Abstract
Previous studies in general people indicated that hypertensive disorders of pregnancy (HDP) increased the risk of subsequent hypertension after delivery. Some studies found that women with gestational diabetes mellitus (GDM) had an increased risk of HDP. However, very few studies have assessed the association between HDP and the risk of postpartum hypertension among GDM women. To evaluate the association between HDP and the risk of postpartum hypertension among GDM women, a retrospective cohort study was conducted in 1261 women with prior GDM at their postpartum 1–5 years using the baseline data from Tianjin Gestational Diabetes Mellitus Prevention Program. Cox regression models were applied to assess the single and joint associations of having a history of HDP, maternal pre-pregnancy Body mass index (BMI) (normal weight, overweight and obesity), and weight change from preconception to post-delivery with the risk of subsequent hypertension among the GDM women. We found that GDM women with a history of HDP, high pre-pregnancy BMI and weight gain more than 7 kg from preconception to post-delivery had an increased risk of postpartum hypertension. Joint effects analysis revealed that the positive association between a history of HDP in the index pregnancy and the risk of postpartum hypertension was consistent in GDM women with different levels of pre-pregnancy BMI or weight gain from preconception to post-delivery. In conclusion, a history of HDP, high pre-pregnancy BMI and weight gain more than 7 kg from preconception to post-delivery increase the risk of subsequent hypertension in postpartum 1–5 years among GDM women.
Keywords: hypertensive disorders of pregnancy, gestational diabetes mellitus, hypertension postpartum, diabetes
INTRODUCTION
Hypertensive disorders of pregnancy (HDP), as a common complication in pregnancy, is one of the leading causes of maternal morbidity and mortality (ref. 1). Furthermore, HDP is associated with the risk of various diseases, such as coronary heart disease (CHD), stroke, kidney disease, diabetes, and chronic hypertension later in life. (ref. 2, 3, 4, 5, 6) Several studies have demonstrated the influence of HDP on the long-term risk of incident hypertension (ref. 2, 3, 4, 5, 7). As said in the Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy, “Little is known of this entity, and, like gestational hypertension, it may be a predictor of future chronic hypertension” (ref. 8). Following the increase of obesity and maternal age in recent decades, the prevalence of gestational hypertension and pre-eclampsia has also increased (ref. 4, 9).
Since 1990s, researchers found that women with gestational diabetes mellitus (GDM) had an increased risk of HDP (ref. 10). GDM, defined as glucose intolerance with onset or first recognition during pregnancy (ref. 11), was associated with 7-fold higher risk in developing Type 2 diabetes (ref. 12). Simultaneously, hypertension often co-exists with diabetes (ref. 13). We do not know if GDM combined with HDP in the pregnancy increases the risk of subsequent hypertension after delivery. Two reviews on GDM/diabetic women with hypertensive pregnancy (ref. 14, 15) have suggested that both GDM and HDP were associated with vascular dysfunction, which may be related with developing later hypertension. However, there still lacks studies regarding to the risk of subsequent hypertension among this GDM/diabetic population. The aim of this study was to evaluate the association between HDP and the risk of hypertension in postpartum 1–5 years among women with GDM.
METHODS
GDM diagnosis process
All pregnant women living in the six urban districts of Tianjin have been screened for GDM at their 26–30 gestational weeks from 1999 (ref. 16). First, they participate in a one-hour oral glucose tolerance test (OGTT) with 50-g glucose load in their community health centers. Then, those who have glucose reading ≥ 7.8 mmol/L are referred to Tianjin Women’s and Children’s Health Center to undergo a 2-hour OGTT with 75-g glucose load. If the pregnant women meet the World Health Organization (WHO)’s criteria of diabetes (fasting glucose ≥ 7 mmol/L or 2-h glucose ≥ 11.1 mmol/L) or impaired glucose tolerance (IGT) (2-h glucose ≥ 7.8 mmol/L and <11.1 mmol/L), they would be diagnosed as GDM (ref. 17).
Study Population
We conducted a retrospective cohort study in 1261 GDM women at their postpartum 1–5 years using the baseline data from the Tianjin Gestational Diabetes Mellitus Prevention Program, (ref. 18) a randomized controlled trial (RCT) conducted among GDM women living in the six urban districts in Tianjin, China. A total of 4644 eligible women, who were diagnosed with GDM between 2005 and 2009, were invited to join the program by mailing. During August 2009 to July 2011, a total of 1263 GDM women finished the baseline survey. The recruitment, inclusion and exclusion criteria have been described in detail elsewhere. (ref. 18) All participants completed a self-administered questionnaire and underwent a physical examination. The questionnaire collected the women’s socio-demographic characteristics, including age, marital status, education (< 13 years, 13–16 years, and ≥16 years), family income (<5000 yuan/month, 5000–8000 yuan/month, and ≥8000 yuan/month), and occupation; family history of hypertension (parents and siblings); medical history of hypertension; history of HDP; pregnancy outcomes (pre-pregnancy weight and the number of births in the index pregnancy); dietary habits (using a self-administered food frequency questionnaire (FFQ) and 3-day 24-hour food records to measure the dietary habits during the past year); and lifestyle in the past year, including smoking status (non-smokers, former smokers, and current smokers), passive smoking, drinking status, sitting time and leisure-time physical activity (0 min/day, 1–29 min/day, and ≥30 min/day). Validation studies of the performance of 3-day 24-hour food records (ref. 19), the FFQ (ref. 19) and the above-mentioned questionnaire assessing physical activity (ref. 20) were conducted in the China National Nutrition and Health Survey in 2002. The physical examination included the measurement of body weight, height, and blood pressure (BP). 1261 women were included in this study after excluding women with chronic hypertension (n=2) at the index pregnancy (Figure 1). We collected written informed consents from all participants and this study was approved by the Human Subjects Committee of Tianjin Women’s and Children’s Health Center.
Figure 1.
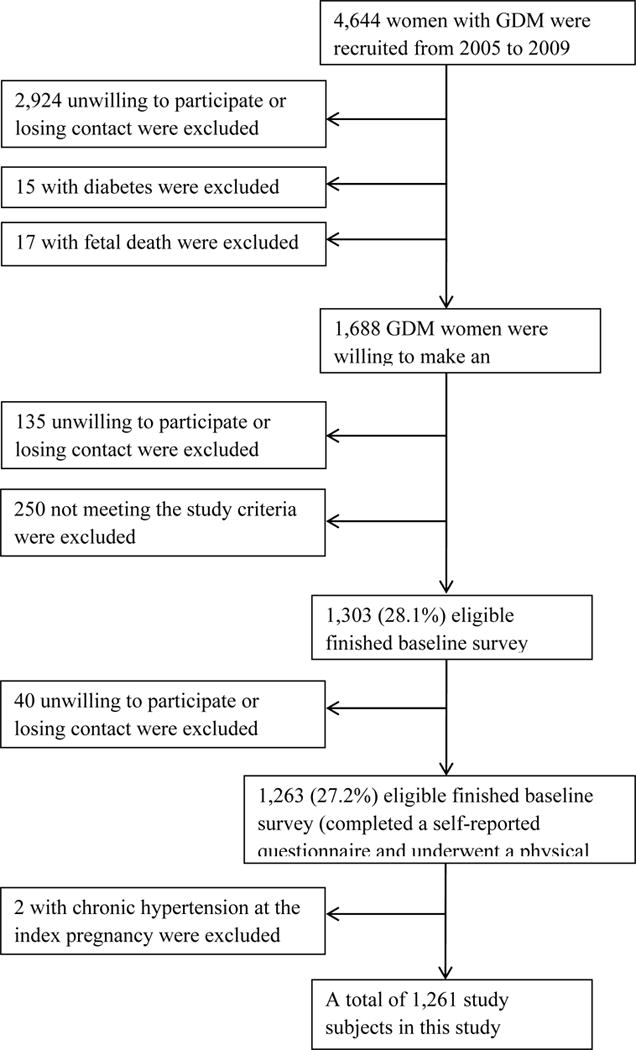
Participant flow chart.
History of hypertensive disorders of pregnancy
In the self-administered questionnaire, women who reported doctors-diagnosed hypertension after 20 gestational weeks, including gestational hypertension, preeclampsia, severe preeclampsia or eclampsia, were classified as having a history of HDP (ref. 21).
Pre-pregnancy BMI and weight change measurement
Using the standardized protocol, all participants’ height and weight were measured in light indoor clothing and without shoes by trained research doctors. Participants’ pre-pregnancy and current BMI were obtained by dividing their pre-pregnancy (self-reported) or current weight in kilograms by the square of height in meters. According to the Chinese BMI cut-offs (ref. 22), pre-pregnancy BMI was categorized as <24, 24 –27.9, and ≥28 kg/m2. Weight change from preconception to post-delivery 1–5 years was calculated based on their current weight and self-reported pre-pregnancy weight and was categorized as 4 groups: weight loss of ≥3 kg, loss of <3 kg to gain of <3 kg, gain of ≥3 kg to 7 kg, and gain of ≥7 kg (ref. 16).
Outcome variable
According to 2007 European Society of Hypertension (ESH)-European Society of Cardiology (ESC) Guidelines (ref. 23), hypertension was defined as systolic BP (SBP) ≥140 mmHg or diastolic BP (DBP) ≥90 mmHg or taking antihypertensive medicines. In the physical examination, we measured the BP twice from the right arm using a standard mercury sphygmomanometer after 5 min of rest with the subject in the sitting position. The mean of two BP measurements was used for the analysis. In addition, those using antihypertensive drugs and having non-hypertension in the physical examination were also included into the cases.
Statistical analysis
The general characteristics of GDM mothers with and without hypertension after delivery were reported as mean ± Standard deviation (SD) or percent, and then compared using T test and chi-square test. The single or joint associations of HDP, pre-pregnancy BMI, and weight change with the risk of hypertension were analyzed by Cox proportional hazards models. Different categories of pre-pregnancy BMI as well as weight change from preconception to post-delivery were included in the models. We also tested the significances of the trend over different categories in the same models with the median of each category as continuous variables. We performed the Cox regressions in three multivariable-adjusted models: model 1 adjusted for age; model 2 adjusted for age, number of births in the index pregnancy, education, family income, family history of hypertension, smoking status, passive smoking, drinking status, leisure-time physical activity, sitting time, total energy intake, dietary fiber intake, dietary sodium intake, dietary potassium intake, carbohydrates intake, and energy percent of monounsaturated fat, polyunsaturated fat and saturated fat intake; model 3 adjusted for the variables in model 2 as well as pre-pregnancy BMI, history of HDP and weight change. All statistical analyses were performed using IBM SPSS Statistics 23.0 (IBM SPSS, Chicago, IL) with a significance level at 0.05.
RESULTS
Table 1 showed the characteristics of the participants by their postpartum hypertension status. A total of 94 (7.45%) women developed an incident hypertension during a mean follow-up of 2.29 years postpartum. Women who developed hypertension during the follow-up were slightly older, were more pre-pregnancy overweight or obese, had a history of HDP (P<0.001) and family history of hypertension (P=0.013) as compared with those who remained free of hypertension.
Table 1.
Demographic characteristics of study subjects at baseline according to incident hypertension
| Hypertension (N=94) |
No hypertension (N=1167) |
P for differences | |
|---|---|---|---|
| Age, y | 33.3±3.8 | 32.3±3.5 | 0.006 |
| Singleton in this index pregnancy, % | 98.9 | 98.8 | 1.000 |
| Pre-pregnancy body mass index, kg/m2 | 25.6±3.77 | 22.9±3.18 | <0.001 |
| Pre-pregnancy body mass index, % | |||
| <24 kg/m2 | 31.9 | 68.0 | <0.001 |
| 24–27.9 kg/m2 | 42.6 | 25.3 | |
| ≥28 kg/m2 | 25.5 | 6.8 | |
| Self-reported hypertensive disorders of pregnancy, % | |||
| No | 74.5 | 94.4 | <0.001 |
| Yes | 25.5 | 5.6 | |
| Weight change from pre-pregnancy to postpartum 1–5 years, kg | 5.12±6.27 | 2.55±5.90 | <0.001 |
| Weight change from pre-pregnancy to postpartum 1–5 years, % | |||
| Loss of ≥3 kg | 7.4 | 10.7 | 0.001 |
| Loss of <3 kg – gain of <3 kg | 37.2 | 47.5 | |
| Gain of ≥3 kg to 7 kg | 20.2 | 23.5 | |
| Gain of ≥7 kg | 35.1 | 18.3 | |
| Education, % | |||
| <13 years | 29.8 | 21.9 | 0.178 |
| 13–16 years | 64.9 | 70.5 | |
| ≥16 years | 5.3 | 7.6 | |
| Income, % | |||
| <5000 yuan/month | 37.2 | 26.7 | 0.067 |
| 5000–8000 yuan/month | 35.1 | 37.1 | |
| ≥8000 yuan/month | 27.7 | 36.2 | |
| Family history of hypertension, % | |||
| No | 38.3 | 51.6 | 0.013 |
| Yes | 61.7 | 48.4 | |
| Smoking status, % | |||
| Non-smoker | 92.6 | 94.6 | 0.631 |
| Former smoker | 5.3 | 3.4 | |
| Current smoker | 2.1 | 2.0 | |
| Passive smoke, % | |||
| No | 43.6 | 46.4 | 0.597 |
| Yes | 56.4 | 53.6 | |
| Current drinking status, % | |||
| No | 76.6 | 78.3 | 0.697 |
| Yes | 23.4 | 21.7 | |
| Leisure-time physical activity, % | |||
| 0 minute/day | 85.1 | 78.2 | 0.138 |
| 1–29 minutes/day | 11.7 | 19.7 | |
| ≥30 minutes/day | 3.2 | 2.1 | |
| Sitting time, hours/day | 3.4±2.1 | 3.2±2.1 | 0.438 |
| Total energy intake, kcal/day | 1718±474 | 1690±438 | 0.549 |
| Dietary fiber intake, g/1000 kcal | 10.4±4.6 | 10.4±4.1 | 0.985 |
| Dietary sodium intake, mg/day | 3041±1429 | 2918±1317 | 0.386 |
| Dietary potassium intake, mg/day | 1827±612 | 1822±554 | 0.939 |
| Carbohydrates intake, g/day | 223±63 | 220±61 | 0.702 |
| Monounsaturated fat, % energy | 13.7±2.7 | 13.5±2.8 | 0.708 |
| Polyunsaturated fat, % energy | 12.2±3.1 | 12.0±3.0 | 0.512 |
| Saturated fat, % energy | 7.9±1.8 | 8.0±2.1 | 0.819 |
Values are means ± SD unless otherwise specified.
The multivariable-adjusted (age, number of births, education, family income, family history of hypertension, smoking status, passive smoking, drinking status, leisure-time physical activity, sitting time, total energy intake, dietary fiber intake, dietary sodium intake, dietary potassium intake, carbohydrates intake, and energy percent of monounsaturated fat, polyunsaturated fat and saturated fat intake – model 2) hazard ratios of hypertension associated with pre-pregnancy BMI (<24, 24–27.9, ≥28 kg/m2) were 1.00, 2.97, and 6.67 (P for trend <0.001) (Table 2). This positive association was still significant after further adjustment for history of HDP and weight change from preconception to post-delivery (P for trend <0.001). Women who reported a history of HDP had a 3.78-fold multivariable-adjusted risk (95% confidence intervals (CI) 2.29–6.24) of hypertension as compared with those who remained free of hypertension during pregnancy. This association remained significant after further adjustment for pre-pregnancy BMI and weight change. Meanwhile, we found a positive association between weight change and the risk of hypertension in the multivariable-adjusted model 2. Compared with women with prior GDM whose body weight remained stable (±3 kg), those with the excessive weight change (≥7 kg) had an 81% (hazard ratio: 1.81, 95% CI: 1.10–2.98) increased risk of incident hypertension.
Table 2.
Hazard ratios of hypertension by pre-pregnancy body mass index, history of hypertensive disorders of pregnancy and weight change from pre-pregnancy to postpartum 1–5 years
| No. of participants (N=1261) |
No. of cases (N=94) |
Person-years | Hazard Ratios (95% CIs)
|
|||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| Pre-pregnancy body mass index | ||||||
| <24 kg/m2 | 823 | 30 | 1881 | 1.00 | 1.00 | 1.00 |
| 24–27.9 kg/m2 | 335 | 40 | 771 | 3.25 (2.02–5.22) | 2.97 (1.82–4.82) | 2.76 (1.68–4.54) |
| ≥28 kg/m2 | 103 | 24 | 232 | 6.60 (3.85–11.3) | 6.67 (3.77–11.8) | 7.16 (3.95–13.0) |
| P for trend | <0.001 | <0.001 | <0.001 | |||
| History of hypertensive disorders of pregnancy | ||||||
| No | 1172 | 70 | 2669 | 1.00 | 1.00 | 1.00 |
| Yes | 89 | 24 | 214 | 3.91 (2.46–6.24) | 3.78 (2.29–6.24) | 2.75 (1.63–4.65) |
| Weight change from pre-pregnancy to postpartum 1–5 years | ||||||
| Loss of ≥3 kg | 132 | 7 | 293 | 0.89 (0.40–2.01) | 0.84 (0.36–1.92) | 0.41 (0.17–0.98) |
| Loss of <3 kg – gain of <3 kg | 589 | 35 | 1309 | 1.00 | 1.00 | 1.00 |
| Gain of ≥3 kg to 7 kg | 293 | 19 | 687 | 0.94 (0.54–1.65) | 0.93 (0.52–1.64) | 0.76 (0.43–1.35) |
| Gain of ≥7 kg | 247 | 33 | 594 | 1.84 (1.14–2.97) | 1.81 (1.10–2.98) | 1.23 (0.73–2.10) |
| P for trend | 0.016 | 0.020 | 0.038 | |||
Model 1 adjusted for age; model 2 adjusted for age, number of births, education, family income, family history of hypertension, smoking, passive smoking, alcohol drinking, leisure-time physical activity, sitting time, total energy intake, dietary fiber intake, dietary sodium intake, dietary potassium intake, carbohydrates intake, energy percent of monounsaturated fat, energy percent of polyunsaturated fat, and energy percent of saturated fat; model 3 adjusted for variables in model 2 and also pre-pregnancy body mass index, history of hypertensive disorders of pregnancy and weight change from pre-pregnancy to postpartum, other than the variable in the analyses.
Moreover, we examined the joint associations of the history of HDP, pre-pregnancy BMI and weight change from preconception to post-delivery with the risk of incident hypertension (Table 3). There was a positive association between a history of HDP and the risk of hypertension, and this association was consistent in the participants with different levels of pre-pregnancy BMI. Compared with GDM women normal weight before pregnancy and free of HDP, those who were obese and reported a history of HDP had a 17.3-fold (95% CI: 7.04–42.7) higher risk of hypertension (Model 2). For GDM women within the same weight change group, those with a history of HDP had a higher risk of hypertension than those without HDP. Compared with GDM women without HDP and weight gain <7 kg from preconception to post-delivery, those with a history of HDP and weight gain ≥7 kg had a 3.90-fold higher risk of developing incident hypertension (95%CI: 1.87–8.14). Meanwhile, the positive association between pre-pregnancy BMI and the risk of postpartum hypertension was persistent in the participants with different levels of weight change (<7 kg and ≥7 kg). Pre-pregnancy obese GDM women (BMI ≥28 kg/m2) with weight change ≥7 kg showed an 11.7-fold (95% CI 4.66–29.3) higher risk of the development of hypertension compared with pre-pregnancy underweight GDM women(BMI <23 kg/m2) with weight gain <7 kg. There was no evidence of effect modification by pre-pregnancy BMI and weight change from preconception to post-delivery with the history of HDP on the postpartum hypertension (all P values for interaction >0.05).
Table 3.
Hazard ratios of hypertension by pre-pregnancy body mass index, history of hypertensive disorders of pregnancy and weight change from pre-pregnancy to postpartum 1–5 years
| Pre-pregnancy body mass index (kg/m2) | History of hypertensive disorders of pregnancy | No. of participants | No. of cases | Person-years | Hazard Ratios (95% CIs)
|
||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||
| <24 | No | 787 | 25 | 1795 | 1.00 | 1.00 | 1.00 |
| <24 | Yes | 36 | 5 | 85 | 3.81 (1.45–10.0) | 3.75 (1.38–10.2) | 3.35 (1.22–9.21) |
| 24–27.9 | No | 297 | 28 | 676 | 3.01 (1.76–5.16) | 2.79 (1.61–4.84) | 2.71 (1.56–4.71) |
| 24–27.9 | Yes | 38 | 12 | 94 | 8.11 (4.07–16.2) | 7.77 (3.72–16.2) | 6.82 (3.22–14.4) |
| ≥28 | No | 88 | 17 | 198 | 6.20 (3.34–11.5) | 6.31 (3.30–12.1) | 6.52 (3.40–12.5) |
| ≥28 | Yes | 15 | 7 | 34 | 15.9 (6.80–37.0) | 17.3 (7.04–42.7) | 15.7 (6.34–39.1) |
| History of hypertensive disorders of pregnancy | Weight change from pre-pregnancy to postpartum 1–5 years | ||||||
| No | <7 Kg | 963 | 47 | 2170 | 1.00 | 1.00 | 1.00 |
| No | ≥7 Kg | 209 | 23 | 499 | 1.94 (1.17–3.20) | 1.95 (1.16–3.26) | 1.94 (1.16–3.27) |
| Yes | <7 Kg | 51 | 14 | 119 | 5.08 (2.78–9.28) | 4.90 (2.62–9.15) | 3.70 (1.97–6.95) |
| Yes | ≥7 Kg | 38 | 10 | 95 | 4.14 (2.08–8.21) | 3.90 (1.87–8.14) | 3.22 (1.53–6.75) |
| Pre-pregnancy body mass index (kg/m2) | Weight change from pre-pregnancy to postpartum 1–5 years | ||||||
| <24 | <7 Kg | 676 | 21 | 1527 | 1.00 | 1.00 | 1.00 |
| <24 | ≥7 Kg | 147 | 9 | 354 | 1.70 (0.78–3.71) | 1.69 (0.76–3.75) | 1.48 (0.66–3.32) |
| 24–27.9 | <7 Kg | 256 | 23 | 578 | 2.95 (1.63–5.34) | 2.62 (1.43–4.80) | 2.41 (1.31–4.45) |
| 24–27.9 | ≥7 Kg | 79 | 17 | 193 | 5.64 (2.97–10.7) | 5.46 (2.81–10.6) | 4.26 (2.15–8.45) |
| ≥28 | <7 Kg | 82 | 17 | 184 | 6.67 (3.51–12.7) | 6.77 (3.47–13.2) | 6.28 (3.21–12.3) |
| ≥28 | ≥7 Kg | 21 | 7 | 48 | 10.6 (4.49–25.0) | 11.7 (4.66–29.3) | 8.43 (3.28–21.7) |
Model 1 adjusted for age; model 2 adjusted for age, number of births, education, family income, family history of hypertension, smoking, passive smoking, alcohol drinking, leisure-time physical activity, sitting time, total energy intake, dietary fiber, dietary sodium intake, dietary potassium intake, carbohydrates intake, energy percent of monounsaturated fat, energy percent of polyunsaturated fat, and energy percent of saturated fat; model 3 adjusted for variables in model 2 and also pre-pregnancy body mass index, history of pregnancy-induced hypertension and weight change from pre-pregnancy to postpartum, other than the variable in the analyses.
DISCUSSION
We found a positive association between a history of HDP in the index pregnancy and the risk of postpartum hypertension among women with a history of GDM, and this positive association was consistent in this population with different levels of pre-pregnancy BMI or weight gain from preconception to post-delivery 1–5 years. Meanwhile, higher pre-pregnancy BMI and excessive weight change from preconception to post-delivery 1–5 years would increase the risk of postpartum hypertension among GDM women.
Several cohort studies in Scotland (ref. 3), Finland (ref. 4), Denmark (ref. 5), Japan (ref. 2) have indicated that a history of HDP increased the risk of hypertension later in life. A meta-analysis revealed that the relative risk for hypertension among pre-eclampsia women was 3.70 times higher after a mean follow-up of 14.1 years compared with that in women who did not develop pre-eclampsia (ref. 24). Another case-control study in Korea also suggested that early onset hypertension with end-organ dysfunction, smoking, and higher pre-pregnancy BMI were significant and independent predictors of chronic hypertension in women after delivery (ref. 25). However, these studies were all conducted among the general population.
Hypertension after GDM is generally described as one of the components of the metabolic syndrome (ref. 26). There is controversy about the impact of GDM combining with hypertensive pregnancy on the long-term hypertension risk. The US Nurses’ Health Study found that women with GDM have an increased risk of developing hypertension after pregnancy, even when considering their history of pregnancy hypertension (ref. 27). Another large longitudinal study in Netherland of 22 265 women indicated that women with a history of HDP were twice more likely to be diagnosed with hypertension in later life, however, they also found that a history of GDM was not associated with a higher prevalence of hypertension in later life after adjusting for HDP (ref. 28). Based on a GDM women cohort, we found a positive association between self-reported HDP and subsequent incident hypertension, even after considering pre-pregnancy BMI and weight change from preconception to post-delivery.
Several studies have assessed the association of pre-pregnancy BMI and weight change with postpartum hypertension among the general population. Hwang et al. indicated that a higher pre-pregnancy BMI was an independent predictor of chronic hypertension in general women after delivery (ref. 25). Moreover, Pirkola’s research suggested that pre-pregnancy overweight was a significant risk in developing hypertension in postpartum 20 years among GDM women (ref. 29). In consistence with previous literatures, our present study found that both pre-pregnancy overweight and large gestational weight gain were associated with an increased risk of postpartum hypertension among GDM women. Additionally, our previous work has already demonstrated that GDM women with large weight gain from pre-pregnancy to post-partum were more likely to develop the metabolic syndrome (ref. 16).
The mechanisms underlying these findings remain to be elucidated. Insulin resistance may be one of the missing links within the association between HDP and GDM on postpartum hypertension risk. Carpenter (ref. 14) reviewed various studies and demonstrated that the single and joint associations between GDM and HDP with the risk of later hypertension could be partly explained by insulin resistance. Solomon and Seely (ref. 30) also pointed out that GDM, obesity and excessive weight gain were associated with an increased risk of insulin resistance, which linked to essential hypertension as well as pregnancy-induced hypertension. Another possible explanation is permanent vascular damage, which may occur during HDP or GDM and subsequently contributes to the development of hypertension and cardiovascular disease in later life (ref. 14, 31).
Both GDM and HDP are common complications that can affect the health of the mother and child. Most of previous studies on the association of HDP with hypertension are conducted among the general population. Our study was a large-scale cohort study of women with GDM during their pregnancy in order to understand the effect of HDP on the hypertension in the postpartum period among this particular population. Additionally, we conducted this study in Tianjin, China, in order to understand the ethnic and cultural influences on the above association. However, the present study still has some limitations. First, HDP history and pre-pregnancy weight in our study was based on self-reported data, which may introduce recall bias. Nevertheless, validation studies in the United States and England have showed good concordance of hypertensive disorder during pregnancy between self-reported information and clinical records (ref. 32). Moreover, Roberts’s study supported that using broad rather than specific categories of the hypertension disorders of pregnancy could improve the ascertainment. (ref. 33) We used this strategy to ensure the accuracy of the self-reported data, but it led to the second limitation that HDP in this study was not further categorized, such as gestational hypertension, preeclampsia, and eclampsia, so we cannot separately investigate the effects of different pathophysiology and severity disorders on the subsequent incident hypertension in the postpartum 1–5 years. Third, the information of assisted reproductive technology pregnancies (ref. 34) and a history of sleep apnea (ref. 35) were not available in the present study; therefore, it should be cautious when generalizing the findings.
In conclusion, a history of HDP was associated with an increased risk of subsequent hypertension in postpartum 1–5 years among Chinese women with GDM, independent of pre-pregnancy BMI and weight change from preconception to post-delivery. Moreover, we found that high pre-pregnancy BMI and weight gain more than 7 kg from preconception to post-delivery were also associated with an increased risk of subsequent hypertension in postpartum 1–5 years among Chinese women with GDM. More attention should be paid to GDM women with pre-pregnancy overweight, a history of HDP and excessive weight gain from preconception to post-delivery.
Summary Table.
What is known about topic?
Hypertensive disorders of pregnancy is associated with the risk of various diseases, such as coronary heart disease, stroke, kidney disease, diabetes, and chronic hypertension later in life among general population.
Women with gestational diabetes mellitus had an increased risk of hypertensive disorders of pregnancy.
Gestational diabetes mellitus and hypertensive disorders of pregnancy were associated with vascular dysfunction.
What this study adds?
A history of hypertensive disorders of pregnancy increases the risk of incident postpartum hypertension among women with gestational diabetes.
High pre-pregnancy Body Mass Index and weight gain more than 7 kg from preconception to post-delivery increase the risk of subsequent hypertension in postpartum 1–5 years among women with gestational diabetes.
Acknowledgments
This work is supported by the grant from European Foundation for the Study of Diabetes (EFSD)/Chinese Diabetes Society (CDS)/Lilly programme for Collaborative Research between China and Europe, Tianjin Women’s and Children’s Health Center, and Tianjin Public Health Bureau. Dr. Hu was supported by grant from National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R01DK100790. We would also like to appreciate all families for participating in Tianjin Gestational Diabetes Mellitus Prevention Program. We would also like to appreciate all families for participating in Tianjin Gestational Diabetes Mellitus Prevention Program.
Sources of Funding
This study is supported by the Tianjin Women’s and Children’s Health Center, Tianjin Public Health Bureau, European Foundation for the Study of Diabetes (EFSD)/Chinese Diabetes Society (CDS)/Lilly programme for Collaborative Research between China and Europe, and the National Institute Of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R01DK100790.
Footnotes
Disclosures: None
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.World Health Organization. WHO Recommendations for prevention and treatment of pre-eclampsia and eclampsia. 2011 [PubMed] [Google Scholar]
- 2.Kurabayashia T, Mizunuma H, Kubota T, Kiyohara Y, Nagai K, Hayashi K. Pregnancy-induced hypertension is associated with maternal history and a risk of cardiovascular disease in later life: A Japanese cross-sectional study. Maturitas. 2013;75(3):227–31. doi: 10.1016/j.maturitas.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. Bmj. 2003;326(7394):845. doi: 10.1136/bmj.326.7394.845. Epub 2003/04/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannisto T, Mendola P, Vaarasmaki M, Jarvelin MR, Hartikainen AL, Pouta A, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127(6):681–90. doi: 10.1161/CIRCULATIONAHA.112.128751. Epub 2013/02/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53(6):944–51. doi: 10.1161/HYPERTENSIONAHA.109.130765. Epub 2009/05/13. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki H, Watanabe Y, Arima H, Kobayashi K, Ohno Y, Kanno Y. Short- and long-term prognosis of blood pressure and kidney disease in women with a past history of preeclampsia. Clinical and experimental nephrology. 2008;12(2):102–9. doi: 10.1007/s10157-007-0018-1. Epub 2008/01/09. [DOI] [PubMed] [Google Scholar]
- 7.Callaway LK, Mamun A, McIntyre HD, Williams GM, Najman JM, Nitert MD, et al. Does a history of hypertensive disorders of pregnancy help predict future essential hypertension? Findings from a prospective pregnancy cohort study. J Hum Hypertens. 2013;27(5):309–14. doi: 10.1038/jhh.2012.45. [DOI] [PubMed] [Google Scholar]
- 8.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. Epub 2013/10/24. [DOI] [PubMed] [Google Scholar]
- 9.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21(5):521–6. doi: 10.1038/ajh.2008.20. Epub 2008/04/26. [DOI] [PubMed] [Google Scholar]
- 10.Bryson CL, Ioannou GN, Rulyak SJ, Critchlow C. Association between gestational diabetes and pregnancy-induced hypertension. Am J Epidemiol. 2003;158(12):1148–53. doi: 10.1093/aje/kwg273. Epub 2003/12/04. [DOI] [PubMed] [Google Scholar]
- 11.Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes care. 1998;21(Suppl 2):B161–7. Epub 1998/08/15. [PubMed] [Google Scholar]
- 12.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–9. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 13.Contreras F, Rivera M, Vasquez J, De la Parte MA, Velasco M. Diabetes and hypertension physiopathology and therapeutics. J Hum Hypertens. 2000;14(Suppl 1):S26–31. doi: 10.1038/sj.jhh.1000983. Epub 2000/06/15. [DOI] [PubMed] [Google Scholar]
- 14.Carpenter MW. Gestational diabetes, pregnancy hypertension, and late vascular disease. Diabetes care. 2007;30:S246–S50. doi: 10.2337/dc07-s224. [DOI] [PubMed] [Google Scholar]
- 15.Kaaja R, Gordin D. Health after pregnancy in the mother with diabetes. Womens Health. 2015;11(4):471–6. doi: 10.2217/WHE.15.29. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Liu H, Qiao Y, Lv F, Zhang S, Wang L, et al. Metabolic syndrome of weight change from pre-pregnancy to 1–5 years post-partum among Chinese women with prior gestational diabetes. Diabet Med. 2015;32(11):1492–9. doi: 10.1111/dme.12790. Epub 2015/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Consultation WHO. Part 1: diagnosis and classification of diabetes mellitus. Geneva: World Health Organisation; 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. [Google Scholar]
- 18.Hu G, Tian H, Zhang F, Liu H, Zhang C, Zhang S, et al. Tianjin Gestational Diabetes Mellitus Prevention Program: study design, methods, and 1-year interim report on the feasibility of lifestyle intervention program. Diabetes research and clinical practice. 2012;98(3):508–17. doi: 10.1016/j.diabres.2012.09.015. Epub 2012/09/27. [DOI] [PubMed] [Google Scholar]
- 19.Li YP, He YN, Zhai FY, Yang XG, Hu XQ, Zhao WH, et al. [Comparison of assessment of food intakes by using 3 dietary survey methods] Zhonghua Yu Fang Yi Xue Za Zhi. 2006;40(4):273–80. Epub 2006/11/14. [PubMed] [Google Scholar]
- 20.Ma G, Luan D, Li Y, Liu A, Hu X, Cui Z, et al. Physical activity level and its association with metabolic syndrome among an employed population in China. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2008;9(Suppl 1):113–8. doi: 10.1111/j.1467-789X.2007.00451.x. Epub 2008/03/01. [DOI] [PubMed] [Google Scholar]
- 21.Yuan X, Liu H, Wang L, Zhang S, Zhang C, Leng J, et al. Gestational hypertension and chronic hypertension on the risk of diabetes among gestational diabetes women. Journal of diabetes and its complications. 2016;30(7):1269–74. doi: 10.1016/j.jdiacomp.2016.04.025. Epub 2016/05/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou B. [Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population] Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 2002;23(1):5–10. Epub 2002/05/17. [PubMed] [Google Scholar]
- 23.Mansia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 ESH-ESC Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Blood Press. 2007;16(3):135–232. doi: 10.1080/08037050701461084. Epub 2007/09/12. [DOI] [PubMed] [Google Scholar]
- 24.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. Bmj. 2007;335(7627):974. doi: 10.1136/bmj.39335.385301.BE. Epub 2007/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang JW, Park SJ, Oh SY, Chang SA, Lee SC, Park SW, et al. The Risk Factors That Predict Chronic Hypertension After Delivery in Women With a History of Hypertensive Disorders of Pregnancy. Medicine. 2015;94(42):0000000000001747. doi: 10.1097/MD.0000000000001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colatrella A, Loguercio V, Mattei L, Trappolini M, Festa C, Stoppo M, et al. Hypertension in diabetic pregnancy: impact and long-term outlook. Best practice & research Clinical endocrinology & metabolism. 2010;24(4):635–51. doi: 10.1016/j.beem.2010.05.003. Epub 2010/09/14. [DOI] [PubMed] [Google Scholar]
- 27.Tobias DK, Hu FB, Forman JP, Chavarro J, Zhang C. Increased risk of hypertension after gestational diabetes mellitus: findings from a large prospective cohort study. Diabetes care. 2011;34(7):1582–4. doi: 10.2337/dc11-0268. Epub 2011/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heida KY, Franx A, van Rijn BB, Eijkemans MJ, Boer JM, Verschuren MW, et al. Earlier Age of Onset of Chronic Hypertension and Type 2 Diabetes Mellitus After a Hypertensive Disorder of Pregnancy or Gestational Diabetes Mellitus. Hypertension. 2015;66(6):1116–22. doi: 10.1161/HYPERTENSIONAHA.115.06005. Epub 2015/10/16. [DOI] [PubMed] [Google Scholar]
- 29.Pirkola J, Pouta A, Bloigu A, Miettola S, Hartikainen AL, Jarvelin MR, et al. Prepregnancy overweight and gestational diabetes as determinants of subsequent diabetes and hypertension after 20-year follow-up. J Clin Endocrinol Metab. 2010;95(2):772–8. doi: 10.1210/jc.2009-1075. Epub 2009/12/03. [DOI] [PubMed] [Google Scholar]
- 30.Solomon CG, Seely EW. Brief review: hypertension in pregnancy : a manifestation of the insulin resistance syndrome? Hypertension. 2001;37(2):232–9. doi: 10.1161/01.hyp.37.2.232. Epub 2001/03/07. [DOI] [PubMed] [Google Scholar]
- 31.Hermes W, Franx A, van Pampus MG, Bloemenkamp KW, van der Post JA, Porath M, et al. 10-Year cardiovascular event risks for women who experienced hypertensive disorders in late pregnancy: the HyRAS study. BMC Pregnancy Childbirth. 2010;10:28. doi: 10.1186/1471-2393-10-28. Epub 2010/06/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dietz P, Bombard J, Mulready-Ward C, Gauthier J, Sackoff J, Brozicevic P, et al. Validation of self-reported maternal and infant health indicators in the Pregnancy Risk Assessment Monitoring System. Matern Child Health J. 2014;18(10):2489–98. doi: 10.1007/s10995-014-1487-y. Epub 2014/04/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts CL, Bell JC, Ford JB, Hadfield RM, Algert CS, Morris JM. The accuracy of reporting of the hypertensive disorders of pregnancy in population health data. Hypertens Pregnancy. 2008;27(3):285–97. doi: 10.1080/10641950701826695. Epub 2008/08/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomopoulos C, Salamalekis G, Kintis K, Andrianopoulou I, Michalopoulou H, Skalis G, et al. Risk of hypertensive disorders in pregnancy following assisted reproductive technology: overview and meta-analysis. J Clin Hypertens (Greenwich) 2017;19(2):173–83. doi: 10.1111/jch.12945. Epub 2017/01/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ip MSM, Lam B, Ng MMT, Lam WK, Tsang KWT, Lam KSL. Obstructive sleep apnea is independently associated with insulin resistance. American journal of respiratory and critical care medicine. 2002;165(5):670–6. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]


