Abstract
The CD103+ subset of lung migratory dendritic cells (DCs) plays an important role in the generation of CD8+ T cell responses following respiratory infection. Here, we demonstrate that the dependence on CD103+ DCs for stimulation of RSV-specific T cells is both epitope and age-dependent. CD103+ DCs in neonatal mice develop two phenotypically and functionally distinct populations following respiratory infection. Neonatal CD103+ DCs expressing low levels of CD103 (CD103lo DCs) and other lineage and maturation markers including costimulatory molecules are phenotypically immature and functionally limited. CD103lo DCs sorted from infected neonates were unable to stimulate cells of the KdM282–90 specificity, which are potently stimulated by CD103hi DCs sorted from the same animals. These data suggest that the delayed maturation of CD103+ DCs in the neonate limits the KdM282–90-specific response and explain the distinct CD8+ T cell response hierarchy displayed in neonatal mice that differs from the hierarchy seen in adult mice. These findings have implications for the development of early-life vaccines, where the promotion of responses with less age bias may prove advantageous. Alternately, specific approaches may be used to enhance the maturation and function of the CD103lo DC population in neonates to promote more adult-like T cell responses.
Keywords: Neonatal immunity, lung dendritic cells, innate immunity, T cell response, respiratory syncytial virus
INTRODUCTION
Recent studies of the ontogeny of dendritic cells (DCs) have dramatically enhanced our understanding of DC subsets and provided a framework for understanding their functional specialization in both the mouse and human (1–5). This framework defines two conventional DC (cDC) lineages originating from a common DC precursor (CDP). The first lineage, now referred to as cDC1, encompasses lymph node resident CD8α+ DCs and a non-lymphoid subset of migratory DCs often characterized by surface expression of the integrin alpha E in peripheral tissues (CD103+ DCs). The cDC1 lineage is dependent on the growth factor Fms-like tyrosine kinase 3 ligand (FLT3L) for differentiation, and signaling through the granulocyte macrophage colony-stimulating factor receptor (GM-CSFR) for cell cycle maintenance (6–8). Several transcription factors are required for development of cDC1, most notably basic leucine zipper transcription factor ATF-like 3 (Batf3) and interferon regulatory factor 8 (IRF8) (9–11). The second lineage of cDCs (cDC2) originating from the CDP consists of lymphoid-resident CD4+ DCs as well as the non-lymphoid, migratory DC population that expresses the integrin alpha M in the lung (CD11b+ DCs). Their development is also dependent on Flt3L and GM-CSFR, but they are less dependent on GM-CSFR than cDC1 (7, 12). Transcription factors governing the development of cDC2 appear more tissue-dependent, with interferon regulatory factor 4 (IRF4) playing a critical role in controlling development, migration, and antigen presentation in CD11b+ DCs (13–16).
The respiratory tract is a critical barrier tissue, and one of the largest interfaces with the outside environment. Here, the maintenance of lung structure and function must be balanced with tolerance to innocuous antigens and the initiation of immune responses to respiratory infection. Following infection, CD103+ DCs and CD11b+ DCs in the lung upregulate CCR7, and carry antigen to the draining mediastinal lymph node (MLN). Antigen is processed and presented in the context of MHC Class I and Class II. This display of viral epitopes, in conjunction with costimulatory factors and cytokines, allows DCs to orchestrate and regulate adaptive immunity. CD103+ DCs are specialized in the cross-presentation of antigen in MHC Class I, and possess a greater ability to drive Th1 effector responses, making them crucial mediators of immunity to intracellular pathogens (10, 17–21). Conversely, CD11b+ DCs more readily present antigen to CD4+ T cells and counter extracellular pathogens. They take up and process extracellular antigens, and can promote both Th2 and Th17 responses (14, 22, 23).
Studies addressing the function and specialization of lung-migratory dendritic cells have primarily focused on adult mice with less understanding of the function, or functional limitations, of these DC subsets in neonates. We have previously shown that neonatal (7 day old) CB6F1 mice infected with respiratory syncytial virus (RSV) develop a distinctly different CD8+ T cell epitope hierarchy than infected adult mice. While adults exhibit a strongly immunodominant response to the KdM282–90 epitope of RSV, the dominance of this epitope does not occur in neonates, which maintain a codominant response to the KdM282–90 epitope and another RSV epitope, DbM187–195 (24). Coincident with the age-dependent CD8+ T cell response, we have demonstrated both qualitative and quantitative deficiencies in CD103+ DCs and CD11b+ DCs in the MLN of RSV-infected neonatal mice. DCs in the MLN are responsible for the induction of adaptive responses, and have lower functionality and expression of the costimulatory molecules CD80 and CD86. Their limited ability to provide CD28-mediated costimulation is related to the codominant CD8+ T cell hierarchy established in neonatal mice, as the KdM282–90 response appears more dependent on costimulation than the response to DbM187–195 (25).
Here, we demonstrate that the neonatal CD103+ DC subset, which most potently stimulates the CD8+ T cell response, partitions into two phenotypically and functionally distinct populations of cells following respiratory infection which can be identified by the level of expression of CD103. Neonatal CD103lo DCs represent the majority of the CD103+ DC population in naïve neonates and during early infection, and express lower levels of lineage-defining markers as well as markers associated with DC maturation. In addition to this phenotypic immaturity, their ability to take up and process antigen is limited, and they demonstrate a striking inability to induce CD8+ T cells of the KdM282–90 specificity. Conversely, neonatal CD103hi DCs display a phenotype and functional profile more akin to their adult counterparts, and most notably, induce a T cell profile that mirrors mature adult CD103+ DCs. Infection of neonatal mice with influenza/PR8 resulted in a similar partitioning of CD103+ DCs, indicating that this is a general feature of the neonatal CD103+ DC response and suggests that the events associated with CD103 expression are concurrent with the maturation of neonatal CD103+ DCs. There is much to be understood about mechanisms for the establishment of these two distinct populations of CD103+ DCs in the neonate and the implications, both for the maintenance of tolerance in the setting of the developing lung and for the ability of these cells to effectively induce adaptive responses during neonatal infection or vaccination.
RESULTS
CD103+ DCs from neonatally-infected mice demonstrate an early impairment in the ability to stimulate KdM282–90-specific T cells
We have previously shown that neonatal CD103+ DCs isolated from the MLN one day post-infection stimulate a distinct CD8+ T cell profile when compared to adult CD103+ DCs (25). To further examine this finding, we assessed the ability of the CD103+ and CD11b+ lung-migratory DC subsets to induce CD8+ T cells at different times following RSV infection. We focused on DCs in the lung-draining posterior MLN, the site of CD8+ T cell induction during primary respiratory infection. CD103+ and CD11b+ DCs were sorted from the MLN of RSV-infected adult or neonatal mice on days 1, 2, 3, and 7 post-infection. All neonatal mice were 7 days old at the time of infection. Timed-matings and infections were performed such that samples from either adults or neonates at all four times points post-infection were acquired, DCs sorted, and T cell cocultures performed on the same day for optimal comparisons between time points. CD8+ T cells of the KdM282–90 and DbM187–195 specificities were isolated from adult, naive TCR transgenic mice, labeled with CFSE, and cocultured with sorted DC populations for three days prior to staining. We determined the frequency of epitope-specific T cells that were stimulated to divide by each DC population using FlowJo proliferation analysis (gating scheme for dendritic cell sorting, and raw data resulting from the coculture of adult and neonatal CD103+ DCs with CD8+ T cells of each specificity are shown in Supplementary Figure S1). Adult CD103+ DCs strongly induced KdM282–90-specific T cells at all times points post-infection (Figure 1a) and stimulated CD8+ T cell proliferation more potently than CD11b+ DCs (Figure 1a vs. Figure 1b). While stimulating fewer CD8+ T cells, adult CD11b+ DCs stimulated both epitope-specific populations more equally. In contrast, CD103+ DCs isolated from neonatally-infected mice during the first two days post-infection stimulated significantly fewer KdM282–90-specific cells than those isolated on or after day 3 post-infection, when the profile of T cell proliferation more closely resembled that of adult CD103+ DCs (Figure 1c). Neonatal CD11b+ DCs stimulated RSV-specific CD8+ T cells in a fashion similar to those of adult CD11b+ DCs with lower induction, and less bias toward the KdM282–90 response than that of CD103+ DC subset (Figure 1d).
Fig 1. Neonatal CD103+ DCs demonstrate an early impairment in the ability to stimulate KdM282–90-specific cells.
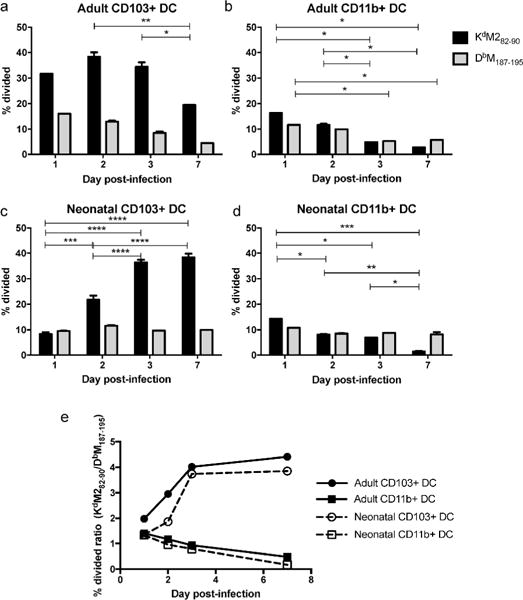
FACS-sorted adult CD103+ DCs (A), adult CD11+ DCs (B), neonatal CD103+ DCs (C) and neonatal CD11b+ DCs (D) from the MLN of RSV-infected mice were cocultured with CFSE-labeled transgenic T cells specific for either the KdM282–90 or DbM187–195 epitope for three days prior to surface staining and FACS analysis. The percent of the original TCR transgenic population that was stimulated to divide was calculated by FlowJo software. Data are representative of two complete experiments for each age group, where MLN were pooled to provide two replicates for each time point post-infection on a single harvest day. E) The percent divided ratio for each antigen presenting cell population on each day post-infection was calculated by dividing the percent of KdM282–90-specific cells that were induced to proliferate by the percent of DbM187–195-specific cells that were induced to proliferate. Data are representative of two independently performed experiments for each age group with two replicates for each dendritic cell subset and CD8+ T cell response at each day post-infection. * indicates p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 by 2way ANOVA with Tukey’s multiple comparisons test.
Of note, neonatal mice showed an early impairment in their ability to stimulate KdM282–90-specific cells when compared to adult CD103+ DCs. This trend is more apparent as a ratio of the percent of CD8+ T cells of each specificity induced to divide (Figure 1e). Adult and neonatal CD11b+ DCs show near equal (ratio of 1) stimulation of CD8+ T cells of both specificities, with stimulation becoming slightly more skewed toward the DbM187–195 response over the course of infection. The clear bias of adult CD103+ DCs toward induction of the KdM282–90 response resulted in the stimulation of twice as many KdM282–90-specific cells as Db M187–195–specific cells on day 1 post-infection, and an increase in ratio as the infection progressed, further favoring this specificity. While neonatal CD103+ DCs isolated on day 1 post-infection stimulated both specificities equally, the bias of these cells toward KdM282–90 increased to more closely resemble the response of adults starting at day 3 post-infection. These data demonstrate that each lung-migratory DC subset elicits a distinct profile of epitope-specific CD8+ T cells, and that the ability of these DC subsets to stimulate CD8+ T cells is dependent on both time post-infection and the age at which mice are infected.
CD103+ DCs are dispensable for the induction and maintenance of the KdM282–90-specific response in neonatal mice
In order to assess the global contribution of the CD103+ DC subset to the generation and maintenance of RSV-specific CD8+ T cell responses, we compared CD8+ T cell responses in neonatal and adult Batf3−/− CB6F1 mice to those of wild-type mice after RSV infection. Batf3−/− mice lack cDC1, and have been shown to exhibit reduced CD8+ T cell responses to multiple pathogens, confirming the importance of this lineage to the development of CD8+ T cell responses (10, 26, 27). CD8+ T cell responses were compared at day 7, the peak of the response in infected mice. Batf3−/− neonatal and adult mice had a significantly lower frequency of CD8+ T cells in the lungs overall (Supplementary Figure S2A), but not in the MLN (Supplementary Figure S2B). While the lungs of adult Batf3−/− mice had a trend toward a lower number of CD8+ T cells than wild-type mice (Supplementary Figure S2C), the CD8+ T cell count in the MLN of adult infected Batf3−/− was significantly increased as compared to wild-type mice (Supplementary Figure S2D). The number of CD8+ T cells was less affected in Batf3-deficient neonates, with no significant difference in the lung or MLN in the absence of CD103+ DCs.
RSV epitope-specific CD8+ T cell responses were altered considerably in Batf3−/− mice. Batf3−/− neonatal and adult mice had a significant reduction in the number and frequency of the DbM187–195-specific response (Figure 2a and Figure 2c, respectively). Responses to this epitope were 3.8-fold reduced in Batf3-deficient adult mice, and reduced by 1.6-fold in neonates. Similar significant reductions in the number and frequency of the DbM187–195-specific response were observed in the MLN (Supplementary Figures S2E and S2G). The KdM282–90-specific response in adult mice was similarly dependent on CD103+ DCs in the lung; the frequency (Figure 2b) and the number (Figure 2d) of cells responding to this epitope were significantly lower in Batf3−/− adults than in wild-type controls. Unexpectedly, the absence of CD103+ DCs in neonates resulted in a significant increase in the frequency (Figure 2b) and number (Figure 2d) of KdM282–90-specific cells in the lung.
Fig 2. CD103+ DCs are dispensable for the induction and maintenance of the KdM282–90-specific CD8+ T cell response in neonates.
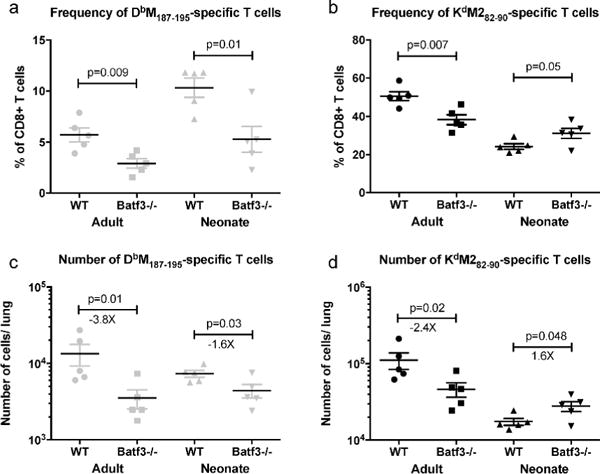
Wild-type and Batf3−/− adult and neonatal mice were infected intranasally with RSV. Seven days post-infection, the frequency and number of DbM187–195-specific (A and C, respectively) and Kd M282–90-specific (B and D) cells in the lung were determined. Data are representative of 3 independent experiments with 5–8 mice/group. P values indicated are from a t-test between wild-type and Batf3−/− mice of the same age.
We have previously demonstrated that the absence of the DbM187–195 epitope leads to an increase in the KdM282–90-specific response in the context of primary RSV infection (28). We hypothesized that the increase in the KdM282–90-specific response in Batf3−/− neonatal mice was due to a reduction in competition from the DbM187–195 response, which was significantly lowered by Batf3-deficiency. To test this hypothesis, neonatal Batf3−/− mice were infected with RSV-N191S, a virus with a mutation in the Db-binding anchor residue of the M187–195 peptide that completely abrogates the T cell response to DbM187–195 (28). Upon infection with this viral mutant, KdM282–90–specific responses were found to be unchanged by the absence of CD103+ DCs, indicating that the increase in the KdM282–90 response in Batf3−/− neonates infected with wild-type RSV was caused, at least in part, by a decrease in competition from the DbM187–195-specific response (Supplementary Figure S3).
Our findings demonstrate that the requirement of CD103+ DCs for the induction and maintenance of CD8+ T cell responses is age and epitope-dependent. Both CD8+ T cell responses in adult mice are heavily reliant on CD103+ DCs and significantly diminished in their absence. While the DbM187–195-specific response of neonates similarly relies on CD103+ DC, the KdM282–90–specific response in neonatal mice does not, indicating that the CD103+ DC subset is entirely dispensable for the generation of this response in neonatal, but not adult mice.
Two distinct populations of lung-migratory CD103+ DCs are present in intranasally-infected neonatal mice
Based on the age-dependency in the requirement of CD103+ DCs for the induction of CD8+ T cell responses, we further explored differences between neonatal and adult CD103+ DCs. We first compared lung-migratory DC populations in naïve adults and neonates (Figure 3a). In addition to possessing a lower frequency of CD11b+ DCs as we have previously described (25), the CD103+ DC subset in neonatal mice was characterized by lower expression of the lineage-defining surface marker CD103. Naïve adult CD103+ DC also presented higher expression levels of CD11b than those of neonates. Throughout primary infection, adult CD103+ DCs form a single population of cells with high expression of CD103 and low, but observable expression of CD11b (Figure 3b). In contrast, neonatal CD103+ DCs segregate into two distinct populations, one reminiscent of CD103+ DCs found in naïve neonatal mice that express low levels of CD103 (CD103lo DCs, Figure 3b). A second population with increased expression of both CD103 and CD11b appears one day post-infection, and forms a second population within the subset through the remainder of primary RSV infection (CD103hi DCs). The partitioning of the neonatal CD103+ DC subset into two distinct populations during respiratory infection occurred in a similar fashion during infection of neonatal mice with influenza/PR8 (Supplemental Figure S4), indicating that this is a common feature of the neonatal CD103+ DC subset following respiratory infection.
Fig 3. Neonatal mice infected with RSV possess two distinct populations of Batf3-dependent CD103+ DCs.
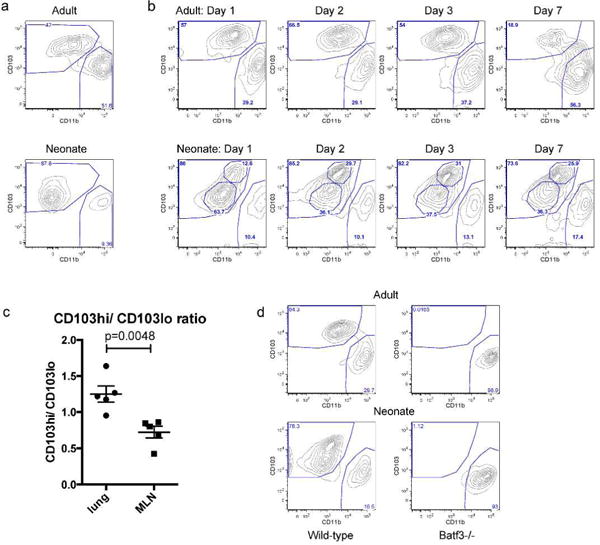
A) Lung-migratory DC populations in the MLN of naïve adult and neonatal mice. B) Lung-migratory DC populations in the MLN of RSV-infected adult and neonatal mice on days 1, 2, 3, and 7 post-infection. Data are representative of two independent experiments with 2–3 samples of pooled lymph nodes each. C) CD103hi DC/CD103lo DC ratio in the lung and MLN of neonatal mice two days post-RSV infection. Data are representative of two independent experiments, each containing 3–4 biological replicates of pooled lymph nodes and lungs. Groups were compared using a t test. D) DC populations in the MLN of wild-type and Batf3−/− adults and neonates 2 days post-RSV infection confirm the dependence of both populations on the transcription factor Batf3. Data are representative of two independent experiments with 2–3 biological replicates from each strain of mice.
We next compared the composition of CD103lo and CD103hi DCs in the lungs and MLN of neonatal mice. On day 2 post-infection, the ratio of CD103hi DCs/CD103lo DCs in the lungs was significantly higher than in the MLN, indicating an enrichment for CD103lo DCs in the MLN (p=0.0048, Figure 3c). To ensure that both populations of the CD103+ DC subset in neonates represent bona fide, Batf-dependent CD103+ lung-migratory DCs, we examined dendritic cells in the MLN two days post-infection in wild-type and Batf3−/− adult and neonatal mice. As expected, adult Batf3−/−mice entirely lacked CD103+ DCs (Figure 3d). Neonatal mice were found to lack both CD103lo and CD103hi DC populations within the CD103+ DC subset, confirming their common, Batf3-dependent origin.
GM-CSF, IL-3, and TGFβ have each been demonstrated to promote the expression of CD103 on dendritic cells (29). In order to investigate differences in the lung environment that may limit CD103 expression, we measured the levels of these cytokines in lung supernatants of adults and neonates on day 0 (naïve adult and 7 day-old mice), and on days 1, 2, 3 and 7 post-infection. Higher expression of GM-CSF was found in the lungs of naïve adults than naïve neonates. The expression level in adults was also significantly higher on days 1, 3, and 7 post-infection (Figure 4a). Conversely, while relatively low overall, expression of IL-3 tended to be higher in neonatal mice, and was significantly higher in neonates on days 1, 2, and 7 post-infection (Figure 4b). TGFβ levels were significantly higher in naïve neonates, and at every time point post-infection (Figure 4c). These data reflect differences in the adult and neonatal lung environment that may play a role in the development and function of lung-migratory dendritic cells. In particular, low steady-state expression of GM-CSF in neonates may play a role in limiting the development of CD103+ DC during early life.
Fig 4. GM-CSF, IL-3 and TGFβ expression levels in the lungs of naïve neonatal (7 day-old) and adult mice, and during acute RSV infection.
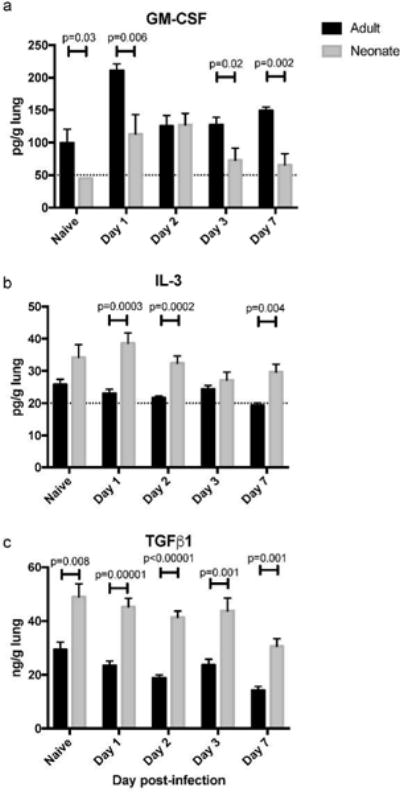
The single left lobe of naïve and infected mice was quick-frozen at the indicated time-point. Post-thawing, lungs were ground on a GentleMACS, and lung supernatants clarified by centrifugation prior to cytokine analysis. Data shown are from an experiment using 5 (Naïve, Day 7), or 10 (Days 1, 2, and 3) mice per group. For statistical analysis, a Student’s t-test was performed comparing samples from each indicated time point. The dashed line indicates the limit of detection for GM-CSF and IL-3, where some samples had undetectable levels of cytokine.
Neonatal CD103lo and CD103hi DCs are phenotypically distinct
We compared the expression of phenotypic markers between the two CD103+ DC populations in neonates, and to adult CD103+ DCs. Two days after infection, neonatal CD103+ DCs from the MLN of neonates and adults were gated as indicated in Figure 3b. Neonatal CD103lo DCs were compared to CD103hi DCs on a forward vs. side scatter plot, revealing that the CD103hi DC population is of greater size and complexity than CD103lo DCs (Figure 5a). The scatter characteristics of the CD103hi DC population were more comparable to those of adult CD103+ DCs (Figure 5b). In addition to CD103, neonatal CD103lo DCs were found to exhibit lower expression of other lineage-defining markers including MHC Class II (I-Ab), CD11b, and CD11c (Figure 5c). The expression of these markers on neonatal CD103hi DCs were indistinguishable from adult CD103+ DCs with the exception of CD11b, which was expressed in lower levels on neonatal CD103hi DCs than on adult CD103+ DCs.
Fig 5. Phenotypic differences between CD103hi and CD103lo DC populations in the MLN of neonatally-infected mice.
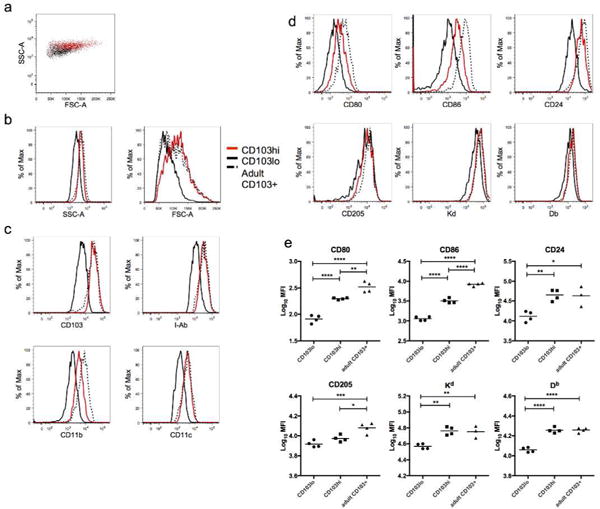
MLN were harvested 2 days post-infection from mice infected at 7 days of age or as adults. A) FSC vs. SSC plot demonstrates size and complexity differences between neonatal CD103lo DCs (black) and CD103hi DCs (red). B) Histograms of side and forward scatter comparing the neonatal CD103lo (black), CD103hi (red), and adult CD103+ (hatched) DC populations. C) Surface staining was performed to measure expression of CD103, I-Ab, CD11b, and CD11c lineage-defining markers on neonatal CD103lo, CD103hi, and adult CD103+ DC populations. D) Representative histograms comparing expression of CD80, CD86, CD24, CD205, Kd, and Db on neonatal CD103hi and CD103lo DCs and adult CD103+ DCs E) Background (fluorescence minus one)-subtracted median fluorescence intensity (MFI) is presented for CD80, CD86, CD24, CD205, and the MHC Class I molecules Kd and Db on neonatal CD103lo, CD103hi, and adult CD103+ DCs. Data are representative of two independent experiments with 3–4 mice/group. * indicates p<0.05, ** p<0.01, *** p<0.001, *** p<0.0001 by one-way ANOVA with Tukey’s multiple comparisons test.
We have previously shown that neonatal CD103+ DCs have lower surface expression of the costimulatory molecules CD80 and CD86 than adult CD103+ DC (25). We compared the expression of these markers on neonatal CD103lo and CD103hi DCs, and adult CD103+ DCs from the MLN. Neonatal CD103hi DCs were found to have significantly higher expression of both costimulatory molecules than CD103lo DCs in the same mouse (Figures 5d and 5e). While their expression is higher than on CD103lo DCs, CD103hi DCs still express significantly lower levels than adult CD103+ DCs, indicating that CD103hi DCs are not equivalent to the adult CD103+ DCs despite having many common phenotypic features with them. We further examined expression of the costimulatory molecule CD24 and CD205 (DEC205), an endocytic receptor important for the recognition of apoptotic or necrotic cells and the MHC Kd and Db Class I molecules. Neonatal CD103lo DCs had significantly lower expression of CD24 than CD103hi and adult CD103+ DCs, while CD205 expression on CD103lo and CD103hi DCs were similar and significantly reduced compared to adult CD103+ DCs. Neonatal CD103lo DCs also had significantly lower expression of both MHC Class I molecules than both CD103hi and adult CD103+ DC, which expressed equivalent levels of each (Figures 5d and 5e). Overall, neonatal CD103lo DCs present a less mature phenotype than CD103hi DCs, with lower surface expression of several proteins important for DC function.
Given the significant phenotypic differences observed within the CD103+ DC subset, neonatal CD11b+ DCs were compared with adult CD11b+ DCs. Neonatal CD11b+ DCs had a similar forward and side scatter profile to adult CD11b+ DCs, in addition to similar expression of lineage-defining markers (Supplemental Figure S5A). As expected based on our previous results, neonatal CD11b+ DCs had significantly lower expression of the costimulatory molecules CD80 and CD86, but their expression of CD24, CD205, and the Class I molecules Kd and Db were indistinguishable from those of adult CD11b+ DCs (Supplemental Figure S5B). While CD11b+ DCs in the neonate have lower CD80 and CD86 expression and are significantly lower in number than adult CD11b+ DCs (25), expression of other lineage-defining and functional markers is similar.
Neonatal CD103lo DCs are functionally limited and demonstrate an inability to stimulate the KdM282–90-specific response
To compare the ability of neonatal CD103lo and CD103hi DCs to take up and transport soluble protein to the MLN, ovalbumin protein conjugated to Alexa647 (Ova Ax647) was co-administered intranasally at the time of RSV infection. Two days post-infection, the frequency of Alexa647+ DCs in the MLN was measured as compared to mice infected with RSV only. Approximately 60% of the neonatal CD103hi DCs in the MLN of mice that received Ova Ax647 were Alexa647+, significantly more than CD103lo DCs, of which only 15% were positive (Figures 6a and 6c). Despite significant functional improvement over the CD103lo DC population, CD103hi DCs were still significantly less able to transport soluble antigen than adult CD103+ DCs (Figures 6b and 6c). Next, we assessed each DC population’s ability to process antigen by co-administration of DQ ovalbumin (Ova DQ) at the time of infection. This protein, heavily labeled with BODIPY dyes, is non-fluorescent but yields brightly fluorescent, dye-labeled peptides when processed. Two days post-infection, neonatal CD103hi DCs in the MLN were more than five times more likely to have processed Ova DQ than CD103lo DCs in the same mice (6.73% vs. 38.3%, Figures 6d and 6f). Again, the function of neonatal CD103hi DCs was significantly lower than adult CD103+ DCs, which were 50% Ova-DQ+ (Figures 6e and 6f). These data demonstrate that while more neonatal CD103hi DCs take up and process antigen than neonatal CD103lo DCs, adult CD103+ DC remain significantly more effective at antigen uptake and processing than either neonatal population.
Fig 6. Neonatal CD103+ DCs are deficient in antigen uptake and processing.
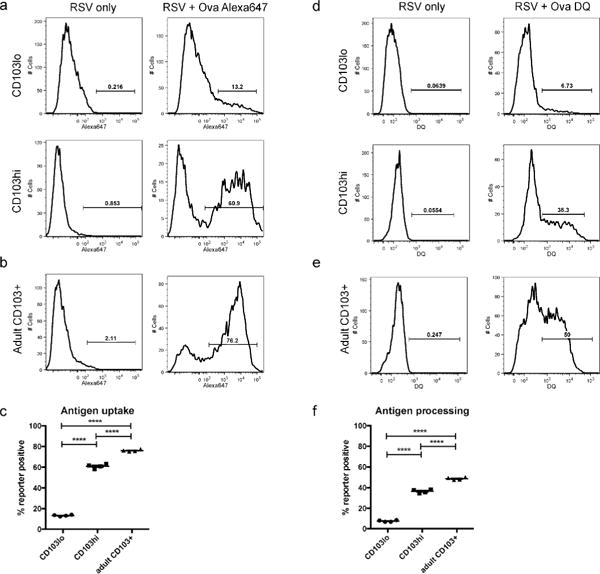
Labeled ovalbumin proteins (Ova Ax647 or Ova DQ) were co-administered at the time of RSV infection, and fluorochrome positive DCs were measured in the MLN 2 days post-infection. A) Alexa647+ neonatal CD103lo and CD103hi DCs were measured in mice that received co-administered Ova Alexa647 at the time of RSV infection. B) Alexa647+ CD103+ DCs measured in the MLN of adult mice two days post-infection. C) A comparison of antigen uptake by neonatal CD103lo and CD103hi DCs, and adult CD103+ DCs. D) DQ+ neonatal CD103lo and CD103hi DCs were measured in mice that received co-administered Ova DQ. E) DQ+ CD103+ DC in the MLN of adult mice two days post-infection. F) A comparison of antigen processing by neonatal CD103lo and CD103hi DCs, and adult CD103+ DCs. Data are representative of two independent experiments with at least two biological replicates of pooled lymph nodes each. **** indicates p<0.0001 by one-way ANOVA with Tukey’s multiple comparisons test.
Finally, we evaluated the ability of neonatal CD103lo and CD103hi DC populations to stimulate RSV-specific CD8+ T cells. Both DC populations were sorted from the MLN of neonates two days post-infection and cocultured with CD8+ T cells specific for KdM282–90 or DbM187–195. Control samples cultured with naïve CB6F1 splenocytes with no peptide, or with 10−6M of specific peptide served as negative and positive controls, respectively. Neonatal CD103hi DCs robustly stimulated proliferation of KdM282–90-specific cells, resulting in proliferation of nearly half of the original population (Figure 7) and were able to stimulate proliferation in approximately 15% of DbM187–195-specific cells. This profile of CD8+ T cell induction by neonatal CD103hi DCs is similar to the profile of induction by adult CD103+ DCs, with a strong bias toward the KdM282–90 response (Supplemental Figure S1B). In striking contrast, CD103lo DCs sorted from the same neonatal mice displayed an inability to stimulate KdM282–90-specific cells, while retaining the ability to stimulate approximately half as many DbM187–195-specific cells as neonatal CD103hi DCs (Figure 7). To rule out a limitation in the ability of CD103lo DC to present the M282–90 peptide in the context of H2-Kd, we pulsed both CD103hi and CD103lo populations with saturating peptide (10−6M) for one hour prior to washing and co-culturing with KdM282–90-specific CD8+ T cells. Both populations equally presented the exogenously provided M282–90 peptide and induced maximum proliferation of the T cells (Supplemental Figure S6). When pulsed with a lower concentration of M282–90 peptide (10−8M), CD103lo DC tended to stimulate fewer CD8+ T cells than CD103hi DC, though we cannot preclude the potential contribution of differences in endogenously presented peptide by the two subsets sorted from RSV-infected mice. These data demonstrate that the neonatal CD103lo DC population has a DbM187–195-biased induction profile, further differentiating the functional profile of the CD103lo and CD103hi DC populations.
Fig 7. Neonatal CD103lo DC cannot stimulate KdM282–90-specific CD8+ T cells.
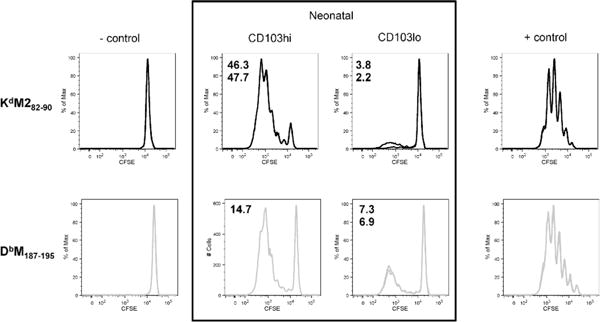
CD103hi and CD103lo DCs were sorted from the MLN two days post-RSV infection of neonatal mice and cocultured with CFSE-labeled KdM282–90 or DbM187–195-specific CD8+ T cells. The percent of transgenic cells induced to proliferate after three days in culture was calculated using Flowjo software. Splenocytes with no peptide, or with 10−6M specific peptide served as antigen presenting cells for the negative and positive controls, respectively. Data are representative of two independent experiments with duplicate samples for each specificity stimulated by CD103lo and CD103hi DCs acquired by FACS-sorting from pooled MLN samples.
Discussion
CD103+ DCs are specialized cross-presenters of viral antigens (13). In accordance with the observations of several others (10, 26, 30), we demonstrate here that the lung-migratory CD103+ DC subset is the most potent stimulator of the anti-viral CD8+ T cell response following respiratory infection. While we have shown direct infection of lung-migratory dendritic cells to be relatively limited (<1%, (25)), these results represent the combined abilities of these dendritic cells to directly present and cross-present antigen. We have previously shown that CD103+ DCs outnumber CD11b+ DCs in the MLN by a factor of nearly 2:1 in adults and 6:1 in neonates following RSV infection (25), indicating that we are likely underestimating the difference in abilities of these lung-migratory DC subsets to stimulate CD8+ T cells. Despite these differences, CD11b+ dendritic cells are capable of eliciting anti-viral CD8+ T cells in Batf3−/− mice, and we routinely measure stimulation of RSV-specific responses by this subset in adults and neonates (Figures 1b and 1d, respectively). Of note, the major factor accounting for the difference in potency between DC subsets is the strong induction of the KdM282–90-specific response by adult CD103+ DCs. The early neonatal CD103+ DC subset does not possess this KdM282–90-bias, and we demonstrate here that the KdM282–90-specific response in neonates is completely independent of CD103+ DCs, in contrast to adults. The limited ability of neonatal CD103+ DCs to support the induction of the KdM282–90-specific response likely belies the distinct CD8+ T cell hierarchy found following RSV infection of neonatal mice (24).
Our data demonstrate that the induction of CD8+ T cell responses is not only APC and epitope-dependent, but also age-dependent. This work additionally cautions against using responses to a single epitope to generalize about the requirements for all CD8+ T cells. Our previous work has shown significant functional differences between the high-affinity DbM187–195–specific response and the dominant, yet lower-affinity KdM282–90-specific response (24, 28, 31). Further understanding of the rules governing the elicitation of these distinct anti-viral responses may offer insight into the unique limitations of neonatal innate and adaptive immunity.
The absence of both the CD103hi and CD103lo DC populations in RSV-infected Batf3−/− neonatal mice confirms the common cDC1 lineage of these populations. In naïve neonatal mice, CD103+ DCs assume a nearly uniform CD103lo phenotype. Rather than present a maturation gradient following infection, neonatal CD103+ DCs appear to partition into either more mature CD103hi DCs or remain immature CD103lo DCs. These immature, underdeveloped CD103lo DCs in the MLN display lower expression of lineage-defining markers, which may be partially due to their smaller size/surface area. Significantly lower expression of Kd, Db, and I-Ab may be indicative of lower antigen processing and presentation via MHC Class I and Class II. Coupled with low expression of the costimulatory molecules CD80, CD86, and CD24, neonatal CD103lo DCs are unlikely to productively stimulate anti-pathogen T cell responses in the lung-draining MLN. This idea is further supported by the coculture experiments in which CD103lo DCs demonstrate a complete deficiency in stimulating KdM282–90-specific CD8+ T cells, and reduction in the ability to stimulate DbM187–195-specific CD8+ T cells. CD24 expression by adult CD103+ DCs in particular has been associated with their ability to promote “lung-tropic” effector CD8+ T cell responses in the lung (26). While neonatal CD103hi DCs display a more mature scatter profile and phenotype, they retain lower expression of CD11b, CD80, CD86, and CD205 than adult CD103+ DCs, indicating continued functional limitations when compared to adult CD103+ DCs. The endocytic receptor CD205 plays a role in antigen uptake and delivery for presentation on MHC Class II and cross-presentation on MHC Class I, and low CD205 expression may limit these functions for both neonatal CD103lo and CD103hi DC populations (32). We have previously shown that following RSV infection adult mice have approximately ten times more CD11b+ DCs in the MLN than neonatal mice (25). Although fewer in number, neonatal CD11b+ DCs appear nearly indistinguishable from adult CD11b+ DCs. Their scatter profile and phenotype appear consistent with those of adult CD11b+ DCs, with the exception of lower CD80 and CD86 expression, which may limit their ability to provide CD28-mediated costimulation.
Our functional comparison of CD103hi and CD103lo DCs in neonates aligns well with the phenotypic differences between these populations; CD103lo DCs in neonates display a lesser ability to take up and process co-administered antigen. Neonatal CD103hi DCs demonstrate improvements in both of these functions, yet have lower functionality than adult CD103+ DCs. It is important to note that uptake and processing are inherently linked, and measuring lower frequencies of DCs processing antigen reflects, at last in part, their limited ability to take up antigen. Despite having lower levels of antigen uptake and processing than adult CD103+ DC, neonatal CD103hi DCs stimulate an “adult-like”, dramatically KdM282–90-skewed CD8+ T cell profile. The marked inability of CD103lo DCs to stimulate the KdM282–90-specific response may be due to their limited ability to take up and process antigen and to provide costimulation, as we have previously shown that the KdM282–90-specific response is more dependent on CD28-mediated costimulation than the response to DbM187–195 (25). The KdM282–90-specific response has a lower avidity than the response to DbM187–195 (24, 31). It is possible that lower levels of DbM187–195 pMHC may be required for induction, particularly in the context of dampened costimulation, and that KdM282–90 presented on CD103lo DCs does not achieve the threshold level for induction of T cell proliferation.
CD103lo DCs in neonates appear to have not yet undergone the final stages of DC development. The environment of the neonatal lung may present a restriction to these final developmental stages. GM-CSF stimulates the activation and proliferation of dendritic cells and regulates CD103 expression, and the addition of GM-CSF in the later phase (day 6) of Flt3L-derived bone marrow-derived DC promotes the expression of high levels of CD103 and increases costimulatory molecule expression (33–36). Recently, Mayer et. al described a protocol to generate large numbers of CD8α-like CD103+ DCs in vitro from mouse bone marrow. They demonstrated that the development of CD103+ DCs in culture required a prolonged culture (15–16 days) with both Flt3L and GM-CSF, with provision of GM-CSF in both early and late phases being required to promote the differentiation and homeostasis of CD103+ DCs (33). Moreover, airway epithelium-produced GM-CSF, which is also critical for the development of alveolar macrophages in early life, spikes at birth and wanes quickly thereafter (37). We demonstrate here that naïve neonatal mice have lower expression levels of GM-CSF in the lungs than adults, and this holds true for most post-infection time points (Figure 4). Access to this growth factor may regulate DC development and could play a role in linking early lung development to the development of tissue-resident DCs. Interestingly, IL-3 and TGFβ, which have also been shown to enhance CD103 expression on dendritic cells (29), are expressed more highly in the lungs of RSV-infected neonates and may play a unique role in the development of the CD103+ DC subset during early life. The concerted maturation of several distinct phenotypic markers and the ability to reproduce the development of CD103+ DC using Flt3L and GM-CSF in vitro suggests there is an overarching developmental program to coordinately regulate several pathways to achieve fully functional CD103+ DCs. We are further exploring early restrictions to DC development in the neonatal lung and whether this phenomenon is unique to the lung microenvironment, or also occurs in other barrier tissues.
It is tempting to speculate about an important developmental role for CD103lo DCs in the neonatal lung. In the steady-state, CD103+ DCs have been shown to play a role in the maintenance and induction of tolerance (17, 18, 38, 39). This function may be particularly important in neonatal mice newly experiencing a world filled with many innocuous antigens. CD103lo DCs would seem a likely population to promote tolerance given their low level of maturation and costimulatory molecule expression. Interestingly, a decreased CD103hi/CD103lo ratio in the MLN compared to the lung suggests that CD103lo DCs preferentially migrate into the MLN. Lower expression of CD103, and therefore weaker binding to E-cadherin on epithelial cells in the lung may account for this preference. Thus, approaches aimed at inducing effective immunity in the lung during early life through promoting maturation of the cDC1 population could potentially interfere with the induction of tolerance. Flt3L treatment improves the innate immune responses to RSV in neonatal mice and limit lung disease upon RSV re-exposure (40). Yet, care should be taken to prematurely force DC maturation and upset a delicate balance, particularly at sensitive barrier sites still developing and establishing long-lasting relationships with commensal bacteria. A better understanding of both the mechanisms of neonatal CD103+DC partitioning and the biological role of the CD103lo DC population will help determine the validity of this type of approach. An alternative approach is the use of antigens to which the neonatal response is as robust as that of adults. Neonates elicit responses to the DbM187–195 epitope as effectively as adults, and this response is highly functional at both ages (24). Understanding the parameters that determine age-dependent differences in the immune response will help define metrics to predict early life antigenicity and improve vaccines designed to elicit anti-pathogen immunity in young infants.
METHODS
Ethics statement
Mice used in these studies were maintained according to the guidelines of the NIH Guide to the Care and Use of Laboratory Animals. Experimental procedures had approval of the Animal Care and Use Committee of the Vaccine Research Center (VRC), National Institute of Allergy and Infectious Diseases at the National Institute of Health. Mice were housed in a facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Animal procedures were conducted in strict accordance with all relevant federal and National Institutes of Health guidelines and regulations.
Mice, RSV infections and other treatments
Adult (8–14 week old) male and female CB6F1 mice were obtained from Jackson Labs (Bar Harbor, ME) or bred in-house. Neonatal CB6F1 mice were bred in-house, the offspring of mating BALB/c female mice with C57Bl/6 males. TCR transgenic mice were produced by NCI-Frederick Laboratory Animal Sciences Program (LASP) transgenic mouse model service and subsequently bred as heterozygotes in-house as previously described (25). Batf3−/− BALB/c and C57Bl/6 mice were obtained from Jackson Labs and bred in-house to obtain both neonatal and adult Batf3-deficient hybrid mice. All mice were housed in our animal care facility at NIAID under specific pathogen-free conditions, and maintained on standard rodent chow and water supplied ad libitum. Neonatal mice were infected at 7 days old. Mice were anesthetized using isoflurane (3%), and infected intranasally with 2×106 PFU of live RSV in 10% EMEM (100 μl for adults, 25 μl for mice infected at 7 days of age). For influenza infection, neonates were infected intranasally with 25μL of PBS containing 600 TCID50 of influenza/PR8 using the same method. All mice were euthanized by lethal overdose of pentobarbital (250 mg/kg). Experiments involving co-administration of either OVA-Alexa647 or OVA-DQ (Invitrogen) were performed by adding 50 μg protein/mouse directly to virus preparations prior to intranasal administration.
Flow cytometry and cytokine analysis
Following euthanasia, MLN and/or lung tissues were harvested and processed at the indicated times post-infection as previously described (25). In most cases, tissues from 3–7 mice were pooled to generate one sample. After isolation, cell preparations were stained with fluorochrome-labeled antibodies to the following cell surface markers: CD11c (N418), CD11b (M1/70), CD103 (2E7), CD8α (53-6.7), I-A/I-E (M5/114.15.2), I-Ab (AF6-120.1), CD3 (145-2C11), CD86 (GL1), CD80 (16-10A1), CD24 (M1/69), CD205 (205yekta) purchased from BD biosciences, eBioscience, or Biolegend. KdM282–90 and DbM187–195-containing tetramers were purchased from either Beckman Coulter or MBL International. All staining was done for 20 minutes at 4°C in FACS staining buffer (PBS with 1% FBS and 0.05% sodium azide). Samples were collected on an LSR-II flow cytometer and data were analyzed using FlowJo version 9.9.10. For cytokine analysis, the single left lobe of naïve or infected mice was collected and snap-frozen in 2mL of MEM supplemented with 2mM glutamine, 10U/mL penicillin, and 10ug/mL streptomycin. After thawing, lung tissue was dissociated using a GentleMACS (on program lung_02) prior to centrifugation to remove cellular debris. Clarified lung supernatants were sent to AssayGate (Ijamsville, MD) for analysis of cytokines using a multiplex bead-based array.
Dendritic cell: T cell coculture
Dendritic cells for DC:T cell coculture were stained using the above markers as previously described and sorted on a FACSAria (25). CD8+ T cells were isolated from splenocytes of TCR transgenic mice with T cells specific for either RSV-KdM282–90 or RSV-DbM187–195 using a CD8α T cell isolation kit (Miltenyi), then labeled with 5μM CFSE for 5 minutes at room temperature followed by three washes with FBS containing media. More than 95% of CD8+ T cells from KdM282–90-transgenic mice are tetramer-binding, epitope-specific cells, while approximately 70% of CD8+ T cells of DbM187–195-transgenic mice are tetramer-binding and epitope-specific due to recognition being mediated by the TCR beta chain paired with endogenous TCR alpha. All comparisons were made between samples set up on the same day, with the same CFSE-labeled responding cell populations. CD8+ T cells of each specificity (100,000) were cocultured with FACSAria-sorted populations of CD103+ DCs or CD11b+ DCs (10,000) from mice infected at 7 days of age or as an adults in lymphocyte media without the addition of exogenous peptide for all experiments except those presented in Supplemental Figure S6, where dendritic cells were pulsed with the indicated concentration of M282–90 peptide (SYIGSINNI) for one hour prior to extensive washing before coculturing with CD8+ T cells. CD8+ T cells were incubated with splenocytes pulsed with 10−6M cognate peptide as a positive control, or splenocytes with no peptide as a negative control. Three days later, all samples were harvested and stained with antibodies to CD8, CD3, and with ViViD (for viability) prior to collection on a LSRII.
Statistical analysis
Statistical analyses were performed using GraphPad prism version 6.00 for Windows, www.graphpad.com, using either a student’s t-test, or one-way or two-way ANOVA with the indicated post-tests for multiple comparisons.
Supplementary Material
Supplemental Figure S1. Induction of epitope-specific T cells by sorted adult CD103+ DCs. A) Gating of CD103+ DCs and CD11b+ DCs in the MLN of adult and neonatal mice. Live singlets were gated prior to gating on the CD11c+, Class IIhi DC population. This population was further broken down into CD103+ DCs and CD11b+ DCs. B) An example of raw data obtained from an in vitro coculture experiment showing the induction of proliferation of KdM282–90 and DbM187–195=specific cells by FACS-sorted CD103+ DCs sorted from the MLN of adult mice on the indicated days post-infection. Mice were infected on different days in order to harvest MLN from all time points post-infection and perform the coculture on one day with the same reporter T cell populations.
Supplemental Figure S2. Frequency and number of CD8+ T cells in RSV-infected adult and neonatal wild-type and Batf3−/− mice. CD8+ T cell frequency and numbers in the lung and MLN 7 days post-infection (A–D) and the frequency and number of CD8+ T cells specific for DbM187–195 and KdM282–90 specific cells in the MLN of wild-type and Batf3-deficient adults and neonates 7 days post-RSV infection (E–H). Data are representative of 3 independent experiments with 5–8 mice/group. P values indicated are from a t-test between wild-type and Batf3−/− mice of the same age.
Supplemental Figure S3. Higher KdM282–90-specific responses in the lungs of Batf3−/− deficient neonates are due to the lack of competition from the DbM187–195-specific response. Wild-type and Batf3-deficient neonatal mice were infected with RSV-N191S, an RSV virus that does not stimulate a response to the DbM187–195 epitope due to a mutation in the P5 anchor residue. The frequency and number of KdM282–90-specific cells were measured by tetramer staining in the lung and MLN 7 days post-infection. Results shown are combined data from two litters of wild-type and two litters of Batf3−/− deficient neonates.
Supplemental Figure S4. Influenza/PR8-infected neonatal mice possess two populations within the CD103+ DC subset. Seven-day-old mice were infected intranasally with 600 TCID50 of influenza/PR8. MLN were harvested from naïve mice, and mice at days 1–3 post-infection for surface staining of lung-migratory dendritic cell populations. The sample shown is representative of several pools of MLN from neonatal mice infected with influenza/PR8.
Supplemental Figure S5. Phenotypic comparison of neonatal CD11b+ DCs and adult CD11b+ DCs in the MLN of mice two days post-infection. A) Scatter characteristics and comparison of expression of lineage-defining markers between neonatal and adult CD11b+ DCs. B) Background (FMO)-subtracted median fluorescence intensity (MFI) is presented for CD80, CD86, CD24, CD205, and the MHC Class I molecules Kd and Db on neonatal and adult CD11b+ DCs. Data are representative of two independent experiments with 3–4 mice/group. * indicates p<0.05, *** indicates p<0.001.
Supplemental Figure S6. Neonatal CD103lo DCs are capable of fully presenting exogenously delivered M282–90 peptide. CD103lo and CD103hi dendritic cells sorted from neonates 2 days post-infection were pulsed with 10−6M or 10−8M of M282–90 (SYIGSINNI) peptide for one hour prior to washing and co-culturing with CFSE-labeled KdM282–90-specific CD8+ T cells. The percent of transgenic cells induced to proliferate after three days in culture was calculated using Flowjo software.
Acknowledgments
This work was supported by intramural funding from the National Institute of Allergy and Infectious Diseases. We thank Richard Nguyen and David Ambrozak for assistance with sorting dendritic cell populations, and Brenda Hartman for help with figure preparation.
Footnotes
AUTHOR CONTRIBUTIONS
TJR, KMM, EB, and DN performed the experiments. TJR, KMM, and BSG designed the experiments, analyzed and interpreted the data, and prepared the manuscript.
DISCLOSURE
The authors declare no conflicts of interest
References
- 1.Desch AN, Henson PM, Jakubzick CV. Pulmonary dendritic cell development and antigen acquisition. Immunol Res. 2013;55(1–3):178–86. doi: 10.1007/s12026-012-8359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14(8):571–8. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilliams M, van de Laar L. A Hitchhiker’s Guide to Myeloid Cell Subsets: Practical Implementation of a Novel Mononuclear Phagocyte Classification System. Front Immunol. 2015;6:406. doi: 10.3389/fimmu.2015.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schraml BU, Reis e Sousa C. Defining dendritic cells. Curr Opin Immunol. 2015;32:13–20. doi: 10.1016/j.coi.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Scott CL, Henri S, Guilliams M. Mononuclear phagocytes of the intestine, the skin, and the lung. Immunol Rev. 2014;262(1):9–24. doi: 10.1111/imr.12220. [DOI] [PubMed] [Google Scholar]
- 6.Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206(13):3115–30. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greter M, Helft J, Chow A, Hashimoto D, Mortha A, Agudo-Cantero J, et al. GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity. 2012;36(6):1031–46. doi: 10.1016/j.immuni.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324(5925):392–7. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207(4):823–36. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322(5904):1097–100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiavoni G, Mattei F, Sestili P, Borghi P, Venditti M, Morse HC, 3rd, et al. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8alpha(+) dendritic cells. J Exp Med. 2002;196(11):1415–25. doi: 10.1084/jem.20021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King IL, Kroenke MA, Segal BM. GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization. J Exp Med. 2010;207(5):953–61. doi: 10.1084/jem.20091844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlitzer A, McGovern N, Ginhoux F. Dendritic cells and monocyte-derived cells: Two complementary and integrated functional systems. Semin Cell Dev Biol. 2015;41:9–22. doi: 10.1016/j.semcdb.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013;38(5):970–83. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki S, Honma K, Matsuyama T, Suzuki K, Toriyama K, Akitoyo I, et al. Critical roles of interferon regulatory factor 4 in CD11b high CD8 alpha-dendritic cell development. Proc Natl Acad Sci U S A. 2004;101(24):8981–6. doi: 10.1073/pnas.0402139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura T, Tailor P, Yamaoka K, Kong HJ, Tsujimura H, O’Shea JJ, et al. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J Immunol. 2005;174(5):2573–81. doi: 10.4049/jimmunol.174.5.2573. [DOI] [PubMed] [Google Scholar]
- 17.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10(5):488–95. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 18.Desch AN, Randolph GJ, Murphy K, Gautier EL, Kedl RM, Lahoud MH, et al. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J Exp Med. 2011;208(9):1789–97. doi: 10.1084/jem.20110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helft J, Manicassamy B, Guermonprez P, Hashimoto D, Silvin A, Agudo J, et al. Cross-presenting CD103+ dendritic cells are protected from influenza virus infection. J Clin Invest. 2012;122(11):4037–47. doi: 10.1172/JCI60659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mashayekhi M, Sandau MM, Dunay IR, Frickel EM, Khan A, Goldszmid RS, et al. CD8alpha(+) dendritic cells are the critical source of interleukin-12 that controls acute infection by Toxoplasma gondii tachyzoites. Immunity. 2011;35(2):249–59. doi: 10.1016/j.immuni.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zelenay S, Keller AM, Whitney PG, Schraml BU, Deddouche S, Rogers NC, et al. The dendritic cell receptor DNGR-1 controls endocytic handling of necrotic cell antigens to favor cross-priming of CTLs in virus-infected mice. J Clin Invest. 2012;122(5):1615–27. doi: 10.1172/JCI60644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hagerbrand K, Marsal J, et al. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 2013;38(5):958–69. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38(2):322–35. doi: 10.1016/j.immuni.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Ruckwardt TJ, Malloy AM, Gostick E, Price DA, Dash P, McClaren JL, et al. Neonatal CD8 T-cell hierarchy is distinct from adults and is influenced by intrinsic T cell properties in respiratory syncytial virus infected mice. PLoS Pathog. 2011;7(12):e1002377. doi: 10.1371/journal.ppat.1002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruckwardt TJ, Malloy AM, Morabito KM, Graham BS. Quantitative and qualitative deficits in neonatal lung-migratory dendritic cells impact the generation of the CD8+ T cell response. PLoS Pathog. 2014;10(2):e1003934. doi: 10.1371/journal.ppat.1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim TS, Gorski SA, Hahn S, Murphy KM, Braciale TJ. Distinct dendritic cell subsets dictate the fate decision between effector and memory CD8(+) T cell differentiation by a CD24-dependent mechanism. Immunity. 2014;40(3):400–13. doi: 10.1016/j.immuni.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montagna GN, Biswas A, Hildner K, Matuschewski K, Dunay IR. Batf3 deficiency proves the pivotal role of CD8alpha dendritic cells in protection induced by vaccination with attenuated Plasmodium sporozoites. Parasite Immunol. 2015 doi: 10.1111/pim.12222. [DOI] [PubMed] [Google Scholar]
- 28.Ruckwardt TJ, Luongo C, Malloy AM, Liu J, Chen M, Collins PL, et al. Responses against a subdominant CD8+ T cell epitope protect against immunopathology caused by a dominant epitope. J Immunol. 2010;185(8):4673–80. doi: 10.4049/jimmunol.1001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sathe P, Pooley J, Vremec D, Mintern J, Jin JO, Wu L, et al. The acquisition of antigen cross-presentation function by newly formed dendritic cells. J Immunol. 2011;186(9):5184–92. doi: 10.4049/jimmunol.1002683. [DOI] [PubMed] [Google Scholar]
- 30.Torti N, Walton SM, Murphy KM, Oxenius A. Batf3 transcription factor-dependent DC subsets in murine CMV infection: differential impact on T-cell priming and memory inflation. Eur J Immunol. 2011;41(9):2612–8. doi: 10.1002/eji.201041075. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Haddad EK, Marceau J, Morabito KM, Rao SS, Filali-Mouhim A, et al. A Numerically Subdominant CD8 T Cell Response to Matrix Protein of Respiratory Syncytial Virus Controls Infection with Limited Immunopathology. PLoS Pathog. 2016;12(3):e1005486. doi: 10.1371/journal.ppat.1005486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shrimpton RE, Butler M, Morel AS, Eren E, Hue SS, Ritter MA. CD205 (DEC-205): a recognition receptor for apoptotic and necrotic self. Mol Immunol. 2009;46(6):1229–39. doi: 10.1016/j.molimm.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayer CT, Ghorbani P, Nandan A, Dudek M, Arnold-Schrauf C, Hesse C, et al. Selective and efficient generation of functional Batf3-dependent CD103+ dendritic cells from mouse bone marrow. Blood. 2014;124(20):3081–91. doi: 10.1182/blood-2013-12-545772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosler B, Herold S. Lung epithelial GM-CSF improves host defense function and epithelial repair in influenza virus pneumonia-a new therapeutic strategy? Mol Cell Pediatr. 2016;3(1):29. doi: 10.1186/s40348-016-0055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unkel B, Hoegner K, Clausen BE, Lewe-Schlosser P, Bodner J, Gattenloehner S, et al. Alveolar epithelial cells orchestrate DC function in murine viral pneumonia. J Clin Invest. 2012;122(10):3652–64. doi: 10.1172/JCI62139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhan Y, Carrington EM, van Nieuwenhuijze A, Bedoui S, Seah S, Xu Y, et al. GM-CSF increases cross-presentation and CD103 expression by mouse CD8(+) spleen dendritic cells. Eur J Immunol. 2011;41(9):2585–95. doi: 10.1002/eji.201141540. [DOI] [PubMed] [Google Scholar]
- 37.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med. 2013;210(10):1977–92. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hintzen G, Ohl L, del Rio ML, Rodriguez-Barbosa JI, Pabst O, Kocks JR, et al. Induction of tolerance to innocuous inhaled antigen relies on a CCR7-dependent dendritic cell-mediated antigen transport to the bronchial lymph node. J Immunol. 2006;177(10):7346–54. doi: 10.4049/jimmunol.177.10.7346. [DOI] [PubMed] [Google Scholar]
- 39.Semmrich M, Plantinga M, Svensson-Frej M, Uronen-Hansson H, Gustafsson T, Mowat AM, et al. Directed antigen targeting in vivo identifies a role for CD103+ dendritic cells in both tolerogenic and immunogenic T-cell responses. Mucosal Immunol. 2012;5(2):150–60. doi: 10.1038/mi.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Remot A, Descamps D, Jouneau L, Laubreton D, Dubuquoy C, Bouet S, et al. Flt3 ligand improves the innate response to respiratory syncytial virus and limits lung disease upon RSV re-exposure in neonate mice. Eur J Immunol. 2015 doi: 10.1002/eji.201545929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Induction of epitope-specific T cells by sorted adult CD103+ DCs. A) Gating of CD103+ DCs and CD11b+ DCs in the MLN of adult and neonatal mice. Live singlets were gated prior to gating on the CD11c+, Class IIhi DC population. This population was further broken down into CD103+ DCs and CD11b+ DCs. B) An example of raw data obtained from an in vitro coculture experiment showing the induction of proliferation of KdM282–90 and DbM187–195=specific cells by FACS-sorted CD103+ DCs sorted from the MLN of adult mice on the indicated days post-infection. Mice were infected on different days in order to harvest MLN from all time points post-infection and perform the coculture on one day with the same reporter T cell populations.
Supplemental Figure S2. Frequency and number of CD8+ T cells in RSV-infected adult and neonatal wild-type and Batf3−/− mice. CD8+ T cell frequency and numbers in the lung and MLN 7 days post-infection (A–D) and the frequency and number of CD8+ T cells specific for DbM187–195 and KdM282–90 specific cells in the MLN of wild-type and Batf3-deficient adults and neonates 7 days post-RSV infection (E–H). Data are representative of 3 independent experiments with 5–8 mice/group. P values indicated are from a t-test between wild-type and Batf3−/− mice of the same age.
Supplemental Figure S3. Higher KdM282–90-specific responses in the lungs of Batf3−/− deficient neonates are due to the lack of competition from the DbM187–195-specific response. Wild-type and Batf3-deficient neonatal mice were infected with RSV-N191S, an RSV virus that does not stimulate a response to the DbM187–195 epitope due to a mutation in the P5 anchor residue. The frequency and number of KdM282–90-specific cells were measured by tetramer staining in the lung and MLN 7 days post-infection. Results shown are combined data from two litters of wild-type and two litters of Batf3−/− deficient neonates.
Supplemental Figure S4. Influenza/PR8-infected neonatal mice possess two populations within the CD103+ DC subset. Seven-day-old mice were infected intranasally with 600 TCID50 of influenza/PR8. MLN were harvested from naïve mice, and mice at days 1–3 post-infection for surface staining of lung-migratory dendritic cell populations. The sample shown is representative of several pools of MLN from neonatal mice infected with influenza/PR8.
Supplemental Figure S5. Phenotypic comparison of neonatal CD11b+ DCs and adult CD11b+ DCs in the MLN of mice two days post-infection. A) Scatter characteristics and comparison of expression of lineage-defining markers between neonatal and adult CD11b+ DCs. B) Background (FMO)-subtracted median fluorescence intensity (MFI) is presented for CD80, CD86, CD24, CD205, and the MHC Class I molecules Kd and Db on neonatal and adult CD11b+ DCs. Data are representative of two independent experiments with 3–4 mice/group. * indicates p<0.05, *** indicates p<0.001.
Supplemental Figure S6. Neonatal CD103lo DCs are capable of fully presenting exogenously delivered M282–90 peptide. CD103lo and CD103hi dendritic cells sorted from neonates 2 days post-infection were pulsed with 10−6M or 10−8M of M282–90 (SYIGSINNI) peptide for one hour prior to washing and co-culturing with CFSE-labeled KdM282–90-specific CD8+ T cells. The percent of transgenic cells induced to proliferate after three days in culture was calculated using Flowjo software.


