Abstract
Transplantation of autologous mesenchymal stem cells (MSCs) has been shown to attenuate renal injury and dysfunction in several animal models, and its efficacy is currently being tested in clinical trials for patients with renal disease. Accumulating evidence indicates that MSCs release extracellular vesicles (EVs) that deliver genes, microRNAs and proteins to recipient cells, acting as mediators of MSC paracrine actions. In this context, it is critical to characterize the MSC-derived EV cargo to elucidate their potential contribution to renal repair. In recent years, researchers have performed high-throughput sequencing and proteomic analysis to detect and identify genes, microRNAs, and proteins enriched in MSC-derived EVs. The present review summarizes the current knowledge of the MSC-derived EV secretome to shed light into the mechanisms mediating MSC renal repair, and discusses preclinical and clinical studies testing the efficacy of MSC-derived EVs for treating renal disease.
Keywords: mesenchymal stem cells, extracellular vesicles, microvesicles, exosomes, kidney
Introduction
Acute kidney injury (AKI) is a common disorder defined by an abrupt loss in renal function that remains an important challenge in developed and developing countries. AKI is responsible for approximately 1.9% of all hospitalizations in the United States [1], and the incidence of severe dialysis-requiring AKI is estimated to be nearly 30 per 100,000 person per year [2]. Moreover, AKI is associated with increased short term mortality and long term risk of Chronic kidney disease (CKD) and other complications [3].
CKD is the progressive loss of renal function that affects over 200 million people worldwide [4], and is associated with increased morbidity and mortality rates [5]. According to the most recent reports of the Global Burden of Disease Study, CKD was ranked as the 25th leading cause of death in 1990, but rose to the 18th place in 2010 [6]. Similarly, total mortality for CKD rose by 31.7% from 937,000 deaths in 2005 to more than 1,234,000 deaths in 2015 [7]. Furthermore, the prevalence of end-stage renal disease (ESRD), the most severe stage of CKD, is estimated to be 8-16% worldwide [5]. Thus, given the continuously rising incidence and prevalence of both diabetes mellitus and hypertension, the most common risk factors for developing CKD, continue to rise, mortality attributable to CKD is predicted to increase in the next decade [8].
Both AKI and CKD also impose a severe economic burden. Costs associated with AKI represent approximately 5% of overall hospital expenses [9]. Similarly, in many developed countries, more than 2-3% of the total annual health-care budget is allotted for the care of patients with ESRD [10]. According to the U.S. Renal Data System, Medicare spent over $29 billion, or 5.9% of its total annual budget, for treatment of patients with ESRD in 2009 [11], but the annual expenditure for patients in stages 2 to 4 of CKD was about $49 billion in 2011 [12].
Current therapeutics options for patients with AKI and CKD are limited. Management of AKI is mostly conservative and there are few measures to change its course and prevent its progression to CKD [13]. Treatment of the underlying cause and preventive measurements, such as blood pressure and glucose control, is the cornerstone in the management of patients with CKD. However, dialysis and kidney transplant are the only viable therapeutic options for patients reaching ESRD [14]. Therefore, the economic burden of both AKI and CKD, the grave prospect of its rising incidence and prevalence, and the limited therapeutic options underscores the need for developing novel interventions to prevent its progression to ESRD and the costs of dialysis or organ transplantation.
Over the last couple of decades, the field of regenerative medicine emerged as a novel promising strategy to modulate the progression of AKI and CKD. Mesenchymal stem/stromal cells (MSCs) have attracted much attention over other stem/progenitor-cell types, due to their self-renewal capacity, multi-lineage differentiation, immunomodulatory properties, and potential for tissue repair. According to the International Society for Cellular Therapy, the minimal criteria for defining MSCs include the evidence of plastic adherence in culture, expression of CD73, D90, and CD105 and lack expression of CD14, CD34, and CD45 surface markers, and the ability to differentiate in vitro into adipocytes, chondrocytes and osteocytes [15]. MSCs are present in many adult tissues and can be easily isolated from different sources, including the bone marrow, adipose tissue, and umbilical cord, becoming an ideal candidate for cell-based therapy [16].
In recent years, several experimental studies have uncovered protective roles of MSCs for both AKI and CKD [17, 18]. For example, a single intravenous injection on MSCs attenuates sepsis-associated AKI and improves survival in mice [19]. Likewise, in rats with partial nephrectomy, an experimental model of CKD, injection of MSCs in the tail vein preserves renal function and attenuates renal injury [20]. In agreement, we have previously shown in swine renovascular disease that a single intrarenal injection of adipose-tissue derived MSCs with or without renal revascularization ameliorates renal injury and improves function in the post-stenotic kidney, underscoring the therapeutic potential of MSCs for preserving the post-stenotic kidney [21-23].
According to the US National Institute of Health database (ClinicalTrials.gov), more than 30 clinical trials worldwide are currently testing the safety and efficacy of MSCs to treat patients with renal-related diseases. A phase-I clinical trial in patients with AKI following cardiac surgery demonstrated that administration of allogenic MSCs into the suprarenal aorta confers early and late protection of kidney function [24]. In addition, several clinical trials are testing the safety and efficacy of autologous and allogenic MSCs to treat CKD, including diabetic nephropathy, renovascular disease, and Lupus nephropathy, among others [25]. Taken together, preclinical and clinical data have illustrated the enormous potential therapeutic value of MSCs to prevent renal injury and promote functional recovery. However, challenges remain in clinical applications as reports have indicated that delivery of live-replicating cells may promote tumor growth, malformation, or microinfarctions [26], underscoring the need of safe and effective alternatives for cell-based therapy.
Although MSCs may contribute to repopulating injured renal tissue by engrafting into renal tubular and endothelial cells [27], their regenerative effects are primarily exerted by their paracrine function [28]. In addition to the release of cytokines, chemokines, and growth factors, these cells produce and secrete extracellular vesicles (EVs), membrane microparticles that transfer mRNAs, microRNAs, and proteins to recipient cells [29]. Previous studies have shown that MSC-derived EVs transfer enhances proliferation, inhibits apoptosis, decreases inflammation, and promotes angiogenesis by altering the gene expression profile of their target cells [28, 30]. Therefore, delivery of MSC-derived EVs may be an attractive cell-free therapy for renal disease. The present review summarizes the current knowledge of the MSC-derived EV secretome to shed light into the mechanisms mediating MSC renal repair, and discusses preclinical and clinical studies testing efficacy of MSC-derived EVs in treatment of renal disease.
MSC-derived EVs
EVs released from MSCs are phospholipid bilayer-enclosed structures which can be visualized by electron microscopy techniques. Generally, EVs appear as membrane surrounded particles emerging from the MSC surface on transmission electron microscopy (Fig. 1), with “cup-like” morphology on negative staining (Fig. 2). Independent of their cell of origin, EVs can be classified by their size in exosomes and microvesicles. Although exosomes (with a diameter of 30-120nm) are generally smaller than microvesicles (ranging from 100nm to 1μm), the main distinction between these EV subgroups reside in their primary mechanisms of biogenesis [31]. Exosomes arise from endosomal compartments, known as multi-vesicular bodies, and are released upon their fusion with the cell membrane [32, 33], whereas microvesicles are formed by outward budding and fission of the plasma membrane in a process dependent on calcium and the cytoskeleton [32, 33]. MSC-derived EVs express characteristics of their parental MSCs, including the surface markers CD44, CD73, CD90, and CD105, as well as specific EV surface markers, such as CD9, CD63, and CD81 [34-36]. Importantly, MSC-derived EVs contain a vast number of mRNAs, microRNAs and proteins, which mediate the paracrine effects of MSCs by modulating several cellular pathways in recipient cells [30].
Figure 1.
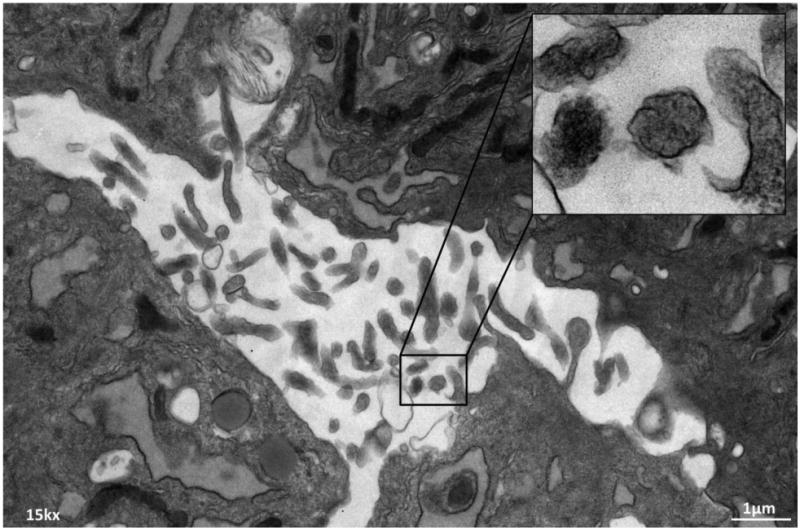
Transmission electron microscopy of culture swine adipose tissue-derived mesenchymal stem cells (MSCs) showing release of multiple extracellular vesicles (EVs).
Figure 2.
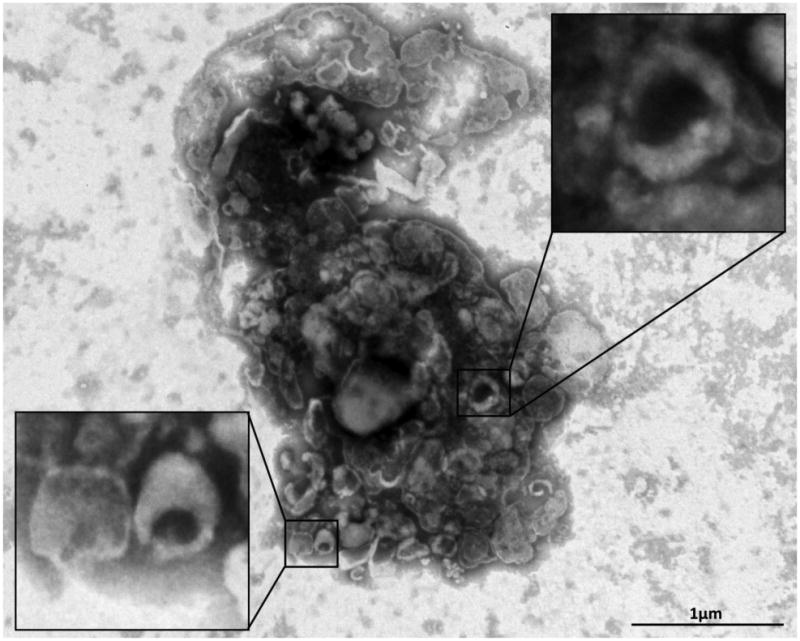
Negative staining of MSC culture supernatants showing EV clusters with the classic “cup-like” morphology.
Genes enriched in MSC-derived EVs
Accumulating evidence indicates that the mRNA cargo of MSC-derived EVs is not merely a reflection of the mRNA pool in their parental MSCs. A defined set of mRNAs are selectively packed in EVs (Table 1 and Figure 3). Characterization of the transcriptome of human bone marrow MSCs and their relative-derived EVs using real-time quantitative polymerase chain reaction (RT-qPCR) arrays revealed that EVs contain a wide range of mRNAs involved in transcription (e.g. TCFP2, RAX2, IRF6), cell cycle regulation (e.g. SENP2, RBL1, CDC14B), immune regulation (e.g. IL1RN, MT1X, CRLF1), extracellular matrix remodeling (e.g. COL4A2, IBSP), cytoskeleton (e.g. DDN, MSN, CTNNA1), and cell differentiation (e.g. RAX2, EPX, SCNN1G) [37]. In another study, RT-qPCR detected a selected pattern of transcripts in EVs versus their parental bone marrow-derived MSCs, including important members of the human insulin signaling pathway, such as Insulin-like Growth Factor-1 receptor (IGF1R) [38]. We have previously characterized the mRNA cargo of EVs from porcine adipose-tissue derived MSCs using high-throughput RNA sequencing (RNA-seq), identifying selective EV enrichment for distinct classes of RNAs. Functional annotation enrichment analysis of genes packed in EVs revealed mRNA for transcription factors (e.g. MDFIC, POU3F1, NRIP1) and genes involved in angiogenesis (e.g. HGF, HES1, TCF4) and adipogenesis (e.g. CEBPA, KLF7) [39]. EVs also contain Golgi apparatus genes (ARRB1, GOLGA4) and genes involved in transforming growth factor (TGF)-β signaling (TGFB1, TGFB3, FURIN, and ENG), whereas mitochondrial, calcium signaling, and cytoskeleton genes are selectively excluded from EVs, possibly because these genes remain sequestered in organelles or intracellular compartments. Thus, these findings indicate that MSC-derived EVs contain a selective cargo of genes with potential to alter the phenotype of recipient cells and exert tissue trophic and reparative effects.
Table 1.
Evidence of mRNAs enriched in MSC-derived EVs.
| Study | Source of EVs | mRNAs |
|---|---|---|
| Tomasoni et al [38], Stem Cells Dev, 2013 | Mouse Bone-marrow MSCs | IGF-1R |
| Bruno et al [37], J Am Soc Nephrol, 2009 | Human Bone-marrow MSCs | RAX2, OR11H12, OR2M3, DDN, GRIN3A, NIN, BMP15, IBSP, MAGED2, CEACAM5, COL4A2, SCNN1G, PKD2L2, HK3, EPX, CLOCK, IRF6, LHX6, RAX2, TCFP2, BCL6B, HMGN4,TOPORS, ESF1, POLR2E, ELP4,HNRPH2, SENP2, RBL1,CDC14B, S100A13, CEACAM5, CLEC2A, CXCR7, ADAM15, FUT3, ADM2, LTA4H, BDH2, RAB5A, CRLF1, IL1RN, MT1X, DDN, MSN, CTNNA1, COL4A2, IBSP |
| Eirin et al [39], Gene, 2014 | Porcine adipose tissue MSCs | ABCC2, ACER2, ACTL10, ADAM8, ADAMTS13, ADAMTS5, AKAP5, AKAP9, ANKAR, ANKRD11, ANKRD12, ANKRD50, ARRB1, ASPN, ATP11A, AXIN2, B3GALNT1, BAZ2B, C11orf74, C1orf27, C6orf163, CACNA1G, CAPS2, CASP12, CCDC88A, CD83, CEBPA, CEP57L1, CLIC2, CLK4, CLMN, CLU, COL27A1, CREG1, CRISPLD2, DGKH, ENG, ERICH1, ERO1LB, F13A1, FBXO30, FGD4, FILIP1L, FMO4, FOXP3, FURIN, GDF1, GFPT2, GOLGA4, GPRIN1, GRM2, GUCY1A3, HES1, HINT3, HIVEP1, HSP70, HSP70, IFN-ALPHA9, IFNAR1, IFT57, IGSF3, INPP4B, ITGA11, ITM2B, IVNS1ABP, JAK, JARID2, JMJD1C, KAT6B, KBTBD11, KCNE4, KCNH6, KDM6B, KLF7, KLHL24, KRCC1, LACC1, LCOR, LDB2, LGALS1, LOC733612, LPAR6, LYRM2, MDFIC, MDM4, MGA, MKLN1, MPZL3, MXD1, MYH3, MYNN, N4BP2, NCALD, NDRG4, NFKBIZ, NIP7, NRIP1, OAF, PAIP2, PCDH7, PDCD4, PDGFRB, PEG3, PEX13, PI15, PLXNC1, PMAIP1, PPAP2B, PPP4R2, PRKAR2B, PROX2, PRR15, PRR5L, PRRX1, PTGER4, RAB39A, RAB5A, RAPGEF5, RARR, ES2, RBAK, RCSD1, REL, RFTN2, RGS1, RGS17, RICTOR, RIT1, RNF138, RNF19A, RUNX1T1, SAMD9, SAV1, SEMA5A, SEMA7A, SETBP1, SH2B3, SH3PXD2A, SIGLEC15, SIKE1, SKIL, SKOR1, SLC15A2, SLC25A36, SLC38A2, SLC47A2, SMAD5, SNAPC1, SNORA11, SNORA21, SNORD16, SNO, RD89, SPAG16, SSBP2, SUFU, SUMO1, TBK1, TBK1, TBK1, TCF4, TET2, TGFB1, TGFB3, TMEM55A, TMF1, TRPS1, TRPS1, TRPS1, UBE2H, UBN2, UFSP2, VANGL2, VPS37B, WDR43, WDR52, YPEL5, ZBED3, ZBTB1, ZFAND5, ZHX1, ZNF217, ZNF238, ZNF280D, ZNF461, ZNF568, ZNF608, ZNF667, ZSWIM6 |
| Eirin et al [78], PloS One, 2017 | Porcine adipose tissue MSCs | SNORD89, C6orf163, SKOR1, VPS37B, HINT3, ACTL10, ACER2, RNF19A, PROX2, NRIP1, TRPS1, TET2, CASP12, IGSF3, ANKRD12, ZSWIM6, RGS1, MDM4, KCNH6, CCDC88A, JARID2, BAZ2B, TRPS1, ZNF667, MYNN, CACNA1G, DGKH, INPP4B, FILIP1L, RGS17, KAT6B, ASPN, COL27A1, ZNF461, F13A1, PEG3, MYH3, PDCD4, GFPT2, CRISPLD2, ATP11A, FMO4, FGD4, ABCC2, UFSP2, CAPS2, RUNX1T1, SAMD9, SH2B3, GDF1, PRR5L, LCOR, N4BP2, ADAMTS5, SIGLEC15, SEMA5A, GOLGA4, NFKBIZ, KLHL24, C1orf27, SPAG16, UBN2, ANKAR, MXD1, ZBTB1, GPRIN1, B3GALNT1, AKAP5, ZHX1, ITM2B, WDR52, KBTBD11, REL, PRR15, RICTOR, KCNE4, PI15, FBXO30, SIKE1, RFTN2, JMJD1C, RBAK, SETBP1, ZNF217, ZNF568, TMF1, LPAR6, LDB2, PPP4R2, MGA, CLMN, LGALS1, PRRX1, LACC1, ZNF280D, SNORD16, RAB39A, PAPOLG, LYSMD2, ETV1, PPP3CA, ZNF131, BRWD1, ZFP30, GLIS3, RSPH9, SLC10A6, EFNA1, TRIP11, ITGB1, PPM1L, JMY, KLF7, LYST, C10orf118, CCNT2, ZNF260, MAP3K1, SENP1, PIGU, RING1, MLXIP, TBC1D15, CEP290, ZBED6, DDI2, FAM171B, ZCCHC6, ARRDC4, BMPR1B, ELK4, LARP1B, ARRDC3, NIPBL, MED13, SEMA6A, EFHB, TSPAN18, ZNF146, SOS2, OMD, STARD9, CARD14, MBTD1, ERBB2IP, SP4, LMBRD2, ZNF292, SLMO2, MYSM1, ERS1, NLRC3, DENND3, USP37, SAMD8, ZFP37, UBASH3B, OPRD1, SLC6A19, GCC2, ATP2A2, ARL5B, IKZF2, CACNB2, KCTD12, SMOC2, OLFML2B, PTAR1, KIAA0825, CSMD2, PITX3, ITGB8, PLAG1, CPEB4, ZNF770, ZNF404, FEM1C, ARID4B, METTL4, ZBTB37, TP53, ZNF701, SPAG9, SLC4A10, ICAM3, EGFLAM, PRKD3, SCARNA15, CDCP1, RASSF8, RSBN1, RPS6KL1, HMGCLL1, ELMOD2, CEP120, RNF183, ESCO1, MAP3K2, CHD1, CEP97, XRN1, RASAL1, SWT1, ZNF33B, ZNF827, FNDC3A, USP34, BICC1, PCMTD1, ZFHX3, NFAT5, MEGF10, FRMD4B, FREM2, NAA16, SNORA55, GALNTL5, SNORA70, PCDHA3, NBEAL1, TRPM7, NKTR, DAZAP1, GABRB1, GPR137B, NIN, BNC1, RNF217, C5orf34, SNORA46, MFI2, LHX9, MDM2, EXOC5, FHAD1, TNFSF10, PHC3, SNX29, NGFR, LRGUK, SF3B1, PNPLA8, SIRT2, ZNF354C, HSPA13, GOLGB1, RYR2, TMEM100, FAM214A, STK17B, CSPP1, DTX1, TPRG1, NTN5, CEP350, THAP5 |
Figure 3.
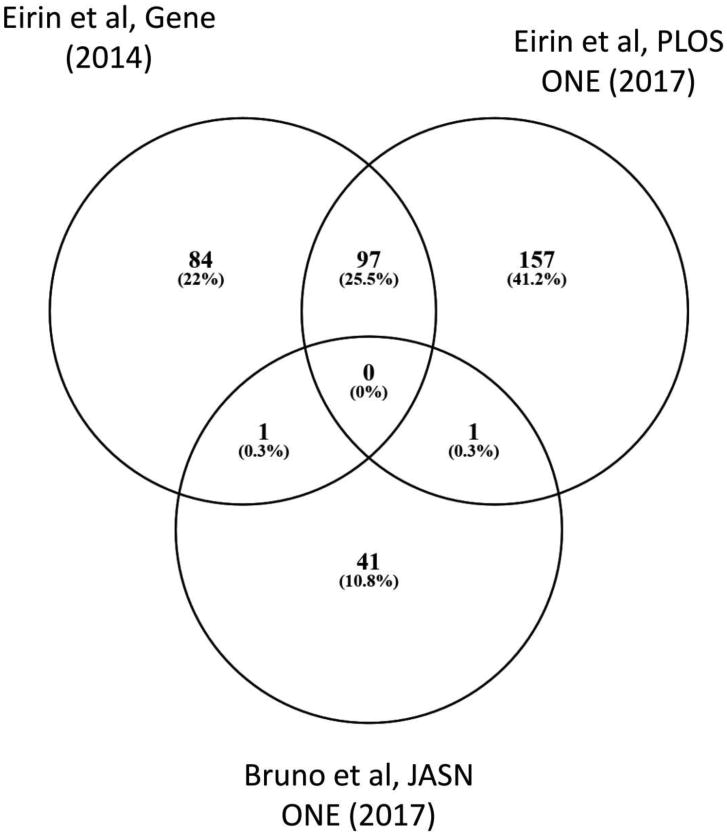
Overlap of mRNAs enriched in MSC-derived EVs among studies.
microRNAs upregulated in EVs
In-vitro studies using RT-qPCR and RNA-seq have shown that a selective group of microRNAs are upregulated in EVs compared to their parent MSCs (Table 2 and 3). For instance, miR-24 was consistently detected in MSC-derived EVs and may mediate regenerative effects of EVs in renal [40] and cardiac [41] cells after ischemia. This microRNA has the potential to modulate both apoptosis [42] and vascular inflammation [43], ameliorating tissue injury in animals treated with MSC-derived EVs. Likewise, miR-29 is preferentially included in MSC-derived EVs [44]. This microRNA has been associated with improved repair of cardiomycoytes in a model of myocardial infarction [41]. miR-29 can also regulate the expression of the anti-apoptotic gene MCL-1 [45], and modulate inflammation by suppressing the expression of ZFP36 [46], which encodes for a protein that regulates tumor necrosis factor (TNF)-α production [47]. In addition, we and others have shown that several members of let-7 family, microRNAs highly conserved across different species [48], are packed in MSC-derived EVs [39, 49]. These microRNAs have the potential to modulate cell cycle and proliferation [50, 51], inflammation [52], cellular repair [53], and osteogenic differentiation [54].
Table 2.
Evidence of microRNAs enriched in MSC-derived EVs.
| Study | Source of EVs | microRNAs |
|---|---|---|
| He et al [44], Nephrology, 2015 | Mouse Bone-marrow MSCs | miRNA-29 (29a-3p, 29b-3p, 29b-1-5p), mi-RNA-30 (30b-3p, 30b-5p, 30d-5p, 30e -3p, 30c-5p), mi-RNA-210-3p |
| Li et al [92], EBioMedicine, 2016 | Human Umbilical cord MSCs | miRNA-181c |
| Shao et al [41], Biomed Res Int, 2017 | Rats bone-marrow MSCs | miRNA-29, miRNA-24 |
| Aliotta et al [93], Cardiovasc Res, 2016 | Mouse bone-marrow MSCs | miRNA-34a, miRNA-122, miRNA-124, miRNA-127 |
| Qin et al [94], Sci Rep, 2016 | Human bone-marrow MSCs | miRNA-196a, miRNA-27, miRNA-206 |
| Muntion et al [95], PLoS One, 2016 | Human bone marrow MSCs | miRNA-10a, miRNA-15a |
| Zhang et al [53], J Am Heart Assoc, 2016 | Rats bone-marrow MSCs | miRNA-147, let-7i-3p, miRNA-503-5p, miRNA-362-3p |
| Cui et al [96], FEBS Lett, 2016 | Mouse bone-marrow MSCs | miRNA-1192, miRNA-680, miRNA-302a |
| Phinney et al [56], Nat Commun, 2015 | Human bone-marrow MSCs | miRNA-451a, miRNA-1202, miRNA-630, miRNA-638 |
| Collino et al [40], J Am Soc Nephrol, 2015 | Mouse bone-marrow MSCs | miRNA-483–5p, miRNA-191, miRNA-28–3p, miRNA-423–5p, miRNA-744, miRNA-129–3p, miRNA-24, miRNA-148a |
| Wang et al [97], Stem Cell Res Ther, 2015 | Rat bone-marrow MSCs | miRNA-133b-3p, miRNA-294 |
| Ti et al [52], J Transl Med, 2015 | Human Umbilical cord MSCs | Let-7b |
| Wang et al [98], Sci Rep, 2015 | Mouse bone-marrow MSCs | miRNA-223 |
| Eirin et al [39], Gene, 2014 | Porcine adipose tissue MSCs | miRNA-148a, miRNA-532-5p, miRNA-378, let-7f |
| Baglio et al [55], Stem Cell Res Ther, 2015 | Human bone-marrow MSCsHuman adipose-tissue MSCs | miRNA-486-5p, miRNA-10a-5p, miRNA-10b-5p, miRNA-191-5p, miRNA-222-3p,miRNA-143-3p, miRNA-10b-5p, miRNA-486-5p, miRNA-22-3p, miRNA-21-5p |
| Nakamura et al [99], FEBS Lett, 2015 | Human bone-marrow MSCs | miRNA-494 |
| Vallabhaneni et al [57], Oncotarget, 2015 | Human bone-marrow MSCs | miRNA-21, miRNA-34a |
| Xu et al [54], PLoS One, 2014 | Human bone-marrow MSCs | let-7a, miR-199b, miR-218, miR-148a, miR-135b, miR-203, miR-219, miR-299-5p, miR-302b |
| Garcia-Contreras et al [49], PLoS One, 2014 | Human adipose tissue MSCs | let-7-a-1, miR-21, miR-143, miR145, miR-451a, miR-338-3p, miR-1260b, miR-1908 |
| Ono et al [100], Sci Signal, 2014 | Human bone-marrow MSCs | miRNA-23b |
| Feng et al [101], PLoS One, 2014 | Mouse bone-marrow MSCs | miRNA-22 |
| Lee et al [102], PLoS One, 2013 | Mouse bone-marrow MSCs | miRNA-16 |
| Xin et al [103], Stem Cells, 2013 | Rat bone-marrow MSCs | miRNA-133b |
Table 3.
List of microRNAs consistently enriched in MSC-derived EVs across studies.
Importantly, the microRNA cargo of MSC-derived EVs depends on the tissue source of MSCs. Characterization of the microRNA content of EVs released from bone marrow-derived and adipose tissue-derived MSCs indicated that despite similarities in the most represented microRNAs, the relative microRNA proportions are different between EVs obtained from different MSC populations, implying that post-transcriptional regulation might differ between bone marrow and adipose tissue MSC-derived EVs [55]. Furthermore, expression of the miR-21 has been reported to be downregulated in MSC-derived EVs in several studies [41, 56], but increases in EVs derived from bone marrow MSCs under stressful conditions, such as serum deprivation [57]. Overall, these studies demonstrate that EV-derived microRNAs are capable of fine-tuning numerous pathways in recipient cells and contribute to the biological actions of MSC-derived EVs.
Proteins enriched in EVs
Previous studies have described the biological signature of MSC-derived EVs from a proteomics perspective. Kim et al profiled the proteome of human bone marrow MSC-derived EVs and identified 730 proteins packed in EVs. Among them were MSC surface markers (e.g. CD44, CD73 and CD105) and proteins involved in pathways related to MSC self-renewal (e.g. platelet-derived growth factor receptor-β, insulin-like growth factor-2, and TGFβ induced) and differentiation (TGFβ, mitogen-activated protein kinases (MAPK), and peroxisome proliferator-activated receptor (PPAR) signaling pathways) [58]. Mass spectrometric analysis of human embryonic MSC-derived EVs detected a significant number of proteins enriched in EVs, including the pro-angiogenic proteins angiopoietin, hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF), as well as proteins that modulate apoptosis (caspase-14), inflammation (interleukin (IL)-10), and fibrosis (matrix metalloproteinase-3, TGFβ-1, TGFβ-2) [59]. In agreement, our recent proteomic studies in porcine MSC-derived EVs detected almost 5,000 proteins included in EVs, 128 exclusively detected in EVs, and 563 only expressed in MSCs [60]. Functionally, proteins enriched in EVs were involved in a wide range of biological activities, including angiogenesis (e.g. VEGF and angiopoietin-related protein-4), apoptosis (e.g. netrin-1), inflammatory response (e.g. TNF-inducible gene 6 protein), and extracellular matrix remodeling (e.g. matrix metalloproteinase-19 and TGFβ-1), whereas proteins excluded from EVs were mostly nuclear proteins, such as those involved in nucleotide binding and RNA splicing. Lastly, a recent study that characterized the proteomic profile of human bone marrow MSC-derived EVs under hypoxic conditions identified 1,927 proteins packed in EVs. Functional analysis revealed high expression of pro-angiogenic proteins and proteins associated with inflammation, TGFβ signaling, and Wnt signaling pathways [61]. Collectively, these studies identified a significant number of proteins that could contribute to the therapeutic efficacy of MSC-derived EVs.
MSC-derived EVs for renal repair
Experimental studies
Recently, several studies evaluated the potential of MSC-derived EVs to regenerate injured renal cells in experimental AKI and CKD (Table 4). Results from these studies suggest that EVs exert their trophic and reparative effects by shuttling their cargo of genes, microRNAs, and proteins to recipient cells in the kidney, attenuating renal injury and improving its recovery competence.
Table 4.
Methodological summary of published studies using MSC-derived EVs for renal repair.
| Study | Isolation procedure | Qualitative and quantitative assays | EV Dose | Route of administration | Mechanism of action |
|---|---|---|---|---|---|
| Bruno et al [37], 2009 | Ultracentrifugation of human bone-marrow MSCs medium | RT-qPCR, RT-PCR, Micro-array data analysis | 15μg | Intravenous injection into tail vein of mice | mRNA transfer |
| Tomasoni et al [38], 2013 | Ultracentrifugation of human bone-marrow MSCs medium | Electron microscopy, RT-qPCR, RT-PCR, IF | In vitro study | No systemic administration | mRNA transfer |
| Collino et al [40], 2015 | Ultracentrifugation of human bone-marrow MSCs medium | RNA-seq, RT-qPCR, Micro-array data analysis, microRNA target sites enrichment analysis | 2.2×10∧8 EVs/mouse | Intravenous injection into tail vein of mice | microRNA transfer |
| He et al [44], 2015 | Ultracentrifugation of mice bone-marrow MSCs medium | Western blot, RT-qPCR, RT-PCR, RNA assays, biochemical and histopathological analysis | 30mg | Intravenous injection into causal vein of mice | microRNA transfer |
| Bruno et al [62], 2012 | Ultracentrifugation of human bone-marrow MSCs medium | Morphological studies, Apoptosis TUNEL assay, IF, RT-PCR, RT-qPCR | 100μg | Intravenous injection | Not studied |
| Choi et al [63], 2015 | Ultracentrifugation of mouse kidney MSCs medium | EMT differentiation, western blot, IHC, IF | 2×10∧7 EVs/mouse | Intravenous injection into tail vein | mRNA transfer |
| Reis et al [64], 2012 | Ultracentrifugation of rat bone-marrow MSCs medium | Flow cytometry, differentiation assays, biochemical and histological analysis, IF, RT-PCR, RT-qPCR, Electron microscopy | Unknown | Intravenous injection | RNA-dependent, but protein independent |
| Gatti et al [65], 2011 | Ultracentrifugation of human bone-marrow MSCs medium | Biochemical and histological analysis, Apoptosis TUNEL assay, RNA micro-assay | 30μg | Intravenous injection | RNA-dependent |
| Lindoso et al [66], 2014 | Ultracentrifugation of human bone-marrow MSCs medium | Apoptosis TUNEL assay, RT-qPCR, RT-PCR, microRNA target prediction | In vitro study | No systemic administration | microRNA transfer |
| Zhang et al [69], 2014 | Ultracentrifugation of human Warton Jelly MSCs medium | Electron microscopy, Surface marker analysis, biochemical analysis, histological analysis, ROS level analysis, IHC, IF, apoptosis TUNEL assay, western blot, RT-qPCR, | 100μg | Intravenous injection into rat causal vein | Not studied |
| Zou et al [70], 2014 | Ultracentrifugation of human Warton Jelly MSCs medium | Electron microscopy, Surface marker analysis, biochemical analysis, histological analysis, ELISA, IHC, apoptosis TUNEL assay, western blot, RT-qPCR, | 100μg | Intravenous injection into rat causal vein | microRNA transfer |
| Zhou et al [71], 2013 | Filtration of human umbilical cord MSCs medium | Histological study, Apoptosis TUNEL assay, IHC, mitochondrial membrane potential assay, western blot | 200μg | Rat renal capsule injection | Not studied |
| Ju et al [73], 2015 | Ultracentrifugation of human umbilical cord MSCs medium | Biochemical analysis, Histological analysis, IHC, RT-PCR, Western blot | 30μg | Intravenous injection into rat causal vein | RNA transfer |
| Wang et al [74], 2014 | Ultracentrifugation of rat bone-marrow MSCs medium | Biochemical analysis, Histological analysis, Apoptosis TUNEL assay, RT-PCR, RT-qPCR, Western blot | 100μg | Unknown | Not studied |
| He et al [75], 2012 | Ultracentrifugation of mice bone-marrow MSCs medium | Biochemical analysis, Histological analysis | 30μg | Intravenous injection into mouse causal vein | Not studied |
| Eirin et al [76], 2017 | Ultracentrifugation of porcine adipose-tissue MSCs medium | Flow cytometry, IF, surface marker analysis, Histological analysis, ELISA, RT-qPCR | 1×10∧10 EVs/pig | Injection into renal artery of pigs | mRNA and protein transfer |
| Lin et al [77], 2016 | The method of harvest unknown, rat adipose-tissue MSCs medium | Biochemical analysis, Histological analysis, western blot, IHC, IF | 100μg | Intravenous administration | Not studied |
| Nassar et al [79], 2016 | Ultracentrifugation of umbilical cord MSCs medium | Flow cytometry, electron microscopy, histologic analysis, IHC, ELISA | 100μg/Kg | Intravenous and intra-arterial injection | Not studied |
Abbreviations: MSC: Mesenchymal stem cell; EV: Extracellular vesicle; RT-qPCR: Real time quantitative polymerase chain reaction; RT-PCR: Reverse transcriptase polymerase chain reaction; IF: Immunofluorescence; RNA-seq: RNA sequencing; TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling; IHC: Immunohistochemistry; ELISA: Enzyme-linked immunosorbent assay
Several biological effects of EVs on the kidney are mediated by their cargo of mRNAs. In an in vitro model of cisplatin-induced AKI, Tomasoni et. al. demonstrated that co-incubation of damaged proximal renal tubular epithelial cells with MSC-derived EVs, which are selectively enriched with IGF1R mRNA, enhanced cell proliferation and repair, suggesting that the transfer of this gene to tubular cells is an important mechanism by which MSCs confer renoprotective effects in experimental AKI [38]. Similarly, Bruno and colleagues have shown that a single intravenous administration of MSC-derived EVs improved mouse survival after injection of a lethal dose of cisplatin, whereas multiple EV injections further decreased mortality, and preserved renal structure and function [62]. Administration of MSC-derived EVs up-regulated the expression of the anti-apoptotic genes BCLX, BCL2, and BIRC8, but down-regulated the expression of the pro-apoptotic genes CASP1, CASP8, and LTA in cisplatin-treated human tubular epithelial cells, suggesting that modulation of programmed cell death may contribute to MSC-derived EV-induced renal repair. Indeed, RNase treatment of EVs abrogated EV-induced in vitro proliferation and resistance to apoptosis, implying that the mRNAs shuttled by EVs activate a transcriptional program of repair in recipient cells [37]. In line with this observation, EVs released from kidney-derived MSCs pre-incubated with RNase failed to ameliorate TGF-β1-induced peritubular capillary rarefaction and tubulo-interstitial fibrosis in mice with unilateral ureteral obstruction (UUO) [63]. Likewise, in rats with gentamycin-induced AKI, bone marrow MSC-derived EVs prevented an increase in serum creatinine and urea, attenuated necrosis, apoptosis, and inflammation, and increased cellular proliferation [64], effects that were blunted when EVs were co-incubated with RNase. Administration of MSC-derived EVs immediately after IRI protected rats from AKI by inhibiting apoptosis and stimulating tubular epithelial cell proliferation, and protected against later development of CKD after AKI [65]. Yet, pretreatment of EVs with RNase abrogated these protective effects, suggesting that the renoprotective effects of EVs are mediated partly by the transfer of mRNA to target cells.
Experimental studies have demonstrated that phenotypic changes induced by MSC-derived EVs may be also mediated by their cargo of microRNAs. Using an in vitro model of ischemia-reperfusion injury (IRI) induced by ATP depletion in renal proximal tubular epithelial cells, Lindoso et. al. found that incorporation of MSC-EVs in damaged cells modulated several microRNAs related to important processes in renal recovery [66]. EV-mediated transfer of miR-410, miR-495, miR-548c-5p, and let-7a down-regulated several coding mRNAs associated with apoptosis, cytoskeletal reorganization and hypoxia, such as CASP3, CASP7, SHC1, and SMAD4. In addition, transfer of miR-375, miR-584c-5p, and miR-561 was associated with decreased expression of SHC1, which encodes for a signaling adapter that contributes to cell death by inhibiting pro-survival pathways [67]. EV transfer of this set of microRNAs was also associated with decreased expression of SMAD4, which encodes for a protein implicated in TGF-β1-mediated fibrosis [68]. In agreement, in mice with UUO, MSC-derived EVs containing selective patterns of microRNAs attenuated renal dysfunction in-vivo and reversed TGF-β1-induced morphological changes in proximal tubular epithelial cells in-vitro [44]. Therefore, these observations suggest that the anti-fibrotic effects of EVs may be, at least in part, mediated by the transfer of microRNAs that regulate targets related to renal fibrosis.
Renal oxidative stress and inflammation may be also modulated by MSC-derived EVs. Renal expression of the NADPH oxidase (NOX)-2 is up-regulated in rats with IRI, but not in those treated with intravenous MSC-derived EVs [69]. Importantly, this intervention not only mitigates oxidative stress, but also reduces apoptosis and enhances renal cell proliferation, suggesting that post-transcriptional regulation of NOX2 in renal recipient cells may be implicated in MSC-derived EVs-induced renal repair. In rats with IRI, MSC-derived EVs alleviated renal inflammation and improved renal function by suppressing the expression of C-X3-C motif ligand-1 (CX3CL1), a potent chemo-attractant protein for macrophages that also promotes interstitial fibrosis [70]. Interestingly, MSC-derived EVs were enriched with miR-16, miR-15b and miR-15a, all of which target CX3CL1, suggesting that post-transcriptional modulation of CX3CL1 is an important mechanism by which MSC-derived EVs mitigate inflammation and renal injury in ischemic AKI. Furthermore, in rats with glycerol-induced AKI, treatment with human bone marrow MSC-derived EVs increased the expression of genes involved in fatty acid metabolism and downregulated the expression of those that modulate inflammation, matrix-receptor interaction, and cell adhesion [40]. However, global down-regulation of microRNAs enriched in MSC-derived EVs halted the renal regenerative effects of these particles, suggesting that EV-mediated transfer of microRNAs is implicated not only in preventing injury, but also in the healing properties of MSC-derived EVs.
Proteins enriched in MSC-derived EVs are important contributors to the renal reparative potency of MSCs. Studies in rats with cisplatin-induced AKI have shown that EVs derived from human umbilical cord MSCs attenuated tubular cellular oxidative stress, apoptosis, necrosis, and renal dysfunction [71]. Notably, MSC-derived EVs promoted cell proliferation, and subsequently renal repair through activation of several protein members of the extracellular signal regulated kinase (ERK)1/2 pathway, a subfamily of the MAPKs involved in relaying extracellular signals into intracellular responses [72]. In line with these observations, studies in rats subjected to IRI have shown that MSC-derived EV delivery upregulated ERK 1/2 protein expression, as well as the expression of the pro-angiogenic factor HGF, promoting tubular cell differentiation and regeneration [73]. Similarly, treatment with bone marrow MSC-derived EVs conferred protection against IRI in rats by decreasing the expression of several pro-inflammatory cytokines including IL-1β and TNF-α [74]. Notably, this intervention decreased the expression of the pro-apoptotic protein caspase-3, in parallel with decreased levels of serum creatinine and blood urea nitrogen (BUN) levels.
The renal anti-inflammatory potential of MSC-derived EVs is often parallel with robust anti-fibrotic properties. He and colleagues previously demonstrated that administration of bone marrow MSC-derived EVs through the caudal vein of mice with subtotal nephrectomy attenuated renal interstitial lymphocyte infiltrates [75]. Importantly, these anti-inflammatory effects were associated with decreased tubular swelling and necrosis, and tubulo-interstitial fibrosis. We have recently shown that intra-renal injection of MSC-derived EVs attenuated renal inflammation and fibrosis, and improved medullary oxygenation and renal function in pigs with coexisting metabolic syndrome and renovascular disease. Interestingly, these renoprotective effects were blunted in pigs treated with IL-10 depleted EVs, suggesting that some of the salutary effects of EVs are mediated by the cargo of this anti-inflammatory cytokine [76]. Also, MSC-derived EVs have been shown to protect rats against IRI by modulating the expression of proteins involved in inflammation (TNF-α, NF-κB, IL-1β, MIF, PAI-1, Cox-2), oxidative-stress (NOX-1 and NOX-2), apoptosis (Bax, caspase-3, PARP), fibrosis (SMAD3, TGF-β), and angiogenesis (CD31, vWF, angiopoietin) [77], suggesting a critical role of proteins packed into MSC-derived in the renal regenerative potential of these particles. Clearly, the anti-fibrotic effects of EVs outweigh potential pro-fibrotic effects that might result from activation of the MAPK pathway.
Taken together, accumulated evidence suggests that the reno-protective effect of MSC-derived EVs is in part mediated by a selective three-component cargo (mRNAs, microRNAs and proteins). Upon release from EVs, mRNAs can be translated, increasing the protein content of recipient cells. microRNAs can inhibit the expression of multiple target genes, suppressing protein translation, whereas proteins packed in EVs can directly exert an immediate biochemical effect in recipient cells. Importantly, interactions among the different types of molecular cargo of EVs may regulate the transcriptional control of cellular function in recipient cells. In a recent comprehensive integrated analysis of the mRNA and microRNA transcriptomes and proteome of porcine MSC-derived EVs, we have found that mRNAs and microRNAs enriched in EVs are predicted to interact and control the activity of transcription factors, whereas EV proteins are capable of modulating multiple cellular phosphorylation pathways. Hence, interactions among mRNA, microRNA, and proteins may be an important mechanism driving MSC-based repair [78]. However, additional studies are needed to elucidate the molecular mechanisms by which genes, microRNAs, and proteins packed in MSC-derived EVs exert tissue trophic and reparative effects.
Clinical studies
Promising results from these experimental studies provided the impetus to apply MSC-derived EVs to address clinical needs of patients with renal disease. However, to date only one clinical trial investigated the safety and therapeutic efficacy of MSC-derived EVs in patients with kidney disease [79]. This single-center, randomized, placebo-controlled, phase II/III clinical pilot study recruited 40 patients with stage III-IV CKD (eGFR between 15-60mg/ml/min), who were randomized to receive either placebo or two doses (first intravenous and second intra-arterial) of MSC-derived EVs, one week apart. After a 12-month follow-up, EV-treated patients exhibited a significant improvement in renal function (improved eGFR and decreased serum creatinine, BUN, and albuminuria). Clinical improvement paralleled changes in plasma levels of several immune inflammatory markers, including TNF-α, TGF-β1, and IL-10. Although kidney biopsy specimens obtained 3 months after therapy did not show significant histologic changes, expression of Ki67 (a marker of regeneration) and the number of CD133 cells (possessing capabilities of clonal expansion and repair) were both upregulated in kidney samples [79]. These observations suggest that MSC-derived EVs are safe and can ameliorate the inflammatory immune reaction and improve the overall kidney function in patients with CKD. Nevertheless, additional studies are needed to confirm these results and provide further insight into the role of MSC-derived EVs in improving renal function in patients with CKD.
Conclusions and future perspectives
In this review, we summarized the evidence available from several studies in animal models of AKI and CKD, identifying a potential for MSC-derived EVs for preservation of renal structure and function. These studies suggest that EVs contribute to renal repair by virtue of their unique gene, microRNA, and protein cargo, which possess potent pro-regenerative properties. However, several aspects need to be carefully considered before moving toward clinical applications.
Both the route of MSC-derived EV delivery and the fate of these membrane particles post-transplantation may affect their efficiency for kidney repair. Experimental studies in murine AKI and CKD models suggest that administration of MSC-derived EVs in the caudal vein is feasible, safe, and effective to confer important reno-protective effects [64, 65, 75]. Likewise, we have recently shown in swine coexisting metabolic syndrome and renovascular disease that a single injection of MSC-derived EVs into the renal artery preserves the structure and function of the post-stenotic kidney [76]. Retention of MSC-derived EVs in the stenotic kidney of treated pigs peaked at 2 days and decreased thereafter, remaining at approximately 2% by 4 weeks after intra-renal injection. Moreover, injected EVs were detected in higher proportions in other organs, including the liver, lung, and spleen. Notably, we found that EVs engrafted in renal proximal and distal tubular cells, as well as in macrophages. In line with this, mice with glycerol-induced AKI, the biologic action of MSC-derived EVs required their CD44- and β1-integrin-dependent incorporation into tubular cells [37]. Further studies are needed to elucidate the precise molecular mechanisms underlying incorporation of EVs on damaged renal cells.
Fewer studies have explored whether multiple MSC-derived EV injections are associated with more pronounced improvements in renal function compared to a single delivery. In mice with AKI, multiple intravenous injections of MSC-derived EVs exerted superior pro-survival and reno-protective effects compared to single-dose regimens [62]. Contrarily, the need for several MSC-derived EV injections was not confirmed in the remnant kidney CKD mouse model [75]. Possibly, the remnant kidney cannot accommodate increased EV engraftment. Additional studies are warranted to identify the most appropriate therapeutic regimen and doses of EVs.
It is also reasonable to speculate on whether administration of MSC-derived EVs would confer more efficient renoprotection compared to delivery of their parent MSCs. Studies in murine models of AKI suggest that administration of MSC-derived EVs recapitulate the beneficial effect in kidney repair of their parent MSCs [74, 75]. Furthermore, MSC and MSC-derived EV delivery has shown similar potential to decrease fibrosis, interstitial lymphocyte infiltrates, and tubular atrophy, and preserve the remnant renal function in mice with 5/6 nephrectomy [75]. A single administration of EVs derived from MSCs immediately after induction of UUO mimicked the effects of their parent cells in protecting mice against renal failure [44]. Yet, EVs were superior to MSCs in some respects, suggesting that the former confers additional renoprotective effects. In a recent study, Lin and colleagues investigated the efficacy of MSC, MSC-derived EV, and combined MSC and MSC-derived EV therapy on protecting the kidneys from acute IR injury [77]. They found that MSCs and MSC-derived EVs were comparably effective for decreasing inflammation and oxidative stress, and preserving kidney function. However, combined MSC and MSC-derived EV therapy was superior to either one alone in reducing proteinuria and preserving kidney function after acute IR injury. Taken together, these studies suggest that MSC and MSC-derived EVs exhibit a comparable and potentially additive effect on reducing renal injury and dysfunction. Nevertheless, further experimental research is needed to select the most appropriate regenerative therapy to improve the damaged kidney.
Employing EVs as a therapeutic tool in large scales faces practical challenges. Currently, there are no standard effective methods for EV mass production, and billions of MSCs need to be cultured in vitro to harvest only few micrograms of EVs [80]. Evidence supports that increasing intracellular calcium levels [81, 82] and modulation of ex-vivo culture conditions (thermal stress [83], hypoxia [84], radiation [85], etc.) might potentiate EV production. Likewise, sulfhydryl-blocking agents, which alter cytoskeletal function, may also enhance the rate of EV release [86].
The quality of harvested EVs, with regard to their cargo and membrane composition, also needs to be carefully controlled and standardized. The ability to MSCs to suppress the immune response raises concerns of immunologic dysfunction [87], and EVs, at least partially, share some membrane characteristics with their parent MSCs. Human bone marrow MSCs [88] and EVs [89] express MHC class I molecules. Thus, using EVs derived from a patient's own MSCs should be sufficient to avoid an immunogenic response [80]. However, EVs derived from different donors may harbor different membrane markers, which may alter their therapeutic efficacy.
Last, but not least, co-morbid conditions, such as aging, smoking, obesity, hypertension, and diabetes may compromise MSC functionality and vitality [90]. Furthermore, progressive accumulation of several uremic toxins, including advanced glycation end products, p-cresylsulfate, and indoxyl sulfate may impair the renoprotective properties of autologous MSCs in patients with CKD [91]. Therefore, whether these factors impact the genetic and protein cargo of MSC-derived EVs and their potential to repair the kidneys needs to be evaluated carefully prior to wide clinical application.
In summary, MSC-derived EVs currently emerge as a promising approach to repair damaged kidneys. However, clinical data supporting the use of MSC-derived EVs in patients with renal disease is limited to a single clinical trial in patients with CKD. No doubt, additional experimental and clinical studies are needed to further explore the mechanisms of MSC-derived EV reno-protection, and develop adequate protocols to treat patients with renal disease.
Acknowledgments
This study was partly supported by NIH grant numbers DK100081, DK104273, HL123160, DK102325, and DK106427, the Mayo Clinic Center for Regenerative Medicine, and the Mayo Clinic Foundation: Mary Kathryn and Michael B. Panitch Career Development Award.
Abbreviations
- AKI
Acute Kidney Injury
- CKD
Chronic Kidney Disease
- ESRD
End-Stage Renal Disease
- MSCs
Mesenchymal Stem/Stromal Cells
- EVs
extracellular vesicles
- RT-qPCR
Real-time quantitative Polymerase chain reaction
- RNA-seq
RNA sequencing
- TGF
Transforming growth factor
- TNF
Tumor necrosis factor
- MAPK
mitogen-activated protein kinases
- HGF
Hepatocyte growth factor
- VEGF
Vascular endothelial growth factor
- IL
Interleukin
- IGF1R
Insulin-like Growth Factor-1 receptor
- IRI
ischemia-reperfusion injury
- NOX
NADPH oxidase
- CX3CL1
C-X3-C motif ligand-1
- ERK
Extracellular signal regulated kinase
- BUN
Blood urea nitrogen
Footnotes
Conflict of interest: Authors declare having no potential competing financial interest.
References
- 1.Liangos O, et al. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1(1):43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 2.Hsu CY, et al. Community-based incidence of acute renal failure. Kidney Int. 2007;72(2):208–12. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doyle JF, Forni LG. Acute kidney injury: short-term and long-term effects. Crit Care. 2016;20(1):188. doi: 10.1186/s13054-016-1353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ojo A. Addressing the global burden of chronic kidney disease through clinical and translational research. Trans Am Clin Climatol Assoc. 2014;125:229–43. discussion 243-6. [PMC free article] [PubMed] [Google Scholar]
- 5.Jha V, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–72. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 6.Lozano R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Mortality and global health estimates: Causes of death; Projections for 2015–2030; Projection of death rates [Google Scholar]
- 9.Chertow GM, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72(3):247–59. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 11.Collins AJ, et al. ‘United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59(1 Suppl 1):A7, e1–420. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Honeycutt AA, et al. Medical costs of CKD in the Medicare population. J Am Soc Nephrol. 2013;24(9):1478–83. doi: 10.1681/ASN.2012040392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 14.Group., K.D.I.G.O.K.C.W. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney inter, Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 15.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 16.Hass R, et al. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, et al. Systematic review and meta-analysis of mesenchymal stem/stromal cells therapy for impaired renal function in small animal models. Nephrology (Carlton) 2013;18(3):201–8. doi: 10.1111/nep.12018. [DOI] [PubMed] [Google Scholar]
- 18.Fleig SV, Humphreys BD, et al. Humphreys, Rationale of mesenchymal stem cell therapy in kidney injury. Nephron Clin Pract. 2014;127(1-4):75–80. doi: 10.1159/000363680. [DOI] [PubMed] [Google Scholar]
- 19.Luo CJ, et al. Mesenchymal stem cells ameliorate sepsis-associated acute kidney injury in mice. Shock. 2014;41(2):123–9. doi: 10.1097/SHK.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 20.Choi S, et al. The role of mesenchymal stem cells in the functional improvement of chronic renal failure. Stem Cells Dev. 2009;18(3):521–9. doi: 10.1089/scd.2008.0097. [DOI] [PubMed] [Google Scholar]
- 21.Eirin A, et al. Adipose tissue-derived mesenchymal stem cells improve revascularization outcomes to restore renal function in swine atherosclerotic renal artery stenosis. Stem Cells. 2012;30(5):1030–41. doi: 10.1002/stem.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebrahimi B, et al. Mesenchymal stem cells improve medullary inflammation and fibrosis after revascularization of swine atherosclerotic renal artery stenosis. PLoS One. 2013;8(7):e67474. doi: 10.1371/journal.pone.0067474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eirin A, et al. Renal vein cytokine release as an index of renal parenchymal inflammation in chronic experimental renal artery stenosis. Nephrol Dial Transplant. 2014;29(2):274–82. doi: 10.1093/ndt/gft305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Initial report on a phase I clinical trial: Prevention and treatment of post-operative Acute Kidney Injury with allogeneic Mesenchymal Stem Cells in patients who require on-pump cardiac surgery. Cellular Therapy and Transplantation. 2008 Dec 24;1(2):31–35. [Google Scholar]
- 25.Peired AJ, Sisti A, Romagnani P. Mesenchymal Stem Cell-Based Therapy for Kidney Disease: A Review of Clinical Evidence. Stem Cells Int. 2016;2016:4798639. doi: 10.1155/2016/4798639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunter U, et al. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol. 2007;18(6):1754–64. doi: 10.1681/ASN.2007010044. [DOI] [PubMed] [Google Scholar]
- 27.Broekema M, et al. Determinants of tubular bone marrow-derived cell engraftment after renal ischemia/reperfusion in rats. Kidney Int. 2005;68(6):2572–81. doi: 10.1111/j.1523-1755.2005.00728.x. [DOI] [PubMed] [Google Scholar]
- 28.Biancone L, et al. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant. 2012;27(8):3037–42. doi: 10.1093/ndt/gfs168. [DOI] [PubMed] [Google Scholar]
- 29.Ratajczak J, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–56. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 30.Koniusz S, et al. Extracellular Vesicles in Physiology, Pathology, and Therapy of the Immune and Central Nervous System, with Focus on Extracellular Vesicles Derived from Mesenchymal Stem Cells as Therapeutic Tools. Front Cell Neurosci. 2016;10:109. doi: 10.3389/fncel.2016.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rani S, et al. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther. 2015;23(5):812–23. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73(10):1907–20. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Gyorgy B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68(16):2667–88. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.T LR, et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun Signal. 2016;14:2. doi: 10.1186/s12964-015-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beyer Nardi N, da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006;174:249–82. [PubMed] [Google Scholar]
- 36.Andreu Z, Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. doi: 10.3389/fimmu.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruno S, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20(5):1053–67. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomasoni S, et al. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013;22(5):772–80. doi: 10.1089/scd.2012.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eirin A, et al. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014;551(1):55–64. doi: 10.1016/j.gene.2014.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collino F, et al. AKI Recovery Induced by Mesenchymal Stromal Cell-Derived Extracellular Vesicles Carrying MicroRNAs. J Am Soc Nephrol. 2015;26(10):2349–60. doi: 10.1681/ASN.2014070710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shao L, et al. MiRNA-Sequence Indicates That Mesenchymal Stem Cells and Exosomes Have Similar Mechanism to Enhance Cardiac Repair. Biomed Res Int. 2017;2017:4150705. doi: 10.1155/2017/4150705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian L, et al. miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J Exp Med. 2011;208(3):549–60. doi: 10.1084/jem.20101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maegdefessel L, et al. miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat Commun. 2014;5:5214. doi: 10.1038/ncomms6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He J, et al. Micro-vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microRNA-dependent repairing. Nephrology (Carlton) 2015;20(9):591–600. doi: 10.1111/nep.12490. [DOI] [PubMed] [Google Scholar]
- 45.Mott JL, et al. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26(42):6133–40. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gebeshuber CA, Zatloukal K, Martinez J. miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO Rep. 2009;10(4):400–5. doi: 10.1038/embor.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281(5379):1001–5. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- 48.Pasquinelli AE, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408(6808):86–9. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Contreras M, et al. Therapeutic potential of human adipose-derived stem cells (ADSCs) from cancer patients: a pilot study. PLoS One. 2014;9(11):e113288. doi: 10.1371/journal.pone.0113288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson CD, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67(16):7713–22. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 51.Boyerinas B, et al. The role of let-7 in cell differentiation and cancer. Endocr Relat Cancer. 2010;17(1):F19–36. doi: 10.1677/ERC-09-0184. [DOI] [PubMed] [Google Scholar]
- 52.Ti D, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015;13:308. doi: 10.1186/s12967-015-0642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Z, et al. Pretreatment of Cardiac Stem Cells With Exosomes Derived From Mesenchymal Stem Cells Enhances Myocardial Repair. J Am Heart Assoc. 2016;5(1) doi: 10.1161/JAHA.115.002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu JF, et al. Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS One. 2014;9(12):e114627. doi: 10.1371/journal.pone.0114627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baglio SR, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127. doi: 10.1186/s13287-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Phinney DG, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vallabhaneni KC, et al. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. 2015;6(7):4953–67. doi: 10.18632/oncotarget.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim HS, et al. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012;11(2):839–49. doi: 10.1021/pr200682z. [DOI] [PubMed] [Google Scholar]
- 59.Lai RC, et al. Proteolytic Potential of the MSC Exosome Proteome: Implications for an Exosome-Mediated Delivery of Therapeutic Proteasome. Int J Proteomics. 2012;2012:971907. doi: 10.1155/2012/971907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eirin A, et al. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci Rep. 2016;6:36120. doi: 10.1038/srep36120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson JD, et al. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling. Stem Cells. 2016;34(3):601–13. doi: 10.1002/stem.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bruno S, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7(3):e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi HY, et al. Mesenchymal stem cell-derived microparticles ameliorate peritubular capillary rarefaction via inhibition of endothelial-mesenchymal transition and decrease tubulointerstitial fibrosis in unilateral ureteral obstruction. Stem Cell Res Ther. 2015;6:18. doi: 10.1186/s13287-015-0012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reis LA, et al. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One. 2012;7(9):e44092. doi: 10.1371/journal.pone.0044092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gatti S, et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26(5):1474–83. doi: 10.1093/ndt/gfr015. [DOI] [PubMed] [Google Scholar]
- 66.Lindoso RS, et al. Extracellular vesicles released from mesenchymal stromal cells modulate miRNA in renal tubular cells and inhibit ATP depletion injury. Stem Cells Dev. 2014;23(15):1809–19. doi: 10.1089/scd.2013.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Natalicchio A, et al. Role of the p66Shc isoform in insulin-like growth factor I receptor signaling through MEK/Erk and regulation of actin cytoskeleton in rat myoblasts. J Biol Chem. 2004;279(42):43900–9. doi: 10.1074/jbc.M403936200. [DOI] [PubMed] [Google Scholar]
- 68.Meng XM, et al. Disruption of Smad4 impairs TGF-beta/Smad3 and Smad7 transcriptional regulation during renal inflammation and fibrosis in vivo and in vitro. Kidney Int. 2012;81(3):266–79. doi: 10.1038/ki.2011.327. [DOI] [PubMed] [Google Scholar]
- 69.Zhang G, et al. The anti-oxidative role of micro-vesicles derived from human Wharton-Jelly mesenchymal stromal cells through NOX2/gp91(phox) suppression in alleviating renal ischemia-reperfusion injury in rats. PLoS One. 2014;9(3):e92129. doi: 10.1371/journal.pone.0092129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zou X, et al. Microvesicles derived from human Wharton's Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther. 2014;5(2):40. doi: 10.1186/scrt428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou Y, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4(2):34. doi: 10.1186/scrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yao Z, Seger R. The ERK signaling cascade--views from different subcellular compartments. Biofactors. 2009;35(5):407–16. doi: 10.1002/biof.52. [DOI] [PubMed] [Google Scholar]
- 73.Ju GQ, et al. Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction. PLoS One. 2015;10(3):e0121534. doi: 10.1371/journal.pone.0121534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang R, et al. Bone marrow mesenchymal stem cell-derived exosome protects kidney against ischemia reperfusion injury in rats. Zhonghua Yi Xue Za Zhi. 2014;94(42):3298–303. [PubMed] [Google Scholar]
- 75.He J, et al. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology (Carlton) 2012;17(5):493–500. doi: 10.1111/j.1440-1797.2012.01589.x. [DOI] [PubMed] [Google Scholar]
- 76.Eirin A, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int. 2017 doi: 10.1016/j.kint.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lin KC, et al. Combination of adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes for protecting kidney from acute ischemia-reperfusion injury. Int J Cardiol. 2016;216:173–85. doi: 10.1016/j.ijcard.2016.04.061. [DOI] [PubMed] [Google Scholar]
- 78.Eirin A, et al. Integrated transcriptomic and proteomic analysis of the molecular cargo of extracellular vesicles derived from porcine adipose tissue-derived mesenchymal stem cells. PLoS One. 2017;12(3):e0174303. doi: 10.1371/journal.pone.0174303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nassar W, et al. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res. 2016;20:21. doi: 10.1186/s40824-016-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ingato D, et al. Good things come in small packages: Overcoming challenges to harness extracellular vesicles for therapeutic delivery. J Control Release. 2016;241:174–185. doi: 10.1016/j.jconrel.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 81.Savina A, et al. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278(22):20083–90. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 82.Miyoshi H, et al. Calpain activation in plasma membrane bleb formation during tert-butyl hydroperoxide-induced rat hepatocyte injury. Gastroenterology. 1996;110(6):1897–904. doi: 10.1053/gast.1996.v110.pm8964416. [DOI] [PubMed] [Google Scholar]
- 83.Hedlund M, et al. Thermal- and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PLoS One. 2011;6(2):e16899. doi: 10.1371/journal.pone.0016899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wysoczynski M, Ratajczak MZ. Lung cancer secreted microvesicles: underappreciated modulators of microenvironment in expanding tumors. Int J Cancer. 2009;125(7):1595–603. doi: 10.1002/ijc.24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zaborowski MP, et al. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience. 2015;65(8):783–797. doi: 10.1093/biosci/biv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang B, et al. Immunotherapeutic potential of extracellular vesicles. Front Immunol. 2014;5:518. doi: 10.3389/fimmu.2014.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Machado Cde V, Telles PD, Nascimento IL. Immunological characteristics of mesenchymal stem cells. Rev Bras Hematol Hemoter. 2013;35(1):62–7. doi: 10.5581/1516-8484.20130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chaput N, Thery C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011;33(5):419–40. doi: 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]
- 90.Efimenko AY, et al. Autologous Stem Cell Therapy: How Aging and Chronic Diseases Affect Stem and Progenitor Cells. Biores Open Access. 2015;4(1):26–38. doi: 10.1089/biores.2014.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hickson LJ, Eirin A, Lerman LO. Challenges and opportunities for stem cell therapy in patients with chronic kidney disease. Kidney Int. 2016;89(4):767–78. doi: 10.1016/j.kint.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li X, et al. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. EBioMedicine. 2016;8:72–82. doi: 10.1016/j.ebiom.2016.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aliotta JM, et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res. 2016;110(3):319–30. doi: 10.1093/cvr/cvw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qin Y, et al. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep. 2016;6:21961. doi: 10.1038/srep21961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Muntion S, et al. Microvesicles from Mesenchymal Stromal Cells Are Involved in HPC-Microenvironment Crosstalk in Myelodysplastic Patients. PLoS One. 2016;11(2):e0146722. doi: 10.1371/journal.pone.0146722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cui Y, et al. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett. 2016;590(1):185–92. doi: 10.1002/1873-3468.12024. [DOI] [PubMed] [Google Scholar]
- 97.Wang Y, et al. Differentially expressed microRNAs in bone marrow mesenchymal stem cell-derived microvesicles in young and older rats and their effect on tumor growth factor-beta1-mediated epithelial-mesenchymal transition in HK2 cells. Stem Cell Res Ther. 2015;6:185. doi: 10.1186/s13287-015-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang X, et al. Exosomal miR-223 Contributes to Mesenchymal Stem Cell-Elicited Cardioprotection in Polymicrobial Sepsis. Sci Rep. 2015;5:13721. doi: 10.1038/srep13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nakamura Y, et al. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;589(11):1257–65. doi: 10.1016/j.febslet.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 100.Ono M, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7(332):ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 101.Feng Y, et al. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 2014;9(2):e88685. doi: 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee JK, et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One. 2013;8(12):e84256. doi: 10.1371/journal.pone.0084256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xin H, et al. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31(12):2737–46. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]


