Abstract
Background
Little is known about the feasibility and impact of lifestyle intervention, determined by change in diet and cardiovascular fitness, on glycemic control in youth who are overweight with type 2 diabetes. This was examined in the TODAY clinical trial cohort from across 15 US centers.
Subjects
TODAY enrolled 699 youth aged 10–17 years with type 2 diabetes <2 years and BMI ≥85th percentile at baseline.
Methods
Dietary data were collected by an interviewer-administered food frequency questionnaire; cardiovascular fitness (CRF) was assessed using a submaximal cycle ergometer test. Change from baseline in these variables was analyzed using generalized linear mixed models for both continuous and categorical measures. Models were adjusted for age, baseline HbA1c, treatment group, and medication adherence. Data were collected at baseline, 6, and 24 months.
Results
At 6 months, ~25% of females and ~33% of males improved CRF. In males, this was related to a decreased HbA1c (p=0.001) and a lower percent experiencing glycemic failure (HbA1c ≥8%; p=0.007). Females who decreased their saturated fat intake and/or increased their fiber intake had lower HbA1c at month 24 (p=0.01 and p=0.007, respectively). Males who increased their sweetened beverage intake at 6 month follow-up were at a 1.6-fold higher risk of experiencing glycemic failure (p=0.04).
Conclusions
Few youth improved fitness and/or diet over time, although those who did showed a beneficial impact on glycemic outcomes. Although lifestyle behaviors are difficult to change in youth with type 2 diabetes, interventions are needed that are feasible (in scope, complexity, and demands), sustainable, and clinically meaningful.
Keywords: Adolescent, Diabetes Mellitus Type 2, glycemia, fitness, diet
INTRODUCTION
The increase in type 2 diabetes in youth, related in part to the high prevalence of childhood obesity, creates a need for management strategies. Clinical guidelines recommend lifestyle interventions in conjunction with medication as the initial treatment approach.1 However, little is known regarding the success of lifestyle intervention efforts in youth with type 2 diabetes in changing diet and physical activity and, subsequently, glycemic control.
TODAY (Treatment Options for type 2 Diabetes in Adolescents and Youth) was the largest intervention trial conducted in youth with type 2 diabetes. This National Institute of Diabetes and Digestive and Kidney Diseases-funded randomized clinical trial examined three treatments in a racially, ethnically, and geographically diverse group of youth with type 2 diabetes.2 Although all three treatment groups were instructed on healthy lifestyle changes, only one included an intensive family-based behavioral weight loss program.3 Results after a mean 3.9 year follow-up indicated that the metformin plus rosiglitazone group was superior to metformin alone in preventing glycemic deterioration. Although not superior in preventing glycemic deterioration, the metformin plus lifestyle group was the only treatment arm showing a short-term decrease in BMI after 6 months of intervention.4 However, the impact of change in lifestyle behaviors on glycemic control among youth independent of TODAY randomized treatment assignment has not been examined.
The purpose of this report was to evaluate the extent to which TODAY youth, irrespective of treatment arm, made lifestyle behavior changes, specifically improvements in fitness and/or diet, over time. We hypothesized that demonstrated improvements in one or more of these important behavioral lifestyle components would be associated with improvements in measures of glycemic control.
METHODS
TODAY was a randomized clinical trial to evaluate the efficacy and safety of three treatments for type 2 diabetes in youth: metformin alone, metformin plus rosiglitazone, and metformin plus an intensive lifestyle program.2 The primary outcome was time to treatment failure, defined as loss of glycemic control (HbA1c ≥8% for 6 months or inability to wean from insulin within 3 months of its initiation due to metabolic decompensation).4
Participants were recruited at 15 centers with enrollment ending in February 2009.2 Participants were 10–17 years old, had been diagnosed with type 2 diabetes for <2 years, had negative pancreatic autoantibodies, and had a BMI at the ≥85th percentile at time of randomization. The protocol was approved by the Institutional Review Boards of participating institutions; parents signed informed consent forms for a minor child, and youth signed informed assent forms according to local practice. Youth who successfully completed a run-in period were randomized and completed baseline measures.5 The TODAY cohort included 699 youth.
Measures were obtained at baseline, 6, and 24 months and included the following:
Anthropometric measures and HbA1c
All anthropometric measures were taken wearing lightweight clothing without shoes by trained/certified staff. Height was measured to the nearest 0.1 cm; weight was measured to the nearest 0.1 kg. BMI was calculated as kg/m2, and BMI z-score was derived from sex-and age-specific standards.6 Percent overweight was calculated as BMI (at assessment) minus BMI at 50th percentile for age and sex, divided by 50th percentile. HbA1c was obtained from blood samples assayed at a central laboratory.2
Medication adherence
Average medication adherence over the first 6 and 24 months was calculated as percent of prescribed study drug taken using pill counts. Since medication adherence was not normally distributed, it was analyzed as above or below the cut-point of 80% (defined as “adequate adherence” per protocol).
Physical work capacity (PWC)-170
Cardiorespiratory fitness (CRF) was assessed using a standardized submaximal cycle ergometer (bike) test (Monark 818E cycle ergometer, Quinton Monark, WA) that determines PWC by predicting the workload (kg*m) at a heart rate of 170 beats per minute (bpm).7–8 The heart rate recorded from the last 5 seconds of each stage was used to extrapolate a PWC at a heart rate of 170 bpm. A best fit line approximated the bike’s workload at a heart rate of 170 bpm, resulting in an estimate of the PWC-170 expressed in kg*m/min. Raw measurements of PWC-170 were divided by participant’s weight (kg) to provide weight-adjusted estimates in m/min. Participants who weighed ≥350 pounds were excluded due to bike weight limits (n=6 at baseline). The test was discontinued for inability to maintain a minimal speed during warm-up (n=22), inability to complete the test (n=92; e.g., leg pain, lack of motivation), or heart rate >170 bpm during the initial stage (n=5).
Assessment of dietary intake
The TODAY food frequency questionnaire (FFQ), a modification of the SEARCH for Diabetes in Youth FFQ,9 was based on the Kids’ Food Questionnaire (validated in children ≥8 years, including low-income African American youth).10 TODAY added foods likely to be important in the ethnically/ regionally diverse TODAY sample.9,11 For each food item, participants were asked if the food was consumed in the past week (“yes/no”); if yes, on how many days and average portions. Portion size was defined as “very small,” “small,” “medium,” “large,” or “very large” relative to pictures. Portion size options were expanded to allow reporting of larger amounts of food than might be expected for adolescents. Also, questions were added to understand whether the recall period (one week) reflected “usual” intake, use of dietary supplements and low-fat products, and frequency of eating out. The nutrient and portion-size databases were modified from SEARCH FFQ databases using the Nutrition Data System for Research (database 3 version 4.05/33, 2002, Nutrition Coordinating Center, University of Minnesota, Minneapolis) and industry sources.
Staff administered the FFQ via interview with direct computer entry of responses using software provided by the University of South Carolina Diet Assessment Center with consistent quality control measures. FFQs with inconsistent or improbable responses12 were excluded.
Thirteen nutrient variables, previously shown to relate to glycemic control and/or body composition, were analyzed in units per day: percent of calories from fat, saturated fat, monounsaturated fat, carbohydrate, and sugar; total fiber in grams; servings of sweetened beverages, grains, red meat, fruits and vegetables, vegetables, dairy, and desserts.13–17
Sample Selection
FFQ and PWC-170 data were collected at visits prior to occurrence of the primary outcome (i.e., loss of glycemic control) while the participant was still receiving the randomized intervention. Data from participants who reached primary outcome or left the study before 6 months were not analyzed. Participants were required to minimally have a baseline and at least one follow-up value to be included in the study.
The analysis sample included PWC-170 data (N=369; 231 females, 138 males) and FFQ data (N=481; 307 females, 174 males). Participants with PWC-170 and FFQ data included in the analysis sample were compared to the rest of the TODAY participants by sex, age at baseline, percent overweight, and treatment group. The only significant difference was for percent overweight; the equipment could not accommodate participants weighing >350 pounds (n=6 at baseline). The 369 participants with PWC-170 data were less overweight at baseline (mean 74.6%, SD 34.4%) than the rest of the cohort (83.8%, 39.8%; p=0.001).
Statistical Analysis
Given the significant differences at baseline in fitness and dietary intake between males and females,8,12 analyses were performed separately by sex. To estimate associations between changes in lifestyle factors (from baseline to 6 and 24 months) and glycemic outcomes, generalized linear mixed models that account for longitudinal repeated measures and accommodate both continuous and categorical outcomes were used. Glycemic outcomes were HbA1c at 6 and 24 months and glycemic failure (primary study outcome) after 6 months. The effect of randomized treatment group on each lifestyle factor was tested but was never statistically and/or clinically significant; therefore, further analyses were pooled across treatment groups. All models were adjusted for the following standard set of covariates: baseline HbA1c, treatment group, age at baseline, and medication adherence. Also, for the nutrition variables, the effect of change in percent overweight was evaluated by comparing models with and without the factor.
Change from baseline was defined as follow-up value minus baseline value. The change from baseline in lifestyle factors was modeled continuously and divided into 3 categories, i.e., improved, worsened, and no change. Without existing clinical standards, fitness was categorized based upon expert evaluation of its distribution in this cohort. Specifically, change in fitness (PWC-170 in kg*m/min) was categorized as >130 increase from baseline, >80 decrease, and no change (i.e., between a decrease of 80 and an increase of 130). Similarly, for fitness defined as PWC-170 adjusted for weight (m/min), categories were >1 increased, >1 decreased, and no change (i.e., between a decrease of 1 and an increase of 1).
Since no clinical standards for nutritional variables exist for youth with type 2 diabetes, nutrition categories of change (improved, worsened, no change) were determined based on 6-month tertiles of change from baseline for all variables except sweetened beverages (specific cut-offs give in Table 2). Since the majority of participants reported consuming 0 servings/day of sweetened beverages at baseline (57.2%) and follow-up (57.4%), sweetened beverage was dichotomized as follows: 1) decreased intake at follow-up or 0 at both baseline and follow-up (improved or stayed at 0); 2) increased intake at follow-up or value >0 at baseline and same value reported at follow-up (worsened or stayed at >0). Change in sweetened beverage consumption was also measured continuously for some analyses.
Table 2.
Association between change in lifestyle factor (categorical1 and continuous2) and glycemic outcomes, by sex and visit3
| A. Association with HbA1c | N | HbA1c Month 6 | HbA1c Δ 6-0 | P-value | N | HbA1c Month 24 | HbA1c Δ 24-0 | P-value |
|---|---|---|---|---|---|---|---|---|
| PWC-170 (m/min) | ||||||||
| Female | ||||||||
|
| ||||||||
| Worsened (Δ <−1) | 64 | 6.0 (0.8) | 0.1 (0.7) | p=ns | 47 | 6.0 (0.9) | 0.4 (0.7) | p=0.01 |
| No change | 105 | 6.1 (1.0) | 0.1 (0.8) | 53 | 5.9 (0.7) | 0.0 (0.6)b | ||
| Improved (Δ >1) (S) | 62 | 5.9 (1.2) | 0.1 (1.0) | 27 | 6.4 (1.8) | 0.7 (1.8)b | ||
| Continuous Δ | β (SE) = −0.019 (0.027) | p=ns | β (SE) = 0.018 (0.039) | p=ns | ||||
|
| ||||||||
| Male | ||||||||
|
| ||||||||
| Worsened (Δ <−1) | 34 | 6.2 (0.9) | 0.4 (0.6)a,c | p=0.001 | 34 | 6.2 (1.3) | 0.5 (1.3)a | p=0.01 |
| No change | 54 | 5.9 (0.8) | −0.1 (0.4)c | 23 | 6.0 (0.9) | 0.2 (0.8) | ||
| Improved (Δ >1) (S) | 50 | 5.7 (0.7) | −0.1 (0.6)a | 21 | 5.6 (0.6) | −0.2 (0.5)a | ||
| Continuous Δ | β (SE) = −0.040 (0.017) | p=0.02 | β (SE) = −0.097 (0.042) | p=0.02 | ||||
|
| ||||||||
| Saturated fat (% of energy/day) | ||||||||
| Female | ||||||||
|
| ||||||||
| Worsened (Δ>1.3) | 109 | 6.2 (1.1) | 0.2 (0.9) | p=ns | 55 | 6.2 (0.9) | 0.5 (0.9)a,c | p=0.01 |
| No change | 100 | 6.0 (1.1) | 0.1 (0.9) | 60 | 6.0 (0.9) | 0.2 (0.8)c | ||
| Improved (Δ< −1.5) (S) | 98 | 6.1 (0.9) | 0.1 (0.8) | 80 | 5.9 (0.8) | 0.1 (0.7)a | ||
| Continuous Δ | β (SE) = 0.012 (0.014) | p=ns | β (SE) = 0.023 (0.015) | p=ns | ||||
|
| ||||||||
| Male | ||||||||
|
| ||||||||
| Worsened (Δ>1.3) | 51 | 6.0 (0.8) | 0.1 (0.5) | p=ns | 27 | 6.0 (0.8) | 0.1 (0.7) | p=ns |
| No change | 61 | 5.9 (1.0) | 0.1 (0.7) | 37 | 6.1 (1.0) | 0.1 (0.8) | ||
| Improved (Δ< −1.5) (S) | 62 | 5.9 (0.9) | −0.0 (0.7) | 38 | 5.9 (1.2) | 0.1 (1.0) | ||
| Continuous Δ | β (SE) = 0.019 (0.014) | p=ns | β (SE) = 0.014 (0.021) | p=ns | ||||
|
| ||||||||
| Fiber (g/day) | ||||||||
| Female | ||||||||
|
| ||||||||
| Worsened (Δ< −2) | 100 | 6.2 (0.9) | 0.2 (0.7) | p=ns | 67 | 6.3 (0.9) | 0.4 (0.9)a,c | p=0.007 |
| No change | 109 | 6.1 (1.1) | 0.1 (0.9) | 58 | 6.0 (0.8) | 0.2 (0.8)c | ||
| Improved (Δ>1.5) (S) | 98 | 6.0 (1.1) | 0.2 (0.9) | 70 | 5.8 (0.8) | 0.1 (0.7)a | ||
| Continuous Δ | β (SE) = −0.007 (0.010) | p=ns | β (SE) = −0.030 (0.011) | p=0.008 | ||||
|
| ||||||||
| Male | ||||||||
|
| ||||||||
| Worsened (Δ< −2) | 60 | 6.0 (0.9) | 0.1 (0.7) | p=ns | 32 | 5.7 (0.6) | 0.0 (0.7) | p=ns |
| No change | 52 | 5.9 (0.9) | 0.0 (0.5) | 25 | 5.9 (0.9) | 0.3 (0.6) | ||
| Improved (Δ>1.5) (S) | 62 | 6.0 (0.9) | 0.1 (0.7) | 45 | 6.1 (0.9) | 0.2 (0.8) | ||
| Continuous Δ | β (SE) = −0.004 (0.009) | p=ns | β (SE) = 0.014 (0.012) | p=ns | ||||
| B. Association with Glycemic Failure | N | % Failed after Month 6 | P-value |
|---|---|---|---|
| PWC-170 (m/min) | |||
| Female | |||
|
| |||
| Worsened (Δ <−1) | 64 | 34% | p=ns |
| No change | 105 | 43% | |
| Improved (Δ >1) (S) | 62 | 29% | |
| Continuous Δ | OR = 1.0 (0.9=1.2) | p=ns | |
|
| |||
| Male | |||
|
| |||
| Worsened (Δ <−1) | 34 | 62%a,c | p=0.007 |
| No change | 54 | 43%c | |
| Improved (Δ >1) (S) | 50 | 26%a | |
| Continuous Δ | OR = 0.8 (0.7–0.9) | p=0.04 | |
|
| |||
| Sweetened beverages (servings/day) | |||
| Female | |||
|
| |||
| Worsened or stayed at >0 | 114 | 42% | p=ns |
| Improved or stayed at 0 (S) | 193 | 34% | |
| Continuous Δ | OR = 1.2 (0.9–1.5) | p=ns | |
|
| |||
| Male | |||
|
| |||
| Worsened or stayed at >0 | 60 | 52% | p=ns |
| Improved or stayed at 0 (S) | 114 | 39% | |
| Continuous Δ | OR = 1.6 (1.1–2.5) | p=0.04 | |
In part A, mean (SD) HbA1c values and change from baseline by sex and visit are shown. In part B, percents of participants who failed to control glycemia on the randomized treatment assignment after 6 months are given. Tests were performed to examine differences in glycemic outcome across categories of change in the lifestyle factor. Lifestyle change categories indicating a beneficial change are labeled with ‘(S)’. If the overall test was significant, pairwise comparisons were performed and significance was denoted by the superscripts (a) comparing worsened vs improved, (b) comparing no change vs improved, and (c) comparing worsened vs no change.
In part A, slope (β) and standard error (SE) represent a change in HbA1c per 1 unit increase in the lifestyle factor (i.e., a positive slope means HbA1c increases as lifestyle factor increases, whereas a negative slope means HbA1c decrease as lifestyle factor increases). In part B, odds ratio (OR) and 95% confidence interval give the risk of glycemic failure; OR<1 indicates greater fitness is protective and OR>1 indicates that more sweetened beverages increases risk of failure.
Only lifestyle factors with significant results are shown; all models were adjusted for the following standard set of covariates: baseline HbA1c, treatment group, age at baseline, and medication adherence. Italic bold values indicate significant associations (p<0.05).
Venn diagrams were used to illustrate groups of TODAY participants who achieved positive behavior change (improved) for single variables and a combination of fitness and nutrition variables. All analyses were considered exploratory, with statistical significance defined as p <0.05. SAS statistical software version 9.3 (SAS Institute Inc., Cary, NC) was used for all analyses.
RESULTS
Baseline TODAY cohort characteristics have been previously reported. 5 At study entry, females were on average 13.7 (SD 2.0) years old with mean HbA1c of 6.1% (SD 0.8), mean BMI of 34.9 kg/m2 (SD 7.8), and mean percent overweight of 78.1% (SD 35.9). Males were slightly older (mean 14.5, SD 2.0; p<.0001) with mean HbA1c of 6.0% (SD 0.7), mean BMI of 35.6 kg/m2 (SD 8.3), and mean percent overweight of 80.4% (SD 39.7), all p-values = ns.
Mean values of lifestyle behaviors and change from baseline are shown by sex and visit in Table 1. Females had a significant (p<.05) decrease in fitness level at month 24 whereas males increased at month 6 (both adjusted and unadjusted). Males increased sweetened beverage intake at month 24, however, 33.6% of all participants reported 0 sweetened beverage intake at baseline and at follow-up visits. Females decreased sweets/desserts and dairy intake at month 24.
Table 1.
Mean (SD) for lifestyle factors (fitness1 and nutrition2) and change from baseline, by sex and visit
| Female | Male | |||||
|---|---|---|---|---|---|---|
| Baseline | Month 6 | Month 24 | Baseline | Month 6 | Month 24 | |
| PWC-170 (kg*m/min) | 622 (199) | 631 (204) | 635 (242) | 862 (269) | 930 (344) | 903 (289) |
|
| ||||||
| Change from baseline | -- | 9.2 (162) | 6.2 (217) | -- | 68.3 (273) | 45.2 (254) |
|
| ||||||
| PWC-170 (m/min) | 7.3 (2.6) | 7.2 (2.5) | 6.8 (2.4) | 8.7 (2.6) | 9.3 (3.2) | 8.6 (2.6) |
|
| ||||||
| Change from baseline | -- | −0.1 (2.0) | −0.5 (2.3) | -- | 0.6 (2.7) | −0.2 (2.7) |
|
| ||||||
| Fat (% of energy/day) | 38.2 (6.6) | 37.6 (7.2) | 37.1 (7.0) | 38.8 (6.6) | 37.8 (6.9) | 37.0 (6.3) |
|
| ||||||
| Change from baseline | -- | −0.5 (8.3) | −1.4 (8.6) | -- | −1.0 (8.5) | −1.6 (7.5) |
|
| ||||||
| Saturated fat (% of energy/day) | 13.1 (2.9) | 13.0 (3.0) | 12.7 (2.9) | 13.4 (2.5) | 13.1 (3.0) | 12.9 (2.5) |
|
| ||||||
| Change from baseline | -- | −0.1 (3.4) | −0.7 (3.8) | -- | −0.3 (3.5) | −0.6 (3.3) |
|
| ||||||
| Monounsaturated fat (% of energy/day) | 14.9 (2.8) | 14.7 (3.1) | 14.4 (3.1) | 15.4 (3.1) | 15.1 (3.0) | 14.6 (2.7) |
|
| ||||||
| Change from baseline | -- | −0.3 (3.7) | −0.7 (3.8) | -- | −0.3 (4.0) | −0.7 (3.5) |
|
| ||||||
| Carbohydrate (% of energy/day) | 45.2 (8.8) | 46.0 (9.5) | 47.2 (9.5) | 43.5 (8.4) | 45.1 (8.9) | 46.4 (8.8) |
|
| ||||||
| Change from baseline | -- | 0.8 (10.9) | 2.3 (11.5) | -- | 1.6 (10.7) | 3.0 (11.6) |
|
| ||||||
| Meat (servings/day) | 1.4 (1.0) | 1.3 (1.0) | 1.2 (1.0) | 1.8 (1.5) | 1.7 (1.3) | 2.0 (1.4) |
|
| ||||||
| Change from baseline | -- | −0.1 (1.2) | −0.2 (1.2) | -- | −0.1 (1.7) | 0.2 (1.7) |
|
| ||||||
| Fiber (g/day) | 9.3 (4.5) | 9.1 (4.7) | 8.9 (4.3) | 10.1 (5.2) | 9.9 (5.0) | 10.6 (5.0) |
|
| ||||||
| Change from baseline | -- | −0.2 (4.9) | 0.0 (4.9) | -- | −0.2 (5.4) | 0.4 (5.7) |
|
| ||||||
| Grain (servings/day) | 9.6 (6.0) | 9.2 (6.4) | 8.7 (6.2) | 10.0 (6.5) | 9.2 (6.5) | 9.5 (7.2) |
|
| ||||||
| Change from baseline | -- | −0.4 (7.9) | −1.1 (8.1) | -- | −0.8 (8.0) | −0.3 (9.1) |
|
| ||||||
| Sweetened beverage (servings/day) | 0.3 (0.6) | 0.4 (0.9) | 0.6 (1.0) | 0.3 (0.8) | 0.4 (0.9) | 0.9 (1.6) |
|
| ||||||
| Change from baseline | -- | 0.1 (0.9) | 0.2 (1.0) | -- | 0.1 (1.0) | 0.6 (1.5) |
| % reporting 0 serv/day | 56.7% | 47.9% | 43.6% | 58.0% | 52.9% | 47.1% |
| % reporting >0 serv/day | 43.3% | 52.1% | 56.4% | 41.9% | 47.1% | 52.9% |
|
| ||||||
| Fruit and vegetable (servings/day) | 2.1 (1.3) | 2.1 (1.4) | 2.1 (1.4) | 2.2 (1.7) | 2.2 (1.6) | 2.5 (1.8) |
|
| ||||||
| Change from baseline | -- | 0.0 (1.5) | 0.1 (1.7) | -- | 0.0 (1.7) | 0.1 (1.8) |
|
| ||||||
| Vegetable (servings/day) | 1.1 (0.9) | 1.1 (0.8) | 1.1 (0.9) | 1.2 (1.0) | 1.1 (1.1) | 1.3 (1.3) |
|
| ||||||
| Change from baseline | -- | 0.0 (1.0) | 0.0 (1.1) | -- | −0.1 (1.0) | 0.1 (1.1) |
|
| ||||||
| Sugar (% of energy/day) | 5.1 (1.9) | 5.3 (2.2) | 5.7 (2.4) | 4.6 (1.9) | 4.8 (2.1) | 5.3 (2.5) |
|
| ||||||
| Change from baseline | -- | 0.2 (2.5) | 0.6 (2.9) | -- | 0.2 (2.5) | 0.5 (2.8) |
|
| ||||||
| Sweets and dessert (servings/day) | 1.6 (2.3) | 1.5 (2.3) | 1.1 (1.5) | 2.2 (3.4) | 1.8 (3.0) | 1.5 (2.0) |
|
| ||||||
| Change from baseline | -- | −0.1 (2.7) | −0.6 (2.7) | -- | −0.4 (3.9) | −0.8 (3.5) |
|
| ||||||
| Dairy (servings/day) | 4.4 (4.1) | 4.6 (4.4) | 3.6 (3.4) | 5.3 (4.6) | 5.2 (5.4) | 4.6 (4.9) |
|
| ||||||
| Change from baseline | -- | 0.1 (4.5) | −1.1 (4.7) | -- | −0.1 (5.6) | −1.0 (6.4) |
Sample with fitness data: 231 female and 138 male participants at baseline and month 6; 127 female and 78 male participants at month 24.
Sample with nutrition data: 307 female and 174 male participants at baseline and month 6; 195 female and 102 male participants at month 24.
Italic bold values indicate where the change from baseline was significantly different from zero (p<0.05).
Examining change in fitness from baseline to month 6 by categories, 28% (of 231) of females worsened, 45% were unchanged, and 27% improved. At month 24, 37% (of 127) of females worsened, 42% were unchanged, and 21% improved. In males at month 6, 24% (of 138) worsened, 39% were unchanged, and 36% improved. At month 24, 43% (of 78) of males worsened, 29% were unchanged, and 26% improved.
Change in Fitness/Nutrition and HbA1c
Changes in fitness and nutrition from baseline to months 6 and 24 were examined relative to glycemic control (Table 2A). All variables from Table 1 having a potential effect on glycemia were analyzed, but only significant results are presented. Results for PWC-170 were similar for both weight-adjusted and unadjusted PWC-170 measures; only weight adjusted is given in Table 2.
In males, there was a significant inverse relationship between PWC-170 change from baseline (analyzed categorically and continuously) and HbA1c at both follow-up time points (i.e., as fitness levels increased, HbA1c decreased). In females, the only significant finding was a higher HbA1c in those who improved compared to those who did not change in fitness from baseline to month 24, although this did not hold when fitness was examined continuously.
With regard to nutrition, females who decreased saturated fat intake by >1.5%/day or kept saturated fat intake stable had significantly lower HbA1c levels at month 24 than those who increased % saturated fat by >1.3%/day (p=0.01). There was a significant positive association between % saturated fat change and percent overweight change (r=0.36, p=0.03), and with change in percent overweight added to the model as a covariate, the findings remained significant (p=0.02). None of the associations between change in continuously measured % saturated fat and HbA1c were significant.
At month 24, females who increased fiber intake by >1.5 g/day or kept fiber intake stable had lower HbA1c than those who decreased fiber intake by >2 g/day (p=0.007). Increases in fiber were related to decreases in percent overweight overall (r=−0.09, p=0.009); with change in percent overweight added to the model as a covariate, the findings remained significant (p=0.02). A change in fiber measured continuously also related to HbA1c; for every gram increase in fiber, HbA1c decreased by 0.03% (slope/SE −0.030/0.011, p=0.008). However, with change in percent overweight in the model, the slope decreased to −0.025 (p=ns). No other changes in the preselected nutrition variables related to HbA1c.
Change in Fitness/Nutrition and Glycemic Failure
Table 2B shows associations between changes in lifestyle factors at month 6 and glycemic failure after month 6. In males, 62% who decreased their fitness levels by more than 1 PWC-170/kg at month 6 reached primary outcome after 6 months, compared to 26% of those who increased fitness level (p<0.001). Similarly, increase in fitness (continuously measured) from baseline to month 6 protected against the risk of reaching glycemic failure after 6 months (OR=0.8, p=0.04). Results in females were not significant.
Other than change in sweetened beverages, 6-month changes in the nutrition variables were not significantly associated with glycemic failure. In males only, an increase in intake or stay at >0 level of sweetened beverages (continuously measured) from baseline to month 6 increased risk of reaching failure after 6 months (OR=1.6, p=0.04).
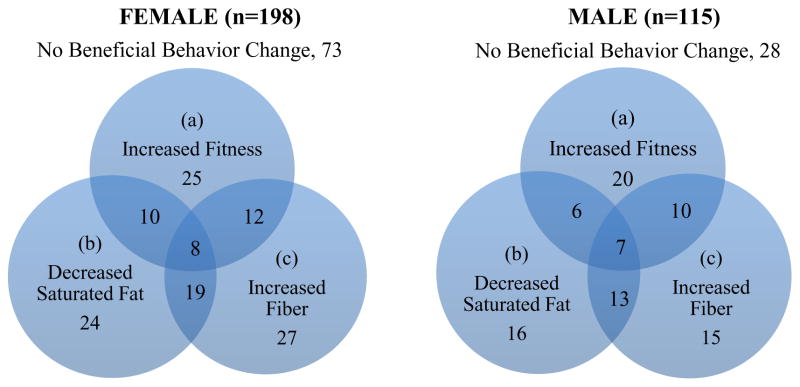
Combination of Nutrition and Fitness Success
Sex-specific Venn diagrams (see Figure 1) show any overlaps between improvements in 3 lifestyle behaviors significantly related to HbA1c (fitness, saturated fat, fiber) at month 6. At month 6, 63% of females and 76% of males improved in at least one of these three lifestyle behaviors. Among those who made a successful fitness gain from baseline to month 6, only around half also improved in at least one of the two nutrition markers (55% females, 53% males). Only 4% of females and 6% of males improved in all 3 lifestyle behaviors. Successful changes were even less evident at month 24 with only 3% of females and 2% of males having improved in all 3 factors (data not shown).
Figure 1.
Venn diagram showing numbers of participants distributed across change from baseline to month 6 in 3 lifestyle behaviors singly and in combination: (a) increased fitness (weight adjusted PWC-170) by >1 m/min; (b) decreased saturated fat intake by >1.5%/day; (c) increased total fiber intake by >1.5 g/day.
DISCUSSION
The TODAY cohort was predominately overweight,5 sedentary and unfit,8 with poor dietary intake relative to national recommendations,12 emphasizing the need for strategies to change lifestyle behaviors in this demographic. This effort sought to characterize the extent of lifestyle behavior change and to evaluate the impact of beneficial changes on glycemic control, regardless of treatment arm.
A recent review of the literature highlighted the fact that structured lifestyle intervention efforts that included both exercise and diet modification resulted in significant improvements in these behaviors in overweight and obese youth without diabetes. 18 In contrast, only a small number of TODAY youth made beneficial changes in both fitness and nutrition. Assessing change in cardiovascular fitness, 27% of females and 36% of males improved at month 6. For males, this gain in fitness was related to an improved HbA1c which was consistent with findings in adults.19 The clinical significance of this improvement in HbA1c is unknown although the additional finding of a lesser likelihood of glycemic failure in males who improved fitness does add some value to its importance. Change in fitness in females did not have as much impact on glycemic outcomes, likely because their improvement was relatively smaller compared to that in males. Physical activity levels in young girls have been identified as a national concern20 and our fitness data confirm consistently lower levels in girls than boys over the course of the study.
Improvements in saturated fat and fiber intake were associated with improvements in both % overweight and glycemic control in females; increased sweetened beverage intake was associated with a greater likelihood of glycemic failure in boys, also consistent with findings in adults.21–23 Sweetened beverage intake in adults has been associated with weight gain and worsening of cardiometabolic profile, as sweetened beverages provide a concentrated source of excess calories and rapidly absorbable sugars.21–23
The number of TODAY youth who made beneficial changes in more than one area was relatively small, and <5% improved in all 3 lifestyle behaviors despite the support of diabetes educators, physicians, and for those randomized to the metformin + lifestyle group, one-on-one health coaches. The inability to achieve successful lifestyle changes may be related to a variety of factors including degree of obesity,5 the self-care burden and regimen complexity (self-monitoring, medication-taking, lifestyle behaviors) related to comorbidities,24–27 and exposure to challenging psychocultural and socioeconomic environments, 28–30 and other family challenges.
Addressing one behavior change at a time using permissive (e.g., eat more foods that are high in fiber) rather than restrictive (e.g., eat less foods that are high in fat) messages may be more effective and reduce regimen complexity and distress. A study in adults with metabolic syndrome comparing a single-component intervention aimed at increasing fiber intake versus a multi-component dietary intervention resulted in clinically meaningful weight loss and improved blood pressure and insulin resistance in the former. 22 Similarly, a pilot study examining a focus on increasing intake of healthy foods (permissive) vs reducing intake of high-energy-dense foods (restrictive) was more effective over time in improving child and parent weight in a family based pediatric obesity study.31 In individuals with type 2 diabetes, reducing regimen distress has been associated with improved medication adherence, physical activity levels, and glycemic control in adults.32
There are limitations to this analysis. The data were collected to address multiple secondary outcomes in a large clinical trial; only the primary outcome was adequately powered. Therefore, the results should be considered exploratory. Also, although the models were adjusted for age and most of our cohort had already reached puberty at baseline based upon the Tanner score, change in puberty status may still have influenced these results. Finally, although the FFQ was administered by trained interviewers and the data underwent quality control methods at a Diet Assessment Center, the instrument is dependent on participant recall and self-report.
In conclusion, our results reflect the difficulty of changing lifestyle behaviors in youth who are dealing with the diagnosis of type 2 diabetes, are sedentary, and have a poor diet. In youth with type 2 diabetes, it is critical to target lifestyle changes that are feasible (in scope, complexity, and demands), sustainable, and clinically meaningful (affect glycemic control). The current findings demonstrated that increasing physical activity/fitness and decreasing sweetened beverage intake in boys and increasing fiber and decreasing saturated fat intake in girls may be suitable initial targets for lifestyle intervention and education programs. Such lifestyle changes, although difficult for these youth with type 2 diabetes to make, are worth the effort as they appeared to have a beneficial impact on glycemia for those who were successful.
Supplementary Material
Acknowledgments
The TODAY Study Group thanks the following companies for their donations: Becton, Dickinson and Company; Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; LifeScan, Inc.; Pfizer; Sanofi Aventis. We also gratefully acknowledge the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service; the opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the respective Tribal and Indian Health Service Institution Review Boards or their members.
Materials developed and used for the TODAY standard diabetes education program and the intensive lifestyle intervention program are available to the public at https://today.bsc.gwu.edu/.
Funding Support This work was completed with funding from NIDDK/NIH grant numbers U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, and U01-DK61254; from the National Center for Research Resources General Clinical Research Centers Program grant numbers M01-RR00036 (Washington University School of Medicine), M01-RR00043-45 (Children’s Hospital Los Angeles), M01-RR00069 (University of Colorado Denver), M01-RR00084 (Children’s Hospital of Pittsburgh), M01-RR01066 (Massachusetts General Hospital), M01-RR00125 (Yale University), and M01-RR14467 (University of Oklahoma Health Sciences Center); and from the NCRR Clinical and Translational Science Awards grant numbers UL1-RR024134 (Children’s Hospital of Philadelphia), UL1-RR024139 (Yale University), UL1-RR024153 (Children’s Hospital of Pittsburgh), UL1-RR024989 (Case Western Reserve University), UL1-RR024992 (Washington University in St Louis), UL1-RR025758 (Massachusetts General Hospital), and UL1-RR025780 (University of Colorado Denver).
ABBREVIATIONS
- TODAY
Treatment Options for type 2 Diabetes in Adolescents and Youth
- BMI
Body mass index
- CRF
Cardiorespiratory fitness
- PWC
Physical work capacity
- bpm
Beats per minute
- FFQ
Food frequency questionnaire
Footnotes
Trial registration ClinicalTrials.gov NCT00081328
Duality of Interest Kenneth C Copeland is a consultant to Daiichi Sankyo Inc. Lorraine E Levitt Katz is a consultant to Takeda Pharmaceuticals. The other authors have no conflicts of interest relevant to this article to disclose.
Author Contributions AK conceptualized and designed the study, worked on data interpretation, drafted the initial manuscript, and approved the final manuscript as submitted. LMD helped in the original design of the study, worked on data interpretation, helped to draft the initial manuscript, and approved the final manuscript as submitted. KCC helped with initial data interpretation, helped draft the initial manuscript, reviewed and revised the manuscript, and approved the final manuscript as submitted. JH helped in the original design of the study, helped with initial data interpretation, helped draft the initial manuscript, and approved the final manuscript as submitted. LE carried out the analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted. CEIL, LELK, PY, and PT helped with initial data interpretation, reviewed and revised the manuscript, and approved the final manuscript as submitted. AW reviewed and revised the manuscript and approved the final manuscript as submitted.
References
- 1.Copeland KC, Silverstein J, Moore KR, et al. American Academy of Pediatrics. Management of newly diagnosed type 2 Diabetes Mellitus (T2DM) in children and adolescents. Pediatrics. 2013;131(2):364–382. doi: 10.1542/peds.2012-3494. [DOI] [PubMed] [Google Scholar]
- 2.TODAY Study Group. Treatment Options for type 2 Diabetes in Adolescents and Youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8(2):74–87. doi: 10.1111/j.1399-5448.2007.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.TODAY Study Group. Design of a family-based lifestyle intervention for youth with type 2 diabetes: the TODAY study. Int J Obes (Lond) 2010;34(2):217–226. doi: 10.1038/ijo.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366:2247–2256. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.TODAY Study Group. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96(1):159–167. doi: 10.1210/jc.2010-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 7.Baumgartner TA. Measurement for Evaluation in Physical Education and Exercise Science. 8. Boston, MA: McGraw-Hill; 2007. [Google Scholar]
- 8.Kriska A, Delahanty L, Edelstein S, et al. Sedentary behavior and physical activity in youth with recent onset of type 2 diabetes. Pediatrics. 2013;131(3):e850–856. doi: 10.1542/peds.2012-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer-Davis EJ, Nichols M, Liese AD, et al. for the SEARCH for Diabetes in Youth Study. Dietary intake among youth with diabetes: the SEARCH for Diabetes in Youth Study. J Amer Diet Assoc. 2006;106(5):689–697. doi: 10.1016/j.jada.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Block G, Murphy M, Roullet J, et al. Pilot validation of a FFQ for children 8–10 years. Presented at Fourth International Conference on Dietary Assessment Methods; September 17, 2000; Tucson, AZ. [Google Scholar]
- 11.Mayer-Davis EJ, Sparks KC, Hirst K, et al. Dietary intake in the Diabetes Prevention Program cohort: baseline and 1-year post-randomization. Ann Epidemiol. 2004;14:763–772. doi: 10.1016/j.annepidem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Delahanty L, Kriska A, Edelstein S, et al. Self-reported dietary intake of youth with recent onset of type 2 diabetes: results from the TODAY study. J Acad Nutr Diet. 2013;113(3):431–439. doi: 10.1016/j.jand.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2014;37(Suppl 1):S120–S143. doi: 10.2337/dc14-S120. [DOI] [PubMed] [Google Scholar]
- 14.Pan A, Sun Q, Bernstein AM, et al. Changes in red meat consumption and subsequent risk of type 2 diabetes mellitus: three cohorts of US men and women. JAMA Intern Med. 2013;173(14):1328–1335. doi: 10.1001/jamainternmed.2013.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fogelholm M, Anderssen S, Gunnarsdottir I, et al. Dietary macronutrients and food consumption as determinants of long-term weight change in adults populations: a systematic literature review. Food and Nutrition Research. 2012;56:1–22. doi: 10.3402/fnr.v56i0.19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malik VS, Pan A, Willett WC, et al. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98(4):1084–1102. doi: 10.3945/ajcn.113.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mozaffarian D, Hao T, Rimm EB, et al. Changes in diet and lifestyle and long-term weight gain in men and women. NEJM. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGavock J, Dart A, Wicklow B. Lifestyle therapy for the treatment of youth with type 2 diabetes. Curr Diab Rep. 2015;15(1):568. doi: 10.1007/s11892-014-0568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakicic JM, Egan CM, Fabricatore AN, et al. Look AHEAD Research Group. Four-year change in cardiorespiratory fitness and influence on glycemic control in adults with type 2 diabetes in a randomized trial: the Look AHEAD Trial. Diabetes Care. 2013;36(5):1297–303. doi: 10.2337/dc12-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Youth Risk Behavior Surveillance – United States, 2009. MMWR CDC Surveillance Summary-Recommendations and Reports. 2010;59(5):1–148. [PubMed] [Google Scholar]
- 21.Evert AB, Riddell MC. Lifestyle intervention: nutrition therapy and physical activity. Med Clin North Am. 2015;99(1):69–85. doi: 10.1016/j.mcna.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y, Olendzi BC, Wang J, et al. Single-component versus multicomponent dietary goals for the metabolic syndrome: a randomized trial. Ann Intern Med. 2015;162:248–257. doi: 10.7326/M14-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292(8):927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 24.TODAY Study Group. Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36:1735–1741. doi: 10.2337/dc12-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.TODAY Study Group. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and beta-cell function in TODAY. Diabetes Care. 2013;36:1749–1757. doi: 10.2337/dc12-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.TODAY Study Group. Lipid and inflammatory cardiovascular risk worsens over 3 years in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36:1758–1764. doi: 10.2337/dc12-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.TODAY Study Group. Retinopathy in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Care. 2013;36:1772–1774. doi: 10.2337/dc12-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.TODAY Study Group. Binge eating, mood, and quality of life in youth with type 2 diabetes: baseline data from the TODAY study. Diabetes Care. 2011;34:858–860. doi: 10.2337/dc10-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson BJ, Edelstein S, Abramson NW, et al. for the TODAY Study Group. Depressive symptoms and quality of life in adolescents with type 2 diabetes: baseline data from the TODAY study. Diabetes Care. 2011;34:2205–2207. doi: 10.2337/dc11-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinstock RS, Trief P, El ghormli L, et al. Parental characteristics associated with outcomes in youth with type 2 diabetes: results from the TODAY clinical trial. Diabetes Care. 2015;38:784–792. doi: 10.2337/dc14-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epstein LH, Paluch RA, Beecher MD, et al. Increasing healthy eating vs. reducing high energy-dense foods to treat pediatric obesity. Obesity. 2008;16(2):318–326. doi: 10.1038/oby.2007.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hessler D, Fisher L, Glasgow RE, et al. Reductions in regimen distress are associated with improved management and glycemic control over time. Diabetes Care. 2014;37:617–624. doi: 10.2337/dc13-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.