Abstract
Background
Patients with psychotic disorders are often treated with numerous medications, many of which have anticholinergic activity. We assessed cognition in relation to the cumulative anticholinergic burden of multiple drugs included in treatment regimens of participants from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study.
Method
Clinically stable participants with schizophrenia (n=206), schizoaffective disorder (n=131), and psychotic bipolar disorder (n=146) were examined. Anticholinergic properties of all scheduled drugs were quantified using the Anticholinergic Drug Scale (ADS). ADS scores were summed across individual drugs to create a total ADS burden score for each participant and examined in relation to the Brief Assessment of Cognition in Schizophrenia (BACS).
Results
Anticholinergic burden aggregated across all medications was inversely related to cognitive performance starting at ADS scores of 4 in participants with schizophrenia. Those with ADS scores ≥4 had lower composite BACS scores compared to those with ADS<4 (p=0.004). Among BACS subtests, Verbal Memory was the most adversely affected by high anticholinergic burden. Despite similar anticholinergic burden scores across groups, a significant effect of anticholinergic burden was not detected in schizoaffective or psychotic bipolar disorder.
Conclusion
We identified an adverse effect threshold of anticholinergic burden on cognition in clinically stable participants with schizophrenia. This relationship was not identified in affective psychoses. Examination of other medications, doses, and clinical measures did not account for these findings. Patients with schizophrenia may have increased cognitive susceptibility to anticholinergic medications and the aggregate effects of one’s medication regimen may be important to consider in clinical practice.
Keywords: Anticholinergic medication burden, cognitive impairments, psychotic disorders
1. Introduction
Neuropsychological impairment is a core feature of schizophrenia (Hill et al., 2004b; Keefe et al., 2007; Lam et al., 2014). Impairments have been reported in many cognitive domains, including verbal learning and memory, verbal fluency, working memory, processing speed, and executive function (Bilder et al., 2002; Hill et al., 2013, 2004a; Saykin et al., 1994). Similar neuropsychological deficits, albeit less severe, are reported in other psychotic disorders (Hill et al., 2013, 2009, 2008; Lee et al., 2016). Cognitive impairment relates directly to functional outcomes in patients such as psychosocial skill acquisition, performing daily activities, and vocational attainment and contributes to poor quality of life (Green et al., 2000; Leifker et al., 2009). Identifying and minimizing factors exacerbating cognitive deficits is essential for enhancing quality of life and compliance to treatments in patients with psychotic disorders.
Medications with high anticholinergic activity may adversely affect cognition. One biological mechanism for this effect relates to the suppression of the central cholinergic system via direct blockade of muscarinic cholinergic receptors which can disrupt memory (Bartus et al., 1982; Everitt and Robbins, 1997). Among the five distinct muscarinic receptor subtypes (M1–M5), antagonism of the muscarinic M1 receptor is thought to be most closely linked to cognitive impairments, especially those involving memory processes (Everitt and Robbins, 1997). These M1 receptor relationships are linked to cognition in multiple central nervous system (CNS) disorders (Gray and Roth, 2007).
The adverse cognitive effects of anticholinergic medications are established from studies primarily in the elderly whereby anticholinergic burden is associated with increases in delirium, falls, and cognitive deficits (Ancelin et al., 2006; Campbell et al., 2009; Risacher et al., 2016). Furthermore, the aggregate contribution of numerous medications in treatment regimen can collectively contribute to these effects (Campbell et al., 2016; Gray et al., 2015). Studies of anticholinergic medication effects on cognition in schizophrenia (Baitz et al., 2012; Baker et al., 1983; Brébion et al., 2004; Fayen et al., 1988; Minzenberg et al., 2004; Mori et al., 2002; Perlick et al., 1986; Strauss et al., 1990; Sweeney et al., 1991; Tune et al., 1982; Wojtalik et al., 2012) typically have smaller sample sizes and focus on specific anticholinergic medications (i.e. benztropine or trihexyphenidyl) (Baitz et al., 2012; Baker et al., 1983; Brébion et al., 2004; Fayen et al., 1988; Mori et al., 2002; Sweeney et al., 1991) used to treat movement disorder side effects of antipsychotic drugs. However, investigations considering other medications with anticholinergic properties in patient regimens are lacking and these relationships in affective psychosis are relatively understudied.
Patients with psychosis-spectrum disorders often take a number of psychotropic medications, which have varying degrees of anticholinergic properties (Chakos et al., 2006). High medical comorbidities in psychosis often result in the utilization of many non-psychotropic medications, some of which have anticholinergic properties (Carnahan et al., 2006; Jeste et al., 1996). Due to known differences in medication utilization, clinical features, and cognitive deficits across psychotic disorders (Hill et al., 2013), it is important to better understand the adverse cognitive implications of net anticholinergic burden and to examine such effects in each of these diagnoses. In the present study, we assessed cognition in relation to anticholinergic burden aggregated across all medications included in individual treatment regimens of clinically stable patients with schiaophrenia, schizoaffective disorder, or psychotic bipolar disorder from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study (Tamminga et al., 2013).
2. Methods
2.1 Participants
Participants in this study were selected from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) consortium, which is a study designed to examine an array of candidate endophenotypes including cognition across psychotic disorders (Tamminga et al., 2013). Inclusion criteria for B-SNIP included: (1) age between 15 and 65; (2) age-corrected Wide Range Achievement Test Fourth Edition (WRAT-IV) Reading Score > 65; (3) sufficient English proficiency to complete cognitive testing; (4) no history of seizures or organic brain insults with loss of consciousness > 10 minutes; (5) no diagnosis of substance abuse in the past 30 days or substance dependence during the previous 6 months; (6) negative urine toxicology screen for commonly abused drugs the day of testing; (7) no history of unstable medical or neurological conditions (see reference (Hill et al., 2013)). We focused on a subgroup of B-SNIP probands (206 schizophrenia, 131 schizoaffective, and 146 psychotic bipolar disorder) who were taking at least one antipsychotic medication and had detailed dosing information available. Given the known relationships of dopamine antagonism properties and cognition (Reilly et al., 2006; Sweeney et al., 1991), we selected patients with antipsychotic exposure that could be consistently examined across diagnoses in our analyses.
DSM-IV diagnoses were established via consensus diagnostic meetings using information obtained from the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (First et al., 1995), available medical charts, and interviews with relatives. Clinical symptom assessments included the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987), the Young Mania Rating Scale (YMRS) (Young et al., 1978), and the Montgomery-Åsberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979). The Schizo-Bipolar Scale (SBS) ranging from 0 (the most bipolar-like disorder) to 9 (the most schizophrenia-like disorder) (Keshavan et al., 2011) was also assessed in relation to medication variables. All patients were clinically stable with no major changes in medication regimen for at least 4 weeks. Institutional review board approvals were obtained at each B-SNIP site (Hartford, Baltimore, Chicago, Dallas, Boston and Detroit). After the study was explained in detail, all participants provided written informed consent.
2.2 Medication assessments
A medication history interview was performed for both prescription and non-prescription medications. Estimated anticholinergic potency was assigned a numerical value for each scheduled medication in regimens using an updated version of the Anticholinergic Drug Scale (ADS) (Carnahan et al., 2006). This is currently the most comprehensive scale available to quantify anticholinergic burden for the majority of medications commonly used to treat psychotic symptoms and has been validated against serum anticholinergic activity (SAA) (Carnahan et al., 2006). Since the initial development of the ADS, additional information about the anticholinergic properties of some older medications (Chew et al., 2008; http://kidbdev.med.unc.edu/databases/kidb.php), as well as newly available medications with anticholinergic properties, were incorporated for the current analyses. Examples include modification of scores for selected medications (i.e. olanzapine, quetiapine, etc.) based on more recent reports of anticholinergic activity (Chew et al., 2008) and available inhibitory constant (Ki) values for muscarinic receptors (http://kidbdev.med.unc.edu/databases/kidb.php). The original ADS is available in Carnahan et al (Carnahan et al., 2006), and the updated items for this analysis are highlighted in Supplement Table 1. Supplement Table 2 shows the number of participants for each total ADS score. Total ADS scores for each patient were calculated by summing the values of all scheduled medications used by each participant. Total ADS scores based on the aggregate accumulation of many medications each with different anticholinergic burden values were not normally distributed (due to many participants having no exposure), and the linear nature of ADS scores in relation to serum anticholinergic activity has not been established. Thus ADS scores were treated as ordinal data (0, 1, 2, 3, 4, 5…12).
Finally, to estimate relative antipsychotic dose, a chlorpromazine dose equivalent (CPZeq) was calculated using the Andreasen method (Andreasen et al., 2010). CPZeq was not normally distributed and required a log transformation to normalize the distribution in each diagnostic group for statistical analyses.
2.3 Neuropsychological performance
Participants completed the Brief Assessment of Cognition in Schizophrenia (BACS) battery to assess neuropsychological function (Keefe et al., 2008, 2004). The BACS consists of six subtests: Verbal Memory, Digit Sequencing, Token Motor, Verbal Fluency, Symbol Coding, and Tower of London. BACS composite and subtest z-scores were derived from age, sex, and race stratified norms as in previous studies (Hill et al., 2013; Keefe et al., 2008). Primary outcomes included BACS total scores, and also the Verbal Memory subtest given the established impact of anticholinergic drugs on this cognitive domain (Brébion et al., 2004; Minzenberg et al., 2004; Sweeney et al., 1991). Other BACS subtests were evaluated as secondary outcomes.
2.4 Statistical analyses
Diagnostic group differences in baseline demographics and clinical characteristics were examined using analysis of variance (ANOVA) for continuous variables and chi-square (χ2) analyses for categorical variables. To examine the relationship of anticholinergic burden with cognitive performance in each diagnostic group, we conducted a series of multivariable linear regression analyses controlling for symptom severity (PANSS total score) and antipsychotic burden (CPZeq). Analyses were stratified by diagnostic group given that the larger B-SNIP sample (Hill et al., 2013) as well as analyses of the current study sample identified significant group differences on a number of demographic and clinical parameters (see Table 1). Linear regression was first used to identify whether ADS scores ≥ 1 were collectively related to BACS scores in each diagnosis. We then examined whether there was a threshold at which anticholinergic burden impacted neuropsychological performance, each ordinal ADS level was compared to no anticholinergic exposure. Findings indicated a threshold of 4+ on the ADS before neuropsychological scores (primary outcomes) were significantly impacted in the schizophrenia group (Supplement Figures 1 and 2). This threshold was then used to set anticholinergic burden strata (None: ADS = 0; Low: ADS = 1–3; High: ADS = 4+) for subsequent analyses.
Table 1.
Demographic and clinical data of participants
| Variable.a | Schizophrenia (N=206) | Schizoaffective Disorder (N = 131) | Psychotic Bipolar Disorder (N=146) | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Maleb, *** | 137 | 66.5 | 53 | 40.5 | 55 | 37.7 |
| Racec | ||||||
| Caucasian*** | 101 | 49 | 74 | 56.5 | 106 | 72.6 |
| African American*** | 94 | 45.6 | 54 | 41.2 | 34 | 23.3 |
| Other | 11 | 5.3 | 3 | 2.3 | 6 | 4.1 |
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 36.37 | 13.21 | 36.83 | 11.84 | 35.08 | 12.20 |
| Education (years) | 12.77 | 2.42 | 13.04 | 2.17 | 13.97 | 2.28 |
| WRAT-IV Readingd, ** | 93.90 | 15.50 | 95.67 | 14.25 | 99.92 | 13.65 |
| PANSS Total Scoree, *** | 64.23 | 17.10 | 69.65 | 15.49 | 53.58 | 13.55 |
| SBS Scoref, *** | 7.96 | 1.16 | 5.24 | 1.46 | 1.15 | 1.13 |
| BACS Composite Scoreg, ** | −1.52 | 1.27 | −1.34 | 1.19 | −1.09 | 1.23 |
| BACS Subtest Score | ||||||
| Verbal Memoryg, ** | −1.00 | 1.19 | −0.82 | 1.33 | −0.55 | 1.21 |
| Digit Sequencing* | −0.94 | 1.06 | −0.70 | 1.06 | −0.68 | 1.01 |
| Token Motorh, * | −1.19 | 1.15 | −1.28 | 1.04 | −0.93 | 1.14 |
| Verbal Fluency* | −0.87 | 1.09 | −0.59 | 1.04 | −0.65 | 1.13 |
| Symbol Coding | −1.40 | 1.18 | −1.36 | 1.13 | −1.21 | 1.17 |
| Tower of London | −0.63 | 1.28 | −0.55 | 1.16 | −0.31 | 1.16 |
| Medications | ||||||
| Total Number of All Medicationsg, ** | 4.21 | 2.57 | 4.93 | 3.35 | 5.40 | 3.63 |
| Total Number of Psychotropic Medicationsi, *** | 2.51 | 1.39 | 3.30 | 1.54 | 3.40 | 1.53 |
| Chlorpromazine Equivalents (mg/day)j, *** | 527.61 | 422.23 | 539.07 | 507.18 | 354.67 | 325.68 |
| Total ADS Score | 2.46 | 2.37 | 2.67 | 2.21 | 2.28 | 2.04 |
P-value < 0.05;
p-value < 0.01;
p-value < 0.001
WRAT-IV Reading=Wide-Range Achievement Test 4th Edition, reading subtest; PANSS=Positive and Negative Syndrome Scale; SBS= Schizo-Bipolar Scale; BACS= Brief Assessment of Cognition in Schizophrenia; ADS=Anticholinergic Drug Scale
Disproportionate number of males across diagnostic groups
Disproportionate number of Caucasian and African-American across diagnostic groups
Psychotic bipolar > schizophrenia and schizoaffective group
Schizoaffective > schizophrenia > psychotic bipolar group
Schizophrenia > schizoaffective > psychotic bipolar group
Psychotic bipolar > schizophrenia group
Psychotic bipolar > schizoaffective group
Psychotic bipolar, schizoaffective > schizophrenia group
Schizophrenia, schizoaffective > psychotic bipolar group
In additional post hoc analyses, we explored other ways to examine the influence of disease or symptom severity and overall medication burden. This included analyses examining the total number of psychotropic medications or total number of all medications as additional covariates as well as focused analyses of ADS relationships with BACS in patients with and without clozapine. We examined the influence of other medication groups (e.g. other psychotropic classes such as sedative-hypnotics, anticonvulsants, stimulants, lithium, antidepressants, etc.) as well as premorbid intelligence. In addition, analyses were repeated stratified by age (two age groups: ≤ 50 years and > 50 years) to examine the potential effect of age. Finally, dosing of benztropine, as a representative highly anticholinergic drug, was compared across diagnosis groups to assess potential dosing differences beyond antipsychotic drugs. In these analyses, all major findings remained consistent with the primary analysis and of similar magnitude.
Lastly, the relationship between neuropsychological performance and SBS scores was examined using Spearman’s correlation within no (ADS=0), low (ADS=1–3), and high (ADS≥4) ADS burden strata in all psychosis participants to examine the cognitive impact of anticholinergic properties in relation to a dimensional assessment of disease presentation, rather than discrete diagnosis categories.
All statistical analyses were performed using SPSS version 23 (IBM Corp, Armonk, NY), and significance was set at a two-tailed p-value < .05 for all analyses.
3. Results
3.1 Participant characteristics
The demographic and clinical data are summarized in Table 1. There were no significant differences in the total ADS scores across diagnostic groups. However, there was a higher frequency of medication use in general, greater psychotropic medication use, and lower antipsychotic dose among the bipolar group compared to the other diagnostic groups (p-values < 0.05).
3.2 Association between anticholinergic burden and neuropsychological performance
When examining the overall effect of any anticholinergic exposure on BACS performance, ADS scores ≥ 1 were collectively associated with composite BACS scores as compared to those with ADS=0 in schizophrenia (p-value 0.022) and schizoaffective disorder (p-value 0.027), but not in psychotic bipolar disorder (p-value 0.508). After further examination of incremental increases in anticholinergic burden, we identified that among schizophrenia participants, the ADS burden score of 4 was the point at which adverse cognitive influence became statistically significant and further that a high (ADS≥4) versus no (ADS=0) or low (ADS=1–3) anticholinergic burden was significantly associated with lower performance on the BACS composite and Verbal Memory (Figure 1 and 2). The unstandardized coefficient (B) of the ADS ≥ 4 for composite BACS was −0.576 [95% confidence interval (CI) −0.964 to −0.189, p-value = 0.004], indicating that schizophrenia patients with high ADS scores had lower composite BACS scores (on average a 0.58 standard deviations (SDs) lower) compared to their counterparts with lower ADS scores. Verbal Memory scores were 0.55 SDs worse in schizophrenia patients with high as compared to no or low anticholinergic burden. Additionally, among schizophrenia participants, there was a significant association between high anticholinergic burden and lower performance on the Token Motor and Symbol Coding (Table 2). Because there were education differences across anticholinergic burden strata (p-value = 0.024) within the schizophrenia group, we repeated these analyses adding years of education as a covariate, which did not change the pattern of results. In addition, there were no differences in the pattern of findings among men and women with schizophrenia. These patterns were not statistically significant among either schizoaffective or psychotic bipolar groups on the BACS composite or any subtest (Table 2). It was noted that higher antipsychotic dose was associated with lower performance on Token Motor performance among all diagnostic groups; however, all other cognitive outcomes were not related to antipsychotic dose in schizophrenia participants.
Figure 1.
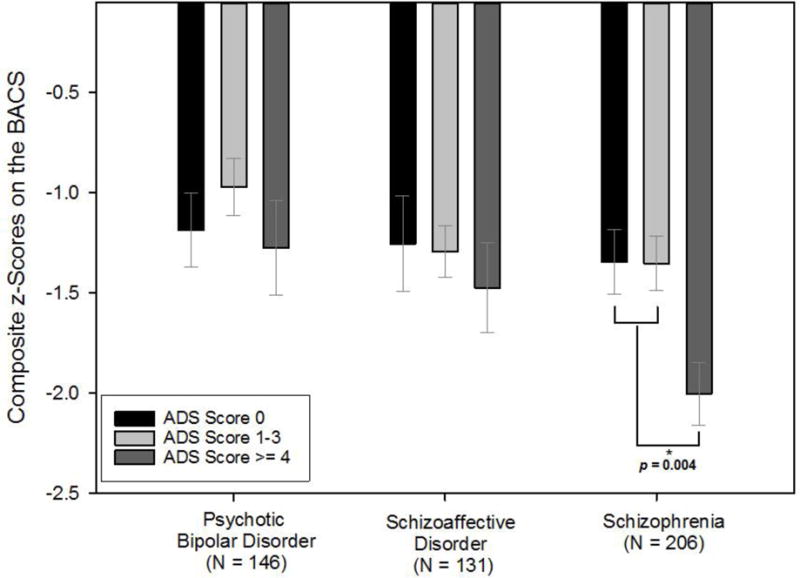
Anticholinergic burden and global neuropsychological performance across psychotic disorders. Abbreviations: BACS=Brief Assessment of Cognition in Schizophrenia; ADS=Anticholinergic Drug Scale.
Figure 2.
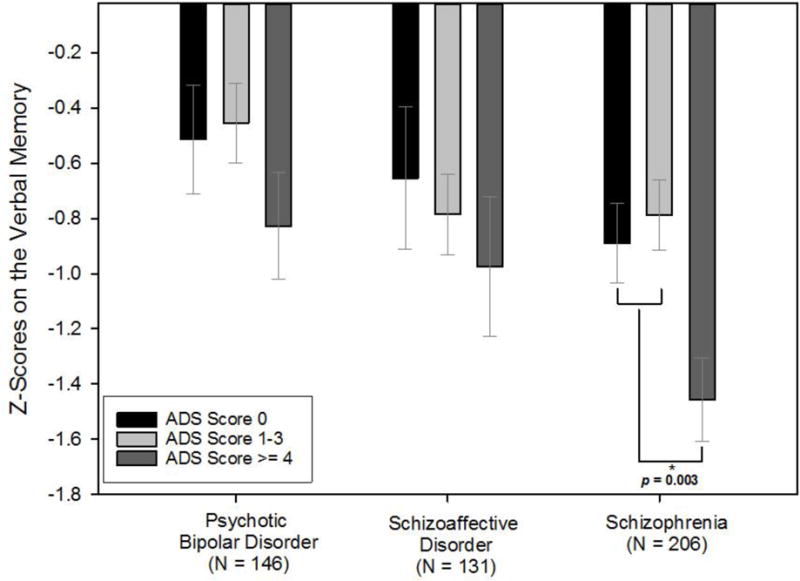
Anticholinergic burden and Verbal Memory performance across psychotic disorders score. Abbreviations: ADS=Anticholinergic Drug Scale.
Table 2.
Unstandardized coefficients of high anticholinergic burden when controlling for current symptom severity (PANSS total score) and antipsychotic dose (CPZeq)a
| High Anticholinergic Burden (ADSb score of ≥ 4) | ||||||
|---|---|---|---|---|---|---|
| Schizophrenia (N=206) | Schizoaffective Disorder (N = 131) | Psychotic Bipolar Disorder (N=146) | ||||
| Unstandardized Coefficients (B) | p-value | Unstandardized Coefficients (B) | p-value | Unstandardized Coefficients (B) | p-value | |
| Composite BACSc | −0.576 | 0.004 | −0.08 | 0.725 | −0.104 | 0.671 |
| Verbal Memory | −0.55 | 0.003 | −0.152 | 0.557 | −0.297 | 0.231 |
| Digit Sequencing | −0.148 | 0.387 | −0.146 | 0.482 | −0.103 | 0.622 |
| Token Motor | −0.523 | 0.003 | 0.044 | 0.823 | −0.061 | 0.791 |
| Verbal Fluency | −0.207 | 0.225 | 0.123 | 0.545 | −0.086 | 0.698 |
| Symbol Coding | −0.512 | 0.006 | −0.299 | 0.163 | 0.142 | 0.536 |
| Tower of London | −0.386 | 0.061 | 0.056 | 0.804 | −0.001 | 0.995 |
Bold: statistically significant (p-value < 0.05)
ADS=Anticholinergic Drug Scale
BACS=Brief Assessment of Cognition in Schizophrenia
3.3 Anticholinergic burden and neuropsychological performance in relation to dimensional assessment of psychotic disorders
The relation of anticholinergic burden and neuropsychological performance was assessed along a psychotic illness dimension. A significant negative correlation was seen for BACS composite in which scores decreased (worsened) as SBS scores increased from most bipolar-like symptoms (SBS score 0) to most schizophrenia-like symptoms (SBS score 9), but only in those within the high anticholinergic burden group (Spearman’s rho = −0.258, p-value = 0.004).
4. Discussion
To our knowledge, this is the first study to identify a potential threshold effect of cumulative anticholinergic burden on neuropsychological performance using medication regimen data from clinically stable patients with schizophrenia. Furthermore, this threshold effect was not observed in participants with schizoaffective or psychotic bipolar disorder. The negative influence of anticholinergic burden on neurocognitive performance in schizophrenia participants was observed when total ADS scores calculated from all currently scheduled medications were 4+. Among the BACS subtests, Verbal Memory, Token Motor, and Symbol Coding were significantly related to total ADS scores exceeding this threshold. The magnitude of this effect was ~0.5–0.6 standard deviation greater cognitive impairment in high anticholinergic schizophrenia patients compared to their low or no anticholinergic burden counterparts.
Verbal Memory was the BACS subtest most robustly influenced by anticholinergic burden and this is consistent with previously established effects of anticholinergic medications on verbal learning and memory in schizophrenia patients (Brébion et al., 2004; Minzenberg et al., 2004; Sweeney et al., 1991) and in the elderly (Bartus et al., 1982); however, the diagnostic sensitivity of this effect is a novel finding. Schizophrenia participants receiving a high anticholinergic load showed deficits in Verbal Memory approximately twice as large as those with lower anticholinergic burden. In contrast, no significant differences in Verbal Memory scores were found among the schizoaffective and psychotic bipolar disorder participants in this study, although trends of smaller but noticeable effects for this subtest may be indicative of sensitivity at higher burden levels (Figure 2). While this does not indicate an absence of effect in non-schizophrenia diagnoses, it is consistent with the observation of cognitive sensitivity at lower anticholinergic burden in patients with schizophrenia compared to the other disorders. Whereas anticholinergic load was the most significant predictor of Verbal Memory in patients with schizophrenia, increasing dose of antipsychotic medications was associated with worse Token Motor scores in all diagnostic categories (similar effect sizes). Other BACS subtests were not significantly associated with antipsychotic dosage in schizophrenia participants.
The notion that there may be an increased cognitive sensitivity to anticholinergic effects in schizophrenia relative to other psychotic disorders is intriguing, as it is consistent with previously described molecular studies. Numerous post-mortem studies measuring M1/M4 selective radio-ligand binding (i.e. [3H]pirenzepine) (Crook et al., 2001, 2000, 1999; Dean et al., 2002, 1996; Deng and Huang, 2005; Gibbons et al., 2013; Zavitsanou et al., 2004), as well as the levels of protein and mRNA (Dean et al., 2002; Mancama et al., 2003), have observed a widespread reduction of muscarinic receptors, notably M1, in postmortem brain samples of schizophrenia patients. Additionally, in vivo analyses identified a 20–35% decrease of muscarinic receptor availability in multiple brain regions in unmedicated patients with schizophrenia compared to the healthy controls (Raedler et al., 2003). Studies comparing M1 and M4 muscarinic receptor density across diagnosis groups identified reductions in patients with schizophrenia, but not in bipolar disorder or major depression (Gibbons et al., 2009; Zavitsanou et al., 2004). Antipsychotic medication use or dose does not appear related to decreased muscarinic receptor density (Crook et al., 2001, 2000, 1999; Dean et al., 2002; Deng and Huang, 2005; Gibbons et al., 2013, 2009; Zavitsanou et al., 2004). Given the already decreased central cholinergic activity through fewer muscarinic receptors in schizophrenia patients, even a small amount of anticholinergic load may cause a significant adverse impact on cognition due to M1 receptor saturation, which may in turn make them more vulnerable to cognitive impairing effects of anticholinergic medication burden compared to those with mood-related psychotic disorders or healthy controls who have greater M1 availability (Tani et al., 2015).
These findings may have significant clinical relevance. O’Reilly et al. recently reported that anticholinergic burden negatively impacted the outcomes of psychosocial treatment focusing on cognitive impairment in patients with schizophrenia (O’Reilly et al., 2016). Because cognitive impairment related to anticholinergic burden may affect skills necessary for independent living and vocational success, it is important for clinicians to appreciate the cumulative effects of anticholinergic drug regimen properties on cognition. Our findings provide some insight into a potential threshold effect of this phenomenon and provide preliminary evidence indicating that clinically accessible tools such as the ADS may be helpful in assessing cumulative anticholinergic burden and its relation to cognitive deficits in specific patients.
When examining this relationship along a disease dimension (SBS), correlations with cognitive performance stratified by anticholinergic burden load groups (no load, low load, high load), we again identified these relationships with participants scoring ‘most schizophrenia like’ most robustly in those with highly anticholinergic drug regimens. Including patients taking at least one antipsychotic drug may have led to including more severely ill bipolar patients in our analyses. However, anticholinergic effects on cognition were not evident in this potentially more severe group of bipolar patients. Furthermore, given different prescribing practices across psychotic disorders, it is possible that bipolar patients might have less anticholinergic exposure and a lower cumulative impact on cognition. However, ADS burden estimates were similar across diagnostic groups. Another consideration is that the increased cognitive sensitivity to anticholinergic burden in schizophrenia may be due to their cognitive impairment, which possibly indicates reduced cognitive reserve. Post hoc exploratory analyses of schizoaffective and bipolar groups stratified by composite BACS score subgroups (Low: BACS ≤ −1.5; Medium: BACS = −1.5~−0.5; High: BACS ≥ −0.5), however, did not identify such effects in the low performance (BACS ≤ 1.5) group. Finally, secondary analyses examining or adjusting for alternative factors (drug types, dosing, clinical ratings, estimated premorbid intelligence, etc.) did not account for anticholinergic-cognition relationships.
Our study has several limitations that need to be considered when interpreting study findings. The cross-sectional nature of our study design limits the ability to establish causal relationships regarding the observed relationship between anticholinergic burden and neuropsychological performance. While we examined many clinical, medication, and other demographic factors, it is difficult to rule out the possibility of disease severity confounding the findings. Nonetheless, adverse drug effect thresholds in clinically stable patients remain intriguing and consistent with prior molecular studies showing muscarinic receptor differences in brains of those with schizophrenia as compared to primary mood disorders. Second, medication dose is not taken into account in ADS scoring assignments, as dose-weighting approaches in the development of this scale did not enhance correlations with serum anticholinergic activity (Carnahan et al., 2006). Nonetheless differences in dosing strategies across diagnostic groups beyond antipsychotic drugs could be a potential confounding source in our analyses. We addressed several potential confounds by looking at differences in dosing for benztropine as a representative anticholinergic agent (data not shown), and did not observe differences in dosing across diagnostic groups. Third, the duration of anticholinergic medication use was not available for our study participants, and thus it may be likely that schizophrenia patients may have longer duration of anticholinergic exposure than the other groups. Although all patients had been exposed to the estimated anticholinergic burden for a minimum of 4 weeks before cognitive assessments, further studies to examine the influence of the duration of anticholinergic exposure on our findings are warranted. Last, the ADS scoring approach has intuitive clinical appeal, but lacks direct in vivo assessment of central anticholinergic drug effect. Thus, our study has advantages in showing a consistency of clinical effect in a large sample, but less direct linkage to CNS biology.
In conclusion, we identified an adverse effect threshold of anticholinergic burden on cognition in patients with schizophrenia. These findings are novel in terms of the methods with which anticholinergic burden was quantified based on overall medication regimens, the assessment of these relationships in clinically stable patients, and the sensitivity of these effects in those with schizophrenia as compared to schizoaffective and bipolar disorder. Prior molecular studies have identified differences in muscarinic receptor expression in patients with schizophrenia as opposed to those with mood disorders, and our findings may represent a differential clinical sensitivity to drug effects consistent with those differences. However, the mechanisms underlying this effect require further investigation. While it is difficult to dissociate illness severity and different baseline of cognitive impairment from pharmacologic effects in the current study, our findings support the hypothesis that patients with schizophrenia may have increased cognitive sensitivity to anticholinergic medications and that the aggregate effects of one’s anticholinergic medication regimen on cognition is sufficiently robust to be important to consider in clinical practice.
Supplementary Material
Acknowledgments
This work was supported in part by funding from the National Institute of Mental Health (MH083888 to J.R.B., MH072767 to S.K.H., MH083126 to J.L.R., MH077851 to C.A.T., MH078113 to M.S.K., MH077945 to G.P., MH077852 to G.T., MH077862 to J.A.S.).
We express gratitude to the patients and families who participated in this study. We also thank Gunvant Thaker, MD, for his scientific contributions to the B-SNIP consortium.
Role of the funding source
The funding agencies had no role in the design and conduct of the study collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
J.R.B. developed the idea for the application of anticholinergic burden estimates to B-SNIP drug regimens to study in relation to cognitive data. Authors S.E. and J.R.B. conducted the analyses and wrote the first draft with conceptual and statistical assistance from S.K.H., L.H.R., and J.A.S. Author R.M.C. developed ADS and assisted with updates and application to medication regimens in this study. Author S.E. applied ADS scoring to all medication data. Authors C.A.T., G.D.P., B.A.C., E.S.G., M.S.K., and J.A.S. are B-SNIP PIs, who designed the parent study, wrote the overarching B-SNIP protocol and phenotyping approaches and oversaw data collection. Authors E.I.I., J.L.R., and J.R.B. evaluated and validated medication assessments of participants. R.S.K. provided the BACS battery. S.K.H. oversaw training and quality control of the BACS throughout the study. All authors contributed to and have approved the final manuscript.
Conflict of interest
C.A.T. has received support from Intracellular Therapies (ITI, Inc.), PureTech Ventrues, Eli Lilly Pharmaceuticals, Sunovion, Astellas, Merck (ad hoc consulting), International Congress on Schizophrenia Research (unpaid volunteer), NAMI (unpaid volunteer), American Psychiatric Association (Deputy Editor), and Finnegan Henderson Farabow Garrett & Dunner, LLP. J.L.R. has received investigator initiated support from Naurex, Inc. R.S.E.K. has received investigator initiated support from the Department of Veteran’s Affair, Feinstein Institute for Medical Research, GlaxoSmithKline, National Institute of Mental Health, Novartis, Psychogenics, Research Foundation for Mental Hygiene, Inc., and the Singapore National Medical Research Council. R.S.E.K. has received honoraria, served as a consultant, or advisory board member for Abbvie, Akebia, Amgen, Astellas, Asubio, AviNeuro/ChemRar, BiolineRx, Biomarin, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, EnVivo, Helicon, Lundbeck, Merck, Mitsubishi, Otsuka, Pfizer, Roche, Shire, Sunovion, Takeda, Targacept. R.S.E.K. is a shareholder in Sengenix and NeuroCog Trials, Inc. and receives royalties from the BACS testing battery and the MATRICS Battery (BACS Symbol Coding). M.S.K. has received support from Forum Pharmaceuticals. J.A.S. has received support from Takeda, BMS, Roche, and Eli Lilly and research funding from Janssen. The other authors report no related conflict of interest.
References
- Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006;332:455–459. doi: 10.1136/bmj.38740.439664.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baitz HA, Thornton AE, Procyshyn RM, Smith GN, MacEwan GW, Kopala LC, Barr AM, Lang DJ, Honer WG. Antipsychotic medications: linking receptor antagonism to neuropsychological functioning in first episode psychosis. J Int Neuropsychol Soc. 2012;18:717–727. doi: 10.1017/S1355617712000343. [DOI] [PubMed] [Google Scholar]
- Baker LA, Cheng LY, Amara IB. The withdrawal of benztropine mesylate in chronic schizophrenic patients. Br J Psychiatry. 1983;143:584–590. doi: 10.1192/bjp.143.6.584. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Kunz M, Chakos M, Cooper TB, Horowitz TL, Lieberman JA. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. Am J Psychiatry. 2002;159:1018–1028. doi: 10.1176/appi.ajp.159.6.1018. [DOI] [PubMed] [Google Scholar]
- Brébion G, Bressan RA, Amador X, Malaspina D, Gorman JM. Medications and verbal memory impairment in schizophrenia: the role of anticholinergic drugs. Psychol Med. 2004;34:369–374. doi: 10.1017/s0033291703008900. [DOI] [PubMed] [Google Scholar]
- Campbell N, Boustani M, Limbil T, Ott C, Fox C, Maidment I, Schubert CC, Munger S, Fick D, Miller D, Gulati R. The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging. 2009;4:225–233. doi: 10.2147/cia.s5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell NL, Perkins AJ, Bradt P, Perk S, Wielage RC, Boustani MA, Ng DB. Association of Anticholinergic Burden with Cognitive Impairment and Health Care Utilization Among a Diverse Ambulatory Older Adult Population. Pharmacotherapy. 2016;36:1123–1131. doi: 10.1002/phar.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481–1486. doi: 10.1177/0091270006292126. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Glick ID, Miller AL, Hamner MB, Miller DD, Patel JK, Tapp A, Keefe RSE, Rosenheck RA. Baseline use of concomitant psychotropic medications to treat schizophrenia in the CATIE trial. Psychiatr Serv. 2006;57:1094–1101. doi: 10.1176/ps.2006.57.8.1094. [DOI] [PubMed] [Google Scholar]
- Chew ML, Mulsant BH, Pollock BG, Lehman ME, Greenspan A, Mahmoud RA, Kirshner MA, Sorisio DA, Bies RR, Gharabawi G. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56:1333–1341. doi: 10.1111/j.1532-5415.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- Crook JM, Dean B, Pavey G, Copolov D. The binding of [3H]AF-DX 384 is reduced in the caudate-putamen of subjects with schizophrenia. Life Sci. 1999;64:1761–1771. doi: 10.1016/s0024-3205(99)00114-9. [DOI] [PubMed] [Google Scholar]
- Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B. Low muscarinic receptor binding in prefrontal cortex from subjects with schizophrenia: a study of Brodmann’s areas 8, 9, 10, and 46 and the effects of neuroleptic drug treatment. Am J Psychiatry. 2001;158:918–925. doi: 10.1176/appi.ajp.158.6.918. [DOI] [PubMed] [Google Scholar]
- Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B. Decreased muscarinic receptor binding in subjects with schizophrenia: a study of the human hippocampal formation. Biol Psychiatry. 2000;48:381–388. doi: 10.1016/s0006-3223(00)00918-5. [DOI] [PubMed] [Google Scholar]
- Dean B, Crook JM, Opeskin K, Hill C, Keks N, Copolov DL. The density of muscarinic M1 receptors is decreased in the caudate-putamen of subjects with schizophrenia. Mol Psychiatry. 1996;1:54–58. [PubMed] [Google Scholar]
- Dean B, McLeod M, Keriakous D, McKenzie J, Scarr E. Decreased muscarinic1 receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2002;7:1083–1091. doi: 10.1038/sj.mp.4001199. [DOI] [PubMed] [Google Scholar]
- Deng C, Huang XF. Decreased density of muscarinic receptors in the superior temporal gyrusin schizophrenia. J Neurosci Res. 2005;81:883–890. doi: 10.1002/jnr.20600. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fayen M, Goldman MB, Moulthrop MA, Luchins DJ. Differential memory function with dopaminergic versus anticholinergic treatment of drug-induced extrapyramidal symptoms. Am J Psychiatry. 1988;145:483–486. doi: 10.1176/ajp.145.4.483. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P) New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Gibbons AS, Scarr E, Boer S, Money T, Jeon WJ, Felder C, Dean B. Widespread decreases in cortical muscarinic receptors in a subset of people with schizophrenia. Int J Neuropsychopharmacol. 2013;16:37–46. doi: 10.1017/S1461145712000028. [DOI] [PubMed] [Google Scholar]
- Gibbons AS, Scarr E, McLean C, Sundram S, Dean B. Decreased muscarinic receptor binding in the frontal cortex of bipolar disorder and major depressive disorder subjects. J Affect Disord. 2009;116:184–191. doi: 10.1016/j.jad.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Roth BL. Molecular targets for treating cognitive dysfunction in schizophrenia. Schizophr Bull. 2007;33:1100–1119. doi: 10.1093/schbul/sbm074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, Yu O, Crane PK, Larson EB. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175:401–407. doi: 10.1001/jamainternmed.2014.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Hill SK, Beers SR, Kmiec JA, Keshavan MS, Sweeney JA. Impairment of verbal memory and learning in antipsychotic-naïve patients with first-episode schizophrenia. Schizophr Res. 2004a;68:127–136. doi: 10.1016/S0920-9964(03)00125-7. [DOI] [PubMed] [Google Scholar]
- Hill SK, Harris MSH, Herbener ES, Pavuluri M, Sweeney JA. Neurocognitive allied phenotypes for schizophrenia and bipolar disorder. Schizophr Bull. 2008;34:743–759. doi: 10.1093/schbul/sbn027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Harris MSH, Rosen C, Marvin RW, Deleon O, Sweeney JA. A comparison of neuropsychological dysfunction in first-episode psychosis patients with unipolar depression, bipolar disorder, and schizophrenia. Schizophr Res. 2009;113:167–175. doi: 10.1016/j.schres.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RSE, Gold JM, Bishop JR, Gershon ES, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry. 2013;170:1275–1284. doi: 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Schuepbach D, Herbener ES, Keshavan MS, Sweeney JA. Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naïve patients with schizophrenia. Schizophr Res. 2004b;68:49–63. doi: 10.1016/S0920-9964(03)00213-5. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Gladsjo JA, Lindamer LA, Lacro JP. Medical comorbidity in schizophrenia. Schizophr Bull. 1996;22:413–430. doi: 10.1093/schbul/22.3.413. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Harvey PD, Goldberg TE, Gold JM, Walker TM, Kennel C, Hawkins K. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS) Schizophr Res. 2008;102:108–115. doi: 10.1016/j.schres.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Sweeney JA, Gu H, Hamer RM, Perkins DO, McEvoy JP, Lieberman JA. Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164:1061–1071. doi: 10.1176/ajp.2007.164.7.1061. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Morris DW, Sweeney JA, Pearlson G, Thaker G, Seidman LJ, Eack SM, Tamminga C. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo-Bipolar Scale. Schizophr Res. 2011;133:250–254. doi: 10.1016/j.schres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam M, Collinson SL, Eng GK, Rapisarda A, Kraus M, Lee J, Chong SA, Keefe RSE. Refining the latent structure of neuropsychological performance in schizophrenia. Psychol Med. 2014;44:3557–3570. doi: 10.1017/S0033291714001020. [DOI] [PubMed] [Google Scholar]
- Lee J, Rizzo S, Altshuler L, Glahn DC, Miklowitz DJ, Sugar CA, Wynn JK, Green MF. Deconstructing Bipolar Disorder and Schizophrenia: A cross-diagnostic cluster analysis of cognitive phenotypes. J Affect Disord. 2016;209:71–79. doi: 10.1016/j.jad.2016.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifker FR, Bowie CR, Harvey PD. Determinants of everyday outcomes in schizophrenia: the influences of cognitive impairment, functional capacity, and symptoms. Schizophr Res. 2009;115:82–87. doi: 10.1016/j.schres.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Mancama D, Arranz MJ, Landau S, Kerwin R. Reduced expression of the muscarinic 1 receptor cortical subtype in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2003;119B:2–6. doi: 10.1002/ajmg.b.20020. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Poole JH, Benton C, Vinogradov S. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry. 2004;161:116–124. doi: 10.1176/appi.ajp.161.1.116. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Mori K, Yamashita H, Nagao M, Horiguchi J, Yamawaki S. Effects of anticholinergic drug withdrawal on memory, regional cerebral blood flow and extrapyramidal side effects in schizophrenic patients. Pharmacopsychiatry. 2002;35:6–11. doi: 10.1055/s-2002-19831. [DOI] [PubMed] [Google Scholar]
- O’Reilly K, O’Connell P, Donohoe G, Coyle C, O’Sullivan D, Azvee Z, Maddock C, Sharma K, Sadi H, McMahon M, Kennedy HG. Anticholinergic burden in schizophrenia and ability to benefit from psychosocial treatment programmes: a 3-year prospective cohort study. Psychol Med. 2016;46:3199–3211. doi: 10.1017/S0033291716002154. [DOI] [PubMed] [Google Scholar]
- Perlick D, Stastny P, Katz I, Mayer M, Mattis S. Memory deficits and anticholinergic levels in chronic schizophrenia. Am J Psychiatry. 1986;143:230–232. doi: 10.1176/ajp.143.2.230. [DOI] [PubMed] [Google Scholar]
- Raedler TJ, Knable MB, Jones DW, Urbina RA, Gorey JG, Lee KS, Egan MF, Coppola R, Weinberger DR. In vivo determination of muscarinic acetylcholine receptor availability in schizophrenia. Am J Psychiatry. 2003;160:118–127. doi: 10.1176/appi.ajp.160.1.118. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Harris MSH, Keshavan MS, Sweeney JA. Adverse effects of risperidone on spatial working memory in first-episode schizophrenia. Arch Gen Psychiatry. 2006;63:1189–1197. doi: 10.1001/archpsyc.63.11.1189. [DOI] [PubMed] [Google Scholar]
- Risacher SL, McDonald BC, Tallman EF, West JD, Farlow MR, Unverzagt FW, Gao S, Boustani M, Crane PK, Petersen RC, Jack CR, Jr, Jagust WJ, Aisen PS, Weiner MW, Saykin AJ, Alzheimer’s Disease Neuroimaging Initiative Association Between Anticholinergic Medication Use and Cognition, Brain Metabolism, and Brain Atrophy in Cognitively Normal Older Adults. JAMA Neurol. 2016;73:721–732. doi: 10.1001/jamaneurol.2016.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Ki Database Psychoactive Drug Screening Program. Available at: http://kidbdev.med.unc.edu/databases/kidb.php (accessed 7.29.16)
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Strauss ME, Reynolds KS, Jayaram G, Tune LE. Effects of anticholinergic medication on memory in schizophrenia. Schizophr Res. 1990;3:127–129. doi: 10.1016/0920-9964(90)90045-9. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Keilp JG, Haas GL, Hill J, Weiden PJ. Relationships between medication treatments and neuropsychological test performance in schizophrenia. Psychiatry Res. 1991;37:297–308. doi: 10.1016/0165-1781(91)90065-w. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Morris DW, Bishop J, Thaker GK, Sweeney JA. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013;170:1263–1274. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- Tani M, Akashi N, Hori K, Konishi K, Kitajima Y, Tomioka H, Inamoto A, Hirata A, Tomita A, Koganemaru T, Takahashi A, Hachisu M. Anticholinergic Activity and Schizophrenia. Neurodegener Dis. 2015;15:168–174. doi: 10.1159/000381523. [DOI] [PubMed] [Google Scholar]
- Tune LE, Strauss ME, Lew MF, Breitlinger E, Coyle JT. Serum levels of anticholinergic drugs and impaired recent memory in chronic schizophrenic patients. Am J Psychiatry. 1982;139:1460–1462. doi: 10.1176/ajp.139.11.1460. [DOI] [PubMed] [Google Scholar]
- Wojtalik JA, Eack SM, Pollock BG, Keshavan MS. Prefrontal gray matter morphology mediates the association between serum anticholinergicity and cognitive functioning in early course schizophrenia. Psychiatry Res. 2012;204:61–67. doi: 10.1016/j.pscychresns.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zavitsanou K, Katerina Z, Katsifis A, Andrew K, Mattner F, Filomena M, Huang XF, Xu-Feng H. Investigation of m1/m4 muscarinic receptors in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression disorder. Neuropsychopharmacology. 2004;29:619–625. doi: 10.1038/sj.npp.1300367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


