Key Clinical Message
High‐dose intravenous thyroxine (T4) is the preferable treatment for myxedema coma. We describe the clinical course of a 69‐year‐old man who presented with myxedema coma and received oral levothyroxine (LT4) therapy (1 mg) in a split dose. This suggests split high‐dose oral LT4 as a therapeutic option in myxedema coma.
Keywords: Coma, hypothyroidism, myxedema, oral administration, thyroxine
Introduction
Myxedema coma is a life‐threatening endocrine emergency. It is a rare presentation of severe hypothyroidism, with an approximate incidence of 0.22 per million people per year. Even with available therapies, the mortality rate of this condition is high, 50–60% 1, 2, 3. In patients with a high index of suspicion, thyroid hormone replacement should be initiated as early as possible. To date, thyroxine (T4) is the mainstay treatment of myxedema coma. Intravenous injection of T4 is the preferable route of administration due to concerns about compromised gastrointestinal (GI) absorption of oral T4 in such patients. However, high‐dose intravenous T4 therapy may result in a deleterious effect on the cardiovascular system, especially in patients with pre‐existing coronary artery disease. In some countries, the use of intravenous T4 is restricted owing to its lack of availability.
Here, we report a case of myxedema coma successfully treated with split high‐dose oral levothyroxine (LT4) therapy, which may be considered as an alternative regimen to the standard treatment, especially in countries where intravenous T4 is not available.
Case Report
A 69‐year‐old man was admitted to the otolaryngology unit due to the presence of an oropharyngeal mass, with difficulty in swallowing for the previous 2 months. The initial examination revealed a mass at the posterior oropharyngeal wall extending to the left tonsil. The pathology report from an incisional biopsy was compatible with well‐differentiated squamous cell carcinoma. He was then admitted for nutritional support and scheduled for gastrostomy.
The patient had a past medical history of laryngeal carcinoma and had undergone total laryngectomy and tracheostomy followed by concurrent chemoradiation 15 years prior. The laryngeal carcinoma was cured. Although he had been breathing through a tracheostomy tube, he was otherwise in good condition and was able to conduct normal activities of daily living. Other personal history included a 40 pack‐years history of smoking, which had been stopped 15 years prior, and no current use of medication. There was no family history of cardiovascular or metabolic diseases.
Clinical features on admission were stable, except for difficulty in swallowing due to mechanical obstruction from the oropharyngeal mass. Initial laboratory investigations revealed normocytic anemia and mild hyponatremia (serum sodium level of 128 mEq/L), which was initially considered to be from hypovolemic hyponatremia owing to his inability to eat. Electrocardiography (ECG) showed atrial fibrillation (AF), probably owing to chronic obstructive pulmonary disease, with a ventricular rate of 70 beats per minute (bpm). An open gastrostomy was performed at day 3 of admission. Intraoperatively, AF with slow ventricular response (heart rate of 46 bpm) occurred after induction of general anesthesia with 4 mg of midazolam, 100 μg of fentanyl, 125 mg of succinylcholine, and 100 mg of propofol, intravenously. However, this bradyarrhythmia responded well to a single dose (0.6 mg) of intravenous atropine. Additional intraoperative medication included 1 g of cefoxitin, intravenously. There were no immediate postoperative complications. Enteral feeding via gastrostomy was initiated afterward.
At day 8 of admission, the patient developed slow responsiveness to arousal and intermittent confusion. His vital signs remained stable, with body temperature 36°C, heart rate 80 bpm, blood pressure 120/80 mmHg, and respiratory rate 16 bpm. Mild puffiness of the face and dry skin were noted. Additional laboratory testing revealed thyroid‐stimulating hormone (TSH) 15.056 mIU/L (0.35–4.94), free T4 (FT4) 0.04 ng/dL (0.70–1.48), undetectable concentration of free triiodothyronine (T3), and morning cortisol 19 μg/dL. Primary hypothyroidism due to laryngectomy and neck radiation was first diagnosed at that time, and a low dose of oral levothyroxine (LT4), 25 μg/day, was given via gastrostomy. At day 14 of admission, chemoradiation for the treatment of oropharyngeal carcinoma was initiated. At day 19 of admission, after 10 days of LT4 initiation, FT4 level was checked and revealed no increment. Over time, the patient's consciousness did not significantly improve. In addition, serum sodium level further decreased to 125 mEq/L. Capillary plasma glucose also progressively declined, ranging 70–100 mg/dL, and he required 10% dextrose solution. The attending physicians were aware of impaired absorption of LT4 because of many medications used (including proton pump inhibitor) and malnutrition. Therefore, the administration time of LT4 was rescheduled to be earlier in the morning (on an empty stomach), and more nutritional support was provided. Nevertheless, myxedema coma was not considered at this time, and the same dosage of LT4 continued to be prescribed.
At day 28 of admission (2 weeks after initiation of the chemoradiation course), the patient's level of consciousness further deteriorated. Endocrinologists were consulted at that time. During clinical evaluation, he did not respond to voice commands. Vital signs showed oral temperature of 33.4°C, pulse rate of 40 bpm with irregularities, respiratory rate of 12 bpm, and blood pressure of 80/40 mmHg. Laboratory testing until day 28 of admission is summarized in Table 1. Other laboratory investigations were as follows: plasma glucose 56 mg/dL, creatine kinase (CK)‐MB 182 U/L (0–24), and troponin T 102 ng/L (<14). ECG showed AF with a ventricular rate of 40 bpm. There was no ECG change suggesting acute myocardial ischemia. Bedside echocardiography revealed global hypokinesia without evidence of myocardial infarction. A small amount of pericardial effusion was documented. Chest radiography showed right upper lung haziness, suggesting pneumonia. The most likely diagnosis of his condition was myxedema coma precipitated by hospital‐acquired pneumonia and neutropenia. The additional differential diagnosis was sepsis. Regarding the treatment of myxedema coma, as intravenous T4 is not available in Thailand, 1 mg oral LT4 therapy was considered as the treatment of choice. Due to a high probability of silent ischemic heart disease in this elderly patient, LT4 was prescribed as follows: a split dose of 0.2 mg LT4 every 8 h for five consecutive doses, followed by a maintenance dose of 0.1 mg/day. The adjustment of LT4 dosage was based on clinical response. Intravenous hydrocortisone was given initially at 300 mg/day. Other supportive therapies included mechanical ventilation, passive rewarming with blankets, empirical antibiotics, intensive fluid therapy, and an inotropic agent. The patient responded well to treatment. Vital signs improved within 48 h, that is normalization of body temperature and improvement of ventricular rate of AF. Blood culture and tracheal suction culture revealed Serratia marcescens infection, which responded well to carbapenem. Hydrocortisone was tapered off after 5 days. The inotropic agent was tapered off, and mechanical ventilation was stopped after 7 days. FT4 level reached the normal reference range after 2 days of high‐dose LT4 initiation and remained in the reference range after 8 days. The changes in FT4 level are summarized in Figure 1. At day 58 of admission, the patient was referred back to his hometown hospital for terminal care of advanced oropharyngeal carcinoma.
Table 1.
Summary of laboratory tests prior to the diagnosis of myxedema coma
| Laboratory tests | Day 1 | Day 8 | Day 14 | Day 19 | Day 28 | Reference range |
|---|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 8.45 | 10.67 | – | 9.1 | 6.4 | 13–18 |
| Hematocrit (%) | 23.2 | 31.81 | – | 26.4 | 19.5 | 40–54 |
| WBC (cells/mm3) | 5,140 | 12,290 | – | 10,340 | 300 | 4,000–10,000 |
| Platelets (cells/mm3) | 297,000 | 242,000 | – | 244,000 | 297,000 | 140,000–450,000 |
| Creatinine (mg/dL) | 1.09 | 1.21 | 0.85 | 0.99 | 1.6 | 0.73–1.18 |
| Sodium (mEq/L) | 126 | 130 | 127 | 125 | 127 | 136–145 |
| Potassium (mEq/L) | 3.89 | 3.55 | 4.08 | 4.36 | 3.7 | 3.5–5.1 |
| Chloride (mEq/L) | 93 | 94 | 84 | 90 | 94 | 98–107 |
| Bicarbonate (mEq/L) | 24.5 | 19.9 | 28.8 | 30.4 | 20.9 | 22–29 |
| Free T3 (pg/mL) | – | <1 | – | – | – | 1.71–3.71 |
| Free T4 (ng/dL) | – | 0.04 | – | 0.04 | 0.09 | 0.7–1.48 |
| TSH (mIU/L) | – | 15.056 | – | – | – | 0.35–4.94 |
| Arterial blood gas | – | – | – | – |
pH 7.3 PaCO2 43 mmHg PaO2 66 mmHg |
|
| Clinical course | Admission | Start LT4 0.025 mg/day | Start chemotherapy | Diagnosis of myxedema coma |
LT4, levothyroxine; T3, triiodothyronine; T4, thyroxine; TSH, thyroid‐stimulating hormone; WBC, white blood cells.
Figure 1.
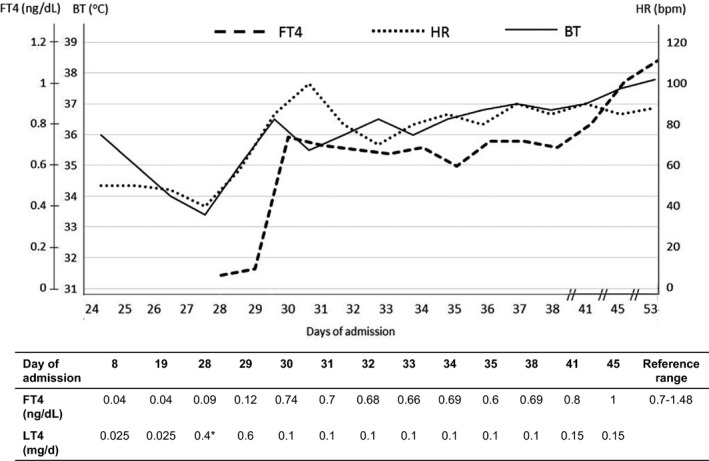
Clinical response after initiation of split high‐dose oral LT4 (at day 28 of admission) with 0.2 mg LT4 orally every 8 h for five consecutive doses, then maintenance with 0.1 mg/day. °C, degree celsius; BT, body temperature; bpm, beats per minute; FT4, free thyroxine; HR, heart rate.
Discussion
The cardinal manifestation of myxedema coma is a deterioration of the patient's mental status, ranging from confusion to lethargy and possibly obtundation 4. Associated symptoms include hypoxemia, hypercapnia, and hypothermia 5. Patients with myxedema coma mostly present with typical features of hypothyroidism, such as dry and coarse skin, alopecia, hoarse voice, periorbital edema, macroglossia, nonpitting edema of the hands and feet, and slow tendon reflex relaxation 2. In patients at risk of primary hypothyroidism – for instance, patients with a history of neck surgery and neck radiation – primary physicians should look for signs and symptoms of hypothyroidism and then provide adequate thyroid hormone replacement if primary hypothyroidism is diagnosed. A delayed diagnosis and inadequate thyroid hormone adjustment in this patient were recognized. Without evidence of myxedema coma at the beginning, initiating 25–50 μg LT4 would be appropriate in this elderly patient who had AF 6. However, in this circumstance where the patient was critically ill, assessment of FT4 levels within a few days to a week is warranted for appropriate adjustment of LT4 dosage. A combination of precipitating events, including surgical intervention and infection, resulted in myxedema coma. This emphasizes the importance of early recognition and awareness of these conditions. When suspicion of myxedema is high, thyroid hormone replacement should not be delayed while waiting for laboratory results of serum T4 and TSH.
The main considerations with thyroid hormone replacement in patients with myxedema coma are the absorption and distribution of the administered hormone preparation, the onset of action, and the efficacy and safety of the treatment regimen. Compared with T3 administration, T4 therapy provides a steadier, smoother, and slower onset of action, which should reduce adverse cardiovascular side effects. Therefore, T4 therapy is considered the mainstay treatment of myxedema coma. Parenteral injection of T4 is the preferable route of administration due to compromised GI function and impaired level of consciousness in such patients.
According to the recommendations of the American Thyroid Association task force on thyroid hormone replacement, initial thyroid hormone replacement for myxedema coma is T4 intravenously with a loading dose of 0.2–0.4 mg. Lower doses would be given to smaller or older patients and those with a history of coronary disease or arrhythmia 7. Close cardiac monitoring in patients on initiation of high‐dose LT4 treatment is crucial. Based on a report suggesting that an estimate of total body T4 is 0.05 mg, such a dose should replace the entire extrathyroidal pool of T4 and restore near‐normal hormonal status. Thereafter, the body's T4 pool is maintained by administration of 0.05–0.1 mg daily, given intravenously or orally 8, 9. However, these recommendations are based solely on expert opinion and case reports due to the rarity of myxedema coma. Regardless of gastric atony in patients with myxedema coma, Read et al. 10 demonstrated that the rate of T4 absorption after oral administration of T4 through a nasogastric tube was similar among hypothyroid and euthyroid (control) subjects. In 1991, Arlot et al. reported on seven patients presenting with myxedema coma. Two of them received treatment with 1 mg intravenous T4, and the rest received 0.5 mg LT4 orally on the first day. The subsequent daily dose was varied for each patient. As expected, plasma T4 and T3 of the oral group increased more slowly than those of the intravenous group. Nonetheless, improvement in clinical outcome occurred within 24–72 h in both groups 11. Similar results were also demonstrated in a study by Dutta et al. 12.
In our patient, we demonstrated the effectiveness of “split high‐dose oral LT4 therapy” as an alternative treatment for myxedema coma. Oral administration of 0.2 mg LT4 every 8 h in five consecutive doses (total dose of 1 mg) resulted in significant restoration of depleted thyroid status and clinical improvement within 48 h after treatment initiation. This regimen should be considered as a promising alternative therapeutic option in light of the risk of adverse cardiovascular effects of single high‐dose LT4 therapy, especially in elderly patients. Using full‐dose T4 therapy has been reported to worsen myocardial ischemia by increasing myocardial oxygen consumption 7. In addition, Yamamoto et al. 3 reported that doses of LT4 of more than 500 μg/day were associated with increased mortality. Of note, receiving an inotropic agent and old age predicted high mortality rate in this patient, regardless of using high‐dose T4 therapy 13. Other fundamental principles are clinical monitoring on a daily basis and measurement of thyroid hormones every 1–2 days 7. Failure of TSH to decrease, or of thyroid hormone levels to increase, suggests the need to increase doses of T4.
Besides thyroid hormone replacement therapy, other general management techniques, including endotracheal intubation or tracheostomy with mechanical ventilation, external warming with an electric blanket, and proper fluid therapy and vasopressor administration, are also vital in determining outcomes of myxedema coma 14. Glucocorticoid therapy is generally deemed prudent because of the possibility of coexisting primary or secondary adrenal insufficiency. Furthermore, there is a high probability of increased cortisol clearance after thyroid hormone therapy, which could aggravate adrenal insufficiency. In our patient, morning serum cortisol of 19 μg/dL before the diagnosis of myxedema coma indicated that the patient's hypothalamic–pituitary–adrenal axis was intact. Correspondingly, glucocorticoids can be stopped safely after the patient has improved. An active search for precipitating causes is also critical and should be performed initially. Urgent empirical antibiotic treatment is warranted in cases of suspicion of infection.
Myxedema coma has mostly been reported in patients with overt primary hypothyroidism, a setting where TSH is extremely increased, and both T4 and T3 are extremely low. In our patient, TSH level was 15.056 mIU/L, and FT4 level was as low as 0.04 ng/dL (Table 1). It is noteworthy that in this setting of very low FT4 level, TSH level was found to be lower than expected. The possible explanation might be the suppressed TSH that sometimes occurs in critically ill patients. Therefore, this phenomenon could be recognized as hypothyroid sick syndrome, in parallel with euthyroid sick syndrome 15.
In myxedema coma, laboratory investigations may reveal hyponatremia, hypoglycemia, anemia, hypercholesterolemia, and elevation of serum lactate dehydrogenase 2, 15, 16, 17, 18, 19, 20. Interestingly, our patient had elevated CK‐MB and troponin T without concomitant clinical or ECG signs of myocardial ischemia. Previous studies have reported that hypothermia in combination with hypothyroidism‐induced reduction in cardiac enzyme turnover might underlie elevated cardiac enzymes 21, 22. Cardiovascular function can be restored after replacement of thyroid hormone. Careful thyroid hormone administration in low doses is essential to reduce the risk of arrhythmia, angina pectoris, and heart failure 23. However, no recommendation is available regarding the proper dose and interval of T4 replacement in patients with myxedema coma and who are at high risk of coronary artery disease.
Conclusion
Early recognition of myxedema coma is crucial, and a high index of suspicion is typically warranted in the elderly with signs of hypothyroidism, hypothermia, hyponatremia, hypercarbia, and/or hypoxemia. Treatment with high‐dose thyroid hormone must be initiated as soon as possible. Regarding the risk of cardiovascular side effects from single high‐dose T4 therapy, especially in the elderly, split high‐dose oral LT4 therapy results in impressive clinical outcomes and should be considered as an alternative therapeutic regimen in myxedema coma.
Conflict of Interest
None declared.
Declarations
The ethical review board of Ramathibodi Hospital, Mahidol University, approved this study. The patient had passed away, while the authors were preparing the manuscript. Therefore, informed consent was provided by the patient's relative.
Authorship
SC and HN: contributed equally to the care of this patient. SC: contributed to the initial conception, drafting, and final approval of the article. CS and HN: gave final approval of the article.
Clinical Case Reports 2017; 5(10): 1706–1711
References
- 1. Rodriguez, I. , Fluiters E., Perez‐Mendez L. F., Luna R., Paramo C., and Garcia‐Mayor R. V.. 2004. Factors associated with mortality of patients with myxoedema coma: prospective study in 11 cases treated in a single institution. J. Endocrinol. 180:347–350. [DOI] [PubMed] [Google Scholar]
- 2. Wartofsky, L. . 2006. Myxedema coma. Endocrinol. Metab. Clin. North Am. 35:687–698, vii–viii. [DOI] [PubMed] [Google Scholar]
- 3. Yamamoto, T. , Fukuyama J., and Fujiyoshi A.. 1999. Factors associated with mortality of myxedema coma: report of eight cases and literature survey. Thyroid 9:1167–1174. [DOI] [PubMed] [Google Scholar]
- 4. Wall, C. R. 2000. Myxedema coma: diagnosis and treatment. Am. Fam. Physician 62:2485–2490. [PubMed] [Google Scholar]
- 5. Reinhardt, W. , and Mann K.. 1997. [Incidence, clinical picture and treatment of hypothyroid coma. Results of a survey]. Med. Klin. (Munich) 92:521–524. [DOI] [PubMed] [Google Scholar]
- 6. Garber, J. R. , Cobin R. H., Gharib H., Hennessey J. V., Klein I., Mechanick J. I., et al. 2012. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr. Pract. 18:988–1028. [DOI] [PubMed] [Google Scholar]
- 7. Jonklaas, J. , Bianco A. C., Bauer A. J., Burman K. D., Cappola A. R., Celi F. S., et al. 2014. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 24:1670–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holvey, D. N. , Goodner C. J., Nicoloff J. T., and Dowling J. T.. 1964. Treatment of myxedema coma with intravenous thyroxine. Arch. Intern. Med. 113:89–96. [DOI] [PubMed] [Google Scholar]
- 9. Nicoloff, J. T. , and LoPresti J. S.. 1993. Myxedema coma. A form of decompensated hypothyroidism. Endocrinol. Metab. Clin. North Am. 22:279–290. [PubMed] [Google Scholar]
- 10. Read, D. G. , Hays M. T., and Hershman J. M.. 1970. Absorption of oral thyroxine in hypothyroid and normal man. J. Clin. Endocrinol. Metab. 30:798–799. [DOI] [PubMed] [Google Scholar]
- 11. Arlot, S. , Debussche X., Lalau J. D., Mesmacque A., Tolani M., Quichaud J., et al. 1991. Myxoedema coma: response of thyroid hormones with oral and intravenous high‐dose L‐thyroxine treatment. Intensive Care Med. 17:16–18. [DOI] [PubMed] [Google Scholar]
- 12. Dutta, P. , Bhansali A., Masoodi S. R., Bhadada S., Sharma N., and Rajput R.. 2008. Predictors of outcome in myxoedema coma: a study from a tertiary care centre. Crit. Care 12:R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ono, Y. , Ono S., Yasunaga H., Matsui H., Fushimi K., and Tanaka Y.. 2017. Clinical characteristics and outcomes of myxedema coma: analysis of a national inpatient database in Japan. J. Epidemiol. 27:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verbalis, J. G. , Goldsmith S. R., Greenberg A., Schrier R. W., and Sterns R. H.. 2007. Hyponatremia treatment guidelines 2007: expert panel recommendations. Am. J. Med. 120(11 Suppl 1):S1–S21. [DOI] [PubMed] [Google Scholar]
- 15. Wartofsky, L. , and Burman K. D.. 1982. Alterations in thyroid function in patients with systemic illness: the “euthyroid sick syndrome”. Endocr. Rev. 3:164–217. [DOI] [PubMed] [Google Scholar]
- 16. Carmel, R. , and Spencer C. A.. 1982. Clinical and subclinical thyroid disorders associated with pernicious anemia. Observations on abnormal thyroid‐stimulating hormone levels and on a possible association of blood group O with hyperthyroidism. Arch. Intern. Med. 142:1465–1469. [PubMed] [Google Scholar]
- 17. Cullen, M. J. , Mayne P. D., and Sliney I.. 1979. Myxoedema coma. Irish J. Med. Sci. 148:201. [DOI] [PubMed] [Google Scholar]
- 18. Das, K. C. , Mukherjee M., Sarkar T. K., Dash R. J., and Rastogi G. K.. 1975. Erythropoiesis and erythropoietin in hypo‐ and hyperthyroidism. J. Clin. Endocrinol. Metab. 40:211–220. [DOI] [PubMed] [Google Scholar]
- 19. Hines, J. D. , Halsted C. H., Griggs R. C., and Harris J. W.. 1968. Megaloblastic anemia secondary to folate deficiency associated with hypothyroidism. Ann. Intern. Med. 68:792–805. [DOI] [PubMed] [Google Scholar]
- 20. Tudhope, G. R. , and Wilson G. M.. 1962. Deficiency of vitamin B12 in hypothyroidism. Lancet 1:703–706. [DOI] [PubMed] [Google Scholar]
- 21. Hickman, P. E. , Silvester W., Musk A. A., McLellan G. H., and Harris A.. 1987. Cardiac enzyme changes in myxedema coma. Clin. Chem. 33:622–624. [PubMed] [Google Scholar]
- 22. Salomo, L. H. , Laursen A. H., Reiter N., and Feldt‐Rasmussen U.. 2014. Myxoedema coma: an almost forgotten, yet still existing cause of multiorgan failure. BMJ Case Rep. 2014: https://doi.org/10.1136/bcr-2013-203223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hylander, B. , and Rosenqvist U.. 1985. Treatment of myxoedema coma – factors associated with fatal outcome. Acta Endocrinol. (Copenh). 108:65–71. [DOI] [PubMed] [Google Scholar]


