Abstract
Changes in the foveal anatomy during infancy are an important component in early development of spatial vision. The present longitudinal study in rhesus monkeys was undertaken to characterize the postnatal maturation of the fovea. Starting at four weeks after birth, the retinas of the left eyes of sixteen infant monkeys were imaged using spectral domain optical coherence tomography (SD OCT). Retinal scans were repeated every 30 days during the first year of life and every 60 days thereafter. Volume scans through the fovea were registered, scaled using a three surface schematic eye, and analyzed to measure foveal pit parameters. The individual layers of the retina were manually segmented and thicknesses were measured over a transverse distance of 1250 microns from the center of the foveal pit. Based on infrared scanning laser ophthalmoscope (IR SLO) images acquired with the SD OCT system, there were significant changes in the extent of the retina scanned as the eyes matured. Using a three-surface schematic eye, the length of each scan could be computed and was validated using image registration (R2 = 0.88, slope = 1.003, p<0.05). Over the first 18 months of life, the mean retinal thickness at the pit center had increased by 21.4% with a corresponding 20.3% decrease in pit depth. The major changes occurred within the first 120 days, but did not stabilize until a year after birth. In Macaca mulatta infants, the primary anatomical maturation of the fovea occurs within the first few months of life, as determined by longitudinal data from SD OCT measurements. The timelines for maturation of the fovea correspond well with the normal development of the lateral geniculate nucleus, cortical neurophysiology, and spatial resolution in monkeys.
Keywords: Fovea, development, optical coherence tomography, ocular magnification
1.0 INTRODUCTION
In primates, the visual system is relatively immature at birth, with high contrast visual acuity often measuring less than 5 cycles per degree (cpd) in both humans and monkeys, compared to acuities of 30–40 cpd in adults (Boothe et al., 1985; Kiorpes and Movshon, 1998; Movshon et al., 2005; Norcia et al., 1990; Ordy et al., 1965; Teller, 1997). The visual system improves rapidly over the first few months of life in both species, at about the same species adjusted relative rate (Boothe et al., 1985; Boothe et al., 1988), until adult-like levels are reached at about five years of age for children (Ellemberg et al., 1999; Mayer and Dobson, 1982; Teller, 1997) or 40 weeks of age for monkeys (Kiorpes, 1992; Kiorpes and Kiper, 1996). The improvement of visual performance early in life has been attributed to changes in the eye’s optics and retinal anatomy, along with neurologic maturation of the of afferent visual pathway (lateral geniculate nucleus and visual cortex, reviewed in Simona and National Research Council Committee on Vision (Simons and National Research Council (U.S.). Committee on Vision., 1993) and Wener and Chalupa (Werner and Chalupa, 2004)). The majority of investigations of early changes in visual optics and development of the fovea pit have been in old world monkeys, because of their similarity to humans.
Optical characteristics of the monkey eye have been measured and modeled by schematic eyes (Jacobs and Blakemore, 1988), double-pass ophthalmoscopy (Williams and Booth, 1981) and wavefront technology (Ramamirtham et al., 2006). These studies have shown that in primate eyes, the optical properties, including clarity and higher order aberrations, continue to improve up to 13 to 21 weeks of age. However, at birth the optics are relatively good and not considered a significant limit on visual resolution (Ramamirtham et al., 2007; Ramamirtham et al., 2006; Williams and Booth, 1981). In contrast, changes in ocular biometry, including axial length, corneal curvature and crystalline lens parameters, all of which have a significant influence on retinal magnification and hence spatial resolution, develop over longer periods of early life. Specifically, a combination of retinal magnification and cone spacing in the fovea pit predict a five-fold increase in spatial resolution during the first two years of life (Hendrickson and Kupfer, 1976; Jacobs and Blakemore, 1988; Packer et al., 1990).
Resolution limits, based on photoreceptor characteristics and density in the fovea pit also have been investigated in the development of visual acuity. Although the future fovea of the monkey eye can be identified as early as fifty days post-conception (Hendrickson and Kupfer, 1976), it is relatively immature at birth, with only a single or bilayer of cuboidal or columnar cone cells in the neonatal macaque foveal pit (Hendrickson, 1992; Provis et al., 1998; Springer and Hendrickson, 2004a). The corresponding peak cone density at birth in monkeys is 31–41% (Hendrickson, 1992), whereas in humans it is only 17% (Yuodelis and Hendrickson, 1986) that of the adult fovea (Hendrickson, 1993). Within the first year after birth, peak cone density for monkeys increases from 43,000 cones/mm2 to 210,000 cones/mm2 (Packer et al., 1990). In humans, cone density has been measured at 36,294 cones/mm2 at 5 days postnatal, and increases to 108,439 cones/mm2 by 45 months of age, but is still not adult like (208,203 cones/mm2) (Yuodelis and Hendrickson, 1986). The increase in cone density does not represent active mitosis (La Vail et al., 1991; Yuodelis and Hendrickson, 1986) but is a direct result of cone migration as is evident by a decrease in the rod free zone (Hendrickson and Kupfer, 1976; Packer et al., 1990). In addition the cone photoreceptors within the fovea become narrower and longer (inner segment diameter of 2 μm and 30–35 μm in length, while outer segments lengthen to 50–65 μm) (Hendrickson and Provis, 2006; Packer et al., 1990). These changes in the photoreceptors are thought to improve both the waveguide characteristics and efficiency of photon capture (Banks and Bennett, 1988; Wilson, 1988).
The retina can be assessed using non-invasive in vivo optical coherence tomography (OCT) imaging (Huang et al., 1991). The feasibility of using this technology for imaging the infant eye has been demonstrated by several investigators (Dubis et al., 2012; Lee et al., 2015; Maldonado et al., 2011; Rosen et al., 2015; Vajzovic et al., 2012; Vinekar et al., 2015), and several of these studies have investigated the maturation of the foveal region. However, the current understanding is primarily based on cross sectional data from histological (Hendrickson and Kupfer, 1976; Kiorpes et al., 2003; Packer et al., 1990; Springer and Hendrickson, 2005) and imaging studies that have only short follow up times. Hence, to establish accurate fovea maturational rates and trends, data from multiple time points for a larger number of subjects are needed. Although OCT imaging in comparison to histology is limited by resolution (i.e. cannot resolve individual cells using conventional imaging), it allows for longitudinal follow up, with accurate change analysis of morphology and retinal layers. As with most optical retinal imaging methodologies, the accuracy of OCT transverse measurements is dependent on the optics of the eye. Although schematic eyes have been used to compute relative retinal magnification (Bengtsson and Krakau, 1992; Bennett et al., 1994; Garway-Heath et al., 1998; Holden and Fitzke, 1988; Jacobs and Blakemore, 1988; Littmann, 1982; Maldonado et al., 2010; Patel et al., 2011; Qiao-Grider et al., 2007), they have not been validated in a growing non-human primate infant eye. The purpose of this study was to document the retinal layer changes in the maturing infant primate eye using OCT technology after compensation for changes in ocular magnification. Some of the findings were reported previously in abstract form (N Patel, et al. IOVS 2009; 50: ARVO E-Abstract 6207)
2.0 METHODS
2.1 Subjects
The subjects for longitudinal follow up were sixteen healthy full-term infant rhesus monkeys (Macaca mulatta). Housing and rearing for the infants have been previously described (Hung et al., 1995; Smith and Hung, 1999; Smith et al., 2013). For histological correspondence to OCT, 2 animals; an infant 10 days of age that was born full-term and a 6yr old young adult with no prior experimental intervention were used. All experimental and animal care procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Houston. The use of animals for these experiments confirmed to National Institutes of Health guidelines for the care and use of laboratory animals.
Animals used for the longitudinal follow up were also subjects for studies of refractive error development (Huang et al., 2009; Smith et al., 2009a; Smith et al., 2009b; Smith et al., 2013) and, therefore, only the left, control eyes were used for analyses, i.e., eyes that had no optical manipulation that could interfere with normal post-natal development of the eye. High myopia secondary to form deprivation has been associated with retinal thinning (Abbott et al., 2011), however, none of the subjects for this study developed excess myopia. Of the 16 animals in this study, 12 had selective hemi-field intervention in the right eye that did not exceed 3D, and 4 were normal controls with no optical intervention (details of refractive outcomes have been presented previously (Smith et al., 2009a; Smith et al., 2013; Smith et al., 2010)). Overall, there were no nasal temporal OCT structural asymmetries noted in any of the findings on the control eye data (see results section). The initial OCT macula scans were acquired around thirty days of age (36 ± 6 days) and, subsequently, every thirty days thereafter for the first year of life. After one year of age the eyes were scanned at sixty-day intervals until the animals were at least 1.5 years of age (627 ± 27 days).
2.2 Animal Preparation
Animals less than a year of age were anesthetized with an intramuscular injection of ketamine (15–20 mg/kg) and acepromazine maleate (0.15–0.2 mg/kg), while animals over a year of age were administered ketamine (20–25 mg/kg) and xylazine (0.4–0.6 mg/kg). Body temperature was maintained between 37 and 38 degrees Celsius with a thermostatically controlled electric blanket (TC1000 temperature controller, CWE, Ardmore, PA). Heart rate and blood oxygen were monitored with a pulse oximeter (model 9847V; Nonin Medical Inc, Plymouth, MN). Prior to imaging the retina, the pupils were dilated with topical tropicamide (1%) and phenylephrine (2.5%), and a plano-powered rigid gas permeable contact lens with similar back surface curvature as the cornea was placed on the eye to maintain optical clarity. Head stabilization was achieved using mouth and occipital bars attached to a rotational mount, enabling appropriate eye alignment for scanning.
2.3 Optical Coherence Tomography
All scans were acquired using the Spectralis OCT+HRA (Heidelberg Engineering, Heidelberg, Germany) system. At high resolution setting, the infra-red scanning laser ophthalmoscope (IR SLO) captures frames at 5 Hz, whereas OCT A-scans are acquired at 40,000 Hz, with 1536 A-scans per 30 degrees. Built in active eye tracking with this system minimizes eye movement artifact and allows for successive scan averaging, increasing the signal-to-noise ratio. The active eye tracking also makes this system ideal for follow up scans scanning identical regions overtime. However, in preliminary studies, due to eye growth and changes in ocular magnification, the instrument algorithm was unable to track the same region after approximately 90 days of age. For this study, images acquired and used for data analyses were high resolution IR SLO images of the optic nerve and fovea captured using a 30 degree scan angle without follow up tracking. Although IR light from imaging systems is generally safe (Delori et al., 2007; Zuclich et al., 2007), to minimize retinal irradiance to the SLO and SLD light sources, OCT scans were limited to a 37 line raster scan centered on the fovea covering a region 15 × 20 degrees, with averaging set at 9 frames.
2.4 Ocular Biometry and Relative Retinal Magnification
The normal development of anterior segment power and eye length of the infant eye results in changes in retinal image size, and transverse scaling of the retinal region scanned by imaging devices (Bennett et al., 1994; Littmann, 1982). Specifically, as the eye lengthens, the area of the retina scanned increases when identical scan parameters are used. Several methodologies have been used to compute transverse retinal scaling (Bennett et al., 1994; Garway-Heath et al., 1998; Littmann, 1982; Sanchez-Cano et al., 2008; Wakitani et al., 2003), but have not been validated in a growing eye. For this study a three surface schematic eye as described by Bennett and Rabbetts (Bennett and Rabbetts, 1989) was constructed for each eye and scan session to determine changes in retinal image size/transverse scaling. In brief, this model includes corneal curvature, axial length, anterior chamber depth and lens thickness. The constructed schematic is then used to quantify the dimensions, in millimeters or micrometers imaged by the IR SLO and SD OCT scans using the scan angle and second nodal point to retinal plane (N′M′) distance, assuming a spherical surface (Equation 1).
| Equation 1 |
Subsequently, the transverse scaling (μm/pixel) for each 20 degree B-scan consisting of 1024 A-scans was computed using equation 2.
| Equation 2 |
2.5 Scaling Validation
Changes in the extent of the retinal area imaged with eye development should correspond with those predicted by the schematic eye. To determine differences between schematic eye quantified and fundus image changes in retinal magnification, 30 degree IR SLO images centered on the optic nerve and fovea from a random scan date for each animal were used as baseline, and a baseline transverse scaling was computed using equation 1. The image series for each animal was subsequently registered to their respective baselines using a generalized dual bootstrap, iterative closest point algorithm (i2k retina, DualAlign, LLC, Clifton, NY) (Stewart et al., 2003). Based on the change in image size (Fig 1A), the scaling for each scan date was determined using equation 3.
| Equation 3 |
Figure 1.
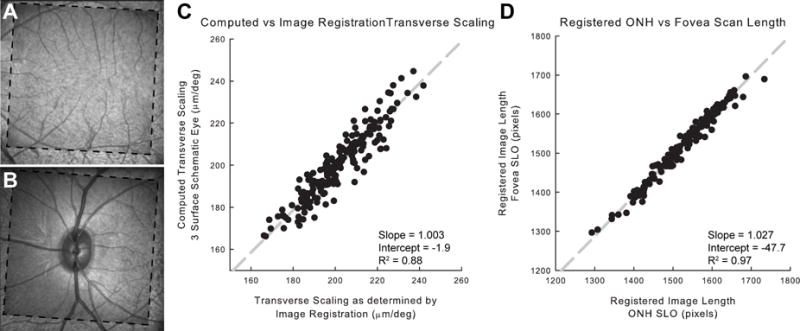
A. Thirty degree IR-SLO images centered on the fovea from 51 days (dashed outline) and 289 days of age in registration. B. IR-SLO images of the optic nerve in registration from the same animal and scan dates as in A. C. Comparison of image scaling using image registration and the three surface schematic eye. D. Validation of image registration technique, comparing IR-SLO image length from corresponding fovea and optic nerve registered scans.
Of the 195 scan dates, 188 scans of both optic nerve and fovea IR SLO images were successfully registered. Image scaling for fovea scans predicted by image registration was compared to that computed by the three surface schematic eye for each scan session (Fig 1C). There was good agreement between the two methodologies (slope = 1.003, intercept = −1.958, R2 = 0.88). In addition, to validate the registration technique, the square root of the registered scan’s pixel content, corresponding to the length/width of the image, of both fovea and optic nerve IR SLO images were compared for all scan dates. Previous models of wide-field retinal imaging would predict minimal differences in retinal scaling for these two regions (Holden and Fitzke, 1988). Similarly, there was good agreement for changes in image size for both optic nerve and fovea scans (Fig 1D, slope = 1.027, intercept = −47.67, R2 = 0.98).
2.6 Segmentation and Foveal Pit Morphology
OCT scans were exported as raw (.vol) files and all image analysis and quantification was performed using MATLAB (The Mathworks, Inc., Natick, MA) algorithms. Total retinal thickness was calculated from Bruch’s membrane (BM) to the inner limiting membrane (ILM). In the majority of cases, instrument segmentation for the ILM was accurate, however, BM had to be manually delineated due to multiple failures in the native instrument based segmentation algorithm.
For purposes for detection and quantification of the foveal pit, thickness maps were considered flattened to the BM. Foveal pit shape characteristics were calculated after rescaling each thickness map to a 1:1 aspect ratio using the computed transverse scaling (equation 2) and the instrument determined axial scaling (3.87μm/pixel). The starting point depth of the pit was determined as the difference between the regional maximum and minimum thickness. To determine the center, points intersecting the pit slope at heights corresponding to one-half to seven-eighths of the pit depth were determined at one micron intervals. For each interval a least squares best fit circle was fit to the intersecting points, and the centroid of these points was used as the center of the pit for all further calculations.
The shape and slope characteristics of the foveal pit were determined using twelve interpolated radial sections through the center of the fovea. The first derivative of the total retinal thickness for each section was used to determine slope information and locate the top and bottom of the pit. The maximum slope was located by the peaks of the thickness differential, while the top and bottom of the pit were determined as points on either side of the maximal slope that approached a slope of zero (Fig 2B). The pit depths reported are the thickness differences between the top and bottom of the pit. Based on the thickness contour, points corresponding to top and bottom of the pit were fit with least squares best fit circles. Similarly, points corresponding to the maximal slope for all radial sections were also fit with a circle (not illustrated in Fig 2).
Figure 2.
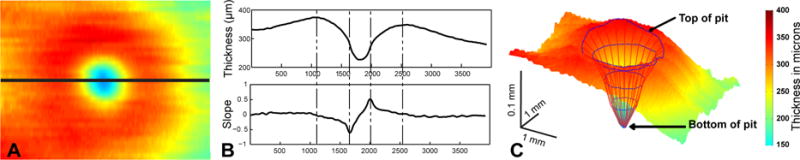
A. Total retinal thickness map for a region 15° × 20° centered on the fovea (256 day old infant), B. Pit characteristics were determined using interpolated thickness data from the total thickness maps. For example, the top plot illustrates the thickness profile for a horizontal radial section through the thickness map as illustrated by the black line though A. Along with the thickness profiles from radial sections centered on the pit, slope data (bottom B) were used to determine pit characteristics and construct a skeleton to describe pit morphology. The dashed lines in B indicate the maximal slope location and top of pit location. C. Illustrated are the thickness map along with a skeletal reconstruction of the pit, including points used to fit the top and bottom of the pit.
2.7 Changes in Overall Retinal Thickness
Although the Spectralis HRA+OCT can align inter-session scans to track changes in retinal thickness, significant changes in relative retinal magnification with age precluded using the eye tracking system to register images across scanning sessions. Instead, commercial software using a generalized dual bootstrap, iterative closest point algorithm (i2k retina, DualAlign, LLC, Clifton, NY) was used to align the 30 degree SLO fundus images from successive scans and the associated total retinal thickness maps (Fig 3A–E). Average total retinal thickness was computed for superior, inferior, nasal and temporal regions for sectors, 0–50 μm, 50–100 μm, 100–250 μm, 250–500 μm, 500–750 μm, 750–1000 μm, 1000–1250 μm, and 1250–1500 μm from the fovea center (Fig 3E). Average thickness measures for sectors were computed only when greater than 75% of the sector overlapped the raster scan derived thickness map. In addition, a difference map was created by subtracting thickness values from baseline scans, and average sector thickness measures were recorded (Fig 3G–J).
Figure 3.
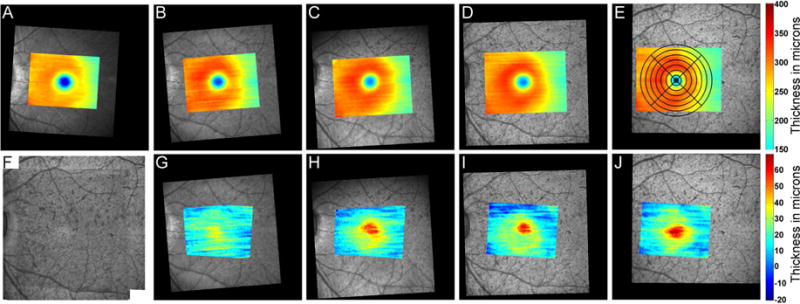
Top row: Registered SLO images and corresponding total retinal thickness maps for one animal (A – 33 days, B – 67days, C – 137days, D – 468 days, and E – 649 days of age). Each scan is in registration, and the montaged IR-SLO image is illustrated in F. Bottom row G–J: Change in total retinal thickness compared to baseline measures at 33 days of age. The series illustrates the increase in total retinal thickness in the central foveal region with age. Notice that as the animal ages, the portion of the retina imaged also increases. A scaled grid was used to determine average thickness measures within quadrants of concentric circular regions were used to determine overall retinal thickness changes with age.
2.8 Correspondence of OCT imaging and Retinal Histology
Several comparative studies have been done to relate OCT B-scan layers to those seen histologically, and overall there is fair agreement between these studies with similar retinal layer identification across species (Abbott et al., 2009; Anger et al., 2004; Drexler and Fujimoto, 2008; Dubis et al., 2012; Gloesmann et al., 2003; Huang et al., 1998; Toth et al., 1997). Because changes in anatomy have been shown to occur histologically, it was important to validate the light reflectivity profile methods used to aid in identifying retinal layers. For this experiment, 2 animals, one 10 days old, and the other 6 years of age were scanned using OCT and subsequently the eyes were enucleated for histological processing. After dissecting the anterior segment, the posterior eye cup was placed in 2% paraformaldehyde and 1% glutaraldehyde, postfixed in osmium tetroxide and embedded in resin. Serial sections were then cut from the foveal region, stained with cresyl violet and imaged. For the best corresponding histological and OCT sections, layers within the retina were manually segmented, and using an iterative cross correlation, the best correspondence of layers was then determined. Although a naming convention has been established for OCT bands (Staurenghi et al., 2014), for consistency and comparison, the outer retinal layers (i.e. myoid zone and ellipsoid zone), were labeled as they have been in previous infant OCT studies. OCT scans, along with longitudinal reflectivity profiles, and histological correspondence from the two animals was used as a reference for analysis of all longitudinal datasets.
2.9 Retinal layer thickness measurements
Although segmentation algorithms can be used to delineate and quantify thickness measures, these often make errors, and most apply smoothing. Hence, to quantify small changes in thickness manual segmentation in targeted small regions was performed for this study. Specifically, the thickness of individual retinal layers were measured at the center of the pit and at eccentricities of 87 microns, 175 microns, and up to 1225 microns in 175 micron steps. A total of 89 locations along six radial regions corresponding to clock hours through the fovea center were sampled (Fig 4). To decrease bias retinal layer thickness measures were performed after all data collection had been completed, and each of the locations was randomly presented for analysis.
Figure 4.
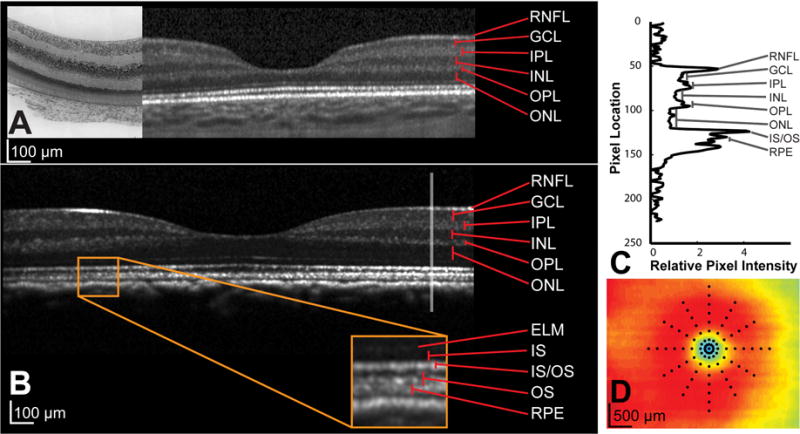
A. OCT section through fovea of a 10 day old infant with corresponding histology stained with Cresyl violet from the same animal. B. OCT section through the foveal region, of a 1.5 year old animal. C. Each layer was manually identified on the OCT B-scan with the assistance of reflectivity profiles, through the region of interest. The reflectivity profile illustrated is generated from the gray vertical line shown in B. D. For each eye, layer thicknesses at 89 points illustrated on the thickness plot were measured and monitored over time.
For each location, a B-scan section corresponding to 75 pixels on either side of the region of interest was interpolated from the raster volume data at one of six radial orientations. This B-scan and the longitudinal reflectivity profile (Fig 4C) through the region of interest were enlarged by a factor of six using bilinear methods to assist with accurate layer identification. The average layer thickness at each eccentricity was used for data analysis. All plots were created using MATLAB, or SigmaPlot (Systat Software, Inc, San Jose, CA), while all statistical analyses were performed using SPSS (IBM, Armonk, NY) and corrected for multiple comparisons. To determine the function that best described changes during the period studied, data from each subject were fit using both linear and non-linear functions. In general, linear, piecewise linear, and a three parameter exponential rise to maximum function (y = a + b(1-exp−cx)) were used to fit the majority of data. Data were binned in 30 day intervals and repeated measures ANOVA with Bonferonni’s correction for multiple comparisons were used to determine timepoints at which there were significant changes in thickness. ANOVA was used to determine thickness differences between retinal thickness measures across eccentricities.
3.0 RESULTS
Over the period studied, a total of 195 scans were acquired and used for data analyses, for the 16 infant rhesus monkeys. The animals all maintained good systemic and ocular health during the period studied, with no major illnesses, ocular injuries or retinal pathology noted.
3.1 Retinal Scaling
Changes in relative retinal image size were best described using a three surface schematic eye. This scaling methodology was validated with predicted scaling changes as determined by image registration of both the fovea and optic nerve IR SLO scans (slope = 1.003, intercept = −1.958, R2 = 0.88, p<0.01, Fig 1C). Overall, retinal image size was linearly related to axial length (Retinal Image/degree = 15.1×Axial Length − 60.2, R2 = 0.97). The relative retinal image size change with age was fit using a three parameter exponential rise to maximum equation (Ret Scaling = 163 + 52(1−e−0.005×Age), R2 = 0.59, p <0.01). For retinal scaling, the asymptote (a+b) along with the 95% confidence interval was calculated as 215 (202, 229) μm/deg.
3.2 Pit Morphology
The foveal pit depth decreased by 25 μm (14.6% from baseline) over the period studied. The group data were fit with an exponential decay model (Pit Depth = 145.8 + 43.8 × exp(−0.015 × Age in Days), R2 = 0.24, p < 0.01, Fig. 5A). Repeated measures ANOVA using Greenhouse-Geisser estimates for sphericity indicated a significant change in depth over the first 150 days of age (F = 21.56, p < 0.01), corresponding to a time point when average pit depth was 150.4 μm.
Figure 5.
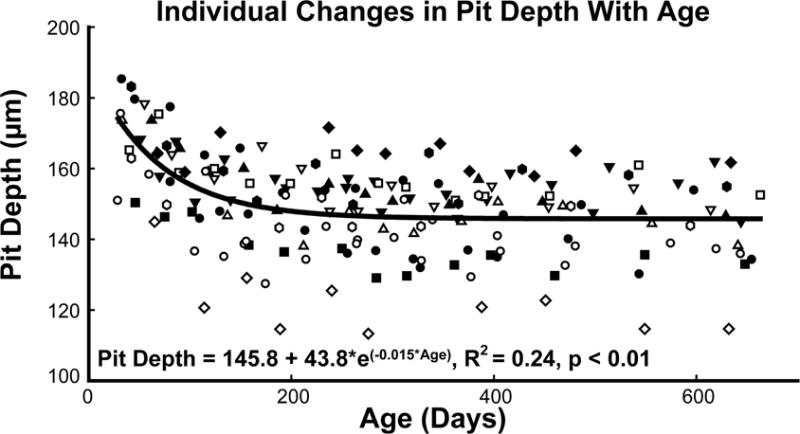
The pit depth decreased rapidly, reaching adult like depths prior to a year of age. Each subject is represented by a different symbol.
The decrease in pit depth should result in corresponding changes in the width of the foveal pit. Although there was a trend in a widening of the pit at the top and narrowing at the bottom, neither the best fit for the top of the pit radius (p=0.28) or the bottom of the pit radius (p=0.07) was statistically significant.
3.3 Total Retinal Thickness
Corresponding with changes in pit depth, the average retinal thickness for measures in the surrounding 500 μm of the pit increased up to 120 days of age (Table 1), with the largest change of 33±9μm (19±6%, Fig 6A, 7) noted within the central 50 μm (Repeated measures ANOVA, F = 26.4, p < 0.01). An analysis of the four quadrants, superior, inferior, nasal, and temporal, did not indicate any statistically significant difference in thickness change at eccentricities up to 1500 μm from the pit center when corrected for multiple comparisons (Fig. 6B, Table 2). Hence, for subsequent analyses of individual retinal layers, only the average layer thickness at each eccentricity was used. To illustrate changes in thickness, for the total retinal thickness (Fig 6C) or individual retinal layers (Fig 8&9), associated with eccentricity and age, a mean thickness plot was constructed using the surface fitting tool in MATLAB (Lowess fitting, Polynomial = linear, Span = 0.1, Robust = Bisquare).
Table 1.
Change in average total retinal thickness within the first 20 months of life (n=16 animals). The reported thicknesses are averages for corresponding regions illustrated in Figure 6B. A significant increase in total retinal thickness was measured for regions within 500μm of the pit center. Significant values for repeated measures ANOVA, after correcting for Bonferroni’s multiple comparison are noted by asterisks.
| Eccentricity (μm) | Mean Thickness (μm) at 36 ± 6 days | Mean Thickness (μm) at 627 ± 27 days | RMANOVA (F) | p |
|---|---|---|---|---|
| 0–50 | 172.01 ± 12.5 | 207.21 ± 13.5 | 26.43 | < 0.001* |
| 50–100 | 174.81 ± 13.3 | 209.87 ± 14.6 | 26.78 | < 0.001* |
| 100–250 | 195.17 ± 24.9 | 226.89 ± 21.9 | 17.54 | < 0.001* |
| 250–500 | 268.72 ± 25.5 | 287.96 ± 19.4 | 8.40 | < 0.001* |
| 500–750 | 318.13 ± 19.9 | 328.34 ± 16.1 | 5.00 | = 0.001 |
| 750–1000 | 328.46 ± 16.4 | 336.24 ± 14.7 | 3.58 | 0.03 |
| 1000–1250 | 323.14 ± 15.2 | 328.98 ± 13.9 | 2.94 | 0.06 |
| 1250–1500 | 312.46 ± 13.2 | 316.97 ± 13.1 | 2.64 | 0.05 |
Figure 6.
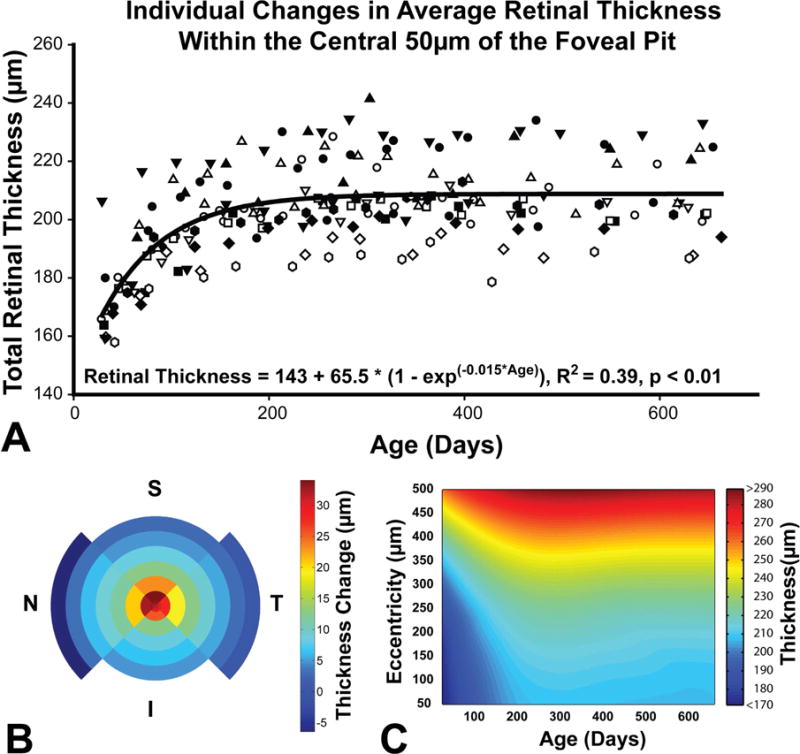
A. The average retinal thickness within 50μm of the pit increased by 20.9% with the majority of change occurring within the first 150 days after birth. The trend was similar for all animals and was best fit with an exponential to maximum function. Each subject is represented by a different symbol. B. The figure illustrates total retinal thickness change for each quadrant at the eccentricities illustrated in figure 3E. C. A surface plot illustrating changes in average thickness as a function of age and eccentricity up to 500 μm.
Figure 7.
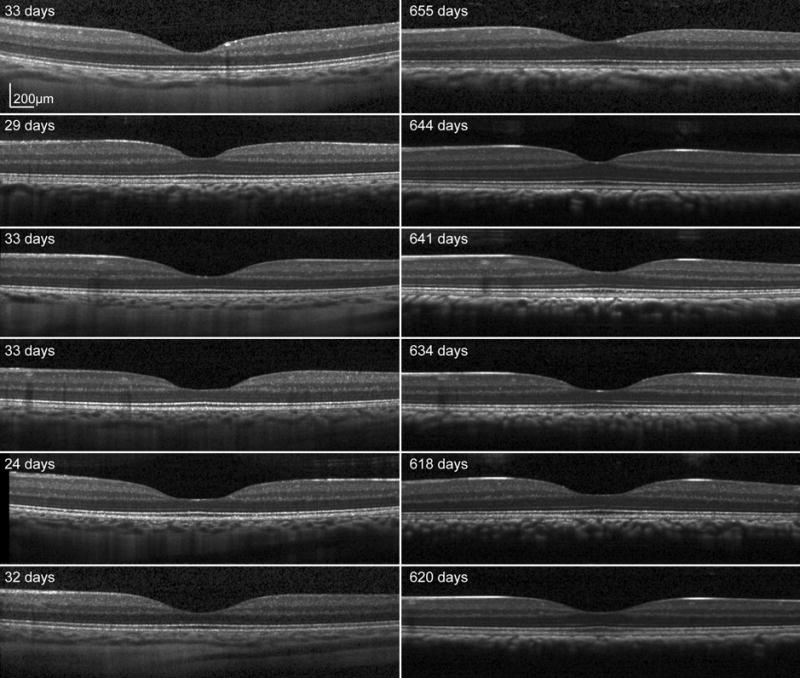
Scaled SD OCT images for six subjects at baseline (left) and endpoint (right) through the fovea center. A well defined foveal pit is seen in all animals at the first scan session. The overall shape of the foveal pit does not change, but there is an increase in outer retinal thickness on the endpoint scans.
Table 2.
Change in overall average retinal thickness in the first 20 months of life (n=16 animals). As indicated by the p values in the left column, changes in quadrant thickness over time for the regions investigated were not statistically significant.
| Average Change in Total Retinal Thickness (μm) | ||||||
|---|---|---|---|---|---|---|
| Eccentricity (μm) | Superior | Inferior | Nasal | Temporal | Overall | P |
| 0–50 | 32.9 | 31.1 | 32.1 | 31.6 | 31.9 | 0.96 |
| 50–100 | 33.6 | 30.2 | 32.9 | 30.6 | 31.8 | 0.72 |
| 100–250 | 34.0 | 25.5 | 32.3 | 27.8 | 29.9 | 0.058 |
| 250–500 | 23.1 | 12.6 | 20.7 | 19.1 | 18.9 | 0.033 |
| 500–750 | 12.7 | 8.2 | 11.6 | 12.7 | 12.7 | 0.259 |
| 750–1000 | 8.4 | 6.5 | 9.0 | 9.3 | 9.3 | 0.659 |
| 1000–1250 | 4.7 | 4.8 | 6.1 | 5.4 | 5.4 | 0.938 |
| 1250–1500 | 2.7 | – | 3.1 | 2.9 | 2.9 | 0.99 |
Figure 8.
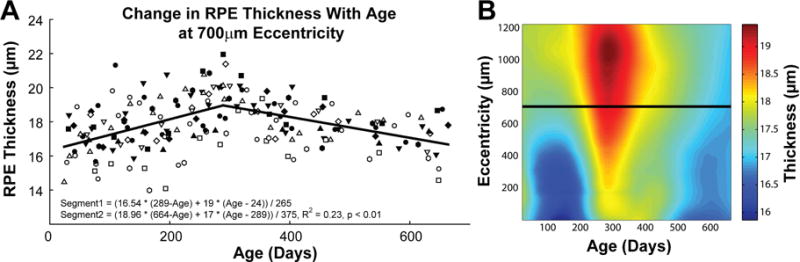
At each eccentricity, the RPE thickness measured increased linearly, reaching peak thickness just prior to a year of age. Thickness after this point decreased linearly. A. Changes in RPE thickness are illustrated for each animal at 700μm eccentricity. The best fit to the data illustrates an increase in thickness till around 289 days of age followed by a reduction in thickness. B. Differences in thickness with age and eccentricity illustrate this trend with age at each eccentricity. The horizontal line through the plot illustrates the location for data presented in plot A.
Figure 9.
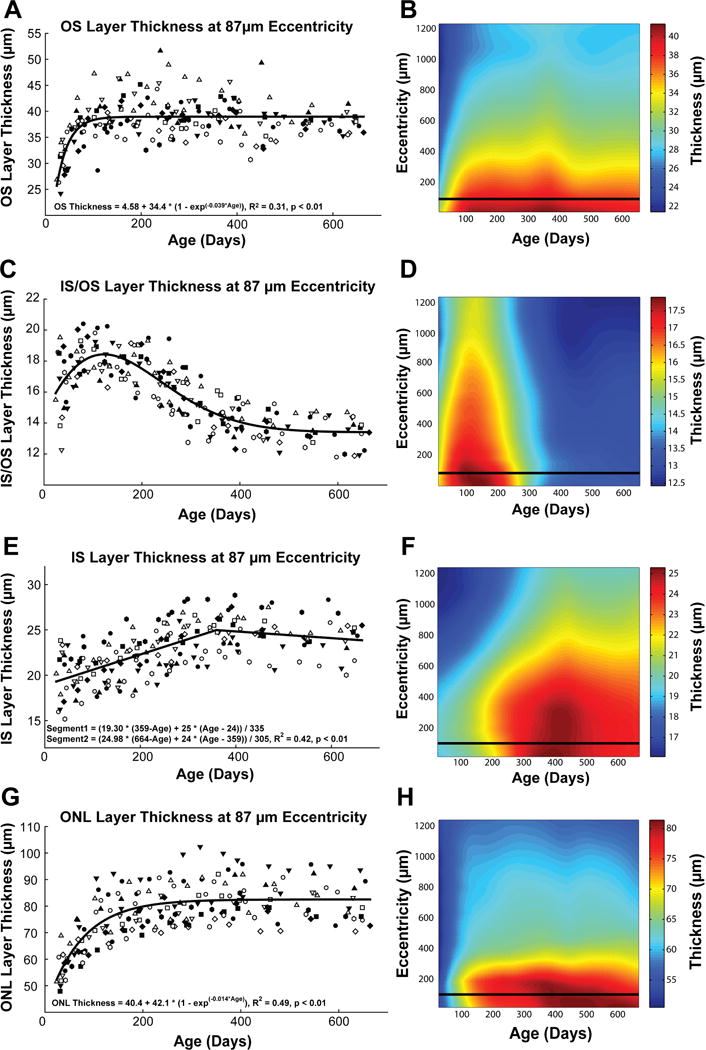
Outer retinal layers including the outer segments, inner segment/outer segment junction, inner segment, and outer nuclear layer, have significant changes in thickness with age. Plots A, C, E, and G, illustrate thickness changes for each of the 16 animals at an eccentricity of 87 μm. Although each of these layers show significant change in thickness, they all mature at different rates, with the outer segment layer thickness stabilizing at an earlier time point. Plots B, D, F, and H illustrate the relationship between thickness, eccentricity and age for each of the layers. The horizontal line through the plots indicate the location of the sampled data in A, C, E, and G.
3.4 Retinal Layer Thicknesses
Thickness measures for the retinal pigment epithelium (RPE) were best fit using a nonlinear piecewise, two segment function, where thickness measures increased for the first 289±24 days of age followed by a steady decrease that continued until the end of the study. Although the maximum thickness change based on the fit is relatively small in magnitude, it was statistically significant at all eccentricities (Repeated measures ANOVA, F > 2.6, p < 0.04, Fig 8).
At baseline, the outer segment layer was thickest within the center of the pit, measuring an average of 33.3±4.5 μm (F = 19.6, p < 0.01, Fig 9A&B). For descriptive purposes, the age-related change in thickness was best fit using an exponential rise to maximum function. The average age to reach 90% of maximal thickness, was used to compare the maturational rates across the regions analyzed. For the center of the fovea, the average age to reach 90% of the maximal outer segment layer thickness was 73.1±37 days (Data for all eccentricities along with repeated measures ANOVA are reported in Table 3). Although the animals all had similar changes in thickness over the period studied, they matured at different rates as demonstrated by the large standard deviations for thickness measures at baseline (4.53 μm at center of the pit), and for the time to reach 90% thickness (SD = 37.6 days at center of the pit).
Table 3.
Thickness change of the outer segment layer (n=16 animals)
| Eccentricity (μm) | Thickness at 36 ± 6 days (μm) | Thickness at 627 ± 27 days (μm) | Repeated measures ANOVA (F) | p | Days to 90% of maximal thickness |
|---|---|---|---|---|---|
| Central | 33.32±4.53 | 39.52±3.86 | 6.897 | < 0.01 | 73.1 ± 37.6 |
| 87 | 31.06±3.86 | 37.69±1.70 | 8.298 | < 0.01 | 70.2 ± 29.1 |
| 175 | 29.73±3.73 | 32.97±1.66 | 8.101 | < 0.01 | 65.5 ± 21.2 |
| 350 | 27.28±3.89 | 32.97±1.79 | 7.026 | < 0.01 | 69.4 ± 25.2 |
| 525 | 24.80±3.71 | 31.19±1.27 | 7.681 | < 0.01 | 78.7 ± 32.2 |
| 700 | 23.51±3.52 | 29.76±1.39 | 9.376 | < 0.01 | 79.0 ± 34.5 |
| 875 | 22.47±3.63 | 29.02±1.35 | 10.351 | < 0.01 | 82.3 ± 33.1 |
| 1050 | 21.40±3.00 | 27.30±1.17 | 11.259 | < 0.01 | 81.6 ± 35.3 |
| 1225 | 21.48±3.53 | 28.18±1.56 | 11.462 | < 0.01 | 91.9 ± 32.7 |
The thickness of the inner segment/outer segment (IS/OS) junction increased over the first hundred days, followed by a gradual reduction in thickness up to around 400 days after birth (Fig 9D). The data for each animal and grouped data for each eccentricity was best fit using a five parameter Weibull function (Fig 9C). Overall, there was a significant decrease in IS/OS thickness at all eccentricities averaging 3.4 ± 2.6 μm (Repeated measures ANOVA, F > 16.1, p < 0.01). Although the central fovea showed a greater reduction in thickness (4.2 ± 2.6 μm), compared to measures at an eccentricity of 1.2 mm (3.2 ± 2.5 μm), the difference was not statistically significant (p = 0.17).
Similarly, the mean thickness of the inner segment layer gradually increased by 4.06±2.1 μm across all eccentricities measured (Fig 9F). Generally, the thickness of this layer increased up to a year or age (mean = 381.2 days), after which the thickness showed a slight thinning (Fig 2–9E&F). Table 4 summarizes the change in thickness, time to reach maximal thickness, along with the repeated measures ANOVA statistic for each eccentricity.
Table 4.
Thickness change of the inner segment layer (n=16 animals)
| Eccentricity (μm) | Thickness at 36 ± 6 days (μm) | Thickness at 627 ± 27 days (μm) | Repeated measures ANOVA (F) | p | Days to maximal thickness |
|---|---|---|---|---|---|
| Central | 20.32±4.36 | 23.67±3.11 | 19.233 | < 0.01 | 394 ± 35 |
| 87 | 19.95±2.91 | 24.34±2.15 | 39.324 | < 0.01 | 359 ± 29 |
| 175 | 20.29±2.68 | 24.38±2.06 | 51.429 | < 0.01 | 385 ± 27 |
| 350 | 19.92±2.50 | 24.31 ±1.86 | 55.345 | < 0.01 | 385 ± 26 |
| 525 | 18.54±2.27 | 22.85±1.90 | 48.501 | < 0.01 | 355 ± 26 |
| 700 | 17.80±2.36 | 21.72±1.97 | 46.767 | < 0.01 | 385 ± 30 |
| 875 | 16.87±2.33 | 20.98±1.92 | 53.851 | < 0.01 | 391 ± 32 |
| 1050 | 16.16±2.09 | 20.24±1.69 | 47.954 | < 0.01 | 392 ± 32 |
| 1225 | 16.11±1.72 | 20.06±1.64 | 25.838 | < 0.01 | 385 ± 39 |
The outer nuclear layer within 525 μm of the pit center had the largest increase in thickness during early post-natal maturation. The increase in thickness (mean = 25.37 μm, repeated measures ANOVA, F = 27.18, p < 0.01) was largest at the center of the pit and within 175 μm of the pit center, while thickness measures outside of 1050 μm were not significant (3.02 μm, repeated measures ANOVA, F = 0.045, p = 0.09, Table 5). Thickness changes for each animal were best fit using an exponential rise to maximum function, which reached 90% of maximal thickness within the pit at 140 ± 52 days of age. The age to reach 90% of maximal thickness, was less for increasing eccentricities.
Table 5.
Thickness change of the outer nuclear layer (n=16 animals)
| Eccentricity (μm) | Thickness at 36 ± 6 days (μm) | Thickness at 627 ± 27 days (μm) | Repeated measures ANOVA (F) | p | Days to 90% of maximal thickness |
|---|---|---|---|---|---|
| Central | 56.54 ± 6.7 | 81.91 ±11.1 | 27.168 | < 0.01 | 140.07 ± 52.4 |
| 87 | 56.64 ± 5.3 | 79.71 ± 6.6 | 51.109 | < 0.01 | 123.19 ± 52.4 |
| 175 | 57.40 ± 5.1 | 75.60 ± 4.8 | 26.156 | < 0.01 | 111.13 ± 43.6 |
| 350 | 51.50 ± 4.8 | 63.53 ± 7.3 | 9.710 | < 0.01 | 89.40 ± 54.5 |
| 525 | 52.32 ± 5.0 | 61.47 ± 7.1 | 8.370 | < 0.01 | 77.63 ± 52.3 |
| 700 | 52.10 ± 4.3 | 60.76 ± 6.4 | 6.900 | < 0.01 | 64.63 ± 19.4 |
| 875 | 51.98 ± 3.9 | 59.72 ± 5.1 | 6.083 | < 0.01 | 64.00 ± 33.9 |
| 1050 | 52.88 ± 4.2 | 57.69 ± 5.8 | 3.996 | < 0.01 | 57.40 ± 31.2 |
| 1225 | 53.15 ± 3.9 | 56.17 ± 5.6 | 0.045 | 0.090 | 64.00 ± 25.5 |
The change in outer plexiform layer thickness with age was only statistically significant at 350 μm from the center of the pit (repeated measures ANOVA = 2.6, p = 0.03). However, the change was only at one eccentricity, and was relatively small in magnitude measuring only 3.75±2.5 μm. Similar to the outer plexiform layer, a slight yet significant change was noted for the inner nuclear layer, inner plexiform layer, and ganglion cell layer within the 350 μm eccentricity region. The inner nuclear layer had significant changes with age at only the 350 μm and 525 μm eccentricities, both showing a linear increase in thickness over the period examined. At 350 μm the inner nuclear layer increased by 3.32±2.3 μm, while at 525 μm the increase measured only 2.52±2.0 μm. The inner plexiform layer within 350 μm of the pit had increased in thickness, while thinning of this layer was noted outside this region. For eccentricities greater than 350 μm, the thickness of the INL showed a two-phase thickness change. From baseline the thickness decreased (at 350 μm, 2.7±0.8 μm at 120 days of age), followed by a transient increase around day 256±24 (at 350 μm, an increase of 2.1±0.5 μm when compared to 120 days of age). Similar to the changes in the inner plexiform layer, the ganglion cell layer showed a slight, increase in thickness with age within 350 μm of the pit, while a decrease in thickness was noted at further eccentricities. At the 350 μm eccentricity, the ganglion cell layer thickness increased by 2.7±0.8 μm. In adult eyes the retinal nerve fiber layer is relatively thin in the macula region, but statistically significant changes at 175 μm (4.7±1.3 μm, p < 0.01) and 350 μm (3.61±0.7 μm, p < 0.01) eccentricities, following similar timelines to the ganglion cell layer, were noted in the current study (Supplemental figure 1).
4.0 DISCUSSION
Although all retinal neural cells responsible for central vision are present at birth (Hendrickson and Kupfer, 1976; Kirby and Steineke, 1996; La Vail et al., 1991; Rapaport et al., 1996), high contrast spatial resolution is relatively poor (5 cpd) in infant primates (Boothe et al., 1985; Kiorpes and Movshon, 1989; Ordy et al., 1965). The rapid improvement in visual acuity over the first year of life has been attributed to both ocular and cortical factors (Banks and Bennett, 1988; Brown et al., 1987; Jacobs and Blakemore, 1988; Kiorpes et al., 2003; Movshon et al., 2005). The current knowledge on retinal maturation during this period has been based on histological data (Hendrickson and Kupfer, 1976; Kiorpes et al., 2003; Packer et al., 1990) which provides high resolution, and cross sectional OCT data (Hendrickson et al., 2012; Lee et al., 2015; Maldonado et al., 2011; Rosen et al., 2015; Vajzovic et al., 2012; Vinekar et al., 2015). However, variability between individuals (Curcio et al., 1987) makes it difficult to establish accurate timelines, especially for subtle and small changes in morphology. In this prospective study, non-invasive in vivo OCT imaging during postnatal maturation was used to investigate changes in retinal image size and fovea characteristics from repeated measurements on infant rhesus monkeys.
The use of OCT measurements on a growing eye requires accurate compensation for the changes in transverse magnification associated with changes in axial length and the optical power of the cornea and crystalline lens. The three surface schematic eye constructed for calculations of retinal image size was verified by comparison to scaling changes required to obtain accurate image registration (Fig 1). In addition, the retinal image size for an average adult eye of the macaque monkey based on this model, was calculated as 215 μm/deg, which is similar to angular sizes previously reported (Lapuerta and Schein, 1995; Perry and Cowey, 1985; Rolls and Cowey, 1970). Over the period studied, the retinal image size increased by an average of 47±16 μm/deg, or 28±9%, with a resulting optical increase in the Nyquist limit. In addition, there was also significant changes in foveal pit morphology and neuronal densities within the central retina that also affect visual resolution.
The important changes in fovea pit morphology involved an increase in total retinal thickness within the foveal pit (65 μm) with a corresponding decrease in pit depth (44 μm). Although this decrease in pit depth should result in a change in pit width, a change in the best fit radius to the top or bottom of the pit was not noted. This discrepancy could be because the shape of the retina was not taken into account for these calculations, and the eye continues to lengthen during this time. The increase in retinal radius of curvature with axial elongation could not be accounted as the instrument applies proprietary non-linear functions to compensate for instrument and ocular optics that were not available at the time of analysis, and other imaging modalities such as MRI or b-scan ultrasound were not acquired. In general, the majority of changes in pit depth and thickness which occurred within the first 120–150 days of age, were in agreement with the histological studies in the rhesus monkey where pit measures are shown to be adult like at 3–5 months of age (Hendrickson and Kupfer, 1976; Packer et al., 1990; Springer and Hendrickson, 2005).
The increase in retinal thickness within the pit region follows a similar timeline to changes in the depth of the pit (Fig 5–7). Such increases in thickness could indicate either an increase in the cell size or cell quantity, but cannot be explained by cell mitosis because the majority of neuronal cells within the central and peripheral retina are differentiated by embryonic day 150 in the Macaca mulatta (La Vail et al., 1991; Rapaport et al., 1996). Hence, this thickness increase is best explained by the centripetal cell migration and changes in cell morphology that were previously shown by histologic observations of age-related increases in photoreceptor cell density and of increasing lengths of the inner and outer segments of cones within the fovea (Curcio, 1991; Hendrickson and Kupfer, 1976; Packer et al., 1990; Springer, 1999; Springer and Hendrickson, 2004a, 2005).
The majority of retinal thickness increase in the foveal pit occurs from changes in the outer nuclear layer, with a similar timeline to that of total retinal thickness, i.e., the rate of change (c) for the best fit exponential function (y = a+b(1-exp−cx)) was similar for both total retinal thickness (0.015) and outer nuclear layer (0.014). In addition, the majority of thickness increase for both total retinal thickness and outer nuclear layer is in the central 1mm of the fovea. Because it contains the cell bodies for photoreceptors, the increase in outer nuclear layer thickness is consistent with an increase in cone density during the period studied. Specifically, included in this area is the 15–20 μm region of highest cone density of 180,000 to 261,000 cones/mm2, which drops off exponentially with increasing eccentricity (Curcio, 1991; Packer et al., 1989, 1990; Springer and Hendrickson, 2005).
There is a discrepancy in thickness measures of the outer nuclear layer using in vivo methods (81.9±11.1μm within the center of the fovea, at 1.5yrs of age) versus those reported in histological sections (< 70μm) (Springer and Hendrickson, 2004b), which is also evident in side-by-side comparisons of histological sections and OCT scans from identical locations of the same animal (Fig. 4A). The incongruity is likely a result of the methodology used for layer identification and characteristics of OCT imaging. Specifically, for OCT scans that are on-axis with the optics of the eye, the border distinction between the outer nuclear layer and Henle’s fiber layer is significantly diminished (Lujan et al., 2010; Otani et al., 2011). Hence, the increased outer nuclear layer thickness is a result of including portions of Henle’s fiber layer. This is also evident by a localized peak sometimes found within the outer nuclear layer band of the reflectivity profile in well aligned scans (Fig. 4C) that is probably the junction between the outer nuclear layer and Henle’s fibers. Nonetheless, the outer nuclear layer measured in OCT scans within the pit center should correspond to increases in peak cone density.
In addition to changes in the outer nuclear layer, there are also significant changes in both outer and inner segment layers, but these layers follow different time courses (Fig 9). For example, the outer segment layer thickness changed at greater than twice the rate of the outer nuclear layer, while changes in inner segment layer thickness were linear with age up to a year after birth. Although the outer segment layer was thickest in the pit region, the change in thickness was similar at all eccentricities measured. These results are in general agreement with histological observations of increase in photoreceptor outer segment length during the first two years after birth (Hendrickson and Kupfer, 1976). Similar to histological reports, the OCT measures reported are assuming scans were acquired on axis with the eye, with minimal or no tilt in the imaging plane.
During the period after birth there is a significant narrowing and lengthening of the photoreceptor outer segments (Yuodelis and Hendrickson, 1986). These changes, especially the lengthening of the photoreceptor allows for more efficient photon capture (Wilson, 1988). For the present study, the cone outer segments at the center of the fovea pit increased in length from 28 μm at 30 days of age to 38 μm at 360 days of age. Based on photon capture probability equations (p = 1 − e(−x/24.8), where x is the length of the outer segment), this change in thickness would increase photon capture by only a factor of 1.16. Thus, although the increase in photon capture cannot explain the observed change in contrast sensitivity functions (Boothe et al., 1988), the timelines for the changes in outer segment thickness and peak contrast sensitivity are very similar.
The inner segment of the photoreceptor in OCT images consists of a portion commonly referred to as the IS/OS junction and the thickness up to the external limiting membrane. Through comparative histology studies, the IS/OS junction is thought to include the ellipsoid and myoid of the photoreceptors (Anger et al., 2004; Fernandez et al., 2008). That this OCT band is more than just a junction is supported by the significant thickness changes noted within the first year of life. Specifically, the IS/OS layer increases in thickness up to 120 days, followed by a gradual decrease and stabilizing after a year of age. The transient increase in thickness peaking at around 120 days of age may represent an increase in metabolic demand as the photoreceptors migrate, lengthen and mature. The inner segment layer measured from the IS/OS junction to the external limiting membrane increased linearly with age up to around 360 days after which a slight decrease in thickness was noted. The maximum thickness of 25.5 μm at the center of the pit is only slightly less than the inner segments length reported using histological methods (26–30 μm) (Hendrickson and Kupfer, 1976). The thinner inner segment layer measures may represent the oblique orientation of these structures within the fovea and the exclusion of the IS/OS junction.
Similar to the inner segment layer, the retinal pigment epithelium also increased in thickness up to 300 days after birth. These changes corresponded with an increase in retinal pigmentation that although not quantified are clearly demonstrated in the reduced resolution of choroidal and deeper structures on OCT B-scans with age (Fig 7). The piecewise fit used to describe changes in this layer could represent changes in metabolic activity associated with maturation and photoreceptor migration. However, the current data cannot be used to determine if there is an associated increase in retinal pigment epithelial cell density within the period studied.
None of the OCT images from animals of any age provided evidence of an inner nuclear, inner plexiform, or ganglion cell layer within the central pit region. Although ganglion cells within the fovea have been reported in some non-human primates (Fukuda et al., 1989; Grunert et al., 1993; Leventhal et al., 1993), this layer was not evident in the Macaca mulatta, although the inability to visualize sparse cells in the infants may be a consequence of the resolution limits of OCT imaging. For the period studied, biomechanical theories on pit maturation using FEA stretch models (Springer and Hendrickson, 2005) would predict a transient thinning of the inner retinal layers within the fovea. Overall, such changes were not measured, during the period studied. Significant thickness changes within the inner retina were confined to a specific retinal layers and eccentricities and common maturational patterns could not be discerned. The minimal changes in the inner retinal layers during foveal maturation would indicate a large displacement between the photoreceptors that have migrated towards the fovea and the location of the ganglion cells receiving its signal. Specifically, although the outer nuclear layer increased in thickness at all eccentricities, the most significant changes were those inside 875 μm, which implies a constraint on the maximal possible displacement of inner retinal neurons, including ganglion cells, that receive input from a photoreceptor. This measure corresponds well with a peak displacement, from inner segment to ganglion cell, of 637 μm measured in human retinas (Drasdo et al., 2007).
There are several differences in the results presented and those from OCT based human foveal development studies in infants (Dubis et al., 2012; Lee et al., 2015; Maldonado et al., 2011; Vajzovic et al., 2012; Vajzovic et al., 2015; Vinekar et al., 2015). Specifically, in preterm and term human infants, the foveal pit region has OCT bands indicating inner retinal components, whereas in the rhesus monkey, no evidence of ganglion cell layer or inner nuclear layer was seen on OCT imaging. One reason for the difference could be that animals in the present study were already a month old when the first scan was acquired on them (36±6 days after birth, n=16). However, even the 10 day old infant scanned had a well-defined foveal pit, with no inner retinal OCT bands (Fig 4A). In fact, c-scan images aligned to the ILM generated from high density OCT scans through the fovea and optic nerve on this infant showed adult like nerve fiber layer arcades and a fairly circular foveal pit region (supplemental video). These observations on OCT are consistent with previous histological sections from 1 day old eyes that show scant inner retinal cells in the fovea of a Macaca, while human and marmoset eyes have a foveal region, but with inner nuclear and ganglion cell layers also present (Hendrickson et al., 2006).
When comparing the results of the present study to those from a large cross sectional human infant cohort, the general timelines for central foveal pit total retinal thickness, photoreceptor and RPE in human infants are similar (Lee et al., 2015). Hence, for acuity and contrast maturation, the study predictions would be similar. However, whereas changes in inner retinal layers in the peri and para fovea are shown in human infants (Lee et al., 2015; Maldonado et al., 2011), only minimal change in these layers was noted in the present study. This discordance could be a reflection of interspecies differences in; 1) foveal development timelines and/or, 2) how OCT images were scaled, and the accuracy of scaling. The longitudinal data collected in the present study allow for accurate and precise, scaled measures that provide relative timelines for OCT band development. These timelines can be useful for monitoring age adjusted maturation of the fovea in human infants, or to establish the effects of ocular intervention/treatment on this area of high acuity.
There are several limitations to this study. To ensure good animal health for all studies, the first scan session on animals was around one month after birth. At this time point, based on prior histological work (Hendrickson, 1992, 1993; Hendrickson and Kupfer, 1976; Hendrickson and Provis, 2006), the foveal region has already changed since birth. Although OCT imaging can be used to quantify retinal layer thickness, current systems are not able to resolve cellular detail. With technological advances, it is possible that future studies will be able to non-invasively investigate the retinal at the cellular level (Jonnal et al., 2016). Such studies will provide useful insight not only to neural maturation, but also in mechanisms that are needed for neural regeneration. While the main purpose of this study was to investigate changes in the fovea, the peripheral retina was not investigated (Hendrickson and Provis, 2006).
In conclusion, using OCT in vivo imaging, detailed timelines for post-natal anatomical maturation of the retina were achieved. In general, these structural changes followed similar timelines to those noted for function measures. However, significant differences in the magnitude of change are clearly evident. With advances in non-invasive imaging technologies, future studies will provide useful information on structural and functional changes in the central and peripheral retina.
Supplementary Material
Supplemental video 1. Enface OCT of 10 day old infant referenced to the inner limiting membrane. The animation illustrates the nerve fiber layer arcades and the location of the foveal pit.
Supplemental Figure 1. There are minimal changes in the OPL, INL, IPL, GCL, and RNFL in the foveal region and time period studied. Eccentricities at which statistically significant changes in the OPL and INL were measures are illustrated by the horizontal lines in the plots. The thickness of both the IPL and GCL decreased with age outside of 350μm (indicated by the dashed horizontal line), but increased in thickness closer to the center of the pit. Only a small change in RNFL thickness was noted at 175μm and 350μm.
Highlights.
-
-
The extent of the retina scanned in infant eyes can be accurately estimated using a three surface schematic eye.
-
-
During the first year and half after birth, the foveal pit of the Macaca mulatta decreases in depth, with a corresponding increase in central foveal thickness.
-
-
Increase in retinal thickness within the foveal region can be attributed to the outer retina.
-
-
There are differences in how each of the SD OCT outer retinal layers change in postnatal maturation, with the outer nuclear layer showing the greatest increase in thickness.
Acknowledgments
Research grant support: NIH/NEI grants R01 EY001139, P30 EY007551, and K23 EY021761, and a John and Rebecca Moores Professorship from the University of Houston. Joe L Wheat and Xunda Luo provided assistance in imaging and monitoring animals. Dr. Earl Smith, gave us access to his research subjects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott CJ, Grunert U, Pianta MJ, McBrien NA. Retinal thinning in tree shrews with induced high myopia: optical coherence tomography and histological assessment. Vision Res. 2011;51:376–385. doi: 10.1016/j.visres.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Abbott CJ, McBrien NA, Grunert U, Pianta MJ. Relationship of the optical coherence tomography signal to underlying retinal histology in the tree shrew (Tupaia belangeri) Investigative ophthalmology & visual science. 2009;50:414–423. doi: 10.1167/iovs.07-1197. [DOI] [PubMed] [Google Scholar]
- Anger EM, Unterhuber A, Hermann B, Sattmann H, Schubert C, Morgan JE, Cowey A, Ahnelt PK, Drexler W. Ultrahigh resolution optical coherence tomography of the monkey fovea. Identification of retinal sublayers by correlation with semithin histology sections. Exp Eye Res. 2004;78:1117–1125. doi: 10.1016/j.exer.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Banks MS, Bennett PJ. Optical and photoreceptor immaturities limit the spatial and chromatic vision of human neonates. Journal of the Optical Society of America. A, Optics and image science. 1988;5:2059–2079. doi: 10.1364/josaa.5.002059. [DOI] [PubMed] [Google Scholar]
- Bengtsson B, Krakau CE. Correction of optic disc measurements on fundus photographs. Graefes Arch Clin Exp Ophthalmol. 1992;230:24–28. doi: 10.1007/BF00166758. [DOI] [PubMed] [Google Scholar]
- Bennett AG, Rabbetts RB. The Schematic Eye. In: Bennett AG, Rabbetts RB, editors. Clinical Visual Optics. Butterworths; London: 1989. pp. 249–274. [Google Scholar]
- Bennett AG, Rudnicka AR, Edgar DF. Improvements on Littmann’s method of determining the size of retinal features by fundus photography. Graefes Arch Clin Exp Ophthalmol. 1994;232:361–367. doi: 10.1007/BF00175988. [DOI] [PubMed] [Google Scholar]
- Boothe RG, Dobson V, Teller DY. Postnatal development of vision in human and nonhuman primates. Annual review of neuroscience. 1985;8:495–545. doi: 10.1146/annurev.ne.08.030185.002431. [DOI] [PubMed] [Google Scholar]
- Boothe RG, Kiorpes L, Williams RA, Teller DY. Operant measurements of contrast sensitivity in infant macaque monkeys during normal development. Vision Res. 1988;28:387–396. doi: 10.1016/0042-6989(88)90181-2. [DOI] [PubMed] [Google Scholar]
- Brown AM, Dobson V, Maier J. Visual-Acuity of Human Infants at Scotopic, Mesopic and Photopic Luminances. Vision Research. 1987;27:1845–1858. doi: 10.1016/0042-6989(87)90113-1. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Hendrickson AE. Chapter 5 Organization and development of the primate photoreceptor mosaic. Progress in Retinal Research. 1991;10:89–120. [Google Scholar]
- Curcio CA, Sloan KR, Jr, Packer O, Hendrickson AE, Kalina RE. Distribution of cones in human and monkey retina: individual variability and radial asymmetry. Science. 1987;236:579–582. doi: 10.1126/science.3576186. [DOI] [PubMed] [Google Scholar]
- Delori FC, Webb RH, Sliney DH, American National Standards, I Maximum permissible exposures for ocular safety (ANSI 2000), with emphasis on ophthalmic devices. Journal of the Optical Society of America. A, Optics, image science, and vision. 2007;24:1250–1265. doi: 10.1364/josaa.24.001250. [DOI] [PubMed] [Google Scholar]
- Drasdo N, Millican CL, Katholi CR, Curcio CA. The length of Henle fibers in the human retina and a model of ganglion receptive field density in the visual field. Vision Res. 2007;47:2901–2911. doi: 10.1016/j.visres.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler W, Fujimoto JG. State-of-the-art retinal optical coherence tomography. Prog Retin Eye Res. 2008;27:45–88. doi: 10.1016/j.preteyeres.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Dubis AM, Costakos DM, Subramaniam CD, Godara P, Wirostko WJ, Carroll J, Provis JM. Evaluation of normal human foveal development using optical coherence tomography and histologic examination. Arch Ophthalmol. 2012;130:1291–1300. doi: 10.1001/archophthalmol.2012.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellemberg D, Lewis TL, Liu CH, Maurer D. Development of spatial and temporal vision during childhood. Vision Res. 1999;39:2325–2333. doi: 10.1016/s0042-6989(98)00280-6. [DOI] [PubMed] [Google Scholar]
- Fernandez EJ, Hermann B, Povazay B, Unterhuber A, Sattmann H, Hofer B, Ahnelt PK, Drexler W. Ultrahigh resolution optical coherence tomography and pancorrection for cellular imaging of the living human retina. Optics express. 2008;16:11083–11094. doi: 10.1364/oe.16.011083. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Sawai H, Watanabe M, Wakakuwa K, Morigiwa K. Nasotemporal overlap of crossed and uncrossed retinal ganglion cell projections in the Japanese monkey (Macaca fuscata) J Neurosci. 1989;9:2353–2373. doi: 10.1523/JNEUROSCI.09-07-02353.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garway-Heath DF, Rudnicka AR, Lowe T, Foster PJ, Fitzke FW, Hitchings RA. Measurement of optic disc size: equivalence of methods to correct for ocular magnification. Br J Ophthalmol. 1998;82:643–649. doi: 10.1136/bjo.82.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloesmann M, Hermann B, Schubert C, Sattmann H, Ahnelt PK, Drexler W. Histologic correlation of pig retina radial stratification with ultrahigh-resolution optical coherence tomography. Investigative ophthalmology & visual science. 2003;44:1696–1703. doi: 10.1167/iovs.02-0654. [DOI] [PubMed] [Google Scholar]
- Grunert U, Greferath U, Boycott BB, Wassle H. Parasol (P alpha) ganglion-cells of the primate fovea: immunocytochemical staining with antibodies against GABAA-receptors. Vision Res. 1993;33:1–14. doi: 10.1016/0042-6989(93)90052-x. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. A morphological comparison of foveal development in man and monkey. Eye (Lond) 1992;6(Pt 2):136–144. doi: 10.1038/eye.1992.29. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. Morphological Development of the Primate Retina. In: Simons K, editor. Early Visual Development, Normal and Abnormal. 1. Oxford University Press; New York: 1993. pp. 287–295. [Google Scholar]
- Hendrickson A, Kupfer C. The histogenesis of the fovea in the macaque monkey. Investigative ophthalmology & visual science. 1976;15:746–756. [PubMed] [Google Scholar]
- Hendrickson A, Possin D, Vajzovic L, Toth CA. Histologic development of the human fovea from midgestation to maturity. Am J Ophthalmol. 2012;154:767–778. e762. doi: 10.1016/j.ajo.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A, Troilo D, Possin D, Springer A. Development of the neural retina and its vasculature in the marmoset Callithrix jacchus. J Comp Neurol. 2006;497:270–286. doi: 10.1002/cne.20996. [DOI] [PubMed] [Google Scholar]
- Hendrickson AE, Provis JM. Comparison of development of the primate fovea centralis with peripheral retina. In: Sernagor E, Eglen S, Harris B, Wong R, editors. Retinal Development. Cambridge University Press; New York: 2006. pp. 126–149. [Google Scholar]
- Holden AL, Fitzke FW. Image size in the fundus: structural evidence for wide-field retinal magnification factor. Br J Ophthalmol. 1988;72:228–230. doi: 10.1136/bjo.72.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Hung LF, Ramamirtham R, Blasdel TL, Humbird TL, Bockhorst KH, Smith EL., 3rd Effects of form deprivation on peripheral refractions and ocular shape in infant rhesus monkeys (Macaca mulatta) Investigative ophthalmology & visual science. 2009;50:4033–4044. doi: 10.1167/iovs.08-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Cideciyan AV, Papastergiou GI, Banin E, Semple-Rowland SL, Milam AH, Jacobson SG. Relation of optical coherence tomography to microanatomy in normal and rd chickens. Investigative ophthalmology & visual science. 1998;39:2405–2416. [PubMed] [Google Scholar]
- Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nature medicine. 1995;1:761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- Jacobs DS, Blakemore C. Factors limiting the postnatal development of visual acuity in the monkey. Vision Res. 1988;28:947–958. doi: 10.1016/0042-6989(88)90104-6. [DOI] [PubMed] [Google Scholar]
- Jonnal RS, Kocaoglu OP, Zawadzki RJ, Liu Z, Miller DT, Werner JS. A Review of Adaptive Optics Optical Coherence Tomography: Technical Advances, Scientific Applications, and the Future. Investigative ophthalmology & visual science. 2016 Oct;57:51–68. doi: 10.1167/iovs.16-19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L. Development of vernier acuity and grating acuity in normally reared monkeys. Vis Neurosci. 1992;9:243–251. doi: 10.1017/s0952523800010658. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Kiper DC. Development of contrast sensitivity across the visual field in macaque monkeys (Macaca nemestrina) Vision Res. 1996;36:239–247. doi: 10.1016/0042-6989(95)00097-j. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Movshon JA. Differential development of two visual functions in primates. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:8998–9001. doi: 10.1073/pnas.86.22.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiorpes L, Movshon JA. Peripheral and central factors limiting the development of contrast sensitivity in macaque monkeys. Vision Res. 1998;38:61–70. doi: 10.1016/s0042-6989(97)00155-7. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Tang C, Hawken MJ, Movshon JA. Ideal observer analysis of the development of spatial contrast sensitivity in macaque monkeys. J Vis. 2003;3:630–641. doi: 10.1167/3.10.6. [DOI] [PubMed] [Google Scholar]
- Kirby MA, Steineke TC. Morphogenesis of retinal ganglion cells: a model of dendritic, mosaic, and foveal development. Perspectives on developmental neurobiology. 1996;3:177–194. [PubMed] [Google Scholar]
- La Vail MM, Rapaport DH, Rakic P. Cytogenesis in the monkey retina. J Comp Neurol. 1991;309:86–114. doi: 10.1002/cne.903090107. [DOI] [PubMed] [Google Scholar]
- Lapuerta P, Schein SJ. A four-surface schematic eye of macaque monkey obtained by an optical method. Vision Res. 1995;35:2245–2254. doi: 10.1016/0042-6989(94)00320-l. [DOI] [PubMed] [Google Scholar]
- Lee H, Purohit R, Patel A, Papageorgiou E, Sheth V, Maconachie G, Pilat A, McLean RJ, Proudlock FA, Gottlob I. In Vivo Foveal Development Using Optical Coherence Tomography. Investigative ophthalmology & visual science. 2015;56:4537–4545. doi: 10.1167/iovs.15-16542. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Thompson KG, Liu D. Retinal ganglion cells within the foveola of New World (Saimiri sciureus) and Old World (Macaca fascicularis) monkeys. J Comp Neurol. 1993;338:242–254. doi: 10.1002/cne.903380208. [DOI] [PubMed] [Google Scholar]
- Littmann H. Determination of the real size of an object on the fundus of the living eye. Klin Monbl Augenheilkd. 1982;180:286–289. doi: 10.1055/s-2008-1055068. [DOI] [PubMed] [Google Scholar]
- Lujan B, Roorda A, Knighton RW, Carroll J. Revealing Henle’s Fiber Layer using Spectral Domain Optical Coherence Tomography. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.10-5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado RS, Izatt JA, Sarin N, Wallace DK, Freedman S, Cotten CM, Toth CA. Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants, and children. Investigative ophthalmology & visual science. 2010;51:2678–2685. doi: 10.1167/iovs.09-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado RS, O’Connell RV, Sarin N, Freedman SF, Wallace DK, Cotten CM, Winter KP, Stinnett S, Chiu SJ, Izatt JA, Farsiu S, Toth CA. Dynamics of human foveal development after premature birth. Ophthalmology. 2011;118:2315–2325. doi: 10.1016/j.ophtha.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer DL, Dobson V. Visual acuity development in infants and young children, as assessed by operant preferential looking. Vision Res. 1982;22:1141–1151. doi: 10.1016/0042-6989(82)90079-7. [DOI] [PubMed] [Google Scholar]
- Movshon JA, Kiorpes L, Hawken MJ, Cavanaugh JR. Functional maturation of the macaque’s lateral geniculate nucleus. J Neurosci. 2005;25:2712–2722. doi: 10.1523/JNEUROSCI.2356-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcia AM, Tyler CW, Hamer RD. Development of contrast sensitivity in the human infant. Vision Res. 1990;30:1475–1486. doi: 10.1016/0042-6989(90)90028-j. [DOI] [PubMed] [Google Scholar]
- Ordy JM, Samorajski T, Collins RL, Nagy AR. Postnatal Development of Vision in a Subhuman Primate (Macaca Mulatta); a Multidisciplinary Study. Arch Ophthalmol. 1965;73:674–686. doi: 10.1001/archopht.1965.00970030676017. [DOI] [PubMed] [Google Scholar]
- Otani T, Yamaguchi Y, Kishi S. Improved visualization of Henle fiber layer by changing the measurement beam angle on optical coherence tomography. Retina (Philadelphia, Pa) 2011;31:497–501. doi: 10.1097/IAE.0b013e3181ed8dae. [DOI] [PubMed] [Google Scholar]
- Packer O, Hendrickson AE, Curcio CA. Photoreceptor topography of the retina in the adult pigtail macaque (Macaca nemestrina) J Comp Neurol. 1989;288:165–183. doi: 10.1002/cne.902880113. [DOI] [PubMed] [Google Scholar]
- Packer O, Hendrickson AE, Curcio CA. Development redistribution of photoreceptors across the Macaca nemestrina (pigtail macaque) retina. J Comp Neurol. 1990;298:472–493. doi: 10.1002/cne.902980408. [DOI] [PubMed] [Google Scholar]
- Patel NB, Luo X, Wheat JL, Harwerth RS. Retinal nerve fiber layer assessment: area versus thickness measurements from elliptical scans centered on the optic nerve. Investigative ophthalmology & visual science. 2011;52:2477–2489. doi: 10.1167/iovs.10-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Cowey A. The ganglion cell and cone distributions in the monkey’s retina: implications for central magnification factors. Vision Res. 1985;25:1795–1810. doi: 10.1016/0042-6989(85)90004-5. [DOI] [PubMed] [Google Scholar]
- Provis JM, Diaz CM, Dreher B. Ontogeny of the primate fovea: a central issue in retinal development. Prog Neurobiol. 1998;54:549–580. doi: 10.1016/s0301-0082(97)00079-8. [DOI] [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL., 3rd Normal ocular development in young rhesus monkeys (Macaca mulatta) Vision Res. 2007;47:1424–1444. doi: 10.1016/j.visres.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamirtham R, Kee CS, Hung LF, Qiao-Grider Y, Huang J, Roorda A, Smith EL., 3rd Wave aberrations in rhesus monkeys with vision-induced ametropias. Vision Res. 2007;47:2751–2766. doi: 10.1016/j.visres.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamirtham R, Kee CS, Hung LF, Qiao-Grider Y, Roorda A, Smith EL., 3rd Monochromatic ocular wave aberrations in young monkeys. Vision Res. 2006;46:3616–3633. doi: 10.1016/j.visres.2006.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport DH, Rakic P, LaVail MM. Spatiotemporal gradients of cell genesis in the primate retina. Perspectives on developmental neurobiology. 1996;3:147–159. [PubMed] [Google Scholar]
- Rolls ET, Cowey A. Topography of the retina and striate cortex and its relationship to visual acuity in rhesus monkeys and squirrel monkeys. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1970;10:298–310. doi: 10.1007/BF00235053. [DOI] [PubMed] [Google Scholar]
- Rosen R, Sjostrand J, Nilsson M, Hellgren K. A methodological approach for evaluation of foveal immaturity after extremely preterm birth. Ophthalmic Physiol Opt. 2015;35:433–441. doi: 10.1111/opo.12221. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cano A, Baraibar B, Pablo LE, Honrubia FM. Magnification Characteristics of the Optical Coherence Tomography STRATUS OCT 3000. Ophthall Physiol Opt. 2008;28:21–28. doi: 10.1111/j.1475-1313.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- Simons K, National Research Council (U.S.) Committee on Vision . Early visual development : normal and abnormal. Oxford University Press; New York: 1993. [Google Scholar]
- Smith EL, Huang J, 3rd, Hung LF, Blasdel TL, Humbird TL, Bockhorst KH. Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Investigative ophthalmology & visual science. 2009a;50:5057–5069. doi: 10.1167/iovs.08-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, Hung LF., 3rd The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999;39:1415–1435. doi: 10.1016/s0042-6989(98)00229-6. [DOI] [PubMed] [Google Scholar]
- Smith EL, Hung LF, 3rd, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res. 2009b;49:2386–2392. doi: 10.1016/j.visres.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, Hung LF, 3rd, Huang J, Arumugam B. Effects of local myopic defocus on refractive development in monkeys. Optometry and vision science : official publication of the American Academy of Optometry. 2013;90:1176–1186. doi: 10.1097/OPX.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL, Hung LF, 3rd, Huang J, Blasdel TL, Humbird TL, Bockhorst KH. Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Investigative ophthalmology & visual science. 2010;51:3864–3873. doi: 10.1167/iovs.09-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer AD. New role for the primate fovea: a retinal excavation determines photoreceptor deployment and shape. Vis Neurosci. 1999;16:629–636. doi: 10.1017/s0952523899164034. [DOI] [PubMed] [Google Scholar]
- Springer AD, Hendrickson AE. Development of the primate area of high acuity. 1. Use of finite element analysis models to identify mechanical variables affecting pit formation. Vis Neurosci. 2004a;21:53–62. doi: 10.1017/s0952523804041057. [DOI] [PubMed] [Google Scholar]
- Springer AD, Hendrickson AE. Development of the primate area of high acuity. 2. Quantitative morphological changes associated with retinal and pars plana growth. Vis Neurosci. 2004b;21:775–790. doi: 10.1017/S0952523804215115. [DOI] [PubMed] [Google Scholar]
- Springer AD, Hendrickson AE. Development of the primate area of high acuity, 3: temporal relationships between pit formation, retinal elongation and cone packing. Vis Neurosci. 2005;22:171–185. doi: 10.1017/S095252380522206X. [DOI] [PubMed] [Google Scholar]
- Staurenghi G, Sadda S, Chakravarthy U, Spaide RF. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN*OCT consensus. Ophthalmology. 2014;121:1572–1578. doi: 10.1016/j.ophtha.2014.02.023. [DOI] [PubMed] [Google Scholar]
- Stewart CV, Tsai CL, Roysam B. The dual-bootstrap iterative closest point algorithm with application to retinal image registration. IEEE transactions on medical imaging. 2003;22:1379–1394. doi: 10.1109/TMI.2003.819276. [DOI] [PubMed] [Google Scholar]
- Teller DY. First glances: the vision of infants. the Friedenwald lecture. Investigative ophthalmology & visual science. 1997;38:2183–2203. [PubMed] [Google Scholar]
- Toth CA, Narayan DG, Boppart SA, Hee MR, Fujimoto JG, Birngruber R, Cain CP, DiCarlo CD, Roach WP. A comparison of retinal morphology viewed by optical coherence tomography and by light microscopy. Arch Ophthalmol. 1997;115:1425–1428. doi: 10.1001/archopht.1997.01100160595012. [DOI] [PubMed] [Google Scholar]
- Vajzovic L, Hendrickson AE, O’Connell RV, Clark LA, Tran-Viet D, Possin D, Chiu SJ, Farsiu S, Toth CA. Maturation of the human fovea: correlation of spectral-domain optical coherence tomography findings with histology. Am J Ophthalmol. 2012;154:779–789.e772. doi: 10.1016/j.ajo.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajzovic L, Rothman AL, Tran-Viet D, Cabrera MT, Freedman SF, Toth CA. Delay in retinal photoreceptor development in very preterm compared to term infants. Investigative ophthalmology & visual science. 2015;56:908–913. doi: 10.1167/iovs.14-16021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinekar A, Mangalesh S, Jayadev C, Maldonado RS, Bauer N, Toth CA. Retinal Imaging of Infants on Spectral Domain Optical Coherence Tomography. BioMed research international. 2015;2015:782420. doi: 10.1155/2015/782420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakitani Y, Sasoh M, Sugimoto M, Ito Y, Ido M, Uji Y. Macular Thickness Measurements in Healthy Subjects with Different Axial Lengths Using Optical Coherence Tomography. Retina, The Journal of Retinal and Vitreous Diseases. 2003;23:177–182. doi: 10.1097/00006982-200304000-00007. [DOI] [PubMed] [Google Scholar]
- Werner JS, Chalupa LM. The visual neurosciences. MIT Press; Cambridge, Mass: 2004. [Google Scholar]
- Williams RA, Booth RG. Development of optical quality in the infant monkey (Macaca nemestrina) eye. Investigative ophthalmology & visual science. 1981;21:728–736. [PubMed] [Google Scholar]
- Wilson HR. Development of spatiotemporal mechanisms in infant vision. Vision Res. 1988;28:611–628. doi: 10.1016/0042-6989(88)90111-3. [DOI] [PubMed] [Google Scholar]
- Yuodelis C, Hendrickson A. A qualitative and quantitative analysis of the human fovea during development. Vision Res. 1986;26:847–855. doi: 10.1016/0042-6989(86)90143-4. [DOI] [PubMed] [Google Scholar]
- Zuclich JA, Lund DJ, Stuck BE. Wavelength dependence of ocular damage thresholds in the near-ir to far-ir transition region: proposed revisions to MPES. Health physics. 2007;92:15–23. doi: 10.1097/01.HP.0000232188.69779.69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental video 1. Enface OCT of 10 day old infant referenced to the inner limiting membrane. The animation illustrates the nerve fiber layer arcades and the location of the foveal pit.
Supplemental Figure 1. There are minimal changes in the OPL, INL, IPL, GCL, and RNFL in the foveal region and time period studied. Eccentricities at which statistically significant changes in the OPL and INL were measures are illustrated by the horizontal lines in the plots. The thickness of both the IPL and GCL decreased with age outside of 350μm (indicated by the dashed horizontal line), but increased in thickness closer to the center of the pit. Only a small change in RNFL thickness was noted at 175μm and 350μm.


