Abstract
Background
Wound healing is a complex and dynamic process and a thorough knowledge of the basics of physiology of wound healing is a must to implement principles of chronic wound care. Understanding wound healing at multiple levels—biochemical, physiologic, cellular and molecular provides the surgeon with a framework for basing clinical decisions aimed at optimizing the healing response.
Objective
This review article describes the classification of wounds and aims to highlight the fundamentals of wound repair, enumerating the dressings used commonly and also, the newer concepts of wound healing.
Materials and methods
Search engines and medical databases were tapped to gather information on the subject. Search words employed were “Wounds”, “wounds in OSMF”, “Wound healing”, “Repair”, “Dressings in OMFS”.
Results
The search resulted in total of 153 articles which we reviewed to add to the literature the concepts of wound healing and to throw some light on recent advances in wound care.
Conclusions
Wound healing remains a challenging clinical problem and correct, efficient wound management is essential to positively influence the wound healing course and reduce potential complications.
Keywords: Wound, Healing, Injury, Dressings, Repair, Debridement
Introduction
The response of tissues to injury forms the foundation of surgical practice. Indeed, from a biologic viewpoint, tissue injury and its sequelae participate in a majority of general medical problems [1].
Wound healing is a complex and dynamic process with the wound environment changing with the changing health status of the individual [2]. A thorough knowledge of basics of physiology of wound healing is a must to implement the principles of chronic wound care.
Definition of a Wound
Any break in the continuity of the skin is called a wound. It has been defined as ‘disruption of normal anatomic structures and function’ [3, 4]. In everyday pathology wounds remain a challenging clinical problem, with early and late complications presenting a frequent cause of morbidity and mortality [5, 6].
Historical Aspects
In 1700 BC, Smith Papyrus described wounds for the first time. Empirically, ancient physicians of Egypt, Greece, India and Europe developed gentle methods of treating wounds including the necessity of removing foreign body, suturing, covering wounds with clean materials and protecting injured tissues from corrosive agents. During fourteenth century, with increasing frequency of bullet wounds, new era of “help wounds heal” emerged. Application of boiling oil, hot cautery, scalding water replaced gentle washing with warmed boiled water and application of mild salves. In mid-sixteenth century, Ambroise Pare, the great French army surgeon, rediscovered gentle methods of wound healing [7]. John hunter, William Stewart, Halsted, Alexis, are a few of the great clinical biologists who demonstrated that minimizing tissue injury produces rapid and effective healing [8].
Classification of Wounds
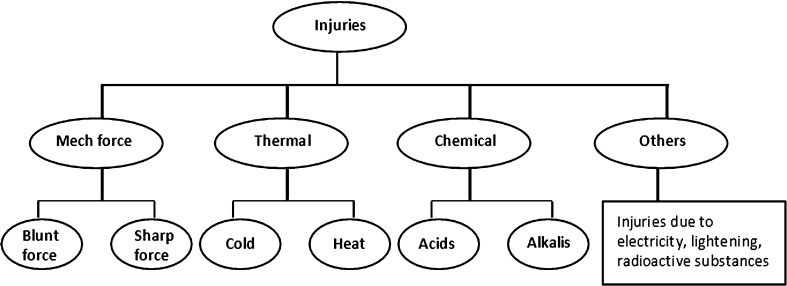
According to Mode of Injury (Fig. 1)
Fig. 1.
Classification according to mode of injury
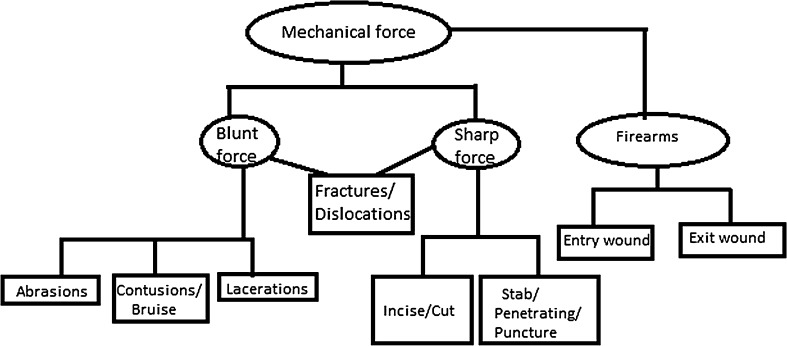
Mechanical injuries are classified with specifications (Fig. 2).
According to Trigger Factor
- Blunt Force Injuries
- Abrasions In this type of injury, the outer layer of the skin is scraped off. Examples are scratches, grazing of the skin caused by dragging.
- Contusions/Bruises This type of injury occurs when blood vessels in the skin or internal organs are ruptured. A bruise heals by destruction and removal of the extravasated blood.
- Lacerations Lacerations are tears or splits of skin, mucous membranes, muscle, internal organs produced by application of blunt force to a broad area of the body.
- Sharp Force Injuries
- Incised/Cuts Injury This type of wound is a superficial injury in which the size of the injury on the surface is larger than the depth of the injury generally made by razor blade, axe or sword.
- Stab/Penetrating/Puncture Injury This type of injury is produced from penetration of pointed/sharp instrument/weapon on to the depth of the body that is deeper than its length, caused generally by knives, broken glass bottles and tools.
-
Firearms InjuryThe injuries produced by firearms vary depending on the projectile, the muzzle velocity, distance, angle of firing and part of the body involved. These wounds are subdivided as, when a bullet, passing through a body, produces a wound at the point of entrance on the skin known as entry wound and another at the point of exit of the bullet known as exit wound. The skin edges are inverted in entry wound but everted in exit wound [9].
Fig. 2.
Classification according to mechanical force
According to Exposure to External Environment [10–12]
Closed wounds are those where the underlying tissue has been traumatized but the skin has not been severed.
Open wounds are those where the skin layer has been damaged with the underlying tissue exposed.
According to Wound Depth [13]
Partial-Thickness Wounds that involve only the epidermal layer of the skin or extend through the epidermis into the dermis with the dermis at least partially intact to generate new epidermis needed to close the wound.
Full-Thickness Wounds penetrate completely through the skin into underlying tissues and may expose adipose tissue, muscle, tendon, or bone. These heal by granulation and contraction, requiring more body resources and time.
According to Time Frame of Healing [3, 4]
Acute wounds are those that repair by themselves and proceed normally by following a timely and orderly healing pathway, with the end result of both functional and anatomical restoration.
Chronic wounds are those that fail to progress through the normal stages of healing and cannot be repaired in an orderly and timely manner.
According to Potential Risk of Infection [14]
Class I/Clean Wounds—An uninfected surgical wound in which no inflammation is encountered and the respiratory, alimentary, genital, or uninfected urinary tracts are not entered. These are primarily closed and, if necessary, drained with closed drainage.
Class II/Clean-Contaminated Wounds—A surgical wound in which the respiratory, alimentary, genital, or urinary tracts are entered under controlled conditions and without unusual contamination, no major break in technique occurs.
Class III/Contaminated Wounds—Open, fresh, accidental wounds. In addition, surgical procedures in which a major break in sterile technique occurs or there is gross spillage from the gastrointestinal tract and incisions in which acute, non-purulent inflammation is encountered are included in this category.
Class IV/Dirty or Infected Wounds—Old traumatic wounds with retained or devitalized tissue and those that involve existing clinical infection or perforated viscera. This definition suggests that the organisms causing postoperative infection were present in the wound before the surgical procedure.
Wound Healing
The healing wound is an overt expression of an intricate sequence of cellular and biochemical responses directed toward restoring tissue integrity and functional capacity following injury [14].
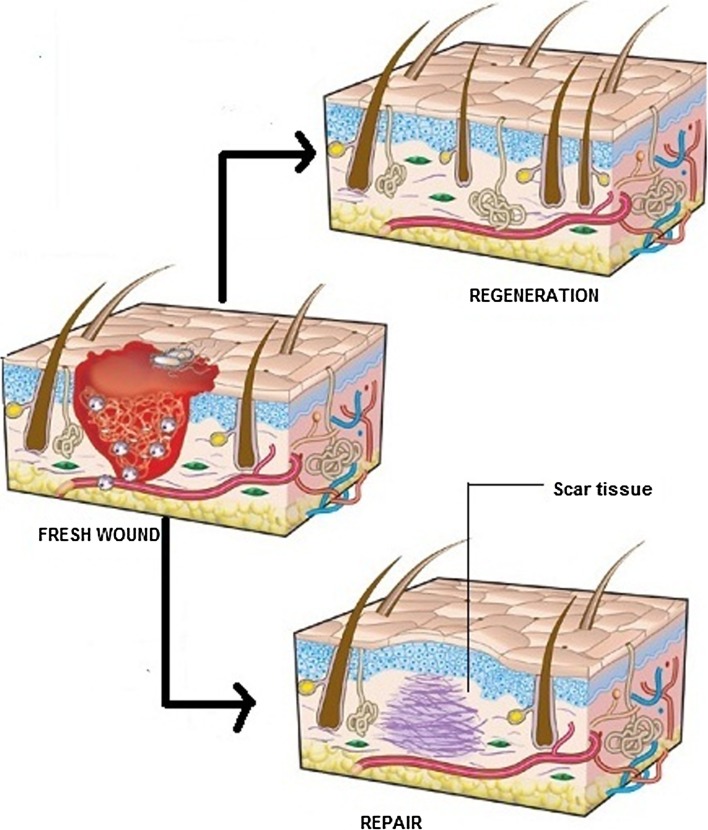
Repair Versus Regeneration (Fig. 3)
Fig. 3.
Repair versus regeneration
When restitution occurs by means of tissue that is structurally and functionally indistinguishable from native tissue, regeneration has taken place. However, if tissue integrity is re-established primarily through the formation of fibrotic scar tissue then repair has occurred [15].
Wound Healing Classification [16]
There are three categories of wound healing—primary, secondary and tertiary wound healing.
Healing by Primary Intention
The tissues approximated by surgical sutures or tapes with minimal loss of tissue are said to heal by primary union or by first intention. Such wounds heal with a clean, neat and thin scar [17]. Primary closure of wounds other than head and neck can be safely done up to a maximum of 19 h after the wound. Wounds of the face and scalp can be primarily closed whenever they are seen, as long as infection is not already present [18]. Within 24 h, neutrophils appear at the margins of incision moving toward the fibrin clot. The epidermal continuity is re-established in 24–48 h. By day 3, neutrophils largely disappear and are replaced by macrophages. By day 5, incisional space is filled with granulation tissue, neovascularisation is maximal. During 2nd week, there is continuous accumulation and proliferation of fibroblasts. By end of 1st month, scar comprises of a cellular connective tissue devoid of inflammatory infiltrate, covered by an intact epidermis.
Healing by Secondary Intention
When there is more extensive loss of cells, or surface wounds that create large defects, the reparative process is more complicated. Granulation tissue grows in from the margins to complete the repair. These wounds heal with an ugly scar [17]. This is referred to as healing by secondary intention. It differs from primary healing in several respects, i.e. in secondary healing:
Inflammatory reaction is more intense.
Much larger amounts of granulation tissue are formed.
Wound contraction is much more.
Healing by Tertiary Intention
Tertiary healing (third intention) is delayed primary wound healing after 4–6 days. This occurs when the process of secondary intention is intentionally interrupted and the wound is mechanically closed. This usually occurs after granulation tissue has formed.
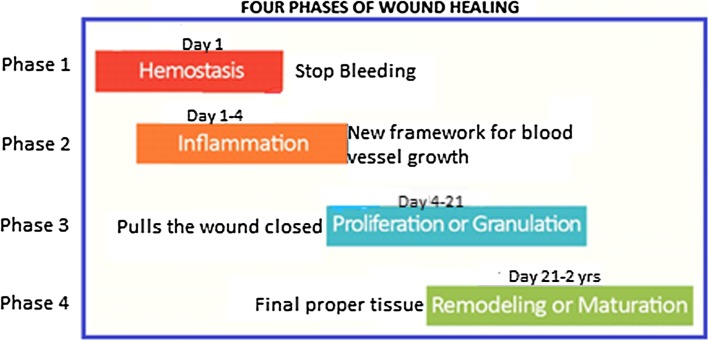
Phases of Wound Healing Cascade
Wound healing has four distinct but overlapping phases: (a) Hemostasis [19], (b) Inflammation [20, 21], (c) Proliferation and (d) Remodelling (Figs. 4, 5 [25]; Table 1 [22–24]).
Fig. 4.
Phases of wound healing
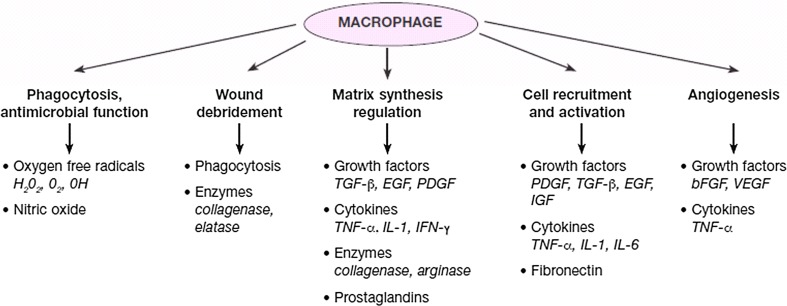
Fig. 5.
The role of macrophage in wound healing
Table 1.
Growth factor families involved in wound repair, cell sources and biological actions
| Growth factor | Cell source | Principle action(s) in wound healing |
|---|---|---|
| EGF family | ||
| EGF | Platelets | Stimulates proliferation of epithelial cells, fibroblasts and vascular endothelial cells |
| TGF-α | Platelets | Similar to EGF, more potent inducer of angiogenesis |
| Macrophages | ||
| Keratinocytes | ||
| Eosinophils | ||
| Hb-EGF | Macrophages | Mitogenic for keratinocytes |
| Amphiregulin | Keratinocytes | Mitogenic for some cells, inhibits others |
| TGF-α-family | ||
| TGF-α (there are five subunits of TGF-α, however only TGF-α1–4 are found in mammalian cells) | Macrophages | Inhibits proliferation of many cell types in vitro, including keratinocytes, endothelial cells and macrophages. May inhibit or stimulate fibroblasts |
| Lymphocytes | ||
| Fibroblasts | ||
| Keratinocytes | ||
| Platelets | ||
| PDGF family | ||
| PDGF | Fibroblasts | Attracts fibroblasts, smooth muscle cells, monocytes and neutrophils into the wound |
| Vascular endothelial cells | ||
| VEGF | Pituitary cells | Mitogen for vascular endothelial cells, stimulates angiogenesis |
| Macrophages | ||
| Keratinocytes | ||
| IGF family | ||
| IGF-1 | Fibroblasts | May promote migration of endothelial cells into the wound. Mitogenic for fibroblasts |
| Macrophages | ||
| Platelets | ||
| FGF family | ||
| aFGF and bFGF (the IGF family includes IGF-1 usually represents this family) | Macrophages | Mitogens for tissues of mesenchymal and neural origin |
| Neural tissue | ||
| Fibroblasts | ||
| Astrocytes | ||
| Endothelial cells | ||
| Bone cells | ||
| Smooth muscle | ||
| KGF | Fibroblasts | Mitogen for epithelial cells |
EGF epidermal growth factor, TGF-α transforming growth factor alpha, Hb-EGF heparin binding epidermal growth factor, VEGF vascular endothelial growth factor, IGF insulin-like growth factor, PDGF platelet derived growth factor, FGF fibroblast growth factor, aFGF acidic FGF, bFGF basic FGF, KGF keratinocyte growth factor
Factors Affecting Wound Healing [26]
Local Factors
Oxygenation
Oxygen delivery is a critical element for the healing of wounds [27–31]. Tissue oxygen tensions are found to be lower in chronic wounds in contrast to the control tissue [32]. Temporary hypoxia after injury triggers wound healing, but chronic hypoxia delays it [33, 34]. Cytokines produced in response to hypoxia are crucial promoters of cell proliferation, migration, chemotaxis and angiogenesis.
Infection
Bioburden on the wound bed which comprises of devitalised tissue, proteinaceous exudates and most specifically micro-organisms may be one of the most important barriers to wound healing. Micro-organisms can impair wound healing by an increase in pathogenic effect due to production of toxins and destructive enzymes, release of free radicals, degradation of growth factors and secretion of immune-evasive factors. Infection also causes down-regulation of immune response, consumption of local oxygen, localized thrombosis, release of vasoconstricting metabolites, interference with collagen formation and degradation of matrix metalloproteinases.
Foreign Body
It is everything that the host’s immune system views as “non self”, including bacteria, dirt, suture material, etc. Foreign body reaction is characterized by the formation of foreign body giant cells and accumulation of exudate at the site of injury, infiltration of inflammatory cells, formation of granulation and thus enhancing chronic inflammation.
Venous Insufficiency [35]
It is associated with the development of chronic wounds and delayed wound healing. It impairs wound healing by forming a barrier surrounding capillaries and decreasing effective diffusion of oxygen and nutrients from capillaries to surrounding tissue.
Systemic Factors
Age
Every phase of healing undergoes characteristic age-related changes, including enhanced platelet aggregation, increased secretion of inflammatory mediators, delayed infiltration of macrophages and lymphocytes [36], impaired macrophage function, decreased secretion of GF, delayed re-epithelialization, delayed angiogenesis and collagen deposition, reduced collagen turnover and remodeling, and decreased wound strength [37].
Sex Hormones
Compared with aged females, aged males have delayed healing of acute wounds. Female estrogens (estrone and 17β-estradiol), male androgens (testosterone and 5α-dihydrotestosterone, DHT), and their steroid precursor dehydroepiandrosterone (DHEA) have significant effects on the wound-healing process [38]. While androgens regulate cutaneous wound healing negatively, estrogen can improve the age-related impairment in healing.
Stress
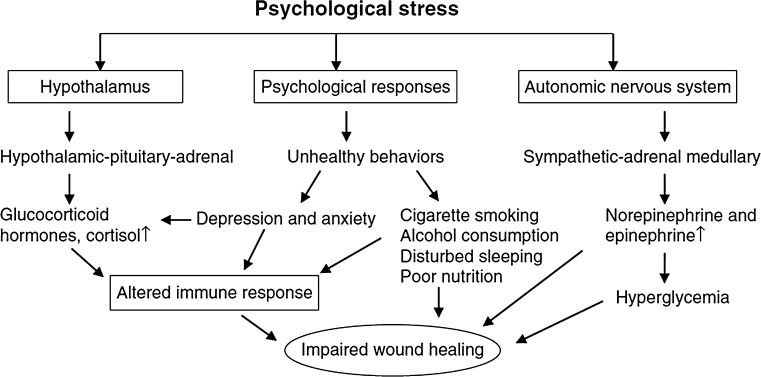
The concept that the mind is important in the treatment of illness is integral to the healing approaches of traditional Chinese and Ayurvedic medicine. Hippocrates in 400 BC recognized the moral and spiritual aspects of healing. Today, principles of mind–body medicine are incorporated in the programs of many major hospitals. The pathophysiology of stress results in the deregulation of the immune system, mediated primarily through the hypothalamic–pituitary adrenal (HPA) and sympathetic-adrenal medullary axes, which regulate the release of pituitary and adrenal hormones [39, 40]. These hormones include the adrenocorticotrophic hormones, cortisol and prolactin, and catecholamines (epinephrine and norepinephrine). Stress up-regulates glucocorticoids (GC) and reduces the levels of the proinflammatory cytokines at the wound site. Stress also reduces the expression of IL-1α and IL-8 at wound sites—both chemo-attractants necessary for the initial inflammatory phase of wound healing. Thus, psychological stress impairs normal cell mediated immunity at the wound site, causing a significant delay in the healing process (Fig. 6).
Fig. 6.
Effect of stress on wound healing
Diabetes [41–44]
Many cellular, metabolic, biochemical factors and microvascular disease contribute to altered tissue repair in diabetes mellitus. The basement membrane thickening makes leukocyte migration more difficult. This extracellular matrix deposition traps inflammatory cells and contributes to the increased tendency for infection. Hyperglycemia also leads to “advanced glycosylation end products” (AGE), which inhibit normal collagen degradation and lead to increased oxidative stress. Edema is a key factor that impairs healing in diabetes.
Thyroid Dysfunction
Thyroid hormone deficiency causes derangements of cardiovascular, pulmonary, renal and central nervous system functions, and alters drug metabolism in ways that could predispose to surgical complications. Many studies also report altered collagen synthesis in patients with thyroid dysfunction [45].
Obesity [46–56]
Several studies describe the inherent decreased vascularity of adipose tissue as leading to its associated wound complications. Obesity also induces a chronic low-grade inflammatory process (Table 2).
Table 2.
Effect of obesity on wound healing
| Local wound conditions | Associated diseases and conditions | Factors altering immune and inflammatory responses |
|---|---|---|
| 1. Decreased vascularity in adipose tissue | 1. Hard to reposition | 1. Adipokines: leptin, adiponectin, resistin |
| 2. Skin folds harbour micro-organisms | 2. Coronary heart disease | 2. Cytokines: TNF-alpha, IL-1, IL-6, IL-8, IL-10 |
| 3. Friction caused by skin on skin | 3. Atherosclerosis | 3. Chemokines: IL-8, MCP-1, IP-10 |
| 4. Increased wound tension | 4. Type 2 diabetes | |
| 5. Increased tissue pressure | 5. Cancer | |
| 6. Hematoma and seroma formation | 6. Hypertension | |
| 7. Venous hypertension | 7. Dyslipidemia | |
| 8. Stroke | ||
| 9. Respiratory problems |
MCP monocyte chemoattractant protein-1, IP-10 interferon-gamma-inducible protein 10
Medications
-
Steroids
GC inhibit wound repair via global anti-inflammatory effects and suppression of cellular wound responses, including fibroblast proliferation and collagen synthesis, causing healing with incomplete granulation tissue, reduced wound contraction and increase in the risk of wound infection [57, 58]. Topical low-dosage corticosteroid treatment of chronic wounds, however, has been found to accelerate wound healing, reduce pain and exudate, and suppress hypergranulation tissue formation in 79 % of cases [59].
-
Nonsteroidal Anti-inflammatory Drugs (NSAID)
NSAID or their selective cyclooxygenase-2 (COX-2) inhibitors, frequently used as analgesics inhibit prostaglandin E2 (PGE2) production, which might exacerbate excessive scar formation, especially when used during the later proliferative phase of wound healing [60].
-
Chemotherapeutic Drugs
Most chemotherapeutic drugs inhibit cellular metabolism, rapid cell division, and angiogenesis thus inhibiting many pathways that are critical to appropriate wound repair. These medications inhibit DNA, RNA, or protein synthesis, resulting in decreased fibroplasia and neovascularization of wounds [61]. They delay cell migration into the wound, decrease early wound matrix formation, lower collagen production, impair proliferation of fibroblasts, and inhibit contraction of wounds. In addition, these agents weaken the immune functions of the patients, and thereby increase the risk of wound infection.
Alcohol [62–66]
Alcohol exposure diminishes host resistance and ethanol intoxication at the time of injury is a risk factor for increased susceptibility to infection in the wound. The most significant impairment seems to be in wound angiogenesis, which is reduced by up to 61 % following a single ethanol exposure. Exposure to ethanol also influences the proliferative phase of healing, re-epithelialization, collagen production and wound closure.
Smoking [67–73]
Post-operatively, patients who smoke show a delay in wound healing and an increase in a variety of complications such as infection, wound rupture, anastomotic leakage, wound and flap necrosis, epidermolysis and a decrease in the tensile strength of wounds [67, 68]. Nicotine induces tissue ischemia, since it can cause decreased tissue blood flow via vasoconstrictive effects [68, 73]. It also increases blood viscosity caused by decreasing fibrinolytic activity and augmentation of platelet adhesiveness. In addition to nicotine, carbon monoxide in cigarette smoke also causes tissue hypoxia.
Nutrition
Nutrition has been recognized as a very important factor that affects wound healing.
-
Carbohydrates, Protein, and Amino Acids
Together with fats, carbohydrates are the primary source of energy in the wound-healing process. Glucose is the major source of fuel used to create the cellular ATP that provides energy for angiogenesis and deposition of the new tissues [74, 75].
Protein is one of the most important nutrient factors affecting wound healing. Its deficiency can impair capillary formation, fibroblast proliferation, proteoglycan synthesis, collagen synthesis, and wound remodelling. It can also affect the immune system and increase the susceptibility to infection [76].
Collagen synthesis requires hydroxylation of lysine and proline, and co-factors such as ferrous iron and vitamin C. Impaired wound healing results from deficiencies in any of these co-factors [77].
-
Fatty Acids
Omega-3 fatty acids have been reported to affect pro-inflammatory cytokine production, cell metabolism, gene expression, and angiogenesis in wound sites [78, 79]. They have the ability to improve the systemic immune function of the host, thus reducing infectious complications and improving survival [75].
-
Vitamins, Micronutrients and Trace Elements
Vitamins C (l-ascorbic acid), A (retinol) and E (tocopherol) show potent anti-oxidant and anti-inflammatory effects. Vitamin C deficiencies result in impaired healing and have been linked to decreased collagen synthesis and fibroblast proliferation, decreased angiogenesis and increased capillary fragility.
The biological properties of vitamin A also includes increased fibroblast proliferation, modulation of cellular differentiation and proliferation, increased collagen and hyaluronate synthesis and decreased MMP-mediated extracellular matrix degradation [80].
Vitamin E maintains and stabilizes cellular membrane integrity by providing protection against destruction by oxidation.
Several micronutrients have been shown to be important for optimal repair. Magnesium, Zinc and Copper function as co-factors for many enzymes involved in protein and collagen synthesis. Iron is required for the hydroxylation of proline and lysine, and, as a result, severe iron deficiency can result in impaired collagen production [74, 75, 81].
Wound Management
Wound Assessment [82]
The following need to be assessed and carefully recorded:
The number and location of wounds
The cause, nature and severity of any pain related to the wound. There are many causes of wound pain, which may be determined using a pain assessment scale [83].
- Tissue Types
-
(A)Necrotic Tissue—dead; non-viable
- Slough—yellow, green, grey, nonviable (necrotic) tissue, usually lighter in colour, thin, wet, stringy.
- Eschar—black, brown, dry, nonviable (necrotic) tissue, usually darker in colour, thicker and hard.
-
(B)Epithelial Tissue—deep pink to pearly pink, light purple from edges in full thickness wounds or may form islands in superficial wounds.
-
(C)Granulation Tissue—beefy red, puffy or mounded bubbly appearance.
-
(D)Hypergranulation Tissue—granulation tissue forms above the surface of the surrounding epithelium and delays epithelialization.
-
(A)
Exudate [84]
Exudate is known to assist healing by preventing the wound bed from drying out, aiding the migration of tissue repairing cells, providing essential nutrients for cell metabolism, enabling the diffusion of immune and growth factors and assisting separation of dead or damaged tissue (autolysis) (Tables 3, 4) [85].
Table 3.
Types of exudate
| Characteristic | Possible cause |
|---|---|
| Clear, amber | Serious exudate, often considered ‘normal’, but may be associated with infection by fibrinolysin-producing bacteria such as Staphylococcus aureus; may also be due to fluid from a urinary or lymphatic fistula |
| Cloudy, milky or creamy | May indicate the presence of fibrin stands (fibrinous exudate—a response to inflammation) or infection (purulent exudate containing white blood cells and bacteria) |
| Pink or Red | Due to the presence of red blood cells and indicating capillary damage (sanguineous or hemorrhagic exudate) |
| Green | May be indicative of bacterial infection, e.g. Pseudomonas aeruginosa |
| Yellow or brown | May be due to the presence of wound slough or material from an enteric or urinary fistula |
Table 4.
Transudate versus exudate
| Feature | Transudate | Exudate |
|---|---|---|
| Definition | Filtrate of blood plasma without changes in endothelial permeability | Oedema of inflamed tissue associated with increased vascular permeability |
| Character | Non-inflammatory oedema | Inflammatory oedema |
| Protein content | Low (less than 3 g/dl) | High (more than 3 g/dl) |
| Glucose | Same as in blood | Low in neoplasms and infections |
| pH | More than 7.3 | Less than 7.3 |
| Cells | Few, mainly mesothelial cells and cellular debris | Many (inflammatory as well as parenchymal) |
Color Description of Wound
RYB Classification
It is a tool to assess the characteristics of tissue in a wound bed [86].
Red—If the wound is red (colour of healthy granulation tissue), the wound is healthy and normal healing is under way.
Yellow—Colour in the wound bed may be due to a film of fibrin on the tissue. Fibrin is a sticky substance that normally acts as glue in tissue rebuilding. However if the wound is unhealthy or too dry, fibrin builds up into a layer that cannot be rinsed off and may require debridement.
Black—A black wound bed signals necrosis. Eschar covers the wound, slowing the healing process and providing microorganisms with a site to proliferate.
Table 5 describes color and description about the color in analyzing the wound [83].
Table 5.
Role of color in analyzing wounds
| Sr. no. | Wound base color | Description |
|---|---|---|
| 1. | Beefy red | Healthy tissue, good blood flow |
| 2. | Pale pink | Poor blood flow, anemia |
| 3. | Purple | Engorged, swelling, high bacteria levels, trauma |
| 4. | Black | Non viable necrotic tissue |
| 5. | Brown | Non viable necrotic tissue |
| 6. | Yellow | Non viable tissue, slough |
| 7. | Green | Infection, non viable tissue |
| 8. | White | Macerated, poor blood flow |
Wound Imaging
It is a modality that can be used to overcome both lack of objectivity and non-invasiveness in the identification, evaluation and assessment of wounds.
These methods may be divided into techniques that measure area and perimeter (2 D) and techniques that measure volumetric information (3 D) [87].
2D Methods
The first method uses a ruler (or a caliper) to measure the major and minor axes of the wound. Based on these two measurements, the area of the wound is estimated as a rectangle or as an ellipse.
In second approach a transparent film is placed over the wound and tracing the outline with a permanent marker is done. Afterwards, the film is placed on a metric grid and the area is calculated by counting the number of squared millimeters contained within the outline. This process is prone to human error.
Digital photography is a practical and low-cost imaging technique, which provides valuable information about the appearance of the tissue under study.
3D Methods
Volume of the wound can be estimated by filling it with liquid or measuring it by using a cast model. This method is highly invasive and not commonly used clinically.
DERMA provides a single and uniform interface to manage patient data, 3D scanning of the wound, and to perform different kinds of measurements and comparisons: geometric (on the 3D model) and colorimetric (on the image).
The Bates–Jensen wound assessment tool (BWAT) and pressure ulcer score of healing (PUSH) provide valuable and reliable means of assessment of pressure ulcer characteristics and prediction of wound healing [88].
This also includes tools which allow for optical characterization of wound healing in different layers of tissue. These include confocal laser scanning microscopy, multi-photon microscopy, optical coherence tomography and hyperspectral imaging [88] (Table 6).
Table 6.
Four types of wound imaging techniques
| Imaging technique | Advantages | Limitations |
|---|---|---|
| Confocal laser scanning microscopy | Controllable depth of field Elimination of out of focus data Ability to collect optical sections—3D reconstruction Portable |
Resolution limited by wavelength of light Photodamage from high intensity laser |
| Multiphoton microscopy | Deeper sectioning than confocal microscopy because infrared excitation results in less scattering Reduced photobleaching Optical section based on fluorescence |
Photodamage at focal point Low efficiency from auto fluorescence excitation |
| Optical coherence tomography | Much larger field of view than CLSM or MPM Deeper tissue regions than MPM 3-D scattering-based section |
Lower resolution than CLSM or MPM High computational demands |
| Hyperspectral imaging | Simple—analogous to taking a picture with a digital camera Quantification of wound healing—areas of different tissue types in healing wound Portable |
Can only acquire reflectance data Region of interest selection subjective |
Wound Bed Preparation
By definition wound bed preparation is ‘the management of a wound in order to accelerate endogenous healing or to facilitate the effectiveness of other therapeutic measures [89, 90].
The TIME acronym was developed in 2002 by a group of wound care experts, as a practical guide for use when managing patients with wounds. It summarizes the four main components of wound bed preparation:
- T:
Tissue management
- I:
Control of infection and inflammation
- M:
Moisture imbalance
- E:
Advancement of the epithelial edge of the wound
T: Tissue
The specific characteristics of the tissue within a wound bed play a very important role in the wound healing continuum. Where tissue is non-viable or deficient, wound healing is delayed. It also provides a focus for infection, prolongs the inflammatory response, mechanically obstructs contraction and impedes re-epithelialisation. In the majority of clinical cases there is a need to remove the devitalised tissue through a process of debridement.
Debridement
Debridement can be achieved by the following methods:
Surgical/Sharp—these are considered to be the quickest ways to debride wounds. These must be performed by a skilled practitioner using sterile, surgical instruments in an operation theatre [91].
Autolytic—it is slower than other debridement methods in which the body uses moisture to shed dead or devitalised tissue. Progress is seen within 72–96 h and black eschar changes to brown/grey in colour, eventually becoming stringy yellow [92].
Biosurgical—this involves using sterile larvae of green bottle fly to debride a wound and provides effective, rapid debridement for sloughy, smelly or infected wounds. It is used as an alternative if rapid debridement is required or wounds have not responded to autolytic debridement [93].
Mechanical—involves using either dry or wet-to-dry gauze dressings, impregnated gauze/tulle dressings or a monofilament fibre pad to remove dead tissue. This procedure is painful and also removes healthy tissue [92, 93].
Advanced Debridement Methods
I: Infection/Inflammation
All wounds contain bacteria at levels ranging from contamination, through critical colonisation, to infection. The increased bacterial burden may be confined to the superficial wound bed or may be present in the deep compartment and surrounding tissue of the wound margins. The classic signs of infection in acute wounds include pain, erythema, oedema, purulent discharge, increased heat and for chronic wounds these are delayed healing, increased exudates, bright red discoloration of granulation tissue, friable and exuberant tissue, new areas of slough, undermining and malodor and wound breakdown.
M: Moisture Imbalance
Creating a moisture balance at the wound interface is essential if wound healing is to be achieved. Exudate is produced as part of the body’s response to tissue damage and the amount of exudate produced is dependent upon the pressure gradient within the tissues. A wound which progresses through the normal wound healing cycle produces enough moisture to promote cell proliferation and supports the removal of devitalised tissue through autolysis. If, however, the wound becomes inflamed and/or stuck in the inflammatory phase of healing, exudate production increases as the blood vessels dilate.
E: Edge
When the epidermal margins of a wound fail to migrate across the wound bed or the wound edges fail to contract and reduce in size, consideration needs to have been given to the T, I, and M first to ensure that all aspects of wound bed preparation have been considered. The final stage of wound healing is epithelialisation, which is the active division, migration and maturation of epidermal cells from the wound margin across the open wound [94]. The wound bed must be full of well vascularised granulation tissue in order for the proliferating epidermal cells to migrate. This also ensures that there is adequate oxygen and nutrients to support epidermal regeneration. There are many reasons why the epidermal margin fails to migrate including hypoxia, infection, desiccation, dressing trauma, hyperkeratosis and callus at the wound margin.
Surgical Dressings [95]
Properties of an Ideal Wound Dressing [96]
Remove excessive exudate from the wound without allowing the wound to dry out thereby maintaining a moist environment.
Allow gaseous exchange so that oxygen, water vapour and carbon dioxide may pass in and out of the dressing.
Be thermally insulating so as to maintain the wound core temperature at approximately 37 °C.
Be impermeable to micro-organisms in order to minimise contamination of the wound from outside the wound itself.
Be free from either particulate or toxic contamination.
Be non-traumatic and not adhere to the wound, so that at dressing change it will not damage granulating tissue.
Classification of Wound Dressings
Functionally [97], the simplest method of classification uses the terms primary and secondary dressing.
Primary Dressing—is the first dressing placed over the wound which directly contacts it. It may provide absorptive capacity and prevents desiccation, infection, and adhesion of the dressing to the wound e.g., plain gauze, impregnated gauze.
-
Secondary Dressing—can be directly placed or placed over a primary dressing, providing further protection e.g., hydrogel dressings.
However, dressings like sofra tulle or paraffin gauze dressings can be used both as primary or secondary.
Another way to classify dressings is based on nature of origin of dressings and the mechanism of action.
- Synthetic Dressings
-
(A)Inert/Passive Dressings—These are traditional dressings, e.g., gauze and tulle dressings which produce a waterproof paraffin cover over the wound, but this may lead to maceration as the water vapour and exudation may not pass through and be trapped within the wound. These dressings are permeable to bacteria, may adhere to the wound and in some cases may cause trauma on removal. Use is limited to simple clean superficial wounds and minor burns. Absorbent pads covered with a perforated plastic film to prevent adhering to wounds are also included in this category.
-
(B)Interactive/Bioactive Dressings—They alter the wound environment and interact with the wound surface to optimize healing. They provide a moist, conducive environment for improved healing when compared with traditional passive dressings. Interactive dressings use the environment provided by the body to encourage normal healing and stimulate the healing cascade.
-
(A)
-
Biological Dressings
This includes amniotic membrane (AM) dressings, collagen membrane or skin graft, fascia. AM is the innermost layer of placenta and consists of a thick basement membrane and an avascular stromal matrix. Its properties such as immunogenicity, fluid loss controlling, pain relieving, re-epithelialisation and granulation and its stimulating, anti-inflammatory, anti-fibrotic, anti-microbial properties make it an ideal biological dressing [95] (Tables 7, 8).
Table 7.
First line bioactive dressings
| Dressing | Brand names | Indications |
|---|---|---|
| Semi-permeable | Aqua Protect Film, Bioclusive, Cutifilm | Non-absorbent; superficial burns, grazes, closed surgical incisions, small skin tears and IV sites; secondary dressing |
| Foams | Cavi-care, Curafoam, Hydrosorb | Moderate to heavily exudating, superficial and cavity wound, venous ulcers (with compression), infected ulcers, skin tears, pressure ulcers, skin grafts or donor site |
| Alginates | Algisite M, Algoderm, Comfeel | Heavily exudating leg ulcers, pressure ulcers and dehisced abdominal wounds |
| Hydrocolloids | Comfeel, DuoDerm | Light to moderately exudating wounds that would benefit from autolytic debridement. Leg ulcers, pressure ulcers, burns and donor sites |
| Hydrogels | Aquaclear, Purilon Gel, Curafil, Curagel | Absorbency is limited; best for minimally exudating or dehydrated wounds such as minor bums, grazes, lacerations, donor sites and pressure ulcers. Indications for the thicker viscosity products include protection of exposed tendon and/or bone from dehydrating and rehydrating eschar prior to debridement. The thinner viscosity products are useful for soothing burns and acute lesions such as chickenpox |
| Hydroactive | Allevya Thin, Biatain, Cutinova Hydro | Highly exudating surface and cavity wounds including leg ulcers, pressure wounds and minor burns. Useful over joints as they expand/contract without causing constriction |
Table 8.
Second line bioactive dressings
| Dressing | Brand names | Indications |
|---|---|---|
| Cadexomer iodine | Lodosorb | Venous leg ulcers, foot ulcers, and diabetic foot ulcers. Contraindicated in patients sensitive to iodine products or any thyroid pathology |
| Capillary wicking | Vacutex | Heavily exudating and infected wounds. Contraindicated in low exudating wounds within close proximity to blood vessels |
| Honey | Medihoney | May be useful in management of sloughy and septic wounds |
| Hydrofibre | Aquacel, Aquacel Ag | Heavily exudating wounds such as dehisced abdominal or pelvic wounds, chronic leg ulcers and infected wounds. Dressing frequency may be reduced depending on level of exudate |
| Hypertonic saline | Curasalt, Hypergel | Moist, necrotic, exudating infected wounds. May be effective in decreasing hypergranulation tissue |
| Interactive wet Silicone | TenderWet | Infected, sloughy and diabetic wounds |
| Silver | Acticoat, Actiocoat Absorbent | Wounds with high microbial burden and moderate to high exudates. Useful in partial and full thickness wounds (burns, donor sites) and for complementary use in infected or contaminated partial thickness wounds |
| Zinc paste | Flexidress, Gelopast | Chronic venous leg ulcers, particularly where venous eczema is present and when used in conjunction with appropriate compression bandaging |
Ancient Remedies
Urine—It is used traditionally in India for the treatment of burns and wounds. It is speculated that the wound healing activity of human urine may be due to combination of its antibacterial, antioxidant and growth promoting effects.
Honey—The use of honey as a wound dressing material, an ancient remedy has been rediscovered. Mechanisms associated with wound cleansing and healing properties of honey include decreased inflammatory edema, attraction of macrophages to further cleanse the wound, accelerated sloughing of devitalized tissue, provision of a local cellular energy source, and formation of a protective layer of protein over the wound and a healthy granulation bed. Honey also has antibacterial properties that have been attributed to its high osmolarity, acidity, and hydrogen peroxide (H2O2) content. The antibacterial factor inhibine has been isolated from honey produced from several different plant sources. In addition to its inhibine component, pure, unpasteurized, commercial honey is composed of approximately 40 % glucose, 40 % fructose, 20 % water and trace amounts of amino acids, vitamins, enzymes and minerals, all of which enhance the rate of formation of granulation tissue and epithelialization of wounds [98–100].
Herbs—India has a rich tradition of plant-based knowledge on healthcare. A large number of plants/plant extracts/decoctions or pastes are equally used by tribals and folklore traditions in India for treatment of cuts, wounds, and burns.
-
Aloe vera—Aloe vera [101–103] gel accelerates epithelialisation, neo-vascularization and increased wound contraction in the later stage of the wound healing process.
It is alleged that sap from Aloe vera eases pain and reduces inflammation. It has antiseptic and antibiotic properties which make it highly valuable in treating cuts and abrasions. It has also been commonly used to treat first and second degree burns, as well as sunburns and eczema. It is very effective on open wounds and is a promising herbal drug.
Centellaasiatica/Brahmi—Clinical studies report that, when it is applied topically thrice daily for 24 days on open wound site, the treated wound epithelized faster and the rate of wound contraction is higher as compared to control wound.
Ocimumsanctum/Tulsi—Traditionally it is used in malarial fevers, gastric disorders, hepatic infections, bronchitis, ringworm and other cutaneous diseases, earache, as a nerve tonic and to sharpen memory and also has a role in wound healing.
Turmeric—Traditionally it has been proved to have anti-inflammatory, anticancer and antiseptic properties.
Neem—Alcoholic extract of neem is useful in eczema, ringworm and scabies. Neem leaf extracts and oil from seeds has proven anti-microbial effect. Clinical studies have also revealed that neem inhibits inflammation as effectively as cortisone acetate.
Nelumbanucifera/Lotus—Significant effect is seen in respect with wound contracting activity, wound closure time, tensile strength, regeneration of tissue at the wound site and lysyl oxidase activity.
Recent Advances in Wound Care
Growth Factors
GF are naturally occurring proteins in the body which control many key cellular activities during normal tissue repair process. Macromolecules present in the wound fluid and bed trap GF within fibrin cuffs in the surrounding capillaries or bind them to the extracellular matrix [104–106]. As a result, there is deficiency of GF in the wound bed thereby affecting the healing process in chronic wounds. Studies have suggested that exogenous application of these factors to wound surface may benefit healing process [107]. GF are available commercially under the trade name of (Regranex) [108].
Skin Substitutes
Bioengineered skin substitutes both biosynthetic skin substitutes and cultured autologous engineered skin is available to provide temporary or permanent coverage with the advantages of availability in large quantities and negligible risk of infection or immunologic issues. The main limitation of these products is their expense. Biobrane is a temporary dressing composed of knitted nylon mesh bonded to a thin silicone membrane and coated with porcine polypeptides and is used in clean superficial and mid-dermal depth burns or as coverage for donor sites in split-thickness skin grafting.
Collagen
Collagen produced by fibroblasts is the most important constituent of connective tissue. Chronic wounds now treated with topical collagen products are thought to be chemotactic for fibroblasts and macrophages. They also provide a temporary scaffold to allow in-growth of tissues. They absorb wound exudates to form a soft biodegradable gel over the wound surface, which maintains wound moisture [109].
Topical Insulin
Topical insulin in combination with zinc has shown to heal wounds faster. It regulates wound inflammatory response by stimulating proliferation and migration of macrophages and keratinocytes in adjacent tissues. However, no suitable method for routine administration of insulin has been reported [110–113].
Topical Antioxidants
Effect of topical vitamin E, which is a major lipid soluble antioxidant on wound healing and scar formation remains inconclusive as some reports claim it is valuable for speeding wound healing and improving outcome of scars and other studies have shown that it has no effect [114–116].
Hyperbaric Oxygen
Hyperbaric oxygen is a treatment modality that has been used as an adjunct in wound healing. It involves placing the patient in a sealed chamber where 100 % oxygen is pressurized to between 1.5 and 3 atmospheres absolute (ATA) for 60–120 min over a course of multiple treatments. The increased atmospheric pressure increases arterial oxygen pressure (PaO2), which in turn causes vasoconstriction which promotes fluid absorption into the venous system thereby reducing edema, as well as causing an increase in hyperoxygenated plasma to the tissues. This effect typically lasts for several hours after the treatment has finished.
Lasers
Low-level laser therapy (LLLT) has been promoted for its beneficial effects on tissue healing and pain relief. However, according to the results of in vivo studies, the effectiveness of this modality varies [117].
Tissue healing, highlighted as one of the main effects of LLLT, is characterized by three main factors. First, there is an increment of ATP production, leading to a boost in mitotic activity and an increase in protein synthesis by mitochondria, resulting in greater tissue regeneration in the repair process. Second, there is a stimulus to microcirculation, which increases the delivery of nutritional elements associated with increased speed of mitosis, facilitating cell multiplication. Finally, new vessels are formed from pre-existing vessels facilitating wound healing [117–120].
Electrical Stimulation (ES) [121]
The rationale for use of this method is based on the fact that the human body has an endogenous bioelectric system that enhances healing of bone fractures and soft-tissue wounds. When the body’s endogenous bioelectric system fails and cannot contribute to wound repair processes, therapeutic levels of electrical current may be delivered into the wound tissue from an external source. Neutrophil, macrophage, fibroblast and epidermal cells involved in wound repair carry either a positive or negative charge. When these cells are needed to contribute to autolysis, granulation tissue formation, anti-inflammatory activities, or epidermal resurfacing, ES may facilitate galvanotaxic attraction of these cells into the wound tissue and thereby accelerate healing.
Negative Pressure Therapy Devices [122]
Controlled negative pressure devices promote vacuum-assisted wound closure. Negative pressure applies non-compressive mechanical forces to the tissue, which allows arterioles to dilate and increases blood flow. Negative pressure is achieved in the wound by positioning a suction tube into the foam dressing and connecting this to a pump. The most widely used is the vacuum assisted closure (VAC), which is portable and comes in different sizes allowing use on different parts of the body.
Tissue Glues [95]
Cyanoacrylate—It is used for skin closure, but it requires perfect hemostasis. They have the advantage of being quick and easy to use.
Fibrin Tissue Glue—It works on the conversion of fibrinogen by thrombin to fibrin. It has been used for neurosurgery in dural tears, in ear, nose and throat and ophthalmic surgery to attach implants.
Ligating Clips [95]
Clearon—These are woven polypropylene delnet tape coated with hypoallergenic adhesive mass. It is extremely porous, ensuring adequate wound ventilation. Being transparent, progress of wound healing can be checked.
Ethistrips—It is a woven rayon acetate tape coated on one side with a hypoallergenic adhesive mass. These are used to buttress the suture and prevent spreading of scar.
Nanoscale Particle Therapy [123]
Nanobiotechnology has arisen from the convergence of engineering and molecular biology leading to development of structures, devices and systems in the atomic, molecular or macromolecular size range. The potential of nanotechnology has been well known since 1959 when Nobel Laureate Richard Feynman predicted the emergence of a new science that deals with structures on a scale of 1–100 nm. Several advantages are offered by small size, such as the ability to enter into the cytoplasmic space like Trojan horses, ferrying nanoparticles across cellular barriers and activating specific endocytic and transcytic transport mechanisms. The nanoparticles have a particle size of 130–300 nm, with a positive surface charge and stability at pH 1.2–2.5. They possess antibiotic properties against gram + and gram − bacteria. Examples include nanoparticles bearing Vancomycin or N-methylthiolated β lactams, silver based nanoparticles and nitric oxide delivery nanoparticles.
Commonly Used Agents in Oral and Maxillofacial Practice
Whitehead’s Varnish
Whitehead’s varnish was first described by Walter Whitehead in 1891 on its use following tongue resection [117]. The composition that is currently used is iodoform 10 g, benzoic acid 10 g, storax 7.5 g, balsam of tolu 5 g and ether 100 ml. Iodoform (tri-iodomethane) is an antiseptic, while benzoic acid is a preservative and disinfectant. Storax and balsam of tolu are resins that are used in the perfume industry [124–126].
Whitehead’s varnish prevents capillary oozing, provides postoperative pain relief and allows the patient to be fed orally. It has been used as a dressing for skin graft donor sites, as a pack for cystic cavities of the jaw, to reduce pain following wisdom tooth removal, in orbital floor reconstruction, in cleft palate surgery, and with the surgical management of osteomyelitis [125–132].
Bismuth Iodoform Paste
Bismuth iodoform paraffin paste (BIPP) was first described by Rutherford Morison in 1917 [132]. The formulation is—iodoform 440 g, bismuth subnitrate 220 g and paraffin base 220 g. It is used as a wound packing and dressing material. It can be used in those at risk of dry socket, to prevent infection after the reduction of nasal fractures, in the treatment of epistaxis and as a dressing after ear surgery [133, 134]. Bismuth toxicity, leading to neurological impairment, has been reported and it should be applied sparingly to open wounds as a result [135–137].
Ozone Therapy (O3)
Ozone therapy has been utilized and heavily studied for more than a century. Its effects are proven, consistent, safe and with minimal and preventable side effects. Medical O3 is used to disinfect and treat disease. Mechanism of action is by inactivation of bacteria, viruses, fungi, yeast and protozoa, stimulation of oxygen metabolism, activation of the immune system. In local applications as in the treatment of external wounds, its application in the form of a transcutaneous O3 gas bath has established itself as being the most practical and useful method. Ozonized water, which is particularly known in dental medicine, is optimally applied as a spray or compress [138].
Zinc Oxide Based Dressings
Zinc oxide can be combined with other materials to form a paste or cement, which is used to cover the gingival tissues or extraction sockets. These materials function to provide a physical barrier against the entry of food or other materials. They may be divided into eugenol-containing (e.g. kalzinol) and non-eugenol-containing (e.g. coe-pak) materials. It is associated with contact allergy at low doses and cytotoxicity at high doses. However, non-eugenol zinc oxide-based materials avoid the potential for eugenol related cytotoxicity and allergy [139].
Iodoform
First prepared in 1822, iodoform, also called triiodomethane is a yellow, crystalline solid belonging to the family of organic halogen compounds, used as an antiseptic component of medications for minor skin wounds [140]. It is manufactured by electrolysis of aqueous solutions containing acetone, inorganic iodides, and sodium carbonate. It is used as a disinfectant and as a healing and antiseptic dressing for wounds and sores.
Sofra Tulle
Sofra-Tulle consists of a cotton leno-weave fabric, impregnated with a base composed of white soft paraffin, anhydrous lanolin, and 1.0 % w/w framycetin sulphate. Framycetin is an antibiotic of the aminoglycoside group with a wide spectrum of antibacterial activity. Organisms sensitive to framycetin include Staphylococcus aureus, Escherichia coli, Klebsiella species, and some strains of Pseudomonas aeruginosa. It is not active against yeasts, fungi or viruses. The dressing is used as a primary wound contact layer in the management of infected wounds, combining low adherence with antimicrobial activity. It is contraindicated in patients who are allergic to lanolin, or who have previously demonstrated a sensitivity to framycetin, neomycin, or any other chemically related product.
Complications of Wound Healing
Dehiscence [141]
Dehiscence is a mechanical failure of wound healing in which local factors play a very important role. For example, infection predisposes a wound to disruption in the early postoperative period. The persistent presence of micro-organisms leads to an increased number of phagocytes that release proteolytic enzymes, free radicals and inflammatory mediators. The effect of these substances is additional tissue injury and wound deterioration. Chronic tissue hypoxia also leads to collagen with inadequate tensile strength which contributes to wound dehiscence. The management of wound dehiscence ranges from simple dressing to further surgery for wherein the wound is laid open, is washed daily and dressing done along with intravenous antibiotics according to culture and sensitivity [142].
Hypertrophic Scars and Keloids
Hypertrophic scars and keloids are the result of an exaggerated response of the skin following injury. These proliferative scars are more common in darker skin types and are characterized by increased collagen, elastin, fibronectin and glycosaminoglycan contents, as well as low collagenase activity [143–147].
Hypertrophic scars are raised and stay within the boundaries of the original wound, usually regressing spontaneously. Keloids also are raised but spread beyond the original wound boundaries, invading the surrounding skin; they also may continue to grow over time and often recur following excision. Hypertrophic scars usually are more responsive to different treatment modalities than keloids [148, 149]. These can be managed by using corticosteroids (softens and flattens scars), topical silicone gel, pressure (in the early phase of hypertrophic scar formation and as prophylaxis in keloid prone patients or keloid prone regions) and surgery which ranges from simple excision and primary closure, Z- or W-plasty to hide the scar within the skin creases (relaxed skin tension lines) and skin graft for wound closure with minimal tension [150].
Infection [151]
Pathogenic organisms causing surgical wound infections vary according to the anatomical site of surgery. Antibiotic resistant organisms, such as methicillin resistant Staphylococcus aureus (MRSA), are more commonly encountered, reflecting the hospital flora. The challenge clinically and microbiologically is to identify those wounds in which healing is impaired as a result of infection or heavy bacterial burden and in which systemic or topical antimicrobial treatment will be of benefit. Staphylococci and streptococci are the most commonly encountered pathogenic organisms in community acquired superficial wounds. Infected wounds are managed by wound toilet and surgical debridement along with antibiotic course.
Scar Contracture [152]
The development of contractures is by definition the pathologic shortening of scar tissue, resulting in deformities as opposed to wound contraction, which occurs in an open wound with the positive outcome of reducing the wound surface area. These disorders represent aberrations in the fundamental processes of wound healing, which include cell migration and proliferation, inflammation, increased synthesis and secretion of cytokines and extracellular matrix (ECM) proteins, and remodelling of the newly synthesized matrix. Scar contracture can be managed through massaging the area locally.
Implantation (Epidermal) Cyst
It may occur due to persistence of epithelial cells in the wound after healing. Asymptomatic epidermoid cysts do not need treatment. Inflammation can be resolved by intralesional injection with triamcinolone. Usual surgical approach is simple excision. Incision and drainage may be performed in an infected cyst.
Pigmentation
Healed wounds may at times have rust-like colour due to staining with haemosiderin. Some coloured particulate material left in the wound may persist and impart colour to the healed wound. Management includes photoavoidance and photoprotection, use of photodynamic therapy and lasers, use of topical agents like hydroquinone 4 %, retinoic acid cream 0.1 % or chemical peel with 20–30 % salicylic acid [153].
Deficient Scar Formation
This may occur due to inadequate formation of granulation tissue.
Conclusion
Wound healing remains a challenging clinical problem and correct, efficient wound management is essential as it involves multiple cell populations, the extracellular matrix and the action of soluble mediators such as growth factors and cytokines. Correct clinical management may positively influence the wound healing course and reduce potential complications.
Compliance with Ethical Standards
Conflict of Interest
Shruti Chhabra, Naveen Chhabra, Avneet Kaur and Niti Gupta declare that they have no conflict of interest.
Ethics Statement/Confirmation of Patient Permission
This article does not contain any studies with human/animal participants performed by any of the authors.
Contributor Information
Shruti Chhabra, Email: drshruti75@gmail.com.
Naveen Chhabra, Email: naveenprisha@yahoo.co.in.
Avneet Kaur, Email: avneetsoni7@gmail.com.
Niti Gupta, Email: neeti0@gmail.com.
References
- 1.Mehra R. Historical survey of wound healing. Bull Indian Inst Hist Med Hyderabad. 2002;32(2):159–175. [PubMed] [Google Scholar]
- 2.Kerstein MD. The scientific basis of healing. Adv Wound Care. 1997;10(3):30–36. [PubMed] [Google Scholar]
- 3.Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Pecoraro RE, Rodeheaver G, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994;130:489–493. doi: 10.1001/archderm.1994.01690040093015. [DOI] [PubMed] [Google Scholar]
- 4.Robson MC, Steed DL, Franz MG. Wound healing: biologic features and approaches to maximize healing trajectories. Curr Probl Surg. 2001;38:72–140. doi: 10.1016/S0011-3840(01)70035-4. [DOI] [PubMed] [Google Scholar]
- 5.Natarajan S, Williamson D, Stiltz AJ, Harding K. Advances in wound care and healing technology. Am J Clin Dermatol. 2000;1:269–275. doi: 10.2165/00128071-200001050-00002. [DOI] [PubMed] [Google Scholar]
- 6.Alonso JE, Lee J, Burgess AR, Browner BD. The management of complex orthopaedic injuries. Surg Clin N Am. 1996;76:879–903. doi: 10.1016/S0039-6109(05)70486-2. [DOI] [PubMed] [Google Scholar]
- 7.Traversa B, Sussman G. The role of growth factors, cytokines and proteases in wound management. Primary Intent. 2001;9(4):161–167. [Google Scholar]
- 8.Sabiston DC. Chap 10: Wound healing: biologic and clinical features. In: Sabiston DC, editor. Textbook of surgery. 14. Philadelphia: WB Saunders; 1991. [Google Scholar]
- 9.Sharma M, Khajja BS, Jha S, Mathur GK, Mathur VN. Forensic interpretation of injuries/wounds found on the human body. J Punjab Acad Forensic Med Toxicol. 2011;11(2):105–109. [Google Scholar]
- 10.Bischoff M, Kinzl L, Schmelz A. The complicated wound. Unfallchirurg. 1999;102:797–804. doi: 10.1007/s001130050483. [DOI] [PubMed] [Google Scholar]
- 11.Degreef H. How to heal a wound fast. Dermatol Clin. 1998;16:365–375. doi: 10.1016/S0733-8635(05)70019-X. [DOI] [PubMed] [Google Scholar]
- 12.Attinger CE, Janis JE, Steinberg J, Schwartz J, Al-Attar A, Couch K. Clinical approach to wound debridement and wound bed preparation including the use of dressings and wound healing adjuvants. Plast Reconstr Surg. 2006;117(7 Suppl):72S–109S. doi: 10.1097/01.prs.0000225470.42514.8f. [DOI] [PubMed] [Google Scholar]
- 13.CCHCS care guide: wound and skin ulcer management. Nov 2012
- 14.Chard R. Wound classifications. Clinical issues. AORN J. 2008;88(1):108–109. [Google Scholar]
- 15.Miloro M, Ghali GE, Larsen PE, Waite PD. Chapter 1 Wound healing. In: Shetty V, Bertolami CN, editors. Peterson’s principles of oral and maxillofacial surgery. 2. Hamilton: BC Decker; 2004. [Google Scholar]
- 16.Kumar V, Abbas AK, Fausto N. Robbins and Cotron Pathologic basis of disease. 7. Philadelphia, PA: Saunders; 2004. [Google Scholar]
- 17.Rajagopal KS. Manipal manual of surgery. 2. New Delhi: CBS Pullisher; 2008. [Google Scholar]
- 18.Peterson AS. The “golden period” for wound repair. J Lanc Gen Hosp. 2010;5(4):134–135. [Google Scholar]
- 19.Diegelmann RF, Evans MC. Wound healing: an overview of acute fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 20.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 21.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 22.McGrath MH. Peptide growth factors and wound healing. Clin Plast Surg. 1990;17:421–432. [PubMed] [Google Scholar]
- 23.Steed D. The role of growth factors in wound healing. Surg Clin N Am. 1997;77(3):575–586. doi: 10.1016/S0039-6109(05)70569-7. [DOI] [PubMed] [Google Scholar]
- 24.Stadlemann W, Digenis A, Tobin G. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg. 1998;176(Suppl 2A):26S–38S. doi: 10.1016/S0002-9610(98)00183-4. [DOI] [PubMed] [Google Scholar]
- 25.Witte MB, Barbul A. General principles of wound healing. Surg Clin N Am. 1997;77(3):509–528. doi: 10.1016/S0039-6109(05)70566-1. [DOI] [PubMed] [Google Scholar]
- 26.Guo S, DiPietro LA. Factors affecting wound healing: critical reviews in oral biology and medicine. J Dent Res. 2010;89(3):219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonsson K, Jensen JA, Goodson WH, 3rd, Scheuenstuhl H, West J, Hopf HW, et al. Tissue oxygenation, anemia and perfusion in relation to wound healing in surgical patients. Ann Surg. 1991;214(5):605–613. doi: 10.1097/00000658-199111000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavan FB, Hunt TK. Oxygen and wound healing. Clin Plast Surg. 1990;17:463–472. [PubMed] [Google Scholar]
- 29.Wu L, Xia YP, Roth SI, Gruskin E, Mustoe TA. Transforming growth factor-beta1 fails to stimulate wound healing and impairs its signal transduction in an aged ischemic ulcer model: importance of oxygen and age. Am J Pathol. 1999;154(1):301–309. doi: 10.1016/S0002-9440(10)65276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt TK, Hopf HW. Wound healing and wound infection. What surgeons and anesthesiologists can do. Surg Clin N Am. 1997;77:587–606. doi: 10.1016/S0039-6109(05)70570-3. [DOI] [PubMed] [Google Scholar]
- 31.Suh DY, Hunt TK. Time line of wound healing. Clin Podiatr Med Surg. 1998;15:1–9. [PubMed] [Google Scholar]
- 32.Tandara AA, Mustoe TA. Oxygen in wound healing— more than a nutrient. World J Surg. 2004;28:294–300. doi: 10.1007/s00268-003-7400-2. [DOI] [PubMed] [Google Scholar]
- 33.Bishop A. Role of oxygen in wound healing. J Wound Care. 2008;17:399–402. doi: 10.12968/jowc.2008.17.9.30937. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez PG, Felix FN, Woodley DT, Shim EK. The role of oxygen in wound healing: a review of the literature. Dermatol Surg. 2008;34:1159–1169. doi: 10.1111/j.1524-4725.2008.34254.x. [DOI] [PubMed] [Google Scholar]
- 35.Grey JE, Harding KG, Enoch S. Venous and arterial leg ulcers. BMJ. 2006;332(7537):347–350. doi: 10.1136/bmj.332.7537.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol. 2001;117:1027–1035. doi: 10.1046/j.0022-202x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- 37.Minimas DA. Ageing and its influence on wound healing. Wounds UK. 2007;3(1):42–50. [Google Scholar]
- 38.Gilliver SC, Ashworth JJ, Ashcroft GS. The hormonal regulation of cutaneous wound healing. Clin Dermatol. 2007;25:56–62. doi: 10.1016/j.clindermatol.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Godbout JP, Glaser R. Stress-induced immune dysregulation: implications for wound healing, infectious disease and cancer. J Neuroimmune Pharmacol. 2006;1:421–427. doi: 10.1007/s11481-006-9036-0. [DOI] [PubMed] [Google Scholar]
- 40.Boyapati L, Wang HL. The role of stress in periodontal disease and wound healing. Periodontology. 2007;2000(44):195–210. doi: 10.1111/j.1600-0757.2007.00211.x. [DOI] [PubMed] [Google Scholar]
- 41.Goodson WH, Hunt TK. Wound healing and the diabetic patient. Surg Gynecol Obstet. 1979;149:600–608. [PubMed] [Google Scholar]
- 42.Kamal K, Powell RJ, Sumpio BE. The pathobiology of diabetes mellitus: implications for surgeons. J Am Coll Surg. 1996;183:271–289. [PubMed] [Google Scholar]
- 43.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 44.Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, et al. Enhanced cellular oxidant stress by the interaction of advanced glycosylation end products with their receptors/binding proteins. J Biol Chem. 1994;269(13):9889–9897. [PubMed] [Google Scholar]
- 45.Kivirikko KI, Laitinen O, Aer J, Halme J. Metabolism of collagen in experimental hyperthyroidism and hypothyroidism in the rat. Endocrinology. 1967;80:1051–1061. doi: 10.1210/endo-80-6-1051. [DOI] [PubMed] [Google Scholar]
- 46.Coutinho W. Latin America consensus on obesity. Arq Bras Endocrinol Metabol. 1999;43:21–67. [Google Scholar]
- 47.Pitanguy I, De Amorim NFG, Radwanski HN. Contour surgery in the patient with great weight loss. Aesthet Plast Surg. 2000;24(6):406–411. doi: 10.1007/s002660010068. [DOI] [PubMed] [Google Scholar]
- 48.Rosen JC, Orosan P, Reiter J. Cognitive behavior therapy for negative body image in obese women. Behav Ther. 1995;26(1):25–42. doi: 10.1016/S0005-7894(05)80081-4. [DOI] [Google Scholar]
- 49.Postlethwait RW, Johnson WD. Complications following surgery for duodenal ulcer in obese patients. Arch Surg. 1972;105(3):438–440. doi: 10.1001/archsurg.1972.04180090043011. [DOI] [PubMed] [Google Scholar]
- 50.Printen KJ, Paulk SC, Mason EE. Acute postoperative wound complications after gastric surgery for morbid obesity. Am Surg. 1975;41(8):483–485. [PubMed] [Google Scholar]
- 51.Matory WE, O’Sullivan J, Fudem G, Dunn R. Abdominal surgery in patients with severe morbid obesity. Plast Reconstr Surg. 1994;94(7):976–987. doi: 10.1097/00006534-199412000-00011. [DOI] [PubMed] [Google Scholar]
- 52.Abdel-Moneim RI. The hazards of surgery in the obese. Int Surg. 1985;70(2):101–103. [PubMed] [Google Scholar]
- 53.Carlson MA. Acute wound failure. Surg Clin N Am. 1997;77(3):607–636. doi: 10.1016/S0039-6109(05)70571-5. [DOI] [PubMed] [Google Scholar]
- 54.Yale CE. Gastric surgery for morbid obesity. Complications and long-term weight control. Arch Surg. 1989;124(8):941–946. doi: 10.1001/archsurg.1989.01410080077012. [DOI] [PubMed] [Google Scholar]
- 55.Thompson WR, Ameral JF, Caldwell MD. Complications and weight loss in 150 consecutive gastric exclusion patients. Critical review. Am J Surg. 1983;146(5):602–612. doi: 10.1016/0002-9610(83)90296-9. [DOI] [PubMed] [Google Scholar]
- 56.Grazer FM, Goldwyn RM. Abdominoplasty assessed by survey, with emphasis on complications. Plast Reconstr Surg. 1977;59(4):513–517. doi: 10.1097/00006534-197759040-00006. [DOI] [PubMed] [Google Scholar]
- 57.Franz MG, Steed DL, Robson MC. Optimizing healing of the acute wound by minimizing complications. Curr Probl Surg. 2007;44:691–763. doi: 10.1067/j.cpsurg.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Wagner AE, Huck G, Stiehl DP, Jelkmann W, Hellwig-Burgel T. Dexamethasone impairs hypoxia-inducible factor-1 function. Biochem Biophys Res Commun. 2008;372:336–340. doi: 10.1016/j.bbrc.2008.05.061. [DOI] [PubMed] [Google Scholar]
- 59.Hofman D, Moore K, Cooper R, Eagle M, Cooper S. Use of topical corticosteroids on chronic leg ulcers. J Wound Care. 2007;16:227–230. doi: 10.12968/jowc.2007.16.5.27047. [DOI] [PubMed] [Google Scholar]
- 60.Su WH, Cheng MH, Lee WL, Tsou TS, Chang WH, Chen CS, et al. Nonsteroidal anti inflammatory drugs for wounds: pain relief or excessive scar formation? Mediat Inflamm. 2010 doi: 10.1155/2010/413238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waldron DR, Zimmerman-Pope N. Superficial skin wounds. In: Slatter DH, editor. Textbook of small animal surgery. New York: Saunders; 2003. pp. 260–271. [Google Scholar]
- 62.Choudhry MA, Chaudry IH. Alcohol intoxication and post burn complications. Front Biosci. 2006;11:998–1005. doi: 10.2741/1857. [DOI] [PubMed] [Google Scholar]
- 63.Radek KA, Matthies AM, Burns AL, Heinrich SA, Kovacs EJ, Dipietro LA. Acute ethanol exposure impairs angiogenesis and the proliferative phase of wound healing. Am J Physiol Heart Circ Physiol. 2005;289:H1084–H1090. doi: 10.1152/ajpheart.00080.2005. [DOI] [PubMed] [Google Scholar]
- 64.Radek KA, Kovacs EJ, DiPietro LA. Matrix proteolytic activity during wound healing: modulation by acute ethanol exposure. Alcohol Clin Exp Res. 2007;31:1045–1052. doi: 10.1111/j.1530-0277.2007.00386.x. [DOI] [PubMed] [Google Scholar]
- 65.Radek KA, Kovacs EJ, Gallo RL, DiPietro LA. Acute ethanol exposure disrupts VEGF receptor cell signaling in endothelial cells. Am J Physiol Heart Circ Physiol. 2008;295:H174–H184. doi: 10.1152/ajpheart.00699.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fitzgerald DJ, Radek KA, Chaar M, Faunce DE, DiPietro LA, Kovacs EJ. Effects of acute ethanol exposure on the early inflammatory response after excisional injury. Alcohol Clin Exp Res. 2007;31:317–323. doi: 10.1111/j.1530-0277.2006.00307.x. [DOI] [PubMed] [Google Scholar]
- 67.Chan LK, Withey S, Butler PE. Smoking and wound healing problems in reduction mammaplasty: is the introduction of urine nicotine testing justified? Ann Plast Surg. 2006;56:111–115. doi: 10.1097/01.sap.0000197635.26473.a2. [DOI] [PubMed] [Google Scholar]
- 68.Ahn C, Mulligan P, Salcido RS. Smoking—the bane of wound healing: biomedical interventions and social influences. Adv Skin Wound Care. 2008;21:227–238. doi: 10.1097/01.ASW.0000305440.62402.43. [DOI] [PubMed] [Google Scholar]
- 69.Levin L, Schwartz AD. The effect of cigarette smoking on dental implants and related surgery. Implant Dent. 2005;14:357–361. doi: 10.1097/01.id.0000187956.59276.f8. [DOI] [PubMed] [Google Scholar]
- 70.Balaji SM. Tobacco smoking and surgical healing of oral tissues: a review. Indian J Dent Res. 2008;19:344–348. doi: 10.4103/0970-9290.44540. [DOI] [PubMed] [Google Scholar]
- 71.Siana JE, Rex S, Gottrup F. The effect of cigarette smoking on wound healing. Scand J Plast Reconstr Surg Hand Surg. 1989;23:207–209. doi: 10.3109/02844318909075119. [DOI] [PubMed] [Google Scholar]
- 72.Goldminz D, Bennett RG. Cigarette smoking and flap and full thickness graft necrosis. Arch Dermatol. 1991;127:1012–1015. doi: 10.1001/archderm.1991.01680060086009. [DOI] [PubMed] [Google Scholar]
- 73.Sorensen LT, Jorgensen S, Petersen LJ, Hemmingsen U, Bulow J, Loft S, et al. Acute effects of nicotine and smoking on blood flow, tissue oxygen, and aerobe metabolism of the skin and subcutis. J Surg Res. 2009;152:224–230. doi: 10.1016/j.jss.2008.02.066. [DOI] [PubMed] [Google Scholar]
- 74.Shepherd AA. Nutrition for optimum wound healing. Nurs Stand. 2003;18:55–58. [PubMed] [Google Scholar]
- 75.Arnold M, Barbul A. Nutrition and wound healing. Plast Reconstr Surg. 2006;117(7 Suppl):42S–58S. doi: 10.1097/01.prs.0000225432.17501.6c. [DOI] [PubMed] [Google Scholar]
- 76.Gogia PP. Physiology of wound healing. In: Gogia PP, editor. Clinical wound management. Slack: Thorofare; 1995. pp. 8–12. [Google Scholar]
- 77.Lanman TH, Ingalls TH. Vitamin C deficiency and wound healing: an experimental and clinical study. Ann Surg. 1937;105(4):616–625. doi: 10.1097/00000658-193704000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McDaniel JC, Belury M, Ahijevych K, Blakely W. Omega-3 fatty acid’s effect on wound healing. Wound Repair Regen. 2008;16:337–345. doi: 10.1111/j.1524-475X.2008.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shingel KI, Faure MP, Azoulay L, Roberge C, Deckelbaum RJ. Solid emulsion gel as a vehicle for delivery of polyunsaturated fatty acids: implications for tissue repair, dermal angiogenesis and wound healing. J Tissue Eng Regen Med. 2008;2:383–393. doi: 10.1002/term.101. [DOI] [PubMed] [Google Scholar]
- 80.Burgess C. Topical vitamins. J Drugs Dermatol. 2008;7(7 Suppl):s2–s6. [PubMed] [Google Scholar]
- 81.Campos AC, Groth AK, Branco AB. Assessment and nutritional aspects of wound healing. Curr Opin Clin Nutr Metab Care. 2008;11:281–288. doi: 10.1097/MCO.0b013e3282fbd35a. [DOI] [PubMed] [Google Scholar]
- 82.Grey JE, Enoch S, Harding KG. Wound assessment. BMJ. 2006;332(7536):285–288. doi: 10.1136/bmj.332.7536.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kumar KS, Reddy BE. Wound image analysis classifier for efficient tracking of wound healing status. Signal Image Process Int J. 2014;5(2):15–27. doi: 10.5121/sipij.2014.5202. [DOI] [Google Scholar]
- 84.World Union of Wound Healing Societies . Principles of best practice: wound exudate and the role of dressings. A consensus document. London: MEP Ltd; 2007. [Google Scholar]
- 85.Robins . Basic pathology. 7. Amsterdam: Elsevier; 2003. [Google Scholar]
- 86.Krasner D. Wound care. How to use red–yellow–black system. AJN. 1995;95(5):44–47. [PubMed] [Google Scholar]
- 87.Manakar NB, Nagdeve UT. Comparison of different imaging techniques used for chronic wounds. IJRET. 2013;2(7):68–70. doi: 10.15623/ijret.2013.0207009. [DOI] [Google Scholar]
- 88.Zhou AH. A survey of optical imaging techniques for assessing wound healing. Int J Intell Control Syst. 2012;17(3):79–85. [Google Scholar]
- 89.Falanga V. Classifications for wound bed preparation and stimulation of chronic wounds. Wound Repair Regen. 2000;8:347–352. doi: 10.1111/j.1524-475X.2000.00347.x. [DOI] [PubMed] [Google Scholar]
- 90.Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K, Romanelli M, Stacey MC, Teot L, Vanscheidt W. Wound bed preparation : a systematic approach to wound management. Wound Repair Regen. 2003;11(2):S1–S27. doi: 10.1046/j.1524-475X.11.s2.1.x. [DOI] [PubMed] [Google Scholar]
- 91.Flanagan M. Principles of wound management. In: Flanagan M, editor. Wound healing and skin integrity: principles and practice. London: Wiley-Blackwell; 2013. pp. 66–83. [Google Scholar]
- 92.Wounds UK (2013) Effective debridement in a changing NHS: a UK consensus. Wounds UK, London. www.wounds-uk.com
- 93.Strohal R, Dissemond J, O’Brien J, Piaggesi A, Rimdeika R, Young T, et al. An updated overview and clarification of the principle role of debridement. EWMA document: debridement. J Wound Care. 2013;22:S1–S52. doi: 10.12968/jowc.2013.22.Sup1.S1. [DOI] [PubMed] [Google Scholar]
- 94.Dodds S, Haynes S. The wound edge, epithelialization and monitoring wound healing. A journey through TIME. Wound bed preparation in practice. Br J Nurs Supplement. 2004;13(18):23–26. [Google Scholar]
- 95.Borle RM. Textbook of oral and maxillofacial surgery. Chapter 2: Sutures. 1. New Delhi: Jaypee Brothers; 2014. [Google Scholar]
- 96.Turner TD. Development of wound management products in chronic wound care. In: Krasner D, Rodeheaver G, Sibbald RG, editors. Chronic wound care: a clinical source book for healthcare professionals. 3. PA: HMP Communications, Wayne; 2001. [Google Scholar]
- 97.https://nstupharma.files.wordpress.com/2012/11/maimum-surgical-dressing1.pdf
- 98.Hyslop PA, Hinshaw DE, Scraufsratter IU. Hydrogen peroxide as a potent bacteriostatic antibiotic: implications for host defense. Free Radic Biol Med. 1995;19(11):31–37. doi: 10.1016/0891-5849(95)00005-I. [DOI] [PubMed] [Google Scholar]
- 99.Shenoy VP, Balla M, Shivananda PG, Bairy I. Honey as an antimicrobial agent against Pseudomonas aeruginosa isolated from infected wounds. J Glob Infect Dis. 2012;4(2):102–105. doi: 10.4103/0974-777X.96770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bergman A, Yanai J, Weiss J, Bell D, David MP. Acceleration of wound healing by topical application of honey: an animal model. Am J Surg. 1983;145(3):374–376. doi: 10.1016/0002-9610(83)90204-0. [DOI] [PubMed] [Google Scholar]
- 101.Atherton P. Aloe vera: magic or medicine? Nurs Stand. 1998;12(49):52–54. doi: 10.7748/ns.12.41.49.s40. [DOI] [PubMed] [Google Scholar]
- 102.Davis RH, Didonato JJ, Hartman GM, Haas RC. Anti-inflammatory and wound healing activity of a growth substance in aloe vera. J Am Podiatr Med Assoc. 1994;84(2):77–81. doi: 10.7547/87507315-84-2-77. [DOI] [PubMed] [Google Scholar]
- 103.Yadav KC, Kumar RJ, Basha S, Deshmukh GR, Gujjula R, Santhamma B. Wound healing activity of topical application of aloe vera gel in experimental animal models. Int J Pharm Bio Sci. 2012;3(2):63–72. [Google Scholar]
- 104.Higley HR, Ksander GA, Gerhardt CO, Falanga V. Extravasation of macromolecules and possible trapping of transforming growth factors-beta in venous ulcerations. Br J Dermatol. 1995;132:79–85. doi: 10.1111/j.1365-2133.1995.tb08629.x. [DOI] [PubMed] [Google Scholar]
- 105.Trengone NJ, Stacey MC, MacAuley S. Analysis of acute and chronic wound environment: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7:442–452. doi: 10.1046/j.1524-475X.1999.00442.x. [DOI] [PubMed] [Google Scholar]
- 106.Trengone NJ, Ohmann HB, Stacey MC. Miogenic activity and cytokine levels in nonhealing and healing chronic leg ulcers. Wound Repair Regen. 2000;8:13–25. doi: 10.1046/j.1524-475x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 107.Robson MC, Smith PD. Topical use of growth factors to enhance healing. In: Falanga V, editor. Cutaneous wound healing. London: Martin Dunitz; 2001. pp. 379–398. [Google Scholar]
- 108.Margolis DJ, Bartus C, Hoffstad O, Malay S, Berlin JA. Effectiveness of recombinant human PDGF for treatment of diabetic neuropathic foot ulcers. Wound Repair Regen. 2005;13:531–536. doi: 10.1111/j.1524-475X.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 109.Hess CT. Wound care. 5. Baltimore: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 110.Zhang X, Wu X, Wolf SE, Hawkins HK, Chinkes DL, Wolfe RR, et al. Local insulin zinc injection accelerated skin closure donor site wound healing. J Surg Res. 2007;142:90–96. doi: 10.1016/j.jss.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 111.Greenway SE, Filler LE, Greenway FL. Topical insulin in wound healing: a randomized double blind, placebo controlled trial. J Wound Care. 1999;8:526–528. doi: 10.12968/jowc.1999.8.10.26217. [DOI] [PubMed] [Google Scholar]
- 112.Rezvani O, Shabbak E, Aslam A, Bidar R, Jafari M, Safanezhad S. A randomized double blind placebo controlled trial to determine the effects of topical insulin on wound healing. Ostomy Wound Manag. 2009;55:22–28. [PubMed] [Google Scholar]
- 113.Chen X, Liu Y, Zhang X. Topical insulin application improves healing by regulating the wound inflammatory response. Wound Repair Regen. 2010;20:425–434. doi: 10.1111/j.1524-475X.2012.00792.x. [DOI] [PubMed] [Google Scholar]
- 114.Baumann LS, Spencer J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol Surg. 1999;25:311–315. doi: 10.1046/j.1524-4725.1999.08223.x. [DOI] [PubMed] [Google Scholar]
- 115.Pinnel SR. Regarding d-alpha tocopherol. Dermatol Surg. 1999;25:827. doi: 10.1046/j.1524-4725.1999.99137-3.x. [DOI] [PubMed] [Google Scholar]
- 116.Mackay D, Miller AL. Nutritional support for wound healing. Altern Med Rev. 2003;8:359–377. [PubMed] [Google Scholar]
- 117.Hopkins JT, McLoda TA, Seegmiller JG, David Baxter G. Low-level laser therapy facilitates superficial wound healing in humans: a triple-blind, sham-controlled study. J Athl Train. 2004;39(3):223–229. [PMC free article] [PubMed] [Google Scholar]
- 118.Silva EC, Filho AH, Musskopf DE. Radiação laser. In: Rodrigues EM, editor. Manual de recursos terapêuticos. Rio de Janeiro: Revinter; 1998. pp. 17–35. [Google Scholar]
- 119.Karu T, Pyatibrat L, Kalendo G. Irradiation with He–Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol, B. 1995;27:219–223. doi: 10.1016/1011-1344(94)07078-3. [DOI] [PubMed] [Google Scholar]
- 120.Bibikova A, Belkin V, Oron U. Enhancement of angiogenesis in regenerating gastrocnemius muscle of the toad (Bufo viridis) by low-energy laser irradiation. Anat Embryol (Berl) 1994;190:597–602. doi: 10.1007/BF00190110. [DOI] [PubMed] [Google Scholar]
- 121.Kloth LC, Mc Culloch MJ. Promotion of wound healing with electrical stimulation. Adv Wound Care. 1996;9(5):42–45. [PubMed] [Google Scholar]
- 122.Weller C, Sussman G. Wound dressings update. J Pharm Pract Res. 2006;36(4):318–324. doi: 10.1002/j.2055-2335.2006.tb00640.x. [DOI] [Google Scholar]
- 123.Tocco I, Zavan B, Bassetto F, Vindigni V. Nanotechnology-based therapies for skin wound regeneration. J Nanomater. 2012 [Google Scholar]
- 124.Whitehead W. A hundred cases of entire excision of the tongue. Br Med J. 1891;1(1583):961. doi: 10.1136/bmj.1.1583.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Davies H, Carr R. Osteomyelitis of the mandible: a complication of routine dental extractions in alcoholics. Br J Oral Maxillofac Surg. 1990;28(3):185–188. doi: 10.1016/0266-4356(90)90086-Z. [DOI] [PubMed] [Google Scholar]
- 126.Stanley D, Emerson D, Daley J. Whitehead’s varnish and Jelonet—a better dressing for skin graft donor sites than Jelonet alone. Ann R Coll Surg Engl. 1988;70(6):369. [PMC free article] [PubMed] [Google Scholar]
- 127.Stoelinga P, Bronkhorst F. The incidence, multiple presentation and recurrence of aggressive cysts of the jaws. J Cranio-Maxillofac Surg. 1988;16:184–195. doi: 10.1016/S1010-5182(88)80044-1. [DOI] [PubMed] [Google Scholar]
- 128.Eyre J, Zakrzewska J. The conservative management of large odontogenic keratocysts. Br J Oral Maxillofac Surg. 1985;23(3):195–203. doi: 10.1016/0266-4356(85)90090-7. [DOI] [PubMed] [Google Scholar]
- 129.Hellem S, Nordenram A. Prevention of postoperative symptoms by general antibiotic treatment and local bandage in removal of mandibular third molars. Int J Oral Surg. 1973;2(6):273–278. doi: 10.1016/S0300-9785(73)80022-5. [DOI] [PubMed] [Google Scholar]
- 130.Rowe-Jones J, Adam E, Moore-Gillon V. Subtle diagnostic markers of orbital floor blow-out fracture on coronal CT scan. J Laryngol Otol. 1993;107(02):161–162. doi: 10.1017/S0022215100122522. [DOI] [PubMed] [Google Scholar]
- 131.Braithwaite F, Maurice D. The importance of the levator palati muscle in cleft palate closure. Br J Plast Surg. 1968;21(1):60–62. doi: 10.1016/S0007-1226(68)80087-6. [DOI] [PubMed] [Google Scholar]
- 132.Morison R. Remarks on the treatment of infected, especially war wounds. Br Med J. 1917;2(2964):503–506. doi: 10.1136/bmj.2.2964.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nakhla V, Takwoingi YM, Sinha A. Myringoplasty: a comparison of bismuth iodoform paraffin paste gauze pack and tri-adcortyl ointment ear dressing. J Laryngol Otol. 2007;121(4):329–332. doi: 10.1017/S0022215106002660. [DOI] [PubMed] [Google Scholar]
- 134.Kotecha B, Fowler S, Harkness P, Walmsley J, Brown P, Topham J. Management of epistaxis: a national survey. Ann R Coll Surg Engl. 1996;78(5):444–446. [PMC free article] [PubMed] [Google Scholar]
- 135.Corbridge RJ, Djazaeri B, Hellier WP, Hadley J. A prospective randomised controlled trial comparing the use of merocel nasal tampons and BIPP in the control of acute epistaxis. Clin Otolaryngol Allied Sci. 1995;20(4):305–307. doi: 10.1111/j.1365-2273.1995.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 136.Flook EP, Uddin FJ, Johnston MN. The need to include BIPP reactions in routine consent. Clin Otolaryngol. 2006;31(2):165–166. doi: 10.1111/j.1749-4486.2006.01179.x. [DOI] [PubMed] [Google Scholar]
- 137.Youngman L, Harris S. BIPP madness: an iatrogenic cause of acute confusion. Age Ageing. 2004;33(4):406–407. doi: 10.1093/ageing/afh103. [DOI] [PubMed] [Google Scholar]
- 138.Elvis AM, Ekta JS. Ozone therapy; a clinical review. J Nat Sci Biol Med. 2011;2(1):66–70. doi: 10.4103/0976-9668.82319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O’Neil T. Antibacterial properties of periodontal dressings. J Periodontol. 1975;46(8):469. doi: 10.1902/jop.1975.46.8.469. [DOI] [PubMed] [Google Scholar]
- 140.Singh V, Das S, Sharma NK. Iodoform—a boon in disguise. Open Journal of Stomatology. 2012 [Google Scholar]
- 141.Hahler B. Surgical wound dehiscence. Medsurg Nurs. 2006;15:296–300. [PubMed] [Google Scholar]
- 142.Amini AQ, Khan NA, Ahmed J, Memon AS. Management of abdominal wound dehiscence: still a challenge. Pak J Surg. 2013;29(2):84–87. [Google Scholar]
- 143.Russell SB, Trupin JS, Kennedy RZ, Russel JD, Davidson JM. Glucocorticoid regulation of elastin synthesis in human fibroblasts: down regulation in fibroblasts from normal dermis but not from keloids. J Invest Dermatol. 1995;105:241–245. doi: 10.1111/1523-1747.ep12612788. [DOI] [PubMed] [Google Scholar]
- 144.Babu M, Diegelmann R, Oliver N. Fibronectin is overproduced by keloid fibroblasts during abnormal wound healing. Mol Cell Biol. 1989;9:1642–1650. doi: 10.1128/MCB.9.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brissett AE, Sherris DA. Scar contractures, hypertrophic scars, and keloids. Facial Plast Surg. 2001;17:263–272. doi: 10.1055/s-2001-18827. [DOI] [PubMed] [Google Scholar]
- 146.Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschkw MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17(1–2):113–125. doi: 10.2119/molmed.2009.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mutalik S. Treatment of keloids and hypertrophic scars. Indian J Dermatol Venereol Leprol. 2005;71(1):3–8. doi: 10.4103/0378-6323.13777. [DOI] [PubMed] [Google Scholar]
- 148.Naeini FF, Najafian J, Ahmadpour K. Bleomycin tattooing as a promising therapeutic modality in large keloids and hypertrophic scars. Dermatol Surg. 2006;32:1023–1029. doi: 10.1111/j.1524-4725.2006.32225.x. [DOI] [PubMed] [Google Scholar]
- 149.Segev F, Jaeger-Roshu S, Gefen-Carmi N, Assia EI. Combined mitomycin C application and free flap conjunctival autograft in pterygium surgery. Cornea. 2003;22:598–603. doi: 10.1097/00003226-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 150.Wolfram D, Tzankov A, Pulzi P, Katzer HP. Hypertrophic scars and keloids. A review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009;35:171–181. doi: 10.1111/j.1524-4725.2008.34406.x. [DOI] [PubMed] [Google Scholar]
- 151.Healy B, Freedman A. Infections. BMJ. 2006;332(7545):838–841. doi: 10.1136/bmj.332.7545.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tregdet EE, Nedelec B, Scott PG, Ghahary A. Hypertrophic scars and contractures: the cellular and molecular basis for therapy. Surg Clin N Am. 1997;77(3):701–730. doi: 10.1016/S0039-6109(05)70576-4. [DOI] [PubMed] [Google Scholar]
- 153.Moioli EK, Bakus AD, Yaghmai D, Hernandez C. Treatment strategies for pigmentation disorders in skin of color. Cosmet Dermatol. 2011;24(11):524–531. [Google Scholar]