Abstract
In this study, Ligustrum lucidum flowers as raw material, the extraction, isolation and coagulatory activity of polysaccharides were carried out for the first time. The crude polysaccharide was obtained by hot water extraction and ethanol precipitation, and preliminarily purified by Sevage method and D101 macroporous resin. Then the polysaccharide was further purified by DEAE-52 cellulose and Sephadex G-100 column chromatography, respectively. The structural characteristics were detected by LC, GC, FT-IR and NMR. Furthermore, the coagulatory activity of the polysaccharides were investigated by APTT, TT, PT and FIB assays in vitro. The results demonstrated that four polysaccharides were isolated from flowers of L. lucidum, named as LLP-1a, LLP-1b, LLP-2 and LLP-3, and the yields were 0.039, 0.0054, 0.0055 and 0.017%, respectively based on the weight of the dried flowers. The four polysaccharides components were free of nucleic acids and proteins, and their average molecular weights were 25,912, 64,919, 3,940,246 and 2,975,091 g/mol, respectively. The monosaccharide compositions of LLp-1a were l-rhamnose, l-arabinose, d-xylose, d-glucose and d-galactose (molar ratio of 3.16: 2.46: 1.00: 7.27: 4.22). Only d-galactose was detected from LLp-1b. LLp-2 was composed of l-arabinose, d-glucose and d-galactose (molar ratio of 1.28:1.32:1.00). LLp-3 was composed of l-rhamnose, l-arabinose, d-xylose, d-glucose and d-galactose (molar ratio of 5.85: 2.21: 2.23: 1.00: 2.25). Coagulation assays indicated that LLp-1a and LLp-3 had good anticoagulant effect in vitro, while LLp-1b showed procoagulant activity.
Keywords: Ligustrum lucidum Ait flowers, Polysaccharides, Coagulatory activity
Background
Ligustrum lucidum, belonging to Ligustrum genus, a flowering plant in the Oleaceae family, is native to the south of the Yangtze River to South China, southwest provinces and autonomous regions, Northwest distribution to Shanxi, Gansu, and naturalized in several other countries including India, Nepal and Korea [1]. At present, “Chinese Materia Medica” records the fruits, leaves, barks and roots of L. lucidum. Its fruit is often called “Nüzhenzi”, as a traditional Chinese medicine. There are more studies on its chemical constituents and pharmacological effects [2–6], but the research on flowers is relatively few, only some reports have studied the chemical composition and pharmacological activity, for example, Yang et al. [7] characterized the chemical composition of essential oil from the its flowers. Long et al. [8], Wang and Hou [9] studied the chemical constituents in flowers, sterols, flavonoids and alcohols were isolated from flowers. Zhang [10] found the anthocyanins in flowers had strong antioxidant activity in vitro. Yao et al. found the total flavonoids in flowers had the activities on scavenging DPPH free radicals and nitrite [11, 12]. About polysaccharides of L. lucidum, only Shi et al., have studied the polysaccharides from its fruit, found the polysaccharide could markedly improve the immune functions of hydrocortisone-induced immunosuppressed model mouse [13].
However, the polysaccharides in flowers are still uncertain without a clear theoretical evidence. Hence, the preliminary identification of the compositions of flowers polysaccharides would be significant and advantageous to be studied for further illustration of their potential bioactivities.
Thrombosis involves local blood clotting of the vascular system that often leads to serious health-related diseases such as heart attacks and strokes. The risk factors for thrombosis are abnormal hyperlipid, hyperglycemia, elevated plasma fibrinogen, high blood pressure and cancer, these thrombotic diseases, have become the primary causes of death and their incidence has been increasing annually [14, 15]. Therefore, effective antithrombotic drugs are urgently needed.
It is well known that polysaccharides have many bio-activities, such as antioxidant [16], laxative [17], hypoglycemic [18], immunomodulating activity [19]. In recent years, the research on the coagulation activity of polysaccharides has also been welcomed by many scholars [20, 21]. Up to now, there is no investigation report on the coagulation active ingredient of L. lucidum flowers.
Based on the above analysis, the objective of this research was to extract and purify the bioactive polysaccharides in flowers of L. lucidum with coagulation activity (Due to the large molecular weight, poor solubility limited sample size of polysaccharides, we only carried out coagulation activity in vitro), which could provide theoretical basis for its further application, and might expand the possibility to find better coagulation drug.
Methods
Plant material
The flowers of L. lucidum were collected in April 2015 from Guiyang City, Guizhou Province, and were identfied by Prof. Qian-jun Zhang. The voucher specimens were deposited in the herbarium of Huanghe Science and Technology College.
Animals
Male rabbit (2.0–2.5 kg), was purchased from the Experimental Animal Center of Henan Province (Zhengzhou, Henan, China, No: 14-3-7).
Reagents
Dextrans with different Mw (T-40, T-64, T-150, T-250 and T-500) were purchased from Sigma-aldrich. Monosaccharide standards including L-rhamnose (Rha), l-arabinose (Ara), d-xylose (Xyl), d-mannose (Man),d-glucose (Glc), d-galactose (Gal) were obtained from Dr. Ehrenstorfer GmbH Co. (Germany). Sephadex G-100 and DEAE-52 cellulose gel were purchased from GE Healthcare Bio-Scinence (Germany). Trifluoroacetic acid (TFA, standard for GC, > 99.8%) was purchased from Aladdin (Shanghai, China). Hydroxylammonium chloride (guarantee reagent) and pyridine were purchased from Tianjin Kemiou chemical reagent co., LTD. Injection breviscapine (Lot: 15141005) was obtained from Hang Sheng Pharmaceutical Co., Ltd. (Hunan, China). Yunanbaioyao (Lot: ZGA1604) was obtained from Yunnan Baiyao Group Co., Ltd. (Yunan, China). APTT (Lot: 1121911), TT (Lot: 121168), PT (Lot: 105295) and FIB (Lot: 132107) assay kits were purchased from Shanghai Sun Biotech Co., Ltd (Shanghai, China).
Extraction, purification of the crude polysaccharides
The dried flowers of L. lucidum (475 g) were crushed and refluxed with petroleum ether twice for 2 h to remove liposoluble constituents, and the polar constituents were removed by the soaking of 70% ethanol for 3 days. The degreased flowers were extracted twice by ultrapure water (W/V 1:12) that prepared with a Mill-Q water purification system (Merck Millipore Germany) at 85 ± 0.5 °C for 5 and 4 h. The extracting solution were merged, filtered and concentrated with rotatory evaporation till a quarter of the total volume. The concentrated solution was mixed with alcohol (2.8 vol) to obtain the crude polysaccharide.
The protein present was removed by Sevage method [22], and due to the dark color, D101 macroporous resin was applied to decolorize crude polysaccharide, followed by centrifugation (6000 rpm for 15 min at 4 °C) and alcohol precipitation (2.8 vol). Then the refined polysaccharide was redissolved in water and dialyzed with dialysis bag (Molecular weight cut-off 8000–14,000 Da) for 24 h in distilled water and another 12 h in ultra-pure water. Finally, the dialyzed polysaccharide solution was dehydrated by freeze-drying using LL-1500 Freeze Dryer (Thermo) to obtain refined polysaccharide.
The refined polysaccharide was further purified by DEAE-52 cellulose gel (2.5 × 60 cm) and was eluted sequentially with 0.0, 0.1, 0.2 and 0.3 mol/L NaCl. The purified fraction showed three main peaks (LL-1, LL-2 and LL-3), after that the Sephadex G-100 column (1.5 × 100 cm) was used to fractionate the three fractions. LL-1 fractionated into two polysaccharides, named as LLp-1a, and LLp-1b, respectively. LL-2 fractionated one polysaccharide, named as LLp-2, and LL-3 fractionated into one polysaccharide, named as LLp-3.
UV–Vis spectrophotometer analysis
The freeze-dried four polysaccharides were mixed with ultrapure water to make concentration of 0.1 mg/mL solution for the analysis. The spectrum was scanned from 200 to 760 nm by Hitachi U-4100 UV–Vis spectrophotometer.
Determination of the average molecular weight and monosaccharide composition
The average molecular weights of four polysaccharides (LLp-1a, LLp-1b, LLp-2 and LLp-3) were determined by liquid chromatograph (Waters) equipped with an differential refraction detector and TSK G4000P WXL chromatographic column (7.8 mm × 300 mm × 17 μm, Japanese east cao co., LTD), and the polysaccharide solutions 10 μL, previously filtered through a membrane (0.22 μm, Millipore), was injected at a concentration of 1 mg/mL, and run with Watsons purified water at 1.0 mL/min as mobile phase. The standard curve was established using using T-40, T-64, T-150, T-250 and T-500 as standard dextrans.
Freeze-dried four polysaccharides (10 mg) were hydrolyzed with 2 mL 2 mol/L of trifluoroacetic acid (TFA) in oven for 3 h at 110 °C in nitrogen sealed ampoule bottles. The soluble fraction was evaporated to dryness under stream of nitrogen to get hydrolysates. The hydrolysates were incubated with 10 mg hydroxylamine hydrochloride and 0.5 mL pyridine in water bath for 30 min at 90 °C, and then were acetylated with 0.5 mL Ac2O at 90 °C for 30 min. The acetylates were filtered through a membrane and readied for GC analysis. GC was used to determine the monosaccharide peak area. GC analysis was equipped with a HP capillary column (30 m × 0.35 mm, 0.25 μm) and a FID detector, and nitrogen was used as carriergas (2 mL/min). The program was isothermal at 100 °C, hold for 1 min, with a temperature gradient of 4 °C/min up to a final temperature of 240 °C, hold for 10 min. The injector temperature was 250 °C, and detector temperature 280 °C. l-rhamnose, l-arabinose, d-xylose, d-mannose, d-glucose, d-galactose were also derivatized as standard.
FT-IR analysis
1 mg of freeze-dried four polysaccharides were mixed with 150 mg of dried potassium bromide (KBr), and pressed into disk for the analysis. The IR spectrum was recorded in the range of 400–4000/cm on a Thermo Scientific Nicolet iS5 Fourier transform infrared spectroscopy (Thermo Electron, USA).
NMR spectral analysis
The samples (20 mg) were freeze-dried with 500 μL D2O (99.9%) three times before dissolution in 500 μL D2O (99.9%), finally transferred into 5-mm NMR tube. The one-dimensional NMR spectra (1H-NMR and 13C-NMR) were conducted on Bruker Avanced III 400 MHz equipment (Billerica, MA, USA). The chemical shifts of 1H-NMR spectra were calibrated with reference to D2O, used as an internal standard at 4.70 ppm.
Coagulation activity test
The coagulation activity of four polysaccharides was evaluated by activated APTT, TT, PT and FIB assays in vitro.
Preparation of sample and positive control
Weigh a certain amount of polysaccharide dissolved in a certain volume solvent (anhydrous ethanol: 1,2-propylene glycol: physiological saline = 1:1:3, volume ratio), and configured to a concentration of 5 mg/mL solution. Breviscapine was configured to a concentration of 13.33 mg/mL, and the concentration of Yunnanbaiyao was 40 mg/mL.
Preparation of plasma
Blood samples were taken at the ear vein of rabbits, and added to centrifuge tubes containing 0.4 mL, 0.109 mol/L of sodium citrate, the mixture was centrifuged to separate the supernatant at 3000 rpm for 15 min.
APTT assay
25 μL polysaccharide solution was added to the test cup, and then add 100 μL of plasma and 100 μL of APTT reagent pre-warmed at 37 °C in the test cup. The above reaction solution was incubated at 37 °C for 5 min, and then 100 μL of 0.025 mol/L CaCl2 solution at 37 °C pre-temperature was added to record the coagulation time by HF6000-4 semi-automatic coagulation analyzer, the time was the APTT value.
TT assay
50 μL of polysaccharide solutions was added to the test cup, and then 200 μL of plasma was added to the test cup. After incubation at 37 °C for 3 min, 200 μL PT reagent was added to record the coagulation time by HF6000-4 semi-automatic coagulation analyzer, the time was the TT value.
PT assay
25 μL of polysaccharide solutions was added to the test cup, and then 100 μL of plasma was added to the test cup. After incubation at 37 °C for 3 min, 200 μL 37 °C pre-warmed PT reagent was added to record the coagulation time by HF6000-4 semi-automatic coagulation analyzer, the time was the PT value.
FIB assay
First of all, according to the requirements of specification to draw the standard curve, and then sample determination. Take 200 μL of plasma and 100 μL of polysaccharide solutions, then add 700 μL of buffer, 200 μL of the above mixture was taken and incubated at 37 °C for 3 min. Finally, 100 μL thrombin solution was added to the above mixture to record the content of fibrinogen, the content was FIB value.
For the four methods, solvent was used as blank control, breviscapine and Yunnanbaiyao were used as positive control.
Results and discussion
Polysaccharide isolation and purification
After removing the protein and pigment, the refined polysaccharides were preliminary purified by DEAE-52 cellulose column chromatography, three main polysaccharide fractions were obtained, named LL-1 eluted with 0.1 mol/L NaCl, LL-2 eluted with 0.2 mol/L NaCl and LL-3 eluted with 0.3 mol/L NaCl, respectively (Fig. 1a). The three polysaccharide fractions isolated by DEAE-52 were further isolated and purified by Sephadex G-100 column chromatography. Finally, two polysaccharides were isolated from LL-1, named as LLp-1a (183.7 mg) and LLp-1b (26 mg) (Fig. 1b), LL-2 and LL-3 eluted two polysaccharides, respectively, named as LLp-2 (25.5 mg) (Fig. 1c) and LLp-3 (83 mg) (Fig. 1d).
Fig. 1.
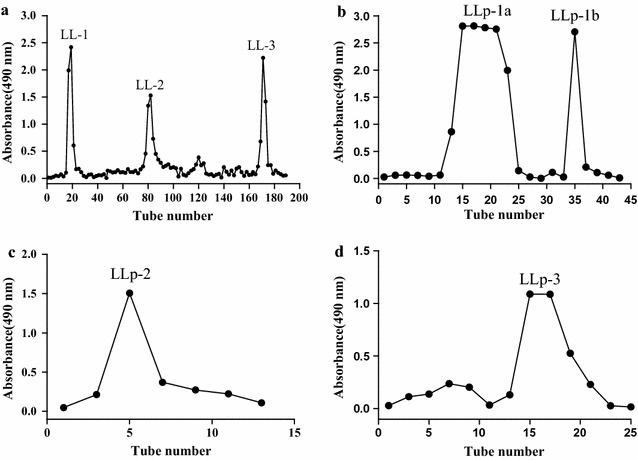
Elution curve of crude polysaccharide by DEAE-52 cellulose column chromatography (a), elution curve of LL-1 on Sephadex G-100 column (b), elution curve of LL-2 on Sephadex G-100 column (c), elution curve of LL-3 on Sephadex G-100 column (d)
UV–Vis spectroscopy analysis
Nucleic acids and proteins have UV absorption at 260 and 280 nm wavelengths, so, UV–visible full-wavelength scanning was used to determine whether polysaccharide solution contained protein and nucleic acid. The scanning result of the four polysaccharides was shown in Fig. 2. The four polysaccharides had no significant absorption peak at 260 and 280 nm, which indicated that the four polysaccharides were free of nucleic acid and protein.
Fig. 2.
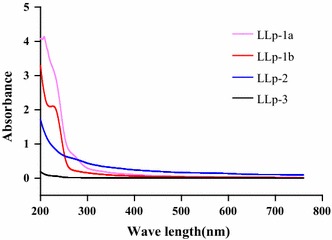
UV-Vis spectra full-wavelength scanning curves of LLp-1a, LLp-1b, LLp-2 and LLp-3
Molecular weight analysis
Most of the polysaccharides were obtained with water extract alcohol precipitation, and the extracted polysaccharides were mostly viscous and unstable colloidal solution. The relative molecular mass of the components contained in the colloidal solution was different, and the pharmacological activity of polysaccharides with different relative molecular weights was quite different, which brought great difficulties for the quality control and further development and utilization of polysaccharide. Therefore, it was necessary to screen the polysaccharides of different molecular segments and determine their molecular weight [23]. At present, the molecular weight of polysaccharides could be measured by several techniques, such as vapor pressure method, end-based analysis, osmotic pressure, viscosity method, high performance liquid chromatography, high performance size-exclusion chromatography (HPSEC) [24], multiple-angle laser light scattering (MALLS) [25], and high-performance gel permeation chromatography (HPGPC) [26, 27]. In our study, the molecular weights were measured by LC equipped with a refractive index detector, with the dextran standards (T-40, T-64, T-150, T-250, and T-500) used for the calibration curve. The equation of the standard curve was: LogMw = − 0.539t + 9.700 (Note: Mw represents molecular weight, while t represents retention time) with a correlation coefficient of 0.988. As it is shown in Table 1, the average molecular weight of LLp-1a, LLp-1b, LLp-2, LLp-3 were estimated to be 25,912, 64,919, 3,940,246 and 2,975,091 g/mol, respectively.
Table 1.
Molecular weight of polysaccharides form Ligustrum lucidum Ait flowers
| Polysaccharide | T (min) | LgMw | Mw | Average Mw (g/mol) |
|---|---|---|---|---|
| LLp-1a | 9.796 | 4.413 | 25,882 | 25,912 |
| 9.794 | 4.414 | 25,941 | ||
| LLp-1b | 9.091 | 4.794 | 62,230 | 64,919 |
| 9.023 | 4.83 | 67,608 | ||
| LLp-2 | 5.762 | 6.591 | 3,899,420 | 3,940,246 |
| 5.745 | 6.6 | 3,981,071 | ||
| LLp-3 | 5.978 | 6.474 | 2,978,516 | 2,975,091 |
| 5.979 | 6.473 | 2,971,666 |
Analysis of monosaccharide composition
Previous studies have shown that the strong biological activity of polysaccharides was strongly related to monosaccharide compositions [28], and the monosaccharide composition of polysaccharides played an important role in further analyzing its physicochemical properties, structure and structure-biological activity. At present, there were many ways to determine the monosaccharide composition, including high performance liquid chromatography [29], reversed-phase high performance liquid chromatography (HPLC) after pre-column derivatization [30], high-performance thin-layer chromatography [31], gas chromatography (GC) [32], high-performance anion-exchange chromatography [33], high performance capillary electrophoresis [34]. In our study, the monosaccharide compositions were measured by GC with good sensitivity, and monosaccharide composition was estimated by comparing retention time (RT). The results were shown Figs. 3, 4. As could be seen from the figures, the peaks of all monosaccharides were sharp and symmetrical. Compared with the standard monosaccharides (Fig. 3), the peaks of the LLp-1a derivatives were identified as l-rhamnose, l-arabinose, d-xylose, d-glucose, d-galactose, LLp-1a was a heteropolysaccharide and in a molar ratio of 3.16: 2.46:1.00: 7.27: 4.22. Only d-galactose was detected from LLp-1b. The monosaccharide compositions of LLp-2 were l-arabinose, d-glucose and d-galactose, and in a molar ratio of 1.28:1.32:1.00. The monosaccharide compositions of LLp-3 were l-rhamnose, l-arabinose, d-xylose, d-glucose and d-galactose, and in a molar ratio of 5.85: 2.21: 2.23:1.00:2.25.
Fig. 3.
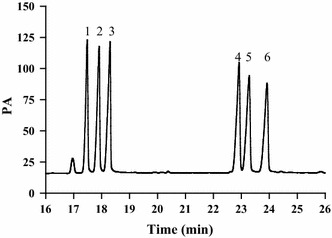
Gas chromatograms of standard monosaccharide mixture solution (1) l-rhamnose (Rha) (2) l-arabinose (Ara) (3) d-xylose (Xyl) (4) d-mannose (Man) (5) d-glucose (Glu) (6) d-galactose (Gal)
Fig. 4.
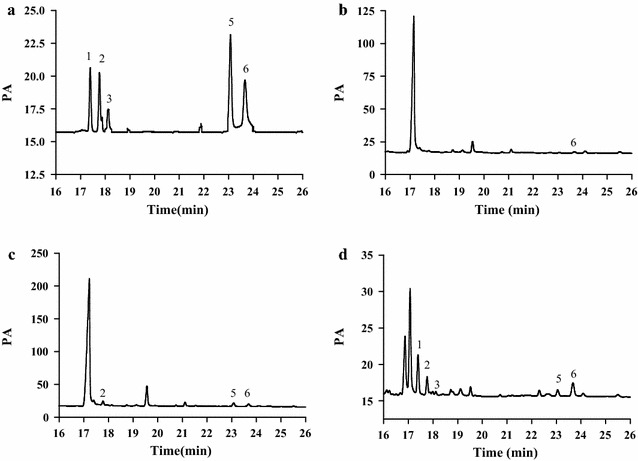
Gas chromatograms of the monosaccharide compositions of polysaccharides LLp-1a (a), LLp-1b (b), LLp-2 (c) and LLp-3 (d) from L. lucidum flowers
FT-IR spectroscopy analysis
The FT-IR spectroscopys of LLp-1a, LLp-1b, LLp-2 and LLp-3 were recorded at the range of 4000–400/cm (Fig. 5). Obviously, it was showed that the IR spectra of four polysaccharides had a strong characteristic absorption band at 3436, 3425, 3436 and 3346 cm−1 for the stretching of hydroxyl, which was common to polysaccharides, then a very weak characteristic absorption appearing at 2947, 2946, 2947 and 1948/cm, respectively, were the absorption peaks of C–H stretching vibration [35]. The strong asymmetrical absorption peak at 1618, 1617, 1617 and 1608/cm, respectively, and weak symmetrical peaks at around 1332–1420/cm were indicative the carboxyl groups and carbonyl groups, which indicated the characteristic IR absorption of uronic acid [36]. According to the study, furanose had two absorption peaks at the range of 1100–1010/cm, and pyranose had three absorption peaks at the range of 1100–1010/cm. Four polysaccharides showed two absorption peaks at 1100–1010/cm, indicating that the four polysaccharides contained furanose rings [37]. Two conformers of carbohydrates, α-and β-conformers, which depended on the types of end carbon-glucoside bonds, could be distinguished based on the anomeric region-vibrational bands from 950 to 750/cm [38], where around 840/cm corresponds to α-conformers, while the β-conformers lie around 890/cm [39].
Fig. 5.
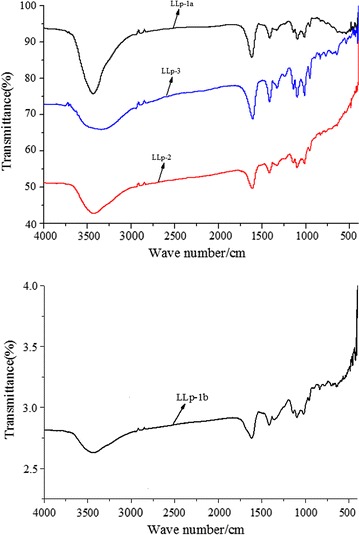
FT-IR spectra of LLp-1a, LLp-1b, LLp-2 and LLp-3
NMR spectral analysis
The 1H-NMR spectra of LLp-1a, LLp-1b, LLp-2, LLp-3 and 13C-NMR spectra of LLp-3 were shown in Fig. 6, respectively. The 1H signal at 4.70 ppm was caused by D2H. General speaking, the signals in the region of 5.60–4.90 ppm was assigned to anomeric protons of α-anomers, and 4.90–4.30 ppm was assigned to anomeric protons of β-anomers, while the region of 4.50–3.00 ppm was contributed to the ring proton region [40]. These data confirmed the backbone had α-glycosidic and β-glycosidic linkages, which were consistent with the results obtained by FT-IR analysis. The region of 4.50–3.00 ppm were assigned to the H-2 to H-6 protons.
Fig. 6.
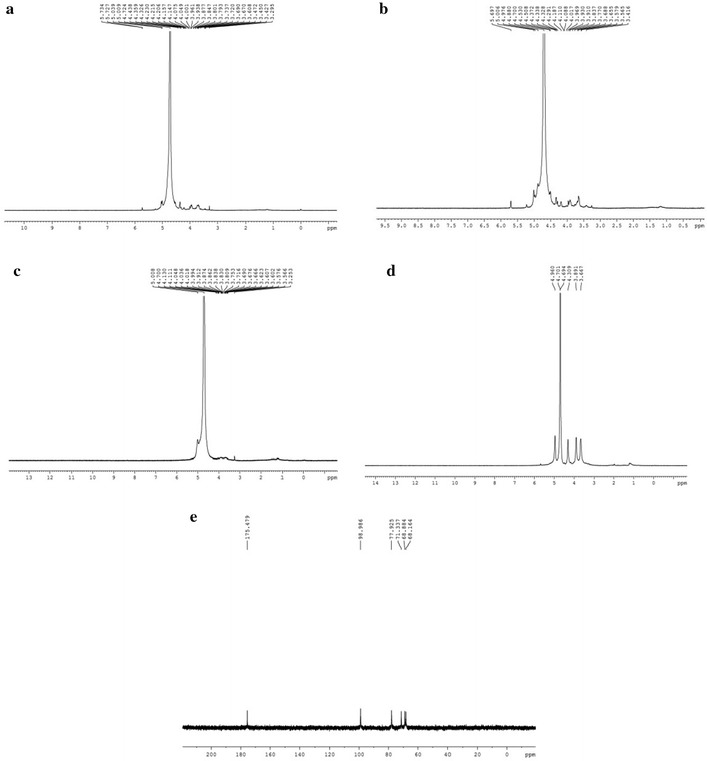
1H NMR spectrum of LLp-1a (a), LLp-1b (b), LLp-2 (c) and LLp-3 (d), 13C NMR spectrum of LLp-3 (e)
The 13C-NMR spectrum of LLp-3 had carboxy carbon signal from 170 to 176 ppm, which illustrated LLp-3 contained uronic acid. Polysaccharide signals generally appeared in the range of 60–110 ppm. Among them, 90–110 ppm for end-based carbon signal, 60–90 ppm for the non-terminal carbon signal. Due to the poor solubility of LLp-1a, LLp-1b and LLp-2, their carbon spectrum signals was not good, but FT-IR spectroscopy analysis indicated that the characteristic IR absorption of uronic acid was existed, which also induced carboxy carbon signal in carbon spectrum, showed the existence of a carboxylic group.
Coagulation activity in vitro
The effects of polysaccharides on plasma coagulation parameters in vitro including APTT, PT, TT and FIB were assayed and the results were described as follows.
As could be seen in the Fig. 7, compared with the control group, LLp-1a and LLp-3 significantly prolonged APTT, PT and TT (p < 0.001 or p < 0.05), and the effects of LLp-1a on prolonging APTT, PT and TT were similar to breviscapine as positive control (p > 0.05), the effects of LLp-3 were significantly weaker than that of breviscapine (p < 0.001). In contrast, compared with the control group, LLp-1b could significantly shorten APTT (p < 0.001), the times of LLp-1b on prolonging PT and TT were shorter than that of control group, but longer than that of Yunnanbaiyao as positive control, the effect of LLp-1b was significantly weaker than that of Yunnan Baiyao (p < 0.001). For FIB, compared with the control group, LLp-1a significantly reduced FIB content (p < 0.001), and LLp-1b and LLp-3 significantly increased FIB content (p < 0.001). From the above data comprehensive analysis, we demonstrated that LLp-1a and LLp-3 had good anticoagulant effect, while LLp-1b had procoagulant activity in vitro.
Fig. 7.
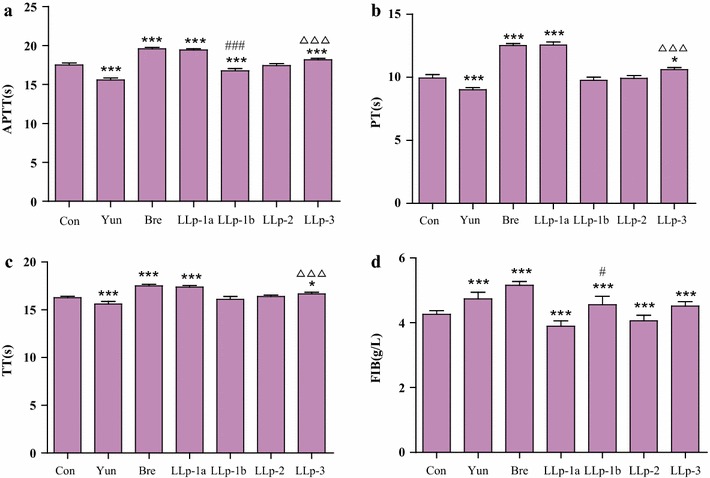
Effects of polysaccharides on plasma coagulation parameters in vitro (a APPT; b PT; c TT; d FIB. n = 6). Compared with control group, *** p < 0.001 < ** p < 0.01 < * p < 0.05; Compared with Yunnan Baiyao, ### p < 0.001 < ## p < 0.01 < # p < 0.05; Compared with breviscapine, △△△ p < 0.001 < △△ p < 0.01 < △ p < 0.05
In clinical tests of blood coagulation, several well-established analyses are used to indicate coagulation activity including APTT, PT, TT and FIB. These assays indicate anti-coagulant activity with respect to the intrinsic and extrinsic pathways in the blood coagulation cascade. PT reflects the extrinsic pathway of the coagulation cascade, whilst APTT reflects changes in the intrinsic pathway of the blood, TT is mainly a reflection of the degree of the conversion of fibrinogen into fibrin and is an important index. FIB mainly reflects the content of fibrinogen [41, 42]. In this study, LLp-1a and LLp-3 could prolong APTT and PT, which suggested that the anticoagulant effect of LLp-1a and LLp-3 might be partially due to altered activity of coagulation factors in both extrinsic and intrinsic clotting pathways [42]. LLp-1a and LLp-3 could prolong TT, but LLp-1a significantly reduced FIB content, LLp-3 significantly increased FIB content. These results showed that LLp-1a could benefit hindering fibrin formation. LLp-1b could significantly shorten APTT and increased FIB content, which indicated that its effects were mediated mainly through the intrinsic coagulation pathway and increasing the content of FIB [15].
Conclusions
In the paper, four polysaccharides were purified from L. lucidum flowers by DEAE-52 cellulose and Sephadex G-100 column chromatography, they were free of nucleic acid and protein. The average molecular weights of LLp-1a, LLp-1b, LLp-2 and LLP-3 were 25,912, 64,919, 3,940,246 and 2,975,091 g/mol, respectively, and their monosaccharide compositions were different, which might affect their activities, LLp-1a and LLp-3 had good anticoagulant effect in vitro, while LLp-1b had procoagulant activity in vitro. The further structural analysis were detected by Fourier transform infrared (FT‑IR) spectrometer and nuclear magnetic resonance spectra (NMR). These results implied these polysaccharides had the potential to be developed as natural medicines or health foods with coagulation activity. However, the structure and mechanism of the biological activity of these polysaccharides still need further study.
Authors’ contributions
Study design and experimental work was by WYK, ZHY and WZ. ZHY was participated in coagulation experiment. JJZ and WZ were participated in extraction, determination of the average molecular weight and monosaccharide composition. ZHY was participated in purification and other experiments. The first draft of the paper was written by ZHY and reviewed by all authors. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by Henan Province University Science and Technology Innovation Team (16IRTSTHN019) and Key Research Projects of Colleges and Universities in Henan province (18A360019).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- LC
liquid chromatograph
- GC
gas chromatography
- FT-IR
fourier transform infrared
- NMR
nuclear magnetic resonance
- ATPP
activated partial thromboplastin time
- TT
thrombin time
- PT
prothrombin time
- FIB
fibrinogen
- TFA
trifluoroacetic acid
- Rha
l-rhamnose
- Ara
l-arabinose
- Xyl
d-xylose
- Man
d-mannose
- Glc
d-glucose
- Gal
d-galactose
Footnotes
Zhenhua Yin and Wei Zhang contributed equally to this work
Contributor Information
Zhenhua Yin, Email: yinzhenhua1000@126.com.
Wei Zhang, Email: zzzwwwqq@126.com.
Juanjuan Zhang, Email: 376621568@qq.com.
Wenyi Kang, Email: Kangweny@hotmail.com.
References
- 1.Editorial Board of Chinese Flora of Chinese Academy of Sciences (1992) Chinese Flora Sci Press 61:153
- 2.Che CT, Wong MS. Ligustrum lucidum and its Constituents: a Mini-Review on the Anti-Osteoporosis Potential. Nat Prod Commun. 2015;10:2189–2194. [PubMed] [Google Scholar]
- 3.Hu B, Du Q, Deng S, An HM, Pan CF, Shen KP, Xu L, Wei MM, Wang SS. Ligustrum lucidum Ait. fruit extract induces apoptosis and cell senescence in human hepatocellular carcinoma cells through upregulation of p21. Oncol Rep. 2014;32:1037–1042. doi: 10.3892/or.2014.3312. [DOI] [PubMed] [Google Scholar]
- 4.Liu Q, Kim SH, Kim SB, Jo YH, Kim ES, Hwang BY, Oh K, Lee MK. Anti-obesity effect of (8-E)-niizhenide, a secoiridoid from Ligustrum lucidum, in high-fat diet-induced obese mice. Nat Prod Commun. 2014;9:1399–1401. [PubMed] [Google Scholar]
- 5.Liu X, Wang CY, Shao CL, Fang YC, We YX, Zheng CJ, Sun LL, Guan HS. Chemical constituents from the fruits of Ligustrum lucidum. Chem Nat Compd. 2010;46:701–703. doi: 10.1007/s10600-010-9719-x. [DOI] [Google Scholar]
- 6.Yang NY, Xu XH, Ren DC, Duan JA, Xie N, Tian LJ, Qian Sh. Secoiridoid constituents from the fruits of Ligustrum lucidum. Helvetica Chim Acta. 2010;93:65–71. doi: 10.1002/hlca.200900144. [DOI] [Google Scholar]
- 7.Yang J, Wei CX, Bian JC. Study on the chemical constituents of essential oil from Ligustrum lucidum flower. Chin Tradit Herbal Drugs. 2006;37(679):752. [Google Scholar]
- 8.Long F, Deng L, Chen Y. Study on the chemical constituents in the flowers of Ligustrum lucium. West China J Pharm Sci. 2011;26:97–100. [Google Scholar]
- 9.Wang JX, Hou GN. Studies on the chemical constituents of the flowers of Ligustrum lucium Ait. China J Chin Mater Med. 1990;15(40–42):63. [PubMed] [Google Scholar]
- 10.Zhang RX. Study on the Extraction of Anthocyanin from Ligustrum lucidum Ait. Flowers and Antioxidant Activity in vitro. Food Ind. 2016;37:132–136. [Google Scholar]
- 11.Yao WH, Li FY, Wang J, Luo Z, Hou T. Study on extraction of total flavonoids in Ligustrum flowers and its scavenging activity on DPPH free radicals. Food Res Dev. 2016;37(42–45):67. [Google Scholar]
- 12.Yao WH, Li FY, Wang J, Luo Z, Ran WG, Hou T. Study on determination and scavenging action of total flavonoids in Ligustrum Flowers. J Qingdao Agric Univ (Nat Sci) 2015;32:194–197. [Google Scholar]
- 13.Shi JJ, Shi B, Miao MS, Li QY. Effect of Ligustrum lucidum polysaccharide on immunity of immunosuppressed mice. Bangladesh J Pharmacol. 2016;11:S68–S71. doi: 10.3329/bjp.v11iS1.26919. [DOI] [Google Scholar]
- 14.Leopold JA, Loscalzo J. Oxidative risk for atherothrombotic cardiovascular disease. Free Radic Biol Med. 2009;47:1673–1706. doi: 10.1016/j.freeradbiomed.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie PY, Zhang Y, Wang XB, Wei JF, Kang WY. Antithrombotic effect and mechanism of Rubus spp. Blackberry. Food Funct. 2017;8:2000–2012. doi: 10.1039/C6FO01717G. [DOI] [PubMed] [Google Scholar]
- 16.Fan J, Feng HB, Yu Y, Sun MX, Liu YR, Li TZ, Sun X, Liu SJ, Sun MD. Antioxidant activities of the polysaccharides of Chuanminshen violaceum. Carbohydr Polym. 2017;157:629–636. doi: 10.1016/j.carbpol.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 17.Luo DD, Qu C, Lin GS, Zhang ZB, Xie JH, Chen HB, Liang JL, Li CL, Wang HF, Su ZR. Character and laxative activity of polysaccharides isolated from Dendrobium officinale. J Funct Food. 2017;34:106–117. doi: 10.1016/j.jff.2017.04.024. [DOI] [Google Scholar]
- 18.Wang S, Lu AX, Zhang L, Shen M, Xu T, Zhan WY, Jin H, Zhang YJ, Wang WM. Extraction and purification of pumpkin polysaccharides and their hypoglycemic effect. Int J Biol Macromol. 2017;98:182–187. doi: 10.1016/j.ijbiomac.2017.01.114. [DOI] [PubMed] [Google Scholar]
- 19.Xie SZ, Liu B, Zhang DD, Zha XQ, Pan LH, Luo JP. Intestinal immunomodulating activity and structural characterization of a new polysaccharide from stems of Dendrobium officinale. Food Funct. 2016;7:2789–2799. doi: 10.1039/C6FO00172F. [DOI] [PubMed] [Google Scholar]
- 20.Wang JM, Lian PL, Yu Q, Wei JF, Kang WY. Purification, characterization and procoagulant activity of polysaccharides from Angelica dahurice roots. Chem Central J. 2017;11:17. doi: 10.1186/s13065-017-0243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ustyuzhanina NE, Bilan MI, Dmitrenok AS, Borodina EY, Stonik VA, Nifantiev NE, Usov AI. A highly regular fucosylated chondroitin sulfate from the sea cucumber Massinium magnum: structure and effects on coagulation. Carbohydr Polym. 2017;167:20–26. doi: 10.1016/j.carbpol.2017.02.101. [DOI] [PubMed] [Google Scholar]
- 22.Navarini L, Gilli R, Gombac Abatangelo A, Bosco M, Toffanin R. Polysaccharides from hot water extracts of roasted Coffea arabica beans: isolation and characterization. Carbohydr Polym. 1990;40:71–81. doi: 10.1016/S0144-8617(99)00032-6. [DOI] [Google Scholar]
- 23.Luo XY. The Purification and Molecular Weight of LanQi Compound Polysaccharide. Guangdong: Guangdong College of Pharmacy; 2015. [Google Scholar]
- 24.Eva GO, Antonio JE, Pilar R. Molecular weight distribution of polysaccharides from edible seaweeds by high-performance size-exclusion chromatography (HPSEC) Talanta. 2012;93:153–159. doi: 10.1016/j.talanta.2012.01.067. [DOI] [PubMed] [Google Scholar]
- 25.Shi YK, Zhao LG, Liu XH, Hu FD, Cui F, Bi YY, Ma YF, Feng SL. Structural characterization of a sulfated glucan isolated from the aqueous extract of Hedysarum polybotrys Hand.-Mazz. Carbohydr Polym. 2012;87:160–169. doi: 10.1016/j.carbpol.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 26.Tang W, Lin LH, Xie JH, Wang ZJ, Wang H, Dong YJ, Shen MY, Xie MR. Effect of ultrasonic treatment on the physicochemical properties and antioxidant activities of polysaccharide from Cyclocarya paliurus. Carbohydr Polym. 2016;151:305–312. doi: 10.1016/j.carbpol.2016.05.078. [DOI] [PubMed] [Google Scholar]
- 27.He SD, Wang X, Zhang Y, Wang J, Sun HJ, Wang JH, Cao XD, Ye YK. Isolation and prebiotic activity of water-soluble polysaccharides fractions from the bamboo shoots (Phyllostachys praecox) Carbohydr Polym. 2016;151:295–304. doi: 10.1016/j.carbpol.2016.05.072. [DOI] [PubMed] [Google Scholar]
- 28.Zhang MJ, Cui SW, Cheung P, Wang Q. Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci Technol. 2007;18:4–19. doi: 10.1016/j.tifs.2006.07.013. [DOI] [Google Scholar]
- 29.Yang XB, Zhao Y, Wang QW, Wang HF, Mei QB. Analysis of the monosaccharide components in angelica polysaccharides by high performance liquid chromatography. Anal Sci. 2005;21:1177–1180. doi: 10.2116/analsci.21.1177. [DOI] [PubMed] [Google Scholar]
- 30.Jiang YP, Qi XH, Gao K, Liu WJ, Li N, Cheng NB, Ding G, Huang WZ, Wang ZZ, Xiao W. Relationship between molecular weight, monosaccharide composition and immunobiologic activity of Astragalus polysaccharides. Glycoconjugate J. 2016;33:755–761. doi: 10.1007/s10719-016-9669-z. [DOI] [PubMed] [Google Scholar]
- 31.Shi LJ, Yimamu H, Kawuli A, Saideaihemati Zhao HJ, Yili A, Morlock GE, Aisa HA. HPTLC study of the monosaccharide composition of a polysaccharide from Apocynum venetum leaves. Chem Nat Compd. 2015;51:130–131. doi: 10.1007/s10600-015-1218-7. [DOI] [Google Scholar]
- 32.Wang H, Liu G, Zhou BH, Hu XM. Monosaccharide compositional analysis of purified polysaccharide from Tricholoma matsutake by capillary gas chromatography. J Med Plant Res. 2012;6:1935–1940. [Google Scholar]
- 33.Xie Jh, Shen MY, Nie SP, Liu X, Zhang H, Xie MY. Analysis of monosaccharide composition of Cyclocarya paliurus polysaccharide with anion exchange chromatography. Carbohydr Polym. 2013;98:976–981. doi: 10.1016/j.carbpol.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Jian LJ, Chang JM, Ablise M, Li GR, He JW. Isolation, purification, and structural elucidation of polysaccharides from Alhagi-honey. J Asian Nat Prod Res. 2014;16:783–789. doi: 10.1080/10286020.2014.898633. [DOI] [PubMed] [Google Scholar]
- 35.Miao M, Bai AJ, Jiang B, Song Y, Cui SW, Zhang T. Characterisation of a novel water-soluble polysaccharide from Leuconostoc citreum SK24.002. Food Hydrocolloids. 2004;36:265–272. doi: 10.1016/j.foodhyd.2013.10.014. [DOI] [Google Scholar]
- 36.Cai WR, Xu HL, Xie LL, Sun J, Sun TT, Wu XY, Fu QB. Purification, characterization and in vitro anticoagulant activity of polysaccharides from Gentiana scabra Bunge roots. Carbohydr Polym. 2016;140:308–313. doi: 10.1016/j.carbpol.2015.12.054. [DOI] [PubMed] [Google Scholar]
- 37.Feng X, Xia Y, Chen GT, Xu JJ, Liao XJ, Zhao LY. Purification and structural analysis of polysaccharides from ginger peels. Food Sci. 2017;38:185–190. [Google Scholar]
- 38.Azmi AF, Mustafa S, Hashim DM, Manap YA. Prebiotic activity of polysaccharides extracted from Gigantochloa Levis (Buluh beting) Shoots. Mol. 2012;17:1635–1651. doi: 10.3390/molecules17021635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Xie MY, Nie SP, Li C, Wang YX. Purification: composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008;107:231–241. doi: 10.1016/j.foodchem.2007.08.021. [DOI] [Google Scholar]
- 40.Sun SN, Yuan TQ, Li MF, Cao XF, Xu F, Liu QY. Structural characterization of hemicelluloses from bamboo culms (Neosinocalamus Affinis) Cellul Chem Technol. 2012;46:165–176. [Google Scholar]
- 41.Sikka P, Bindra VK. Newer antithrombotic drugs. Indian journal of critical care medicine: peer-reviewed. Off Publ Indian Soc Crit Care Med. 2010;14:188–195. doi: 10.4103/0972-5229.76083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan KC, Yin MC, Chao WJ. Effect of diallyl trisulfide-rich garlic oil on blood coagulation and plasma activity of anticoagulation factors in rats. Food Chem Toxicol. 2007;48:502–507. doi: 10.1016/j.fct.2006.10.005. [DOI] [PubMed] [Google Scholar]


