Abstract
Glioblastoma (GBM) is the most common primary malignant astrocytoma characterized by extensive invasion, angiogenesis, hypoxia and micrometastasis. Despite the relatively leaky nature of GBM blood vessels, effective delivery of anti-tumor therapeutics has been a major challenge due to the complications caused by the blood brain barrier (BBB) and the highly torturous nature of newly formed tumor vasculature (blood tumor barrier-BTB). External beam radiation therapy was previously shown to be an effective means of permeabilizing central nervous system (CNS) barriers. By using targeted short ranged radionuclides, we show for the first time that our targeted actinium-225 labeled αvβ3-specific liposomes (225Ac-IA-TLs) caused catastrophic double stranded DNA breaks and significantly enhanced the permeability of BBB and BTB in mice bearing orthotopic GBMs. Histological studies revealed characteristic α-particle induced double strand breaks within tumors but was not significantly present in normal brain regions away from the tumor where BBB permeability was observed. These findings indicate that the enhanced vascular permeability in these distal regions did not result from direct α-particle induced DNA damage. Based on these results, in addition to their direct anti-tumor effects, 225Ac-IA-TLs can potentially be used to enhance the permeability of BBB and BTB for effective delivery of systemically administered anti-tumor therapeutics.
Keywords: Alpha particle radiotherapy, Blood brain-tumor barrier, Glioblastoma, Integrin αvβ3, Drug delivery
INTRODUCTION
Glioblastoma (GBM) is the most common and aggressive primary malignant astrocytoma which accounts for nearly 15,000 deaths annually (1). GBMs are characterized by infiltrating margins, angiogenesis and micrometastasis which result in residual disease that persists even after treatment, giving rise to aggressive tumors which are often resistant to standard therapy (2–4). Surgical resection, external beam radiotherapy and conventional chemotherapy only improve patient survival by a few months. Importantly, many chemotherapies and systemically administered anti-GBM therapies which have been shown to be very effective in vitro are not effective in the clinic due to their poor and non-uniform distribution within the central nervous system (CNS) (5,6).
The Blood Brain Barrier (BBB) is a protective vascular architecture shown to complicate the effective diffusion of systemically administered therapies into the CNS for treating various neurological disorders (7–13). Therefore, significant efforts are being made to design safe and sophisticated strategies to bypass such barriers for efficient drug delivery (14–17). Multiple recent studies have shown that exposure to external beam radiation enhances BTB permeability in mice bearing orthotopic GBMs (18–22). These studies highlight the effect of ionizing radiation on the permeability of the BTB and the potential of using targeted molecular radiotherapeutics for this purpose with the added benefit of limiting collateral damage to healthy brain tissue. α-particle irradiation is characterized by a short range (50–100μm) and high linear energy transfer (~80keV/μm) which causes irreparable DNA double strand breaks. Actinium (225Ac) is effective for use in such strategies due to its 10 day half-life and the release of four α-particles upon its decay (23). Targeting α-particles towards tumor specific cell surface receptors can spare healthy brain tissue surrounding tumors (24). While we and others have demonstrated the potential of using targeted β-emitting radiotherapy (25), its relatively long range energy and significantly lower killing potential make β-emitting radioisotopes less attractive than α-particle emitters in resistant brain cancers that exist in the immediate vicinity of normal brain.
Tumors are characterized by extensive accumulation of fluid due to leaky blood vessels resulting from disorganized angiogenic endothelial cells and lack effective lymphatic drainage (26). This property of tumors, termed Enhanced Permeability and Retention (EPR), allows nanoparticles to passively accumulate within tumor tissue. By targeting liposomes, we can not only deliver nanoparticles to tumor tissue passively by EPR, but also actively target them to tumor specific cell surface receptors resulting in their retention and internalization (27–29).
The purpose of this work is to demonstrate the effectiveness of 225Ac-labeled targeted liposomes (225Ac-TL) to both enhance the BBB/BTB permeability to systemically delivered agents as well as to elicit characteristic double stranded DNA breaks in tumors. To accomplish these goals, we targeted liposomes to the well characterized integrin alpha-V beta-3 (αvβ3), which is not only highly expressed on angiogenic endothelial cells but also on GBM cells at margins of invasion (30,31). We therefore developed a small molecule integrin antagonist (IA) that we previously reported to strongly bind αvβ3 integrin and is now in early stage clinical trials (32–38). We subsequently showed the specificity of our targeted liposomes towards integrin αvβ3 using human glioma cell lines and human umbilical cord vascular endothelial cells in vitro. This specificity was reinforced by demonstrating binding of IA-TLs to the αvβ3 expressing M21 human melanoma cell line but lack of binding to the control matched M21L cell line, which is a stable variant of M21 cell line lacking αvβ3 expression (39). Therefore, for this work, we targeted 225Ac-labeled liposomes with IA and examined their effects on enhancing BBB/BTB permeability by evaluating the extravasation of systemically administered albumin binding dye (Evans Blue dye) and MR contrast (Gadopentetic acid) into neural and GBM tissue. Extravasation of Evans Blue dye and gadopentetic acid which have a molecular weight that is similar or higher than that of commonly used chemotherapeutic agents such as temozolomide indicates that our alpha particle based strategy could potentially be used to deliver systemic therapies more effectively to GBMs.
MATERIALS AND METHODS
Animals
Male immunocompetent and athymic nude mice were purchased from Taconic Farms. All colonies were housed in a pathogen-free facility of the Animal Research Program at Wake Forest School of Medicine under a 12:12-h light/dark cycle and fed ad libitum. The institutional IACUC guidelines for the care and use of laboratory animals were followed for all in vivo experiments.
Cell culture
U87 MG human GBM cell line was acquired from ATCC in 2008 and cultured in DMEM media (Corning, NY) supplemented with 10% FBS (Invitrogen) and 1% Antibiotic-Antimycotic (Gibco). Early passage cells were cryopreserved in freezing medium containing 5% DMSO (ThemoFisher Scientific). For our experiments, previously cryopreserved early passage U87 MG cells were resuscitated and cultured for 2 weeks before being implanted in vivo in athymic nude mice.
Partially polymerized liposomes and Actinium-225
The lipids 1,2-di-(10Z,12Z-tricosadiynoyl)-sn-glycero-3-phosphoethanolamine (DiynePE), 1,2-di-(10Z,12Ztricosadiynoyl)-sn-glycero-3-phosphocholine (DiynePC), and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) were purchased from Avanti Polar Lipids. Inc. (Alabaster, AL) and used without further purification. The radioactive tracer Actinium-225 was obtained from Washington State University at Saint Louis, MO. Functional lipids, 1,2-di-(10Z,12Z-tricosadiynoyl)-sn-glycero-3-phosphoethanolamine with conjugated DOTA (DiynePE-DOTA), 1,2-di-(10Z,12Z-tricosadiynoyl)-sn-glycero-3-phosphoethanolamine-PEG-integrin antagonist (DiynePE-PEG-IA) were synthesized and confirmed by mass spectrometry.
Synthesis of IA- and DOTA-attached polymerizable lipids
To synthesize the monovalent polymerizable IA-lipid, PEG-di(carboxylic acid) linker (HOOC(CH2CH2O)n=2–45COOH) (0.21 mmol) in 15 ml dry dichloromethane was treated with 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) to form NHS ester, which was treated with 1,2-di-(10Z,12Z-tricosadiynoyl)-sn-glycero-3-phosphoethanolamine (DiynePE, 0.1 mmol, Avanti Polar Lipids). The resulted solution was washed with cold brine and purified by a flash chromatography to give pure DiynePE-PEGn-NHS. To the lipid solution of DiynePE-PEGn-NHS (1 mmol) in anhydrous acetonitrile (5 mL), anhydrous dichloromethane (2 mL), and triethylamine (1 mL), IA (1.2 mmol) in DMF was added with continuous stirring in the dark for 24 h. The reaction was complete after stirring at room temperature for 24 hours according to TLC analysis. The reaction solution was evaporated, diluted with water and dialyzed against water using a dialysis bag (cut off size 1000 Da) to give pure DiynePE-PEGn-IA. To synthesize DOTA-attached polymerizable lipid, DiynePE (22.9 μmol) and triethylamine (22.9 μmol) were dissolved in 12 ml of methanol/dichloromethane (10:2, v/v), and S-2-(4-Isothiocyanatobenzyl)-1,4,7,10-tetraazacyclododecane tetraacetic acid (pSCN-Bn-DOTA) (23.0 μmol, Macrocyclics) was added. The reaction mixture was kept under stirring for 24 hours while being protected from light. The reaction solution was washed with brine, dried and precipitate in cold methanol to give pure DiynePE-DOTA.
Radiolabeling of αvβ3 targeted IA-TLs with 225Ac
The lipids used in this study include filler lipids (DiynePC and DPPC) and functionalized lipids (DiynePE-DOTA, DiynePE-PEG-IA). TLs were prepared using the same procedure as previously described and the prepared liposomes had a mean diameter of ~ 100 nm. Briefly, lipids were dissolved in 5 ml of chloroform in a 50 ml flask, evaporated under reduced pressure to form thin lipid film. The lipid film was rehydrated to form large vesicles. The lipid solution was then transferred to a 10 ml LIPEX extruder (Northern Lipids Inc., Canada) equipped with two stacked polycarbonate membranes (100 nm pore size) and extruded 10 times at 45°C. The resulting liposomes were then transferred onto a petri dish sitting on ice and irradiated under a 254 nm UV light for an hour to produce liposomes. The liposomes were then filtered using a 220 nm filter followed by purification with Sephadex G50 columns. The nanoparticle size was obtained by dynamic light scattering (DLS) using a Nanosizer (Malvern, UK). For 225Ac labeling, the prepared IA-TL-DOTA was incubated with 225Ac at 70⁰C for 50 minutes.
Intracranial injection of orthotopic glioblastomas and 225Ac labeled IA-TLs into rodents
Orthotropic GBMs were implanted by stereotactic implantation of 1×105 actively growing U87 MG human GBM cells in athymic nude mice. Mice were weighed and anesthetized with a mixture of 114 mg/kg Ketamine and 17 mg/kg xylazine. Mice were then placed in a stereotactic setup and a hole was made 2.0mm lateral and 0.5mm posterior to the bregma in the right cerebral hemisphere through a scalp incision. Stereotactic injection was performed using a Just for Mice stereotaxic apparatus (Harvard Apparatus), with injection of 10-μl syringe (Hamilton) through the hole to a depth of 3.2mm. A Nanomite programmable syringe pump (Harvard Apparatus) delivered constant infusion at a rate of 0.5 μl/min to a total volume of 5 μl.
10 days post tumor implantation, mice received 1μCi of 225Ac-IA-TLs/5μl and 5μg of unlabeled IA-TLs/5μl intracranially using the same procedures that are mentioned above. All mice received an analgesic (Ketaprofen) and were monitored for body weight, ambulatory, feeding and grooming activities.
Small animal MR imaging
Mice were transported to the Wake Forest small animal imaging facility from the Wake Forest animal housing facility following IACUC approved protocols and anesthetized using a mixture of 114 mg/kg Ketamine and 17 mg/kg xylazine. Mice were then placed on a heating pad and injected with gadopentetic acid intravenously. The mice were then placed on a bed and scanned using a 7 Tesla (7T) MRI scanner. Respiratory rate and temperature of all animals was constantly monitored during the procedure.
Intravenous injection of Evans blue dye and PGLA nanoparticles
Athymic nude rats and mice were anesthetized using isoflurane and placed on a heating pad. The rodent tails were warmed for a few minutes under a heat lamp and Evans Blue dye (2%) diluted in 1X PBS was injected intravenously along with (1μg/μl) of green fluorescing PGLA nanoparticles. The animals were then allowed to recuperate under the heat lamp for a few minutes. After a period of 6 hours, the rodents were anesthetized with a mixture of 114 mg/kg Ketamine and 17 mg/kg xylazine and perfused using IACUC approved protocol.
Extraction and cryopreservation of rodent brains
Anesthetized mice were transcardially perfused with PBS followed by 4% paraformaldehyde (PFA). Extracted brains were postfixed in 4% PFA, cryoprotected in gradients of sucrose, covered with tissue embedding medium, snap frozen in liquid nitrogen and stored at −80 °C.
Tissue Sectioning
Serial, 20 μm thick, transverse sections of the frozen tissues were obtained using a cryostat (Microm HM 500, Zeiss, Germany) at −20°C and were mounted on Super Frost Plus microscope slides (Thermo Scientific) in series of three and were stored at −20 °C.
Immunohistochemistry
Sections were dried at room temperature for an hour, rehydrated in PBS, permeabilized with 0.5 % Triton X-100 (Sigma) in PBS solution and blocked to saturate non-specific antigen sites using 5% (vol/vol) goat serum-PBS (Jackson Immunoresearch Labs) overnight at 4°C. On the next day, the sections were incubated with 0.05% Tween 20 and incubated in anti-γH2A.X antibody (abcam) for 4 hours. Later, the sections were washed using PBS-T and incubated with anti-rabbit Alexa flour 647 for 2 hours in the dark. Later, the sections were imaged on incubated with Hoechst 33342 (nuclear marker). The sections were then mounted using mounting medium and observed under an inverted fluorescence microscope.
Microscopy
An inverted motorized fluorescent microscope (Olympus IX81) with an Orca-R2 Hamamatsu CCD camera (Hamamatsu) and a laser scanning confocal microscope (Olympus FluoView1200) were used for image acquisition. Camera drive and acquisition were performed using a MetaMorph Imaging System (Olympus, Japan) and Fluo View Viewer 4.2 software (Olympus, Japan) were used for image acquisition.
Image analysis
Images of PLGA fluorescence indicating the permeabilization of the BTB were analyzed using Image J software. ROIs for measuring the area and intensity of extravasated Evans blue dye, PLGA nanoparticles and γH2A.X fluorochrome signal were drawn using Image J software. Olympus Fluo View Viewer 4.2 imaging software was used to analyze fluorescence images.
Statistical analysis
Data are represented as means +/−standard error of mean (SEM). One-way ANOVA (for three or more experimental groups) and Student’s t-test were performed to assess if experimental groups were significantly different from each other. All statistical analyses and graphs were generated using GraphPad Prism software. p<0.05 was considered to be statistically significant (*).
RESULTS
Development and radiolabeling of IA targeted Liposomes (IA-TLs)
We utilized the liposome formulation previously developed by our group (39). To target our liposomes to GBM and associated vasculature, we conjugated a novel small molecule integrin antagonist (IA) that has been shown to target αvβ3 with a high affinity and a more optimal biodistribution compared to peptides (Figure 1A). We used standard DOTA chelation methods to radiolabel our targeted liposome with 225Ac, as we previously reported (Figure 1B) (23,24). Our radiolabeled 225Ac-IA-TL demonstrated uniform size and radiochemical purity (RCP) of 99.6% (Figure 1C).
Figure 1. Development of 225Ac labeled αvβ3 targeted liposomes (225Ac-IA-TLs).
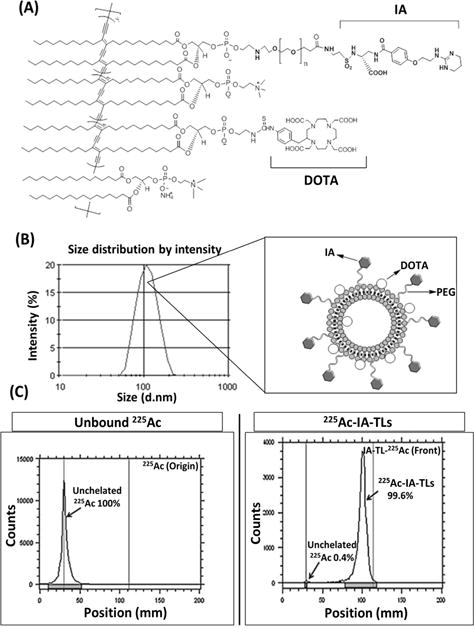
(A) PEG-di (carboxylic acid) linker (HOOC(CH2CH2O)n=2-45COOH) was conjugated to 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) to yield pure DiynePE-PEGn-NHS. Monovalent polymerizable IA-lipid was produced by conjugating IA with lipid solution of DiynePE-PEGn-NHS. (B) TLC analysis showing production of pure DiynePE-PEGn-IA along with diagrammatic representation of IA-TLs; prepared liposomes had a mean diameter of ~100 nm. (C) DiynePE was mixed with S-2-(4-Isothiocyanatobenzyl)-1,4,7,10-tetraazacyclododecane tetraacetic acid (pSCN-Bn-DOTA) to produce pure DiynePE-DOTA. For 225Ac labeling, prepared IA-DOTA-TLs were incubated with 225Ac at 70⁰C for 50 minutes. Radio-TLC analysis showed effective radiolabeling of targeted liposomes with a radiochemical purity of 99.6%.
Cy5.5 labeled IA-TLs show tumor specificity in vivo
To demonstrate GBM-specific targeting of our 225Ac-IA-TLs, groups of mice (n=3) bearing αvβ3-expressing orthotopic GBMs were intracranially infused with cy5.5 labeled targeted and untargeted liposomes. In vivo binding was measured using fluorescence imaging performed 2 days (Figure 2A) and 5 days (Figure 2B) post liposome infusion. Intracranially infused Cy5.5 labeled αvβ3 targeted IA-TL accumulated significantly greater within tumor tissue in mice bearing orthotopic GBMs when compared to cy5.5 labeled untargeted liposomes (p< 0.05) (Figure 2C). Importantly, Cy5.5-IA-TLs were found to be abundant only within GBM tissue but not in the surrounding normal brain (Figure 2D). This result demonstrated the specificity of targeted IA-TLs towards integrin αvβ3 expressing orthotopic tumors and their suitability to test the effects of targeted α-particle radiation on the BBB/BTB permeability.
Figure 2. in vivo specificity of cy5.5-IA-TLs towards αvβ3.
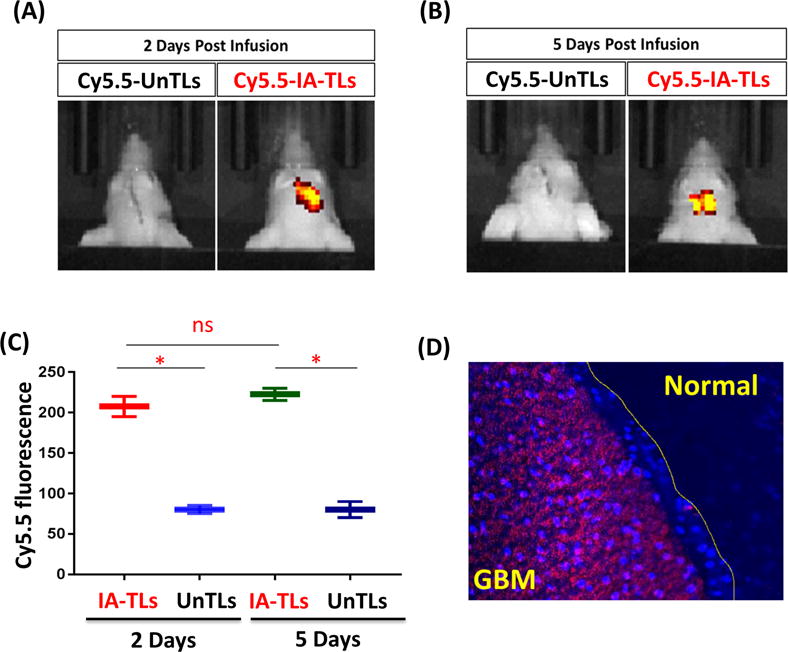
in vivo fluorescence imaging showing tumor accumulation of intracranially infused cy5.5 labeled αvβ3 targeted nanoparticles (cy5.5-IA-TL) (n=3) and cy5.5 labeled untargeted nanoparticles (n=3) (cy5.5-UnTLs) 2 days (n=3) (A) and 5 days (n=3) (B) post intracranial infusion. (C) Cy5.5-IA-TLs accumulated within GBM tissue significantly greater than cy5.5-UnTLs 2 days and 5 days post intracranial infusion. (D) Brain sections from mice revealed Cy5.5-IA-TLs to be abundant within GBM tissue. Negligible presence of cy5.5-IA-TLs was found within normal regions of the brain surrounding GBM tissue. Data represented as mean +/− SEM. Student’s t-test was performed to assess difference between experimental groups (*p< 0.05(significant); ns= not significant).
225Ac-IA-TLs mediates BBB permeability
To examine the targeted α-particle mediated BBB permeability over a period of days, groups of mice (n=3) intracranially infused with 225Ac-IA-TLs were sacrificed 2 days, 5 days and 10 days post-infusion after they were intravenously injected with Evans blue dye. Systemically injected Evans Blue dye does not normally penetrate the BBB and is therefore used to measure permeability of systemically injected agents. We observed modest BBB permeabilization after 2 days and more extensive Evans blue dye extravasation after 5 days, which steadily increased until 10 days post-surgery (Figure 3A). The area of distribution and intensity of Evans blue dye were significantly higher in mice intracranially infused with 225Ac-IA-TLs when compared to control mice (Figure 3B and 3C). Control mice intracranially infused with saline (n=3) only showed minimal Evans Blue dye extravasation after 2 days, likely due to surgical disruption, which completely disappeared at 10 days (Figure 3). These results indicate that 225Ac-IA-TLs cause BBB opening when intracranially infused in vivo.
Figure 3. 225Ac-IA-TLs causes BBB opening in immunocompetent mice.
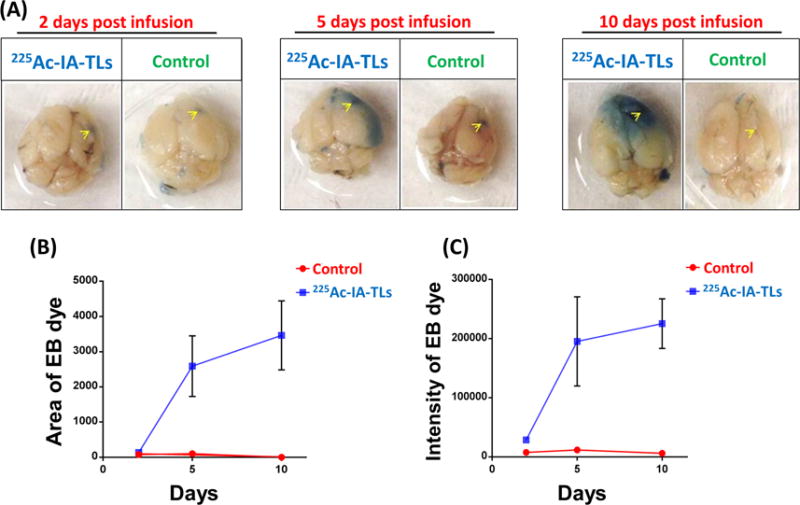
(A) Extravasation of intravenously injected Evans blue dye shows clear BBB opening in immunocompetent mice infused with 225Ac-IA-TLs 2 days, 5 Days and 10 days (n=3) when compared to immunocompetent mice that were infused with saline (n=3). Yellow arrows indicate site of intracranial injection. (B) and (C) Area and intensity of extravasated Evans blue dye was also found to be greater in mice intracranially infused with 225Ac-IA-TLs when compared to mice intracranially infused with saline upto 10 days post infusion.
To examine if there was a dose dependent permeability response, we intracranially infused groups of mice (n=5/dose) with 0.05μCi, 0.1μCi or 0.5μCi of 225Ac-IA-TLs. We observed a dose dependent increase in the extravasation of intravenously injected gadopentetic acid (Figure 4A) and Evans Blue dye (Figure 4B). This confirms the potential of using α-particle mediated BBB permeability to deliver systemically administered agents through the BBB.
Figure 4. 225Ac-IA-TLs permeabilized BBB in immunocompetent mice in a dose dependent manner.

(A) Dose escalation study showed a dose dependent effect of 225Ac-IA-TLs on BBB permeability in immunocompetent mice intracranially infused with 225Ac-IA-TLs (n=15; n=5/dose) indicated by contrast enhancement on MRI. (B) Dose dependent effect of 225Ac-IA-TLs on BBB permeability was also confirmed by the extravasation of intravenously injected Evans Blue dye. Immunocompetent mice intracranially infused with saline showed no BBB opening (n=5). Data represented as mean +/− SEM. Student’s t-test and One-way ANOVA was performed to assess differences between experimental groups (*p< 0.05(significant)).
225Ac labeled IA-TLs enhance BTB permeability in mice bearing orthotopic GBMs
To examine whether α-particle mediated BBB permeability extends to the BTB, we intracranially infused 225Ac-IA-TLs, unlabeled IA-TLs and saline into tumor tissue of mice bearing orthotopic GBMs. Groups of mice (n=3) were sacrificed 2 days and 5 days post 225Ac-IA-TLs infusion, after intravenous injection of Evans Blue dye. We observed increased BTB permeability in mice infused with 225Ac-IA-TLs when compared to mice infused with unlabeled IA-TLs (n=3) (Figure 5A). Upon sectioning the brains, we confirmed the greater area of extravasation of Evans Blue dye in mice intracranially infused with targeted α-particle therapy compared to mice intracranially infused with either unlabeled IA-TLs or saline (Figure 5B and 5C).
Figure 5. 225Ac-IA-TLs cause significant enhancement of blood-tumor barrier permeability.
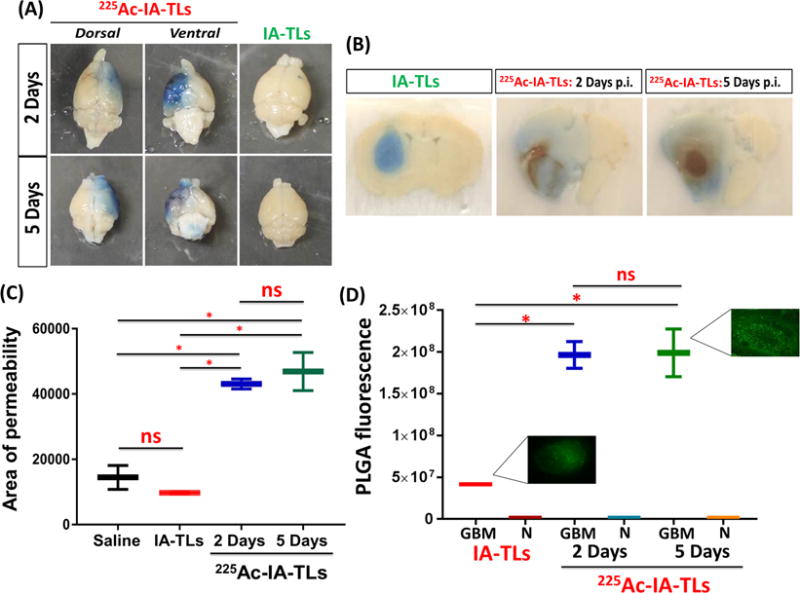
(A) Extravasation of intravenously injected Evans blue dye showed clear enhancement of blood-tumor barrier permeability in nude mice bearing orthotopic glioblastomas that were intracranially infused with 225Ac-IA-TLs 2 days (n=3) and 5 days (n=3) post infusion when compared to mice bearing orthotopic glioblastomas that were intracranially infused with unlabeled IA-TLs. (B) Brain sections showed extensive enhancement of BTB indicated by the extravasation of Evans blue dye in mice intracranially infused with 225Ac-IA-TLs for a period of 5 days when compared to mice intracranially infused with unlabeled IA-TLs. (C) BTB permeability within untreated GBMs, measured using Evans Blue dye permeabilization was found to be similar in mice intracranially infused with saline and unlabeled IA-TLs. Area of extravasation of Evans Blue dye was significantly greater in brains of mice treated with 225Ac-IA-TLs than in untreated mice. (D) Quantification of systemically injected fluorescent PLGA nanoparticles showed that α- particle enhanced BTB could potentially be exploited to deliver diagnostic and therapeutic agents (n=3/group). PLGA fluorescence in and around GBM tissue was measured. GBM= GBM tissue; N= Normal brain region surrounding GBM tissue. Data represented as mean +/− SEM. Student’s t-test and One-way ANOVA was performed to assess differences between experimental groups (*p< 0.001(significant)).
Systemic delivery of fluorescent PLGA nanoparticles (300nm) to exploit α-particle enhanced BTB permeabilization
To examine if the α-particle enhanced BTB permeability could be exploited to systemically deliver larger sized agents, we injected fluorescent PLGA nanoparticles (300nm in diameter) into orthotopic GBM-bearing mice treated intratumorally with 225Ac-IA-TLs. Fluorescent PLGA nanoparticles were intravenously injected prior to sacrifice to quantify the accumulation of PLGA nanoparticles within GBMs upon BTB permeabilization. We observed significantly higher accumulation of PLGA nanoparticles within GBM tissue in mice infused with 225Ac-IA-TLs when compared to mice infused with unlabeled IA-TLs (n=3/time point) (p <0.05) (Figure 5D). PLGA fluorescence in and around GBM tissue was normalized to fluorescence signal from contralateral brain regions before analysis. These results demonstrate that 225Ac-IA-TLs also significantly enhance BTB permeability to larger systemically injected agents.
Alpha particle-induced double-stranded DNA breaks observed at infusion site but not in regions further away
To examine the biological effect of targeted α-particle therapy on GBMs at the site of injection and in the surrounding normal brain where we observed enhanced BBB permeability, we infused mice with 225Ac-IA-TLs and stained for double stranded DNA breaks, a hallmark of α-particle therapy. We observed an abundance of double stranded DNA breaks (manifested by γ H2A.X staining) at the site of infusion in all mice intracranially infused with 225Ac-IA-TLs (n=3/time point) but did not observe significant double-stranded DNA breaks in the surrounding regions of normal brain (0.5mm from GBM border) that demonstrated increased BBB/BTB permeability away from infusion site (Figure 6A and 6B). γ H2A.X nuclear signal was found to be significantly higher around infusion sites when compared to regions further away (0.5mm from GBM border) at 2 day and 5 day time points (p <0.05) (Figure 6C). In contrast, we observed negligible presence of γ H2A.X nuclear signal within untreated orthotopic GBMs in mice (Figure 6C). These results indicate that the catastrophic double stranded DNA damage only occurred at the site of infusion (within tumors) but not in the surrounding normal brain where we observed enhanced BBB permeability, demonstrating the potential of safely exploiting this property for systemically administered therapies to target the areas of brain surrounding GBMs that contain infiltrating cells responsible for inevitable recurrences.
Figure 6. Double strand DNA breaks present within tumor tissue but absent within surrounding tissue showing enhanced vascular permeability.
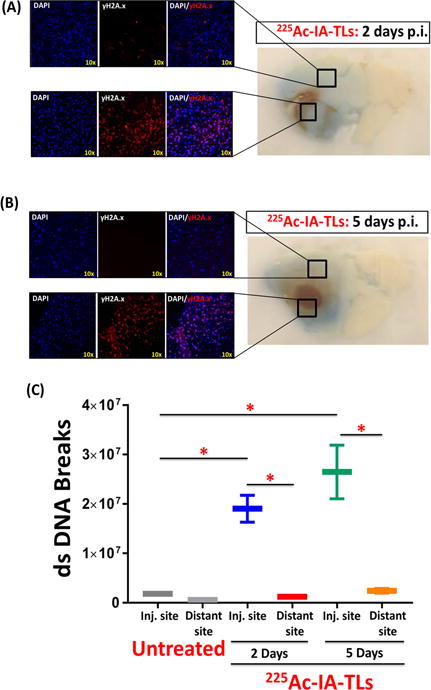
Immunohistochemical staining of sectioned mouse brains revealed the presence of γH2A.X within tumor tissue, indicating α-particle induced tumor cell killing 2 days (n=3) (A) and 5 days (n=3) (B) post intracranial infusion (p.i.) of 225Ac-IA-TLs. (C) Immunohistochemical staining of sectioned mouse brains also revealed negligible presence of γH2A.X in normal brain tissue surrounding tumors (0.5mm from GBM border) (n=3), indicating absence of double strand (ds) DNA breaks within these regions where enhancement in vascular permeability (extravasation of Evans Blue dye) was observed. Data represented as mean +/− SEM. Student’s t-test and One-way ANOVA was performed to assess difference between experimental groups (*p< 0.001(significant)).
DISCUSSION
Effective delivery of systemically administered therapeutics to GBMs is a formidable challenge due to the blood-brain/tumor barrier, with most small molecules unable to cross CNS barriers (40–42). Recurrent disease due to micrometastases that escape exposure to therapeutics is a major driver of patient mortality. Designing strategies that allow drugs to reach micrometastatic sites and the tumor microenvironment is therefore imperative (43,44). In this work, we developed a targeted α-particle platform that we demonstrated can target GBM with lethal α-particles and simultaneously enhance BBB and BTB permeability towards systemically administered agents.
In this study, we showed the in vivo specificity of our intracranially infused nanoparticles towards integrin αvβ3 expressing orthotopic GBMs. Furthermore, we successfully labeled our nanoparticles with actinium-225, a potent α-particle emitter. After intracranially infusing our 225Ac labeled αvβ3 targeting nanoparticles (225Ac-IA-TL) using convection enhanced delivery (CED), we observed enhanced BBB/BTB permeability to systemically administered small molecules to larger nanoparticles. CED is a delivery technique that increases the effective penetration of drugs into GBM by forcing fluid into the parenchyma and does not rely on the limited diffusion of drug after bolus injection. This technique has greatly improved over the past few years as novel catheters have been manufactured that overcome the shortcomings of early CED clinical trials (45). In addition to the added permeability to the BTB after delivering 225Ac-IA-TL via CED, we also observed permeability within surrounding normal brain where the micrometastatic invasive disease often remains and causes recurrence. Importantly, these areas of normal brain showing added permeability did not demonstrate widespread DNA damage caused by targeted α-particles, indicating translational relevance. Although tumors are characterized by disorganized and leaky vasculature, the highly torturous nature of the tumor vessels and rigidity of tumor associated stroma make it difficult for drugs to extravasate and diffuse into tumor tissue (46). Our results increase our knowledge of the effects of molecular targeted radiation on BBB/BTB permeability. Furthermore, we are currently studying the translational potential of using targeted α-particles to enhance the delivery of systemically administered drugs which do not cross the BBB, that are currently being used in clinic for other malignancies.
When we examined for the presence of double strand DNA breaks, we observed greater sub-nuclear γ H2A.X accumulation in GBM cells around the infusion site and an absence of γ H2A.X in distal regions of normal brain (0.5mm from GBM border) where enhanced vascular permeability was observed. This demonstrates that even though the presence of double strand DNA breaks are limited to GBM tissue, its effect on BTB/BBB permeability is extended even to areas further away within the normal brain. While not the focus of this work, this demonstrates the potential therapeutic effect of our strategy of using 225Ac-IA-TLs to elicit double strand DNA breaks in GBM tissue but not within normal brain. Further studies are now underway to evaluate the direct therapeutic effect of 225Ac-IA-TLs alone and in combination with systemically administered therapies.
CONCLUSIONS
225Ac-IA-TLs enhanced the permeability of BBB and BTB in vivo and elicited double strand DNA breaks within GBMs. These characteristics justify further ongoing studies to validate 225Ac-IA-TLs as a candidate therapeutic that can potentially be exploited to effectively deliver other systemic therapeutics across the BBB and BTB to GBM.
Acknowledgments
We would like to thank Ms. Thuy Smith and Ms. Stephanie Rideout Danner for their help with animal studies and their expertise with tissue extraction and processing. We would also like to thank Ken Grant (Wake Forest cellular imaging shared resource) for his help and expertise.
Financial support for the work: This work was supported by the American Cancer Society mentored research scholar grant 124443-MRSG-13-121-01-CDD (Mintz), the National Institutes of Health grants 1R01CA179072-01A1 (Mintz), R01 CA184091-01 (Li) and P30 CA012197 (Pasche, Comprehensive Cancer Center of Wake Forest University (CCCWFU).
References
- 1.Reardon DA, Wen PY. Therapeutic advances in the treatment of glioblastoma: rationale and potential role of targeted agents. The oncologist. 2006;11(2):152–164. doi: 10.1634/theoncologist.11-2-152. [DOI] [PubMed] [Google Scholar]
- 2.Bissell MJ, Radisky D. Putting tumours in context. Nature reviews Cancer. 2001;1(1):46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8(8):610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 4.Price SJ, Jena R, Burnet NG, et al. Improved delineation of glioma margins and regions of infiltration with the use of diffusion tensor imaging: an image-guided biopsy study. AJNR American journal of neuroradiology. 2006;27(9):1969–1974. [PMC free article] [PubMed] [Google Scholar]
- 5.Frosina G. DNA repair and resistance of gliomas to chemotherapy and radiotherapy. Molecular cancer research : MCR. 2009;7(7):989–999. doi: 10.1158/1541-7786.MCR-09-0030. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez YP, Weatherbee JL, Wheelhouse RT, Ross AH. Glioblastoma multiforme therapy and mechanisms of resistance. Pharmaceuticals (Basel, Switzerland) 2013;6(12):1475–1506. doi: 10.3390/ph6121475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. Journal of anatomy. 2002;200(6):629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiology of Disease. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal S, Manchanda P, Vogelbaum MA, Ohlfest JR, Elmquist WF. Function of the Blood-Brain Barrier and Restriction of Drug Delivery to Invasive Glioma Cells: Findings in an Orthotopic Rat Xenograft Model of Glioma. Drug Metabolism and Disposition. 2013;41(1):33–39. doi: 10.1124/dmd.112.048322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawkins BT, Davis TP. The Blood-Brain Barrier/Neurovascular Unit in Health and Disease. Pharmacological Reviews. 2005;57(2):173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 11.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2005;2(1):3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood–brain Barrier: Structural Components and Function Under Physiologic and Pathologic Conditions. Journal of Neuroimmune Pharmacology. 2006;1(3):223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 13.Wolburg H, Lippoldt A. Tight junctions of the blood–brain barrier: development, composition and regulation. Vascular Pharmacology. 2002;38(6):323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 14.Etame AB, Diaz RJ, Smith CA, Mainprize TG, Kullervo HH, Rutka JT. Focused ultrasound disruption of the blood brain barrier: a new frontier for therapeutic delivery in molecular neuro-oncology. Neurosurgical focus. 2012;32(1):E3–E3. doi: 10.3171/2011.10.FOCUS11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leinenga G, Gotz J. Scanning ultrasound removes amyloid-beta and restores memory in an Alzheimer’s disease mouse model. Science translational medicine. 2015;7(278):278ra233. doi: 10.1126/scitranslmed.aaa2512. [DOI] [PubMed] [Google Scholar]
- 16.Liu H-L, Hua M-Y, Chen P-Y, et al. Blood-Brain Barrier Disruption with Focused Ultrasound Enhances Delivery of Chemotherapeutic Drugs for Glioblastoma Treatment. Radiology. 2010;255(2):415–425. doi: 10.1148/radiol.10090699. [DOI] [PubMed] [Google Scholar]
- 17.Xiong X, Sun Y, Sattiraju A, et al. Remote Spatiotemporally Controlled and Biologically Selective Permeabilization of Blood-Brain Barrier. Journal of controlled release: official journal of the Controlled Release Society. 2015;217:113–120. doi: 10.1016/j.jconrel.2015.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumann BC, Kao GD, Mahmud A, et al. Enhancing the Efficacy of Drug-loaded Nanocarriers against Brain Tumors by Targeted Radiation Therapy. Oncotarget. 2013;4(1):64–79. doi: 10.18632/oncotarget.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.d’Avella D, Cicciarello R, Angileri FF, Lucerna S, La Torre D, Tomasello F. Radiation-induced blood-brain barrier changes: pathophysiological mechanisms and clinical implications. Acta neurochirurgica Supplement. 1998;71:282–284. doi: 10.1007/978-3-7091-6475-4_82. [DOI] [PubMed] [Google Scholar]
- 20.Gaber MW, Sabek OM, Fukatsu K, Wilcox HG, Kiani MF, Merchant TE. Differences in ICAM-1 and TNF-alpha expression between large single fraction and fractionated irradiation in mouse brain. International journal of radiation biology. 2003;79(5):359–366. doi: 10.1080/0955300031000114738. [DOI] [PubMed] [Google Scholar]
- 21.Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: A review. Frontiers in oncology. 2012;2:73. doi: 10.3389/fonc.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong JH, Chiang CS, Campbell IL, Sun JR, Withers HR, McBride WH. Induction of acute phase gene expression by brain irradiation. International journal of radiation oncology, biology, physics. 1995;33(3):619–626. doi: 10.1016/0360-3016(95)00279-8. [DOI] [PubMed] [Google Scholar]
- 23.Pandya DN, Hantgan R, Budzevich MM, et al. Preliminary Therapy Evaluation of (225)Ac-DOTA-c(RGDyK) Demonstrates that Cerenkov Radiation Derived from (225)Ac Daughter Decay Can Be Detected by Optical Imaging for In Vivo Tumor Visualization. Theranostics. 2016;6(5):698–709. doi: 10.7150/thno.14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wadas TJ, Pandya DN, Solingapuram Sai KK, Mintz A. Molecular Targeted α-Particle Therapy for Oncologic Applications. American Journal of Roentgenology. 2014;203(2):253–260. doi: 10.2214/AJR.14.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen V, Conyers JM, Zhu D, et al. A novel ligand delivery system to non-invasively visualize and therapeutically exploit the IL13Ralpha2 tumor-restricted biomarker. Neuro-oncology. 2012;14(10):1239–1253. doi: 10.1093/neuonc/nos211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65(1):71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Advanced Drug Delivery Reviews. 2011;63(3):170–183. doi: 10.1016/j.addr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotech. 2015;33(9):941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi H, Watanabe R, Choyke PL. Improving Conventional Enhanced Permeability and Retention (EPR) Effects; What Is the Appropriate Target? Theranostics. 2014;4(1):81–89. doi: 10.7150/thno.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang R, Harmsen S, Samii JM, et al. High Precision Imaging of Microscopic Spread of Glioblastoma with a Targeted Ultrasensitive SERRS Molecular Imaging Probe. Theranostics. 2016;6(8):1075–1084. doi: 10.7150/thno.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnell O, Krebs B, Wagner E, et al. Expression of Integrin α(v)β(3) in Gliomas Correlates with Tumor Grade and Is not Restricted to Tumor Vasculature. Brain Pathology (Zurich, Switzerland) 2008;18(3):378–386. doi: 10.1111/j.1750-3639.2008.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnett CA, Xie J, Quijano J, et al. Synthesis, in vitro, and in vivo characterization of an integrin αvβ3-targeted molecular probe for optical imaging of tumor. Bioorganic & Medicinal Chemistry. 2005;13(11):3763–3771. doi: 10.1016/j.bmc.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 33.Chen K, Chen X. Integrin Targeted Delivery of Chemotherapeutics. Theranostics. 2011;1:189–200. doi: 10.7150/thno/v01p0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu AR, Veeravagu A, Cai W, Hou LC, Tse V, Chen X. Integrin alpha v beta 3 antagonists for anti-angiogenic cancer treatment. Recent patents on anti-cancer drug discovery. 2007;2(2):143–158. doi: 10.2174/157489207780832469. [DOI] [PubMed] [Google Scholar]
- 35.Kim YS, Li F, Kong R, et al. Multivalency of non-peptide integrin alphaVbeta3 antagonist slows tumor growth. Molecular pharmaceutics. 2013;10(10):3603–3611. doi: 10.1021/mp400096z. [DOI] [PubMed] [Google Scholar]
- 36.Lim EH, Danthi N, Bednarski M, Li KC. A review: Integrin alphavbeta3-targeted molecular imaging and therapy in angiogenesis. Nanomedicine : nanotechnology, biology, and medicine. 2005;1(2):110–114. doi: 10.1016/j.nano.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Liu Z, Wang F, Chen X. Integrin α(v)β(3)-Targeted Cancer Therapy. Drug development research. 2008;69(6):329–339. doi: 10.1002/ddr.20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qin G, Li Z, Xia R, et al. Partially polymerized liposomes: stable against leakage yet capable of instantaneous release for remote controlled drug delivery. Nanotechnology. 2011;22(15):155605. doi: 10.1088/0957-4484/22/15/155605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong X, Fung S, Tung C-H, Li KC. Partially Polymerized Liposomes for Targeted Drug Delivery, Controlled Drug Release and Molecular Imaging. WMIS annual meeting. 2013 [Google Scholar]
- 40.Dubois LG, Campanati L, Righy C, et al. Gliomas and the vascular fragility of the blood brain barrier. Frontiers in Cellular Neuroscience. 2014;8(418) doi: 10.3389/fncel.2014.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy. 2015;19:1–12. doi: 10.1016/j.drup.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Wolburg H, Noell S, Fallier-Becker P, Mack AF, Wolburg-Buchholz K. The disturbed blood-brain barrier in human glioblastoma. Molecular aspects of medicine. 2012;33(5–6):579–589. doi: 10.1016/j.mam.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Hurst RE, Bastian A, Bailey-Downs L, Ihnat MA. Targeting dormant micrometastases: rationale, evidence to date and clinical implications. Therapeutic Advances in Medical Oncology. 2016;8(2):126–137. doi: 10.1177/1758834015624277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rulseh AM, Keller J, Klener J, et al. Long-term survival of patients suffering from glioblastoma multiforme treated with tumor-treating fields. World Journal of Surgical Oncology. 2012;10(1):1–6. doi: 10.1186/1477-7819-10-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Debinski W, Tatter SB. Convection-enhanced delivery for the treatment of brain tumors. Expert review of neurotherapeutics. 2009;9(10):1519–1527. doi: 10.1586/ern.09.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson JC, McFarland BC, Gladson CL. New molecular targets in angiogenic vessels of glioblastoma tumours. Expert reviews in molecular medicine. 2008;10:e23. doi: 10.1017/S1462399408000768. [DOI] [PMC free article] [PubMed] [Google Scholar]


