Abstract
Some patients with otherwise treatment-resistant Hodgkin lymphoma (HL) could benefit from chimeric antigen receptor T cell (CART) therapy. However, HL lacks CD19 and contains a highly immunosuppressive tumor microenvironment (TME). We hypothesized that in HL, CART should target both malignant cells and the TME. We demonstrated CD123 on both HL cells and TME, including tumor-associated macrophages (TAM). In vitro, HL cells convert macrophages towards immunosuppressive TAM that inhibit T cell proliferation. In contrast, anti-CD123 CART recognized and killed TAM thus overcoming immunosuppression. Finally, we showed in immunodeficient mouse models that CART123 eradicate HL, and establish long-term immune memory. A novel platform that targets malignant cells and the microenvironment may be needed to successfully treat malignancies with an immunosuppressive milieu.
Introduction
Despite multi-modality therapy, a subset of patients with Hodgkin lymphoma (HL) succumbs to this disease. In particular, 10–15% of patients presenting with localized disease and 20–40% with advanced stage disease will experience relapse. (1) Furthermore, additional 10–15% of HL patients have disease that is refractory to first-line therapy. (2) Recent developments in biological therapy for relapsing/refractory (r/r) HL include the anti-CD30 antibody-drug conjugate brentuximab vedotin (BV) and PD1 inhibition. (3) Although BV induces objective responses in 75% of patients with r/r HL after autologous stem cell transplantation (SCT), responses are not durable and the median progression-free survival is only 5.6 months. (4) Similarly, PD1 inhibition leads to high overall response rates but most of these are partial. (5) Thus, despite the profusion of novel agents for HL, there is still a need for therapies with a curative potential.
The role of adoptive cellular therapy in HL has been demonstrated by the effectiveness of allogeneic SCT in a subset of HL patients and, more recently, by the promising clinical results of the infusion of EBV-specific T cells (6). However, allogeneic SCT carries a high treatment-related mortality and only approximately 30–40% of HL expresses the EBV-encoded antigens. (7, 8) Thus, T cell-related therapies already display an important and growing role in HL. Chimeric antigen receptor T cells (CART) represent an exciting recent development in cancer immunotherapy. (9) Our group and others have demonstrated the clinical efficacy of anti-CD19 chimeric antigen receptor redirected T cells (CART19) for refractory B-cell malignancies. (10–14) However, despite the B-cell origin of Hodgkin Reed-Sternberg (HRS) cells, B-cell antigens including CD19 are rarely expressed in HL. (15) While the CD30 antigen is known to be commonly expressed on HRS cells, outcomes of patients treated with anti-CD30 CART have been relatively disappointing, with reported 1 in 8 complete responses (CR), 4/8 stable disease (SD) and 3/8 progressive disease (PD) in r/r HL patients. (16–18) In HL malignant cells represent only approximately 1–2% of cellularity, with the majority of the tumor-microenvironment cells (TME) being comprised of infiltrating immune cells (macrophages and myeloid-derived suppressive cells, basophils, mast cells, eosinophils, B and T lymphocytes, stromal cells and fibroblasts). The TME and in particular tumor-associated macrophages (TAM) play a key role in promoting tumor growth while also inhibiting the anti-tumor immune response. High frequency of CD68+ TAM in HL biopsies has been correlated with poor overall survival. (19–25) Immunosuppressive and pro-tumor macrophages have been defined as M2 type, as opposed to inactivated (M0) or anti-infection/tumor macrophages (M1). (26–28) Thus, HL represents a unique opportunity to study the impact of the TME on immunotherapy. The development of an approach that could target the malignant cells as well as the supportive TME would likely represent an important advance in the field of CART immunotherapy, by providing robust stimulation of the CAR T cells while avoiding T-cell inhibition.
In this context we studied CD123, the α chain of the receptor for interleukin-3 (IL-3), whose expression has been previously described on Hodgkin HRS cells. (29–33) In addition, in vitro data show that IL-3 rescues HL cells from apoptosis and promotes HL cell line growth. (34) Furthermore, since CD123 is expressed on myeloid cells, including macrophages, plasmacytoid dendritic cells, eosinophils, basophils and mast cells, we hypothesized that CD123 would be expressed within HL tumor masses on both the malignant cells and the supportive TME. (35, 36) A recent studies show that CD123 is expressed also in a subset of myeloid-derived suppressor cells (MDSC). (37) The objective of this study was therefore to develop an anti-HL CART immunotherapy that would also be able to overcome the immunosuppression of the HL microenvironment by targeting the TME. We confirmed the presence of CD123 on HRS cells and found that many immune cells in the HL TME, in particular immunosuppressive M2-type TAM, express CD123. We previously developed anti-CD123 CAR T cells for the treatment of AML (38) and we now find that CART123 cells can eradicate disseminated HL tumor xenografts leading to durable remissions and the development of immune memory. Furthermore, through co-targeting of immunosuppressive CD123-expressing TAM, CART123, are shown to be resistant to microenvironmental immunosuppression.
Results
The IL-3 receptor α, CD123, is expressed in Hodgkin lymphoma cells and in tumor-associated macrophages
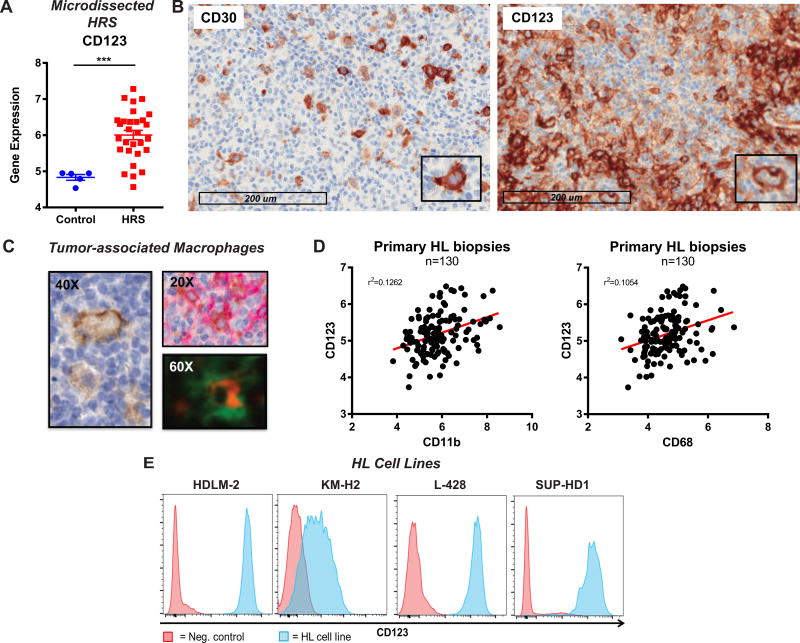
We sought to identify a tumor-associated antigen expressed in the HRS cells and in cells of the HL tumor microenvironment. For this purpose we analyzed RNA expression on microdissected HRS cells from 29 HL biopsies (dataset GSE-39133) (39) and we found high expression of the IL-3 receptor α, CD123, in the vast majority of patients compared to control B cells (Figure 1 A similarly to what observed for CD30 Figure S1 A. To confirm this finding and to study the expression of CD123 in the TME, we evaluated the expression of CD123 and CD30 on histological specimens from patients with HL (Figure 1 B). As expected, in 10/10 of the HL tissue biopsies HRS were positive for CD30, the hallmark biomarker of HRS. Importantly, in 5/10 specimens we also detected expression of CD123 on the HRS cells by immunohistochemistry (IHC). IHC revealed that CD30 was sparsely distributed on infiltrating immune cells, while CD123 was highly expressed on the TME (Figure 1 B); in particular we found that TAM expressed CD123, as shown by dual-color immunofluorescence and IHC (Figure 1 C). We confirmed the expression of CD123 in the HL TME by analyzing RNA expression data of whole biopsies from 130 HL patients (dataset GSE17920) (20) and found a correlation between CD123 expression with the two standard macrophage markers, CD68 and CD11b (Figure 1 D). To define an appropriate human tumor model for further studies we evaluated four HL cell lines (HDLM-2, KM-H2, SUP-HD1, and L-428) and found CD123 to be highly expressed by these cells (CD30 used as positive control) at both the mRNA (GSE-39133) (39) (Figure S1 B and protein level (Figure 1 E and S1 C respectively).
Figure 1. The IL-3 receptor α, CD123, is expressed in Hodgkin Lymphoma cells and in tumor-associated macrophages.
A. Gene expression analysis of microdissected HRS (GSE-39133) reveals high expression of CD123 as compared to controls (microdissected germinal center B cells) B. Primary samples of Hodgkin lymphoma were stained for CD30 and CD123 by immunohistochemistry (IHC). Expression of CD123 was found of the HL Reed-Sternberg cells but also in the tumor microenvironment, as opposed to CD30 that was only positive on HRS. C. CD123 was expressed on tumor-associated macrophages by IHC (left), dual IHC (right, top; brown CD68 and red CD123) and immune fluorescence (right, bottom; red CD123 and green CD68. D. RNA expression of CD123 and CD68 (left) and CD11b (right) in 130 whole HL biopsies (GSE17920): a strong correlation between macrophage markers (CD68, CD11b) and CD123 expression was observed. E. High CD123 protein expression on 4 standard HL cell lines (HDLM-2, KM-H2, SUP-HD1 and L428) by flow cytometry.
HL cells polarize normal macrophages to an M2-like phenotype
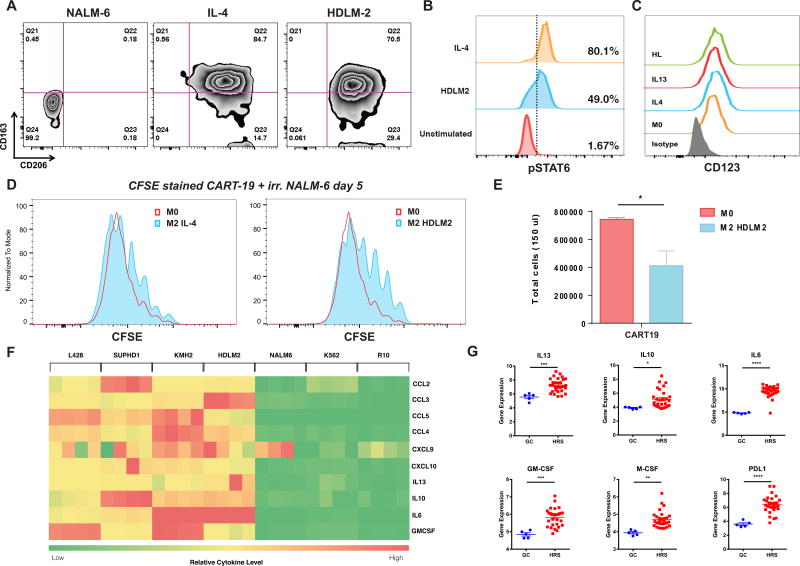
Since TAM are involved in HL pathogenesis and impact its prognosis (20, 25), we sought to examine whether HL cells can directly mediate conversion of macrophages to an immunosuppressive phenotype. Human normal donor macrophages differentiated from peripheral blood monocytes (see macrophage differentiation protocol in Figure S2 A) were co-cultured with HDLM-2 cells, IL-4 (a standard M2-polarizing cytokine), or a control non-HL cell line (the B-cell acute lymphoblastic leukemia cell line NALM-6). After 24 hours of co-culture macrophage phenotype was analyzed by flow cytometry using a panel of 6 markers (CD206, CD163, CD86, CD80, PD-L1 and PD-L2. HDLM-2-primed macrophages showed an M2-like phenotype, with expression of CD206 and CD163 similar to that of IL-4-primed macrophages (Figure 2 A). (40–44) In particular, PD-L1 was significantly increased in macrophages exposed to HL cells (and Figure S2 B and C). As a control, macrophages co-cultured with NALM-6 showed a non-M2-like phenotype (M0). Notably, exposure of macrophages to HDLM-2 supernatants led to phosphorylation of STAT6, as observed during IL-4 activation (Figure 2 B). Importantly, CD123 expression was maintained in both IL-4 and HL-polarized macrophages (Figure 2 C).
Figure 2. HL cells polarize normal macrophages to a M2-like phenotype.
A. Human normal donor macrophages differentiated from peripheral blood monocytes were cultured with a control acute lymphoblastic leukemia cell line (NALM-6), IL-4 (M2 positive control), or HL cell line HDLM-2 conditioned supernatant. HDLM-2 cells can polarize macrophages toward an M2 phenotype (CD163+CD206+) after a 24-hour culture. B. HDLM2 supernatants trigger phosphorylation of STAT6 on macrophages, similarly to IL-4. C. M0, M2-polarized (IL-4 or IL-13), and HL polarized macrophages express CD123 by flow cytometry. D. M2-polarized (IL-4) and, to a greater extent, HL-polarized macrophages can reduce anti-CD19 chimeric antigen receptor T cell proliferation in response to irradiated CD19+ NALM-6 target cells, as shown by CFSE (Carboxyfluorescein succinimidyl ester - a fluorescent cell stained dye that is diluted with cell proliferation) dilution assay. E. HL-polarized macrophages reduce CART19 proliferation in response to irradiated NALM-6 target cells, as shown by absolute T cell numbers at day 5 F. Heatmap demonstrating that HL cell lines HDLM2, KMH2, SUPHD1, and L428 secreted significantly more myeloid cell recruiting chemokines (CCL2, CCL3, CCL4, CCL5, CXCL9, CXCL10), M2 polarizing cytokines (IL13, IL10, IL6) and macrophage supporting factor GM-CSF than control non-HL cell lines K562 and NALM6 or media alone. G. RNA expression analysis of 29 microdissected HRS cells (dataset GSE-39133) confirmed the expression of M2 polarizing cytokines (IL13, IL10, IL6), macrophage supporting factors (GM-CSF, M-CSF), and the immunosuppressive checkpoint ligand PDL1 in primary samples.
In order to test the function of the phenotypically-defined immunosuppressive macrophages, we co-cultured human CART cells with macrophages under M0, M2 (IL-4 induced), or HL-polarized macrophages. In this experiment we used the anti-CD19 CART as the reference “responder” cells and the CD19+ acute leukemia B-cell line NALM-6 as the “stimulator” cells. (45) As expected, CART19 strongly proliferated in the presence of the target cell line, but this proliferation was slightly reduced in the presence of M2 macrophages and further inhibited by HL-TAMs (Figure 2 D–E). In order to probe the mechanism of macrophage polarization by HL cells, we analyzed the concentration of cytokines and chemokines in the supernatant of four HL cell lines and two control non-lymphoma cell lines (NALM-6, K562) using the Luminex assay (Figure 2 F). HL cells secreted cytokines and chemokines capable of stimulating and recruiting myeloid cells, such as GM-CSF, CCL2, CCL3, CCL4, CCL5, CXCL9, and CXCL10, as well as M2 polarizing cytokines IL-13, IL-6, and IL-10. The expression of M2 polarizing cytokines (IL13, IL10, IL6), macrophage supporting factors (GM-CSF, M-CSF), and the immunosuppressive checkpoint PDL1 were confirmed by RNA expression analysis of microdissected HRS cells (n=29, dataset GSE-39133) (Figure 2 G).
Anti-CD123 chimeric antigen receptor T cells kill HL cells in vitro and in vivo
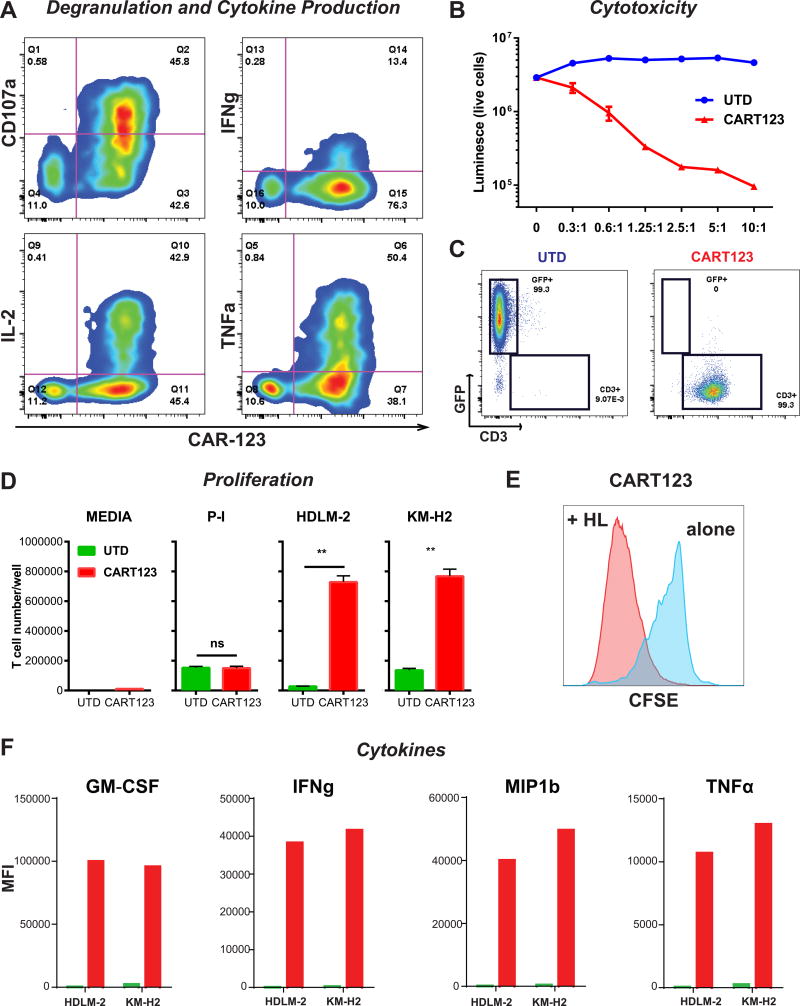
Having demonstrated the presence of CD123 in the HRS cells, we sought to demonstrate the extent to which anti-CD123 CAR T cells (CART123) (see CAR123 construct on Figure S3 A) can recognize HL cells, as measured by antigen-dependent CART proliferation, cytokine production and specific tumor lysis. We used the HL cell line as a model, given that it is impossible to propagate primary HL in culture and due to the lack of reliable primary HL xenograft models. In vitro, CAR123+ cells but not CAR123- demonstrated specific CD107a degranulation and intracellular expression of cytokines (IFNγ, IL-2, and TNFα) when co-cultured with HL cells for 4–6 hours (Figure 3 A). At 24 hours, potent cytotoxicity against HDLM-2 is exerted by CART123, but not by the control untransduced T cells (UTD) (Figure 3 B). Complete eradication of HL cells by day 4 is associated with massive CART123 expansion (day 20) (Figure 3 C). Notably, additional HL cell lines L-428, KM-H2 and SUP-HD1 were also efficiently killed in vitro by CART123 (Figure S3 B). CART123 proliferated when co-cultured with HL cells (HDLM-2 or KM-H2), as demonstrated by absolute T cell number after 5 days (Figure 3 D) and CFSE dilution (Figure 3 E). Importantly, CART123 cells secrete effector cytokines such as GM-CSF, IFNγ, MIP1b and TNFα in the presence of HL cells (Figure 3 F).
Figure 3. Anti-CD123 chimeric antigen receptor T cells exert potent effector function against Hodgkin lymphoma in vitro.
A. HL cells (HDLM-2) were co-cultured with CART123 for 4–6 hours. CAR+ but not CAR- T cells expressed high levels of the degranulation marker CD107A and produced intra-cellular cytokines like IFNγ, IL-2 and TNFα. B. CART123, but not UTD, exert potent cytotoxicity (luciferase-based killing assay) against HL cells in a dose-dependent manner. C. HL cells (HDLM-2) were co-cultured at long term with CART123 or control UTD. At day 20, CART123 but not UTD killed HL cells and proliferated. D. CART123 or UTD were co-cultured with media, PMA/Ionomycin (positive control) or two HL cell lines (HDLM-2 and KM-H2) for 5 days; CART123 but not UTD controls showed significant proliferation as absolute number and CFSE dilution (E). F. HL cells stimulated CART123 but not UTD cells to release multiple cytokines including GM-CSF, IFNγ, MIP1β and TNFα. E:T= effector : target ratio.
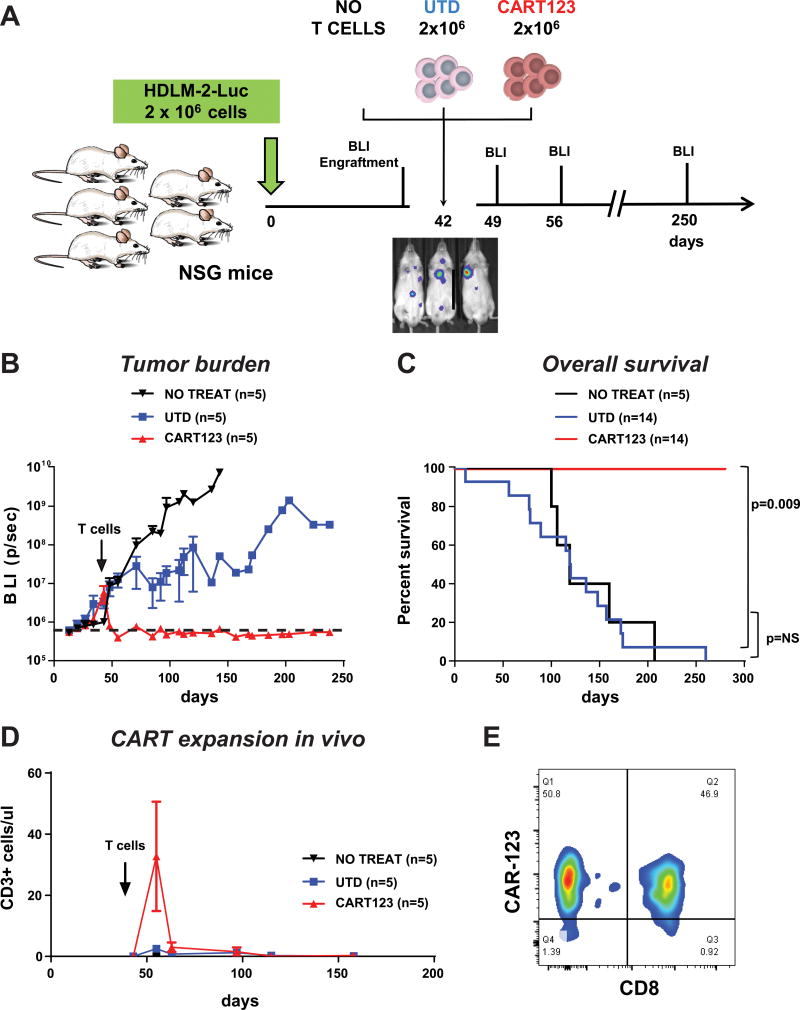
We then developed a xenograft model of progressive HL by injecting 1×106 luciferase+ HDLM-2 cells intravenously in NSG mice on day 0 (Figure 4 A). Serial bioluminescent imaging (BLI) demonstrated tumor engraftment by day 7, which was followed by gradual increase in tumor burden over approximately 6 weeks. At day 42 when the tumor burden was 20-fold higher than the baseline, mice were treated with 1.5×106 CART123 cells or control T cells. CART123 induced complete and durable eradication of disseminated tumor within 14 days, leading to 100% relapse-free and 100% overall survival at 6 months (Figure 4 B and C). Mice were followed up for almost 1 year and no relapses were observed in CART123-treated mice, while mice treated with control T cells had a median survival of 128 days (p=0.009). Minimal reduction in tumor progression was observed in UTD mice; however the OS of UTD vs. no treatment was not statistically different. Tumor elimination was associated with extensive CART expansion in the peripheral blood, including both CD8+ and CD4+ cells as detected by flow cytometry in serial peripheral blood analyses, as seen in clinical studies of anti-CD19 CAR T cells (Figure 4 D and E). Importantly, the in vivo efficacy of CART123 against HL was confirmed in a second xenograft model where NSG mice were injected with luciferase positive SUP-HD1 cells and treated with CART123 at day 21. CART123-treated mice reach complete remission while controls progressed (Figure S3 C).
Figure 4. Anti-CD123 chimeric antigen receptor T cells exert potent effector function against Hodgkin lymphoma in vivo.
A Experiment schema: 2 ×106 Luciferase-positive HDLM-2 cells were injected i.v. in NSG mice and tumor engraftment was monitored by bioluminescence imaging. At day 42 mice were randomized to receive no treatment, 2 ×106 control untransduced T cells (UTD) or 2 ×106 CART123. B. Mice receiving CART123, but not controls, experienced complete response with long term remission of disease (>250 days), representative experiment. C. CART123-treated mice have a significantly longer overall survival as compared to controls (3 experiments combined). D. CAR123 T cells engraft, expand and disappear from the peripheral blood after clearing the tumor. E. T cells in the PB of CART123-treated mice were both CD8 and CD4 with high expression of the CAR.
CART123 establish long-term immunological memory in mice with HL
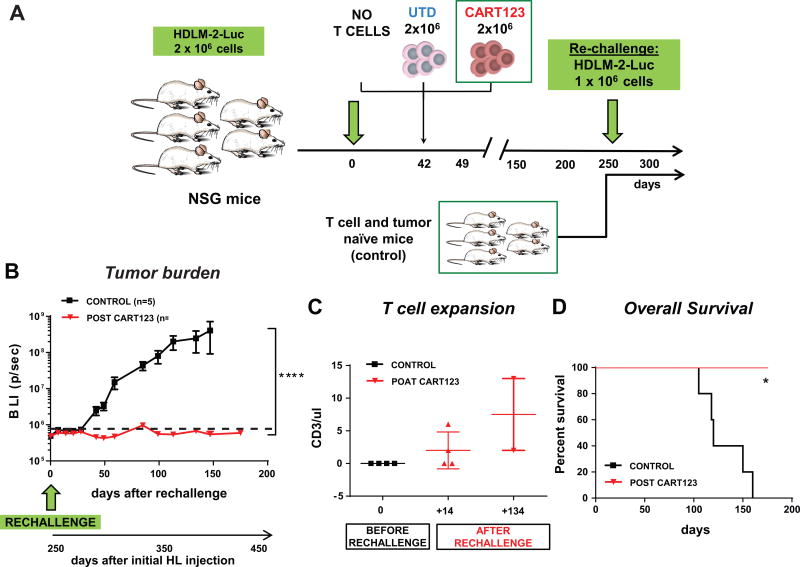
Long-term persistence and T cell memory play an important role in immunosurveillance and prevention of relapse. In order to demonstrate the formation of immunological memory, at a late time point (day 250) when CART123 treated mice were in remission, we re-challenged them with the HDLM-2 HL cells (see experiment schema Figure 5 A). As a control we used tumor and T cell-naïve mice. Notably, in the mice previously treated with CART123 the tumor was rejected and there was no sign of long term relapse; on the contrary, in control mice HL engrafted and progressed overtime (Figure 5 B). The rejection was associated with a re-expansion of the previously undetectable CART123 cells in the peripheral blood (~10 months after T cell injection) (Figure 5 C). In contrast, in control mice HL cells engrafted and led to the death of these mice (Figure 5 D).
Figure 5. CART123 establish long-term immunological memory in mice with HL.
A. Experiment schema: mice previously treated with CART123 and experiencing a long-term remission were rechallenged at day 250 with HL cells (HDLM-2). As a control a tumor-naïve group of mice were also injected with the same HL cells. B. HL cells only engrafted and grew in tumor-naïve mice while long-term surviving mice (post-CART123) were able to control disease growth. C. A re-expansion of CART123 cells was observed in mice previously treated with CART123. D. An improved overall survival was observed in mice with previous exposure to CART123.
CART123 are resistant to the inhibition of M2-macrophages
Lastly, as we have demonstrated that CAR T cells can be inhibited by M2 and HL-polarized macrophages and that these macrophages are CD123-positive, we sought to determine if CART123 were also susceptible to macrophage inhibition, or conversely, due to the expression of CD123 in HL-macrophages, they would receive additional stimulation.
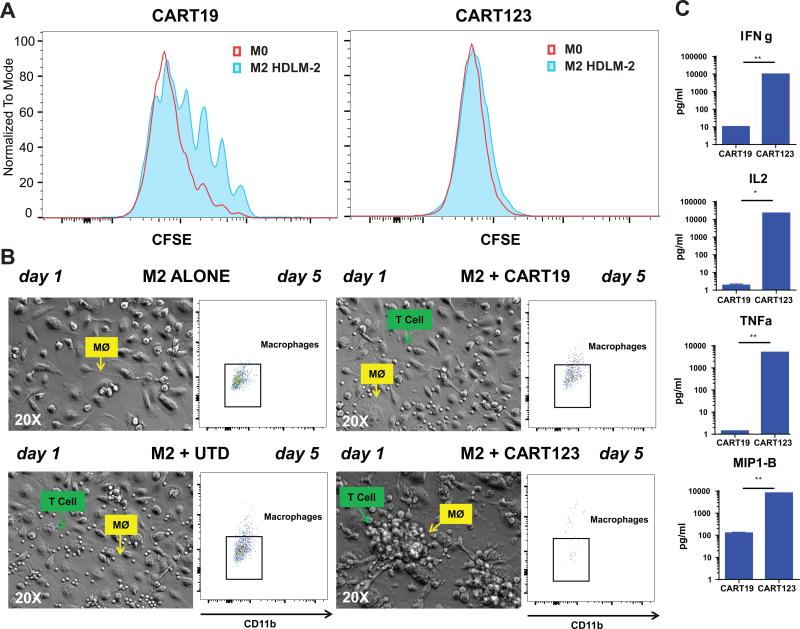
We generated immunosuppressive M2 macrophages by culturing them with HDLM-2 cells (see Figure S2 A). Using the pre-established CART19 and B-cell acute lymphoblastic leukemia model, we showed that HL-polarized TAM can reduce CART19 proliferation following CAR stimulation at day 5 but, in stark contrast, CART123 were not affected (Figure 6 A). Importantly, CART123 actively recognize M2 (both IL-4 polarized or HL-TAMs) macrophages and form aggregates around them as shown by microscopy at 24 hours (Figure 6 B left) and exert significant cytotoxicity by day 5 as shown by flow cytometry (Figure 6 B right). Moreover, cytokine production by T cells was inhibited by M2 macrophages in CART19 but not in CART123 (Figure 6 C). We demonstrated that the ability of CART123 to overcome immunosuppression relies in their capacity to recognize and efficiently kill macrophages as shown in Figure S4 A and B. CART123 thereby overcome TAM-mediated inhibition by simultaneous targeting of CD123 on HRS cells and macrophages.
Figure 6. CART123 are resistant to inhibition by M2-macrophages.
A. In a 5-day CFSE proliferation assay, CART123 are completely resistant to HL-polarized macrophage mediated inhibition. B. CART123 cells rapidly (day 1) recognize M2-macrophages, forming clusters around macrophages and clearing them by day 5 as shown by phase contrast microscopy (20X) and flow cytometry, respectively. C. CART19 but not CART123 cytokine secretion was significantly reduced in the presence of M2 (IL-4 polarized) macrophages in vitro.
Discussion
HL is usually associated with a good prognosis, but a subset of patients experiences multiple relapses or is primarily refractory. Recent advances in the care of HL including the antibody-drug conjugate brentuximab vedotin or the immune checkpoint antagonists nivolumab and pembrolizumab confer high overall response rates in this patient population but the rate of complete responses is relatively low. (3) CART cell therapy has recently come to the fore as a potent antigen-specific modality in the treatment of B cell malignancies. (46) Since CD19 is not expressed on the malignant HRS cells, CART cell therapy against the commonly expressed CD30 antigen has been attempted but the results have been disappointing to date. (18) One possibility is that the heavy microenvironmental infiltrate in HL, composed of numerous immunosuppressive cells such as tumor-associated macrophages (TAM), inhibits the activity of CART cells. Indeed, CD68+ macrophages infiltration correlates with a poor prognosis in HL. (20) To our knowledge, none of the currently approved therapies against HL specifically target the TME, although it is distinctly possible that the PD1 antagonists block the interaction between PD1 on T cells and PD-L1 on HRS as well as on TAM. In this context, we sought an antigen that is co-expressed on HRS cells and in the TME and hypothesized that a co-targeting approach would circumvent immunosuppressive stimuli from the TME.
Using several orthogonal techniques including analysis of publicly available gene expression profiling of microdissected primary HRS cells, RNA sequencing of primary HL lesions, IHC and flow cytometry, we found and confirmed previous reports that CD123 is expressed on the majority of HRS cells and in the TME. (29, 33) We have previously described the activity of CART123 in human acute myeloid leukemia (38). Here, we show that CART123 specifically degranulate, proliferate, produce cytokines and kill target HL cells in vitro. In vivo, we show that human CD123-redirected T cells display potent therapeutic activity against disseminated HL, persist long-term after eradication of disease and are capable of mounting a robust recall response to tumor challenge. Since the xenograft system does not permit an evaluation of the role of the tumor microenvironment (TME), we turned to an in vitro system to investigate the role of the TME in resistance to CAR T cells.
We showed that HL cell lines produce several macrophage-attracting chemokines (CCL2-5 and CXCL9-10) and immunosuppressive cytokines, including IL-13, IL-10, and IL-6, and we demonstrated activation of the STAT6 signaling pathway in response to HL conditioned media, which is a known IL4/IL13 signaling pathway. IL-13 has previously been reported to play a role in autocrine growth stimulation of HRS cells (47, 48) and has been postulated to mediate recruitment of immune cells into HL TME. We showed that exposure to HL supernatant polarizes macrophages towards the “M2” phenotype, leading to a consequent immunosuppressive effect on CART. We used anti-CD19 CAR T cells (CART19) stimulated by the B-cell leukemic cell line NALM-6 as a “gold standard” for potent antigen-specific stimulation. (45, 49) We found that HL-exposed macrophages, as a model of tumor-associated macrophages (TAM), are able to partially inhibit CART19 stimulation by CD19. Although CD19 is not expressed on HL and CART19 therapy is not expected to have activity in HL, there is literature on clonotypic CD19+ B cells in HL and leading to B cell-directed therapies in HL (50, 51) including a trial at our institution evaluating CART19 in this malignancy (NCT02277522). More importantly, if our findings are generalizable to TAM in other malignancies, TAM may have a similar effect on CART19 in bone fide CD19-expressing B-cell malignancies.
CD123 is expressed in other hematologic neoplasms, including acute myeloid leukemia (AML), plasmacytoid dendritic cell neoplasm (52), hairy cell leukemia (53) and B-cell acute lymphoblastic leukemia. (54–57) Due to these characteristics, multiple modalities to target CD123 in hematological neoplasms (including HL (58, 59)) have been developed, in particular the IL3-diphtheria toxin fusion protein (SL-401, SL-501, DT388IL3) (60–62), unconjugated anti–CD123 monoclonal antibodies (CSL-360, CSL-362) (63), antibody-drug conjugates (64), bi-specific antibodies (65, 66) or CD3Fv-IL3 fusion constructs (67), and more recently, anti-CD123 chimeric antigen receptor T cells. (30, 38, 68, 69) Notably, there are currently 13 clinical trials investigating CD123 as a target for hematological cancers (clinicaltrials.gov). Our previous publication on the anti-leukemia efficacy of anti-CD123 CAR T cells also highlighted the potential for myeloablation resulting from targeting CD123 on normal hematopoietic precursors. (38) Thus, our current clinical trial utilizes short-acting mRNA-electroporated CART rather than permanently modified lentivirally transduced CAR T cells. (NCT02623582). A recent case report described one patient treated with lentivirally-transduced CART123 showing the feasibility of this approach, supported by preliminary results from other CD123-targeted agents. (70–72). If the predicted CART123 myelotoxicity is confirmed in ongoing trials, we propose that CART123 could be depleted once remission is obtained (using clinically available anti-T cell antibodies) (73) prior to a subsequent stem cell transplant. Since SCT is already a standard strategy for r/r HL and metabolic remission prior to SCT is an important prognostic factor, it may be possible to use adoptive cellular therapy with potent CART123 to achieve a complete remission prior to salvage SCT.
In summary, we found that HL cells can polarize macrophages to an immunosuppressive phenotype. These “M2”-like TAM are present within the TME, express CD123, and are targetable by CD123-redirected CAR T cells. CART123 display potent therapeutic activity against disseminated HL in two xenograft models. Thus, this approach could be used for depleting both malignant cells and the surrounding immunosuppressive milieu.
Methods
Cell lines and primary samples
Cell lines were originally obtained from ATCC (Manassas, VA) (K-562) or DSMZ (Braunschweig, Germany) (HDLM-2, KMH2, L428, SUPHD1, MOLM-14 and NALM-6). Cell line authentication was based on extensive flow cytometry characterization (data not shown). Cell lines were obtained between 2009 and 2013 and were used for experiments within 15–20 passages. All cell lines were tested for the presence of mycoplasma contamination (MycoAlert™ Mycoplasma Detection Kit, LT07-318, Lonza, Basel, Switzerland). For some experiments, cell lines were transduced with luciferase (click-beetle green) or eGFP and then sorted to obtain a >99% positive population. MOLM-14 and K562 were used as controls as indicated in the relevant figures. The cell lines were maintained in culture with RPMI media 1640 (Gibco, 11875-085, LifeTechnologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS, Gemini, 100–106, West Sacramento, CA), and 50 UI/ml penicillin/streptomycin (Gibco, LifeTechnologies, 15070-063). For all functional studies, primary cells were thawed at least 12 hours before experiment and rested at 37°C. De-identified formalin-fixed primary human HL specimens were obtained from the clinical practices of University of Pennsylvania/Children’s Hospital of Philadelphia under an Institutional Review Board (IRB)-protocol.
Immunohistochemistry
Immunohistochemistry (IHC) of formalin fixed paraffin embedded tissue was performed using antibodies against human CD68 (Leica PA0273, clone 514H12) RTU and CD123 (Leica NCL-L-CD123) at 1:150 dilution. For the dual IHC staining was done sequentially on a Leica Bond-IIITM instrument using the Bond Polymer Refine Detection System (Leica Microsystems DS9800-DAB for CD68 and DS9390-Red for CD123 ). Heat-induced epitope retrieval was done for 20 minutes with ER2 solution (Leica Microsystems AR9640). Incubation time with the CD68 antibody was 15 minutes followed by 8 min post-primary step and 8 min incubation with polymer HRP, then block endogenous peroxidase for 5 min. followed by 10 min DAB. After the first antibody staining was completed, the slides were incubated with the anti-CD123 antibody for 15 min., followed by post primary AP for 20 min and post polymer for 20 min Subsequently, the slides were stained with Fast red for 7 min Slides were washed three times between each step with bond wash buffer or water. All the experiments were done at room temperature.
Immunofluorescence
Immunofluorescence of formalin fixed paraffin embedded tissue was performed using antibodies against human CD30 (DakoM0751) at 1:60 and CD123 (Leica NCL-L-CD123) at 1:400 plus CD68 (Dako M0814) at 1:16K double staining sequentially. Staining was done manually by using Pekin Elmer Opal 7 TIL Panel B Kit (Opal 7B). Heat-induced epitope retrieval was done for 15 minutes with AR9 buffer. Incubation time with the primary antibody was 30 min. followed by 30 min incubation with polymer HRP, then add on opal 520 (FITC) for 10min. for CD30, CD123 and opal 570 (Texas Red) for CD68. All the experiment was done at room temperature. Slides are washed three times between each step with 1XTBS buffer or water.
Gene expression analysis
To interrogate gene expression in primary HL and in multiple HL derived cell lines, the BioGPS web-tool was used (http://biogps.org/). For primary HL, gene expression values of micro-dissected HRS cells (n=29 patients) were compared to normal micro-dissected germinal center B cells (n=5 patients) from Affymetrix GeneChip data (dataset GSE-39133) (39). For HL derived cell line gene expression assessment, five HL cell lines (KM-H2, L428, L-540, L-1236, and HDLM2) were compared to micro-dissected germinal center B cells from five donors (dataset GSE-39132) (39). Expression data of diagnostic non-microdissected biopsy samples from 130 HL patients (dataset GSE17920) (20) was used to correlate expression of CD123 to macrophage markers CD68 and CD11b. Statistical significance was determined by Student’s t-test.
Multiparametric flow cytometry
Flow cytometry was performed as previously described (74). Anti-human antibodies were purchased from BioLegend, eBioscience, or Becton Dickinson. For cell number quantitation, Countbright (Invitrogen) beads were used according to the manufacturer’s instructions. In all analyses, the population of interest was gated based on forward vs. side scatter characteristics followed by singlet gating, and live cells were gated using Live Dead Fixable Aqua (Invitrogen). Time gating was included for quality control. Detection of CAR123 was performed using goat-anti-mouse antibody (Jackson Laboratories) or CD123-Fc/His (Sino Biologicals) and anti-His-APC (R&D) or PE (AbCam) or directly PE-conjugated CD123 protein (Sino Biologicals and AbCam). Flow cytometry was performed on a four-laser Fortessa-LSR II cytometer (Becton-Dickinson) and analyzed with FlowJo X 10.0.7r2 (Tree Star).
Human macrophage differentiation
As shown in Figure S1 A, human macrophages (M0) were generated by differentiating positively selected CD14+ normal donor monocytes (Human CD14 MicroBeads, Miltenyi Biotech) for 7 days in RPMI-1640 (Gibco) supplemented with 10% fetal bovine serum (Gemini BioProducts), 1x Glutamax (Gibco), 10ng/mL recombinant human GM-CSF (Peprotech) and 1x penicillin/streptomycin (Lonza). Macrophages were polarized to M2 by adding either 20ng/mL human IL-4 or IL-13 (Peprotech) to the differentiation media for an additional 24 hours. HL-TAMs were generated by culturing macrophages with HDLM-2 cells or adding HDLM2 conditioned media for 24 hours post-differentiation.
Macrophage killing assay
Primary human monocyte derived macrophages were differentiated and polarized as described above. Autologous macrophages were co-cultured with untransduced (UTD) T cells, CART123, or media alone for 7 days at an E:T ratio of 1:1 in a 96-well plate. All conditions were tested in triplicate. At the end of a 7 day co-culture period, killing was assessed by 10x phase microscopy and FACS analysis. Live macrophages were identified as CD11b+LiveDeadAqua. Absolute macrophage counts were determined using CountBright Absolute Counting Beads (Thermo Fisher) and statistical analysis was done using Student’s T test.
Generation of CAR constructs and CAR T cells
The 2nd generation anti-CD123 chimeric antigen receptor (CAR123) features an anti-CD123 scFv (clone 32716), CD8 hinge, 4-1BB costimulatory domain and CD3-ζ signaling domain (Figure S3 A). (38) This construct is currently used in a clinical trial for acute myeloid leukemia at the University of Pennsylvania (NCT02623582). The murine anti-CD19 chimeric –antigen receptor (CD8 hinge, 4-1BB co-stimulatory domain and CD3 zeta signaling domain) was generated as previously described. (75, 76) This is the same construct currently used in the CART19 (CTL019) clinical trials at the University of Pennsylvania. Production of CAR-expressing T cells was performed as previously described. (38) Normal donor CD4 and CD8 T cells or PB mononuclear cells (PBMC) were obtained from the Human Immunology Core of the University of Pennsylvania. Prior to all experiments, T cells were thawed and rested overnight at 37° C.
In vitro T-cell effector function assays
Degranulation, CFSE proliferation, cytotoxicity assays and cytokine measurements were performed as previously described. (10, 38, 77) Phase contrast images of human macrophage and T cell co-culture (at 24 hours) were generated using the 20x lens on a Nikon Eclipse Ti-S microscope (Nikon Instruments, Inc.)
Animal experiments
In vivo experiments were performed as previously described. (74) Schemas of the utilized xenograft models are discussed in details in the relevant figures. NOD-SCID gamma chain deficient (NSG) mice originally obtained from Jackson Laboratories were purchased from the Stem Cell and Xenograft Core of the University of Pennsylvania. Cells (HL cell lines or T cells) were injected in 100–200 ul of PBS at the indicated concentration into the tail veins of mice. Bioluminescent imaging was performed using a Xenogen IVIS-200 Spectrum camera and analyzed with LivingImage software v. 4.3.1 (Caliper LifeSciencies). Animals were euthanized at the end of the experiment or when they met pre-specified endpoints according to the IACUC protocols.
Study approval
Animal experiments were performed according a protocol (#803230) approved by the Institutional Animal Care and Use Committee (IACUC) that adheres to the NIH Guide for the Care and Use of Laboratory Animals.
Statistical Analysis
All statistics were performed as indicated using GraphPad Prism 6 for Windows, version 6.05 (La Jolla, CA). Two-tailed Student’s t-test was used to compare two groups; in analysis where multiple groups were compared, one-way analysis of variance (ANOVA) was performed with Holm-Sidak correction for multiple comparisons. Linear regression was used to correlate CD123 RNA expression with CD11b or CD68. When multiple groups at multiple time points/ratios were compared, Student’s t-test or ANOVA for each time points/ratios was used. Survival curves were compared using the log-rank test. In the figures asterisks are used to represent p-values (*=<0.05, **=<0.01, ***=<0.001, ****=<0.0001) and “ns” means “not significant” (p>0.05).
Supplementary Material
Statement of significance.
Anti-CD123 chimeric antigen receptor T cells target both the malignant cells and tumor-associated macrophages in Hodgkin lymphoma, thereby eliminating an important immunosuppressive component of the tumor microenvironment.
Acknowledgments
The authors would like to thank Simon Lacey, Jos Melenhorst, Fang Chen and Natalka Kengle from the University of Pennsylvania, Translational and Correlatives Studies Laboratory for performing the Luminex cytokine assays. One of the chimeric antigen receptor used in this study (CART19) was obtained under MTA from Dr. Dario Campana, Dr. Chihaya Imai and St. Jude Children’s Research Hospital and was subsequently modified by cloning into a lentiviral vector and expressed with a eukaryotic promoter. (76)
Funding: This work was supported by grants from the Univ. of Pennsylvania-Novartis Alliance (PI: C.H.J.), the NIH 5R01CA120409 grant (PI: C.H.J.), the EMD-Serono Cancer Immunotherapy Clinical Fellowship by the Society for Immunotherapy of Cancer (SITC) (PI: M.R.), the Bristol-Myers Squibb Oncology Fellowship in Clinical Cancer Research by the American Association for Cancer Research (AACR) (PI: M.R.), the Gabrielle’s Angel Foundation (PI: M.R.), the SIES-AIL fellowship by the Italian Society for Experimental Hematology and the Italian Leukemia Association (PI: M.R.) and an award from the National Cancer Institute (K12 CA090628) (PI: S.S.K.). Imaging was performed at the University of Pennsylvania Small Animal Imaging Facility (SAIF) Optical/Bioluminescence Core, supported by NIH grant CA016520. M.K. was funded by a NIH T32-GM008076.
Footnotes
Competing interests: The authors work under a research collaboration involving the University of Pennsylvania and the Novartis Institutes of Biomedical Research, Inc. M.R., S.S.K., M.W., C.H.J. and S.G. are inventors of intellectual property licensed by the University of Pennsylvania to Novartis.
References
- 1.Josting A, Franklin J, May M, Koch P, Beykirch MK, Heinz J, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin's lymphoma registered in the database of the German Hodgkin's lymphoma study group. J Clin Oncol. 2002;20:221–30. doi: 10.1200/JCO.2002.20.1.221. [DOI] [PubMed] [Google Scholar]
- 2.Santoro A, Bonadonna G, Valagussa P, Zucali R, Viviani S, Villani F, et al. Long-term results of combined chemotherapy-radiotherapy approach in Hodgkin's disease: superiority of ABVD plus radiotherapy versus MOPP plus radiotherapy. J Clin Oncol. 1987;5:27–37. doi: 10.1200/JCO.1987.5.1.27. [DOI] [PubMed] [Google Scholar]
- 3.Borchmann S, von Tresckow B. Novel agents in classical Hodgkin lymphoma. Leukemia & lymphoma. 2017:1–12. doi: 10.1080/10428194.2017.1300898. [DOI] [PubMed] [Google Scholar]
- 4.Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30:2183–9. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. The New England journal of medicine. 2015;372:311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bollard CM, Gottschalk S, Torrano V, Diouf O, Ku S, Hazrat Y, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol. 2014;32:798–808. doi: 10.1200/JCO.2013.51.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staal SP, Ambinder R, Beschorner WE, Hayward GS, Mann R. A survey of Epstein-Barr virus DNA in lymphoid tissue. Frequent detection in Hodgkin's disease. American journal of clinical pathology. 1989;91:1–5. doi: 10.1093/ajcp/91.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Weiss LM, Movahed LA, Warnke RA, Sklar J. Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin's disease. The New England journal of medicine. 1989;320:502–6. doi: 10.1056/NEJM198902233200806. [DOI] [PubMed] [Google Scholar]
- 9.Ruella M, Kalos M. Adoptive immunotherapy for cancer. Immunological reviews. 2014;257:14–38. doi: 10.1111/imr.12136. [DOI] [PubMed] [Google Scholar]
- 10.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science translational medicine. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014;371:1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and Toxicity Management of 19-28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Science translational medicine. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. The Journal of clinical investigation. 2016 doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–28. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbst H, Tippelmann G, Anagnostopoulos I, Gerdes J, Schwarting R, Boehm T, et al. Immunoglobulin and T-cell receptor gene rearrangements in Hodgkin's disease and Ki-1-positive anaplastic large cell lymphoma: dissociation between phenotype and genotype. Leukemia research. 1989;13:103–16. doi: 10.1016/0145-2126(89)90134-3. [DOI] [PubMed] [Google Scholar]
- 16.Savoldo B, Rooney CM, Di Stasi A, Abken H, Hombach A, Foster AE, et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30zeta artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007;110:2620–30. doi: 10.1182/blood-2006-11-059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Stasi A, De Angelis B, Rooney CM, Zhang L, Mahendravada A, Foster AE, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113:6392–402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramos CA, Ballard B, Liu E, Dakhova O, Mei Z, Liu H, et al. Chimeric T Cells for Therapy of CD30+ Hodgkin and Non-Hodgkin Lymphomas. Blood. 2015;126:185. [Google Scholar]
- 19.Steidl C, Connors JM, Gascoyne RD. Molecular pathogenesis of Hodgkin's lymphoma: increasing evidence of the importance of the microenvironment. J Clin Oncol. 2011;29:1812–26. doi: 10.1200/JCO.2010.32.8401. [DOI] [PubMed] [Google Scholar]
- 20.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. The New England journal of medicine. 2010;362:875–85. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Aguilera A, Montalban C, de la Cueva P, Sanchez-Verde L, Morente MM, Garcia-Cosio M, et al. Tumor microenvironment and mitotic checkpoint are key factors in the outcome of classic Hodgkin lymphoma. Blood. 2006;108:662–8. doi: 10.1182/blood-2005-12-5125. [DOI] [PubMed] [Google Scholar]
- 22.Devilard E, Bertucci F, Trempat P, Bouabdallah R, Loriod B, Giaconia A, et al. Gene expression profiling defines molecular subtypes of classical Hodgkin's disease. Oncogene. 2002;21:3095–102. doi: 10.1038/sj.onc.1205418. [DOI] [PubMed] [Google Scholar]
- 23.Mizuno H, Nakayama T, Miyata Y, Saito S, Nishiwaki S, Nakao N, et al. Mast cells promote the growth of Hodgkin's lymphoma cell tumor by modifying the tumor microenvironment that can be perturbed by bortezomib. Leukemia. 2012;26:2269–76. doi: 10.1038/leu.2012.81. [DOI] [PubMed] [Google Scholar]
- 24.Huber S, Hoffmann R, Muskens F, Voehringer D. Alternatively activated macrophages inhibit T-cell proliferation by Stat6-dependent expression of PD-L2. Blood. 2010;116:3311–20. doi: 10.1182/blood-2010-02-271981. [DOI] [PubMed] [Google Scholar]
- 25.Guo B, Cen H, Tan X, Ke Q. Meta-analysis of the prognostic and clinical value of tumor-associated macrophages in adult classical Hodgkin lymphoma. BMC Med. 2016;14:159. doi: 10.1186/s12916-016-0711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez FO, Helming L, Milde R, Varin A, Melgert BN, Draijer C, et al. Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood. 2013;121:e57–69. doi: 10.1182/blood-2012-06-436212. [DOI] [PubMed] [Google Scholar]
- 27.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 28.Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Frontiers in immunology. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fromm JR. Flow cytometric analysis of CD123 is useful for immunophenotyping classical Hodgkin lymphoma. Cytometry B Clin Cytom. 2011;80:91–9. doi: 10.1002/cyto.b.20561. [DOI] [PubMed] [Google Scholar]
- 30.Liu K, Zhu M, Huang Y, Wei S, Xie J, Xiao Y. CD123 and its potential clinical application in leukemias. Life sciences. 2015;122:59–64. doi: 10.1016/j.lfs.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Hassanein NM, Alcancia F, Perkinson KR, Buckley PJ, Lagoo AS. Distinct expression patterns of CD123 and CD34 on normal bone marrow B-cell precursors ("hematogones") and B lymphoblastic leukemia blasts. American journal of clinical pathology. 2009;132:573–80. doi: 10.1309/AJCPO4DS0GTLSOEI. [DOI] [PubMed] [Google Scholar]
- 32.Djokic M, Bjorklund E, Blennow E, Mazur J, Soderhall S, Porwit A. Overexpression of CD123 correlates with the hyperdiploid genotype in acute lymphoblastic leukemia. Haematologica. 2009;94:1016–9. doi: 10.3324/haematol.2008.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aldinucci D, Poletto D, Gloghini A, Nanni P, Degan M, Perin T, et al. Expression of functional interleukin-3 receptors on Hodgkin and Reed-Sternberg cells. The American journal of pathology. 2002;160:585–96. doi: 10.1016/S0002-9440(10)64878-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aldinucci D, Olivo K, Lorenzon D, Poletto D, Gloghini A, Carbone A, et al. The role of interleukin-3 in classical Hodgkin's disease. Leukemia & lymphoma. 2005;46:303–11. doi: 10.1080/10428190400013712. [DOI] [PubMed] [Google Scholar]
- 35.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–70. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tudor CS, Bruns H, Daniel C, Distel LV, Hartmann A, Gerbitz A, et al. Macrophages and dendritic cells as actors in the immune reaction of classical Hodgkin lymphoma. PloS one. 2014;9:e114345. doi: 10.1371/journal.pone.0114345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gustafson MP, Lin Y, Maas ML, Van Keulen VP, Johnston PB, Peikert T, et al. A method for identification and analysis of non-overlapping myeloid immunophenotypes in humans. PloS one. 2015;10:e0121546. doi: 10.1371/journal.pone.0121546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gill S, Tasian SK, Ruella M, Shestova O, Li Y, Porter DL, et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123:2343–54. doi: 10.1182/blood-2013-09-529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steidl C, Diepstra A, Lee T, Chan FC, Farinha P, Tan K, et al. Gene expression profiling of microdissected Hodgkin Reed-Sternberg cells correlates with treatment outcome in classical Hodgkin lymphoma. Blood. 2012;120:3530–40. doi: 10.1182/blood-2012-06-439570. [DOI] [PubMed] [Google Scholar]
- 40.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 41.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators of inflammation. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Georgoudaki AM, Prokopec KE, Boura VF, Hellqvist E, Sohn S, Ostling J, et al. Reprogramming Tumor-Associated Macrophages by Antibody Targeting Inhibits Cancer Progression and Metastasis. Cell reports. 2016;15:2000–11. doi: 10.1016/j.celrep.2016.04.084. [DOI] [PubMed] [Google Scholar]
- 45.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17:1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruella M, June CH. Chimeric Antigen Receptor T cells for B Cell Neoplasms: Choose the Right CAR for You. Curr Hematol Malig Rep. 2016 doi: 10.1007/s11899-016-0336-z. [DOI] [PubMed] [Google Scholar]
- 47.Trieu Y, Wen XY, Skinnider BF, Bray MR, Li Z, Claudio JO, et al. Soluble interleukin-13Ralpha2 decoy receptor inhibits Hodgkin's lymphoma growth in vitro and in vivo. Cancer research. 2004;64:3271–5. doi: 10.1158/0008-5472.can-03-3764. [DOI] [PubMed] [Google Scholar]
- 48.Skinnider BF, Kapp U, Mak TW. The role of interleukin 13 in classical Hodgkin lymphoma. Leukemia & lymphoma. 2002;43:1203–10. doi: 10.1080/10428190290026259. [DOI] [PubMed] [Google Scholar]
- 49.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nature medicine. 2003;9:279–86. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 50.Younes A, Oki Y, McLaughlin P, Copeland AR, Goy A, Pro B, et al. Phase 2 study of rituximab plus ABVD in patients with newly diagnosed classical Hodgkin lymphoma. Blood. 2012;119:4123–8. doi: 10.1182/blood-2012-01-405456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kasamon YL, Jacene HA, Gocke CD, Swinnen LJ, Gladstone DE, Perkins B, et al. Phase 2 study of rituximab-ABVD in classical Hodgkin lymphoma. Blood. 2012;119:4129–32. doi: 10.1182/blood-2012-01-402792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hwang K, Park CJ, Jang S, Chi HS, Huh JR, Lee JH, et al. Immunohistochemical analysis of CD123, CD56 and CD4 for the diagnosis of minimal bone marrow involvement by blastic plasmacytoid dendritic cell neoplasm. Histopathology. 2013;62:764–70. doi: 10.1111/his.12079. [DOI] [PubMed] [Google Scholar]
- 53.Del Giudice I, Matutes E, Morilla R, Morilla A, Owusu-Ankomah K, Rafiq F, et al. The diagnostic value of CD123 in B-cell disorders with hairy or villous lymphocytes. Haematologica. 2004;89:303–8. [PubMed] [Google Scholar]
- 54.Munoz L, Nomdedeu JF, Lopez O, Carnicer MJ, Bellido M, Aventin A, et al. Interleukin-3 receptor alpha chain (CD123) is widely expressed in hematologic malignancies. Haematologica. 2001;86:1261–9. [PubMed] [Google Scholar]
- 55.Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777–84. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- 56.Riccioni R, Rossini A, Calabro L, Diverio D, Pasquini L, Lococo F, et al. Immunophenotypic features of acute myeloid leukemias overexpressing the interleukin 3 receptor alpha chain. Leukemia & lymphoma. 2004;45:1511–7. doi: 10.1080/104281090310001646031. [DOI] [PubMed] [Google Scholar]
- 57.Testa U, Torelli GF, Riccioni R, Muta AO, Militi S, Annino L, et al. Human acute stem cell leukemia with multilineage differentiation potential via cascade activation of growth factor receptors. Blood. 2002;99:4634–7. doi: 10.1182/blood.v99.12.4634. [DOI] [PubMed] [Google Scholar]
- 58.Brooks CL, Cirrito TP, Hoberman K, Rowinsky E. SL-101, a Novel Monoclonal Antibody-Conjugate That Targets Interleukin-3 Receptor Alpha (CD123), Possesses Preclinical Anti-Tumor Activity Against Hodgkin's Lymphoma. Blood. 2012;120:2768. [Google Scholar]
- 59.Diefenbach CS, Sabado R, Brooks CL, Baquero-Buitrago J, Cruz C, Vengco I, et al. Hodgkin's Lymphoma Cell Lines Have up-Regulated IL-3 Receptor α (IL-3Rα) Expression and Are Sensitive to SL-401, An IL-3Rα Targeted Drug. Blood. 2011;118:3737. [Google Scholar]
- 60.Angelot-Delettre F, Roggy A, Frankel AE, Lamarthee B, Seilles E, Biichle S, et al. In vivo and in vitro sensitivity of blastic plasmacytoid dendritic cell neoplasm to SL-401, an interleukin-3 receptor targeted biologic agent. Haematologica. 2015;100:223–30. doi: 10.3324/haematol.2014.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohen KA, Liu TF, Cline JM, Wagner JD, Hall PD, Frankel AE. Safety evaluation of DT388IL3, a diphtheria toxin/interleukin 3 fusion protein, in the cynomolgus monkey. Cancer immunology, immunotherapy : CII. 2005;54:799–806. doi: 10.1007/s00262-004-0643-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frolova O, Benito J, Brooks C, Wang RY, Korchin B, Rowinsky EK, et al. SL-401 and SL-501, targeted therapeutics directed at the interleukin-3 receptor, inhibit the growth of leukaemic cells and stem cells in advanced phase chronic myeloid leukaemia. British journal of haematology. 2014;166:862–74. doi: 10.1111/bjh.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He SZ, Busfield S, Ritchie DS, Hertzberg MS, Durrant S, Lewis ID, et al. A Phase 1 study of the safety, pharmacokinetics and anti-leukemic activity of the anti-CD123 monoclonal antibody CSL360 in relapsed, refractory or high-risk acute myeloid leukemia. Leukemia & lymphoma. 2014:1–10. doi: 10.3109/10428194.2014.956316. [DOI] [PubMed] [Google Scholar]
- 64.Zereshkian A, Leyton JV, Cai Z, Bergstrom D, Weinfeld M, Reilly RM. The human polynucleotide kinase/phosphatase (hPNKP) inhibitor A12B4C3 radiosensitizes human myeloid leukemia cells to Auger electron-emitting anti-CD123 (1)(1)(1)In-NLS-7G3 radioimmunoconjugates. Nuclear medicine and biology. 2014;41:377–83. doi: 10.1016/j.nucmedbio.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 65.Kuo SR, Wong L, Liu JS. Engineering a CD123xCD3 bispecific scFv immunofusion for the treatment of leukemia and elimination of leukemia stem cells. Protein engineering, design & selection : PEDS. 2012;25:561–9. doi: 10.1093/protein/gzs040. [DOI] [PubMed] [Google Scholar]
- 66.Chichili GR, Huang L, Li H, Burke S, He L, Tang Q, et al. A CD3xCD123 bispecific DART for redirecting host T cells to myelogenous leukemia: Preclinical activity and safety in nonhuman primates. Science translational medicine. 2015;7:289ra82. doi: 10.1126/scitranslmed.aaa5693. [DOI] [PubMed] [Google Scholar]
- 67.Fan D, Li Z, Zhang X, Yang Y, Yuan X, Zhang X, et al. AntiCD3Fv fused to human interleukin-3 deletion variant redirected T cells against human acute myeloid leukemic stem cells. Journal of hematology & oncology. 2015;8:18. doi: 10.1186/s13045-015-0109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mardiros A, Dos Santos C, McDonald T, Brown CE, Wang X, Budde LE, et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122:3138–48. doi: 10.1182/blood-2012-12-474056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tettamanti S, Magnani CF, Biondi A, Biagi E. Acute myeloid leukemia and novel biological treatments: monoclonal antibodies and cell-based gene-modified immune effectors. Immunology letters. 2013;155:43–6. doi: 10.1016/j.imlet.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 70.Luo Y, Chang L-J, Hu Y, Dong L, Wei G, Huang H. First-in-Man CD123-Specific Chimeric Antigen Receptor-Modified T Cells for the Treatment of Refractory Acute Myeloid Leukemia. Blood. 2015;126:3778. [Google Scholar]
- 71.Frankel AE, Woo JH, Ahn C, Pemmaraju N, Medeiros BC, Carraway HE, et al. Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood. 2014;124:385–92. doi: 10.1182/blood-2014-04-566737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He SZ, Busfield S, Ritchie DS, Hertzberg MS, Durrant S, Lewis ID, et al. A Phase 1 study of the safety, pharmacokinetics and anti-leukemic activity of the anti-CD123 monoclonal antibody CSL360 in relapsed, refractory or high-risk acute myeloid leukemia. Leukemia & lymphoma. 2015;56:1406–15. doi: 10.3109/10428194.2014.956316. [DOI] [PubMed] [Google Scholar]
- 73.Tasian SK, Kenderian SS, Shen F, Ruella M, Shestova O, Kozlowski M, et al. Optimized Depletion of Chimeric Antigen Receptor T-Cells in Murine Xenograft Models of Human Acute Myeloid Leukemia. Blood. 2017 doi: 10.1182/blood-2016-08-736041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kenderian SS, Ruella M, Shestova O, Klichinsky M, Aikawa V, Morrissette JJ, et al. CD33 Specific Chimeric Antigen Receptor T Cells Exhibit Potent Preclinical Activity against Human Acute Myeloid Leukemia. Leukemia. 2015 doi: 10.1038/leu.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17:1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–84. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 77.Ruella M, Kenderian SS, Shestova O, Fraietta JA, Qayyum S, Zhang Q, et al. The Addition of the BTK inhibitor Ibrutinib to Anti-CD19 Chimeric Antigen Receptor T Cells (CART19) Improves Responses against Mantle Cell Lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016 doi: 10.1158/1078-0432.CCR-15-1527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.