Abstract
Obesity is a serious health problem in the US and is associated with increased risks of various human diseases. To date, the mechanisms by which obesity increases the risks of a wide range of human diseases are not well understood. Here we used a LC–MS/MS-based lipidomics, which can analyze >100 bioactive lipid mediators produced by cyclooxygenase, lipoxygenase, and cytochrome P450 enzymes, to analyze plasma profiles of lipid mediators in high-fat diet induced obesity in C57BL/6 mice. Our results show that the plasma concentrations of epoxyoctadecenoic acids (EpOMEs, also termed as leukotoxins) are significantly increased in plasma of high-fat diet-fed mice, in addition, EpOMEs are among the most abundant lipid mediators detected in mouse plasma. Since substantial studies have shown that EpOMEs and their metabolites have a large array of detrimental effects on health, enhanced levels of EpOMEs could contribute to the pathology of obesity
1. Introduction
Obesity is growing at an alarming rate in the US: currently, more than 35% adults and nearly 17% children are obese [1,2]. Individuals with obesity have significantly increased risks of developing various diseases, including cardiovascular diseases, lung and respi- ratory diseases, diabetes, hypertension, and certain types of cancers [3]. To date, the mechanisms by which obesity increases the risks of such diverse range of human diseases are not well understood. While most previous mechanistic research of obesity has focused on proteinous mediators [4], the roles of non-proteinous regulators, such as eicosanoids and associated lipid mediators (LMs), are largely unknown.
Eicosanoids and associated LMs are enzymatic metabolites of polyunsaturated fatty acids (PUFAs) produced by cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP) enzymes [5–7]. The enzymatic metabolism of PUFAs leads to formation of several major classes of LMs, including prostaglandins produced by COX enzymes, leukotrienes and hydroxyl fatty acids produced by LOX enzymes, and epoxy and dihydroxy fatty acids produced by CYP enzymes [5–7]. Multiple PUFAs, including linoleic acid (LA, 18:2w-6), a-linolenic acid (ALA, 18:3ω-3), dihomo-')'-linolenic acid (DGLA, 20:3w-6), arachidonic acid (ARA, 20:4 ω-6), eicosapentaenoic acid (EPA, 20:5 ω-3), and docosahexaenoic acid (DHA, 22:6 ω-3), are efficient substrates of these PUFA metabolizing enzymes [7,8]. Together, this leads to formation of a large array of LMs, which have diverse chemical structures and biological actions. Previous studies have shown that LMs play central roles in regulating inflammation and hemostasis, and play important roles in the pathology of obesity [9–14]. Most previous studies of eicosanoids in obesity have focused on profiles of LMs in adipose tissues, few studies have characterized the profiles of LMs in plasma. Since the circulating LMs could play an important role in systematic bio- logical responses, here we used a LC–MS/MS-based lipidomics to analyze the plasma profiles of LMs in high-fat diet (HFD)-induced obesity in mice (see list of LMs covered by our LC–MS/MS method in Supplemental Table S1) [15].
2. Materials and methods
2.1. Obesity experiment
C57BL/6 male mice (6-week age, purchased from Charles River) were maintained on a high-fat diet (60% kcal% fat, purchased from Research Diet Inc., catalog # D12492) and a control diet (10 kcal% fat, catalog # D12450J from Research Diet Inc.) for 8 weeks. After 8 weeks of dietary feeding, the mice were sacrificed and the plasma was collected for LC–MS/MS analysis. The animal experiment was conducted in accordance with the protocols approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Amherst.
2.2. Extraction of lipid metabolites from plasma and colon tissues
To extract LMs from mouse plasma, 250 µL mouse plasma was mixed with deuterated internal standards (500 nM of d4-6-keto PGF1a, d4-TXB2, d4-PGE2, d4-LTB4, d11-14,15-DHET, d4-9-HODE, d8-5-HETE, d11-11,12-EET), then loaded onto pre-washed Waters® Oasis solid phase extraction cartridges, washed with 95:5 water/methanol with 0.1% acetic acid, the analytes were eluted with methanol and ethyl acetate, dried using a centrifugal vacuum evaporator, then reconstituted in methanol for LC–MS/MS analysis, as we described [15].
2.3. LC–MS/MS measurement of lipid metabolites
The LC–MS/MS analysis was carried out on an Agilent 1200SL HPLC system (Agilent, Santa Clara, CA) coupled to a 4000 QTRAP MS/MS (AB Sciex, Foster City, CA) as described in our previous report [15]. The peaks were identified according to the retention time and specific multiple reaction monitoring transitions of the standards of lipid metabolite. The concentrations of the lipid metabolites were calculated against the calibration curve with standards.
2.4. Data analysis
Data are expressed as mean ± standard error of the mean (SEM). For the comparison between control group and HFD group, Shapiro- Wilk test was used to verify the normality of data. When data were normally distributed, statistical significance was calculated using two-side t-test; otherwise, significance was determined by Mann-Whitney U test. All of these data analysis was performed by using SigmaPlot software (San Jose, CA). P values less than 0.05 are reported as statistically significant.
3. Results
3.1. Profiles of CYP-derived LMs in plasma
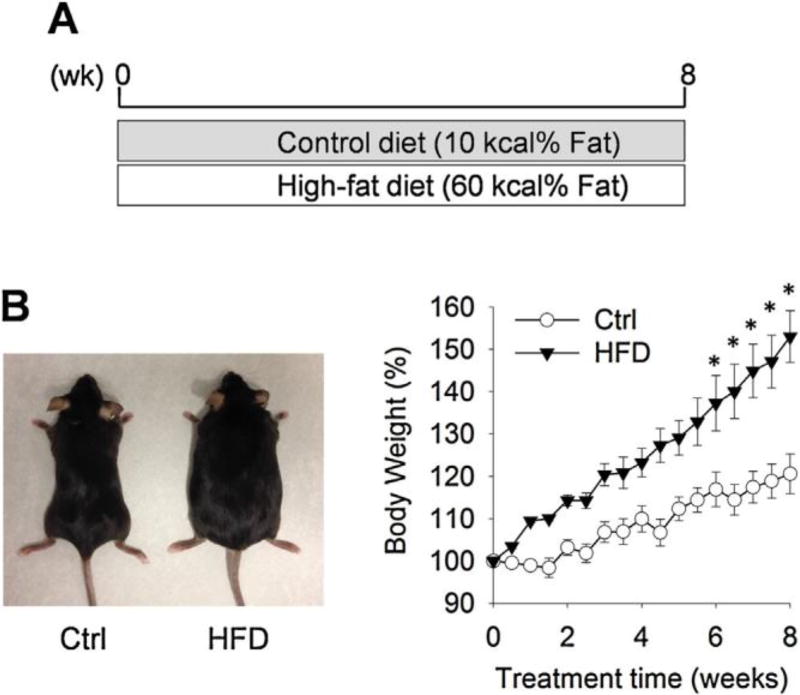
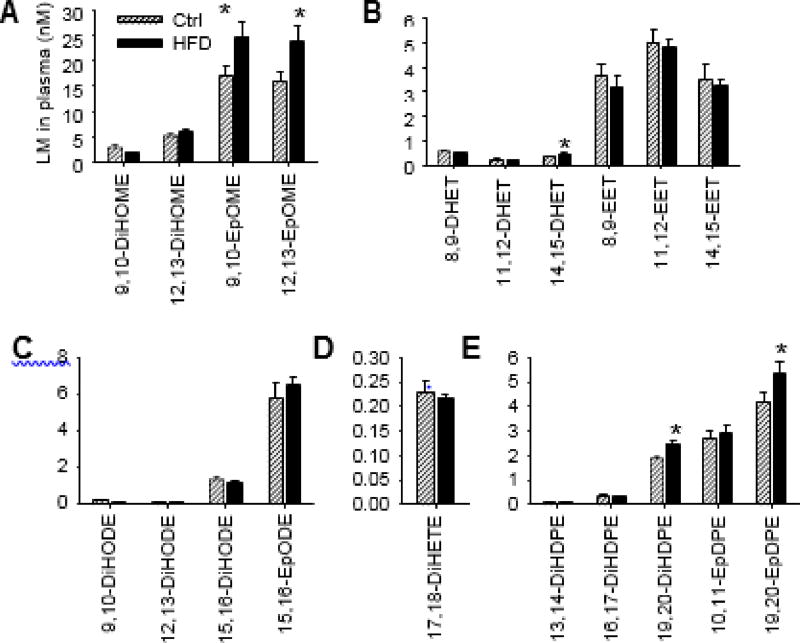
Dietary feeding of HFD significantly increased body weight in C57BL/6 mice (Fig. 1), which is in agreement with previous studies of HFD-induced obesity in mice [16]. We used a LC–MS/MS- based lipidomics to compare the profiles of LMs in the plasma of mice maintained on HFD or control diet. For the CYP-derived LM, we detected 20 fatty acid epoxides and diols in mouse plasma, which are metabolites of LA, ARA, ALA, EPA, and DHA produced by CYP enzymes. Among the detected fatty acid epoxides and diols, the concentrations of 9,10- and 12,13-EpOMEs were significantly increased in the plasma of HFD-fed mice: the plasma concentration of 9,10-EpOME was increased from 17.2 ±1.9 nM (mean ± SEM, control group) to 24.6 ± 2.8 nM (HFD group), and the plasma concentration of 12,13-EpOME was increased from 15.9 ±1.7 nM (control group) to 23.9 ± 2.8 nM (HFD group). In addition, among the detected CYP-derived fatty acid epoxides and diols, 9,10- and 12,13-EpOMEs were the most abundant LMs in plasma (Fig. 2 and supplemental information Fig. S1). Previous studies have shown that EpOMEs (termed as leukotoxins), which are metabolites of LA produced by CYP enzymes, have an array of detrimental effects on health, inducing inflammation, chemotaxis, lung and cardiovascular diseases, and death [17–26], suggesting that increased concentrations of EpOMEs could contribute to pathology of obesity. Besides EpOMEs, we also observed other CYP-derived LMs, including 19,20-DiHOPE and 19,20-EpDPE derived from DHA, and 14,15-DHET derived from ARA, were significantly increased in plasma of HFD-fed mice (Fig. 2).
Fig. 1.
HFD-induced obesity in C57BL/6 mice. (A) Scheme of animal experiment. (B) Body weight of mice maintained on control diet and HFD diet. n = 12 mice per group, the results are mean ± SEM, *P < 0.05.
Fig. 2.
Effects of HFD on Plasma profiles of CYP-derived LMs, including fatty acid epoxides and diols derived from LA (A), ARA (B), ALA (C), EPA (D), and DHA (E). n = 12 mice per group, the results are mean ± SEM, *P < 0.05.
3.2. Profiles of COX-derived LMs in plasma
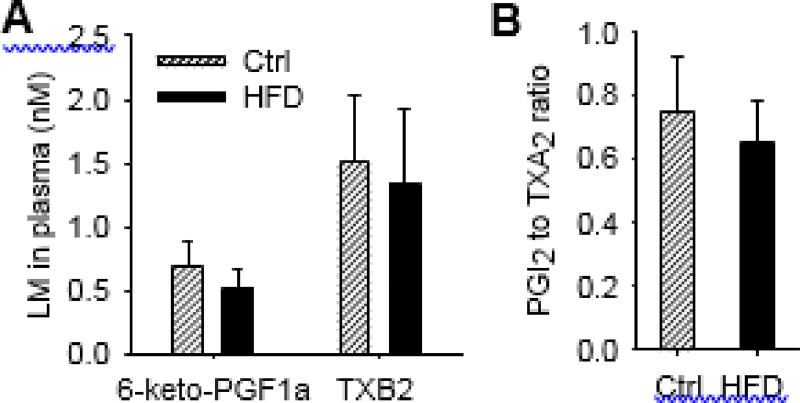
Our LC–MS/MS method can detect multiple COX-derived LMs (see complete list of LMs covered in our LC–MS/MS method in Table S1), while we only detected two LMs in plasma of the treated mice, including 6-keto prostaglandin F1a (6-keto-PGF1a, a stable metabolite of prostacyclin, PGI2) and thromboxane B2 (TXB2, a stable metabolite of thromboxane A2, TXA2). The concentrations of these two metabolites were not changed (Fig. 3A). The relative balance of vasodilative PGI2 (as measured by its stable metabolite 6-keto-PGF1a) and vasoconstrictive TXA2 (as measured by its stable metabolite TXB2) plays critical role in regulating vascular tone, and the ratio of PGI2 to TXA2 is an important biomarker of cardiovascular function [6,27]. In our experiment, dietary feeding of HFD did not change the ratio of PGI2 to TXA2 in plasma (Fig. 3B). Together, these results suggest that COX pathway is not likely to play a major role in the pathology of obesity.
Fig. 3.
Effects of HFD on plasma profiles of COX-derived LMs. (A) Plasma concentrations of 6-keto-PGF1a and TXB2. (B
3.3. Profiles of 5-LOX-derived LMs in plasma
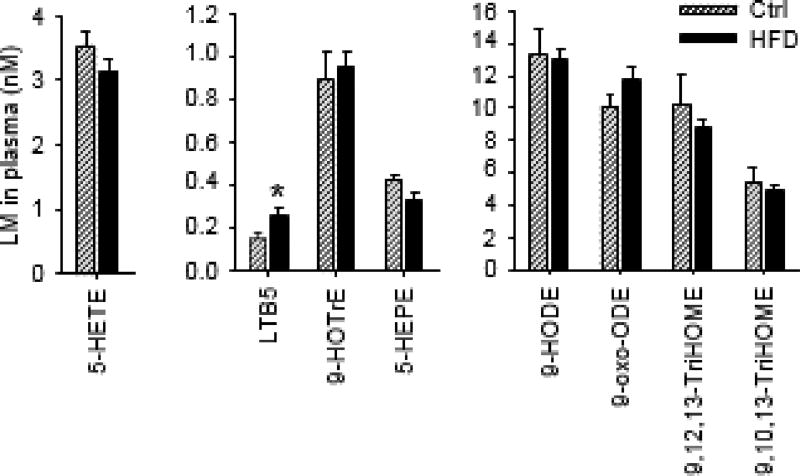
5-LOX pathway generates several LMs which play central roles in regulating inflammation [6]. Our LC–MS/MS analysis showed that in the detected 5-LOX-derived LMs, only the concentration of leukotriene B5 (LTB5), which is a metabolite derived from EPA and is a structural analog of ARA-derived leukotriene B4 (LTB4), was significantly increased in plasma of HFD-fed mice: the plasma con- centration of LTB5 was increased from 0.2 ± 0.03 nM (mean ± SEM, control group) to 0.3 0.04 nM (HFD group). The concentrations of other 5-LOX-derived LMs, such as 5-hydroxyeicosatetraenoic acid (5-HETE), 9-hydroxyoctadecadienoic acid (9-HODE), and 5-hydroxyeicosapentaenoic acid (5-HEPE), were not changed (Fig. 4).
Fig. 4.
Effects of HFD on plasma profiles of 5-LOX-derived LMs. n = 12 mice pergroup, the results are mean ± SEM, *P < 0.05.8-
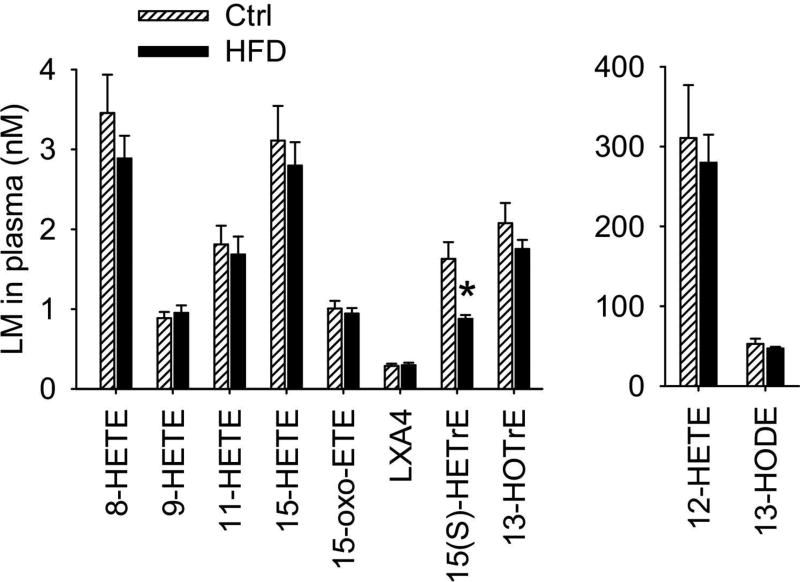
3.4. Profiles of 12/15-LOX-derived LMs in plasma
Among the detected 12/15-LOX-derived LMs, the concentration of 15(S)-hydroxyeicosatrienoic acid (15(S)-HETrE), which is the metabolite of DGLA produced by 15-LOX, was significantly reduced in plasma of HFD-fed mice: the plasma concentration of 15(S)-HETrE was reduced from 1.6 0.21 nM (mean ± SEM, control group) to 0.9 ± 0.05 nM (HFD group). Previous studies have shown that 15(S)-HETrE has anti-inflammatory effect [28,29], therefore it is likely that reduced level of anti-inflammatory 15(S)-HETrE could contribute to the pathology of obesity (Fig. 5).
Fig. 5.
Effects of HFD on plasma profiles of 12/15-LOX-derived LMs. n = 12 mice per group, the results are mean ± SEM, *P < 0.05.
3.5. Profiles of lipid peroxidation products in plasma
We also analyzed the plasma concentration of 12,13-epoxy- 9-keto-10(trans)-octadecenoic acid (EKODE), which is a lipid peroxidation product derived from LA [30]. LC–MS/MS showed that the plasma concentration of EKODE was not changed, suggesting that dietary feeding of HFD may not change systematic marker of redox stress (Supplemental information Fig. S2).
4. Discussion
Obesity is a serious health problem in US and it is associated with increased risks of various diseases, such as cardiovascular diseases, lung and respiratory diseases, diabetes, hypertension, and certain types of cancers. To date, the mechanism by which obesity increases a wide range of human diseases is not well understood. Here we used a LC–MS/MS-based lipidomics to analyze the plasm profiles of LMs in HFD-induced obesity in mice. Our central findings are that the plasma concentrations of EpOMEs were significantly increased in plasma of HFD-fed mice, in addition, EpOMEs are among the most abundant LMs detected in mouse plasma. Together, these results support a potential role of EpOMEs in the pathology of obesity.
EpOMEs have been shown to have a large array of detrimental effects on health, and are termed as “leukotoxins” since leukocytes have been shown to be a major cell type to produce EpOMEs [31–34]. Previous studies have shown that EpOMEs have an array of detrimental effects on health, inducing chemotaxis, inflammation, cardiovascular disease and pulmonary diseases [17–26]. In cell culture studies, a dose as low as 10 nM, EpOME enhanced neutrophil chemotaxis, suggesting potent pro-inflammatory effects of EpOMEs [21]. In animal studies, treatment with exogenous leukotoxins induced pulmonary edema, lung injury, and cardio- depression [19,22,32]. Administration of high-dose leukotoxins caused rapid animal death: 100% of dogs treated with i.v. injection of 50 mg/kg leukotoxin died within 45 min [22]. In human studies, high plasma levels of leukotoxins were observed in patients with severe burns, and are associated with multiple organ failure and adult respiratory distress syndrome in these patients [18,20,24,26]. Therefore, it is likely that increased levels of EpOMEs in circulation could contribute to obesity-induced health problems. A limitation of this study is that we did not perform experiments to study the functional roles of EpOMEs in the pathology of obesity. Further studies are needed to better characterize the biological effects of EpOMEs, since most previous studies used high doses of EpOMEs in cell culture and animal experiments, which were designed to reflect the doses of EpOMEs observed in severe burn patients, few studies have studied the effects of EpOMEs at physiological concentrations. It remains to determine the specific tissues and cell types involved in production of EpOMEs. It is not likely that the increased concentrations of EpOMEs in plasma are derived from adipose tissues, since studies by us and others have shown that there is a dramatic reduction of the expressions of CYP enzymes involved in biosynthesis of fatty acid epoxides, and reduced levels of CYP-derived fatty acid epoxides in adipose tissues of HFD-fed mice [10,35]. In our study, we also analyze the ratio of fatty acid epoxides to diols, which is a potential biomarker of sEH [5]. We found that there is a significantly increased ratio of 9,10-EpOME to 9,10-DiHOME in plasma of HFD-fed mice, while the ratios of 12,13- EpOME to 12,13-DiHOME, and 14,15-EET to 14,15-DHET were not significantly changed (supplemental information Fig. S3). Further studies are needed to characterize whether sEH plays a role in the changed plasma concentration of EpOMEs.
To date, it remains debatable whether the observed adverse effects of EpOMEs were caused by EpOMEs themselves or their sEH-derived diol metabolites. In cell culture studies, DiHOMEs, the diol metabolites of EpOMEs, have been shown to have more potent pro-inflammatory effects: at a dose of 1 nM, DiHOMEs caused a 2-fold increase of neutrophil chemotaxis, while EpOMEs had no effect [21]. In addition, DiHOMEs have been shown to have enhanced toxic effects in cultured cells and animals [17,25]. Pharmacological inhibition of sEH, which blocks metabolic conversion of EpOMEs to DiHOMEs, attenuated the toxic effects of EpOMEs [17]. Together, these results support that the observed detrimental effects of EpOMEs could be at least in part mediated by their diol metabolites. Since tissue levels of EpOMEs (as well as their diol metabolites DiHOMEs) are in part determined by amount of LA in the diet [5], our study may suggest a novel mechanistic linkage of dietary LA and obesity. Recent studies showed that LA-rich soybean oil is more obesogenic and diabetogenic than saturated fatty acid- rich coconut oil and fructose, suggesting a potential role of LA in promoting the obesity epidemic [36]. More studies are needed to better understand the biological actions of EpOMEs and DiHOMEs. Besides EpOMEs, our results showed that the plasma concentrations of most LMs were not significantly changed. Among all detected LMs in plasma, only seven LMs showed significant changes in HFD-induced obesity, including LTB5, 15(S)-HETrE, 14,15-DHET, 19,20-EpDPE, 19,20-DiHDPE, 9,10-EpOME, and 12,13-EpOME. In addition, we did not detect the presence of several important pro-inflammatory LMs, such as prostaglandin E2 (PGE2) and LTB4, in mouse plasma. These results are largely in agreement with previous studies which showed that obesity induced a low-degree inflammation [37]. The profiles of LMs in adipose tissues and plasma in HFD-induced obesity are very different [10,35]. This is feasible, since most LMs are short-lived, locally produced and locally acting in response to cellular stimulation, followed with well-controlled degradation to maintain homeostasis [5,6]. Therefore, the profiles of LMs could be highly different in different tissues. A limitation of this study is that since this is an exploratory study, we did not per- form statistical analysis with correction for multiple comparisons. In summary, here we used a LC–MS/MS-based lipidomics to analyze plasma profiles of LMs in HFD-induced obesity in C57BL/6 mice. Our results demonstrate that the plasma concentrations of EpOMEs were significantly increased in plasma of HFD-fed mice, in addition, EpOMEs are among the most abundant LMs detected in mouse plasma. Since previous studies have shown that EpOMEs and their diol metabolites have a large array of detrimental effects on human health [17–26], enhanced levels of EpOMEs could contribute to pathology of obesity.
Supplementary Material
Acknowledgments
This work was partially supported by a new faculty start-up fund, Armstrong Fund of Science Award from UMass-Amherst, and USDA NIFA2016-67017-24423.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.prostaglandins.2016.11.003.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 4.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 5.Zhang G, Kodani S, Hammock BD. Stabilized epoxygenated fatty acids regulate inflammation, pain, angiogenesis and cancer. Prog. Lipid Res. 2014;53(0):108–123. doi: 10.1016/j.plipres.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 7.Buczynski MW, Dumlao DS, Dennis EA. Thematic Review Series: proteomics. An integrated omics analysis of eicosanoid biology. J. Lipid Res. 2009;50(6):1015–1038. doi: 10.1194/jlr.R900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W, Zhu J, Lyu F, Panigrahy D, Ferrara KW, Hammock B, Zhang G. Omega-3 Polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer. Prostaglandins Other Lipid Mediat. 2014;113(115C):13–20. doi: 10.1016/j.prostaglandins.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hetu PO, Riendeau D. Down-regulation of microsomal prostaglandin E2 synthase-1 in adipose tissue by high-fat feeding. Obesity (Silver Spring) 2007;15(1):60–68. doi: 10.1038/oby.2007.514. [DOI] [PubMed] [Google Scholar]
- 10.Zha W, Edin ML, Vendrov KC, Schuck RN, Lih FB, Jat JL, Bradbury JA, DeGraff LM, Hua K, Tomer KB, Falck JR, Zeldin DC, Lee CR. Functional characterization of cytochrome P450-derived epoxyeicosatrienoic acids in adipogenesis and obesity. J. Lipid Res. 2014;55(10):2124–2136. doi: 10.1194/jlr.M053199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horrillo R, Gonzalez-Periz A, Martinez-Clemente M, Lopez-Parra M, Ferre N, Titos E, Moran-Salvador E, Deulofeu R, Arroyo V, Claria J. 5-lipoxygenase activating protein signals adipose tissue inflammation and lipid dysfunction in experimental obesity. J. Immunol. 2010;184(7):3978–3987. doi: 10.4049/jimmunol.0901355. [DOI] [PubMed] [Google Scholar]
- 12.Claria J, Dalli J, Yacoubian S, Gao F, Serhan CN. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J. Immunol. 2012;189(5):2597–2605. doi: 10.4049/jimmunol.1201272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virtue S, Masoodi M, de Weijer BA, van Eijk M, Mok CY, Eiden M, Dale M, Pirraco A, Serlie MJ, Griffin JL, Vidal-Puig A. Prostaglandin profiling reveals a role for haematopoietic prostaglandin D synthase in adipose tissue macrophage polarisation in mice and humans. Int. J. Obes. (Lond.) 2015;39(7):1151–1560. doi: 10.1038/ijo.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodnett BL, Dearman JA, Carter CB, Hester RL. Attenuated PGI2 synthesis in obese Zucker rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296(3):R715–21. doi: 10.1152/ajpregu.90330.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal. Chem. 2009;81(19):8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010;23(2):270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 17.Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat. Med. 1997;3(5):562–566. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosaka K, Suzuki K, Hayakawa M, Sugiyama S, Ozawa T. Leukotoxin, a linoleate epoxide: its implication in the late death of patients with extensive burns. Mol. Cell. Biochem. 1994;139(2):141–148. doi: 10.1007/BF01081737. [DOI] [PubMed] [Google Scholar]
- 19.Hu JN, Taki F, Sugiyama S, Asai J, Izawa Y, Satake T, Ozawa T. Neutrophil-derived epoxide, 9,10-epoxy-12-octadecenoate, induces pulmonary edema. Lung. 1988;166(6):327–337. doi: 10.1007/BF02714065. [DOI] [PubMed] [Google Scholar]
- 20.Hanaki Y, Kamiya H, Ohno M, Hayakawa M, Sugiyama S, Ozawa T. Leukotoxin, 9, 10-epoxy-12-octadecenoate: a possible responsible factor in circulatory shock and disseminated intravascular coagulation. Jpn. J. Med. 1991;30(3):224–228. doi: 10.2169/internalmedicine1962.30.224. [DOI] [PubMed] [Google Scholar]
- 21.Totani Y, Saito Y, Ishizaki T, Sasaki F, Ameshima S, Miyamori I. Leukotoxin and its diol induce neutrophil chemotaxis through signal transduction different from that of fMLP. Eur. Respir. J. 2000;15(1):75–79. doi: 10.1183/09031936.00.15107500. [DOI] [PubMed] [Google Scholar]
- 22.Fukushima A, Hayakawa M, Sugiyama S, Ajioka M, Ito T, Satake T, Ozawa T. Cardiovascular effects of leukotoxin (9, 10-epoxy-12-octadecenoate) and free fatty acids in dogs. Cardiovasc. Res. 1988;22(3):213–218. doi: 10.1093/cvr/22.3.213. [DOI] [PubMed] [Google Scholar]
- 23.Ozawa T, Nishikimi M, Sugiyama S, Taki F, Hayakawa M, Shionoya H. Cytotoxic activity of leukotoxin, a neutrophil-derived fatty acid epoxide, on cultured human cells. Biochem. Int. 1988;16(2):369–373. [PubMed] [Google Scholar]
- 24.Hayakawa K, Kosaka S, Sugiyama K, Yokoo H, Aoyama Y. Proposal of leukotoxin, 9,10-epoxy-12-octadecenoate, as a burn toxin. Biochem. Int. 1990;21(3):573–579. [PubMed] [Google Scholar]
- 25.Zheng J, Plopper CG, Lakritz J, Storms DH, Hammock BD. Leukotoxin-diol: a putative toxic mediator involved in acute respiratory distress syndrome. Am. J. Respir. Cell Mol. Biol. 2001;25(4):434–438. doi: 10.1165/ajrcmb.25.4.4104. [DOI] [PubMed] [Google Scholar]
- 26.Ozawa T, Hayakawa M, Kosaka K, Sugiyama S, Ogawa T, Yokoo K, Aoyama H, Izawa Y. Leukotoxin, 9,10-epoxy-12-octadecenoate, as a burn toxin causing adult respiratory distress syndrome. Adv. Prostaglandin Thromboxane Leukot. Res. 1991;21B:569–572. [PubMed] [Google Scholar]
- 27.Cheng Y, Austin SC, Rocca B, Koller BH, Coffman TM, Grosser T, Lawson JA, FitzGerald GA. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science. 2002;296(5567):539–541. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- 28.Petrich K, Ludwig P, Kühn H, Schewe T. The suppression of 5-lipoxygenation of arachidonic acid in human polymorphonuclear leucocytes by the 15-lipoxygenase product (15S)-hydroxy-(5Z, 8Z, 11Z, 13E)-eicosatetraenoic acid: structure-activity relationship and mechanism of action. Biochem. J. 1996;314(Pt. 3):911–916. doi: 10.1042/bj3140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haviv F, Ratajczyk JD, DeNet RW, Martin YC, Dyer RD, Carter GW. Structural requirements for the inhibition of 5-lipoxygenase by 15-hydroxyeicosa-5,8,11,13-tetraenoic acid analogues. J. Med. Chem. 1987;30(2):254–263. doi: 10.1021/jm00385a005. [DOI] [PubMed] [Google Scholar]
- 30.Wang R, Kern JT, Goodfriend TL, Ball DL, Luesch H. Activation of the antioxidant response element by specific oxidized metabolites of linoleic acid. Prostaglandins Leukot. Essent. Fatty Acids. 2009;81(1):53–59. doi: 10.1016/j.plefa.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozawa T, Sugiyama S, Hayakawa M, Taki F, Hanaki Y. Neutrophil microsomes biosynthesize linoleate epoxide (9, 10-epoxy-12-octadecenoate), a biological active substance. Biochem. Biophys. Res. Commun. 1988;152(3):1310–1318. doi: 10.1016/s0006-291x(88)80428-5. [DOI] [PubMed] [Google Scholar]
- 32.Ozawa T, Hayakawa M, Takamura T, Sugiyama S, Suzuki K, Iwata M, Taki F, Tomita T. Biosynthesis of leukotoxin, 9,10-epoxy-12 octadecenoate, by leukocytes in lung lavages of rat after exposure to hyperoxia. Biochem. Biophys. Res. Commun. 1986;134(3):1071–1078. doi: 10.1016/0006-291x(86)90360-8. [DOI] [PubMed] [Google Scholar]
- 33.Hayakawa M, Sugiyama S, Takamura T, Yokoo K, Iwata M, Suzuki K, Taki F, Takahashi S, Ozawa T. Neutrophils biosynthesize leukotoxin, 9,10-epoxy-12-octadecenoate. Biochem. Biophys. Res. Commun. 1986;137(1):424–430. doi: 10.1016/0006-291x(86)91227-1. [DOI] [PubMed] [Google Scholar]
- 34.Ozawa T, Sugiyama S, Hayakawa M. Leukocytes biosynthesize leukotoxin (9,10-epoxy-12-octadecenoate)–a novel cytotoxic linoleate epoxide. Adv. Prostaglandin Thromboxane Leukot. Res. 1989;19:164–167. [PubMed] [Google Scholar]
- 35.Wang W, Yang J, Qi W, Yang H, Wang C, Tan B, Hammock B, Park Y, Kim D, Zhang G. Lipidomic profiling of high-fat diet-induced obesity in mice: importance of cytochrome P450-derived fatty acid epoxides. Obesity. 2016 doi: 10.1002/oby.21692. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deol P, Evans JR, Dhahbi J, Chellappa K, Han DS, Spindler S, Sladek FM. Soybean oil is more obesogenic and diabetogenic than coconut oil and fructose in mouse: potential role for the liver. PLoS One. 2015;10(7):e0132672. doi: 10.1371/journal.pone.0132672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereira SS, Alvarez-Leite JI. Low-grade inflammation, obesity, and diabetes. Curr. Obes. Rep. 2014;3(4):422–431. doi: 10.1007/s13679-014-0124-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.