Abstract
Objective
Weight-loss surgery results in significant changes in the anatomy, function and intraluminal environment of the gastrointestinal tract affecting the gut microbiome. While bariatric surgery results in sustained weight loss, decreased appetite and hedonic eating; it is unknown if the surgery-induced alterations in gut microbiota play a role in the observed changes in hedonic eating. We explored the following hypotheses 1) Laparoscopic sleeve gastrectomy results in changes in gut microbial composition ; 2) Alterations in gut microbiota are related to weight loss; 3) Alterations in gut microbiome are associated with changes in appetite and hedonic eating.
Methods
Eight obese women underwent LSG. Their BMI, body fat mass, food intake, hunger, hedonic eating scores, and stool samples were obtained at baseline and 1-month post-surgery. 16S ribosomal RNA gene sequencing was performed on stool samples. DESeq2 for changes in microbial abundance. Multilevel-sparse partial least squares discriminant analysis was applied to genus level abundance for discriminative microbial signatures.
Results
LSG resulted in significant reductions in BMI, food intake and hedonic eating. A microbial signature comprised of 5 bacterial genera discriminated between pre and post-surgery status. Several bacterial genera were significantly associated with weight loss (Bilophila (q=3E-05), Faecalibacterium (q=4E-05)), lower appetite (Enterococcus, q=3E-05) and reduced hedonic eating (Akkermansia, q=0.037) after surgery.
Conclusions
In this preliminary analysis, changes in gut microbial abundance discriminated between pre and postoperative status. Alterations in gut microbiome were significantly associated with weight loss and with reduced hedonic eating after surgery, however a larger sample is needed to confirm these findings.
Keywords: bariatric surgery, gut microbiome, hedonic eating, sleeve gastrectomy, appetite, food addiction
INTRODUCTION
Bariatric surgery is currently the most effective intervention for the treatment of obesity (1). The mechanisms underlying bariatric surgery- induced weight loss are complex, and are poorly understood. Implicated mechanisms include alterations in the anatomy, physiology and microenvironment of the digestive tract, which in turn result in changes in gut microbial composition and function (2–5). Remarkably, the transplantation of fecal microbiota from a post-bariatric surgery donor is able to confer weight and fat mass reduction to germ-free recipient mice (6,7). These findings support the idea that inherent properties of the post-bariatric gut microbiome are able to influence weight and metabolism. While multiple mechanisms may be involved in mediating these microbiome related effects, the possible role of changes in gut-microbiota-brain interactions affecting ingestive behaviors and hedonic eating have not been studied.
The brain-gut-microbiome axis is a bidirectional communication system which plays a central role in the maintenance of homeostasis through neuronal pathways involving the brain, the vagus and/or spinal nerves and the enteric nervous system, as well as through signaling pathways involving hormones, neuropeptides, cytokines and gut and microbe-derived metabolites. Comprehensive reviews of the brain-gut-microbiome axis structure and function are available elsewhere (8–10). There is a large body of preclinical evidence supporting a role of the gut microbiota in the regulation of anxiety, mood and appetite (11–15). In addition, fecal transplantation was able to transfer changes in eating behaviors (hyperphagia) in an animal model of metabolic syndrome (16), suggesting that the gut microbiota may also influence complex human behaviors such as hedonic eating. Increased hedonic responses to highly-palatable foods have been reported in obese individuals (17–19). Several bariatric procedures including gastric bypass (RYGB), duodenal-jejunal bypass and vertical sleeve gastrectomy have shown to decrease hedonic responses to highly-palatable foods, and to affect dopamine release in the striatum (20–23).
By assessing changes in food intake and hedonic eating after bariatric surgery and their relationship with the gut microbiome, we aimed to test the following hypotheses: 1) Laparoscopic sleeve gastrectomy (LSG) results in distinct changes in gut microbial composition that are able to discriminate between pre and post surgical status; 2) Alterations in gut microbial composition are related to weight loss and; 3) These gut microbial changes are associated with changes in ingestive behaviors that are conducive to weight loss.
METHODS
Study sample
Eight adult obese right-handed female subjects (age: 39.5±8.7yrs, BMI: 44.1±5.6 kg/m2) were recruited before undergoing LSG at the UCLA Ronald Reagan Medical Center in Los Angeles, from June 2014 to June 2015. All selected subjects underwent a preoperative evaluation by a multidisciplinary team including nutritionists, bariatric surgeons, gastroenterologists, and psychologists at the Center for Obesity and Metabolic Health -COMET- following the American Gastrointestinal and Endoscopic Surgeons guidelines for bariatric surgery (24). We excluded subjects with prior major gastrointestinal surgery, history of neurological/mental disease including eating disorders, or who were using medications known to affect appetite or psychotropic medications, who were pregnant, had any contraindication for undergoing MRI, or had received antibiotics or probiotics within 3 months of inclusion. LSG was performed by one of the two bariatric surgeons (ED, YC) according to a standardized procedure. Postoperative diet progressed gradually from liquid to soft diet with a return to solid foods around 7 weeks after surgery.
The study was approved by the Biomedical Institutional Review Board at the University of California at Los Angeles (UCLA). All participants provided written consent before study participation.
Anthropometrics and Body Composition
A certified nutritionist at the UCLA Clinical & Translational Research Center obtained measures for height, weight, BMI and waist circumference using techniques and methods described in NHANES III at baseline and at 1 month after surgery. A wall-mounted standard stadiometer (model PE-WM-60-84; Perspective Enterprises, Inc.) was used to measure standing height to the nearest 0.1 cm. An electronic scale (5002 Stand-On Scale; Scale-Tronix, Inc.) was used obtain body weight to the nearest 0.1 kg. Body mass index (BMI) was determined by dividing weight in kg by height in meters squared. All measurements were taken in duplicate for better accuracy.
In addition, all subjects underwent a Bioelectrical impedance analysis (BIA) using a body composition analyzer (BIA Model 450 Bioimpedance Analyzer; Biodynamics, Inc.) to assess body composition using electrical tissue conductivity and provide estimates of percent body fat and lean body mass.
Dietary Assessment
Dietary intake data were measured prospectively to obtain information about eating habits, energy, and nutrients. Subjects were provided detailed instructions to record all food and beverage consumed over a three-day period both at baseline (before surgery) and 1 month after surgery. Subject entries were reviewed for accuracy by a trained nutrition professional and entered into a nutrition analysis software (Food Processor 10.15, Esha Research) to generate a report of estimated energy and macronutrient values.
Hunger and satiety ratings
All subjects completed two validated visual analog scales (VAS) for hunger and satiety after overnight fasting. The VAS was a 100-mm line with a phrase at each end describing the extremes. Subjects were told to make a mark across the line corresponding to their perceptions of hunger and satiety and quantification was done by measuring the distance from the left end of the scale to the mark.
Hedonic eating assessment
All subjects filled out a questionnaires designed to measure food addiction including the Yale Food Addiction Scale (YFAS), a 25-item scale developed to measure food addiction by assessing signs of substance-dependence symptoms (e.g., tolerance, withdrawal, loss of control) in eating behavior. The YFAS has displayed a good internal reliabitlity (Kuder–Richardson α = .86) (25). They also rated their desire to eat or “wanting” that reflects the motivational aspect of ingestive behavior. All subjects were shown pictures that depicted 4 different types of foods (high-calorie: sweets and savory food; and low calorie: fruits and salads), shown as 3 runs of each type of food and each run consisted of 4 pictures. After each run, subjects were asked to rate their experience as “how much did you want to eat what you just saw?”, on a scale from 0 to 100, zero being ‘not at all’ and 100 being ‘very much. Ratings were averaged across all runs from each condition to obtain an overall desire to eat rating for each stimuli type (high- vs. low-calorie foods; and sweets vs. savory foods vs. fruit vs. salads) for each participant, pre- and post surgery.
Microbiome composition assessment: 16S ribosomal RNA gene sequencing
Stool samples were collected at baseline and at 1-month after surgery. The frozen fecal samples were ground with mortar and pestle in the presence of liquid nitrogen, then aliquoted. Genomic DNA was extracted using the Powersoil kit as per then manufacturer’s instructions (MoBio). The V4 region of 16S ribosomal RNA genes was amplified and underwent paired end sequencing on an Illumina HiSeq 2500 as previously described (26). The 253 base pair reads were processed using QIIME v1.9.1 with default parameters (27). Observable Taxonomic Units (OTUs) were picked against the May 2013 version of the Greengenes database, pre-filtered at 97% identity. Sequence depth ranged from 499,588 to 1,070,571.
Diversity analysis
Alpha diversity (i.e. bacterial diversity within a sample) and beta diversity (differences in composition across samples) were calculated in QIIME v1.9.1 using data rarefied to 499,588 sequences. Alpha diversity metrics included Faith’s phylogenetic diversity metric, Chao1, and Shannon index. Pre-post surgery differences in alpha diversity were calculated using a nonparametric t-test and 1000 Monte Carlo permutations were used to calculate the nonparametric p-value. Beta diversity was calculated using unweighted and weighted UniFrac for all pairwise combinations of pre- and post-surgery samples. Adonis analysis, a permutational analysis of variance, was performed to test for differences in overall microbial composition pre- and post-surgery.
Differences in taxonomic abundance of the gut microbiome
Differences in phylum and genus abundance between pre- and post- surgery samples were evaluated using DESeq2 in R (28). Unrarefied 16S rRNA count data were first filtered to remove OTUs present in only one sample then fitted to multivariate negative binomial models with surgery status (before and after) and subject as covariates. P-values for differential abundance between preoperative vs. postoperative status were converted to q-values to correct for multiple hypothesis testing (< 0.05 for significance) (29). The significance of the Firmicutes/Bacteroidetes ratio was determined using the Mann-Whitney U test.
Multi-level sparse Partial Least Square-Discriminant Analysis (sPLS-DA) was applied to identify genus-level taxa specific able to discriminate the effects of surgery within individuals. This method accounts for repeated measurements from pre- and post- treatment assays and highlights the effect of treatment within subject separately from the biological variation between subjects (30). This projection-based technique simultaneously performs feature selection and modelling and achieves sparsity using lasso penalization (31). sPLS-DA operates using a supervised framework to find linear combinations of a limited set of variables, here genus level taxa, that predict pre- and post-surgery status. We refer to each linear combination or component as discriminatory “taxa signature”. sPLS-DA is our method of choice given its good classification performance and its ability to deal with a large number of predictors, small sample size, and high co-linearity among predictors (32,33). sPLS-DA was performed using the R package mixOmics (http://www.R-project.org).
As recommended, the number of components to identify was fixed at 1 (32.33). To select the optimal number of features for this component we estimated the leave-one-out classification error with respect to a range of number of features (5 to 200 by units of 5). This process was repeated 50 times and the results averaged. This so-called “tuning” procedure indicated that 1 component model comprised of 5 features would be optimal.
The microbial signatures were summarized using variable loadings on the components and VIP coefficients. Each selected variable has an associated “loading” indexing the relative importance of that variable in the component for group discrimination (32). Variable importance in projection (VIP) scores is a standardized measure that represents contribution of each feature relative to the variance explained by all components (33). As a rule of thumb, predictors with VIP coefficients greater than one are considered particularly important for the discrimintation (32). We also use graphical displays to illustrate the discriminative abilities of the algorithms (33).
Statistical analyses
Statistical analyses were calculated using R or SPSS version 23 (SPSS Inc., USA). The Shapiro-Wilks test was used to check for normality, and paired t-tests were used for changes in measures of obesity (BMI and body fat mass), appetite (fasting and postprandial hunger levels), food addiction (YFAS symptom count score, reported desire to eat high and low-calorie foods), and food intake (daily calorie intake: total, from fats, from carbohydrates and from proteins) and measures of hedonic eating (YFAS, want to eat sweets. Associations between baseline and surgically induced changes in the clinical parameters described above and gut microbiota abundance were explored using Pearson’s and Spearman’s correlations, for normally distributed variables vs. non-parametric variables (bacterial genera abundance), respectively. Statistical computations were considered significant when the resulting p values were <0.05.
RESULTS
Effects of bariatric surgery on measures of obesity, appetite and food intakeIn this sample, the mean average excess weight loss achieved at 1 month after surgery was 27.4±11.4%, corresponding to a mean weight loss of 12.6 kg. LSG resulted in a marked reduction in all measures of obesity, and in measures ofappetite, food intake and hedonic eating as described in Table 1. Before surgery, subjects gave higher ratings to high-calorie foods and sweet-foods than to low-calorie foods when rating their subjective desire to eat these types of foods. Baseline YFAS scores were associated with the reported desire to eat high-calorie foods (r=0.869, p=0.011) and displayed an even stronger but inverse correlation with the reported desire to eat low-calorie foods (r=-0.925, p<0.001). These associations between YFAS scores and desire to eat either high or low-calorie food were attenuated after surgery (r=-0.352, p=0.393 for high-calorie foods and r=-0.400, p=0.326 for low-calorie foods). In this sample, no associations were found between measures of food addiction and measures of appetite at baseline.
TABLE 1.
Effect of bariatric surgery on measures of obesity, appetite and hedonic eating.
| Variable | Before surgery (mean±SD) N=8 |
After surgery (mean±SD) N=8 |
P value |
|---|---|---|---|
| Obesity measures | |||
| BMI (kg/m2) | 43.4±6.0 | 38.8±6.3 | **<0.001 |
| Body fat mass (kg) | 52.9±14.1 | 44.8±13.5 | *0.009 |
| Appetite measures | |||
| Hunger at fast | 72.5±10.3 | 41.25±24.7 | *0.003 |
| Food intake (calories/day) | 1837.5±514.7 | 676.5±350.7 | *0.006 |
| Fat intake (calories/day) | 658.9±257.2 | 201.8±169.8 | *0.012 |
| Carbohydrates intake (calories/day) | 830.2±249.4 | 262.8±183.0 | *0.007 |
| Hedonic eating measures | |||
| YFAS score (symptom count) | 4.3±2 | 1.1±0.3 | *0.015 |
| Desire to eat high-calorie foods | 7.5±1.2 | 4.5±2.0 | *0.018 |
| Desire to eat low-calorie foods | 5.1±1.7 | 5.5±1.6 | 0.734 |
Data were collected at baseline and 1-month after surgery. Surgery had a profound effect in measures of obesity, appetite and hedonic eating. YFAS (Yale Food Addiction Scale)
Significant, p<0.05
Significant, p<0.001
Early effects of bariatric surgery on gut microbiota composition
To investigate the early effects of Laparoscopic Sleeve Gastrectomy on the gut microbiota, we obtained fecal samples from 6 of the subjects, 1 month before and 1 month after the procedure. These stool samples underwent sequencing of the 16S ribosomal RNA gene, a marker of bacterial taxonomy that can be used to characterize the composition of the microbiome. The significance of differences before and after surgery in taxonomic profiles at the phylum and genus levels was determined using DESeq2. This algorithm normalizes the sequence data across samples, shrinks dispersion of the count data, and fits the data to negative binomial models (one model for each taxon). The results of this analysis are expressed as a magnitude of change (log2 fold change = log2FC) and as significance (p-values are adjusted to q-values to correct for multiple hypothesis testing). LSG produced changes in the gut microbiota abundance at the phylum level, with a highly significant increase in Fusobacteria (log2FC=4.0, q=4.1E-07) and a significant reduction in the Firmicutes/Bacteroidetes ratio (p=0.008) due to decreased Firmicutes (log2FC=-0.71, q=0.0055) after surgery (Fig. 1). There was no change in alpha diversity post-surgery. Beta diversity analysis using weighted UniFrac showed a trend towards a difference after surgery with p=0.056 by Adonis. Multivariate models demonstrated differential abundance of four genera after surgery, including decreased unclassified Bifidobacteriaceae (log2FC=-8.4, q=0.0003) and increased Fusobacterium (log2FC=4.3, q=0.0005), Atopobium (log2FC=4.1, q=0.01), and Bulleidia (log2FC=3.8, q=0.04).
Figure 1. Changes in the relative abundance of gut microbiota after Sleeve Gastrectomy.
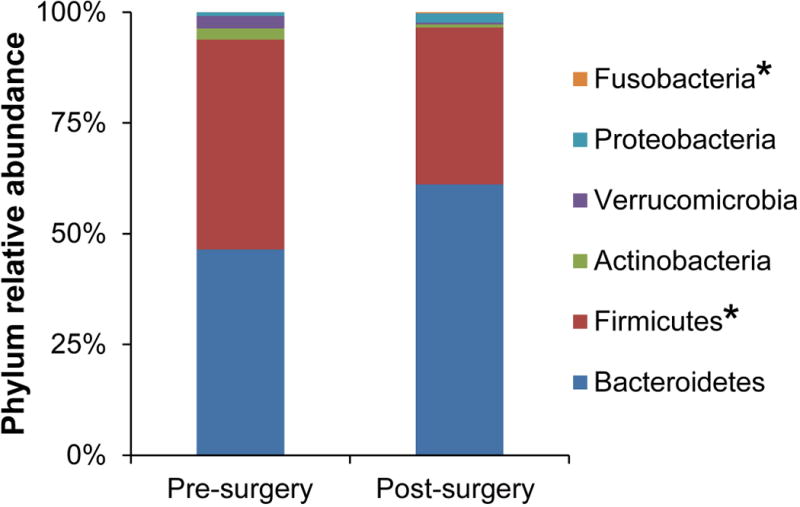
LSG results in changes in the relative abundance at the phylum level. The relative abundance of only 2 of the major phyla (Firmicutes and Fusobacteria) changed significantly after surgery. *q<0.05
Gut microbial signature as related to surgical status and changes in weight, and eating behaviors
Supervised learning methods were applied to identify a distinctive microbial signature comprised of 5 genus level taxa that differentiated between preoperative and postoperative status (Fig. 2). In this preliminary analysis, the signature accounted for 90% of variance in the discrimination and was comprised of 5 bacteria including Atopobium (Actinobacteria phylum), Bacteroides (Bacteroidetes), Bulleidia (Firmicutes), Epulopiscium (Firmicutes), and TG5 (Synergistetes). The variable loadings, and their VIP coefficients for the signature are summarized in Table 2. These are measures of how much each genus contributes to differentiating pre and post-surgical status. Noticeably all members of this signature experienced a rise in their abundance after surgery.
Figure 2. Gut microbial signature discriminates surgical status.
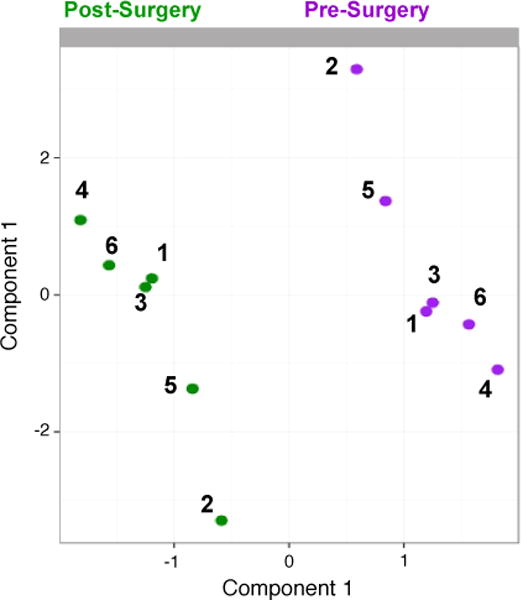
Sample representation from multilevel sPLS-DA. Samples were projected onto a subspace spanned by the first and second sPLS-DA component, based on the 5 genus-level taxa selected on the components. Dots and numbers represent individuals, purple color codes for pre-operative status and green color for post-operative status.
TABLE 2. List of the genera comprising the discriminative microbial signature.
A microbial signature that discriminates between pre- and postsurgical status was found after analyzing stool samples from 6 participants. The discriminative microbial signature is summarized in this table using variable loadings and variable importance in projection (VIP) coefficients. Each variable loading indexes the relative importance of that genu in the signature for group discrimination between pre and post-surgical status and VIP scores represent contribution of each feature relative to the variance explained by all selected signatures. Negative loadings indicate increase in relative abundance of that genus after surgery.
| Genus | VIP | Load |
|---|---|---|
| Atopobium | 10.19953 | −0.89802 |
| Bacteroides | 4.653624 | −0.40973 |
| Bulleidia | 1.32918 | −0.11703 |
| TG5 | 1.069011 | −0.09412 |
| Epulopiscium | 0.635539 | −0.05596 |
Postoperative changes in the relative abundance of members of the microbial signature correlated with changes in clinical variables. For example, Atopobium was associated with changes in BMI (rho=-0.943, p=0.004) and body fat mass (rho=-0.828, p=0.041) and similar findings were seen for TG5 (BMI: rho=-0.885, p=0.012; body fat mass: rho=-0.886, p=0.019), a genus within the Dethiosulfovibrionaceae family. Reduced desire to eat sweets post-surgery was inversely correlated with changes in the abundance of Bulleidia (rho=-0.841, p=0.036) and TG5 (rho=-0.812, p=0.049).
Microbial interactions with weight loss and eating behaviors
We explored whether intestinal bacterial profiles were associated with BMI, body composition (body fat mass), hunger levels and measures of food addiction (YFAS and reported desire to eat sweets) using multivariate models adjusting for subject’s ID and surgical status. Twenty bacterial genera displayed at least one significant association at a rate for false discovery less than 5% (q<0.05). Figure 3 summarizes the findings for the bacteria belonging to a known genus. Noticeably, Enterococcus showed a broad spectrum of associations including direct correlations with BMI (log2FC= 0.13, q=0.044), body fat mass (log2FC= 0.094, q=0.034) and hunger levels (log2FC= 0.17, q=3E-05).
Figure 3. Associations between gut microbial genera and changes in clinical variables at 1 month after LSG.
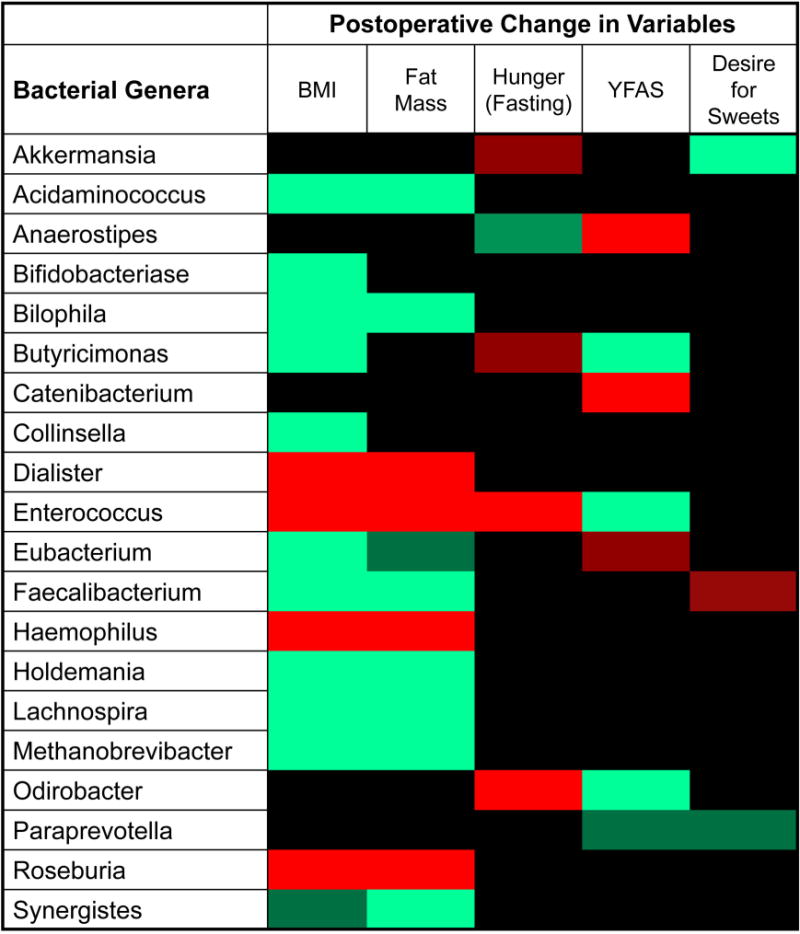
This heatmap shows the significant associations between bacterial genera and post-surgical changes in clinical variables after LSG. Correlations were adjusted for multiple hypothesis testing and considered significant at a false discovery rate of 5%. The red color indicates significant positive correlations, the green color indicates significant negative correlations, and the black color indicates non-significant correlations. Darker shades of red or green indicate correlations with false discovery rate between 5 and 10%.
In this study, gut bacteria profiles associated with surgically induced changes in BMI and body fat mass were similar but not identical. The postoperative drop in BMI was significantly associated with 20 bacterial genera, several of them with strong inverse associations such as Bilophila (log2FC= -0.25, q=3E-05), Faecalibacterium (log2FC= -0.249, q=4E-05), Lachnospira (log2FC= -0.205, q=0.003), and Acidaminococcus (log2FC=- 0.187, q=0.003). Reduction in body fat mass was related to 16 bacterial genera, with Bilophila again the most significant (log2FC=- 0.145, q=3E-05). Hunger levels were associated with Enterococcus (log2FC= 0.172, q=3E-05), Odoribacter (log2FC= 0.125, q=0.044), and Anaerostipes (log2FC=- 0.112, q=0.051). Gut microbial profiles were also associated with hedonic eating behaviors as shown in Figure 3.
DISCUSSION
The main findings of this preliminary analysis were: 1) Laparoscopic Sleeve Gastrectomy results in distinct changes in gut microbiota composition which were able to discriminate between the pre- and post-surgery state. 2) Surgically induced alterations in gut microbial profiles were related to weight loss and reductions in body fat mass. 3) Gut microbial profiles were associated with surgically induced reductions in appetite and in food addiction measures.
Effect of sleeve gastrectomy on microbial signatures
Consistent with published data, we did not find large changes in diversity of the gut microbiota one month after sleeve gastrectomy, suggesting less dramatic alteration of the gut microbiome by sleeve gastrectomy, possibly related to the limited anatomical derangement of the GI tract when compared to gastric bypass (34,35). Remarkably, by using supervised machine learning methods we were able to identify a group of bacteria which changes in relative abundance discriminated between the pre and post-surgery status. This microbial signature was comprised of Bacteroides, Atopobium, TG5, Bulleidia and Epulopiscium, all of which bloomed after LSG. It remains to be determined if this microbial signature is sustained over time and whether it is the result of adaptive changes to the “new anatomy”, or a response to postoperative changes in diet. Several members of the Bacteroides genus are highly adapted to live in the colonic mucus layer. This characteristic confers them resilience and stability within the gut microbiome by adaptively directing its glycan-foraging behavior to the mucus when dietary sources of polysaccharide are scarce (36–38). It is possible, that the observed prominence of Bacteroides after surgery reflects changes in diet and a decreased availability of intraluminal nutrients. The other members of the signature were either not present or present at very low counts before surgery; little information about their role in obesity and/or weight loss is available. Sulfate-reducing bacteria, such as Desulfovibrio spp, are important in the utilization of mucin as substrate by other bacteria. Glycan-foraging bacteria, such as Bacteroides, release sulfate during mucin degradation, enabling sulfate reducing bacteria to compete for H2 or organic compounds, like lactate, as electron donors for reduction of sulfate (36,38–40). Thus, the postoperative bloom in Bacteroides and TG5 (a member of the Dethiosulfovibrionaceae family) may be an indication of a mutualistic relationship between these two members of the gut microbiome.
Role of the gut microbiota in the effect of Sleeve Gastrectomy on hunger
Fasting hunger levels were rapidly and dramatically reduced by sleeve gastrectomy, and this reduction was strongly associated with Enterococcus, a lactobacillus known to ferment dietary fiber producing short-chain-fatty acids (SCFA). In an animal model of diet induced obesity, fiber supplementation resulted in a bloom of Enteroccocus and Bifidobacterium, an increased production of SCFA, decreased body weight gain and fat deposition, and changes in neuronal activation at the hypothalamus suggesting a satiated state (15,16). Akkermansia, which was weakly associated with decreased appetite in the present study, has been linked to activation of the endocannabinoid system which in turn stimulates the secretion of GLP-1 by the intestinal L-cells and reduces ghrelin levels (16,41,42).
Role of the gut microbiota in the effect of Sleeve Gastrectomy on food preferences
To our knowledge, this is the first study to explore whether the gut microbiota play a role in the changes in food preferences seen in humans after bariatric surgery. In this small sample, we found that ratings for hedonic eating decreased after LSG and that the change was associated with alterations of the gut microbiome. While subjects showed a preference for high-calorie foods over low-calorie foods at baseline, this preference was lost after surgery. These results parallel changes in high-hedonic value food preferences seen after RYGB, the most commonly studied bariatric procedure (21,43,44). In both preclinical and human studies, RYGB is characterized by a decreased response to food cues in brain regions of the reward system and a decrease in the palatability of highly-hedonic foods (21–23). However, the mechanisms behind surgery-related changes in hedonic eating are still elusive. Gastric bypass and sleeve gastrectomy result in an incretin profile that is “anorexinogenic” and thought to contribute in the postoperative drop in hedonic eating; however changes in hedonic eating have been shown to be independent from plasma levels of incretins or bile acids, symptoms of dumping syndrome and hunger levels (21,43,45). On the other hand, taste receptors, in the mouth and in the gut, have been implicated in appetite control, taste preferences and intake of preferred foods (43,46). For example, in an animal model with preference for sweets, the surgical diversion of the duodenum caused a decrease in dopamine release at the striatum and in the amount of sweet solution consumed by the mice (24). Authors speculated that these findings were related to bypassing sweet-receptors present in the small bowel (24). However, this theory will not apply to changes in hedonic eating seen after LSG since this procedure preserves the continuity of the GI tract. But, expression and function of sweet receptors in the gut is modulated by gut microbiota; moreover germ-free animals display an exaggerated preference for high-sucrose solutions (47). Along this, the present study found that the decline in preference for sweets after LSG was associated with alterations in the gut microbiome; specifically the Akkermansia genu, was significantly but inversely associated with the postoperative drop in the reported desire to eat sweets. The mechanism behind this alteration in hedonic eating is unclear and studies in larger samples should be conducted to confirm these findings. Of note, a study in rodents with high and low preference for saccharin consumption phenotypes, showed differential gut microbial composition independently of the saccharin intake suggesting a diet-independent interaction between scaccharin preference and gut microbiome (48).
This study shows that sleeve gastrectomy results in a significant reduction in scores of another measure of hedonic eating, the Yale Food Addiction scale. The postoperative drop in food addiction scores was directly associated with members of the gut microbial community including Catenibacterium and Anaerostipes and inverse correlations with Butyricimonas, Enterocococcus and Odoribacter. Of note, alterations in abundance of metabolically active genera including, amongst others, Enterococcus, Akkermansia and Odoribacter have been associated with autistic behaviors in children (49). Whether or not the effect of the identified bacteria on food addiction measures is mediated by microbial production of metabolites or neuroactive substances remains to be tested. Our findings merit further assessment to confirm them in a larger sample and to try to elucidate the mechanisms behind the associations with hedonic eating.
Interactions between gut microbiome, sleeve gastrectomy and weight loss
Results showed a strong inverse correlations between BMI and fat mass with a group of microbes. The most significant association was with Bilophila, a sulfate-reducing-bacteria which blooms in response to increased bile acid secretion induced by diets rich in animal fats and in saturated fats from milk (50,51). Bilophila, as a sulfate-reducing-bacteria, produces hydrogen sulfide which has been associated with inflammatory bowel disease and disruptions in immune regulation (51, 52). The inverse correlation with weight loss measures seen in the present study may be indicative of changes in diet during the postoperative period which led to a significant drop in the consumption of fats. Other genera negatively associated with changes in BMI and fat mass include Faecalibacterium, Lachnospira, and Acidaminococcus. Lachnospira and Faecalibacterium have been associated with complications of obesity, including metabolic syndrome and diabetes and decrease in their abundance after RYGB correlated with a reduction in inflammatory markers (35,53).
An alternative or complementary explanation of the interaction between gut microbiome and the effect of bariatric surgery on weight and on remodeling of the body fat mass is the association between white fat tissue (WAT) genes and gut microbiome. In an animal model of RYGB, significant associations were found between several WAT genes and Lactobacillus, Escherichia, Blautia, Alistipes, Bacteroides and Bifidobacterium amongst others (54).
LIMITATIONS
The current report presents results from an interim analysis of a long term prospective study, and the present sample size is small. The small sample size raises concerns about the reliability and consistency of the present results which will need to be addressed in a larger sample. Because of the small sample size, we were not able to control for the effect of confounders including changes in diet after surgery, which is known to be a major factor determining gut microbiota composition and function. Potential variations across the menstrual cycle were also not taken into account. Furthermore, our results show only the early effects of sleeve gastrectomy on weight, appetite, hedonic eating and gut microbial composition. Longitudinal follow-up is necessary to evaluate whether these findings are sustained over time. Although the instruments used here to measure food addiction including YFAS have been validated in lean and obese populations and are used broadly to study hedonic eating, subjects may feel uncomfortable reporting truthfully their struggle with high-hedonic value foods.
CONCLUSIONS
Laparoscopic sleeve gastrectomy results in early and dramatic reductions in weight, body fat mass, food intake and hunger levels. In addition, the procedure significantly reduced measures of hedonic eating including food addiction scales, and preference for high-calorie foods. In this preliminary analysis, the postoperative changes in weight, appetite and hedonic eating were associated with alterations of the gut microbial composition suggesting that surgically induced perturbations in gut microbiota-brain interactions axis may play an important role as mediators of the effects of bariatric surgery. Further analysis in a larger cohort with additional time points and assessment of metabolite changes are required to fully characterize the molecular mechanisms underlying sustained weight loss following bariatric surgery.
Acknowledgments
This study is funded in part by the NIH/NCATS UCLA CTSI -UL1TR000124
Abbreviations
- BMI
Body Mass Index
- Log2FC
Log2 fold change
- LSG
Laparoscopic Sleeve Gastrectomy
- NHANES
National Health and Nutrition Examination Survey
- OTUs
Observable Taxonomic Units
- SCFAs
Short Chain Fatty Acids
- sPLS-DA
Partial Least Square-Discriminant Analysis (sPLS-DA)
- UCLA
University of California Los Angeles
- VAS
Visual Analogue Scale
- VIP
Variable importance in projection
- YFAS
Yale Food Addiction Scale
Footnotes
Conflict of interest: None of the listed authors have any conflict of interest
References
- 1.Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013 Oct 22;347:f5934. doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aron-Wisnewsky J, Doré J, Clement K. The importance of the gut microbiota after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2012 Oct;9(10):590–8. doi: 10.1038/nrgastro.2012.161. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, Krajmalnik-Brown R. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365–70. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li JV, Ashrafian H, Bueter M, Kinross J, Sands C, le Roux CW, Bloom SR, Darzi A, Athanasiou T, Marchesi JR, Nicholson JK, Holmes E. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut. 2011;60(9):1214–23. doi: 10.1136/gut.2010.234708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graessler J, Qin Y, Zhong H, Zhang J, Licinio J, Wong ML, Xu A, Chavakis T, Bornstein AB, Ehrhart-Bornstein M, Lamounier-Zepter V, Lohmann T, Wolf T, Bornstein SR. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013 Dec;13(6):514–22. doi: 10.1038/tpj.2012.43. [DOI] [PubMed] [Google Scholar]
- 6.Liou AP, Paziuk M, Luevano JM, Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013 Mar 27;5(178):178ra41. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tremaroli V, Karlsson F, Werling M, Ståhlman M, Kovatcheva-Datchary P, Olbers T, Fändriks L, le Roux CW, Nielsen J, Bäckhed F. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015 Aug 4;22(2):228–38. doi: 10.1016/j.cmet.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009 May;6(5):306–14. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012 Nov;10(11):735–42. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 10.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012 Oct;13(10):701–12. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 11.Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain-Raspaud S, Trotin B, Naliboff B, Mayer EA. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013 Jun;144(7):1394–401. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, Berger B, Huizinga JD, Kunze W, McLean PG, Bergonzelli GE, Collins SM, Verdu EF. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arora T, Loo RL, Anastasovska J, Gibson GR, Tuohy KM, Sharma RK, Swann JR, Deaville ER, Sleeth ML, Thomas EL, Holmes E, Bell JD, Frost G. Differential effects of two fermentable carbohydrates on central appetite regulation and body composition. PLoS One. 2012;7(8):e43263. doi: 10.1371/journal.pone.0043263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everard A, Cani PD. Gut microbiota and GLP-1. Rev Endocr Metab Disord. 2014 Sep;15(3):189–96. doi: 10.1007/s11154-014-9288-6. [DOI] [PubMed] [Google Scholar]
- 16.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328(5975):228–31. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Geliebter A, Ladell T, Logan M, Schneider T, Sharafi M, Hirsch J. Responsivity to food stimuli in obese and lean binge eaters using functional MRI. Appetite. 2006;46:31–35. doi: 10.1016/j.appet.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Jansen A, Theunissen N, Slechten K, Nederkoorn C, Boon B, Mulkens S, Roefs A. Overweight children overeat after exposure to food cues. Eat Behav. 2003 Aug;4(2):197–209. doi: 10.1016/S1471-0153(03)00011-4. [DOI] [PubMed] [Google Scholar]
- 20.Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM, Ismail NA, Durighel G, Ahmed AR, Olbers T, Vincent RP, Alaghband-Zadeh J, Ghatei MA, Waldman AD, Frost GS, Bell JD, le Roux CW, Goldstone AP. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut. 2014 Jun;63(6):891–902. doi: 10.1136/gutjnl-2013-305008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL, Berthoud HR. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2009 Nov;297(5):R1273–82. doi: 10.1152/ajpregu.00343.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin AC, Zheng H, Pistell PJ, Berthoud HR. Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes (Lond) 2011 May;35(5):642–51. doi: 10.1038/ijo.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han W, Tellez LA, Niu J, Medina S, Ferreira TL, Zhang X, Su J, Tong J, Schwartz GJ, van den Pol A, de Araujo IE. Striatal Dopamine Links Gastrointestinal Rerouting to Altered Sweet Appetite. Cell Metab. 2016 Jan 12;23(1):103–12. doi: 10.1016/j.cmet.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guidelines for Clinical Application of Laparoscopic Bariatric Surgery. Practice/Clinical Guidelines published on: 06/2008 by the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) http://www.sages.org/publication/id/30/
- 25.Gearhardt AN, Corbin WR, Brownell KD. Preliminary Validation of the Yale Food Addiction Scale. Appetite. 2009 Apr;52(2):430–6. doi: 10.1016/j.appet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Tong M, Jacobs JP, McHardy IH, Braun J. Sampling of intestinal microbiota and targeted amplification of bacterial 16S rRNA genes for microbial ecologic analysis. Curr Protoc Immunol. 2014 Nov 3;107:7, 41.1–11. doi: 10.1002/0471142735.im0741s107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010 May;7(5):335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–5. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liquet B, Lê Cao KA, Hocini H, Thiébaut R. A novel approach for biomarker selection and the integration of repeated measures experiments from two assays. BMC Bioinformatics. 2012 Dec 6;13:325. doi: 10.1186/1471-2105-13-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lê Cao KA, Martin PG, Robert-Granié C, Besse P. Sparse canonical methods for biological data integration: application to a cross-platform study. BMC Bioinformatics. 2009;10:34. doi: 10.1186/1471-2105-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Cao KARD, Robert-Granie C, Besse P. A sparse PLS for variable selection when integrating omics data. Stat Appl Genet Mol Biol. 2008;7:35. doi: 10.2202/1544-6115.1390. [DOI] [PubMed] [Google Scholar]
- 33.Le Cao KA, Boitard S, Besse P. Sparse PLS discriminant analysis: biologically relevant feature selection and graphical displays for multiclass problems. Bmc Bioinformatics. 2011;12:253. doi: 10.1186/1471-2105-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Damms-Machado A, Mitra S, Schollenberger AE, Kramer KM, Meile T, Königsrainer A, Huson DH, Bischoff SC. Effects of Surgical and Dietary Weight Loss Therapy for Obesity on Gut Microbiota Composition and Nutrient Absorption. BioMed Research International. 2015:806248. doi: 10.1155/2015/806248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Doré J, Henegar C, Rizkalla S, Clément K. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–57. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006 Feb 24;124(4):837–48. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 37.Martens EC, Chiang HC, Gordon JI. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe. 2008;4:447–57. doi: 10.1016/j.chom.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, Weatherford J, Buhler JD, Gordon JI. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–9. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 39.Gibson GR, Cummings JH, Macfarlane GT. Use of a three-stage continuous culture system to study the effect of mucin on dissimilatory sulfate reduction and methanogenesis by mixed populations of human gut bacteria. Appl Environ Microbiol. 1988;54:2750–5. doi: 10.1128/aem.54.11.2750-2755.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rey FE, Gonzalez MD, Cheng J, Wu M, Ahern PP, Gordon JI. Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc Natl Acad Sci U S A. 2013;110:13582–7. doi: 10.1073/pnas.1312524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–71. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cani PD, Montoya ML, Neyrinck AM, Delzenne NM, Lambert DM. Potential modulation of plasma ghrelin and glucagon-like peptide-1 by anorexigenic cannabinoid compounds, SR141716A (rimonabant) and oleoylethanolamide. Br J Nutr. 2004;92(5):757–61. doi: 10.1079/bjn20041256. [DOI] [PubMed] [Google Scholar]
- 43.Hajnal A, Kovacs P, Ahmed T, Meirelles K, Lynch CJ, Cooney RN. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol. 2010;299:G967–79. doi: 10.1152/ajpgi.00070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saeidi N, Nestoridi E, Kucharczyk J, Uygun MK, Yarmush ML, Stylopoulos N. Sleeve gastrectomy and Roux-en-Y gastric bypass exhibit differential effects on food preferences, nutrient absorption and energy expenditure in obese rats. Int J Obes (Lond) 2012;36:1396–402. doi: 10.1038/ijo.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sclafani A, Ackroff K. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1119–33. doi: 10.1152/ajpregu.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swartz TD, Duca FA, de Wouters T, Sakar Y, Covasa M. Up-regulation of intestinal type 1 taste receptor 3 and sodium glucose luminal transporter-1 expression and increased sucrose intake in mice lacking gut microbiota. Br J Nutr. 2012 Mar;107(5):621–30. doi: 10.1017/S0007114511003412. [DOI] [PubMed] [Google Scholar]
- 48.Lyte M, Fodor AA, Chapman CD, Martin GG, Perez-Chanona E, Jobin C, Dess NK. Gut Microbiota and a Selectively Bred Taste Phenotype: A Novel Model of Microbiome-Behavior Relationships. Psychosom Med. 2016 Jun;78(5):610–9. doi: 10.1097/PSY.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 49.De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, Cristofori F, Guerzoni ME, Gobbetti M, Francavilla R. Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder Not Otherwise Specified. PLoS ONE. 2013;8:e76993. doi: 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Devkota S, Chang EB. Interactions between Diet, Bile Acid Metabolism, Gut Microbiota, and Inflammatory Bowel Diseases. Dig Dis. 2015;33(3):351–6. doi: 10.1159/000371687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turnbaugh PJ. Microbiology: fat, bile and gut microbes. Nature. 2012 Jul 4;487(7405):47–8. doi: 10.1038/487047a. [DOI] [PubMed] [Google Scholar]
- 53.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 54.Kong LC, 1, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot JL, Zucker JD, Doré J, Clément K. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98:16–24. doi: 10.3945/ajcn.113.058743. [DOI] [PubMed] [Google Scholar]


