Abstract
Psychosocial stress contributes to the development of anxiety and depression. Recent clinical studies have reported increased inflammatory leukocytes in circulation of individuals with stress-related psychiatric disorders. Parallel to this, our work in mice shows that social stress causes release of inflammatory monocytes into circulation. In addition, social stress caused the development of prolonged anxiety that was dependent on inflammatory monocytes in the brain. Therefore, we hypothesize that chronic stress drives the production of inflammatory monocytes that are actively recruited to the brain by microglia, and these monocytes augment neuroinflammatory signaling and prolong anxiety. Here we show that repeated social defeat stress in mice activated threat appraisal centers in the brain that spatially coincided with microglial activation and endothelial facilitation of monocyte recruitment. Moreover, microglial depletion with a CSF1R antagonist prior to stress prevented the recruitment of monocytes to the brain and abrogated the development of anxiety. Cell-specific transcriptional profiling revealed that microglia selectively enhanced CCL2 expression, while monocytes expressed the pro-inflammatory cytokine IL-1β. Consistent with these profiles, the recruited inflammatory monocytes with stress adhered to IL-1R1+ neurovascular endothelial cells and this interaction was blocked by microglial depletion. Furthermore, disruption of IL-1β signaling by caspase-1KO specifically within bone marrow-derived cells revealed that monocytes promoted anxiogenesis through stimulation of neurovascular IL-1R1 by IL-1β. Collectively, the development of anxiety during stress was caused by microglial recruitment of IL-1β-producing monocytes that stimulated brain endothelial IL-1R1. Thus, monocyte IL-1β production represents a novel mechanism that underlies behavioral complications associated with stress-related psychiatric disorders.
Keywords: Repeated Social Defeat, Social Stress, Anxiety, Monocytes, Endothelial cells, Microglia, Interleukin-1 beta
Introduction
Psychosocial stress is a substantial risk factor in the occurrence of mood and anxiety disorders1,2. Moreover, recent reports indicate that neuroinflammatory cytokine signaling contributes to the pathophysiology of stress-related psychiatric disorders (reviewed in3–5). For example, a recent neuroimaging study in depressed patients revealed that symptom severity correlated with microglial activation within brain regions implicated in mood regulation, including the prefrontal, anterior cingulate, and insular cortices6. Furthermore, postmortem studies of depressed suicide victims revealed microglial activation and macrophage accumulation within the anterior cingulate cortex7,8. Notably, circulating Ly6Chi monocytes were increased in populations exposed to chronic stress9,10. This is relevant because brain macrophage trafficking originates from circulating Ly6Chi monocytes11,12. These clinical findings are consistent with observations that systemic inflammation promotes depression and anxiety. For instance, cytokine therapies caused the development of major depressive disorder in up to half of all treated patients (reviewed in13). In addition, cytokine therapy elicited side-effects that include fatigue, anxiety, and anhedonia. Related to this, a recent clinical trial using a cytokine antagonist significantly improved mood and behavior in treatment-resistant depressed patients with elevated inflammatory biomarkers14,15. Taken together, inflammatory signaling contributes to behavioral complications associated with stress-related psychiatric disorders.
The immune and behavioral responses to stress observed in humans are recapitulated in rodent models of psychosocial stress, such as repeated social defeat (reviewed in16,17). For example, repeated social defeat increased the production and release of Ly6Chi monocytes that exhibited a proinflammatory profile similar to the human conserved transcriptional response to adversity18. Furthermore, repeated social defeat caused these Ly6Chi monocytes to be recruited to brain regions implicated in threat appraisal, differentiate into macrophages, and augment inflammatory cytokine signaling19. The threat appraisal regions of the brain are critical to the regulation of fear, anxiety and depressive-like behaviors20,21. The recruitment of Ly6Chi monocytes to the brain during stress was dependent on key chemokine ligand and receptor interactions, including CCL2/CCR222,23. For instance, genetic deletion of CCR2 prevented the recruitment of monocytes to the brain in mice exposed to repeated social defeat19. Notably, blockade of monocyte recruitment during stress prevented the development of anxiety and attenuated cortical IL-1β expression19. Related to this, several rodent models of stress provide evidence that increased IL-1β in the brain promotes fear and anxiety24–27.
Our previous results indicate that the development of anxiety during repeated social defeat was caused by the recruitment of inflammatory monocytes to the brain19. Nonetheless, the mechanisms underlying monocyte recruitment and anxiogenesis during stress are unclear. Thus, the purpose of this study was to 1) identify the cells responsible for the recruitment of monocytes to threat appraisal centers in the brain and 2) determine the signaling pathways by which monocytes recruited to the brain during stress promote anxiety. Here we show novel data that the development of anxiety during stress was caused by microglial recruitment of inflammatory monocytes that stimulated brain endothelial IL-1R1 via IL-1β.
Methods
Mice
Male C57BL/6 resident mice were housed in cohorts of three per cage, while CD-1 aggressors were singly housed. Mice were randomly distributed to groups upon arrival from supplier. Animal and replicate are indicated in figure legends. All procedures were in accordance with the NIH Guidelines and were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee.
Repeated Social Defeat
Mice were subjected to repeated social defeat stress as previously reported28 and as described in Supplementary Materials. In brief, an aggressive intruder male CD-1 mouse was introduced into cages of established male cohorts for two hours (17:00 to 19:00) per night for six consecutive nights. During each cycle, submissive behaviors were observed to ensure that the resident mice showed subordinate behavior.
Minocycline Treatment
Minocycline (Sigma-Aldrich) was administered in vivarium drinking water at 90 mg/kg. Oral minocycline treatment was started two days prior to the beginning of social defeat and was terminated on the last day of social defeat.
Clonazepam Treatment
Clonazepam (Sigma-Aldrich) was administered intraperitoneally (50 μg/kg). Clonazepam treatment was started two days prior to the beginning of social defeat and was terminated on the last day of social defeat.
Plexxikon Oral administration
PLX5622 was provided by Plexxikon Inc and formulated in standard AIN-76A rodent chow by Research Diets at a concentration of 1200 ppm. Control diet consisted of the standard AIN-76A rodent chow. Mice were provided ad libitum access to PLX5622 or control diet for 14 days to deplete microglia prior to exposure to social defeat.
Anxiety-like behavior
Anxiety-like behavior was determined using open-field activity as previously reported28 and as described in Supplementary Materials.
Isolation of cells from bone marrow, blood, and brain
Tissues were collected immediately following CO2 asphyxiation. Cells from bone marrow and blood were isolated as previously described28. CD11b+ brain cells were enriched by Percoll density gradient.
nanoString and nCounter Analysis of mRNA Copy Number
After technical normalization of positive and negative controls, RNA was normalized to reference gene expression. Data are expressed as messenger RNA (mRNA) copy numbers.
Casp1−/− Bone Marrow (BM)-chimera
To establish chimerism, recipient C57BL/6 CD45.1 male mice were injected intraperitoneally once daily for two consecutive days with busulfan (30 mg/kg/100 μL). Donor BM-derived cells were obtained from either male C57BL/6 CD45.2 (WT) mice or Casp1 KO (CD45.2) mice. BM-derived cells (1 × 106) were transferred to recipient mice by tail vein injection (100 μL) 48 h after the second dose of busulfan.
Statistical analysis
Data were analyzed using two-way ANOVA using the General Linear Model procedures of SPSS (IBM) that also provides feedback regarding data normality and variance. Individual data points more than two standard deviations above and below the mean were counted as outliers, and were excluded in the subsequent analyses. Main effects of experimental treatment or treatment interaction effects were evaluated by an F-protected t-test using the Least-Significant Difference procedure of SPSS. All data are expressed as treatment means ± SEM.
Results
Threat appraisal during stress promotes microglial activation and monocyte recruitment
Our first objective was to determine if neuronal activation within threat appraisal centers after stress was spatially related to the cellular events that facilitate monocyte recruitment to the brain. Here, repeated social defeat increased the number of ΔFosB+ cells within threat appraisal regions of the brain (Fig.S1A–C) including the prelimbic cortex (PrL), central amygdala (CeA), and CA3 region of the hippocampus (CA3). This increase spatially coincided with increased microglial Iba-1+ percent area (Fig.S1D&E) and morphological restructuring (Fig.S1D inset). Two key monocyte cell adhesion molecules (CAMs: VCAM-1 & ICAM-1) were increased on brain endothelial cells following repeated social defeat within these same regions (Fig.S1F–I). Furthermore, repeated social defeat increased the number of CD45+ monocytes that adhered specifically to the CAM-expressing endothelial cells (Fig.S1F&H insets; Fig.S1J–L). It is important to note that these cellular responses to stress were absent in the primary motor cortex (MCX), a brain region not involved in threat appraisal (Fig.S1A-1). The spatial overlap between these cellular events suggested that neuronal activation caused by threat appraisal during stress may underlie regional microglial restructuring and neurovascular adhesion of monocytes.
To attenuate threat appraisal, clonazepam was administered during repeated social defeat29. Consistent with its function as a benzodiazepine that modulates GABAergic signaling30, clonazepam prevented neuronal activation within threat appraisal regions in stress-exposed mice (Fig.1A&B; Fig.S2A). Notably, clonazepam also blocked subsequent microglial restructuring (Fig.1C&D; Fig.S2B), vascular endothelial CAM induction (Fig.1E&F; Fig.S2C–E), and monocyte recruitment (Fig.1G–I) within threat appraisal regions. Thus, inhibition of threat appraisal with clonazepam during stress prevented all of the neuroinflammatory cellular responses to stress, including microglial activation, endothelial CAM induction, and monocyte recruitment.
Figure 1. Monocyte recruitment to threat appraisal centers is dependent on neuronal and microglial activation.
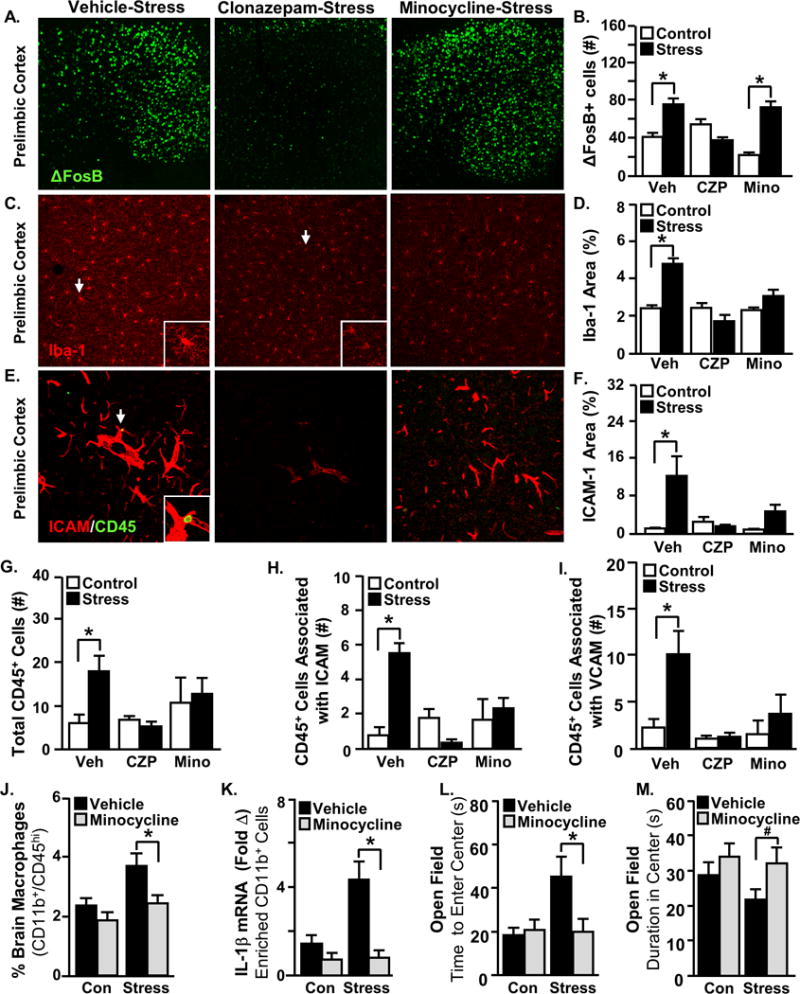
Male C57BL/6 mice received either minocycline (Mino) in their drinking water, clonazepam (CZP) injections i.p., or vehicle treatment daily two days before and during repeated social defeat (Stress). Mice were perfused and the brain was PFA fixed 14 h after the last cycle of stress. Neuronal activation (ΔFosB), microglial activation (Iba-1), vascular endothelial activation (ICAM-1), and the presence of monocytes (CD45+) in the vasculature (ICAM-1/CD45) were assessed. Representative images within the prelimbic cortex of A) ΔFosB, C) Iba1, and E) ICAM-1/CD45 labeling. Arrows indicate the cells used in insets. B) The number of ΔFosB+ cells (Stress × Intervention interaction, F(2,33)=12.02, p=0.0002), D) Iba-1 proportional area (Stress × Drug interaction, F(2,36)=11.21, p=0.0002), and F) ICAM-1 proportional area (Stress × Intervention interaction; F(2,30)=5.23, p=0.012). G) The number of CD45+ cells in threat appraisal centers (Stress × Intervention interaction; F(2,35)=3.31, p=0.05). Number of CD45+ cells associated with H) ICAM-1 positive blood vessels (Stress × Intervention interaction; F(2,35)=17.57, p<0.0001) and I) VCAM-1 positive blood vessels (Stress × Intervention interaction; F(2,35)=2.95, p=0.067). In a separate experiment, male C57BL/6 mice received minocycline or vehicle in their drinking water two days before and during social defeat (Stress). Anxiety-like behavior was determined 14 h after the last cycle of stress and then samples (bone marrow, blood, and brain) were collected for cell or mRNA analyses. J) CD45hi macrophages in the brain (main effect of Stress; F(1,49)=7.37, p=0.0093 & main effect of Minocycline; F(1,49)=7.91, p=0.0072). K) IL-1β mRNA levels in enriched CD11b+ cells from the brain (Stress × Minocycline interaction; F(1,25)=7.76, p=0.011). L) Latency to enter the center of the open field (Stress × Minocycline interaction; F(1,76)=4.043, p=0.048) and M) time spent in the center of the open field (main effect of minocycline; F(1,75)=4.094, p=0.047). Total number of samples per group (n) and number of replicates (rep): A-I (n=4–6, rep=2), J (n=12–16, rep=2), K (n=6, rep=2), L-M (n=18–20, rep=4). Bars represent the mean ± SEM. Means with asterisk (*) are significantly different from the corresponding control mice (p<0.05), and means with (#) tended to be different from control mice (p<0.1), according to F-protected post hoc analysis.
Monocyte recruitment to the brain during stress depends on microglia
Next, minocycline was used to assess the role of microglial activation in the recruitment of monocytes to the brain. Treatment with minocycline during repeated social defeat had no effect on neuronal activation within threat appraisal regions (Fig.1A&B; Fig.S2A). Consistent with its established role as a microglial inhibitor31, minocycline prevented microglial restructuring during repeated social defeat (Fig.1C&D; Fig.S1B). Minocycline, however, did not affect the induction of adhesion molecules on endothelial cells in these same brain regions (Fig.1E&F; Fig.S2C–E). Nonetheless, inhibition of microglia with minocycline prevented stress-induced monocyte recruitment to the brain vasculature (Fig.1G–J). Furthermore, minocycline prevented the induction of IL-1β mRNA expression in enriched brain CD11b+ cells (Fig.1K). Consistent with our previous report32, minocycline did not prevent the development of social avoidance behavior following repeated social defeat (Fig.S3A&B). Notably, minocycline prevented stress-induced anxiety-like behavior in the open field (Fig.1L&M). Collectively, inhibition of microglia with minocycline prevented brain monocyte recruitment and anxiety following stress.
As an improved strategy to further define the role of microglia, a series of experiments using the specific CSF1R antagonist Plexxikon 5622 (PLX) was completed. Consistent with previous reports33, two weeks of dietary administration of PLX eliminated microglia from the brain (Fig.2A&B). The mRNA expression of CX3CR1, a microglia-specific receptor, was also reduced by PLX (Fig.2C). Depletion of microglia with PLX during repeated social defeat did not affect neuronal activation within threat appraisal brain regions (Fig.2D&E; Fig.S4A). PLX eliminated microglial Iba-1+ percent area in both control and stress mice (Fig.2F&G; Fig.S4B). Nonetheless, the induction of endothelial CAM expression by repeated social defeat was maintained despite microglia depletion by PLX (Fig.2H&I; Fig.S4C–E).
Figure 2. Microglial depletion with CSF1R antagonist does not prevent stress-induced threat appraisal or endothelial activation.
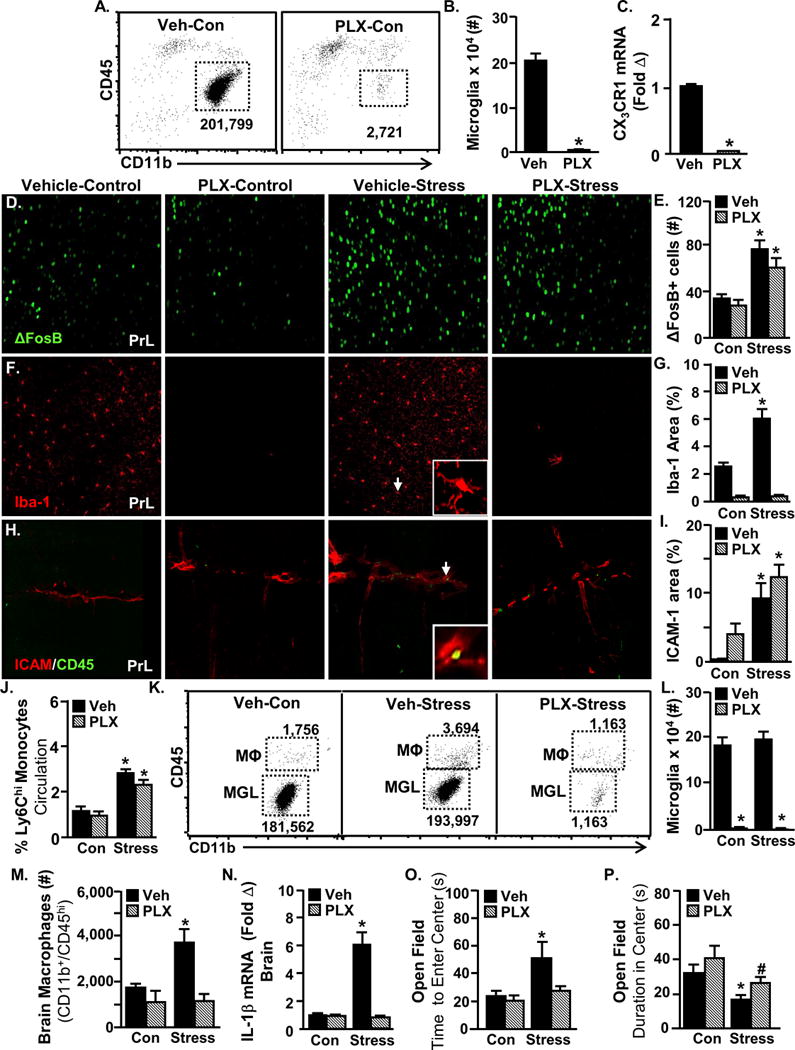
Male C57BL/6 mice were provided ad libitum diets of PLX5622 (1200 ppm chow) or vehicle chow for 14 days. Brain samples were collected to determine microglial ablation. A) Representative flow bivariate dot plots of CD11b and CD45 labeling on enriched microglia and macrophages. B) Number of CD11b+CD45lo microglia (main effect of PLX, F(1,7)=123.30, p<0.0001). C) CX3CR1 mRNA levels in a coronal brain section (main effect of PLX, F(1,18)=896.65, p<0.0001). A separate set of mice provided with PLX5622 or vehicle chow for 14 days were then exposed to repeated social defeat (Stress). Mice were perfused and brains were PFA fixed 14 h after the last cycle of stress. Neuronal activation (ΔFosB), microglial activation (Iba-1) and vascular endothelial activation (ICAM-1) were assessed. Representative images within the prelimbic cortex (PrL) of D) ΔFosB, F) Iba-1, H) ICAM-1/CD45 labeling. Arrows indicate the cells used in insets. E) Number of ΔFosB+ cells (main effect of Stress; F(1,15)=26.00, p=0.0003), G) Iba-1 proportional area (Stress × PLX interaction; F(1,15)=21.37, p=0.0006), and I) ICAM-1 proportional area (main effect of Stress; F(1,16)=27.19, p=0.0002). In a separate experiment, mice were provided with a diet of PLX5622 (1200 ppm chow) or vehicle chow for 14 days and then exposed to social defeat (Stress). Anxiety-like behavior was determined 14 h after the last cycle of stress and then samples (blood and brain) were collected for cell or mRNA analyses. J) Percentage of circulating CD11b+/Ly6Chi monocytes (main effect of Stress; F(1,30)=44.18, p<0.0001). K) Representative flow bivariate dot plots of CD11b and CD45 labeling on enriched brain microglia and macrophages. L) Number of CD11b+CD45lo microglia (main effect of PLX; F(1,44)=164.89, p<.0001) and M) CD11b+CD45hi macrophages (Stress × PLX interaction; F(1,38)=3.33, p=0.0767) in the brain. N) IL-1β mRNA levels in the brain (Stress × PLX interaction; F(1,28)=40.95, p<0.0001). O) Latency to enter the center of the open field (main effect of Stress; F(1,46)=4.78, p=0.0343) and P) time spent in the center of the open field (main effect of Stress; F(1,46)=9.69, p=0.0033 & main effect of PLX; F(1,46)=3.99, p=0.0522). Total number of samples per group (n) and number of replicates (rep): B (n=4, rep=1), C (n=4, rep=1), D-I (n=4–5, rep=1), J-M (n=8–12, rep=2), and O&P (n=8–12, rep=2). Bars represent the mean ± SEM. Means with asterisk (*) are significantly different (p<0.05), and means with (#) tended to be different (p=0.1) from the corresponding control mice, according to F-protected post hoc analysis.
Next, the effect of microglial depletion on monocyte recruitment to the brain and anxiety was tested. Microglial depletion with PLX had no effect on the peripheral production or release of Ly6Chi monocytes in either control or stress mice (Fig.2J). Flow cytometry confirmed the microglia depletion in both control and stress mice treated with PLX (Fig.2K&L). Depletion of microglia by PLX prevented monocyte recruitment to the brain during repeated social defeat (Fig.2M). Furthermore, PLX prevented stress-induced IL-1β mRNA expression in the brain (Fig.2N). Similar to minocycline, PLX prevented the development of anxiety-like behavior (Fig.2O&P) but not social avoidance (Fig.S3C&D). Thus, anxiety and social avoidance are dichotomously regulated by neuroinflammatory signaling during stress. Collectively, microglia were necessary for the recruitment of monocytes to the brain and the development of anxiety during stress.
Microglia chemoattract IL-1β-expressing monocytes to interact with IL-1R1+ endothelium in the brain
We next aimed to determine the relative contribution of microglia and brain macrophages to the enhanced neuroinflammatory milieu promoted by repeated social defeat. Here, microglia (CD11b+/CD45lo) and brain macrophages (CD11b+/CD45hi) were FAC-sorted, and mRNA counts were analyzed by nanoString array for >45 genes related to microglia or macrophages (Fig.3A). These were classified as genes associated with cell signature (Fig.3B), cytokines/chemokines (Fig.3C), adrenergic signaling (Fig.3D), cytokine receptors (Fig.3E), glucocorticoid signaling (Fig.3F), and immune signaling (Fig.3G). Genes that were significantly enriched by cell type are highlighted in grey (Fig.3). Numerous signature genes were differentially expressed between microglia and macrophages in the brain. For instance, CSF1R, CX3CR1, and CD14 mRNA expression were robustly enriched in microglia compared to brain macrophages (Fig.3B), while CD45, LysM, CCR2, CXCR2, and Ly6C were highly enriched in brain macrophages compared to microglia. Stress-dependent differences are highlighted and bolded (Fig.3). For example, CCL2 expression was increased in microglia, while IL-1β was specifically increased in brain macrophages following repeated social defeat (Fig.3C). Notably, adrenergic receptors (Fig.3D), cytokine receptors (Fig.3E), and glucocorticoid signaling (Fig.3F) genes were highly enriched in microglia. Repeated social defeat also reduced glucocorticoid receptor (GCrec) expression in brain macrophages. Other immune signaling genes, such as Cox2 and Nos2, were unaffected by stress in either cell type (Fig.3G).
Figure 3. Transcriptional profiling of FAC-Sorted microglia and monocytes.
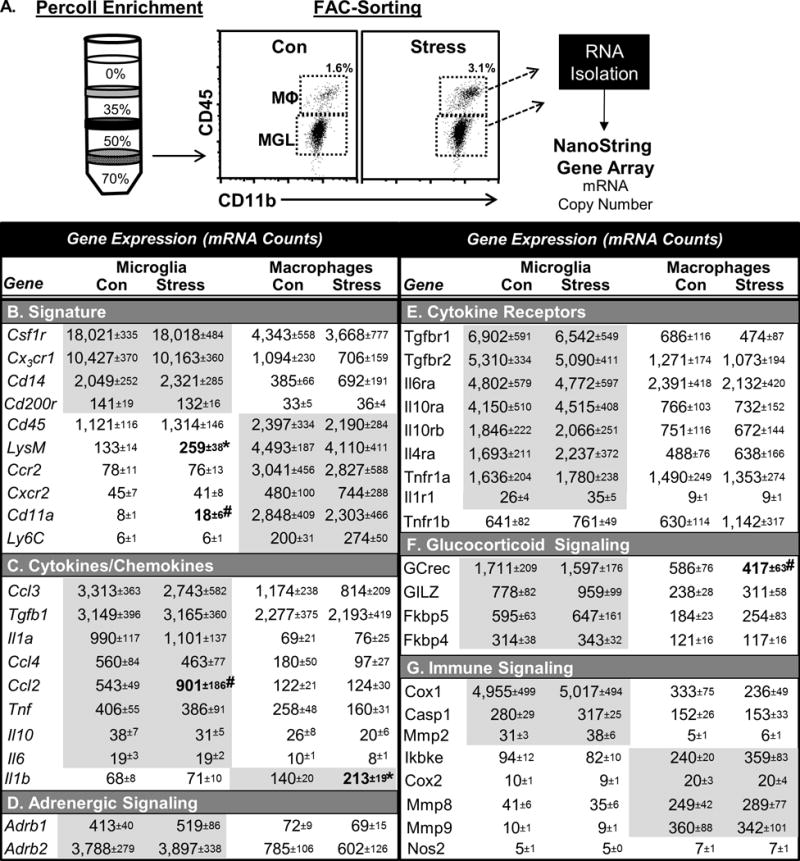
Male C57BL/6 mice were subjected to 6 cycles of repeated social defeat (stress) or left undisturbed as controls. A) Microglia (CD11b+/CD45lo) and brain macrophages (CD11b+/CD45hi) were Percoll-enriched and FAC-Sorted 14 h after the last cycle of stress. Then, mRNA copy number was determined by nanoString analysis. Cell-specific mRNA copy number for microglia or monocytes is shown for B) signature transcripts, C) cytokines/chemokines, D) adrenergic receptors, E) cytokine receptors, F) glucocorticoid signaling, and G) immune signaling. Total number of samples per group (n) and number of replicates (rep): n=6, rep=2). Bolded and grey shaded groups of transcripts indicate significant main effect of cell type (p<0.05 from MANOVA). All groups, n= 6. Means with asterisk (*) are significantly different (p<0.05), and means with (#) tended to be different (p<0.1) from the corresponding control mice.
These mRNA profiling results revealed that monocytes propagated IL-1β in the brain during repeated social defeat. This finding is important because increased IL-1β signaling is associated with the development of anxiety in several stress models24–27. Thus, our next objective was to determine the cell type-specific expression of the IL-1β receptor (IL-1R1). To avoid artifacts in the detection of in situ IL-1R1 expression in the brain, IL-1R1GR/GR reporter mice were used34. These mice have an IRES site followed by tdTomato (tdTom) sequence inserted in exon 11 of the IL-1R1 gene, and have three hemagglutinin (HA) sequences tagged to exon 11 of IL-1R1 (Fig.4A). Thus, tdTom reports IL-1R1 transcription and HA is tagged to IL-1R1 protein. Co-localization of tdTom and HA was limited to brain vascular endothelial cells (Fig.4B) and was undetected in other cell types. Furthermore, the IL-1R1 antibody used here was validated by the co-localization of IL-1R1 labeling and tdTom expression (Fig.4C).
Figure 4. Microglial activation increases brain endothelial IL-1R1 expression during stress.
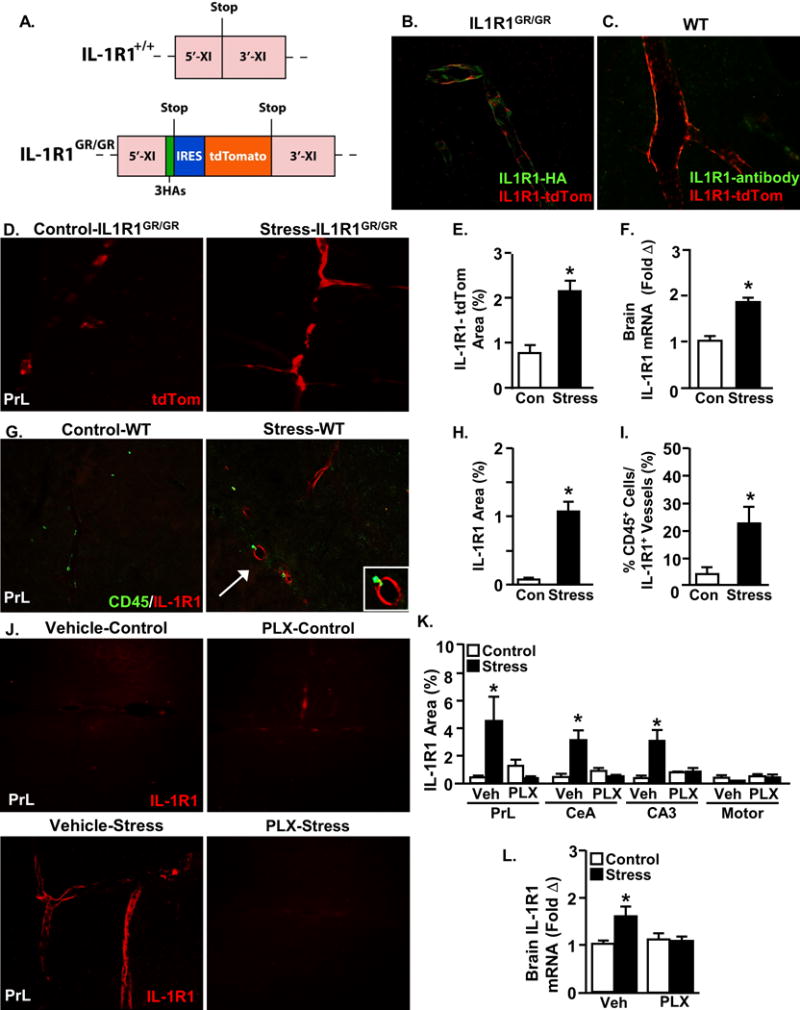
A) Diagram of exon XI of the wild type IL-1R1+/+ allele (top) and the IL-1R1GR/GR targeted mutation allele. IL-1R1GR/GR allele includes 3 hemagglutinin (HA) tagging sequences within exon XI and includes an internal ribosomal entry sequence (IRES) followed by tdTomato sequence. IL-1R1 transcription results in tdTomato fluorescence and translated IL-1R1 proteins detectable by antibody labeling of HA. Representative images showing tdTomato fluorescence (red) co-labeled with B) anti-HA antibody (green) and C) anti-IL1R1 antibody (green). Male IL-1R1GR/GR mice were subjected to social defeat (Stress) or left undisturbed as controls. Mice were perfused and the brain was PFA fixed 14 h after the last cycle of stress. D) Representative images of tdTomato fluorescence in the prelimbic cortex. E) tdTomato proportional area in the PrL after stress (F(1,4)=17.33, p=0.0252). In related experiments, male C57B/L6 wild type mice were subjected to social defeat (Stress) or left undisturbed as controls. Samples were collected 14 h later and either brain IL-1R1 mRNA or IL-1R1+ cells and CD45+ cells were determined in the PrL. F) IL-1R1 mRNA levels in a coronal section after stress (F(1,7)=33.75, p=0.0011). G) Representative images of CD45 and IL-1R1 antibody labeling within the prelimbic cortex. H) IL-1R1 proportional area after stress (F(1,7)=51.06, p=0.0004), and I) Percentage of CD45 positive cells associated with IL-1R1 positive blood vessels (F(1,7)=8.05, p=0.0297). Next, male C57B/L6 mice were provided ad libitum diets of PLX5622 (1200 ppm chow) or vehicle chow for 14 days and then exposed to repeated social defeat (Stress). J) Representative images of IL-1R1 antibody labeling within the PrL. K) IL-1R1 proportional area in the PrL (Stress × PLX interaction; F(1,15)=15.88, p=0.0018), CeA (Stress × PLX interaction; F(1,16)=11.61, p=0.0047), CA3 (Stress × PLX interaction; F(1,16)=8.23, p=0.0132) and Motor cortex. L) IL-1R1 mRNA levels in a coronal brain section (Stress × PLX interaction; F(1,38)=4.86, p=0.0339). Total number of samples per group (n) and number of replicates (rep): E (n=3, rep=1), F (n=4, rep=1), H-I (n=4, rep=1), K (n=4, rep=1), and L (n=8–10, rep=3). Bars represent the mean ± SEM. Means with asterisk (*) are significantly different (p<0.05), and means with (#) tended to be different (p<0.1) from the corresponding control mice, according to F-protected post hoc analysis.
IL-1R1 mRNA tracking by tdTom showed that social stress increased IL-1R1 expression on brain endothelial cells (Fig.4D&E). Repeated social defeat increased IL-1R1 mRNA in whole brain (Fig.4F) and upregulated IL-1R1 protein expression specifically on brain endothelial cells (Fig.4G&H). Furthermore, IL-1R1 and CD45 co-labeling revealed that stress preferentially recruited monocytes to adhere to IL-1R1-expressing brain endothelial cells (Fig.4I). Moreover, PLX prevented the induction of IL-1R1 expression on endothelial cells within threat appraisal centers after repeated social defeat (Fig.4J&K). Consistent with these data, microglial depletion prevented the induction of brain IL-1R1 mRNA (Fig.4L). Collectively, microglial activation during stress led to the selective recruitment of monocytes to IL-1R1+ endothelial cells.
Monocyte production of active IL-1β protein in the brain mediates anxiety
Our results indicate that IL-1β-expressing monocytes were preferentially recruited to IL-1R1+ brain endothelial cells during repeated social defeat. This is important because increased IL-1β in the brain during stress is implicated in anxiogenesis24–27. To determine the necessity of IL-1β specifically from peripherally-derived immune cells, caspase-1 (Casp1) knockout (KO) bone marrow-chimeric mice were generated. Casp1 is the enzyme necessary for the cleavage of pro-IL-1β into active IL-1β. Deletion of Casp1 prevented the secretion of active IL-1β protein from bone marrow cells (Fig.5A) without preventing the induction of IL-1β mRNA (Fig.5B). Thus, Casp1 serves a post-translational role in the release of active IL-1β.
Figure 5. Bone marrow-derived monocyte production of active IL-1β protein in the brain mediates anxiety.
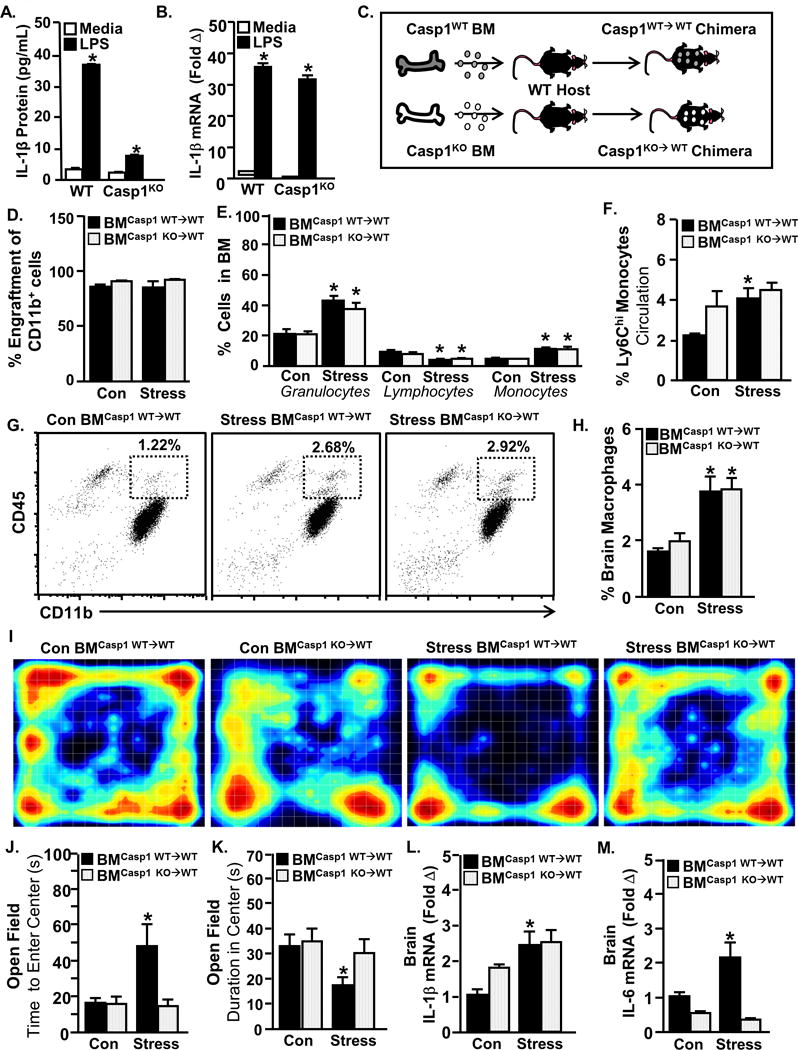
Bone marrow cells were collected from male C57BL/6 WT and Caspase-1KO (Casp1−/−) mice and treated with ex vivo LPS (1μg/mL) for 18 hours. A) IL-1β protein levels (Genotype × Treatment interaction; F(1,22)=488.48, p<0.0001) and B) IL-1β mRNA levels were determined (main effect of Genotype; F(1,23)=5.12, p=0.0350 and main effect of LPS; F(1,23)=1197.51, p<0.0001). C) In another experiment, Casp1−/− bone marrow-chimeric mice were generated. C57BL/6 CD45.1 mice were reconstituted with bone marrow from wildtype CD45.2 mice or Casp1−/− CD45.2 mice to produce WT BM chimera (BMCasp1 WT→WT) and Casp1−/− BM chimera (BMCasp1 KO→WT), respectively. The chimeric mice were then exposed to repeated social defeat (Stress) or left undisturbed as controls. D) Percentage of CD11b+CD45.2+ blood cells in WT chimera and Casp1−/− chimera mice after exposure to repeated social defeat (Stress). E) Percentage of bone marrow monocytes (main effect of Stress; F(1,44)=34.44, p<.0001), granulocytes (main effect of Stress; F(1,43)=30.71, p<.0001), lymphocytes (main effect of Stress; F(1,45)=26.39, p<.0001) and erythrocytes (main effect of Stress; F(1,44)=29.82, p<.0001). F) Percentage of circulating Ly6Chi monocytes in the blood (main effect of Stress; F(1,42)=7.25, p=0.0104). G) Representative bivariate dot plots of CD11b and CD45 labeling on enriched brain macrophages and microglia. H) Percentage of CD45hi macrophages in the brain (main effect of Stress; F(1,35)=21.22, p<.0001). I) Heat-map representing activity in the open field box. J) Latency to enter the center of the open field (Stress × Genotype interaction; F(1,66)=3.98, p< 0.05) and K) time spent in the center (main effect of Stress; F(1,66)=6.56, p < 0.01). L) IL-1β mRNA (main effect of Stress; F(1,26)=9.67, p < 0.005) and M) IL-6 mRNA levels in a coronal brain section (Stress × Genotype interaction; F(1,22)=11.06, p=0.0036 & main effect of Genotype; F(1,22)=32.31, p<0.0001). Total number of samples per group (n) and number of replicates (rep): A-B (n=5–6, rep=1), D-H (n=6, rep=1), J&K (n=19–23, rep=3), L&M (n=6, rep=1). Bars represent the mean ± SEM. Means with asterisk (*) are significantly different from the corresponding control mice (p<0.05), and means with (#) tended to be different from control mice (p<0.1), according to F-protected post hoc analysis.
To generate chimera mice, Casp1 WT or KO bone marrow was transferred into myeloablated host mice. This resulted in Casp1WT→WT or Casp1KO→WT chimera mice (Fig.5C). Engraftment of donor cells was >85% within circulating CD11b+ cells, and engraftment was unaffected by repeated social defeat (Fig.5D). Deletion of Casp1 in bone marrow-derived cells had no effect on the peripheral production (Fig.5E) or release (Fig.5F) of Ly6Chi monocytes during repeated social defeat. Furthermore, the lack of active IL-1β within bone marrow-derived cells did not prevent the accumulation of brain macrophages (Fig.5G&H). The development of anxiety during stress, however, was prevented by deletion of Casp1 in bone marrow-derived cells (Fig.5I–K). Consistent with its role in post-translational modification of IL-1β, the deletion of Casp1 in bone marrow-derived cells had no effect on stress-induced IL-1β mRNA expression in the brain (Fig.5L). The induction of IL-6 mRNA in the brain after repeated social defeat, however, was prevented in casp1KO→WT chimera mice (Fig.5M). It is important to highlight that these collective effects of stress in chimera mice were recapitulated in global caspase-1 KO mice (Fig.S5). Taken together, recruitment of IL-1β–producing monocytes to the brain promotes the development of anxiety during stress.
Discussion
Mounting evidence indicates that dynamic bidirectional communication between the brain and immune system contributes to behavioral responses to psychosocial stress3,4,35. Here we aimed to identify specific mechanisms of neuroimmune signaling that underlie stress-induced behaviors. Our first key finding was that microglia actively recruited monocytes to the brain specifically within threat appraisal regions during stress. Furthermore, these inflammatory monocytes upregulated IL-1β and stimulated IL-1R1+ brain endothelial cells to promote anxiety. Therefore, monocyte stimulation of brain endothelial IL-1R1 is a key pathway that links immune activation to anxiety during stress.
Several approaches were used to assess the role of microglia in the neuroimmune and behavioral responses to social stress. Both microglia inhibition (minocycline) and elimination (PLX) during stress prevented increased brain cytokine production, monocyte recruitment, and the development of anxiety. Additionally, stress selectively increased CCL2 mRNA expression in microglia that may have mediated the chemoattraction of monocytes to the brain. For instance, monocyte recruitment to the brain and anxiogenesis during stress were both dependent on monocyte expression of CCR219, the receptor for CCL2. These findings revealed that inflammatory monocytes were actively recruited by microglia to the brain during stress, and that this was necessary for the development of anxiety. Taken together, microglia mediated the development of anxiety by actively recruiting inflammatory monocytes to the brain.
The principle finding of this study was that the development of anxiety during stress depended on monocyte IL-1β production at the blood-brain interface. IL-1β-expressing monocytes were recruited to be within close proximity to IL-1R1+ endothelial cells in the brain during stress. Notably, both monocyte recruitment and increased endothelial IL-1R1 expression depended on microglial activation. Relevant to this, we previously showed that endothelial IL-1R1 knock-down mice did not develop anxiety following social defeat25. This suggested that monocyte stimulation of endothelial IL-1R1 via IL-1β contributed to anxiety during stress. To address this, caspase-1 KO bone marrow (BM)-chimera mice were produced. As expected, caspase-1 KO prevented the secretion of mature IL-1β protein from BM-derived cells. Similar to endothelial IL-1R1 knockdown, BM caspase-1 deficiency prevented the development of anxiety without preventing the recruitment of monocytes to the brain. Thus, monocyte-derived IL-1β mediated anxiogenesis via endothelial IL-1R1 during stress.
Critical to our study, IL-1β production by inflammatory monocytes was necessary for endothelial IL-1R1 stimulation and the propagation of anxiogenic signals into the brain. For example, the development of anxiety following stress was prevented in caspase 1 KO BM-chimera mice. The activation of endothelial cells by monocyte IL-1β was blocked in these mice. It is unlikely, however, that monocytes are the only source of IL-1β in the brain during stress. For example, peripheral vascular endothelial cells also produce IL-1β to propagate inflammatory signaling37. Nonetheless, a secondary signal from endothelial cells is required to influence neurons to cause anxiety. For instance, we previously showed that endothelial-specific stimulation of IL-1R1 with IL-1β is sufficient to induce potent neuroinflammatory mediators (e.g., prostaglandins) in the brain36. Furthermore, prostaglandins and other endothelial-derived inflammatory mediators directly alter neuronal signaling to promote behaviors such as anxiety, anhedonia, and sickness (reviewed in38).
Another finding was that threat appraisal is a necessary initiator of neuroimmune and behavioral responses to repeated social defeat. For instance, clonazepam blocked the region-specific induction of neuronal ΔFosB. We interpret this to mean that clonazepam abrogated threat appraisal during stress. In support of our hypothesis, clonazepam blocked microglial activation, endothelial CAM induction, monocyte recruitment, social avoidance, and anxiety29. It should also be noted that microglia did not contribute to threat appraisal during stress. For instance, neither microglial depletion nor microglial inhibition attenuated neuronal activation within stress-responsive brain regions. Thus, threat appraisal is a necessary initial step in the neuroimmune and behavioral responses to stress.
Our findings highlight the complex cellular interactions required to recruit monocytes to the brain during stress. Stress increased neuronal activation (ΔFosB), microglial activation (Iba-1), endothelial CAM expression (selectins39, ICAM-1, and VCAM-1), and monocyte recruitment all within the same threat appraisal brain regions. Notably, induction of monocyte adhesion molecules was mediated by neuronal activation during threat appraisal and was independent of microglial activation. Selectins, ICAM-1, and VCAM-1 are all necessary for the slowing down and adhesion of monocytes to vascular endothelium40. Here we show that concurrent microglial activation with stress was required to recruit monocytes to the brain, likely via chemokine gradient (e.g., CCL2). Thus, CAM induction was necessary but not sufficient for increased monocyte recruitment during stress. Overall, our data suggest that monocyte recruitment during stress requires both endothelial expression of adhesion molecules (neuronal-mediated) and a parenchymal chemokine gradient (e.g., CCL2 from microglia). Thus, the convergence of neuronal activation and microglial activation with stress leads to the development of a reactive endothelium that facilitates monocyte recruitment within close proximity to IL-1R1+ endothelial cells. This signaling cascade ultimately results in the activation of endothelial IL-1R1 within threat responsive brain regions that promotes anxiogenesis. This dynamic signaling pathway is graphically summarized in Figure 6.
Figure 6. Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety.
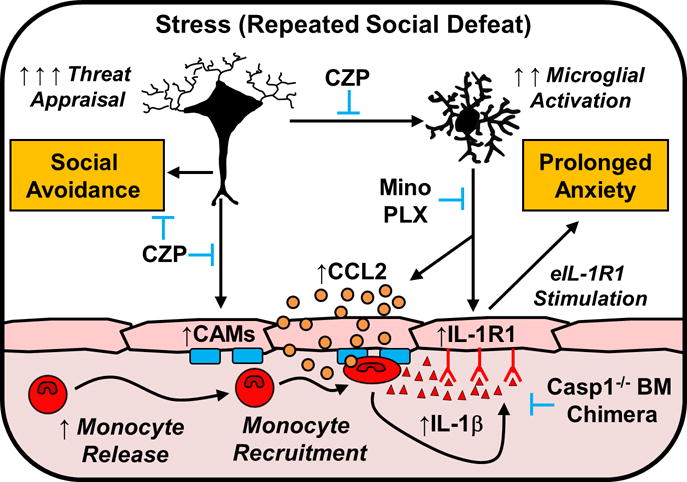
This figure illustrates the key findings of this study. First, exposure to repeated social defeat (Stress) in mice caused significant neuronal activation (ΔFosB) within threat appraisal regions of the brain (e.g., frontal cortex, amygdala, and hippocampus). This threat appraisal was associated with increased endothelial cell adhesion molecule (CAM) expression, activation of microglia, and the release of monocytes into circulation. Second, inhibition of threat appraisal with clonazepam (CZP) prevented endothelial CAM induction, microglial activation, and monocyte recruitment to the brain. Third, stress-induced microglial activation was associated morphological restructuring, increased chemokine expression (CCL2), and induction of IL-1R1 on endothelial cells. For example, inhibition (minocycline; Mino) and depletion (Plexxikon 5622; PLX) of microglia prevented stress-induced monocyte recruitment, endothelial IL-1R1 induction, and anxiety-like behavior. Notably, induction of social avoidance with repeated social defeat was blocked by CZP, but was unaffected by the microglial interventions, PLX or Mino. Fourth, monocytes recruited to the brain with stress expressed a high level of IL-1β. Inflammatory monocytes recruited to the brain with stress stimulated IL-1R1+ endothelial cells (eIL-1R1) to promote anxiety. For example, the inability of monocytes to make functional IL-1β in caspase-1 KO bone marrow (Casp1−/− BM) chimeras prevented the augmentation of neuroinflammatory signaling and the development of anxiety in response to stress. Overall, monocyte IL-1β production represents a novel cellular mechanism by which the immune system communicates with the brain to influence behavior.
Another notable observation was that anxiety but not social avoidance depended on inflammatory signaling in the brain. Here we showed that PLX and minocycline both prevented the development of anxiety but did not prevent the development of social avoidance. This observation was unexpected because previous studies revealed that peripheral production of IL-6 mediated the development of social avoidance in susceptible animals in a similar, albeit less intense, model of social defeat stress41. Collectively, we showed here that social avoidance occurred independent of microglial activation and brain monocyte recruitment.
Here, microglia were depleted with the specific CSF1R inhibitor Plexxikon 5622 (PLX). We confirmed microglial elimination using flow cytometry (CD11b and CD45), immunohistochemistry (Iba-1), and mRNA analysis (CX3CR1). Other studies have also confirmed microglial elimination by PLX33,42. It is important to note that similar CSF1R inhibitors have been reported to influence peripheral innate immune populations43, which is likely due to non-selective inhibition of c-Kit receptor kinases. The compound used here (Plexxikon 5622) does not inhibit c-Kit receptor33. This ensured that our intervention was specifically depleting microglia and not peripheral monocytes. Moreover, findings from microglial elimination studies were recapitulated using the purported microglial inhibitor minocycline. Taken together, PLX and minocycline were successfully used here to determine the specific contributions of microglia to the neuroimmune and behavioral responses to stress.
Collectively, we conclude from these findings that threat appraisal during psychosocial stress caused microglial activation and endothelial facilitation of monocyte recruitment to the brain. Moreover, the development of anxiety during stress was caused by microglial recruitment of IL-1β–producing monocytes that stimulated IL-1R1 on brain endothelial cells. Taken together, monocyte-derived IL-1β may represent a novel therapeutic target in the treatment of anxiety associated with chronic stress.
Supplementary Material
Acknowledgments
This study was supported by National Institute of Health (NIMH) grants R01-MH-093473 and R01-MH093472 to JFS. DBM, MDW, CMS, BLJ were supported by NIDCR Training Grant T32-DE014320. DBM was supported by F31-MH-109234. Authors thank Brian West at Plexxikon Inc for the use of PLX5622. We thank The Ohio State University Comprehensive Cancer Center’s (OSUCCC) Analytical Cytometry and Nucleic Acid Shared Resources.
Footnotes
Authors report no conflict of interest.
References
- 1.Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Arch Gen Psychiat. 2003;60(8):789–796. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- 2.Kendler KS, Karkowski LM, Prescott CA. Stressful life events and major depression: risk period, long-term contextual threat, and diagnostic specificity. The Journal of nervous and mental disease. 1998;186(11):661–669. doi: 10.1097/00005053-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37(1):137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beumer W, Gibney SM, Drexhage RC, Pont-Lezica L, Doorduin J, Klein HC, et al. The immune theory of psychiatric diseases: a key role for activated microglia and circulating monocytes. Journal of leukocyte biology. 2012;92(5):959–975. doi: 10.1189/jlb.0212100. [DOI] [PubMed] [Google Scholar]
- 6.Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA psychiatry. 2015;72(3):268–275. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castillo-Richmond A, Schneider RH, Alexander CN, Cook R, Myers H, Nidich S, et al. Effects of stress reduction on carotid atherosclerosis in hypertensive African Americans. Stroke; a journal of cerebral circulation. 2000;31(3):568–573. doi: 10.1161/01.str.31.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakobsson J, Bjerke M, Sahebi S, Isgren A, Ekman CJ, Sellgren C, et al. Monocyte and microglial activation in patients with mood-stabilized bipolar disorder. Journal of psychiatry & neuroscience : JPN. 2015;40(4):250–258. doi: 10.1503/jpn.140183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20(7):754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci U S A. 2011;108(7):3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239(4837):290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 12.Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10(12):1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 13.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA psychiatry. 2013;70(1):31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinberger JF, Raison CL, Rye DB, Montague AR, Woolwine BJ, Felger JC, et al. Inhibition of tumor necrosis factor improves sleep continuity in patients with treatment resistant depression and high inflammation. Brain Behav Immun. 2015;47:193–200. doi: 10.1016/j.bbi.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wohleb ES, McKim DB, Sheridan JF, Godbout JP. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci. 2014;8:447. doi: 10.3389/fnins.2014.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuzcu EM, Kapadia SR, Tutar E, Ziada KM, Hobbs RE, McCarthy PM, et al. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation. 2001;103(22):2705–2710. doi: 10.1161/01.cir.103.22.2705. [DOI] [PubMed] [Google Scholar]
- 18.Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A. 2013;110(41):16574–16579. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33(34):13820–13833. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10(9):1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ataka K, Asakawa A, Nagaishi K, Kaimoto K, Sawada A, Hayakawa Y, et al. Bone marrow-derived microglia infiltrate into the paraventricular nucleus of chronic psychological stress-loaded mice. PLoS One. 2013;8(11):e81744. doi: 10.1371/journal.pone.0081744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brevet M, Kojima H, Asakawa A, Atsuchi K, Ushikai M, Ataka K, et al. Chronic foot-shock stress potentiates the influx of bone marrow-derived microglia into hippocampus. J Neurosci Res. 2010;88(9):1890–1897. doi: 10.1002/jnr.22362. [DOI] [PubMed] [Google Scholar]
- 24.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31(17):6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J Neurosci. 2014;34(7):2583–2591. doi: 10.1523/JNEUROSCI.3723-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwata M, Ota KT, Li XY, Sakaue F, Li N, Dutheil S, et al. Psychological Stress Activates the Inflammasome via Release of Adenosine Triphosphate and Stimulation of the Purinergic Type 2X7 Receptor. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 27.Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, et al. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. 2014 doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- 28.McKim DB, Patterson JM, Wohleb ES, Jarrett BL, Reader BF, Godbout JP, et al. Sympathetic Release of Splenic Monocytes Promotes Recurring Anxiety Following Repeated Social Defeat. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirez K, Niraula A, Sheridan JF. GABAergic modulation with classical benzodiazepines prevent stress-induced neuro-immune dysregulation and behavioral alterations. Brain Behav Immun. 2016;51:154–168. doi: 10.1016/j.bbi.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrarese C, Appollonio I, Bianchi G, Frigo M, Marzorati C, Pecora N, et al. Benzodiazepine receptors and diazepam binding inhibitor: a possible link between stress, anxiety and the immune system. Psychoneuroendocrinology. 1993;18(1):3–22. doi: 10.1016/0306-4530(93)90051-l. [DOI] [PubMed] [Google Scholar]
- 31.Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, et al. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKim DB, Niraula A, Tarr AJ, Wohleb ES, Sheridan JF. Neuroinflammatory Dynamics Underlie Memory Impairments after Repeated Social Defeat. 2016;36(9):2590–2604. doi: 10.1523/JNEUROSCI.2394-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dagher NN, Najafi AR, Kayala KM, Elmore MR, White TE, Medeiros R, et al. Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice. Journal of neuroinflammation. 2015;12:139. doi: 10.1186/s12974-015-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capotondo A, Milazzo R, Politi LS, Quattrini A, Palini A, Plati T, et al. Brain conditioning is instrumental for successful microglia reconstitution following hematopoietic stem cell transplantation. P Natl Acad Sci USA. 2012;109(37):15018–15023. doi: 10.1073/pnas.1205858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maier SF. Bi-directional immune-brain communication: Implications for understanding stress, pain, and cognition. Brain Behav Immun. 2003;17(2):69–85. doi: 10.1016/s0889-1591(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Yamashita T, Chen Q, Belevych N, McKim DB, Tarr AJ, et al. Interleukin 1 type 1 receptor restore: a genetic mouse model for studying interleukin 1 receptor-mediated effects in specific cell types. J Neurosci. 2015;35(7):2860–2870. doi: 10.1523/JNEUROSCI.3199-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.An Y, Chen Q, Quan N. Interleukin-1 exerts distinct actions on different cell types of the brain in vitro. Journal of inflammation research. 2011;2011(4):11–20. doi: 10.2147/JIR.S15357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quan N. In-depth conversation: spectrum and kinetics of neuroimmune afferent pathways. Brain Behav Immun. 2014;40:1–8. doi: 10.1016/j.bbi.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawicki C, McKim D, Wohleb E, Jarrett B, Reader B, Norden D, et al. Social defeat promotes a reactive endothelium in a brain region-dependent manner with increased expression of key adhesion molecules, selectins and chemokines associated with the recruitment of myeloid cells to the brain. Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41(5):694–707. doi: 10.1016/j.immuni.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A. 2014;111(45):16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82(2):380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chitu V, Nacu V, Charles JF, Henne WM, McMahon HT, Nandi S, et al. PSTPIP2 deficiency in mice causes osteopenia and increased differentiation of multipotent myeloid precursors into osteoclasts. Blood. 2012;120(15):3126–3135. doi: 10.1182/blood-2012-04-425595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


