Abstract
Glutamate receptor interacting proteins 1 and 2 (GRIP1/2) play an important role in regulating synaptic trafficking of AMPA receptor 2/3 (GluA2/3) and synaptic strength. Gain-of-function GRIP1 mutations are implicated in social behavioral deficits in autism. To study mechanisms of Grip1/2-mediated AMPA signaling in the regulation of social behaviors, we performed social behavioral testing on neuron-specific Grip1/2-double knockout (DKO) and wild type (WT) mice that are matched for age, sex, and strain background. We determined the expression profile of key signaling proteins in AMPAR, mGluR, mTOR, and GABA pathways in frontal cortex, striatum, and cerebellum of DKO mice. Compared to WT mice, DKO mice show increased sociability in a modified three-chamber social behavioral test [mean ± sem for interaction time in seconds; WT: 44.0 ± 5.0; n = 10; DKO: 81.0 ± 9.0; n = 9; two factor repeated measures ANOVA: F(1,37) = 14.45; p < 0.01 and planned t-test; p < 0.01] and in a dyadic male–male social interaction test (mean ± sem for total time in seconds: sniffing, WT-WT, 18.9 ± 1.1; WT-DKO, 42.5 ± 2.1; t-test: p < 0.001; following, WT-WT, 7.7 ± 0.72; WT-DKO,14.4 ± 1.8; t-test: p < 0.001). Immunoblot studies identified an increase in phosphorylation at GluA2-Serine 880 (GluA2-pS880) in frontal cortex (mean ± sem; WT: 0.69 ± 0.06, n = 5; DKO: 0.96 ± 0.06, n = 6; t-test; p < 0.05) and reduced GABAβ3 expression in striatum (mean ± sem; WT: 1.16 ± 0.04, n = 4; DKO: 0.95 ± 0.06, n = 4; t-test; p < 0.05) in DKO mice. GluA2-S880 phosphorylation is known to regulate GluA2synaptic recycling, AMPA signaling strength and plasticity. GABAβ3 has been implicated in the etiology and pathogenesis in autism. These data support an important role of Grip1/2-mediated AMPA signaling in regulating social behaviors and disturbance of glutamate-and GABA-signaling in specialized brain regions in autism-related social behavioral deficits.
Keywords: Autism, GRIPs, GluA2-S880, GABAβ3, Social interaction, Frontal cortex
1. Introduction
Severe deficits in reciprocal social interactions are core features of several neuropsychiatric disorders including Autism spectrum disorders (ASDs). Both genetic and environmental risk factors are known to contribute to behavioral phenotypes in ASDs [1,2]. Despite extensive studies in recent years, neural mechanisms of social behavioral regulation and pathogenesis of social deficits in autism remain poorly understood.
Glutamate mediates the majority of excitatory neurotransmission in central nerve system through its families of receptors including ionotropic (AMPA, NMDA, Kainate) and metabotropic glutamate (mGluR) receptors [3]. AMPA receptors cycle between postsynaptic membrane and endosomal vesicles at basal state and in response to synaptic activities [4]. These dynamic changes in receptor availability at postsynaptic membrane play an important role in determining synaptic strength and plasticity [4]. Synaptic trafficking of AMPA receptors is tightly regulated by neural scaffolding proteins such as glutamate receptor interacting proteins 1 and 2 (GRIP1/2) [5–7] and PICK1 [8]. GRIP1/2 are neuron-enriched scaffolding proteins with 7 PDZ domains [5,6], bind to the c-terminal domains of AMPA receptors 2 and 3 (GluA2/3) via its PDZ4-6, and play an important role in GluA2/3 trafficking, synaptic organization and transmission [5]. GRIP1/2 are abundantly expressed in both glutamatergic and GABAergic synapses [9], suggesting a role in regulating both excitatory and inhibitory synaptic functions. Loss of GRIP1/2 expression in neurons results in decelerated recycling of GluA2 to synaptic membrane in hippocampal neurons [10] and lack of LTD expression in cerebellum [7].
Disturbances in glutamate signaling and glutamatergic synaptic functions have been implicated in ASDs. Studies have identified an increase in transcript levels of AMPA glutamate receptor 1, glial glutamate transporter 1, and GRIP1 in postmortem tissues of cerebellum from autism patients [11]. Rare point mutations and/or de novo microdeletions or duplications were found in numerous genes including NLGN3/4, SHANK3, NRXN1, and CNTNAP2 that are known to involve in glutamate receptor clustering, as well as synaptogenesis, dendritic development and maturation in glutamatergic synapsis [12–16]. At the brain circuit levels, abnormal functional connectivity between cortical–cortical and cortical-striatal areas have been identified in autism patients [17,18]. For example, abnormal functional connectivity in frontal lobe circuits was detected in patients with CNTNAP2 mutations [19]. An altered balance of excitatory and inhibitory synaptic functions was found in well-established autism mouse models including Fragile X syndrome [20], Rett syndrome [21], and Neuroligin 3 mutant mice [22]. Furthermore, growing evidence has linked dysfunction in GABAergic signaling pathways to ASDs. For instance, studies have identified a reduced expression of GABAergic signaling proteins and altered GABAergic neurons in brain tissues from autism patients [23,24]. These pathological changes in the GABA-signaling pathway could be a direct effect or a consequence of mutations in other signaling or scaffolding proteins that affect GABA synapses. Together, these results support that dysregulation of both excitatory and inhibitory synaptic function, brain circuits, and network synchrony contributes to behavioral deficits in certain forms of ASDs [25–27].
Recent studies from our laboratories implicated AMPA glutamate signaling disturbance in autism’s social deficits [28]. We identified multiple missense mutations clustered at PDZ4-6 of GRIP1 in a cohort of patients with autism [28,29]. These autism-associated mutations showed a gain-of-function effect manifesting an increase in binding with GluA2 in yeast-two-hybrid assay and an accelerated recycling of GluA2 in hippocampal neurons [28]. Correspondingly, loss of Grip1/2 expression causes a decelerated recycling of GluA2 in neurons [10]. Genotype-phenotype correlation studies of affected sib-pairs in proband families supported that presence of these GRIP1 mutations contribute to an increase in the severity of autism-related social behaviors as measured by cumulative ADI-R scores in reciprocal social interactions [28].
To understand the roles of GRIP1/2 in modulating social behaviors and pathogenesis of social deficits in autism, we systematically studied social behaviors in neuron-specific Grip1/2 double knockout (DKO) mice and examined key AMPA- and GABA-signaling proteins in brain regions that are implicated in autism [30–32]. We found that DKO mice with a complete loss of Grip1/2 neuronal expression exhibit an increase in social interactions, which is consistent with our prior finding that GRIP1 gain-of-function mutations contribute to social deficits in autism [28]. Further, our studies showed that GluA2-S880 phosphorylation (GluA2-pS880) is increased in frontal cortex and cerebellum while GABAβ3 expression is reduced in striatum of DKO mice. These results implicate that GRIP1/2-mediated GluA2/3 trafficking play a role in the modulation of social behaviors and that dysregulation of glutamatergic and GABAergic signaling contribute to the pathogenesis of social behavioral deficits in autism.
2. Methods
2.1. Animals
Grip1/2 double knockout (DKO) mice were generated by crossing Grip2 conventional KO mice with conditional Grip1 KO mice (Grip1 flox/flox) to circumvent embryonic lethality of conventional Grip1 KO mice [7,33]. Neuron-specific Grip1 deletion was achieved by crossing with Nestin-Cre transgenic mice. The cre-dependent Grip1 deletion in neurons was verified in lysates in multiple brain regions by immunoblot (Supplementary Fig. S1). Adult male DKO mice and WT controls (WT) were generated by breeding littermates heterozygous for Grip1/2 deletions and were matched for age, sex, and strain background. WT and DKO mice were genotyped using PCR of tail DNA following a published protocol [28]. All mice were housed in temperature-controlled rooms with 12-h light/dark cycle (9:00 and 21:00) and had free access to water and standard mouse chow. Animal breeding and procedures were conducted in strict accordance with the NIH Guide for Care and Use of Laboratory Animals. The Animal Care and Use Committee (ACUC) at the Johns Hopkins University approved this study protocol.
2.2. Mouse behavioral testing
Mouse behavioral tests were conducted at the Animal Behavioral Core of the Johns Hopkins University School of Medicine following standard protocols from Animal Behavioral Core User Manual (http://www.brainscienceinstitute.org/index.php/cores) [34,35] and published protocols from our laboratories [28,36]. The test order and age of the study cohorts of mice for individual test (in parenthesis) are as follow: Open Field (2 month), elevated plus maze (2 month), rotarod (3 month), sociability and preference for social novelty (3 month), dyadic male–male interaction (4 month), resident-intruder test (4 month), general olfaction (5 month). For individual test, WT and DKO mice were always tested together to minimize variations. Test animals have at least one week break in between these tests. The average ambient lighting (lux) for each tests: open field (318), elevated plus maze (492), dyadic male–male interaction, sociability, preference for social novelty, and resident-intruder test (595) as described in previous published studies [28,36].
2.2.1. Open-field test
Each individual test mouse was placed in a photo-beam (n = 16 at equal spacing of 2.5 cm) equipped clear plastic chamber (45 × 45 cm) and was allowed to explore free from interference for 30 min. The peripheral area (425 sq cm) was defined by the two side-photo beams, #1–2 and #15–16 while the central area (1600 sq cm) was defined by photo beams #3–14 at each direction. Movements in the chamber were tracked using a SDI Photobeam Activity System (San Diego Instruments). The patterns of ambulatory movement, fine movement, and rearing behavior at central and peripheral areas were automatically recorded and analyzed.
2.2.2. Elevated-plus maze
The elevated plus maze, made of stainless steel, consists of two closed arms measuring 48 cm (L) × 10 cm (W) × 38 cm (H) and two open arms measuring 48 cm (L) × 10 cm (W) (San Diego Instruments). The four arms were connected by a middle 10 cm × 10 cm platform. Each test mouse was placed on the middle platform and remained in the maze during the 5 min session. The total time spent and number of entries into the closed and open arms were recorded and analyzed.
2.2.3. Rotarod test
A test mouse was placed on a rotating rod that is accelerated from 5 to 30 rpm during a 5 min session in a standard testing apparatus (Rotamex-5 from Columbus Instruments with mouse spindle). The performance was graded by the time a mouse stays on the rotating rod. Each mouse was tested under the same parameters three times each day for three consecutive days of a total of 9 sessions. Data from all nine sessions was obtained and analyzed for each test mouse.
2.2.4. General olfaction test
Each test mouse was placed in a fresh, clean cage with new bedding for the olfaction test. After five minutes of free exploration, three drops of vanilla extract and three drops of water were placed at the opposite corners of the cage. The time spent sniffing either vanilla or water was recorded over the next two minutes and compared between WT and DKO mice.
2.2.5. Sociability and preference for social novelty
The test was carried out in a 45 cm × 45 cm × 37.5 cm (H) clear plastic chamber divided equally into four quadrants. Two small mesh cages (10 cm in diameter, 15 cm high) were placed at the opposite corners of two quadrants. The test mouse was allowed to explore the chamber freely for 10 min with the small empty mesh cages before starting test trials. For trial 1, a wild-type stranger male mouse was placed inside one of the mesh cages and the test mouse was allowed to explore the chamber freely for 5 min. For trial 2, a second wild-type stranger mouse was placed in the other mesh cage and the test mouse was allowed to explore freely for another 5 min. Total time of direct interaction as defined by sniffing and contact through the wired cage between the test and the stranger mouse or between the test mouse and the empty mesh cage were scored.
2.2.6. Dyadic male–male interaction in neutral field
The test was carried out in a 45 cm × 45 cm × 37.5 cm (H) clear plastic box that is unfamiliar to both test mouse and reference stranger mouse. A test mouse and a reference stranger male mouse of comparable age and strain background were placed in the box that was separated by a divider. The mice were allowed to explore half of the box freely for 5 min. The divider was then removed and the mice were allowed to interact for 10 min free from interference. Aggressive behavior (attacks and tail rattles) and nonaggressive social behavior (sniffing and following) of the test mouse were video-recorded and analyzed.
2.2.7. Resident-intruder test
The test was carried out in the individual home cage of the testing male after seven days of isolation. On the eighth day, a young and unfamiliar intruder male was placed in the home cage of the test mouse. The mice were then allowed to interact free from interference for 10 min. The intruders were 2 month-old male mice of C57BL/6J strain without significant fighting experience. They were housed in separate cages from resident mice. Each intruder mouse was used only once in this test on any given test day. The entire interactions were video-recorded. Aggressive behavior (attacks and tail rattles) and nonaggressive social behavior (sniffing and following) were scored and analyzed by two independent observers.
2.3. Immunoblot analysis
Adult DKO and WT control mice were sacrificed by overdoses of trifluoroethane. Brain cortex, cerebellum and striatum were quickly dissected on ice and snap frozen in liquid nitrogen. Then the tissues were homogenized in a lysis buffer (0.5% Triton, 5 mM EDTA in PBS) supplemented with a mixture of protease inhibitors (Roche) and phosphatase inhibitors (Roche). After incubation on ice for 30 min, the homogenized samples were centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatants were used for Western blotting. Briefly, equal amounts of protein (20 μg) were loaded and separated in 8% Tris-Tricine SDS-PAGE gels. The resolved proteins were transferred onto PVDF membranes (Bio-rad). The membranes were blocked in 5% nonfat milk for 1 h at RT and incubated overnight at 4 °C with rabbit anti-GluA2 pS880 (1:250; Dr. Richard Huganir’s Lab), mouse anti-GluA2 (1:500; NeuroLab), rabbit anti-mTOR 2448 (1:1000; Cell Signaling), rabbit anti-mTOR (1:1000; Cell Signaling), mouse anti-GABA β3 (1:5000; NeuroLab), rabbit anti-mGluR5 (1:500; upstate), rabbit anti-PICK1 (1:250; Dr. Richard Huganir’s Lab) or mouse anti-tubulin (1:100000; Santa Cruz) primary antibody. The blots were then incubated with the secondary antibody, goat anti-mouse or anti-rabbit IgG conjugated with horseradish peroxidase (1:5000; Perkin Elmer), for 1 h at RT. Signals were finally visualized using enhanced chemiluminescence (GE Healthcare), and the blots were exposed to X-ray film. All immune-blot analyses were performed in three or more replicates to obtain consistent results. Immunoblot signals were quantified using NIH-Image J. After normalization with β-tubulin, levels of GluA2, GABAβ3, mGluR5 or PICK1 were expressed as fold changes that are compared to WT mice. GluA2 pS880 and mTOR pS2448 levels were normalized against total GluA2 and total mTOR, respectively, and were expressed as fold changes that are compared with WT mice.
2.4. Statistical analysis
Videos from mouse behavioral testing were analyzed by two investigators who are blinded to the genotype. Two-tailed t-test was used for comparison of the means of two independent samples in open field test, elevated plus maze, rotarod, dyad male–male social interactions, resident intruder test, olfaction test, and immunoblot. Two-factor repeated measures ANOVA followed by planned t-test were used for analysis of data from the sociability and preference for social novelty tests. All data were presented as mean ± SEM and p < 0.05 was considered as statistically significant.
3. Results
3.1. DKO mice show normal physical growth, general activities, and absence of Grip1/2 expression in neurons
To study the role of Grip1/2 in modulating social behaviors, we produced a cohort of male neuron-specific Grip1/2 double-knockout (DKO) mice and wild type control mice (WT) matched for age, sex, and strain background by breeding littermates. Neuron-specific Grip1 deletion was confirmed by a complete lack of Grip1/2 expression in brain cortex, cerebellum, and striatum of DKO mice (Fig. S1). DKO mice were born following expected Mendelian ratios. Their general physical growth, fur color, ambulatory activities, and reproduction are indistinguishable from WT control mice during the period of behavioral testing in this study (Fig. S2).
3.2. DKO mice show normal ambulatory activities, anxiety, and general olfaction
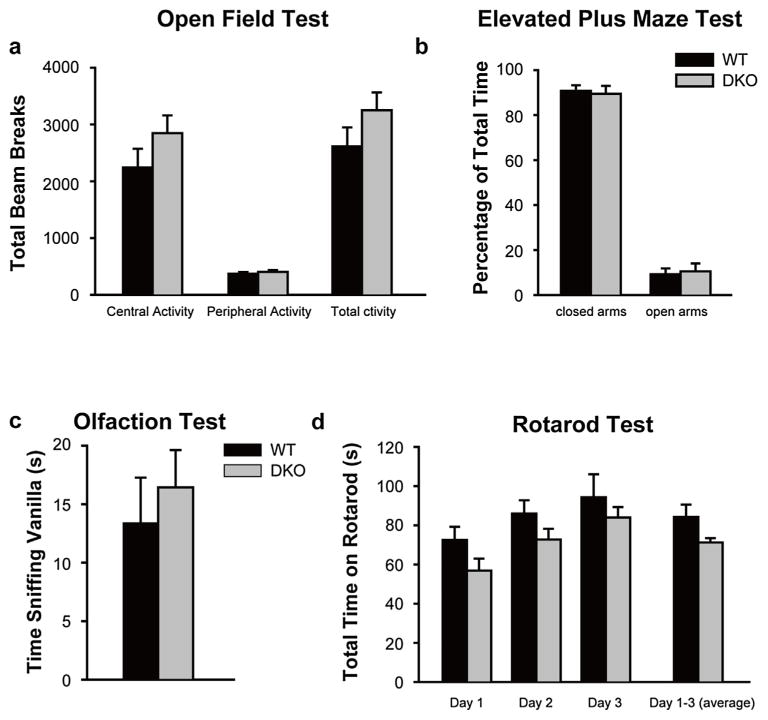
Since defects in ambulatory activities and anxiety levels are known to influence social behaviors in mice, we studied total ambulatory and exploratory activities of DKO mice using open field test and anxiety behavior using elevated plus maze (EPM) test. Compared to WT controls, DKO mice were found to have normal total exploratory activities in the open field test (total number of beam-breaks in 30 min, WT, 2613 ± 335; DKO, 3251 ± 313; mean ± sem; p > 0.05; n = 10 for each cohort) (Fig. 1a). DKO mice were found to have no significant change in their anxiety levels as reflected by the similar fraction of time spent in open and closed arms in EPM test as compared to WT (% of time in closed arms/open arms, WT, 91 ± 2.6; DKO, 89 ± 3.5; mean ± sem; p > 0.05; n = 10 for each cohort) (Fig. 1b). Since normal olfactory function is essential for social interactions in mice, we tested general olfactory function of DKO and WT control mice and found no significant difference between the groups (time spent sniffing vanilla in seconds, WT, 13.4 ± 3.9; DKO, 16.4 ± 3.2; mean ± sem; p > 0.05; n = 10 for each study cohort) (Fig. 1d).
Fig. 1.
Grip1/2 DKO mice show normal ambulatory activity, anxiety, and general olfaction.
Panel a: Open Field Test: Grip1/2 DKO mice showed normal central activity and peripheral activity as compared with WT control mice. Panel b: Elevated Plus Maze: Grip1/2 DKO mice exhibited similar time spent in the open and closed arms in EPM test as compared to WT control mice. Panel c: Rota-rod: Grip1/2 DKO mice showed similar motor function, balance and coordination, and motor learning as compared to WT control mice. Panel d. Olfaction: Grip1/2 DKO mice showed similar general olfactory function as compared to WT control mice. Data are presented as mean ± SEM. Adult WT (n = 10) and Grip1/2 DKO (n = 10) mice were generated from littermate breeding and were matched for age, sex, and strain background.
3.3. DKO mice show normal general motor function and balance, and motor learning
We performed an accelerating rotarod test to evaluate motor function, coordination and balance of DKO mice since significant defects in these functional areas could affect social interaction in mice. No significant difference was found between DKO and WT control mice in these tests (time staying on rotarod over 300 s, WT, 84.3 ± 6.3; DKO, 71.2 ± 2.3; mean ± sem; p > 0.05; n = 10 for each cohort) (Fig. 1c). Furthermore, DKO mice demonstrated an appropriate level of motor learning in rotarod test as compared to WT controls (Fig. 1c)
3.4. DKO mice show increased sociability and preference for social novelty
We first performed standard rodent behavioral tests including sociability and preference for social novelty to assess social behaviors of DKO and matched WT control mice and validate our prior finding that DKO mice manifest an increase in sociability [28]. Sociability and preference for social novelty in mice were determined by documenting time spent in direct social interactions between test and stranger mice [36]. In the sociability test, the test mouse was first allowed a free exploration of an open chamber with two small empty mesh cages at opposite corners. A stranger male (stranger mouse) was then put in one of mesh cages. The test mouse was given a choice of spending time with this stranger male versus an object (empty cage). Preference for social novelty test was followed immediately after sociability test. A novel stranger male (novel mouse) was put into the empty mesh cage. The entire test was video recorded and analyzed by two investigators who are blind to the genotype.
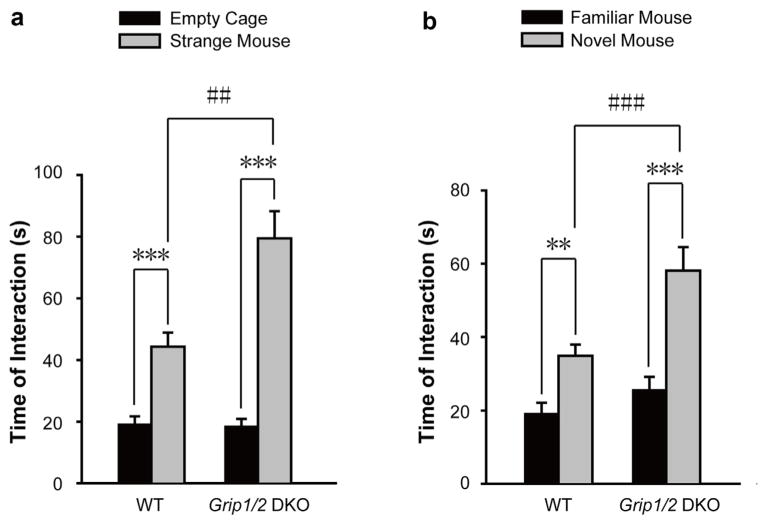
As expected, WT mice showed normal sociability by spending significantly more time in social interaction with the mesh cage containing the first stranger mouse versus the empty cage (total time of social interaction in seconds; mean ± sem for empty cage, 19.0 ± 3.0; stranger mouse, 44.0 ± 5.0; t-test; p < 0.001; n = 10). Intriguingly, DKO mice appeared to show increased levels of sociability as compared to WT mice (total time of social interaction in seconds; mean ± sem for empty cage, 19.0 ± 2.0; stranger mouse, 81.0 ± 9.0; t-test; p < 0.00001; n = 9 for each cohort) (Fig. 2a). To investigate this phenomenon further, we performed two factor repeated measures ANOVA which revealed a significant genotype effect [WT versus KO; F(1,18) = 9.82; p < 0.01], a significant effect due to interacting objects [non-social empty cage versus social object of novel mouse; F(1,19) = 80.05; p < 0.0001], and a significant interaction [F(1,37) = 14.45; p < 0.01]. Next, we performed a planned t-test, which identified no difference in total time of interaction with nonsocial object (empty cage) between WT and KO mice (total time of interaction in seconds; mean ± sem; WT: 19.0 ± 3.0; n = 10; KO: 19.0 ± 2.0, n = 9; t-test; p > 0.05). Interestingly, the analysis identified a significant increase in total time of social interaction with the social object (novel mouse) for DKO as compared to WT mice (total time of interaction in seconds; mean ± sem; WT: 44.0 ± 5.0; n = 10; KO: 81.0 ± 9.0; n = 9; t-test; p < 0.01). These results indicate a significant increase in sociability for DKO mice as compared to WT control mice.
Fig. 2.
Grip1/2 DKO mice show increased sociability and preference for social novelty.
Panel a. Sociability. During the first 10 min, individual test mouse was allowed to freely interact with an empty cage versus a cage with a stranger mouse in the testing chamber. Total time of social interaction (in second) of the test mouse with these two cages was measured. Both WT and Grip1/2 DKO mice spent significantly more time interacting with the stranger mouse versus empty cage (t-test, p < 0.001). Grip1/2 DKO mice exhibit a significant increase in sociability as compared to WT control mice (two-way ANOVA, (F(1,72) = 11.34, p < 0.01). Panel b. Preference for Social Novelty. During the second 10 min, individual test mouse was allowed to freely interact with the cage with a familial mouse and cage with a stranger mouse in the testing chamber. Total time of social interaction (in second) of the test mouse with these two cages was measured. The mean and SEM for WT (n = 10) and Grip1/2 DKO (n = 10) were shown. Both WT and Grip1/2 DKO mice spent significantly more time in social interaction with the novel mouse than with the familiar mouse (p < 0.01 and p < 0.001). Grip1/2 DKO mice exhibit a significant increase in the preference of social novelty as compared to WT control mice (two-way ANOVA test, F(1,72) = 13.04, p < 0.001). Data presents as mean ± SEM. **, p < 0.01; ***, p < 0.001)
Both WT and DKO mice showed a preference for social novelty by spending significantly more time in social interaction with the cage containing a novel mouse as compared to the cage containing the already investigated, familial mouse (total time of social interaction in seconds; mean ± sem. WT: familial mouse, 19.0 ± 3.0 and novel mouse, 35.0 ± 3.0; t-test; p < 0.01; n = 10; DKO: familial mouse, 27.0 ± 3.0 and novel mouse, 59.0 ± 6.0; t-test; p < 0.001; n = 9) (Fig. 2b). Intriguingly, DKO mice appeared to show an increase in preference for social novelty compared to WT. To further investigate these effects, we performed a two factor repeated measures ANOVA which revealed a significant effect of genotype [WT versus DKO; F(1,18) = 10.35; p < 0.01], a significant effect of interacting social subjects [familial versus novel mouse; F(1,19) = 44.85; p < 0.0001], and a significant interaction [F(1,37) = 5.61; p < 0.05]. We next performed a planned t-test to evaluate the genotype effect. The analysis identified no significant difference in the total time of social interaction with the familial mouse between WT and DKO mice (total time of interaction in seconds; mean ± sem; WT: 19.0 ± 3.0; n = 10; DKO: 27.0 ± 3.0, n = 10; t-test; p > 0.05). Intriguingly, the analysis revealed a significant increase in total time of social interaction with the novel social object (novel mouse) for DKO as compared to WT mice (total time of interaction in seconds; mean ± sem; WT: 35.0 ± 3.0; n = 10; DKO: 59.0 ± 6.0; n = 9; t-test; p < 0.01). Together, these results support a significant increase in preference for social novelty in DKO as compared to WT mice.
3.5. DKO mice show increased dyadic male–male social interactions in a neutral arena
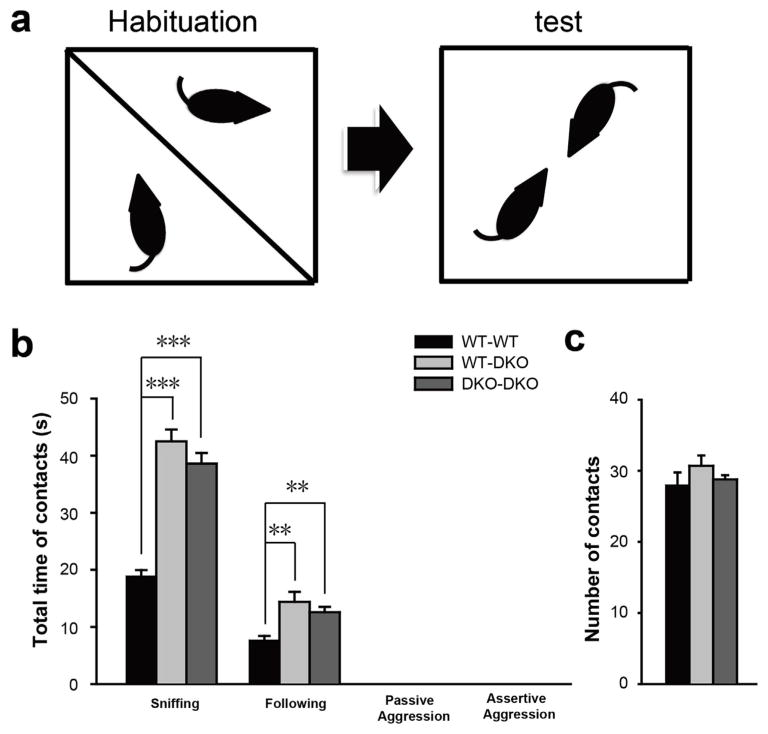
The increase in sociability and preference for social novelty in the DKO mice are supported by results from testing these mice in dyadic male–male interaction in a neutral arena. The test was carried out in an open chamber that is unfamiliar to both the test and stranger mice (Fig. 3a). The entire test was video recorded and total time of social interactions (sniffing and following) between test and stranger mice were scored. Total time of interactions between the WT–WT and WT-DKO cohorts and between the WT–WT and DKO–DKO cohorts show a significant increase in nonaggressive social behaviors (sniffing in seconds: WT-WT, 18.9 ± 1.1; WT-DKO, 42.5 ± 2.1; DKO-DKO, 38.6 ± 1.9; mean ± sem; p < 0.001 for WT–WT versus WT-DKO; p < 0.001 for WT–WT versus DKO-DKO; and following in seconds: WT-WT, 7.7 ± 0.72; WT-DKO, 14.4 ± 1.8; DKO-DKO, 12.6 ± 0.93; mean ± sem; p < 0.001 for WT–WT versus WT-DKO; p < 0.001 for WT–WT versus DKO-DKO; n = 10 pairs for WT-WT; n = 10 pairs for WT-DKO; n = 5 pairs for DKO-DKO) (Fig. 3b). There was no difference in total number of interaction between the three cohorts (number of interactions: WT-WT, 28 ± 1.8; WT-DKO, 30.7 ± 1.5; DKO-DKO, 28.8 ± 0.58; mean ± sem; p > 0.05 for WT–WT versus WT-DKO; p > 0.05 for WT–WT versus DKO-DKO; n = 10 pairs for WT-WT; n = 10 pairs for WT-DKO; n = 5 pairs for DKO-DKO) (Fig. 3c). Thus, total social interaction time for each episode is significantly increased for WT-DKO and DKO–DKO cohorts as compared to the WT–WT cohort. Similar number of encounters support normal ambulatory activities without significant anxiety in the mutant mice. Longer interaction time per episode for the mutant mice potentially implicate an enhanced and sustaining interest to interact with stranger mice.
Fig. 3.
Grip1/2 DKO mice show increased dyadic male–male social interactions.
Panel a. The test and stranger mice were allowed to freely interact for 5 min in a test chamber. The total time of nonaggressive social interactions including sniffing and following was measured. Three testing cohorts including WT–WT (10 pairs), WT-DKO (10 pairs) and DKO–DKO (5 pairs) were studied. Panel b. Total events of interaction for the three testing cohorts were determined. Note the WT-DKO and DKO–DKO cohorts of mice show a significant increase in nonaggressive social interaction as compared to the WT–WT cohort (t-test, p < 0.01). Data presents as mean ± SEM. **, p < 0.01; ***, p < 0.001)
3.6. DKO mice show normal aggressive social behaviors
We performed a resident–intruder test to evaluate aggressive social behaviors in DKO mice in their home cage. This test was carried out in the home cage of the test mouse to evaluate its interaction with a younger, unfamiliar intruder male mouse over 10 min. Compared to WT, DKO mice show no significant difference in their aggressive social behaviors including tail flicking for passive aggression (total time in seconds, WT, 2.5 ± 2.3; DKO, 0.4 ± 0.2; mean ± sem; p > 0.05; n = 10 for each cohort), biting and fighting for assertive aggression (total time in seconds, WT, 0.9 ± 0.8; DKO, 0.5 ± 0.5; mean ± sem; p > 0.05; n = 10 for each cohort), and latency to first attack (total time in seconds; mean ± sem; WT, 227.5 ± 33.3; DKO, 423.3 ± 43.2; p > 0.05; n = 10 for each cohort) (Table 1).
Table 1.
Aggressive Social Behaviors of Grip1/2 DKO Mice and WT Controls in Resident-Intruder Testa.
| Resident Mice | Aggressive Social Behaviors | |||
|---|---|---|---|---|
|
|
|
|||
| Genotype | Number | Passive Aggression (Tail flicking) | Assertive Aggression (Fighting, biting, chasing) | Latency to First Attack (Second) |
| WT | 10 | 2.5 ± 2.3 | 0.9 ± 0.8 | 227.5 ± 33.3 |
| DKO | 10 | 0.4 ± 0.2 | 0.5 ± 0.5 | 423.3 ± 43.2 |
The test was carried out in the individual home cage of a resident male mouse after seven days of isolation. On the eighth day, a young and unfamiliar intruder male was placed in the home cage of the test mouse. These mice were then allowed to interact freely without interference for 10 min. These intruder mice of similar strain background were housed in separate cages and have not been exposed to the resident mice before the test. Each intruder mouse was used only once in this test on any given test day. Isolation induced aggressive behaviors (attacks, biting, and tail rattles) of the resident mouse toward the intruder mouse were video-recorded and analyzed individually. The episodes of each attack as well as the latency to first attack were compared. Mean +/− SEM for each aggressive behavior was shown. Student t-test was performed for statistical analysis.
3.7. DKO mice show increased phosphorylation at GluA2-S880 in frontal cortex and cerebellum as well as reduced GABAβ3 expression in striatum
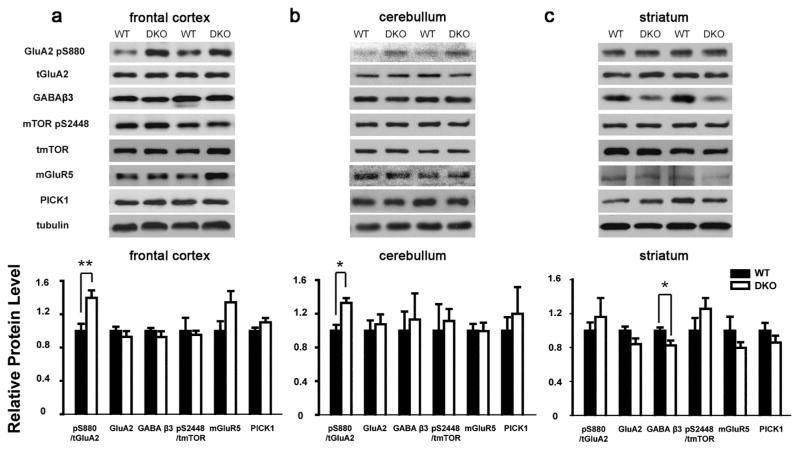
To study neural mechanisms of loss of neuronal Grip1/2 expression, we determined expression levels of Grip1/2 interacting proteins and key signaling proteins in AMPA, mGluR, mTOR, and GABA pathways in brain regions known to related to social behaviors in DKO mice. The levels of phosphorylated GluA2 at serine 880 (GluA2-pS880) in front cortex were found significantly increased in DKO mice (ratio of band intensity for GluA2-pS880/total GluA2; WT: 0.69 ± 0.06, n = 5; DKO: 0.96 ± 0.06, n = 6; mean ± sem; t-test; p < 0.05) while levels of total GluA2, GABAβ3, mTOR 2448, mGluR5, and PICK1 were not altered compared to WT (Fig. 4a). Similar increase of GluA2-pS880 was found in cerebellum (ratio of band intensity for GluA2-pS880/total GluA2; WT: 1.14 ± 0.08, n = 6; DKO: 1.52 ± 0.06, n = 4; mean ± sem; t-test; p < 0.05) but not in striatum of DKO compared to WT mice (Fig. 4b, c). Interestingly, the levels of GABAβ3 in striatum were found significantly reduced in DKO mice (ratio of band intensity for GABAβ3/β tubulin; WT: 1.16 ± 0.04, n = 4; DKO: 0.95 ± 0.06, n = 4; mean ± sem; t-test; p < 0.05) but not in frontal cortex or cerebellum compared to WT mice (Fig. 4b, c).
Fig. 4.
Grip1/2 DKO mice show altered GluA2-Serine880 phosphorylation and GABAβ3 levels in brain tissues. 20 μg of tissue lysates from respective brain regions of WT and Grip1/2 DKO mice were immunoblotted using antibodies specific for the indicated proteins. Expression levels of these proteins were then normalized using tubulin. Note an increase in phosphorylated GluA2 at serine 880 (GluA2-pS880) in brain cortex (Panel a) and cerebellum (Panel b) and reduced levels of GABAβ3 in striatum (Panel c) of Grip1/2 DKO mice. N = 4–6 for each study cohort. Data presents as mean ± SEM. t-test was used to compare the mean between study cohorts; *, p < 0.05 **, p < 0.01.
4. Discussion
Severe deficits in the reciprocal social interactions are a core feature of autism spectrum disorders. However, neural mechanisms that regulate social behaviors are poorly understood. Our recent studies identified autism-associated mutations that alter conserved amino acids in GRIP1-PDZ4-6, the domains that directly bind GluA2 and mediate its synaptic trafficking [28]. Interestingly, these autism-associated mutations show a gain-of-function effect as evident by increased binding to GluA2 in a yeast two-hybrid assay. These mutations result in accelerated GluA2 recycling in primary neurons [28] while loss of Grip1/2 expression results in delayed GluA2 recycling in primary neurons [10]. Genotype and phenotype correlation studies of affected sibs support that these GRIP1 mutations contribute to increased severity of deficits in reciprocal social interactions in autism [28].
Our prior studies identified increased sociability in Grip1/2 KO mice using a modified three chamber social behavioral tests [28]. We first tested an independent cohort of Grip1/2 KO and WT control mice from littermate breeding for sociability and preference for social novelty to validate our initial finding using a modified three chamber method [28]. The results corroborated our prior finding that Grip1/2 KO mice exhibit an increase in sociability and further identified an increase preference for social novelty in mutant mice. To characterize social behaviors in these mice systematically, we completed additional two social behavioral tests in rodent (1) dyadic male–male social interaction and (2) resident-intruder test. These tests take advantage of different motivations for rodents to engage in robust social interactions. Dyadic male–male social interaction test is based on natural exploratory instinct of rodents while resident-intruder test is based on the nature of defending their-own territories. In these tests, Grip1/2 KO mice consistently show increased social interactions but normal aggressive behaviors. Furthermore, these mice show normal ambulation and exploration, motor and balance, anxiety levels, and olfaction. These functions are essential for rodents to engaging in normal social interactions.
To identify disrupted neural signaling pathways in brain of Grip1/2-DKO mice, we studied expression levels and phosphorylation of key signaling proteins in AMPA-, GABA-, mGluR-, and mTOR-pathways in frontal cortex, striatum, and cerebellum. These brain regions are implicated in social deficits in autism [37–39]. The increase in GluA2-S880 phosphorylation in frontal cortex and cerebellum of Grip1/2-DKO mice is very interesting. GluA2 phosphorylation at serine-880 is a well-recognized mechanism regulating AMPA receptor trafficking to post-synaptic membrane in response to glutamate release [40]. Specifically, phosphorylation at this specific serine residue disrupts GluA2 binding to a C-terminal domain of GRIP1 but retains its binding to PICK1. PICK1 is another neural scaffolding protein that regulates GluA2 synaptic trafficking via binding to its C-terminal domain [40,41]. Lack of Grip1/2 expression results in delayed GluA2 recycling following NMDA-induced endocytosis in neurons [10]. Similarly, the rate of NMDA-induced GluA2 recycling was delayed in an artificial GluA2 mutant that could not bind GRIP1 [42]. Since PICK1 expression levels are not altered in brain tissues DKO mice, we speculate that increased GluA2 phosphorylation together with lack of Grip1/2 expression cause a preferential binding of GluA2 with PICK1 over GRIP1. This change in balance in binding kinetics of GluA2 with its trafficking proteins, GRIP1 and PICK1, results in an accelerated synaptic recycling of GluA2 in response to synaptic activities and glutamate release [10,40,41].
How loss of Grip1/2 expression results in increased GluA2-S880 phosphorylation is not known. GluA2-S880 phosphorylation is controlled by activities of PKC and protein phosphatases 1/2 in response to synaptic activities while the status of GluA2-S880 phosphorylation directly influences GluA2 binding kinetics and balance with GRIP1 and PICK1. We speculate that lack of Grip1/2 expression result in a pool of unbound GluA2 that are subjected to phosphorylation by PKC. Once phosphorylated, GluA2-pS880 binds preferentially with PICK1 versus GRIP1. However, the regulatory processes for GluA2 trafficking in neurons without Grip1/2 are likely more complex. At least three additional proteins, KIBRA [43], NEEP21 [44] and Sec8 [10] are also known to affect GluA2 recycling in neurons.
GRIP1/2 express abundantly in both glutamatergic and GABAergic synapses suggesting a role in the regulation of both excitatory and inhibitory synaptic functions [5,9]. Reduced GABAβ3 expression specifically in striatum is of great interesting. GABAβ3 has been implicated in the risks and/or pathogenesis of developing ASDs by genetic linkage and association studies as well as studies of 15q duplication syndrome in which a cluster of GABAnergic genes including GABAβ3 are duplicated [45,46]. Furthermore, defects in striatal functional connectivity have been recognized as a pathogenic mechanism in ASDs [37,47]. Despite these finding, the precise nature of these signaling changes and how these changes relate to social behavioral phenotype in ASDs remain poorly understood. Data from our current study provide valuable insights into the locations and expression levels of GABAβ3 that are potentially relevant to social behavioral regulation. Our data support that disturbances in GABAergic signaling, particularly GABAβ3, in striatum, play a role in the risks and/or pathogenesis of developing social deficits in autism.
Disturbances of the excitatory and inhibitory neurotransmission have been reported in established mouse models of autism including Rett syndrome [21] and Neuroligin 3 KO mice [22]. These data support altered excitatory and inhibitory balance in brain cortex [25] or altered network synchrony [27,48] in social deficits in certain forms of ASDs. As a scaffolding protein in both excitatory and inhibitory synapses, GRIP1/2 likely plays a role in regulating the balance between excitatory and inhibitory synaptic functions. Together, our studies provide supporting evidence for a role of GRIP1/2-mediated AMPA signaling defects in social behavioral deficits in autism.
Supplementary Material
HIGHLIGHTS.
Neuron-specific Grip1/2-knockout mice show increased social interactions.
Increased GluA2-pS880 in frontal cortex and decreased GABAβ3 expression in striatum were identified.
Results support a role of Grip1/2-mediated AMPA signaling in regulating social behavior.
Disturbances of AMPA- and GABA-signaling are implicated in autism social deficits.
Acknowledgments
This work was supported in part by research grants from Simons Foundation (SFARI) [#206683] and Autism Speaks [#2487].
Abbreviations
- GRIP1/2
glutamate receptor interacting proteins 1 and 2
- AMPA
2-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid
- GluA2
AMPA glutamate receptor 2
- GABAβ3
gamma-aminobutyric acid receptor beta 3 subunit
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bbr.2016.12.042.
Footnotes
Competing interest
The authors declare no conflict of interest in the study
Authors’ contribution
RM, MH, TW, and RH designed the study. MH, RM, RR, AA, and TW performed experiments and/or analyzed data. S-L C and RH contribute reagents and data analysis. MH, RM, and TW wrote the paper. All authors read and approved the manuscript.
References
- 1.Huguet G, Ey E, Bourgeron T. The genetic landscapes of Autism spectrum disorders. Ann Rev Genomics Hum Genet. 2013;14:191–213. doi: 10.1146/annurev-genom-091212-153431. [DOI] [PubMed] [Google Scholar]
- 2.Devlin B, Scherer S. Genetic architecture in autism spectrum disorder. Curr Opin Genet Dev. 2012;22:229–237. doi: 10.1016/j.gde.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Seeburg P. The TINS/TiPS Lecture. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993;16(9):359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- 4.Anggono V, Huganir R. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol. 2012;22:461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong H, O’Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386(6622):279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- 6.Dong H, Zhang P, Song I, Petralia R, Liao D, Huganir R. Characterization of the glutamate receptor interacting proteins GRIP1and GRIP2. J Neurosci. 1999;19:6930–6941. doi: 10.1523/JNEUROSCI.19-16-06930.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takamiya K, Mao L, Huganir RL, Linden DJ. The glutamate receptor-interacting protein family of GluR2-binding proteins is required for long-term synaptic depression expression in cerebellar Purkinje cells. J Neurosci. 2008;28(22):5752–5755. doi: 10.1523/JNEUROSCI.0654-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu W, Ziff E. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron. 2005;47:407–421. doi: 10.1016/j.neuron.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Li R, Serwanski D, Miralles C, Li X, Echarych, Riquelme R, et al. GRIP1 in GABAergic synapses. J Comp Neurol. 2005;488:11–27. doi: 10.1002/cne.20566. [DOI] [PubMed] [Google Scholar]
- 10.Mao L, Takamiya K, Thomas G, Lin DT, Huganir RL. GRIP1 and 2 regulate activity-dependent AMPA receptor recycling via exocyst complex interactions. Proc Natl Acad Sci U S A. 2010;107(44):19038–19043. doi: 10.1073/pnas.1013494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purcell A, Jeon O, Zimmerman A, Blue M, Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57:1618–1628. doi: 10.1212/wnl.57.9.1618. [DOI] [PubMed] [Google Scholar]
- 12.Jamain S, Ouach H, Betancur C, Rastam M, Colineaux C, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durand C, Betancur C, Boeckers T, Bockmann J, Chaste P, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associatd with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto D, Pagnamenta A, Klei L, Anney R, Merico D, Regan R, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varea O, Martin-de-Saavedra M, Kopeikina K, Schürmann B, Fleming H, Fawcett-Patel J, et al. Synaptic abnormalities and cytoplasmic glutamate receptor aggregates in contactin associated protein-like 2/Caspr2 knockout neurons. Proc Natl Acad Sci U S A. 2015;112:6176–6181. doi: 10.1073/pnas.1423205112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geschwind D, Levitt P. Autism spectrum disroders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Di Martino A, Kelly C, Grzadzinski R, Zuo X, Mennes M, Mairena M, et al. Aberrant striatal functional connectivity in children with autism, Biol. Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.10.029. (EPub) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott-Van Zeeland A, Abrahams B, Alvarez-Retuerto A, Sonnenblick L, Rudie J, Ghahremani D, et al. Altered functional connectivity in frontal lobe circuits is associated with variation in the autism risk gene CNTNAP2. Sci Transl Med. 2010;2:56ra80. doi: 10.1126/scitranslmed.3001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibson J, Bartley A, Hays S, Huber K. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of Fragile X syndrome. J Neurophysiol. 2008;100:2615–2626. doi: 10.1152/jn.90752.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dani V, Chang Q, Maffei A, Turrigiano G, Jaenisch R, Nelson S. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2005;102:12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabuchi K, Blundell J, Etherton M, Hammer RXL, Powell C, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oblak A, Gibbs T, Blatt G. Reduced GABAA receptors and benzodiazepine binding sites in the posterior cingulate cortex and fusiform gyrus in autism. Brain Res. 2011;1380:218–228. doi: 10.1016/j.brainres.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmen S, van Engeland H, Hof P, Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- 25.Rubenstein J, Merzenich M. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uhlhaas P, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Tsilson, Rojas D, Reite M, Teale P, Rogers S. Children and adolescents with autism exhibit reduced MEG steady-state gamma responses. Biol Psychiatry. 2007;62:192–197. doi: 10.1016/j.biopsych.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mejias R, Adamczyk A, Anggono V, Niranjan T, Thomas G, Sharma K, et al. Gain-of-function glutamate receptor interacting protein 1 variants alter GluA2 recycling and surface distribution in patients with autism. Proc Natl Acad Sci U S A. 2011;108:4920–4925. doi: 10.1073/pnas.1102233108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niranjan T, Adamczyk A, Bravo H, Taub M, Wheelan S, Irizarry R, et al. Effective detection of rare variants in pooled DNA samples using cross-pool tail curve analysis. Genome Biol. 2011;12:R93. doi: 10.1186/gb-2011-12-9-r93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Just M, Keller T, Malavea V, Kana R, Varmac V. Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci Biobehav Rev. 2012;36:1292–1313. doi: 10.1016/j.neubiorev.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Martino A, Kelly C, Grzadzinski R, Zuo X, Mennes M, Mairena M, et al. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry. 2011;69:847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker E, Stoodley C. Autism spectrum disorder and the cerebellum. Int Rev Neurobiol. 2013;113:1–34. doi: 10.1016/B978-0-12-418700-9.00001-0. [DOI] [PubMed] [Google Scholar]
- 33.Takamiya K, Kostourou V, Adams S, Jadeja S, Chalepakis G, Scambler PJ, et al. A direct functional link between the multi-PDZ domain protein GRIP1 and the Fraser syndrome protein Fras1. Nat Genet. 2004;36(2):172–177. doi: 10.1038/ng1292. [DOI] [PubMed] [Google Scholar]
- 34.Pletnikov M, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov M, Huang H, et al. Indicible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2008;13:173–186. doi: 10.1038/sj.mp.4002079. [DOI] [PubMed] [Google Scholar]
- 35.Ayhan Y, Abazyan B, Nomura J, Kim R, Ladenheim B, Krasnova I, et al. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence of neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry. 2011;16:293–306. doi: 10.1038/mp.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adamczyk A, Mejias R, Takamiya K, Yocum J, Krasnova N, Calderon J, et al. GluA3-deficiency in mice is associated with increased social and aggressive behavior and elevated dopamine in striatum. Behav Brain Res. 2012;229:265–275. doi: 10.1016/j.bbr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Striatal Fuccillo MV. Circuits as a common node for autism pathophysiology. Front Neurosci. 2016;10:27. doi: 10.3389/fnins.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann E, Bruck C, Kreifelts B, Ethofer T, Wildgruber D. Reduced functional connectivity to the frontal cortex during processing of social cues in autism spectrum disorder. J Neural Transm (Vienna) 2016;123(8):937–947. doi: 10.1007/s00702-016-1544-3. [DOI] [PubMed] [Google Scholar]
- 39.Jaber M. The cerebellum as a major player in motor disturbances related to Autistic Syndrome Disorders. Encephale. 2016 doi: 10.1016/j.encep.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 40.Chung H, Xia J, Scannevin R, Zhang X, Huganir R. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim C, Chung H, Lee H, Huganir R. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci U S A. 2001;98:11725–11730. doi: 10.1073/pnas.211132798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin D, Huganir R. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. J Neurosci. 2007;27:13903–13908. doi: 10.1523/JNEUROSCI.1750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makuch L, Volk L, Anggono V, Johnson R, Yu Y, Duning K, et al. Regulation of AMPA receptor function by the human memory-associated gene KIBRA. Neuron. 2011;71:1022–1029. doi: 10.1016/j.neuron.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steiner P, Stefano A, Kulangara K, Yersin A, Sarria J, Regulier E, et al. Interactions between NEEP21, GRIP1 and GluR2 regulate sorting and recycling of the glutamate receptor subunit GluR2. EMBO J. 2005;24:2873–2884. doi: 10.1038/sj.emboj.7600755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urraca N, Cleary J, Brewer V, Pivnick EK, McVicar K, Thibert RL, et al. The interstitial duplication 15q11.2-q13 syndrome includes autism, mild facial anomalies and a characteristic EEG signature. Autism Res. 2013;6(4):268–279. doi: 10.1002/aur.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conant KD, Finucane B, Cleary N, Martin A, Muss C, Delany M, et al. A survey of seizures and current treatments in 15q duplication syndrome. Epilepsia. 2014;55(3):396–402. doi: 10.1111/epi.12530. [DOI] [PubMed] [Google Scholar]
- 47.Di Martino A, Kelly C, Grzadzinski R, Zuo XN, Mennes M, Mairena MA, et al. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry. 2011;69(9):847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uhlhaas P, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.