Abstract
Background and study aims
Dye-based chromoendoscopy (DBC) is the preferred method for endoscopic dysplasia surveillance in patients with inflammatory bowel disease (IBD). We sought to examine the uptake of, and perception toward DBC among academic gastroenterologists.
Methods
We conducted an online survey of academic members of the Canadian Association of Gastroenterology to assess their current dysplasia surveillance practice, uptake of DBC, and perceived barriers to adoption of DBC.
Results
Of the 150 physicians contacted, 49 (32.7 %) responded to the survey. The majority of respondents reported subspecialty training in IBD (71.4 %), and the median number of years in practice was 12. White-light endoscopy with random colonic biopsies was the preferred dysplasia screening method (73.5 %). Only 26.5 % of respondents routinely used DBC, despite institutional availability of over 60 %. The major barriers to adoption of DBC were concerns about procedure duration (46.9 %), concerns about cost (44.9 %), and inadequate training (40.8 %).
Conclusion
There is low uptake of DBC for dysplasia surveillance in IBD patients among academic gastroenterologists practicing in Canada. Additional studies should be completed to determine how to improve the uptake of DBC.
Introduction
The recognition that patients with inflammatory bowel disease (IBD) are at higher risk of developing colorectal cancer (CRC) relative to the general population 1 2 3 has led multiple national societies to develop specific recommendations for dysplasia surveillance in this patient population 4 5 6 7 . Central to these recommendations is the need to perform dysplasia surveillance at regular intervals (every 1 to 3 years in North American guidelines, and every 1 to 5 years in British guidelines, depending on risk), starting at 8 to 10 years following disease diagnosis in patients with extensive colonic disease, as well as taking multiple random biopsies at regular intervals throughout the colon to screen for flat undetectable neoplastic lesions 4 5 6 . The past decade has also witnessed the introduction of multiple advanced image-enhanced endoscopy (IEE) modalities, such as dye-based chromoendoscopy (DBC), which have demonstrated improved dysplasia detection in patients with IBD compared with conventional approaches 8 9 10 11 . Recognizing these merits, the latest consensus guidelines have recommended DBC as an alternate to white-light colonoscopy with multiple random biopsies for dysplasia surveillance 12 13 .
Adherence to current recommendations and uptake of newer techniques with proven effectiveness is important to providing optimal CRC prevention in patients with IBD. Physician uptake of consensus recommendations has been shown to be poor among gastroenterologists in the United Kingdom and the United States, which may partially explain the persistently higher rate of CRC observed in this patient population 14 15 16 . A recent US study further reported that a uniform approach to surveillance and treatment is lacking in IBD patients 17 . To date, no studies have evaluated adherence by gastroenterologists in Canada to the current guidelines for dysplasia surveillance in IBD patients. Furthermore, access to and implementation of IEE in clinical practice has not been evaluated. The aim of this paper is to describe the uptake of these modalities by Canadian academic gastroenterologists.
Methods
A questionnaire was developed to assess the attitudes and opinions of Canadian academic gastroenterologists toward IBD surveillance guidelines and newer IEE modalities to detect colorectal dysplasia, as well as utilization, access, and barriers to newer imaging modalities. IBD specialists from various practice settings reviewed and provided input to increase the validity of the survey. A combination of contingency, matrix, and closed-ended questions were used. The questionnaire was administered using an online survey engine (Novi Survey; Novi Systems, Waltham, Massachusetts, USA).
We collected information about practice characteristics (years in practice, subspecialty training in IBD, and proportion of IBD patients in respondent’s practice), as well as colonoscopy surveillance patterns (annual volume of dysplasia surveillance colonoscopies for IBD patients, adherence to any recent major society screening guideline, preferred screen interval, duration of colonoscopy, and number of biopsies taken per colonoscopy). We also assessed uptake of several IEE modalities, including DBC, dye-less chromoendoscopy, and confocal laser endomicroscopy. Respondents were asked to identify barriers to uptake for each of these IEE modalities. Finally, respondents were asked to rate their degree of agreement with nine statements pertaining to dysplasia screening in IBD patients.
The survey was sent by email to a directory of gastroenterologists affiliated with Canadian universities. Following the initial invitation, two subsequent reminders were sent a month apart. Before being able to access the survey, physicians were screened for suitability to participate in the study. Participants were required to be practicing physicians who had completed postgraduate training in gastroenterology and who had cared for IBD patients within the preceding 12 months. Consent was implied if participants proceeded with the survey. All data were collected anonymously.
The study protocol was approved by the Research Ethics Board at Mount Sinai Hospital, Toronto, Ontario. Statistical analyses were conducted using SPSS (v 17.0; SPSS Inc., Chicago, Illinois, USA). The frequency and distribution of the study population was determined using descriptive analyses.
Results
The SCREEN IBD survey was electronically mailed to 150 practicing academic gastroenterologists across Canada, 49 of whom completed the survey (response rate 32.7 %). All respondents met study participation criteria and their responses were included in the final analysis. Practice characteristics and colonoscopy surveillance patterns of study respondents are shown in Table 1 .
Table 1. Practice characteristics and colonoscopy surveillance patterns of study respondents (n = 49).
| Practice characteristics | |
| Time in practice, median (IQR), years | 12 (17) |
| Subspecialty training in IBD, n (%) | 35 (71.4) |
| Proportion of IBD in practice, n (%) | |
|
17 (34.7) |
|
15 (30.6) |
|
17 (34.7) |
| Colonoscopy surveillance patterns | |
| Annual number of IBD surveillance colonoscopies, n (%) | |
|
14 (28.6) |
|
23 (46.9) |
|
12 (24.5) |
| Adherence to any major society screening guidelines, n (%) | |
|
8 (16.3) |
|
6 (12.2) |
|
35 (71.4) |
| Preferred screening interval, n (%) | |
|
9 (18.4) |
|
38 (77.5) |
|
2 (4.1) |
| Duration of surveillance colonoscopies, median (IQR), minutes | 30 (20) |
| Number of biopsies per surveillance biopsy, median (IQR) | 35 (4.1) |
| Preferred surveillance methods, n (%) | |
|
36 (73.5) |
|
13 (26.5) |
IQR, interquartile range; IBD, inflammatory bowel disease.
The median number of years in practice was 12 (interquartile range [IQR] = 17) and the majority of respondents completed subspecialty training in IBD (71.4 %). Twelve respondents were identified as high-volume endoscopists (defined as performing over 100 IBD dysplasia screening colonoscopies annually). The majority of respondents used a major guideline to direct their approach to dysplasia surveillance in IBD patients (71.4 %). Most respondents indicated that the preferred screening interval was every 2 – 3 years (77.5 %). The median time of surveillance colonoscopy completion was 30 minutes (IQR = 20), and the median number of biopsies performed per surveillance colonoscopy was 35 (IQR = 4.1).
The primary endoscopic dysplasia surveillance method reported was white-light colonoscopy with random colonic biopsies performed in each quadrant every 10 cm (36/49, 73.5 %). Only 13 respondents used DBC as their primary surveillance method (26.5 %). Fig. 1 shows the reported availability of IEE modalities at the respondents’ institution. Compared with confocal laser endomicroscopy, both DBC and dye-less chromoendoscopy were more widely available (22.4 % vs. 61.2 % and 63.3 %, respectively). Despite the availability of DBC and dye-less chromoendoscopy, only 22.4 % and 30.6 % of respondents reported training and/or previous use of these modalities, respectively. Over half of respondents (55.1 %) believed that the adoption of DBC by their institution would improve dysplasia detection outcomes, whereas only 26.5 % and 18.4 % of respondents indicated that adoption of dye-less chromoendoscopy and confocal laser endomicroscopy, respectively, would improve detection outcomes.
Fig. 1.
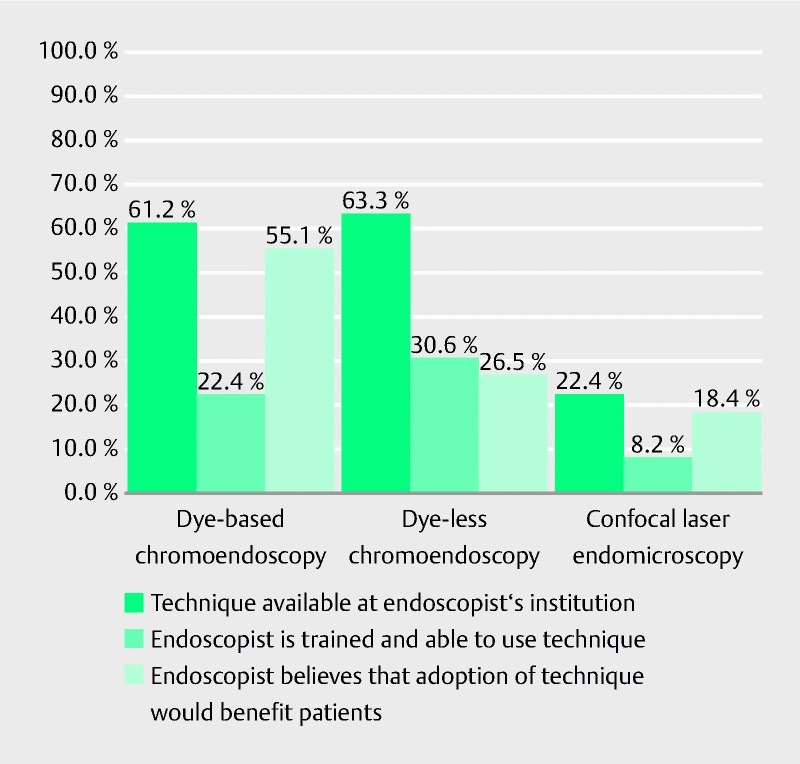
Availability of, and attitudes toward, image-enhanced endoscopy modalities.
Reported barriers to the adoption of IEE modalities are shown in Fig. 2 . Respondents reported the least barriers to adoption with dye-less chromoendoscopy, with 20.4 % reporting concerns about the length of the procedure, 30.6 % reporting concerns about cost, and 38.8 % reporting concerns about inadequate training. Over 40 % of respondents cited time restraints, cost, and inadequate training as barriers to adoption of DBC.
Fig. 2.
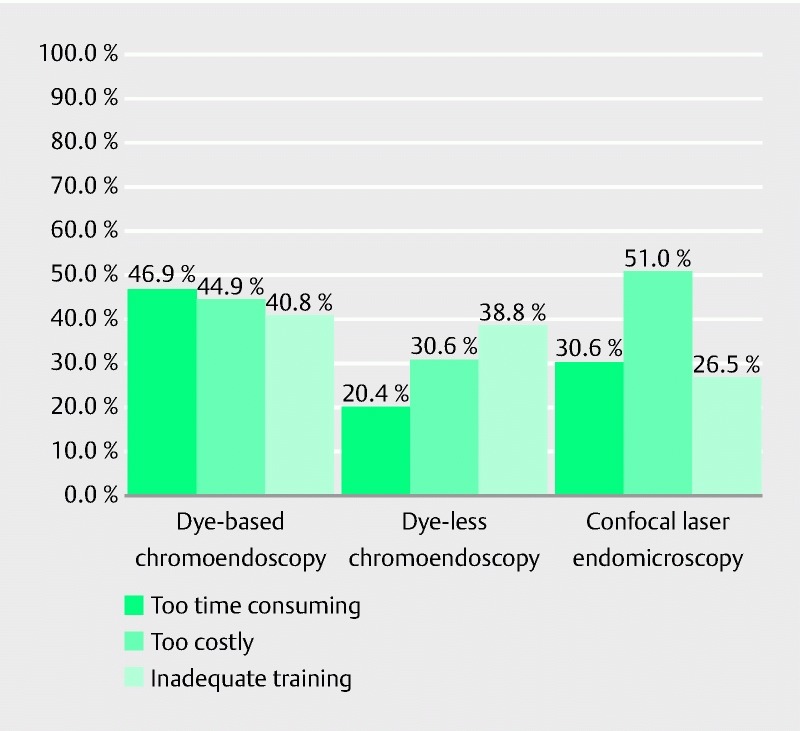
Barriers to adoption of image-enhanced endoscopy modalities.
The results of the beliefs and perceptions survey are shown in Table 2 . Whereas the majority of respondents agreed that white-light endoscopy with random biopsies is an ineffective method for dysplasia surveillance in patients with IBD, there was uncertainty about the alternatives. Less than half of respondents recognized that multiple gastroenterological societies have endorsed the use of DBC as the preferred dysplasia surveillance method, and only 44.9 % agreed that multiple randomized controlled trials have demonstrated increased dysplasia detection with use of DBC. Only 26.5 % of respondents felt that patients would receive better care if novel endoscopic techniques became available at their institution. Financial constraints (42.9 %) and inadequate training opportunities (24.5 %) were identified as barriers to adoption of novel IEE modalities.
Table 2. Gastroenterologists’ beliefs and perceptions regarding surveillance colonoscopy (n = 49).
| Statement | Agree or strongly agree, n (%) |
| My patients would receive better care if more endoscopic techniques were available at my institutions | 13 (26.5) |
| Financial constraints are the biggest barrier to bringing new endoscopic imaging modalities to my institution | 21 (42.9) |
| My institution is open to training its gastroenterologists in new endoscopic imaging modalities | 12 (24.5) |
| Multiple randomized controlled trials have shown an increase in neoplasia detection with the use of chromoendoscopy compared with random biopsies | 22 (44.9) |
| Multiple major organizations have endorsed the use of chromoendoscopy over multiple random biopsies when possible | 24 (49.0) |
| It is difficult to achieve proper bowel preparation for many of these new endoscopic imaging modalities | 8 (16.3) |
| Provincial health ministries should consider focusing efforts toward funding programs to help introduce new endoscopic screening modalities at institutions with a high volume of patients with IBD | 18 (36.7) |
| A Cochrane review demonstrated that there is no clear evidence that surveillance colonoscopy increases survival | 17 (34.7) |
| Random biopsies are an effective method of detecting neoplasia when screening patients with IBD | 4 (8.2) |
IBD, inflammatory bowel disease.
Discussion
Colonoscopy is the gold standard examination used to screen for colorectal cancer in patients without IBD. The examination relies on careful examination of the mucosa to identify visible suspicious lesion. In contrast, colonoscopy for surveillance of dysplasia in IBD has historically relied on extensive random biopsy to identify invisible lesions. Older guidelines published by the American Gastroenterological Association in 2010 recommended obtaining at least 32 random colonic biopsies 5 . Random sampling of colonic mucosa for foci of dysplasia is likely to be associated with significant sampling error, with one study estimating that 40 jumbo forceps biopsies sampled less than 0.05 % of the colonic mucosal surface area 18 . Multiple studies in patients with ulcerative colitis have further shown that only a minority of neoplastic lesions (0 to 30 % across studies) are detected via random biopsies, and that less than 0.6 % of random biopsy specimens actually identify neoplastic foci 8 10 11 .
Novel IEE methodologies ( Fig. 3 ) have been developed to address this problem. These modalities have been reviewed in detail in a recent review article 19 . The most relevant modality for patients with IBD is DBC with targeted biopsies of suspicious lesions, which has been shown to be superior to the conventional method in terms of dysplasia detection 9 11 . A recent meta-analysis demonstrated an absolute increase in the detection of dysplasia with chromoendoscopy compared with white light of 7 % (95 % confidence interval [CI] 3.2 – 11.3), with a number-needed-to-treat of 14.3 in this patient population 20 . Guidelines from several major gastroenterological societies have been published in the past year to recommend DBC using methylene blue or indigo carmine with targeted biopsies of visualized lesions as the preferred method for dysplasia surveillance. Most notably, an expert panel recently released the SCENIC guidelines (Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients: International Consensus Recommendations), which recommended a shift in the methods used for dysplasia surveillance colonoscopies performed on patients with IBD. Among the many new points in the guidelines, one of the most significant was the recommendation for the routine use of DBC as an adjunct to high definition colonoscopy. Overall, 84 % of the expert panel were in agreement with this recommendation, despite low-grade evidence. Interestingly, the panelists were unable to reach an agreement about random biopsies, with only 60 % of the panel members suggesting random biopsies were not necessary when high definition chromoendoscopy was performed 13 . In addition to the Canadian Association of Gastroenterology, the SCENIC Consensus statement has been endorsed by the American Gastroenterological Association, the American Society of Gastrointestinal Endoscopy, the Asian Pacific Association of Gastroenterology, the British Society of Gastroenterology, the European Society of Gastrointestinal Endoscopy, and the Japanese Gastroenterological Endoscopy Society.
Fig. 3.
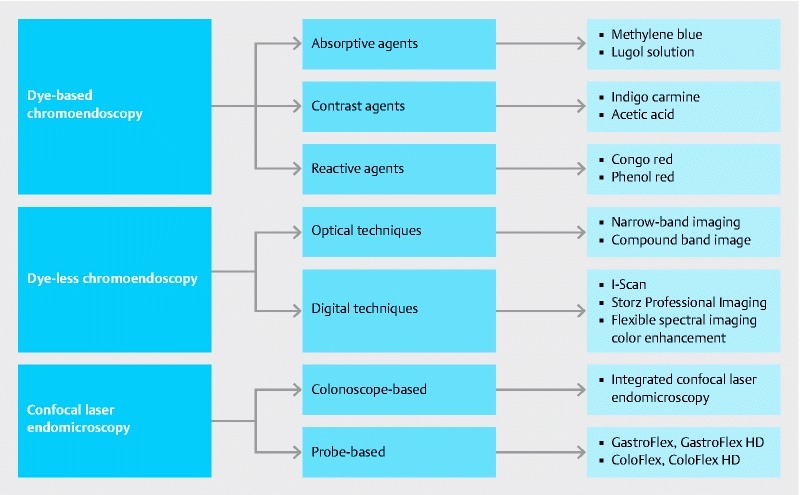
Available image-enhanced endoscopy techniques for dysplasia surveillance in patients with inflammatory bowel disease.
Apart from one retrospective study that questioned the validity of the SCENIC recommendations 21 , it is increasingly accepted that DBC is the new paradigm in dysplasia detection in patients with IBD. Despite this, barriers to uptake of this technology and other IEE modalities have been reported in the literature. Our study adds to this growing body of literature, and is the first study to describe current practices and opinions of Canadian gastroenterologists toward dysplasia surveillance in patients with IBD. Despite the existence of multiple national guidelines on this topic, we found that there was only moderate adherence to guidelines suggesting adoption of DBC, and very little uptake of other IEE modalities by survey respondents. Despite institutional availability of over 60 %, less than 30 % of respondents ever used DBC for dysplasia surveillance in IBD patients. Prolonged procedure time, limited financial resources, and lack of training were reported as being significant barriers to the adoption of newer endoscopic screening modalities, and most gastroenterologists felt that increased funding should be devoted to these areas. Notably, a majority of gastroenterologists reported not being up-to-date with the latest guidelines regarding dysplasia surveillance in patients with IBD.
Several factors may contribute to suboptimal uptake of published guidelines with respect to dysplasia surveillance in IBD. The existence of varying national recommendations potentially contributes to confusion among gastroenterologists with regard to optimal surveillance strategies 4 5 12 . Physician training and experience, as well as practice location, may also influence the extent to which surveillance guidelines are adopted 22 . Additionally, a lack of robust evidence demonstrating benefit of currently recommended approaches to dysplasia surveillance in IBD patients may give rise to skepticism among many gastroenterologists 14 15 . Despite this, it is noteworthy that a majority of respondents to our survey felt that taking random biopsies was an ineffective method of dysplasia surveillance in IBD patients.
In addition to concerns about cost, our study shows that the suboptimal adoption of DBC as a surveillance modality by Canadian gastroenterologists may relate to inadequate training and experience with this technique, and extended procedure time in inexperienced hands. Survey respondents highlighted training as a limitation to the adoption of DBC. Given the significant discrepancy between the growing evidence base supporting DBC for dysplasia surveillance and the limited use of this modality by Canadian gastroenterologists, it is important for health institutions to provide adequate resources and training opportunities to facilitate incorporation of this technique into gastroenterologists’ practices. However, in the current fiscal environment, it is unclear whether it is feasible to implement DBC into routine academic or community practice, potentially leaving IBD patients without the recommended standard of care for surveillance. It is equally important that government funding agencies and hospital administrations coordinate their efforts to ensure equal access to both community and academic institutions such that patients receive uniform care. Centralization of dysplasia surveillance in centers with expertise in DBC may be a solution to this problem.
Furthermore, an appropriate interval between surveillance colonoscopies remains unclear. Our study demonstrated that most Canadian gastroenterologists perform surveillance colonoscopy every 2 to 3 years. This is consistent with most American guidelines 5 6 . The European Crohn’s and Colitis Organisation and the British Society of Gastroenterology recommend that colonoscopy surveillance intervals should be based upon the risk of developing CRC 4 6 . These guidelines suggest that patients at low risk for CRC undergo colonoscopy every 5 years, those at intermediate risk undergo colonoscopy every 3 years, and those at highest risk undergo colonoscopy annually 23 . Recent modification to the nomenclature used for classifying dysplasia (from dysplasia-associated lesions or masses to endoscopically resectable and nonendoscopically resectable lesions), has resulted in changes to surveillance interval guidelines 24 . As these changes are implemented, further studies will be required to determine whether risk stratification is routinely adopted by Canadian gastroenterologists in assigning surveillance intervals to individual patients. One potential prospective study could assess outcomes following educational training of gastroenterologists in the latest surveillance modalities and guidelines.
In addition to DBC, narrow-band imaging (NBI) is the only IEE modality that has been studied in patients with IBD. Meta-analysis of four randomized trials showed an absolute difference of 6 % (95 %CI – 1 % to 16 %) for DBC over NBI in the proportion of patients with dysplasia 25 . The SCENIC guidelines therefore recommend that NBI should not be used in place of DBC because meaningful benefit of NBI over DBC is unlikely. Despite this, many gastroenterologists prefer NBI owing to the “push-of-a-button” nature of this technique, which makes it less time- and labor-intensive, and potentially cheaper. These opinions were mirrored by our study respondents. Other novel IEE modalities, such as confocal laser endomicroscopy, require a certain level of expertise and training. Less than 10 % of respondents in our study reported proficiency in these techniques. Furthermore, these techniques have not been studied thoroughly in patients with IBD.
There are several limitations to our study. Despite an acceptable response rate, our study only includes gastroenterologists in academic practices. Consequently, this study reflects the practices of IBD specialists who work in academic settings and not in community settings. Follow-up studies should assess the practice of community gastroenterologists, and consider comparing differences between practitioners. We did not collect data pertaining to practice geography or access to endoscopy time, which limits the generalizability of our results. Future research may consider investigating provincial disparities between these variables.
In summary, we have shown in this survey study of academic Canadian gastroenterologists that dysplasia surveillance strategies in patients with IBD still rely heavily on white-light endoscopy with random colonic biopsies. Despite institutional availability of DBC of over 60 %, only a minority of respondents reported routinely using DBC as their primary dysplasia surveillance method in patients with IBD. The major barriers to uptake of DBC were cost, time commitment, and lack of training. Improved physician education and increased access to newer endoscopy resources may improve adherence to recommendations and reduce heterogeneity in physicians’ practices, with overall improvement in the care provided to patients with IBD.
Footnotes
Competing interests None
References
- 1.Eaden J A. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein C N, Blanchard J F, Kliewer E et al. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Ekbom A, Helmick C, Zack M et al. Ulcerative colitis and colorectal cancer. A population-based study. New Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 4.Cairns S R, Scholefield J H, Steele R J et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–689. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 5.Farraye F A, Odze R D, Eaden J et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:738–745. doi: 10.1053/j.gastro.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 6.Practice Parameters Committee of the American College of Gastroenterology . Kornbluth A, Sachar D B. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–523. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 7.Leddin D, Hunt R, Champion M et al. Canadian Association of Gastroenterology and the Canadian Digestive Health Foundation: guidelines on colon cancer screening. Can J Gastroenterol. 2004;18:93–99. doi: 10.1155/2004/983459. [DOI] [PubMed] [Google Scholar]
- 8.Hurlstone D P, Sanders D S, Lobo A J et al. Indigo carmine-assisted high-magnification chromoscopic colonoscopy for the detection and characterisation of intraepithelial neoplasia in ulcerative colitis: a prospective evaluation. Endoscopy. 2005;37:1186–1192. doi: 10.1055/s-2005-921032. [DOI] [PubMed] [Google Scholar]
- 9.Kiesslich R, Fritsch J, Holtmann M et al. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880–888. doi: 10.1053/gast.2003.50146. [DOI] [PubMed] [Google Scholar]
- 10.Marion J F, Waye J D, Present D H et al. Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol. 2008;103:2342–2349. doi: 10.1111/j.1572-0241.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 11.Rutter M D, Saunders B P, Schofield G et al. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut. 2004;53:256–260. doi: 10.1136/gut.2003.016386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Annese V, Daperno M, Rutter M D et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982–1018. doi: 10.1016/j.crohns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Laine L, Kaltenbach T, Barkun Aet al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease Gastrointest Endosc 201581489–501.e426 [DOI] [PubMed] [Google Scholar]
- 14.Rutter M D, Saunders B P, Wilkinson K H et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030–1038. doi: 10.1053/j.gastro.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 15.Collins P D, Mpofu C, Watson A J et al. Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease. Cochrane Database Syst Rev. 2006;(2):CD000279. doi: 10.1002/14651858.CD000279.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Herrinton L J, Liu L, Levin T R et al. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology. 2012;143:382–389. doi: 10.1053/j.gastro.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 17.Reddy S I, Friedman S, Telford J J et al. Are patients with inflammatory bowel disease receiving optimal care? Am J Gastroenterol. 2005;100:1357–1361. doi: 10.1111/j.1572-0241.2005.40849.x. [DOI] [PubMed] [Google Scholar]
- 18.Itzkowitz S H, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634–1648. doi: 10.1053/j.gastro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 19.Tontini G E, Rath T, Neumann H. Advanced gastrointestinal endoscopic imaging for inflammatory bowel diseases. World J Gastroenterol. 2016;22:1246–1259. doi: 10.3748/wjg.v22.i3.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian V, Mannath J, Ragunath K et al. Meta-analysis: the diagnostic yield of chromoendoscopy for detecting dysplasia in patients with colonic inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:304–312. doi: 10.1111/j.1365-2036.2010.04525.x. [DOI] [PubMed] [Google Scholar]
- 21.Mooiweer E, van der Meulen-de Jong A E, Ponsioen C Y et al. Chromoendoscopy for surveillance in inflammatory bowel disease does not increase neoplasia detection compared with conventional colonoscopy with random biopsies: results from a large retrospective study. Am J Gastroenterol. 2015;110:1014–1021. doi: 10.1038/ajg.2015.63. [DOI] [PubMed] [Google Scholar]
- 22.Hudson B, Green J T, Lockett M. Updated BSG guidelines for cancer surveillance in IBD – improving consensus and changing practice? Gut. 2011;60:A203–A204. [Google Scholar]
- 23.Beaugerie L, Itzkowitz S H. Cancers complicating inflammatory bowel disease. New Engl J Med. 2015;372:1441–1452. doi: 10.1056/NEJMra1403718. [DOI] [PubMed] [Google Scholar]
- 24.Gallinger Z R, Weizman A V. Colorectal cancer in inflammatory bowel disease: a shift in risk? Expert Rev Anticancer Ther. 2014;14:847–856. doi: 10.1586/14737140.2014.895936. [DOI] [PubMed] [Google Scholar]
- 25.Efthymiou M, Allen P B, Taylor A C et al. Chromoendoscopy versus narrow band imaging for colonic surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2132–2138. doi: 10.1097/MIB.0b013e31829637b9. [DOI] [PubMed] [Google Scholar]


