effector T cell activity in the tumor. [DOI] [PubMed] [Google Scholar]
- 26.Thevenot PT, Sierra RA, Raber PL, Al-Khami AA, Trillo-Tinoco J, Zarreii P, Ochoa AC, Cui Y, Del Valle L, Rodriguez PC. The stress-response sensor CHOP regulates the function and accumulation of myeloid-derived suppressor cells in tumors. Immunity. 2014;41:389–401. doi: 10.1016/j.immuni.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmgaard RB, Zamarin D, Li Y, Gasmi B, Munn DH, Allison JP, Merghoub T, Wolchok JD. Tumor-expressed IDO recruits and activates MDSCs in a Treg-dependent manner. Cell reports. 2015;13:412–424. doi: 10.1016/j.celrep.2015.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldszmid RS, Dzutsev A, Trinchieri G. Host immune response to infection and cancer: unexpected commonalities. Cell Host Microbe. 2014;15:295–305. doi: 10.1016/j.chom.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:638–652. doi: 10.1016/j.ccell.2014.09.007. This study identifies a rare population of tumor-associated DCs that are capable of cross-presentation of tumor antigens and activation of T cell responses. Unlike the much larger populations of defective and suppressive APC normally found in tumors, these appear to be important cross-priming cells, which may be beneficial to expand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest. 2011;121:4746–4757. doi: 10.1172/JCI58814. This report shows that enforced over-expression of IL-12 in the tumor microenvironment can convert the normally tolerogenic local APCs into immune-stimulatory APCs. While this study depended on artificial delivery of IL-12, the important finding was the plasticity of the suppressive tumor microenvironment under the right signals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38:729–741. doi: 10.1016/j.immuni.2013.03.003. This study used anthracycline chemotherapy to induce immunogenic cell death in tumor cells, and showed that this allowed maturation (or perhaps recruitment) of activated APCs into the tumor (again demonstrating that the suppressive milieu is not immuntable, and can be altered) [DOI] [PubMed] [Google Scholar]
- 33.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 34.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Ravishankar B, Liu H, Shinde R, Chandler P, Baban B, Tanaka M, Munn DH, Mellor AL, Karlsson MC, McGaha TL. Tolerance to apoptotic cells is regulated by indoleamine 2,3-dioxygenase. Proc Natl Acad Sci USA. 2012;109:3909–3914. doi: 10.1073/pnas.1117736109. This is an important study showing that tolerogenic signals delivered by IDO-expressing cells in the spleen were required to enforce tolerance to intravenous challenge with apoptotic cells. The significance of this lies in the fact that apoptotic cells (even normal self cells) can become immunogenic when certain tolerogenic signals such as IDO are blocked. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med. 2013;210:1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim CK, Tobias A, Cheng Y, Kim JW, Qiao J, Zhang L, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20:5290–5301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J Immunother Cancer (JITC) 2014;2:3. doi: 10.1186/2051-1426-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ninomiya S, Narala N, Huye L, Yagyu S, Savoldo B, Dotti G, Heslop HE, Brenner MK, Rooney CM, Ramos CA. Tumor indoleamine 2,3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood. 2015;125:3905–3916. doi: 10.1182/blood-2015-01-621474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang L, Li L, Lemos H, Chandler PR, Pacholczyk G, Baban B, Barber GN, Hayakawa Y, McGaha TL, Ravishankar B, et al. Cutting edge: DNA sensing via the STING adaptor in myeloid dendritic cells induces potent tolerogenic responses. J Immunol. 2013;191:3509–3513. doi: 10.4049/jimmunol.1301419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J Exp Med. 2013;210:2435–2466. doi: 10.1084/jem.20130762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Ali K, Soond DR, Pineiro R, Hagemann T, Pearce W, Lim EL, Bouabe H, Scudamore CL, Hancox T, Maecker H, et al. Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510:407–411. doi: 10.1038/nature13444. This study demonstrates that Tregs protecting tumors can be dependent on certain specific activation pathways, with a sufficient degree of selectivity that blocking these pathways can enhance anti-tumor immune responses without creating lethal autoimmunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, Kang C, Geddes JE, Allison JP, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–1224. doi: 10.1126/science.1233913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501:252–256. doi: 10.1038/nature12428. This study demonstrates another important tumor-induced activation pathway that creates suppressive Tregs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Sharma MD, Shinde R, McGaha T, Huang L, Holmgaard RB, Wolchok JD, Mautino MR, Celis E, sharpe A, francisco LM, et al. The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. Science Advances. 2015 doi: 10.1126/sciadv.1500845. in press This study identifies PTEN phosphatase as an important signaling pathway in tumor-associated Tregs, which lies downstream of multiple upstream Treg-activation signals. Blocking PTEN in the Tregs allowed downregulation of multiple suppressive mechanisms in the tumor microenvironment. [DOI] [PMC free article] [PubMed]
- 50.Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, Townamchai N, Gerriets VA, Rathmell JC, Sharpe AH, et al. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol. 2015 doi: 10.1038/ni.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain Th1 and Tfh cell responses. Nat Immunol. 2015;16:178–187. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bezu L, Gomes-de-Silva LC, Dewitte H, Breckpot K, Fucikova J, Spisek R, Galluzzi L, Kepp O, Kroemer G. Combinatorial strategies for the induction of immunogenic cell death. Front Immunol. 2015;6:187. doi: 10.3389/fimmu.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X, Pu Y, Cron K, Deng L, Kline J, Frazier WA, Xu H, Peng H, Fu YX, Xu MM. CD47 blockade triggers T cell-mediated destruction of immunogenic tumors. Nat Med. 2015;21:1209–1215. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J Clin Invest. 2015;125:3347–3355. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]