Abstract
Objective
Our goal was to identify risk factors for acute kidney injury (AKI) in children surviving cardiac arrest (CA).
Design
Retrospective analysis of a public-access dataset.
Setting
Fifteen children’s hospitals associated with the Pediatric Emergency Care Applied Research Network.
Patients
Two hundred ninety-six subjects between 1 day and 18 years of age who experienced in-hospital or out-of-hospital CA between July 1, 2003, and December 31, 2004.
Interventions
None.
Measurements and Main Results
Our primary outcome was development of AKI as defined by the Acute Kidney Injury Network (AKIN) criteria. An ordinal probit model was developed. We found 6 critical explanatory variables, including total number of epinephrine doses, post-CA blood pressure, arrest location, presence of a chronic lung condition, pH, and presence of an abnormal baseline creatinine. Total number of epinephrine doses received as well as rate of epinephrine dosing impacted AKI risk and severity of AKI.
Conclusions
This study is the first to identify risk factors for AKI in children after CA. Our findings regarding the impact of epinephrine dosing are of particular interest and suggest potential for epinephrine toxicity with regard to AKI. The ability to identify and potentially modify risk factors for AKI after CA may lead to improved morbidity and mortality in this population.
Keywords: cardiac arrest, children, pediatric, outcome, acute kidney injury, epinephrine
INTRODUCTION
More than 10,000 children suffer cardiac arrest (CA) annually in the United States (1). Children who experience CA suffer a high rate of mortality and significant morbidity, although outcomes for in-hospital CA (IHCA) are improving (1–3). Acute kidney injury (AKI) is an established independent predictor of both mortality and morbidity in critically ill adults and children (4–15); there are, however, no prior studies of AKI after CA in children. Reliable identification of children most at risk for AKI after CA is necessary to devise strategies for prevention and therapeutic intervention.
Others have attempted to define AKI risk factors in critically ill pediatric populations, including post-cardiopulmonary bypass (CPB) and in a mixed pediatric intensive care unit (PICU) population (12, 16–19). These studies have identified diverse variables that may impact AKI risk, including age, weight, intra-operative events (including CPB time), and features of critical illness (e.g., hypoxemia, hypotension, sepsis, thrombocytopenia, and coagulopathy). Children who experience CA share features with these populations, but with important differences. The uncontrolled, often unmonitored, and abrupt interruption of circulation and oxygen delivery in CA make it distinct from both CPB and other states of pediatric critical illness and organ dysfunction characterized by impaired tissue perfusion and hypoxia. Thus, it is problematic to extrapolate findings from either of these populations to children who have experienced CA.
In adults after CA, total epinephrine dose, history of hypertension, congestive heart failure (CHF), cumulative fluid balance, presence of shock, age, sex, time to return of spontaneous circulation (ROSC), and chronic renal disease have all been identified as independent risk factors for AKI, while out-of-hospital CA (OHCA), experiencing CA in a public setting, and having a shockable initial rhythm were protective (15, 20–21). Although these data inform study of post-CA AKI in children, important differences between the populations limit generalization. Children differ in the prevalence and type of comorbid or pre-existing conditions, in age-related changes in baseline renal function, and in important features of the CA itself, such as etiology and initial rhythm (3).
Thus, despite appreciation of the negative impact of AKI on acutely ill patients, we lack adequate evaluation of pediatric post-CA AKI risk. Here, in a large, multicenter, observational cohort of children who have experienced CA, we aim to identify the incidence and possible risk factors for development of AKI, as defined by the Acute Kidney Injury Network (AKIN) criteria (22).
METHODS
Study Population
We employed a public access dataset from the Pediatric Emergency Care Applied Research Network (PECARN), created with support from the NICHD (HD044955). The database (1, 23–26) is derived from a retrospective cohort study of pediatric CA (July 1, 2003 – December 31, 2004), at 15 PECARN children’s hospitals. Subjects were eligible for inclusion in the database if they were 1 day to 18 years old, experienced CA requiring ≥1 minute of chest compressions, and had ROSC (either spontaneous or mechanically assisted circulation) for ≥20 minutes. OHCA classification was assigned if chest compressions were initiated prior to hospital arrival; IHCA classification was assigned if chest compressions were initiated in the emergency department or other hospital setting. Subjects with CA in a neonatal ICU and those who had planned circulatory arrest in the operating room as part of surgical repair for congenital heart disease were excluded.
Variables collected within the database include subjects’ baseline clinical characteristics, CA event characteristics, physiologic and laboratory data, information on hospital course, and outcome data. We considered over 40 candidate variables, chosen on the basis of availability within the database and either prior published evidence or physiologic plausibility (see supplement). Our candidate variables were prioritized for testing within the ordered probit model based on demonstrated association in the published literature (variables that were consistently found to be associated with AKI risk were given the highest priority) and then according to expert consensus. Variable definitions were based on the Utstein Style Guidelines, where possible (27).
Development of post-CA AKI was the primary outcome variable of interest and was defined and staged according to AKIN creatinine criteria, which specify (1) an increase in serum creatinine of ≥ 0.3 mg/dl or ≥ 150%–200% from baseline for AKIN Stage 1; (2) > 200%–300% for AKIN Stage 2; and (3) > 300% or ≥ 4.0 mg/dl (with an acute increase of at least 0.5 mg/dl) or new requirement for renal replacement therapy (RRT) for AKIN Stage 3 (22). Urine output data for AKIN staging was not available within the dataset. Baseline creatinine was defined as either (1) creatinine level obtained within the 2 hours prior to CA (available only for some IHCA subjects), or (2) the lowest measured creatinine in the 6 hours post-CA. The database did not include information on subject creatinine levels prior to 2 hours preceding the CA event. The peak creatinine in the 12–48 hours post-CA was used to calculate the percent change from baseline, which determined AKIN Stage. Subjects were identified as having an abnormal baseline creatinine if the baseline creatinine was >2 standard deviations above the published mean for age and sex (28). Published standards for individuals 1 year of age were used as the reference range for subjects under 1 year of age.
Systolic blood pressure (SBP) was analyzed as percent deviation from the 50th percentile for age and sex, assuming the 50th percentile for height (29). The impact of both pre-arrest (within 2 hours prior to CA) and post-arrest (within 6 hours after CA) SBP was assessed. Normative 50th percentile blood pressure data does not exist for children under one month of age; no value was assigned to those subjects. For subjects 1 month to 1 year of age, the normative standard for children 1 year of age was used (1).
Subjects were excluded if they did not survive ≥ 24 hours post-CA or if they lacked sufficient creatinine data (appropriate baseline measurement and at least one follow-up level 12–48 hours post-CA).
Statistical Analysis
We analyzed AKIN Stage as an ordinal outcome variable taking on 4 levels, corresponding to no AKI, AKIN Stage 1, AKIN Stage 2, and AKIN Stage 3. We therefore specified an ordinal probit model using the proportional odds regression function (polr) in the R statistical environment (http://www.r-project.org, replication data and code available upon request) to link a set of explanatory variables to AKIN Stage. This model specification provides standard regression parameters (where positive values push cases higher for AKIN Stage, and negative values push cases lower for AKIN Stage), as well as estimates of the “cutpoints” between categories on an underlying continuous metric.
Independent of the ordered probit model, we investigated cross-correlations between the explanatory variables and did not identify a degree of collinearity among the variables to alter our findings.
RESULTS
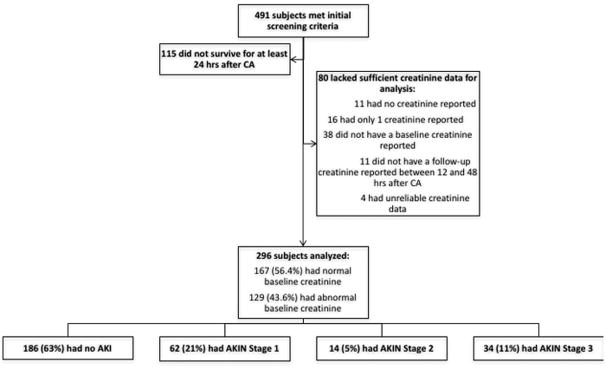
The parent dataset included 491 subjects who met the specified CA criteria; after exclusions, we identified 296 subjects with adequate data for analysis. Demographic data for our cohort is shown in Table 1; univariate comparison of our model variables is shown in Table 2. Within our cohort of 296 subjects, 186 (63%) did not meet AKI criteria. Among those who did meet AKI criteria, 62 (56%) were AKIN Stage 1, 14 (13%) were AKIN Stage 2, and 34 (31%) were AKIN Stage 3 (Figure 1). Nineteen subjects (6.4%) received dialysis within 48 hours post-CA.
Table 1.
Demographic Data of Infants and Children Experiencing Cardiac Arrest in our Cohort
| Demographics | No AKI | AKI | p |
|---|---|---|---|
| No. of subjects | 186 | 110 | |
| Age (months) (SD) | 47.3 (± 63.4) | 61.5 (± 77.1) | 0.0878 |
| Weight (kg) (SD)a | 16.1 (± 17.1) | 20.6 (± 23.1) | 0.0574 |
| Male (%) | 64.5 | 56.4 | 0.1648 |
| Race, n (%)b | |||
| American Indian | 2 (1.3%) | 0 (0%) | |
| Asian | 3 (1.9%) | 2 (2.1%) | |
| Black | 55 (35%) | 28 (29.8%) | |
| Pacific Islander | 1 (0.6%) | 0 (0%) | |
| White | 80 (51%) | 51 (54.2%) | |
| Other | 16 (10.2%) | 13 (13.8%) | |
| Ethnicity, n (%)c | |||
| Hispanic | 15 (15.6%) | 7 (17.9%) | |
| Non-Hispanic | 81 (84.4%) | 32 (82.1%) | |
The p value is calculated using the Student’s t-test.
n = 184 subjects in the No AKI category
n = 157 subjects in the No AKI category and 94 subjects in the AKI category
n = 96 subjects in the No AKI category and 39 subjects in the AKI category.
Table 2.
Univariate Comparison of Model Variables
| Variable | No AKI | AKI | p | |
|---|---|---|---|---|
| Total time to ROSC (min) | n | 140 | 84 | |
| mean (SD) | 21.5 (± 24.3) | 25.9 (± 32.2) | 0.2433 | |
| SEM | 2.1 | 3.5 | ||
| Arrest Location, n (%) | 186 | 110 | ||
| In-hospital (IH) | 128 (68.8%) | 92 (83.6%) | 0.0004 | |
| Out-of-hospital (OH) | 58 (31.2%) | 18 (16.4%) | ||
| Total Epinephrine Doses | n | 174 | 106 | |
| mean (SD) | 2.3 (± 1.9) | 3.4 (± 3.2) | 0.0001 | |
| SEM | 0.1 | 0.3 | ||
| Chronic Lung Condition, n (%) | 186 | 110 | ||
| 57 (30.6%) | 14 (12.7%) | 0.0057 | ||
| Post-CA SBP deviation from 50th %ile (mmHg) | n | 163 | 87 | |
| mean (SD) | −15.5 (+ 26.5) | −29.1 (± 30.3) | 0.0003 | |
| SEM | 2.1 | 3.2 | ||
| Minimum pH | n | 169 | 105 | |
| mean (SD) | 7.17 (± 0.24) | 7.11 (± 0.22) | 0.0475 | |
| SEM | 0.02 | 0.02 |
Fisher’s exact t-test was used for dichotomous variables, and Student’s t-test was used for continuous variables. The number of subjects with data available for analysis within the dataset for each variable is shown.
Figure 1.
Study subject identification and AKIN classification.
The model fit very well, with 6 of the 8 explanatory variables reaching the standard threshold for statistical reliability (Table 3). Predicted AKIN Stage increased as the total number of epinephrine doses increased, with a moderate strength of effect. When we further evaluate the total number of epinephrine doses indexed to minutes to ROSC, we observe risk progression from no AKI to AKIN Stage 2, but an unexpected lack of further progression in the risk for AKIN Stage 3. However, when we separate AKIN Stage 3 subjects into those who did and those who did not receive dialysis in the 48 hours post-CA, we observe the expected risk progression for AKIN Stage 3 in those who did receive dialysis (Figure 2). The numbers of subjects in these categories are small – 15 in AKIN Stage 3 without dialysis, and 19 with dialysis – and the decision to initiate dialysis in the original study was left to the individual physicians so that the timing and indications for dialysis initiation are likely to be highly variable.
Table 3.
Ordered Probit Model for AKIN Stage
| Estimate | Std. Error | t value | P value | |
|---|---|---|---|---|
| Variables | ||||
| log1p(CPR to ROSC (min)) | −0.03670 | 0.08516 | −0.43098 | 0.33402 |
| Total number of epinephrine doses | 0.06676 | 0.03215 | 2.07671 | 0.02076 |
| BP deviation from 50th %ile, pre-cardiac arrest | 0.00185 | 0.00309 | 0.60066 | 0.27535 |
| BP deviation from 50th %ile, post-cardiac arrest | −0.00644 | 0.00290 | −2.22195 | 0.01501 |
| Arrest Location in Hospital (vs. out-of-hospital) | −0.66929 | 0.21874 | −3.05975 | 0.00152 |
| Chronic Lung Condition | −0.53354 | 0.19920 | −2.67839 | 0.00394 |
| pH nadir | −0.92655 | 0.37212 | −2.48990 | 0.00753 |
| Abnormal Baseline Creatinine | 0.26176 | 0.15197 | 1.72244 | 0.04340 |
| Threshold Estimates | ||||
| No AKI to AKIN Stage 1 | −6.21765 | 2.68454 | −2.31609 | 0.01166 |
| AKIN Stage 1 to AKIN Stage 2 | −5.46853 | 2.68103 | −2.03971 | 0.02247 |
| AKIN Stage 2 to AKIN Stage 3 | −5.22834 | 2.67922 | −1.95144 | 0.02739 |
Figure 2.
Increasing number of epinephrine doses per minutes to ROSC increases the likelihood of AKIN Stage 3 with dialysis but not for AKIN Stage 3 without dialysis, suggesting an important difference in these two categories of severe AKI that requires further exploration. As demonstrated here, we observed that, when epinephrine dosing rate is low, the likelihood of developing severe AKI – AKIN Stage 2 and Stage 3 with dialysis – is correspondingly low, and either No AKI or AKIN Stage 1 is more likely after CA. The situation is reversed when epinephrine dosing rates are high, favoring development of more severe AKI.
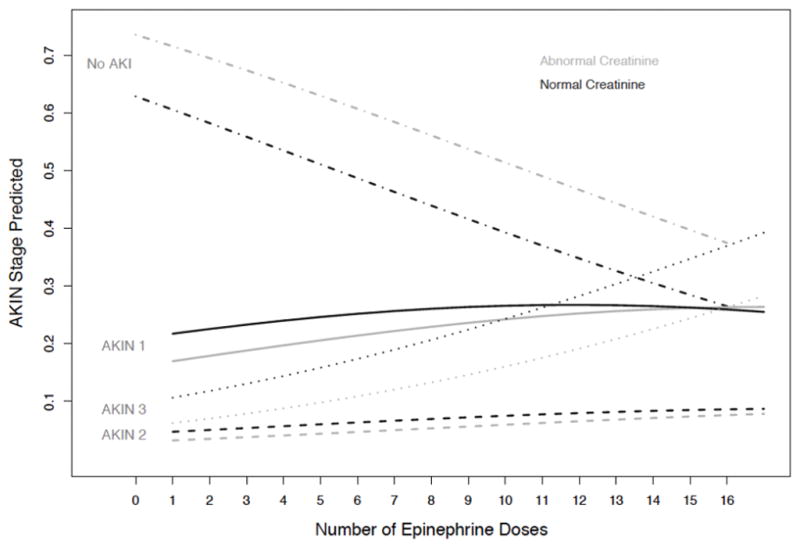
Baseline creatinine influenced the relationship between expected AKIN Stage and epinephrine dosing, most clearly altering the likelihood of no AKI as a function of epinephrine dosing (Figure 3). For AKIN Stage 1 or 2, the effects of total epinephrine dose and baseline creatinine were less pronounced, particularly after 8–9 epinephrine doses. The AKIN Stage 2 group was comprised of only 14 subjects, making a detailed understanding of the relationships among variables in this group difficult to achieve. The combined effects of total epinephrine doses and abnormal baseline creatinine were striking for AKIN Stage 3 risk; we observed an increase in AKIN Stage 3 likelihood with each epinephrine dose, with risk acceleration after 8–9 epinephrine doses.
Figure 3.
Expected AKIN stage in relation to number of epinephrine doses received for subjects with and without abnormal baseline creatinine. The likelihood of No AKI decreases while likelihood of AKIN Stages 1–3 increases with increasing number of epinephrine doses. This effect is reliably influenced by baseline creatinine, favoring more AKI when baseline creatinine is abnormal. Expected AKIN Stage 3 accelerates in likelihood after 8–9 epinephrine doses. The effect of having an abnormal versus normal baseline creatinine on likelihood of AKIN Stages 1 and 2 appears attenuated after 8–9 epinephrine doses.
In comparison to subjects whose post-CA blood pressure remained at or around the 50th percentile for age and sex, those with greater deviations in blood pressure were less likely to reach a higher AKIN Stage. This effect, while statistically reliable and important within the model, is extremely small.
Among the remaining variables in the final model, pH, presence of a chronic lung condition (CLC), and arrest location had a reliable effect on AKIN Stage. Subjects with lower pH (observed pH range was 6.4 to 7.64) had a greater likelihood of higher AKIN Stage. The presence of a CLC was associated with lesser likelihood of higher AKIN Stage (i.e., was “protective”). Subjects who experienced IHCA were more likely to reach a higher AKIN Stage than those who experienced OHCA.
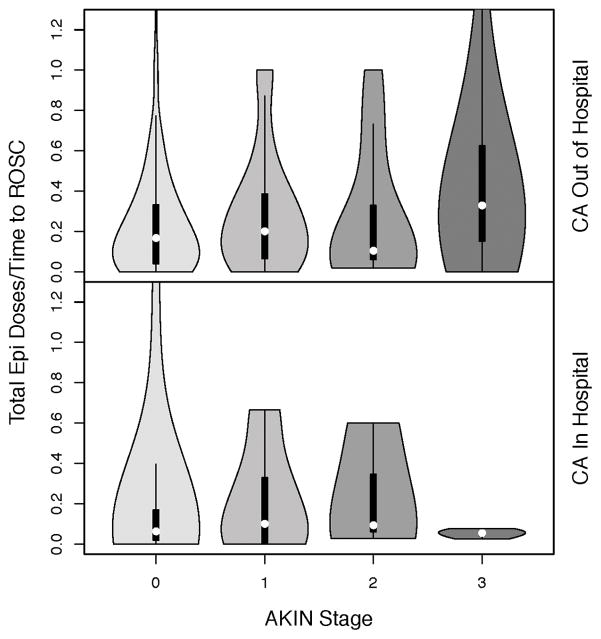
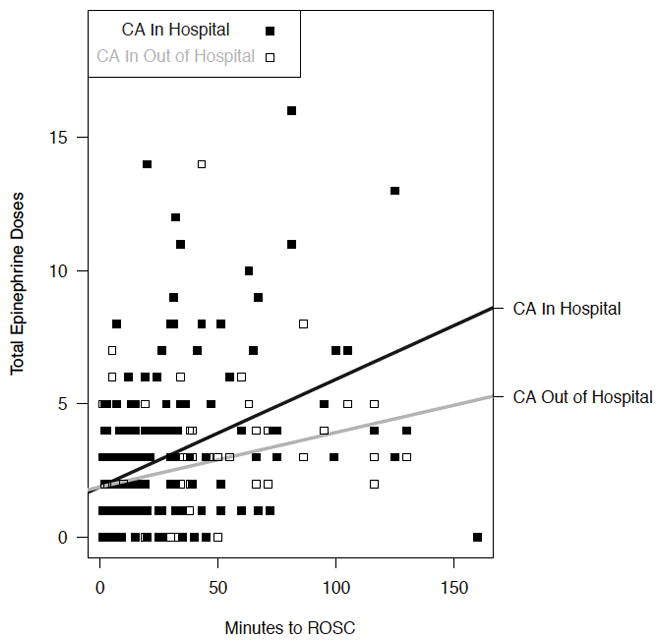
We identified a relationship among the total number of epinephrine doses received per minute to ROSC, arrest location, and AKIN Stage. The higher AKI risk subjects in our model experienced IHCA and received more epinephrine at a higher rate (Figure 4a). Subjects experiencing OHCA and receiving epinephrine at the highest rate reached AKIN Stage 3 more frequently, while those experiencing IHCA demonstrated a subtle increase in AKI of any stage (Figure 4b).
Figure 4.
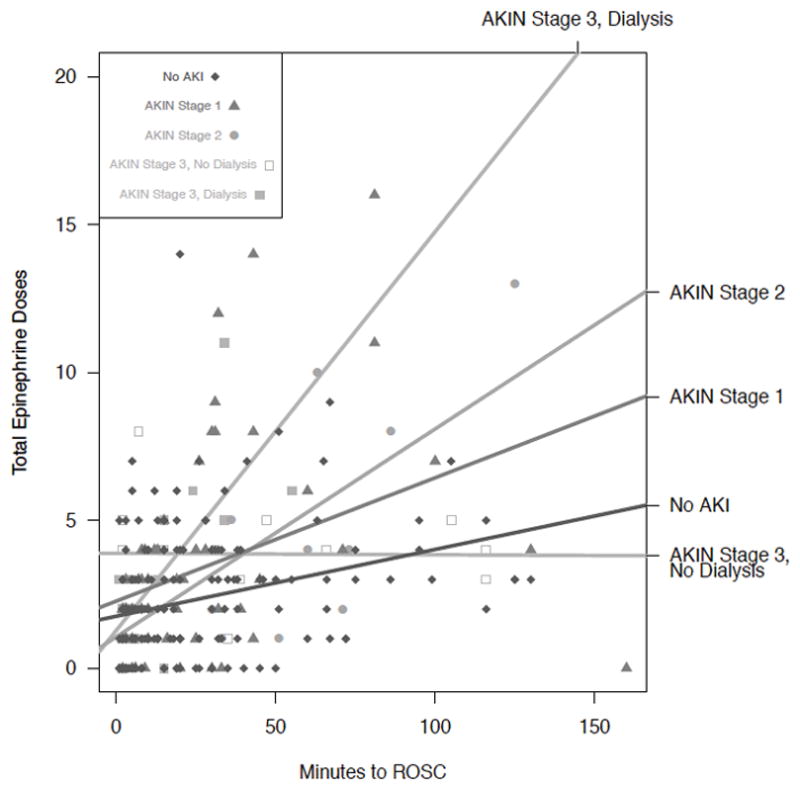
Figure 4a. The rate at which epinephrine was administered differed by arrest location in the cohort. We observed a higher predicted AKIN Stage for children with IHCA than those with OHCA.
Figure 4b. Violin plot demonstrating the relationships among epinephrine dosing rate, CA location, and AKIN Stage. Violin plots are similar to box plots but, by combining box plots with kernel density plots, are able to demonstrate the full distribution of the data rather than summary statistics alone. In our cohort, subjects experiencing IHCA and receiving a greater total epinephrine dose per time to ROSC reached AKIN Stage 3 AKI at a higher rate. For subjects experiencing OHCA, epinephrine dose per time to ROSC was slightly higher for those subjects who developed AKI of any stage.
To further illustrate the model, it is useful to examine exemplary hypothetical cases (Table 4). For each, we examine the influence of single variables while setting all others at the means observed in our cohort. For example, a hypothetical individual (#1, Table 4) with a normal baseline creatinine, the mean time to ROSC, and without a CLC who received 3 epinephrine doses during resuscitation has a 0.63747 probability of having no AKI and a 0.08996 probability of having AKIN Stage 3. If the number of epinephrine doses is doubled (#2, Table 4) the probability of no AKI is 12% lower, while that for AKIN Stage 3 is 41% greater; tripling the number of epinephrine doses (i.e., 9 doses) (#3, Table 4) further exacerbates this effect, with a probability of no AKI that is 25% lower and that for AKIN Stage 3 that is 93% greater than the original case. Within our model, the effect of CPR duration (time to ROSC), while important to the model, was not found to be independently statistically reliable. Thus, the effects of doubling and tripling the time to ROSC (# 4 and #5 in Table 4, respectively), demonstrate comparatively small effects on probability of no AKI and probability of AKIN Stage 3. Doubling the time to ROSC appears to increase the probability of no AKI by 3% and decrease the probability of AKIN Stage 3 by 8%, while tripling the time to ROSC appears to increase the probability of no AKI by 3.5% and decrease the probability of AKIN Stage 3 by 10%. If, on the other hand, we compare the first hypothetical individual to one with an abnormal baseline creatinine (#6, Table 4), the probability of no AKI is 16% lower, and that for AKIN Stage 3 is 56% greater. Thus, an abnormal baseline creatinine increases AKI risk in a degree comparable to increasing the number of epinephrine doses from 3 to 6. Alternatively, compared to the first case, a hypothetical individual with a CLC (#7, Table 4) has a probability of no AKI that is 27% higher and that for AKIN Stage 3 that is 66% lower. Finally, having both an abnormal baseline creatinine and exposure to 9 epinephrine doses (#8, Table 4) yields a probability of no AKI that is 41% lower and that for AKIN Stage 3 that is 176% higher than the first individual. The likelihood of no AKI and of AKIN Stage 3 outcomes for the lowest and highest risk cases in our examples differs by approximately 2-fold and 8-fold, respectively.
Table 4.
Expected AKIN Stage under Hypothetical Conditions
| No. | Baseline creatinine | Time to ROSC (min) | Chronic lung condition | No. of epinephrine doses | Probability of no AKI | Probability of AKIN Stage 3 |
|---|---|---|---|---|---|---|
| 1 | Normal | 24.67 | No | 3 | 0.63747 | 0.08996 |
| 2 | Normal | 24.67 | No | 6 | 0.56018 | 0.12699 |
| 3 | Normal | 24.67 | No | 9 | 0.48051 | 0.17349 |
| 4 | Normal | 49.34 | No | 3 | 0.65447 | 0.08276 |
| 5 | Normal | 74.01 | No | 3 | 0.65995 | 0.08051 |
| 6 | Abnormal | 24.67 | No | 3 | 0.53583 | 0.14024 |
| 7 | Normal | 24.67 | Yes | 3 | 0.81198 | 0.03043 |
| 8 | Abnormal | 24.67 | No | 9 | 0.37804 | 0.24867 |
DISCUSSION
This is the first analysis of incidence and potential risk factors for AKI after pediatric CA. In our cohort, 37% of subjects met AKI criteria within the first 48 hours post-CA, similar to the incidence reported in adults (15). The AKIN creatinine criteria were chosen here, recognizing this as the most conservative criteria for AKI among the consensus definitions (14). Use of the pRIFLE criteria would likely have identified more subjects with a less severe degree of AKI (i.e., the “Risk” category), while use of the KDIGO criteria would have yielded similar results, as we limited our time frame to 48 hours after CA in order to minimize confounding by post-CA factors that could contribute to AKI (14). The addition of urine output criteria, if available, would likely have led to a further increase in the detected AKI in our cohort (4).
Risk factors associated with AKI were higher number of epinephrine doses, abnormal baseline creatinine, in-hospital location of CA, lower pH, and post-CA BP deviation from the reference range. Presence of a chronic lung condition was protective. The model further demonstrates the interrelated nature of these variables in modifying AKI risk after CA.
We identified the total number of epinephrine doses as an important association with AKIN Stage after pediatric CA. Number of epinephrine doses correlates with total epinephrine dosage; as such, our findings generally affirm those in adult studies (15, 20). Similarly, there is a correlation between the number of epinephrine doses and the duration of resuscitation, a risk factor reported by Geri, et al (21). In our model, however, we failed to demonstrate a statistically reliable relationship between CPR duration and AKIN Stage. This apparent dominance of epinephrine effects over time to ROSC may have several explanations. The rate of epinephrine dosing may have been increased for those subjects perceived to be less likely to achieve ROSC. If the perception was accurate, CA survivors with a differing AKI risk profile may have been added to the cohort (they would otherwise have died). If the perception was inaccurate, some survivors may have been exposed to high epinephrine doses who would have attained ROSC with less aggressive epinephrine administration, resulting in increased exposure to epinephrine at the cost of increased end-organ injury – in this case AKI – as previously demonstrated in studies of high-dose epinephrine administration during pediatric resuscitation (30). It remains to be determined whether, in the setting of CA, total epinephrine dose itself – mediated through vasoconstrictive effects or other local or systemic toxic effects (31–34) – epinephrine dosing rate, or CPR duration is the more salient feature as we continue efforts to implement rapid deployment of high-quality CPR to optimize outcomes (35–36). Our observations, however, suggest the hypothesis that epinephrine dosing rate may be linked to AKI risk accrual. The associations among epinephrine dosing rates, AKI, and subsequent morbidity deserve more study, particularly as epinephrine exposure is a potentially modifiable risk factor during CA.
Our finding that abnormal baseline creatinine is associated with higher post-CA AKIN Stage is in agreement with the identification of chronic renal disease as a risk factor for AKI after CA (15). The presence of chronic renal failure (CRF), however, was not included in our final model. There are subtle but important differences between “abnormal baseline creatinine” and CRF. First, it is not clear that the baseline creatinine chosen for each subject is an accurate representation of pre-illness and pre-CA renal function. Since some subjects were hospitalized and others had already experienced CA at the time of their baseline creatinine measurement, the “baseline creatinine” may reflect some degree of acute change in healthy, steady-state renal function. Thus, we cannot reliably infer that subjects whose baseline creatinine exceeded the normal range would meet criteria for CRF or chronic renal disease. Second, CRF definition within the dataset is not clear and may not have included subjects with mild to moderate chronic kidney disease (CKD) and, consequently, an abnormal baseline creatinine. Moreover, the presence of CRF may not have been known at the time of data entry. As a general principle, however, we observed that diminished renal reserve prior to CA appears to increase the risk of further decrements in renal function thereafter.
Our study agrees with Tujjar, et al., in identifying OHCA as protective with respect to development of post-CA AKI (15). This finding may reflect differences in CA etiology, pre-arrest health status, comorbid conditions, in-hospital versus out-of-hospital resuscitative practices, and/or nephrotoxic exposures (unrelated to CA) between subjects in the outpatient versus inpatient setting. Of note, however, the OHCA protective effect was lost beyond a threshold of epinephrine exposure (8–9 doses). Thus, the importance of the subject’s baseline state may be outweighed by the impact of CA event characteristics such as epinephrine exposure.
Our findings differ from published adult data in several respects. Risk factors in adult subjects that were considered as candidate variables in our study but were not found to be explanatory in the final model included initial documented rhythm and history of CHF. Age and sex – while independently predictive in adult studies – were not found to be explanatory in our model but are indirectly present as part of the creatinine and blood pressure variables, as normative ranges for both are age-and sex-specific in children. The dataset did not include information either on prior history of chronic hypertension or on fluid balance, precluding analysis here.
Shock has been identified as an AKI risk factor in adults after CA (15, 21). Topjian, et al., recently demonstrated that early post-resuscitation hypotension was associated with increased mortality and poorer neurologic outcomes after pediatric CA, while Laverriere, et al., showed that longer duration of hypotension after pediatric CA was associated with increased mortality (1, 37 (abstract)). We demonstrated a reliably protective effect – though the strength of the effect was extremely small – in having a SBP with a greater degree of deviation from “normal.” This seems counterintuitive, and we were unable to demonstrate a statistically reliable relationship differentiating the effects of hypotension vs hypertension in our cohort. However, this difference could have important implications for post-arrest clinical management and should be studied in more detail in a larger cohort.
Our finding that subjects with a chronic lung condition (CLC) fared better in terms of expected AKIN Stage is novel. We propose several possible explanations. First, subjects with CLCs may be more likely to experience CA due to primary respiratory failure rather than other causes – etiology of the CA may be the important factor. Second, subjects with CLCs are more likely to experience chronic hypoxemia, possibly leading to “ischemic preconditioning,” which may be protective (38). Third, subjects with CLCs are commonly exposed to specific medication classes, such as corticosteroids, that may confer advantage with regard to AKI risk. Alternatively, the management of subjects with respiratory disease before, during, and after CA may significantly differ from that of other subjects, with either enhanced protective effect or reduced toxicity. This association should be verified, with particular attention to features of the CA, peri-arrest medical management, and pre-CA respiratory function.
Our study has several limitations. First, it is a post-hoc, retrospective review of a larger dataset, which was not designed to address questions about changes in renal function in association with CA; one of the effects of this is that nearly 40% of subjects from the parent dataset had to be excluded from our study, introducing the potential for ascertainment bias. Second, the dataset was generated over 10 years ago, and in that time, there have been significant changes in both post-CA and AKI care, including changes to AHA resuscitation guidelines, renewed emphasis on improving resuscitation quality, increased awareness of AKI, and improving post-CA outcomes. For this reason, evaluation in a more recent cohort is needed. Third, the challenges in using creatinine as a marker of renal function in non-steady state conditions are well-described and include a significant time lapse between renal injury and a rise in serum creatinine (39). The aforementioned difficulty in identifying true baseline renal function further limits analysis of changes in creatinine after CA. The comparisons between IHCA and OHCA subjects are also complicated by an unavoidable time delay in data collection for the OHCA subjects that is not equally present for the IHCA subjects. Our findings with regard to epinephrine dosing rate are descriptive only of an average rate of epinephrine dosing over the duration of resuscitation and may not accurately reflect moment-to-moment epinephrine dosing rates; given the retrospective nature of the dataset, there is also a possibility that recorded rates of epinephrine administration differ from the rate at which epinephrine was actually administered during resuscitation, a hypothesis which we are unable to adequately explore here. This is an area for future study. Also, some variation in epinephrine dosing rate may be attributable to intermittent return of pulse prior to sustained ROSC. Such cases may have had better renal perfusion during the CA as well as fewer doses of epinephrine and so may bias our model results. Finally, the limitations arising from small sample sizes within the AKIN Stages have been previously noted. Our findings should be viewed as hypothesis-generating rather than conclusive, and these challenges should inform a larger, prospectively designed study.
CONCLUSION
AKI after CA in children is a common event with important implications for both morbidity and mortality. Identifying risk factors for AKI, particularly those that may be subject to intervention or modification, may significantly improve outcomes in this challenging population. We have identified several factors, including subject baseline clinical characteristics, CA event characteristics – cumulative epinephrine exposure in particular – and physiologic and laboratory parameters, as important modifiers of AKI risk following CA in children. Further studies to verify our findings, to clarify relationships among variables, and to identify additional important modifiers are needed.
Supplementary Material
Footnotes
Financial Support used for Study: None.
Copyright form disclosure: Dr. Gill received funding from Corning Corporation. Drs. Dean, Moler, and Doctor received support for article research from the National Institutes of Health (NIH). Dr. Dean’s institution received funding from the NIH. Dr. Moler’s institution received funding from the NIH/National Heart, Lung, and Blood Insititution and the NIH/National Institute of Child Health and Human Development. Dr. Doctor’s institution received funding from the NIH, DoD, and Children’s Discovery Institute. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Work Performed at: Washington University School of Medicine, St. Louis, MO
Contributor Information
Tara M. Neumayr, Division of Critical Care Medicine and Division of Nephrology, Hypertension, and Pheresis, Department of Pediatrics, Washington University School of Medicine, St. Louis, MO.
Jeff Gill, Department of Surgery, Department of Political Science, and Department of Biostatistics, Washington University School of Medicine, St. Louis, MO.
Julie C. Fitzgerald, Division of Critical Care Medicine, Department of Anesthesiology and Critical Care Medicine, Children’s Hospital of Philadelphia, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
Avihu Z. Gazit, Division of Critical Care Medicine, Department of Pediatrics, Washington University School of Medicine, St. Louis, MO.
Jose A. Pineda, Division of Critical Care Medicine, Department of Pediatrics and Department of Neurology, Washington University School of Medicine, St. Louis, MO.
Robert A. Berg, Division of Critical Care Medicine, Department of Anesthesiology and Critical Care Medicine, Children’s Hospital of Philadelphia, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA.
J. Michael Dean, Department of Pediatrics, University of Utah, Salt Lake City, UT.
Frank W. Moler, Division of Critical Care Medicine, Department of Pediatrics, CS Mott Children’s Hospital, University of Michigan, Ann Arbor, MI.
Allan Doctor, Division of Critical Care Medicine, Department of Pediatrics and Department of Biochemistry and Molecular Biophysics, Washington University School of Medicine, St. Louis, MO.
References
- 1.Topjian AA, French B, Sutton RM, et al. Early postresuscitation hypotension is associated with increased mortality following pediatric cardiac arrest. Pediatr Crit Care Med. 2014;42(6):1518–1523. doi: 10.1097/CCM.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkins DL, Everson-Stewart S, Sears GK, et al. Epidemiology and outcomes from out-of-hospital cardiac arrest in children: the Resuscitation Outcomes Consortium Epistry - Cardiac Arrest. Circulation. 2009;119:1484–1491. doi: 10.1161/CIRCULATIONAHA.108.802678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girotra S, Spertus JA, Li Y, et al. Survival trends in pediatric in-hospital cardiac arrests: an analysis from Get WIth The Guidelines-Resuscitation. Circ Cardiovasc Qual Outcomes. 2013;6(1):42–49. doi: 10.1161/CIRCOUTCOMES.112.967968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaddourah A, Basu RK, Bagshaw SM, et al. Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults. NEJM. 2017;376(1):11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clermont G, Acker CG, Angus DC, et al. Renal failure in the ICU: comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomes. Kidney International. 2002;62:986–996. doi: 10.1046/j.1523-1755.2002.00509.x. [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 7.Akcan-Arikan A, Zappitelli M, Loftis LL, et al. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney International. 2007;71:1028–1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 8.Plotz FB, Bouma AB, van Wijk JAE, et al. Pediatric acute kidney injury in the ICU: an independent evaluation of pRIFLE criteria. Intensive Care Med. 2008;34:1713–1717. doi: 10.1007/s00134-008-1176-7. [DOI] [PubMed] [Google Scholar]
- 9.Hasper D, von Haehling S, Storm C, et al. Changes in serum creatinine in the first 24 hours after cardiac arrest indicate prognosis: an observational cohort study. Crit Care. 2009:13. doi: 10.1186/cc8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thakar CV, Christianson A, Freyberg R, et al. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med. 2009;37(9):2552–2558. doi: 10.1097/CCM.0b013e3181a5906f. [DOI] [PubMed] [Google Scholar]
- 11.Askenazi DJ, Ambalavanan N, Hamilton K, et al. Acute kidney injury and renal replacement therapy independently predict mortality in neonatal and pediatric noncardiac patients on extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12(1):e1–e6. doi: 10.1097/PCC.0b013e3181d8e348. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Krawczeski CD, Zappitelli M, et al. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery - a prospective multicenter study. Crit Care Med. 2011;39(6):1493–1499. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blinder JJ, Goldstein SL, Lee V, et al. Congenital heart surgery in infants: effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg. 2012;143:368–374. doi: 10.1016/j.jtcvs.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland SM, Byrnes JJ, Kothari M, et al. AKI in hospitilized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. 2015:10. doi: 10.2215/CJN.01900214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tujjar O, Mineo G, Dell’Anna A, et al. Acute kidney injury after cardiac arrest. Crit Care. 2015;19:169–181. doi: 10.1186/s13054-015-0900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey D, Phan V, Litalien C, et al. Risk factors of acute renal failure in critically ill children: a prospective descriptive epidemiological study. Pediatr Crit Care Med. 2007;8(1):29–35. doi: 10.1097/01.pcc.0000256612.40265.67. [DOI] [PubMed] [Google Scholar]
- 17.Chiravuri SD, Riegger LQ, Christensen R, et al. Factors associated with acute kidney injury or failure in children undergoing cardiopulmonary bypass: a case-controlled study. Pediatric Anesthesia. 2011;21:880–886. doi: 10.1111/j.1460-9592.2011.03532.x. [DOI] [PubMed] [Google Scholar]
- 18.Sethi SK, Goyal D, Yadav DK, et al. Predictors of acute kidney injury post-cardiopulmonary bypass in children. Clin Exp Nephrol. 2011;15:529–534. doi: 10.1007/s10157-011-0440-2. [DOI] [PubMed] [Google Scholar]
- 19.Morgan CJ, Zappitelli M, Robertson CMT, et al. Western Canadian Complex Pediatric Therapies Follow-Up Group. Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. J Pediatr. 2013;162(1):120–127. doi: 10.1016/j.jpeds.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 20.Domanovits H, Schillinger M, Mullner M, et al. Acute renal failure after successful cardiopulmonary resuscitation. Intensive Care Med. 2001;27:1194–1199. doi: 10.1007/s001340101002. [DOI] [PubMed] [Google Scholar]
- 21.Geri G, Guillemet L, Dumas F, et al. Acute kidney injury after out-of-hospital cardiac arrest: risk factors and prognosis in a large cohort. Intensive Care Med. 2015;41:1273–1280. doi: 10.1007/s00134-015-3848-4. [DOI] [PubMed] [Google Scholar]
- 22.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007:11. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moler FW, Meert K, Donaldson AE, et al. Pediatric Emergency Care Applied Research Network. In-hospital versus out-of-hospital pediatric cardiac arrest: a multicenter cohort study. Crit Care Med. 2009;37(7):2259–2267. doi: 10.1097/CCM.0b013e3181a00a6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meert KL, Donaldson A, Nadkarni V, et al. Pediatric Emergency Care Applied Research Network. Multicenter cohort study of in-hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2009;10(5):544–553. doi: 10.1097/PCC.0b013e3181a7045c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moler FW, Donaldson A, Meert K, et al. Pediatric Emergency Care Applied Research Network. Multicenter cohort study of out-of-hospital pediatric cardiac arrest. Crit Care Med. 2011;39(1):141–149. doi: 10.1097/CCM.0b013e3181fa3c17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett KS, Clark AE, Meert KL, et al. Pediatric Emergency Care Medicine Applied Research Network. Early oxygenation and ventilation measurements after pediatric cardiac arrest: lack of association with outcome. Crit Care Med. 2013;41(6):1534–1542. doi: 10.1097/CCM.0b013e318287f54c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs I, Nadkarni V. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update and simplification of the Utstein Templates for resuscitation registries. Circulation. 2004;110:3385–3397. doi: 10.1161/01.CIR.0000147236.85306.15. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz GJ, Haycock GB, Spitzer A. Plasma creatinine and urea concentration in children: normal values for age and sex. J Pediatr. 1976;88(5):828–830. doi: 10.1016/s0022-3476(76)81125-0. [DOI] [PubMed] [Google Scholar]
- 29.Falkner B, et al. L. National Heart, and Blood Institute; National Institutes of Health; U.S. Department of Health and Human Services, editor. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. 2005. [Google Scholar]
- 30.Perondi MBM, Reis RAAG, Paiva EF, et al. A comparison of high-dose and standard-dose epinephrine in children with cardiac arrest. NEJM. 2004;350:1722–1730. doi: 10.1056/NEJMoa032440. [DOI] [PubMed] [Google Scholar]
- 31.Rivers EP, Wortsman J, Rady MY, et al. The effect of the total cumulative epinephrine dose administered during human CPR on hemodynamic, oxygen transport, and utilization variables in the postresuscitation period. Chest. 1994;106(5):1499–1507. doi: 10.1378/chest.106.5.1499. [DOI] [PubMed] [Google Scholar]
- 32.Tang W, Weil MH, Sun S, et al. Epinephrine increases the severity of postresuscitation myocardial dysfunction. Circulation. 1995;92:3089–3093. doi: 10.1161/01.cir.92.10.3089. [DOI] [PubMed] [Google Scholar]
- 33.Arrich J, Sterz F, Herkner H, et al. Total epinephrine dose during asystole and pulseless electrical activity cardiac arrests is associated with unfavourable functional outcome and increased in-hospital mortality. Resuscitation. 2012;83:333–337. doi: 10.1016/j.resuscitation.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 34.Dumas F, Bougouin W, Geri G, et al. Is epinephrine during cardiac arrest associated with worse outcomes in resuscitated patients? J Am Coll Cardiol. 2014;64(22):2360–2367. doi: 10.1016/j.jacc.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 35.Institute of Medicine. Strategies to Improve Cardiac Arrest Survival: A Time to Act. Washington, DC: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 36.Neumar RW, Eigel B, Callaway CW, et al. American Heart Association Response to the 2015 Institute of Medicine Report on Strategies to Improve Cardiac Arrest Survival. Circulation. 2015;132:1049–1070. doi: 10.1161/CIR.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 37.Laverriere E, French B, Sanchez S, et al. Longer duration of hypotension is associated with worse outcomes after pediatric cardiac arrest. Critical Care Congress; 2016; Orlando, FL. [Google Scholar]
- 38.Zarbock A, Kellum JA. Remote ischemic preconditioning and protection of the kidney - a novel therapeutic option. Crit Care Med. 2016;44:607–616. doi: 10.1097/CCM.0000000000001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen MT, Devarajan P. Biomarkers for the early detection of acute kidney injury. Pediatr Nephrol. 2007 doi: 10.1007/s00467-007-0470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.