Abstract
Cohesin is one of three multi-protein Structural Maintenance of Chromosomes (SMC) complexes that regulate eukaryotic chromosome dynamics. It forms a ring-shaped structure that embraces sister chromatids through interphase to promote their pairing. In preparation for mitosis, most cohesin is stripped from the chromosome arms in prophase by a poorly defined process that is associated with cohesin phosphorylation. In the fission yeast Schizosaccharomyces pombe this prophase pathway is dependent on the cohesin-related Smc5/6 complex, and this requirement is heightened in Smc5/6 hypomorphs by DNA damage, replication stress and Topoisomerase II (Top2) dysfunction. Cohesin interacts with chromosomes immediately upon mitotic exit, and becomes cohesive coincident with DNA replication. Cohesiveness is promoted by acetylation of the Smc3 subunit by an acetyltransferase, known as Eso1 in the S. pombe, which counteracts the anti-cohesive function(s) of the cohesin regulators Pds5 and Wpl1. We recently showed that Eso1 and Smc5/6 antagonize each other, and concurrent inactivation restores sister chromatid separation following genotoxic stress. Here, we have investigated the relationship between Top2 and Eso1 in successful completion of mitosis. We observe that partial inactivation of both results in a synthetic lethal mitotic block, but this is not overcome by deleting pds5 or wpl1. However, analysis of both acetyl-blocking and mimetic mutations in Smc3 indicates that the cycling of cohesin acetyl-regulation is more important than acetyl-status per se, highlighting the non-linear nature of the cohesin cycle.
Keywords: Cohesin, Topoisomerase, Mitosis, Eso1, Smc3 acetylation
Introduction
Structural Maintenance of Chromosomes (SMC) complexes and DNA Topoisomerases maintain the dynamic structure and organization of chromosomes. There are three SMC complexes: condensin (Smc2/4), cohesin (Smc1/3) and Smc5/6 (Hirano 2002, Hirano 2006). Condensin is primarily associated with chromosomes in mitosis, and is required for the full compaction of mitotic chromosomes, where it functions in conjunction with Topoisomerase II (Top2) (Hirano 2005, Iwasaki and Noma 2016). Cohesin and Smc5/6 are primarily associated with chromosomes throughout interphase, and there is significant overlap in the localization of cohesin and Smc5/6 (Lindroos, et al. 2006). Several studies implicate Smc5/6 in the cohesin cycle (Copsey, et al. 2013, Outwin, et al. 2009, Tapia-Alveal, et al. 2014a, Tapia-Alveal, et al. 2014b, Tapia-Alveal, et al. 2010), though the mechanism(s) remain obscure.
By forming a ring-shaped structure, cohesin embraces sister chromatids throughout interphase (Nasmyth 2011, Nasmyth and Haering 2009). This both facilitates DNA repair by homologous recombination and also ensures equal segregation into daughter cells at anaphase. In most eukaryotes, cohesin is loaded onto chromosomes on mitotic exit, and remains on them through to early mitosis. The cohesiveness of these complexes is established coincident with DNA replication, and enforced by acetylation of the Smc3 subunit on two Nterminal lysines by an acetyltransferase known as Eco1 in S. cerevisiae, Eso1 in S. pombe, and Esco1/2 in humans. This acetylation counteracts anti-cohesiveness functions for two cohesin regulators, Pds5 and Rad61/Wpl1/Wapl (in the three systems mentioned above). At prophase, most cohesin is stripped from the chromosome arms by an ill-defined process associated with cohesin phosphorylation. At anaphase, the kleisin subunit (Scc1/Rad21) within kinetochore-associated cohesin is cleaved by the protease Separase. This opens the ring, enabing chromosome segregation to occur. Reloading of cohesin then restarts the cycle. A variation on this two-step model occurs in S. cerevisiae, where all cohesin is cleaved by separase, and so reloading requires new synthesis and assembly of cohesin, and subsequently occurs later in the cell cycle (Nasmyth 2011, Nasmyth and Haering 2009, Tapia-Alveal, et al. 2014a).
As the original Smc5/6 gene, the rad18 gene of S. pombe (now named smc6) came from a screen for mutants defective in the recombinational repair (Lehmann, et al. 1995), most studies have focused on a DNA repair function for Smc5/6. As the core Smc5/6 genes are essential for cell viability, such studies have relied on the use of damage-sensitive hypomorphic alleles. Null mutants, however, arrest in mitosis with incompletely segregated chromosomes. The same phenotype is seen with damage-sensitive Smc5/6 hypomorphs such as smc6-74 following DNA damage, replication stress, or in combination with Top2 dysfunction conferred by the top2-191 mutant (Harvey, et al. 2004, Verkade, et al. 1999). This phenotype is caused by a failure of the separase-independent (or prophase) pathway of arm cohesin removal (Outwin, et al. 2009).
In S. pombe, eso1 is an essential gene (Tanaka, et al. 2000), and has been studied mostly with a temperature sensitive allele, eso1-H17. At restrictive temperature, eso1-H17 cells die in mitosis. However, in contrast to the essential nature of Smc3 acetylation in S. cerevisiae (Rowland, et al. 2009, Unal, et al. 2008), mutation of the acetylation sites on Psm3, the S. pombe Smc3 homolog, is not lethal (Feytout, et al. 2011). The lethality of eso1-H17 is due to the triggering of the spindle assembly checkpoint by premature centromere separation. In this context, Eso1 dysfunction is not related to cohesin acetylation or recruitment, but does result in a failure of centromeric cohesion. Interestingly, smc6-74 eso1-H17 double mutants co-suppress: smc6-74 suppresses the premature centromere separation seen in eso1-H17, while eso1-H17 suppresses the retention of cohesin on chromosome arms seen in smc6-74 (Lin, et al. 2016).
A temperature sensitive (at 36°C) allele of top2, top2-191 (Holm, et al. 1985, Uemura and Yanagida 1984), is synthetically lethal with Smc5/6 mutants at 30°C (Harvey, et al. 2002, Tapia-Alveal, et al. 2014b, Verkade, et al. 1999). This lethality is also due to cohesin retention, with no DNA damage above background levels (Outwin, et al. 2009). The defect conferred by top2-191 at 30°C is a non-catalytic one, but Top2 is also a structural element of mitotic chromosomes (Maeshima and Laemmli 2003), as judged by catenation assays and the ability of rescue by a catalytically-dead Top2 mutant (Outwin, et al. 2009, Tapia-Alveal, et al. 2010). Similarly, overexpression of catalytically inactive Top2 rescues temperature sensitive (ts) pds5 mutants in S. cerevisiae (Aguilar, et al. 2005). Here we have investigated functional interactions between top2-191 and eso1-H17. The phenotypes indicate a close connection between Top2 and Eso1 dysfunction for mitotic progression, and that this is linked (at least in part) to the regulation of cohesin by reversible Psm3 acetylation.
Materials and Methods
S. pombe genetics
Standard media and methods of propagation were used throughout (Moreno, et al. 1991). All S. pombe strains were constructed by tetrad analysis, and double mutants were selected as non-parental ditypes. For each genotype, multiple progeny were tested and backcrossed to exclude the presence of suppressor mutations occurring in the genome.
For chronic growth assays, cells were streaked on agar plates containing supplemented yeast extract plus glucose (YES) containing phloxin B. The plates were incubated at 25°C for 5 days, or 30°C for 4 days.
Microscopy
For assessing mitotic progression, exponentially growing liquid cultures were shifted from 25°C to 30°C for 4 hours before harvesting cells. 10μM Latrunculin B was added in the culture before shifting temperature to prevent lethal cutting of the nucleus by the division septum, which is associated with mitotic failure. The cells were fixed in 3.7% formaldehyde and DNA was stained with 1μg/ml 4′, 6-diamino-2-phenylindole (DAPI).
Microscopy was performed on a Nikon E800 microscope with a 100x 1.40-numerical-aperture Plan-Apo objective lens, together with differential interference contrast (DIC). Images were captured on a Q-Click camera by using the Q-capture suite plus software and prepared for publication using Adobe Photoshop.
Results
Top2 and Eso1 dysfunction is synthetically lethal
Double mutants between Smc5/6 hypomorphs and top2-191 are synthetically lethal at 30°C because of a failure of the prophase pathway of arm cohesin removal. We therefore investigated functional interactions between top2-191 and two alleles of eso1: the ts lethal eso1-H17 allele (Tanaka, et al. 2000), which has undetectable Psm3 acetylation at all temperatures (Feytout, et al. 2011, Lin, et al. 2016), and the non-ts allele eso1-1, that is severely compromised, though not ablated for Psm3 acetylation (Lin, et al. 2016). Both double mutant combinations were synthetically lethal at 30°C (Fig 1). As top2-191 potentiates cohesin retention, this was a somewhat unexpected result, as these eso1 alleles are predicted to lower the overall level of sister chromatid cohesion, and indeed eso1-H17 suppresses the cohesin-retention defects of smc6-74 (Lin, et al. 2016).
Figure 1. Synthetic lethality between top2-191 and cohesin regulator mutants: eso1- and pds5Δ.
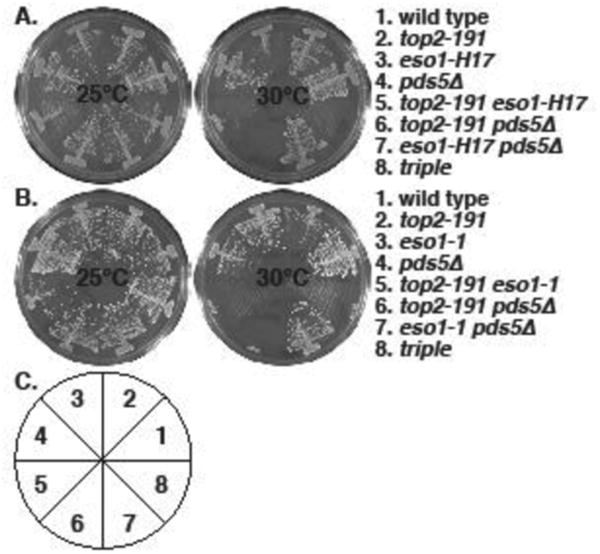
The indicated strains were streaked on YES plates and allowed to form colonies for 4 (30°C) or 5 (25°C) days. A. top2-191 is synthetic lethal with eso1-H17 and pds5Δ , and pds5Δ does not rescue top2-191 eso1-H17 lethality. B. The same phenotypes were seen with eso1-1. C. The pie chart depicts the position of strains in the plates in A and B.
The lethality of eso1-H17 in S. pombe is suppressed by the deletion of two cohesin regulators, pds5 (Tanaka, et al. 2001) and wpl1 (Feytout, et al. 2011). We tested interactions between these genes and top2-191 and found that both pds5Δ (Fig 1) and wpl1Δ (Fig 2) are synthetically lethal with top2-191. This is consistent with the anti-cohesive functions of Pds5 and Wpl1 homologs (Rowland, et al. 2009, Sutani, et al. 2009) and the cohesin-retention proffered by top2-191 (Outwin, et al. 2009), though Pds5 also has the property of promoting cohesion (Vaur, et al. 2012). However, balance to the cohesin cycle that is sufficient to suppress the synthetic lethality of top2-191 eso1-H17 and top2-191 eso1-1 was not provided by either pds5Δ (Fig 1) or wpl1Δ (Fig 2). Thus we conclude that Top2 function is essential when Eso1 function is compromised, even when Eso1 is not antagonized by either Pds5 or Wpl1 in the null mutants.
Figure 2. Synthetic lethality between top2-191 and cohesin regulator mutants: eso1- and wpl1Δ.
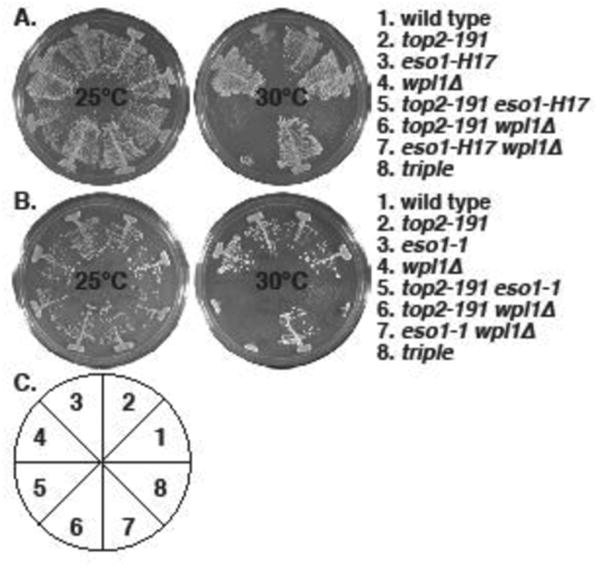
The indicated strains were streaked on YES plates and allowed to form colonies for 4 (30°C) or 5 (25°C) days. A. top2-191 is synthetic lethal with eso1-H17 and wpl1Δ , and wpl1Δ does not rescue top2-191 eso1-H17 lethality. B. The same phenotypes were seen with eso1-1. C. The pie chart depicts the position of strains in the plates in A and B.
Synthetic lethality of top2-191 with cohesin regulator mutants is due to mitotic failure
We next asked what was cause of the synthetic lethality of top2-191 with eso1-H17, eso1-1, pds5Δ and wpl1Δ . To this end, cultures were grown at 25°C, and then shifted to the semi-permissive temperature of 30°C for 4 hours. As seen before (Verkade, et al. 1999), 30°C has no effect on top2-191 alone (Fig 3A), and the same is true for the eso1 alleles (Lin, et al. 2016). All double mutant combinations with top2-191, however, phenocopied double mutants between top2-191 and Smc5/6 genes (Fig 3A): ∼50% cells with gross mitotic failure characterized by incomplete chromosome segregation and bisection of the nucleus by the division septum. As Eso1 is proposed to promote cohesion, and Wpl1 to antagonize this, the common phenotypes suggest there are greater perturbations to the cohesin cycle than this simple model, and is more in keeping with the duality of functions ascribed to Pds5. Moreover, this highlights that cohesin dynamics are a cycle, and not a linear pathway.
Figure 3. Synthetic lethalities are associated with mitotic failure.
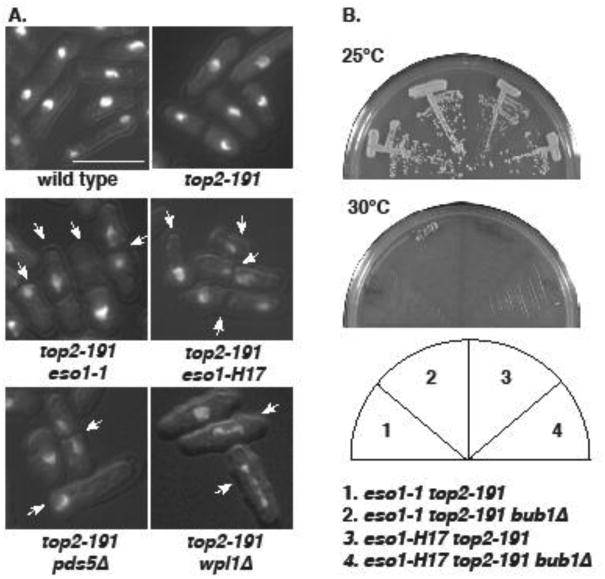
A. The indicated strains were grown at 25°C, and then shifted to 30°C for 4 hours. Cells were then fixed and stained with DAPI. Arrows indicate cells with mitotic failure. Bar = 10μm. B. bub1Δ does not suppress the synthetic lethal interactions between top2-191 and eso1 alleles.
We attempted to directly test cohesin retention in these strains using Chromatin Immunoprecipitation (ChIP)-based protocols. As cohesin is off chromosomes for such a short time, the only way we can assay this is by inactivated the cohesin loader Mis4 using the ts mis4-242 mutant in G2 synchronized cells. On this background, pre-loaded cohesin is stripped from the arms in prophase but cannot be reloaded in wild-type cells, but is retained in top2-191 smc6-74 cells (Outwin, et al. 2009). However, combining mis4-242 with these cohesin regulator mutants resulted in a significant synthetic slow growth phenotype (not shown), which made interpreting these data meaningless as aberrant cohesin retention is mimicked by slow cycling where cohesin has yet to have been removed by the prophase pathway.
As activation of the spindle checkpoint dictates the lethality of eso1-H17 (but not eso1-1) at 36°C (Lin, et al. 2016), we next investigated whether this could be a source of synthetic lethality. In no case did short spindles accumulate (not shown). Moreover, the synthetic lethalities were not suppressed by spindle checkpoint inactivation in bub1Δ cells (Fig 3B, and not shown).
Combining these data with an absence of a centromeric defect in eso1-1, spindle checkpoint activation is not the cause of the synthetic lethalities with top2-191. Thus, the blockade to full sister chromatid separation lies in the arms, and while likely due to cohesin retention, the negative genetic interactions we observed need to be overcome before this can be definitively proven.
Eso1 inactivation does not suppress mitotic failure in top2-191 smc6-74 cells
We recently found that eso1-H17 suppresses the cohesin retention defect conferred by DNA damage and replication stress in smc6-74 cells, presumably through the cohesion-loosening effects of Psm3 hypoacetylation. (Lin, et al. 2016). We next asked whether eso1-H17 had a similar suppressive effect on the potentiation of the same phenotype in top2-191 smc6-74 cells shifted to 30°C. For this experiment, we included the actin poison Latrunculin B to prevent lethal cutting of unresolved chromosomes by the division septum. Unlike with DNA damage or replication stress, eso1-H17 did not suppress the failure to complete mitosis in top2-191 smc6-74 (Fig 4). We also tested eso1-1, and it too showed no rescue of the segregation defects. Thus either the underlying mechanism leading to cohesin retention must be different when potentiated by top2-191, or the mechanism is the same but more severe and above a threshold that can be suppressed by cohesion loosening effects of Psm3 hypoacetylation caused by eso1-H17.
Figure 4. eso1-H17 does not rescue the mitotic failure of smc6-74 top2-191.
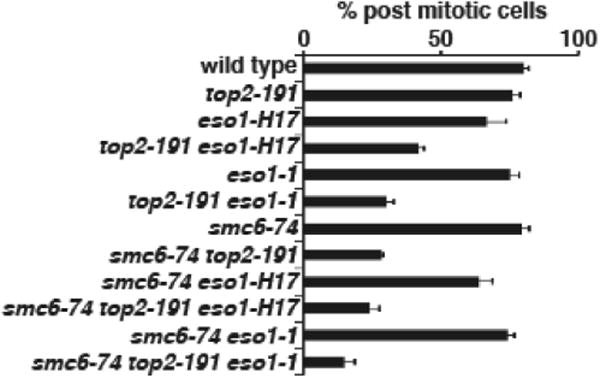
The indicated strains were grown at 25°C, and then shifted to 30°C in the presence of the actin poison latrunculin B. Cells were fixed and stained with DAPI. The percent of cells successfully completing mitosis and becoming binucleate were counted from 3 samples of 100 cells. Data are mean±SD.
Reversible cohesin acetylation is critical in top2-191 cells
To gauge whether the interactions between top2 and eso1 involved the acetylation status of Psm3, we next constructed double mutants between top2-191 and the non-acetylatable (psm3-RR) and acetyl-mimetic (psm3-NN) alleles of psm3 (Feytout, et al. 2011). Consistent with top2-191 potentiating cohesin retention, the top2-191 psm3-NN double was synthetic lethal at 30°C (Fig 5A) and died in lethal mitoses (Fig 5B). However, the same was true for top2-191 psm3-RR (Fig 5). This duality is reminiscent of top2-191 being synthetically lethal with mutations in both pro-cohesive (eso1 and pds5) and anti-cohesive (wpl1 and pds5) genes. These data further highlight the cyclic nature of cohesin dynamics, and show Top2 function likely to be important throughout the cohesin cycle.
Figure 5. top2-191 is synthetic lethal with acetylation site mutants of psm3.
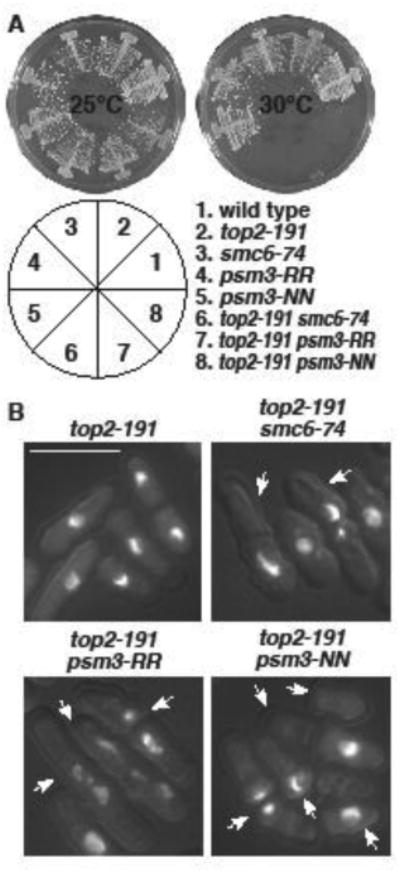
A. The indicated strains were streaked on YES plates and allowed to form colonies for 4 (30°C) or 5 (25°C) days. The pie chart depicts the position of strains in the plates. B. The indicated strains were grown at 25°C, and then shifted to 30°C. Cells were fixed and stained with DAPI. Arrows indicate cells with mitotic failure. Bar = 10μm.
Discussion
Tightly regulated chromosome dynamics control gene expression, DNA replication and repair, as well as sister chromatid separation. Much has been learned in recent years regarding the mechanisms at play in this biology, where the SMC complexes and DNA Topoisomerases are clearly central players. A lot of this biology in ancient in origin and highly conserved, though not all regulatory mechanisms are universal among eukaryotes. Variation exists in the evolution of additional mechanisms with increased biological complexity, as well as additional layers of regulation over a baseline of controls. The work we present here, and elsewhere recently (Lin, et al. 2016), extend functional relationships and regulatory mechanisms, at least in S. pombe, showing functional interactions between the cohesin regulator Eso1 and DNA topoisomerase II (Fig 6). These observations set the framework to revisit interactions between SMC complexes and Topoisomerases throughout the cell cycle, and to extend these observations to other experimental systems that utilize the two-step method of cohesin removal, with the ultimate goal of determining precise molecular mechanisms.
Figure 6. Regulation of the cohesin cycle.
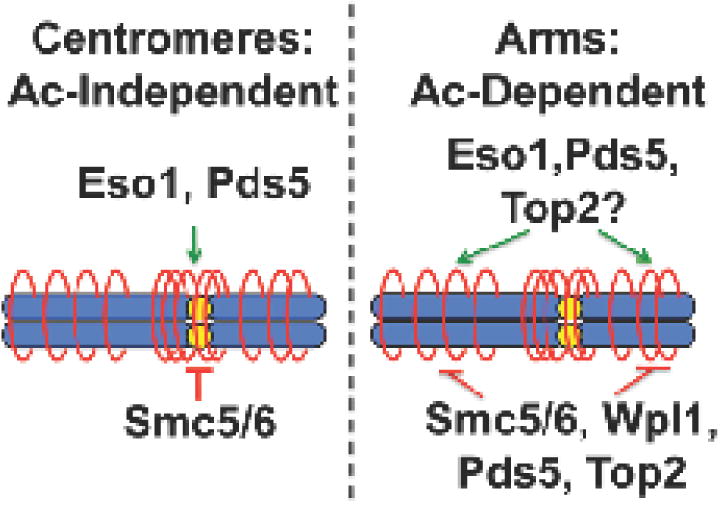
Previous work shows that centromeric cohesion is independent of Smc3/Psm3 acetylation, and associated with Pds5 localization to the centromeres. Cohesion on the arms is acetylation dependent, and antagonized by Wpl1. Pds5 has both pro- and anti-cohesive functions on the arms. Smc5/6 antagonizes both modes of cohesin regulation. Top2 dysfunction affects the acetylation-dependent arm pathway, and any perturbation to Smc3/Psm3 acetylation results in a dependence on Top2 function for survival, suggesting a dynamic role in the acetylation-dependent cohesin cycle.
Clearly cohesin, Smc5/6 and Top2 (plus their regulators) form a functional axis that is necessary for progression through mitosis in S. pombe. However, data in S. cerevisiae functionally link both Top1 (Mahendrawada, et al. 2016) and Top2 (Kanno, et al. 2015) to the Smc5/6 complex. While the precise defects conferred by the mutant alleles used may differ between systems, these findings are provocative and suggest the functional axis may be a more general feature of mitotic progression that is not limited to the prophase pathway, which does not control cohesin dynamics in S. cerevisiae.
Of particular note in these recent studies is the realization that the function of the Eso1 acetyltransferase in S. pombe is broader than enforcing the cohesiveness of cohesin via Smc3/Psm3 acetylation (Feytout, et al. 2011, Lin, et al. 2016, Vaur, et al. 2012). The newly defined centromeric function for Eso1 appears to be non-catalytic, or at least not through Psm3 acetylation. That this additional function exists is clear, but precise details of mechanism are clearly lacking, as is consideration of whether this is a phenomenon specific to S. pombe, which seems unlikely but is formally possible. One implication of recent findings is that the functional repertoire of Eso1 (and its homologs) may extend to further events, and perhaps will only be uncovered by generating detailed allelic series in multiple organisms. Moreover, temporal regulation is critical here, as the events at play are cyclic in nature and involve large scale re-engineering of many molecules and cellular structures.
Here, we have reported additional findings regarding Eso1/Top2 interactions. The synthetic lethalities we have observed are indicative of close functional interaction. However, such genetic observations can suffer from over-interpretation, and can in some cases represent unrelated and/or additive effects on cellular fitness. In the case of synthetic lethalities with top2-191, however, we do not believe this to be the case. Evidence to support this is that all genetic interactions with this top2 allele (but not others that are mutant for decatenation activity (Tapia-Alveal, et al. 2010)) are restricted to the Cohesin-Smc5/6 interactome. Further, they are not related to the catalytic activity of Top2 in chromosomal decatenation, and can be rescued by a Top2 mutant in which the catalytic tyrosine is mutated to phenylalanine (Outwin, et al. 2009). This is reminiscent of the high-copy suppression of ts alleles of PDS5 in S. cerevisiae by catalytically inactive Top2 (Aguilar, et al. 2005). However, reversible Psm3 acetylation does seem to be relevant to these observations given the interactions between top2-191 and both psm3-RR and psm3-NN. This duality also highlights the futility in considering these data in a linear sense, akin to a biosynthetic pathway with the sequential interplay between enzymes and substrates.
Motivation to extend the study of Eso1 and its homologs comes from the fact that mutations in homologous genes and other components of the cohesin cycle are associated with cancers and developmental syndromes (Ball, et al. 2014, Barbero 2013, Bose and Gerton 2010, Cucco and Musio 2016, Gerkes, et al. 2010, McNairn and Gerton 2008, Musio and Krantz 2010, Parenti, et al. 2016, Skibbens, et al. 2013). Not all these need be explained by Smc3 hypoacetylation, and indeed the biology of these diseases, let alone interventional therapies, would benefit greatly by a better understanding of the underlying biology.
Acknowledgments
We thank Jean-Paul Javerzat for the provision of strains. The authors declare no competing financial interests. This work was supported by NIH grants R01-GM088162 and T32-CA078207.
References
- Aguilar C, Davidson C, Dix M, Stead K, Zheng K, Hartman T, Guacci V. Topoisomerase II suppresses the temperature sensitivity of Saccharomyces cerevisiae pds5 mutants, but not the defect in sister chromatid cohesion. Cell Cycle. 2005;4:1294–1304. doi: 10.4161/cc.4.9.1997. doi: [DOI] [PubMed] [Google Scholar]
- Ball AR, Jr, Chen YY, Yokomori K. Mechanisms of cohesin-mediated gene regulation and lessons learned from cohesinopathies. Biochim Biophys Acta. 2014;1839:191–202. doi: 10.1016/j.bbagrm.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbero JL. Genetic basis of cohesinopathies. The application of clinical genetics. 2013;6:15–23. doi: 10.2147/TACG.S34457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose T, Gerton JL. Cohesinopathies, gene expression, and chromatin organization. J Cell Biol. 2010;189:201–210. doi: 10.1083/jcb.200912129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copsey A, Tang S, Jordan PW, Blitzblau HG, Newcombe S, Chan AC, Newnham L, Li Z, Gray S, Herbert AD, Arumugam P, Hochwagen A, Hunter N, Hoffmann E. Smc5/6 coordinates formation and resolution of joint molecules with chromosome morphology to ensure meiotic divisions. PLoS Genet. 2013;9:e1004071. doi: 10.1371/journal.pgen.1004071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucco F, Musio A. Genome stability: What we have learned from cohesinopathies. American journal of medical genetics Part C, Seminars in medical genetics. 2016;172:171–178. doi: 10.1002/ajmg.c.31492. [DOI] [PubMed] [Google Scholar]
- Feytout A, Vaur S, Genier S, Vazquez S, Javerzat JP. Psm3 acetylation on conserved lysine residues is dispensable for viability in fission yeast but contributes to Eso1-mediated sister chromatid cohesion by antagonizing Wpl1. Mol Cell Biol. 2011;31:1771–1786. doi: 10.1128/MCB.01284-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerkes EH, van der Kevie-Kersemaekers AM, Yakin M, Smeets DF, van Ravenswaaij-Arts CM. The importance of chromosome studies in Roberts syndrome/SC phocomelia and other cohesinopathies. European journal of medical genetics. 2010;53:40–44. doi: 10.1016/j.ejmg.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Harvey SH, Krien MJ, O'Connell MJ. Structural maintenance of chromosomes (SMC) proteins, a family of conserved ATPases. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-2-reviews3003. REVIEWS3003 doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SH, Sheedy DM, Cuddihy AR, O'Connell MJ. Coordination of DNA damage responses via the Smc5/Smc6 complex. Mol Cell Biol. 2004;24:662–674. doi: 10.1128/MCB.24.2.662-674.2004. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T. The ABCs of SMC proteins: two-armed ATPases for chromosome condensation, cohesion, and repair. Genes Dev. 2002;16:399–414. doi: 10.1101/gad.955102. doi: [DOI] [PubMed] [Google Scholar]
- Hirano T. Condensins: organizing and segregating the genome. Curr Biol. 2005;15:R265–275. doi: 10.1016/j.cub.2005.03.037. doi: [DOI] [PubMed] [Google Scholar]
- Hirano T. At the heart of the chromosome: SMC proteins in action. Nat Rev Mol Cell Biol. 2006;7:311–322. doi: 10.1038/nrm1909. doi: [DOI] [PubMed] [Google Scholar]
- Holm C, Goto T, Wang J, Bostein D. DNA topoisomerase II is required at the time of mitosis in yeast. Cell. 1985;41:553–563. doi: 10.1016/s0092-8674(85)80028-3. doi: [DOI] [PubMed] [Google Scholar]
- Iwasaki O, Noma KI. Condensin-mediated chromosome organization in fission yeast. Curr Genet. 2016;62:739–743. doi: 10.1007/s00294-016-0601-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T, Berta DG, Sjogren C. The Smc5/6 Complex Is an ATP-Dependent Intermolecular DNA Linker. Cell reports. 2015;12:1471–1482. doi: 10.1016/j.celrep.2015.07.048. [DOI] [PubMed] [Google Scholar]
- Lehmann AR, Walicka M, Grittiths DJF, Murray JM, Watts FZ, McCready S, Carr AM. The rad18 gene of Schizosaccharomyces pombe defines a new subgroup of the SMC superfamily involved in DNA repair. Mol Cell Biol. 1995;15:7067–7080. doi: 10.1128/mcb.15.12.7067. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Tapia-Alveal C, Jabado OJ, Germain D, O'Connell MJ. An acetyltransferase-independent function of Eso1 regulates centromere cohesion. Mol Biol Cell. 2016;27:4002–4010. doi: 10.1091/mbc.E16-08-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindroos HB, Strom L, Itoh T, Katou Y, Shirahige K, Sjogren C. Chromosomal association of the Smc5/6 complex reveals that it functions in differently regulated pathways. Mol Cell. 2006;22:755–767. doi: 10.1016/j.molcel.2006.05.014. doi: [DOI] [PubMed] [Google Scholar]
- Maeshima K, Laemmli UK. A two-step scaffolding model for mitotic chromosome assembly. Dev Cell. 2003;4:467–480. doi: 10.1016/s1534-5807(03)00092-3. doi: [DOI] [PubMed] [Google Scholar]
- Mahendrawada L, Rai R, Kothiwal D, Laloraya S. Interplay between Top1 and Mms21/Nse2 mediated sumoylation in stable maintenance of long chromosomes. Curr Genet. 2016 doi: 10.1007/s00294-016-0665-4. doi:10. 1007/s00294-016-0665-4. [DOI] [PubMed] [Google Scholar]
- McNairn AJ, Gerton JL. Cohesinopathies: One ring, many obligations. Mutat Res. 2008;647:103–111. doi: 10.1016/j.mrfmmm.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Method Enzymol. 1991;194:795–723. doi: 10.1016/0076-6879(91)94059-l. doi: [DOI] [PubMed] [Google Scholar]
- Musio A, Krantz ID. Am J Med Genet A; Cohesin biology and the cohesinopathies: Abstracts from the Second Biennial Conference; Pontignano, Italy. 2009; 2010. pp. 1630–1640. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. Cohesin: a catenase with separate entry and exit gates? Nat Cell Biol. 2011;13:1170–1177. doi: 10.1038/ncb2349. [DOI] [PubMed] [Google Scholar]
- Nasmyth K, Haering CH. Cohesin: its roles and mechanisms. Annu Rev Genet. 2009;43:525–558. doi: 10.1146/annurev-genet-102108-134233. [DOI] [PubMed] [Google Scholar]
- Outwin EA, Irmisch A, Murray JM, O'Connell MJ. Smc5-Smc6-dependent removal of cohesin from mitotic chromosomes. Mol Cell Biol. 2009;29:4363–4375. doi: 10.1128/MCB.00377-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenti I, Gervasini C, Pozojevic J, Graul-Neumann L, Azzollini J, Braunholz D, Watrin E, Wendt KS, Cereda A, Cittaro D, Gillessen-Kaesbach G, Lazarevic D, Mariani M, Russo S, Werner R, Krawitz P, Larizza L, Selicorni A, Kaiser FJ. Broadening of cohesinopathies: exome sequencing identifies mutations in ANKRD11 in two patients with Cornelia de Lange-overlapping phenotype. Clin Genet. 2016;89:74–81. doi: 10.1111/cge.12564. [DOI] [PubMed] [Google Scholar]
- Rowland BD, Roig MB, Nishino T, Kurze A, Uluocak P, Mishra A, Beckouet F, Underwood P, Metson J, Imre R, Mechtler K, Katis VL, Nasmyth K. Building sister chromatid cohesion: smc3 acetylation counteracts an antiestablishment activity. Mol Cell. 2009;33:763–774. doi: 10.1016/j.molcel.2009.02.028. doi: [DOI] [PubMed] [Google Scholar]
- Skibbens RV, Colquhoun JM, Green MJ, Molnar CA, Sin DN, Sullivan BJ, Tanzosh EE. Cohesinopathies of a feather flock together. PLoS Genet. 2013;9:e1004036. doi: 10.1371/journal.pgen.1004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutani T, Kawaguchi T, Kanno R, Itoh T, Shirahige K. Budding yeast Wpl1(Rad61)-Pds5 complex counteracts sister chromatid cohesion-establishing reaction. Curr Biol. 2009;19:492–497. doi: 10.1016/j.cub.2009.01.062. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Hao Z, Kai M, Okayama H. Establishment and maintenance of sister chromatid cohesion in fission yeast by a unique mechanism. Embo J. 2001;20:5779–5790. doi: 10.1093/emboj/20.20.5779. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Yonekawa T, Kawasaki Y, Kai M, Furuya K, Iwasaki M, Murakami H, Yanagida M, Okayama H. Fission yeast Eso1p is required for establishing sister chromatid cohesion during S phase. Mol Cell Biol. 2000;20:3459–3469. doi: 10.1128/mcb.20.10.3459-3469.2000. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia-Alveal C, Lin SJ, O'Connell MJ. Functional interplay between cohesin and Smc5/6 complexes. Chromosoma. 2014a;123:437–445. doi: 10.1007/s00412-014-0474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia-Alveal C, Lin SJ, Yeoh A, Jabado OJ, O'Connell MJ. H2A.Z-Dependent Regulation of Cohesin Dynamics on Chromosome Arms. Mol Cell Biol. 2014b;34:2092–2104. doi: 10.1128/MCB.00193-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia-Alveal C, Outwin EA, Trempolec N, Dziadkowiec D, Murray JM, O'Connell MJ. SMC complexes and topoisomerase II work together so that sister chromatids can work apart. Cell Cycle. 2010 doi: 10.4161/cc.9.11.11734. [DOI] [PubMed] [Google Scholar]
- Uemura T, Yanagida M. Isolation of type I and II DNA topoisomerase mutants from fission yeast: single and double mutants show different phenotypes in cell growth and chromatin organization. Embo J. 1984;3:1737–1744. doi: 10.1002/j.1460-2075.1984.tb02040.x. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal E, Heidinger-Pauli JM, Kim W, Guacci V, Onn I, Gygi SP, Koshland DE. A molecular determinant for the establishment of sister chromatid cohesion. Science. 2008;321:566–569. doi: 10.1126/science.1157880. doi: [DOI] [PubMed] [Google Scholar]
- Vaur S, Feytout A, Vazquez S, Javerzat JP. Pds5 promotes cohesin acetylation and stable cohesin-chromosome interaction. EMBO Rep. 2012;13:645–652. doi: 10.1038/embor.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkade HM, Bugg SJ, Lindsay HD, Carr AM, O'Connell MJ. Rad18 is required for DNA repair and checkpoint responses in fission yeast. Mol Biol Cell. 1999;10:2905–2918. doi: 10.1091/mbc.10.9.2905. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]


