Abstract
An effective adaptive immune response hinges on the rapid clonal expansion of T cells in response to antigen. The sensitivity of these T cells to programmed cell death (i.e. apoptosis) is carefully calibrated at various stages to ensure a robust yet measured reaction that resolves without inflicting unintended damage to host tissues. To meet bioenergetic demands associated with vigorous proliferation, acquisition of effector functions, and memory formation, T cells also undergo dynamic changes in their metabolism at every stage of this response. In this review, we focus on relatively recent studies that illuminate intimate links between metabolic programs and apoptosis sensitivity in T cells. We then examine how these connections ultimately influence T cell survival and function within the metabolically taxing environs of the tumor microenvironment.
Keywords: immunometabolism, apoptosis, T cells, restimulation-induced cell death, cytokine withdrawal-induced cell death, CWID
Introduction
The adaptive immune response is intricately linked to the cellular metabolism of lymphocytes. Many recent studies have underscored the importance of dynamic metabolic reprogramming over the course of the immune response [1, 2]. Basic properties associated with effector T and B cells, including proliferation, differentiation, migration, and effector functions (e.g. cytokine production), have different metabolic demands and therefore require flexibility in metabolic programming. This metabolic adaptability is also vital for responding T cells that encounter challenging anatomical niches, including the tumor microenvironment or certain sites of infection such as Mycobacterium tuberculosis (Mtb)-induced granulomas
Although advances in our understanding of both immunology and cancer biology have empowered progress in immunometabolism research, the extent to which changes in metabolic programs shape the T cell memory response requires further elucidation. Moreover, the magnitude and potency of any T cell response is also ultimately shaped by the sensitivity of those T cells to programmed cell death at various phases. However, the question of how cellular metabolism contributes to T cell apoptosis during the immune response has only just started to be investigated.
As the adaptive immune response unfolds, responding T cells become sensitized to various apoptotic signals in order to maintain immune homeostasis. Following activation and several days of clonal expansion, effector T cells become increasingly sensitive to apoptosis in the presence of cytokines [3, 4]. Specifically, at the peak of an adaptive immune response when antigen is still abundant, T cells are subject to a “negative feedback” apoptotic mechanism mediated by restimulation of the T cell receptor (TCR) [5]. This TCR-induced apoptosis, also known as restimulation-induced cell death (RICD), constrains the magnitude of effector T cell proliferation to prevent damaging immunopathology [6]. As pathogens and/or transformed cells are eliminated from the system, antigens are cleared and cytokines such as interleukin-2 (IL-2) that promote T cell expansion also decline. Waning cytokines and growth factors induce an intrinsic apoptosis program known as cytokine withdrawal-induced death (CWID), also referred to as activated cell autonomous death (ACAD) [7, 8]. CWID culls most of the effector T cell population during this contraction phase, leaving only a small pool of T cells that persist as memory T cells. Both RICD and CWID are critical for maintaining immune homeostasis; therefore, this review will focus on how these death programs are influenced by T cell metabolism (Figure 1).
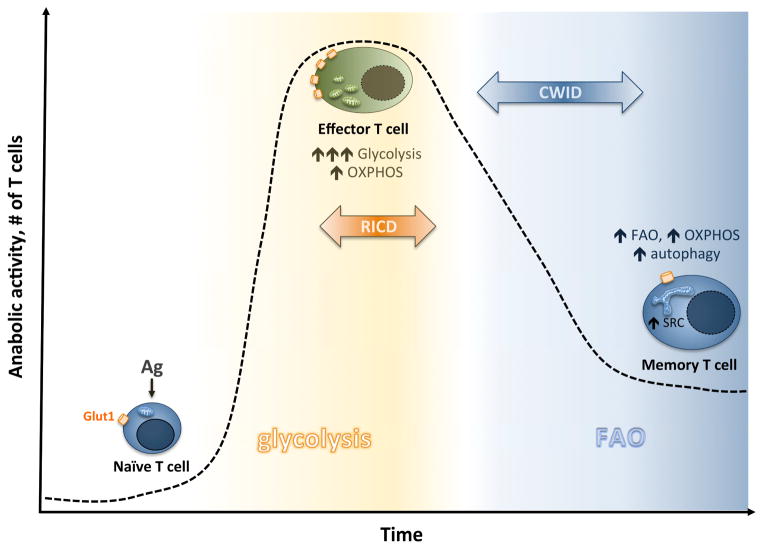
Figure 1. Sensitivity to critical apoptosis pathways correlates with metabolic reprogramming over the course of the T cell response.
Naïve T cells exist in a metabolically quiescent state until antigen (Ag stimulation), which triggers increased Glut1-mediated glucose uptake and an abrupt increase in aerobic glycolysis (as well as OXPHOS). Enhanced glycolysis sensitizes effector T cells to RICD by enabling TCR-induced FASL induction upon Ag re-encounter. As Ag is cleared, most effector T cells are culled through CWID. Those T cells that persist into the memory pool likely escape both RICD and CWID by inducing protective autophagy and turning off glycolysis in favor of FAO-direct OXPHOS. This metabolic switch returns memory T cells to a more quiescent state, “primed” for rapid recall responses via increased spare respiratory capacity.
Changes in T cell metabolism impact apoptosis sensitivity
Intricate links between metabolism and apoptosis have been detailed in various eukaryotic cell types, often with mitochondria as the central players. Studies establishing connections between apoptosis and glycolysis, the tricarboxylic acid cycle (TCA cycle), and the pentose phosphate pathway (PPP) have been explored in the context of cancer, diabetes, and neurodegenerative disorders [9]. With the recent explosion of research into immunometabolism, particularly in T cells, links to apoptosis sensitivity are just now garnering attention.
Apoptosis is a particularly important regulator of immune homeostasis because either a paucity or overaccumulation of lymphocytes can have detrimental consequences, giving rise to immunodeficiency or autoimmunity, respectively. If control is lost through apoptotic defects, an overactive immune response to pathogens can pose danger to the host in the form of lymphoproliferation and immunopathological damage [6, 10]. Furthermore, chronically activated T and B lymphocytes undergoing rapid proliferation are at increased risk of sustaining mutations, which can contribute to the development of lymphoma [8]. Alternatively, premature or excessive apoptosis could result in a lackluster T cell response that leaves the host susceptible to infectious diseases and/or cancer. Overall, T cell apoptosis sensitivity is carefully calibrated over the course of an adaptive immune response to ensure both effective clearance of pathogen and disposal of activated cells that are no longer needed and potentially dangerous to the host if left unchecked.
Naïve T cells are metabolically quiescent, relying largely on oxidative phosphorylation (OXPHOS) for ATP generation prior to antigen engagement [11]. Upon TCR/CD28 stimulation, activated T cells upregulate their anabolic metabolism to meet higher demands required for energy production, rapid cell proliferation and acquisition of effector functions. This burst of activity is associated with rapid uptake of nutrients like glucose and amino acids that jumpstart a profound increase in both glycolysis and OXPHOS, although the increase in glycolysis is much larger [12, 13]. Activated T cells upregulate cell surface expression of the glucose transporter Glut1, which is dependent on CD28 costimulation [13, 14]. Glut1-mediated glucose import and subsequent processing through the glycolytic machinery provides an abundant carbon source for building macromolecules for clonal expansion, as well as ATP. This shift to glucose dependency can also protect T cells from intrinsic apoptotic stimuli, such as osmotic stress in mouse T cell lymphomas [15]. Similarly, the viability of proliferating Jurkat and primary human T cells in culture decreases as glucose availability is limited, highlighting the importance of glucose as a metabolic substrate for activated T cell survival [13] [16]. Increased T cell death during glucose deprivation was linked to mitochondrial integrity, which is normally controlled by a balance of pro- and anti-apoptotic B cell lymphoma 2 (Bcl-2) family proteins. Specifically, increased expression of pro-apoptotic Bcl-2 domain homology 3 (BH3)-only proteins such as Bim, Puma and Noxa can initiate programmed cell death by binding and counteracting the function of anti-apoptotic Bcl-2, Bcl-xL, and Mcl-1 proteins [17]. In fact, knockdown of Noxa improved survival of activated T cells experiencing glucose limitation [16], establishing a specific role for Noxa in eliminating glucose-starved T cells. The pro-survival effect of cytokines like IL-2 and IL-7 for expanding T cells is also linked to enhanced glucose influx. Specifically, IL-7-mediated survival conferred by STAT5-dependent Akt activation was partly dependent on increased Glut-1 expression and glucose uptake; IL-7 alone was insufficient to rescue cells from apoptosis in glucose-limiting conditions [18]. Moreover, DNA synthesis inhibitors like 6-mercaptopurine block Jurkat T cell proliferation via inhibition of glycolytic (and glutaminolytic) flux [19]. Therefore, glucose not only provides a primary fuel source for activated T cells, but also ensures their persistence as the adaptive immune response takes off.
As glucose is processed through the glycolytic machinery, several molecular intermediates are shuttled into biosynthetic pathways (e.g. PPP, serine biosynthesis) that contribute to DNA replication and proliferation [1]. Pyruvate is ultimately formed as a key intermediate at the crossroads of several metabolic pathways. During clonal T cell expansion, excess pyruvate is fermented into lactate and secreted despite abundant oxygen (i.e. aerobic glycolysis) – a common feature of rapidly dividing cells [1]. However, pyruvate continues to enter the TCA cycle as acetyl-CoA to drive both lipid synthesis and OXPHOS – the most efficient pathway for ATP production. Increased influx of essential amino acids (e.g. glutamine) triggered by T cell activation also provides substrates for the TCA cycle to maintain OXPHOS. Although enhanced glycolysis clearly impacts cell death sensitivity in expanding effector T cells (see next section), recent research suggests OXPHOS constitutes a critical T cell survival signal as well. In studying patients with mitochondrial disease resulting from genetic defects in cytochrome c oxidase (COX), OXPHOS dysfunction was linked to increased apoptosis after activation, which contributed to immunodeficiency [20]. To ascertain the significance of COX function in T cells, the gene encoding COX10, a critical assembly factor for complex IV, was conditionally deleted in murine CD4+ T cells. The resulting COX-deficient T cells demonstrated an increase in both dead and actively dying cells at 72 hours post activation in vitro compared to wild type cells. The enhanced apoptosis sensitivity did not appear to be due to differences in Fas ligand (FasL) expression nor differential oxidative stress. Although the mechanism linking OXPHOS deficiency to increased apoptosis remains unclear, these studies suggest a vital role for COX activity and OXPHOS in effector T cell survival.
Another major component of T cell metabolism is fatty acid synthesis and/or oxidation. Importantly, fatty acids are not only nutrients for energy production, but also can act as signaling molecules in T cells. In addition, the composition of fatty acids that make up cell membrane phospholipids contribute to the physical properties of the membrane and its signaling components [21]. Literature connecting fatty acid metabolism to T cell differentiation and memory development has been reviewed previously [22]. However, nascent exploration of links between fatty acid metabolism and apoptosis sensitivity in T cells has yielded interesting insights. For example, palmitic acid exposure induced apoptosis in a dose-dependent manner in the Jurkat T-cell line and primary human T cells [23]. However, activated CD4+ T cells succumb to apoptosis when fatty acid synthesis or fatty acid uptake is inhibited in vitro [24]. Furthermore, fatty acid oxidation (FAO) plays a particularly important role in the formation and persistence of memory T cells. Unlike effector T cells, memory T cells utilize FAO to fuel OXPHOS instead of glycolysis to meet their energy demands [1, 25]. Indeed, limiting glycolysis via inhibition with rapamycin or RNAi-mediated knockdown of mTORC1 enhances memory T cell development [26, 27]. The FAO-rich metabolism of memory T cells is associated with a higher mitochondrial mass and therefore a higher spare respiratory capacity (SRC) than effector T cells [28], which is critical for surviving energetically stressful periods [29]. Interestingly, this difference can be reduced to dynamic remodeling of the mitochondrial architecture, which is required for optimal OXPHOS efficiency [30]. Ultimately, memory T cells are much longer-lived than effector T cells. Therefore, it appears that adapting a different type of metabolism can result in survival advantages, and likely impacts secondary effector T cell function during a recall response [31].
The size of the responding effector T cell pool is shaped by the interplay between metabolic reprogramming and specific apoptosis pathways
Restimulation-induced cell death (RICD), previously known as activation-induced cell death (AICD), is a critical pre-programmed death pathway that constrains the size of a responding effector T cell pool [6]. This apoptosis pathway occurs in cycling effector T cells that are strongly restimulated through the TCR in the presence of IL-2. Defects in RICD perturb immune homeostasis by allowing uncontrolled T cell expansion and extensive immunopathology, as illustrated by X-linked lymphoproliferative disease (XLP-1). In the absence of SAP, a small adaptor molecule required for signaling lymphocyte activation molecule (SLAM) family receptor signaling, attenuated TCR signal strength in XLP-1 patient T cells manifests in poor induction of pro-apoptotic molecules like FasL, Bim, Nur77 and NOR-1, and ultimately impaired RICD [32]. Defining the molecular determinants of RICD can therefore provide valuable therapeutic targets for controlling T cell responses by adjusting RICD sensitivity – a paradigm we recently demonstrated in the context of XLP-1 [33]. RICD can even be further exploited as a mechanism for peripheral tolerance induction via precise clonal deletion of autoreactive T cells [34].
Lipid metabolism, primarily of fatty acids, has also been coupled to changes in RICD sensitivity. Indeed, T cell hybridomas treated with myriocin, an inhibitor of serine palmitoyl-CoA transferase, exhibited a reduction in RICD sensitivity [35]. The inhibition of sphingolipid synthesis seemingly impacted apoptosis through modulation of CD95 death receptor signaling and caspase activation. However, preliminary data from our lab has shown that myriocin treatment on primary human CD8+ T cells does not impact RICD. On the other hand, inhibition of fatty acid synthase (FASN) with cerulenin or C75 decreased RICD significantly (unpublished data), suggesting different nodes in lipid metabolism are indeed critical for this apoptosis pathway and require further study.
In the context of cancer, cell growth has previously been linked to an inverse correlation between apoptosis sensitivity and nutrient-dependent metabolic activity [36]. Although effector T cells follow a parallel path by relying upon aerobic glycolysis for accelerated proliferation, new research suggests that enhanced glycolytic flux also renders these T cells more prone to apoptosis as clonal expansion peaks. Specifically, we recently demonstrated a direct correlation between RICD sensitivity and glycolytic flux in human CD8+ T cells [37]. Reducing either glycolytic activity or exogenous glucose availability rendered effector T cells significantly less sensitive to RICD. Interestingly, this study demonstrated that active glycolysis specifically facilitates the induction of pro-apoptotic FasL upon TCR restimulation, accounting for enhanced RICD sensitivity in highly glycolytic T cells. Collectively, these data indicate that RICD susceptibility is linked to metabolic reprogramming and represents an elegant mechanism of maintaining immune homeostasis, such that only activated effector T cells undergoing aerobic glycolysis are licensed to die through this pathway. Further studies are needed to identify other temporal factors required to render effector T cells competent to die via RICD well after initial clonal expansion has occurred. It also has not been determined whether switching back to a more metabolically quiescent state helps to shield T cells from RICD as they transition into the memory pool.
T cell metabolism and death sensitivity can influence immunological memory
The size of the responding effector T cell pool is not only important for the acute adaptive response, but also has implications for memory formation. Indeed, the number of effector CD8+ T cells that expand in response to infection directly correlates with the subsequent size of the memory T cell compartment [38]. Similarly, the dynamics of cytokine withdrawal and ensuing apoptosis impacts the size of the memory T cell pool. T cells entering the memory pool must curtail glycolysis (perhaps to escape RICD) and return to a more catabolic state, largely dependent on FAO to fuel OXPHOS, with enhanced SRC for rapid recall responses. In fact, genetic and pharmacological interventions that augment this switch (e.g. deoxyglucose, rapamycin, etc.) also enhance memory T cell formation [39–41]. However, glucose uptake is still required for memory T cell survival in a manner dependent on Notch signaling [42].
During the contraction phase of the T cell response triggered by cytokine withdrawal, T cells also increase autophagic activity to cope with the stress of growth factor deprivation [43, 44]. Macroautophagy, referred to here as autophagy, is a catabolic process by which cellular proteins and organelles are degraded to provide free amino acids and allows T cells to maintain ATP production. Importantly, the link between autophagy and changes in metabolism may induce or protect cells from apoptosis during stressful periods, depending on the context [45–49]. However, autophagy is clearly required for T cell activation, and plays a critical role in the survival and differentiation of memory and regulatory T cells [47, 50, 51]. Inhibition of NOTCH1 signaling induces metabolic shutdown and triggers autophagy for survival in T cell acute lymphoblastic leukemia (T-ALL); co-inhibition of autophagy synergistically enhanced anti-NOTCH treatment efficacy in eliminating T-ALL cells [52]. Additionally, we recently demonstrated that secondary effector T cells derived from sorted human memory CD8+ T cell subsets (central and effector memory) are differentially sensitive to CWID [43]. Effectors derived from the central memory (CM) pool are more resistant to RICD than their effector memory (EM)-derived counterparts, due to more sustained protective autophagy and reduced BIM expression [43]. Hence, disparate rates of autophagy and subsequent apoptosis in memory T cell subsets likely influence the magnitude of a secondary immune response, which is an important consideration for adoptive T cell transfer strategies.
The tumor microenvironment alters lymphocyte metabolism and the anti-tumor response by tuning apoptosis sensitivity
Tumor infiltrating lymphocytes (TIL) advance into extremely hostile territory upon entering the tumor microenvironment. Cancer cells evade T cell immunosurveillance by both escaping antigen recognition altogether (“immunoediting”) [53], and maintaining an immunosuppressive microenvironment that severely compromises T cell effector functions. Although recent efforts for improving anti-tumor immunity have rightly focused on restoring residual, functionally hobbled TIL via immune checkpoint blockade therapies [54, 55], many effector T cells simply die on the battlefield [56]. In some cases, self-antigen bearing immature or tolerogenic APCs found in tumor draining lymph nodes, or within the tumor microenvironment, can contribute to suboptimal CD8+ T cell activation, anergy, and apoptosis –traditionally termed “cross-tolerance” [57]. This form of T cell depletion is likely BIM-dependent [58], suggesting apoptosis is triggered by a dearth of critical nutrients and growth factors. On the other hand, robustly activated effector Th1 CD4+ and CD8+ T cells can succumb to death in response to chronic antigen stimulation, bolstered by toxic metabolites (e.g. lactate) and tumor-derived effector molecules such as galectins and FasL itself [59, 60]. Ultimately those T cells that survive despite growth factor withdrawal and/or chronic antigen exposure exhibit a transcriptionally distinct “exhausted” state [61]. T cell exhaustion is associated with increased expression of programmed death-1 (PD-1) and other co-inhibitory receptors (e.g. CTLA-4, LAG3) that render T cells hyporesponsive to antigen stimulation and therefore ineffective at eliminating tumor cells.
So how might the distinct metabolic environment within tumors, often distinguished by hypoxia and aerobic glycolysis (a.k.a. the “Warburg effect”), influence apoptosis sensitivity of responding T cells? Recent studies demonstrate that fierce competition by tumor cells for oxygen, glucose, and other key metabolites directly hobbles T cell expansion and effector functions by hindering their own metabolic needs, forcing TIL to adopt an “exhausted” phenotype [2, 62, 63]. In fact, co-inhibitory receptors like PD-1 enforce exhaustion by suppressing glycolysis and promoting fatty acid oxidation (FAO), which increases harmful reactive oxygen species (ROS) that can impact T cell survival [65]. Components of the “cancer secretome” may also directly hinder TIL function, including high concentrations of lactate and tryptophan metabolites [2]. However, the effects of many tumor-secreted factors on immunometabolism and apoptosis sensitivity require further exploration. Interestingly, regulatory T cells (Tregs) adapt a metabolic phenotype reliant on OXPHOS and FOXP3-dependent transcriptional regulation in order to function in low glucose, lactate-rich environments [66]. Indeed, progressive depletion of lipid reserves via FAO and induction of autophagy may also help FOXP3− TIL persist for a while under these conditions [64]. Over time, however, many of those TIL likely perish in response to prolonged metabolic stress, with apoptosis eventually triggered in response to severe deprivation of glucose, essential amino acids (e.g. tryptophan, glutamine, etc.) and/or autocrine/paracrine IL-2 [56]. Mitochondrial dysfunction and buildup of reactive oxygen species likely contribute to CWID/ACAD as PD-1 expression increases on exhausted T cells [67]. At the same time, those exhausted TIL that remain alive but functionally crippled within the tumor may also escape RICD by modulating TCR signal strength and glycolysis. Work from our group and others has demonstrated that optimal RICD sensitivity requires strong TCR restimulation, IL-2 driven cell cycling, and glycolysis; the magnitude of glycolytic flux directly correlates with FasL induction and RICD sensitivity in human CD8+ effector T cells [6, 37]. Thus it appears that although decreased metabolic drive in exhausted TIL dampens anti-tumor effector functions, it can promote survival in the short term by deterring those apoptosis pathways normally responsible for constraining (RICD) or contracting (CWID) the effector T cell pool in any adaptive immune response. We propose that while “rationing” of available fuels via catabolic metabolism spares TIL from acute growth factor withdrawal, modulation of both TCR signal strength and glycolytic metabolism via inhibitory receptors protects against death from chronic TCR restimulation (Figure 2).
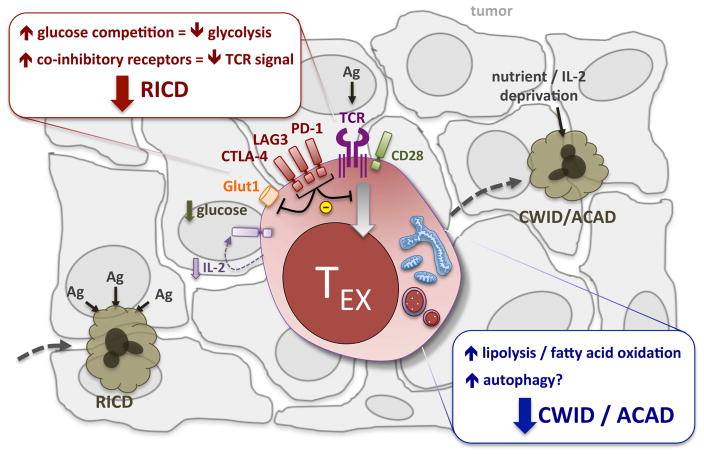
Figure 2. Exhausted TIL (TEX) escape apoptosis via altered metabolism.
Highly glycolytic T cells that infiltrate a solid tumor may be susceptible to RICD immediately upon repeated Ag encounter. However, the upregulation of co-inhibitory receptors like PD-1 attenuate TCR signal strength and promote FAO over glycolysis. This metabolic switch likely helps exhausted TEX escape RICD despite chronic Ag exposure, and survive despite fierce completion for glucose, amino acids, and other nutrients inside the harsh tumor microenvironment. Eventually, severely exhausted T cells exhibit profound metabolic stress and likely become susceptible to CWID/ACAD in response to prolonged nutrient and cytokine deprivation.
Checkpoint blockade therapy, including anti-PD-1/PD-L1 and anti-CTLA-4 blocking antibodies, rescue TIL effector functions in part by restoring glucose availability in the tumor microenvironment and reinvigorating their anabolic drive [62]. However, not all TIL respond to these interventions. “Severely” exhausted TIL exhibiting a more terminally-differentiated state (e.g. PD-1hi Tim3+) do not respond to PD-1/PD-L1 checkpoint blockade [68, 69], perhaps because they are already committed to an apoptotic fate [70]. Indeed, engagement of Tim3 by pro-apoptotic ligands (e.g. galectin-9) may promote death via RICD ([71], unpublished observations). This is consistent with the general idea that improved survival and self-renewal of less terminally-differentiated T cells provide superior protection against tumors [72] and even persistent infections like Mtb [73]. Those TIL that do respond to checkpoint blockade are re-energized in the short term to eliminate tumor cells via enhanced cytotoxicity and effector functions, but paradoxically may be rendered more sensitive to RICD as glycolysis ramps up. In the context of viral infection, the small fraction of effector T cells that end up populating the long-lived memory pool are those that downregulate glycolysis and maintain FAO and protective autophagy [2, 51]. The fate of such “memory precursor” cells may be determined as early as the first asymmetric division of a newly stimulated T cell, during which mTORC1, c-Myc, and other drivers of glycolysis are selectively partitioned into the daughter cell destined for rapid clonal expansion and enhanced but short-lived effector function [74, 75]. It is tempting to speculate that those T cells capable of tempering glycolytic drive and shifting to a more quiescent metabolic state survive to become memory T cells in part by evading both RICD and CWID. If true, we posit that evolving strategies for the optimization checkpoint blockade immunotherapies should be mindful of tuning relative apoptosis sensitivity of tumor-specific T cells via metabolic manipulations [76]. Optimized TIL responses must strike a temporal balance between acute restoration of glycolytic flux for short-term effector function, followed by a gradual switch to FAO and/or autophagy that ushers cells into the long-lived memory pool, maintained by healthy mitochondrial architecture for robust OXPHOS. Such strategies are currently under investigation and yielding promising results [41, 77]. The importance of apoptosis sensitivity extends to adoptive T cell therapies for tumor eradication, for which the selection of appropriate T cell subsets for expansion and autologous transfer is imperative for sustained anti-tumor responses. Our finding that human effector CD8+ T cells derived from the CM vs. EM subset survive better after IL-2 withdrawal may help to explain their superior expansion and protective capacity in vivo [43] and may help explain why CM T cells give rise to longer-lived, more robust and efficacious effector T cell responses. Furthermore, RICD sensitization must also be considered carefully when engineering such T cells for precision targeting using chimeric antigen receptors (CARs). CARs with the strongest activating potential in vitro often compromise the anti-tumor potency of those T cells in vivo due to enhanced susceptibility to FasL-dependent RICD [78], which correlates with enhanced glycolysis. Hence the targeting of specific T cell subsets and/or design of CARs that enforce balanced metabolic reprogramming could promote stronger and more sustained anti-tumor responses by recalibrating T cell apoptosis sensitivity.
Conclusion
In summary, immunometabolism plays a critical role in governing the scale and efficiency of effector and memory T cell responses, in part by calibrating apoptosis sensitivity properly over the duration of the immune response. Further elucidation of these mechanistic conditions will inform and improve translational applications that rely on a robust but regulated T cell attack, particularly in stressful niches like the tumor microenvironment.
Highlights.
T cells demonstrate dynamic reprogramming of their metabolism as an adaptive immune response unfolds
The magnitude of any T cell response is governed both by proliferation and temporal changes in susceptibility to specific apoptosis signals
Changes in T cell metabolism directly influence apoptosis sensitivity, which helps to maintain both immunological memory and homeostasis
Stressful conditions of the tumor microenvironment force T cells to succumb to apoptosis or adapt their metabolism to escape this fate
Understanding these metabolic adaptations and their influence on T cell death may inform and improve new immunotherapies
Abbreviations
- AICD
Activation-induced cell death
- Bcl-2
B cell lymphoma 2
- BH3
Bcl-2 domain homology 3
- CM
Central memory
- COX
Cytochrome c oxidase
- CWID
Cytokine withdrawal-induced death
- EM
Effector memory
- FasL
Fas ligand
- FAO
Fatty acid oxidation
- FASN
Fatty acid synthase
- IL-2
Interleukin-2
- IL-7
Interleukin-7
- Mtb
Mycobacterium tuberculosis
- OXPHOS
Oxidative phosphorylation
- PPP
Pentose phosphate pathway
- PD-1
Programmed death-1
- ROS
Reactive oxygen species
- Tregs
Regulatory T cells
- RICD
Restimulation-induced cell death
- SLAM
Signaling lymphocyte activation molecule
- SRC
Spare respiratory capacity
- T-ALL
T cell acute lymphoblastic leukemia
- TCR
T cell receptor
- TCA cycle
Tricarboxylic acid cycle
- TIL
Tumor infiltrating lymphocytes
- XLP-1
X-linked lymphoproliferative disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pearce EL, et al. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342(6155):1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buck MD, et al. Metabolic Instruction of Immunity. Cell. 2017;169(4):570–586. doi: 10.1016/j.cell.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehme SA, Lenardo MJ. Propriocidal apoptosis of mature T lymphocytes occurs at S phase of the cell cycle. European journal of immunology. 1993;23(7):1552–1560. doi: 10.1002/eji.1830230724. [DOI] [PubMed] [Google Scholar]
- 4.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353(6347):858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 5.Russell JH, et al. Receptor-stimulated death pathway is opened by antigen in mature T cells. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(6):2151–2155. doi: 10.1073/pnas.88.6.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snow AL, et al. The power and the promise of restimulation-induced cell death in human immune diseases. Immunol Rev. 2010;236:68–82. doi: 10.1111/j.1600-065X.2010.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner D, Krammer PH, Arnold R. Concepts of activated T cell death. Crit Rev Oncol Hematol. 2008;66(1):52–64. doi: 10.1016/j.critrevonc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Strasser A, Pellegrini M. T-lymphocyte death during shutdown of an immune response. Trends Immunol. 2004;25(11):610–5. doi: 10.1016/j.it.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Andersen JL, Kornbluth S. The tangled circuitry of metabolism and apoptosis. Mol Cell. 2013;49(3):399–410. doi: 10.1016/j.molcel.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su HC, Lenardo MJ. Genetic defects of apoptosis and primary immunodeficiency. Immunol Allergy Clin North Am. 2008;28(2):329–51. ix. doi: 10.1016/j.iac.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212(9):1345–60. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson MO, et al. Nutrients and the microenvironment to feed a T cell army. Semin Immunol. 2016;28(5):505–513. doi: 10.1016/j.smim.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maciver NJ, et al. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol. 2008;84(4):949–57. doi: 10.1189/jlb.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs SR, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180(7):4476–86. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bortner CD, et al. T-cell development of resistance to apoptosis is driven by a metabolic shift in carbon source and altered activation of death pathways. Cell Death Differ. 2016;23(5):889–902. doi: 10.1038/cdd.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alves NL, et al. The Noxa/Mcl-1 axis regulates susceptibility to apoptosis under glucose limitation in dividing T cells. Immunity. 2006;24(6):703–16. doi: 10.1016/j.immuni.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9(5):505–12. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- 18.Wofford JA, et al. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. 2008;111(4):2101–11. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Ramos AA, et al. 6-mercaptopurine promotes energetic failure in proliferating T cells. Oncotarget. 2017;8(26):43048–43060. doi: 10.18632/oncotarget.17889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarasenko TN, et al. Cytochrome c Oxidase Activity Is a Metabolic Checkpoint that Regulates Cell Fate Decisions During T Cell Activation and Differentiation. Cell Metab. 2017;25(6):1254–1268 e7. doi: 10.1016/j.cmet.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. 2008;79(3–5):101–8. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Lochner M, Berod L, Sparwasser T. Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 2015;36(2):81–91. doi: 10.1016/j.it.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi HK, et al. Activation of survival and apoptotic signaling pathways in lymphocytes exposed to palmitic acid. J Cell Physiol. 2012;227(1):339–50. doi: 10.1002/jcp.22740. [DOI] [PubMed] [Google Scholar]
- 24.Angela M, et al. Fatty acid metabolic reprogramming via mTOR-mediated inductions of PPARgamma directs early activation of T cells. Nat Commun. 2016;7:13683. doi: 10.1038/ncomms13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–83. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollizzi KN, et al. mTORC1 and mTORC2 selectively regulate CD8(+) T cell differentiation. J Clin Invest. 2015;125(5):2090–108. doi: 10.1172/JCI77746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460(7251):103–7. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Windt GJ, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36(1):68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholls DG. Spare respiratory capacity, oxidative stress and excitotoxicity. Biochem Soc Trans. 2009;37(Pt 6):1385–8. doi: 10.1042/BST0371385. [DOI] [PubMed] [Google Scholar]
- 30.Buck MD, et al. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell. 2016;166(1):63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Windt GJ, et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc Natl Acad Sci U S A. 2013;110(35):14336–41. doi: 10.1073/pnas.1221740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snow AL, et al. Restimulation-induced apoptosis of T cells is impaired in patients with X-linked lymphoproliferative disease caused by SAP deficiency. J Clin Invest. 2009;119(10):2976–89. doi: 10.1172/JCI39518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruffo E, et al. Inhibition of diacylglycerol kinase alpha restores restimulation-induced cell death and reduces immunopathology in XLP-1. Sci Transl Med. 2016;8(321):321ra7. doi: 10.1126/scitranslmed.aad1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng L, Li J, Lenardo M. Restimulation-induced cell death: new medical and research perspectives. Immunol Rev. 2017;277(1):44–60. doi: 10.1111/imr.12535. [DOI] [PubMed] [Google Scholar]
- 35.Solomon JC, et al. A novel role for sphingolipid intermediates in activation-induced cell death in T cells. Cell Death Differ. 2003;10(2):193–202. doi: 10.1038/sj.cdd.4401136. [DOI] [PubMed] [Google Scholar]
- 36.Mason EF, Rathmell JC. Cell metabolism: an essential link between cell growth and apoptosis. Biochim Biophys Acta. 2011;1813(4):645–54. doi: 10.1016/j.bbamcr.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsen SE, et al. Sensitivity to Restimulation-Induced Cell Death Is Linked to Glycolytic Metabolism in Human T Cells. J Immunol. 2017;198(1):147–155. doi: 10.4049/jimmunol.1601218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3(7):619–26. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 39.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–12. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pollizzi KN, Powell JD. Integrating canonical and metabolic signalling programmes in the regulation of T cell responses. Nat Rev Immunol. 2014;14(7):435–46. doi: 10.1038/nri3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sukumar M, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest. 2013;123(10):4479–88. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maekawa Y, et al. Notch controls the survival of memory CD4+ T cells by regulating glucose uptake. Nat Med. 2015;21(1):55–61. doi: 10.1038/nm.3758. [DOI] [PubMed] [Google Scholar]
- 43.Larsen SE, et al. Differential cytokine withdrawal-induced death sensitivity of effector T cells derived from distinct human CD8+ memory subsets. Cell Death Discov. 2017;3:17031. doi: 10.1038/cddiscovery.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lum JJ, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120(2):237–48. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 45.Booth LA, et al. The role of cell signalling in the crosstalk between autophagy and apoptosis. Cell Signal. 2014;26(3):549–55. doi: 10.1016/j.cellsig.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marino G, et al. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15(2):81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLeod IX, Jia W, He YW. The contribution of autophagy to lymphocyte survival and homeostasis. Immunol Rev. 2012;249(1):195–204. doi: 10.1111/j.1600-065X.2012.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rathmell JC. Metabolism and autophagy in the immune system: immunometabolism comes of age. Immunol Rev. 2012;249(1):5–13. doi: 10.1111/j.1600-065X.2012.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh R, Cuervo AM. Lipophagy: connecting autophagy and lipid metabolism. Int J Cell Biol. 2012;2012:282041. doi: 10.1155/2012/282041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei J, et al. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat Immunol. 2016;17(3):277–85. doi: 10.1038/ni.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu X, et al. Autophagy is essential for effector CD8(+) T cell survival and memory formation. Nat Immunol. 2014;15(12):1152–61. doi: 10.1038/ni.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herranz D, et al. Metabolic reprogramming induces resistance to anti-NOTCH1 therapies in T cell acute lymphoblastic leukemia. Nat Med. 2015;21(10):1182–9. doi: 10.1038/nm.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vesely MD, Schreiber RD. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann N Y Acad Sci. 2013;1284:1–5. doi: 10.1111/nyas.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dammeijer F, et al. Rationally combining immunotherapies to improve efficacy of immune checkpoint blockade in solid tumors. Cytokine Growth Factor Rev. 2017 doi: 10.1016/j.cytogfr.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 55.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36(4):265–76. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu B, Finn OJ. T-cell death and cancer immune tolerance. Cell Death Differ. 2008;15(1):70–9. doi: 10.1038/sj.cdd.4402274. [DOI] [PubMed] [Google Scholar]
- 57.Kurts C, et al. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J Exp Med. 1997;186(2):239–45. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davey GM, et al. Peripheral deletion of autoreactive CD8 T cells by cross presentation of self-antigen occurs by a Bcl-2-inhibitable pathway mediated by Bim. J Exp Med. 2002;196(7):947–55. doi: 10.1084/jem.20020827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9(5):338–52. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 60.Whiteside TL. Tumor-induced death of immune cells: its mechanisms and consequences. Semin Cancer Biol. 2002;12(1):43–50. doi: 10.1006/scbi.2001.0402. [DOI] [PubMed] [Google Scholar]
- 61.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–99. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang CH, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162(6):1229–41. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ho PC, et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162(6):1217–28. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patsoukis N, et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692. doi: 10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tkachev V, et al. Programmed death-1 controls T cell survival by regulating oxidative metabolism. J Immunol. 2015;194(12):5789–800. doi: 10.4049/jimmunol.1402180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Angelin A, et al. Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments. Cell Metab. 2017;25(6):1282–1293 e7. doi: 10.1016/j.cmet.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bengsch B, et al. Bioenergetic Insufficiencies Due to Metabolic Alterations Regulated by the Inhibitory Receptor PD-1 Are an Early Driver of CD8(+) T Cell Exhaustion. Immunity. 2016;45(2):358–73. doi: 10.1016/j.immuni.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Im SJ, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015;6:e1792. doi: 10.1038/cddis.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang CW, et al. Apoptosis of tumor infiltrating effector TIM-3+CD8+ T cells in colon cancer. Sci Rep. 2015;5:15659. doi: 10.1038/srep15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu C, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–52. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 72.Klebanoff CA, Gattinoni L, Restifo NP. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J Immunother. 2012;35(9):651–60. doi: 10.1097/CJI.0b013e31827806e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lindenstrom T, et al. Control of chronic mycobacterium tuberculosis infection by CD4 KLRG1- IL-2-secreting central memory cells. J Immunol. 2013;190(12):6311–9. doi: 10.4049/jimmunol.1300248. [DOI] [PubMed] [Google Scholar]
- 74.Pollizzi KN, et al. Asymmetric inheritance of mTORC1 kinase activity during division dictates CD8(+) T cell differentiation. Nat Immunol. 2016;17(6):704–11. doi: 10.1038/ni.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verbist KC, et al. Metabolic maintenance of cell asymmetry following division in activated T lymphocytes. Nature. 2016;532(7599):389–93. doi: 10.1038/nature17442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herbel C, et al. Clinical significance of T cell metabolic reprogramming in cancer. Clin Transl Med. 2016;5(1):29. doi: 10.1186/s40169-016-0110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patel CH, Powell JD. Targeting T cell metabolism to regulate T cell activation, differentiation and function in disease. Curr Opin Immunol. 2017;46:82–88. doi: 10.1016/j.coi.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kunkele A, et al. Functional Tuning of CARs Reveals Signaling Threshold above Which CD8+ CTL Antitumor Potency Is Attenuated due to Cell Fas-FasL-Dependent AICD. Cancer Immunol Res. 2015;3(4):368–79. doi: 10.1158/2326-6066.CIR-14-0200. [DOI] [PubMed] [Google Scholar]