Abstract
Human cytomegalovirus (HCMV) establishes a lifelong chronic latent infection and often reactivates in immunocompromised patients. In addition, HCMV reactivates in patients with sepsis or other critical illnesses, particularly in patients with poor prognoses. However, the immunological characteristics of sepsis patients with HCMV reactivation have not been elucidated. In the present study, we examined T-cell responses in severe sepsis patients with and without HCMV reactivation. First, HCMV pp65-specific T-cell functions were assessed by intracellular cytokine staining (ICS) for IFN-γ, TNF-α, and MIP-1β and by CD107a staining. We analyzed the ICS data for each function individually and found no difference between the patient groups. However, the relative frequency of polyfunctional CD8+ T cells was significantly decreased in sepsis patients with HCMV reactivation. Next, we examined programmed cell death protein 1 (PD-1) expression. It was significantly increased in the CD8+ T-cell population in severe sepsis patients with HCMV reactivation, indicating CD8+ T-cell exhaustion. Interestingly, the frequency of PD-1+ cells in the CD8+ T-cell population was inversely correlated with the relative frequency of polyfunctional CD8+ T cells. Herein, we demonstrate that HCMV reactivation in severe sepsis patients is associated with PD-1 expression and impaired polyfunctionality of CD8+ T cells.
Introduction
Human cytomegalovirus (HCMV) is a member of the β-herpesvirus family and is widely recognized as a common opportunistic pathogen of immunocompromised hosts, such as solid organ transplantation recipients, patients with hematologic malignant disorders and human immunodeficiency virus-1-infected individuals.1, 2, 3 Primary HCMV infection is usually asymptomatic, but HCMV establishes lifelong latency similar to other herpesviruses. HCMV often reactivates in immunocompromised hosts, and the lytic reactivation of HCMV is known to worsen the clinical outcomes of various diseases that result in immunosuppression.4, 5
HCMV reactivation has also been studied in patients with sepsis or other critical illnesses without predisposing immunocompromised conditions. HCMV often reactivates in critically ill patients and is correlated with increased mortality and morbidity (that is, longer hospital stays and frequent or longer mechanical ventilation).6, 7, 8, 9, 10, 11, 12, 13, 14, 15 HCMV reactivation is thought to be caused by excessive inflammatory cytokine release, epigenetic alterations of viral DNA and immune suppression.16, 17, 18, 19 HCMV reactivation has been suggested to indicate critical illness-induced immunosuppression, which worsens the clinical outcomes by itself.11 In addition, it has been suggested that HCMV could play important pathological roles in the clinical course of patients with critical illness by lung injury,20 enhanced responsiveness of antigen-presenting cells21 and increased activity of T cells and NK cells.22, 23, 24 However, the mechanism by which HCMV reactivation is associated with increased morbidity and mortality in patients with sepsis or other critical illness is unknown. To address this issue, T-cell immunity needs to be examined in critically ill patients with or without HCMV reactivation.
In immunocompromised patients, T-cell immunity is crucial for the control of HCMV.25, 26 However, the role of T-cell immunity in the control of HCMV among critically ill patients without predisposing immunocompromised conditions has not been clearly elucidated. In septic shock patients with HCMV reactivation, the HCMV-specific CD4+ T-cell response has been shown to be maintained or somewhat increased.23 Other studies have also shown that the HCMV-specific T-cell response is preserved despite HCMV reactivation in critically ill patients,22 although the absence of initial T-cell immunity against HCMV seemed to be correlated with later HCMV reactivation.27, 28 However, these studies examined HCMV-specific T-cell responses by simply measuring IFN-γ production without studying additional details.
In the present study, we investigated whether the HCMV-specific T-cell response was impaired in severe sepsis patients with HCMV reactivation. We addressed this question by analyzing the function (particularly the polyfunctionality) of HCMV-specific T cells in newly diagnosed severe sepsis patients with HCMV reactivation. We found that PD-1 expression and reduced polyfunctionality of CD8+ T cells were associated with HCMV reactivation in severe sepsis patients without known predisposing immunocompromised factors.
Materials and methods
Study subjects and definitions
Severe sepsis was defined as sepsis with at least one organ dysfunction using the diagnostic criteria of the 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definition Conference: arterial hypoxemia, acute oliguria, creatinine increase, coagulation abnormalities, ileus, thrombocytopenia and hyperbilirubinemia.29 We prospectively enrolled forty-eight participants with positive HCMV IgG results, as determined by a quantitative enzyme-linked fluorescent assay (BioMérieux VIDAS, Lyon, France), among newly diagnosed severe sepsis patients arriving at the emergency department (ED) of Severance Hospital (Seoul, Korea) from January 2013 to June 2014. Patients with the following circumstances were not eligible for this study and were excluded from final enrollment: use of antiviral agents to treat HCMV within the last 3 months, known or suspected history of immune-dysregulating disease, use of systemic immune-modulating medications such as prednisone, cyclosporine, sirolimus and tacrolimus, and recent history of any critical illness other than severe sepsis such as trauma, major surgery or intensive care unit (ICU) admission within 3 months. The plasma HCMV viremia was assessed by quantitative real-time polymerase chain reaction (CMV Real-Time PCR kit, Biocore, Seoul, Korea; one copy/ml in this CMV real-time PCR kit can be converted to 0.98 IU ml−1) from a blood sample obtained within 3 days after ED arrival. We defined HCMV reactivation in severe sepsis patients with HCMV seropositivity as HCMV DNAemia of more than 500 copies per ml of plasma.30 Clinical information was collected through a prospective review of electronic medical records until death or discharge. In addition, we assessed the Sequential Organ Failure Assessment score (SOFA score) and Charlson comorbidity index of the measured plasma HCMV level as previously described.31, 32, 33 This study was carried out in accordance with the ethical guidelines of the Declaration of Helsinki and was approved by Severance Hospital, and written informed consent was obtained from all of the participants.
Collection of peripheral blood mononuclear cells
Whole blood was obtained in EDTA tubes within 3 days after ED arrival. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using lymphocyte separation medium (Corning, Manassas, VA, USA) and cryopreserved in liquid nitrogen until use. Analyses of PBMCs and HCMV DNA titers were performed with samples acquired on the same day.
Intracellular cytokine staining
Thawed PBMCs were incubated overnight in complete RPMI 1640 medium (WelGENE, Daegu, Korea). The next morning, the cells were stimulated with a mix of CMV pp65 overlapping peptides (PepTivator CMV pp65, Miltenyi Biotec, Auburn, CA, USA) in the presence of anti-CD107a-PE (H4A3, BD Biosciences, San Jose, CA, USA) for 6 h. Brefeldin A and monensin were added 1 h after stimulation. Then, the cells were stained with Live/Dead Fixable Red Stain dye (Life Technologies, Gaithersburg, MD, USA) and fluorochrome-conjugated monoclonal antibodies for cell surface proteins. The stained cells were fixed and permeabilized using a fixation/permeabilization buffer kit and stained for intracellular cytokines. The antibodies used are as follows: anti-CD3-V500 (UCHT1, BD Biosciences), anti-CD8-APC-H7 (SK1, BD Biosciences), anti-CD4-V450 (RPA-T4, BD Biosciences), anti-IFN-γ-APC (B27, BD Biosciences), anti-TNF-α-PE-Cy7 (MAb11, eBioscience, San Diego, CA, USA), and anti-MIP-1β-PerCP-Cy5.5 (D21–1351, BD Biosciences). The stained cells were analyzed with an LSR II instrument (BD Biosciences) and FlowJo v10.7 software (FlowJo, Ashland, OR, USA) according to the gating strategy in Supplementary Figure 1. The percentage of cytokine+ cells in the unstimulated tubes was subtracted from the percentage in the peptide mix-stimulated tubes to obtain the percentage of CMV-specific cytokine-producing cells. T-cell polyfunctionality was analyzed with SPICE v5.35 software (courtesy of Mario Roederer and Joshua Nozzi, NIAID, NIH).
Immunophenotype analysis
Cryopreserved PBMCs were thawed and incubated with FcR Blocking Reagent (Miltenyi Biotec). The FcR-blocked PBMCs were stained with Live/Dead Fixable Red Stain dye and fluorochrome-conjugated monoclonal antibodies for cell surface proteins. The antibodies used are as follows: anti-CD3-V500, anti-CD8-APC-H7, anti-CD14-PE-eFluor610 (61D3, eBioscience), anti-CD19-PE-eFluor610 (HIB19, eBioscience) and anti-PD-1-PerCP-Cy5.5 (EH12.2H7, Biolegend, San Diego, CA, USA). The stained cells were analyzed with an LSR II instrument and FlowJo v10.7 software according to the gating strategy in Supplementary Figure 2.
Statistical analysis
Differences between two groups were analyzed by a nonparametric t-test (Mann–Whitney U-test) and Fisher’s exact test. Association between two parameters was tested by Pearson’s correlation. The statistical analysis was performed using the software program SPSS version 20.0 (SPSS, Chicago, IL, USA). To determine the differences in the pie charts, permutation tests in the SPICE software were used (10,000 permutations). Two-sided P-values were determined in all analyses, and a P-value <0.05 was considered significant.
Results
Clinical characteristics of the study population
Among the 48 enrolled patients with severe sepsis, 19 (39.6%) patients exhibited HCMV reactivation. The demographic and clinical characteristics of all participants are summarized in Table 1. In both groups, with or without HCMV reactivation, the major infection sites of severe sepsis were the lungs and urinary tract, and most of the causative organisms were bacteria. We assessed the Charlson comorbidity index, which weights the comorbid conditions in patients, and the SOFA score, which estimates the degree of organ dysfunction, although no significant difference was found between the two groups. However, the group with HCMV reactivation had a significantly higher frequency of mechanical ventilation use and longer lengths of stay in the hospital and ICU than the group without HCMV reactivation. The count of each leukocyte subset in the peripheral blood was not different between the two groups (data not shown).
Table 1. Demographic and clinical characteristics of the enrolled severe sepsis patients.
|
HCMV reactivation |
|||
|---|---|---|---|
| Characteristics | Yes (n=19) | No (n=29) | P-value |
| Age, year | 71.5 (49–95) | 70 (21–89) | 0.540a |
| Sex, male | 13 (68.4) | 16 (55.2) | 0.364b |
| HCMV DNA titer (copies per ml) | 1830 (555–>5 × 106) | <500 | |
| Infection site | |||
| Lung | 11 (57.9) | 10 (34.5) | 0.130b |
| Urinary tract | 3 (15.8) | 7 (24.1) | 0.719b |
| Abdomen | 3 (15.8) | 6 (20.7) | 1.000b |
| Others | 1 (5.3) | 6 (20.7) | 0.225b |
| Causative organism | |||
| Bacteria | 15 (83.2) | 28 (96.6) | 0.150b |
| Fungus | 1 (5.6) | 1 (3.4) | 1.000b |
| Virus | 1 (5.6) | 0 (0.0) | 0.383b |
| Mixed | 1 (5.6) | 0 (0.0) | 0.383b |
| Charlson comorbidity index | 1.5 (0–8) | 1 (0–4) | 0.078a |
| SOFA score | 7.5 (0–18) | 5 (0–16) | 0.355a |
| Clinical outcomes | |||
| Mechanical ventilationc | 8 (42.1) | 3 (10.3) | 0.020a |
| Total admission duration, day | 21 (6–133) | 14 (1–75) | 0.031a |
| ICU care | 9 (47.4) | 7 (24.1) | 0.072a |
| Duration of ICU care, day | 3 (0–75) | 0 (0–12) | 0.023a |
| In-hospital all-cause mortality | 9 (47.4) | 6 (20.7) | 0.064a |
Abbreviations: HCMV, human cytomegalovirus; ICU, intensive care unit; SOFA score, Sequential Organ Failure Assessment score.
The data were expressed as median (range) and number (percent).
Mann–Whitney U-test.
Fisher’s exact test.
The number of patients who had ever received the mechanical ventilation during hospitalization.
The relative frequency of single cytokine-producing T cells did not differ between the groups
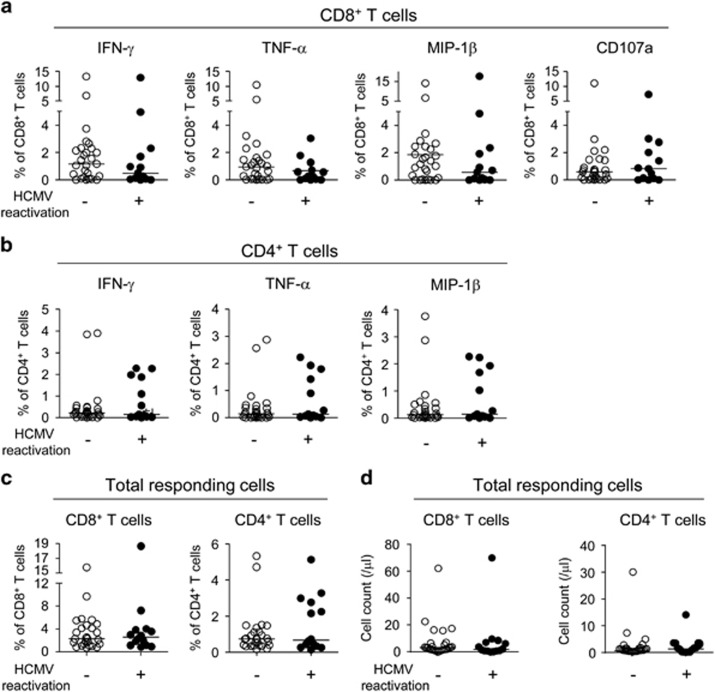
First, we examined the functions of the HCMV-specific CD8+ and CD4+ T cells by intracellular cytokine staining for IFN-γ, TNF-α and MIP-1β using flow cytometry. For the intracellular cytokine staining, PBMCs were stimulated with HCMV pp65 overlapping peptides. Fluorochrome-conjugated anti-CD107a was also added to the culture to assess antigen-specific degranulating activity of cytotoxic T cells.34 When we analyzed the data by each function individually, there were no differences in the percentage of IFN-γ-, TNF-α- or MIP-1β-producing CD8+ T cells or CD107a+-degranulating CD8+ T cells between the groups (Figure 1a). There were also no differences in the percentage of IFN-γ-, TNF-α- or MIP-1β-producing CD4+ T cells between the groups (Figure 1b). Finally, there were no differences between the groups when we analyzed the percentage (Figure 1c) or the absolute counts (Figure 1d) of CD8+ or CD4+ T cells with any type of function (total responding cells).
Figure 1.
The relative frequency of single cytokine-producing T cells in severe sepsis patients. Peripheral blood mononuclear cells (PBMCs) from severe sepsis patients without (n=27) and with (n=13) human cytomegalovirus (HCMV) reactivation were stimulated with HCMV pp65 overlapping peptides, and intracellular cytokine staining for IFN-γ, TNF-α and MIP-1β was performed. Fluorochrome-conjugated anti-CD107a was also added to the culture when the PBMCs were stimulated to assess the antigen-specific degranulating activity of the T cells. The data were analyzed for single cytokine+ cells or CD107a+ cells in the total CD8+ T-cell population (a) and for single cytokine+ cells in the total CD4+ T-cell population (b). The data were also analyzed for the percentage of the total CD8+ or CD4+ T-cell population (c) or the absolute counts (d) of T cells with any type of function (total responding cells). The horizontal lines represent the median values.
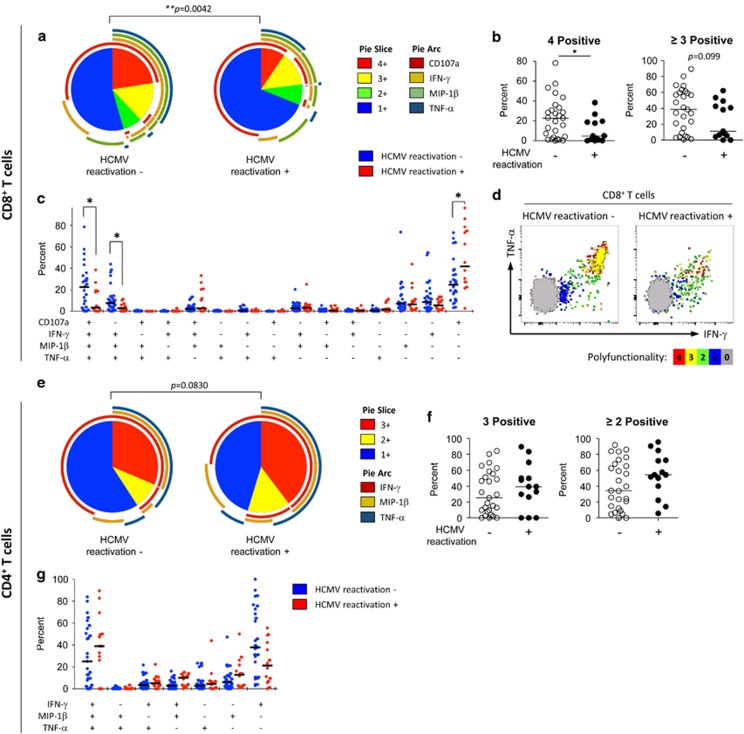
Polyfunctionality of CD8+ T cells was impaired in patients with HCMV reactivation
We also analyzed the intracellular cytokine staining data based on the number of T-cell functions. In CD8+ T cells, the percentage of quadruple-positive cells among the total responding cells was significantly decreased in patients with HCMV reactivation (Figure 2a and b). In the detailed analysis of polyfunctionality, the percentages of both quadruple-positive (IFN-γ+, TNF-α+, MIP-1β+ and CD107a+) and triple-positive (IFN-γ+, TNF-α+, MIP-1β+ and CD107a−) cells were significantly decreased in patients with HCMV reactivation (Figure 2c). As presented in Figure 2d showing representative polychromatic dot plots, the CD8+ T-cell population in patients with HCMV reactivation exhibited a low frequency of polyfunctional cells as well as reduced fluorescence intensity for IFN-γ and TNF-α compared to the population in patients without HCMV reactivation. In particular, reduced fluorescence intensity of IFN-γ or TNF-α was observed in monofunctional (single-positive) cells compared to polyfunctional (quadruple-positive or triple-positive) cells (Figure 2d). In contrast, polyfunctionality and fluorescence intensity were well-maintained in the CD8+ T-cell population in patients without HCMV reactivation (Figure 2d). In the CD4+ T cells, the percentage of polyfunctional (3 functions or ⩾2 functions) cells among the total responding cells did not differ between the groups (Figure 2e and f). A detailed analysis of the polyfunctionality with every possible combination of functions also showed no difference between the groups (Figure 2g). Polyfunctionality of T cells was also analyzed by absolute counts of responding T cells (Supplementary Figure 3), and the data showed a similar tendency with those based on the percentage.
Figure 2.
Polyfunctionality of human cytomegalovirus (HCMV) pp65-specific T cells in severe sepsis patients. The data from Figure 1 were analyzed for the polyfunctionality of T cells according to the number of T-cell functions. CD8+ T cells were analyzed for IFN-γ, TNF-α, MIP-1β and CD107a (a–d), and CD4+ T cells were analyzed for IFN-γ, TNF-α and MIP-1β (e–g). The pie graphs show the fraction of T cells positive for a given number of functions among T cells with any type of function (total responding cells) (a, e). The percentage of T cells positive for a given number of functions was compared between severe sepsis patients without (n=27) and with (n=13) CMV reactivation (b, f). Detailed analyses of polyfunctionality were presented with every possible combination of functions, and the data were compared between the groups (c, g). Representative polychromatic dot plots for IFN-γ and TNF-α are shown. T cells positive for a given number of functions are marked by different colors to show their distribution in the dot plots for IFN-γ and TNF-α (d). The horizontal lines represent the median values. *P<0.05, **P<0.01.
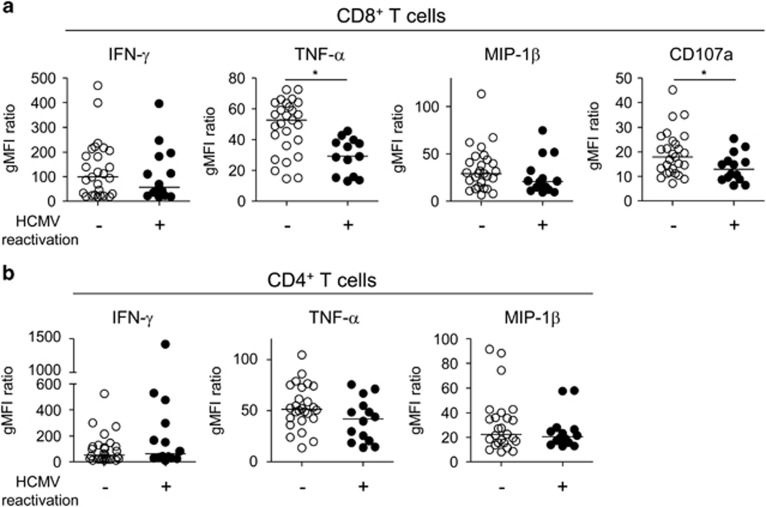
These findings led us to analyze the intracellular cytokine staining data by geometric mean fluorescence intensity (gMFI). When we analyzed the data for each single function by gMFI, we found that the gMFI values of TNF-α and CD107a were significantly reduced in the CD8+ T-cell population in patients with HCMV reactivation (Figure 3a). However, the difference was not observed in the CD4+ T-cell population between the groups (Figure 3b).
Figure 3.
Geometric mean fluorescence intensity (gMFI) of cytokine-producing T cells in severe sepsis patients. The data from Figure 1 were analyzed for the gMFI of each function. The gMFI of the cytokine-producing cell population was divided by the gMFI of the cytokine-negative cell population to normalize the data. The data were analyzed for single cytokine+ cells or CD107a+ cells in the CD8+ T cells (a) and for single cytokine+ cells in the CD4+ T cells (b). The horizontal lines represent the median values. *P<0.05.
PD-1 expression in the CD8+ T-cell population was increased in patients with severe sepsis with HCMV reactivation
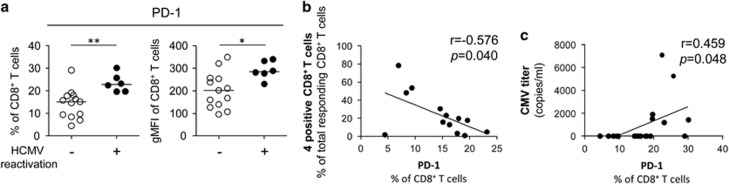
Next, we performed immunophenotype analysis to examine the expression of a T-cell exhaustion marker, programmed cell death protein 1 (PD-1), in the CD8+ T-cell population. In the CD8+ T-cell population, PD-1 expression was significantly increased in patients with HCMV reactivation in terms of both the percentage of PD-1+ cells and gMFI of PD-1 (Figure 4a). These findings suggested that CD8+ T cells are exhausted in severe sepsis patients with HCMV reactivation. We also analyzed the correlation between PD-1 expression and CD8+ T-cell polyfunctionality and found that the percentage of PD-1+ cells in the CD8+ T-cell population was inversely correlated with the percentage of polyfunctional (quadruple-positive) cells among the total responding CD8+ T cells (Figure 4b). Moreover, the percentage of cells expressing PD-1 in the CD8+ T-cell population was positively correlated with HCMV DNA titers (Figure 4c).
Figure 4.
Correlation between PD-1 expression and the polyfunctionality of CD8+ T cells in severe sepsis patients. (a) The percentage of PD-1+ cells and gMFI of PD-1 in the CD8+ T-cell population were compared between severe sepsis patients without (n=13) and with human cytomegalovirus (HCMV) reactivation (n=6). (b) Correlational analysis was performed between the percentage of PD-1+ cells in the total CD8+ T-cell population and the percentage of highly polyfunctional (quadruple-positive) cells among the CD8+ T cells with any type of function (total responding CD8+ T cells). (c) Correlational analysis was performed between the percentage of PD-1+ cells in the total CD8+ T-cell population and HCMV DNA titers. The horizontal lines represent the median values. *P<0.05, **P<0.01.
Taken together, these findings demonstrate that the CD8+ T-cell population in patients with HCMV reactivation has a comparable percentage of cytokine-producing cells but reduced polyfunctionality compared to the CD8+ T-cell population in patients without HCMV reactivation. Furthermore, reduced polyfunctionality is associated with PD-1 expression in CD8+ T cells in patients with severe sepsis.
Discussion
As the first report of HCMV reactivation in non-immunocompromised critically ill patients was published,6 it has become widely accepted that HCMV reactivation is correlated with increased morbidity and mortality in critically ill patients.7, 8, 9, 10, 11, 12, 13, 14 However, the immunological characteristics of critically ill patients with HCMV reactivation have not been clearly elucidated. In the present study, we enrolled severe sepsis patients with or without HCMV reactivation and examined T-cell responses in detail using intracellular cytokine staining and polyfunctionality analysis. We found that the polyfunctionality of HCMV-specific CD8+ T cells was impaired in patients with HCMV reactivation and that PD-1 expression in the CD8+ T cells was increased in patients with HCMV reactivation, suggesting the occurrence of sepsis-induced T-cell immunosuppression.35 In addition, the relative frequency of the polyfunctional CD8+ T cells was inversely correlated with the relative frequency of PD-1+ cells in the CD8+ T-cell population. This result is consistent with a previous finding that the polyfunctionality of CD8+ T cells against HIV is inversely correlated with the expression of PD-1, CD160 and 2B4 in HIV-infected patients.36 Moreover, the percentage of PD-1-expressing cells in the CD8+ T-cell population is positively correlated with HCMV DNA titers.
In the case of immunocompromised patients, PD-1 elevation has been reported to correlate with HCMV reactivation.37, 38, 39 We found that PD-1 expression was increased in sepsis patients with HCMV reactivation in the present study, and these data are consistent with previous findings that PD-1 expression is increased in severe sepsis.35 Based on our current findings and previous findings, it is plausible that severe sepsis may cause clinically relevant immunosuppression and that the sepsis-induced immunosuppression represented by PD-1 upregulation results in HCMV reactivation. In addition, we suggest that HCMV reactivation could be a surrogate marker for severe immunosuppression among sepsis patients. However, the clinical relevance of HCMV reactivation needs to be further investigated in the context of the management of severe sepsis patients.
In the intracellular cytokine staining experiments, the percentage of each subset of single cytokine+ cells among the CD8+ or CD4+ T-cell population did not differ between patients with or without HCMV reactivation. These data are in accordance with previous findings,22, 23, 27 which showed that IFN-γ-producing HCMV-specific T cells are maintained in septic and critically ill patients with HCMV reactivation. However, in our current study, CD8+ T-cell polyfunctionality was significantly reduced in patients with HCMV reactivation, and the gMFIs of TNF-α and CD107a in CD8+ T cells was significantly decreased in patients with HCMV reactivation. A previous study using mouse models reported that septic stimuli induce a transient contraction of murine cytomegalovirus (MCMV)-specific CD8+ T cells and MCMV reactivation.19 In this previous study, the number of MCMV-specific CD8+ T cells was decreased upon lipopolysaccharide administration. However, the percentage of single cytokine-positive T cells was maintained even with HCMV reactivation in our study, while CD8+ T cell polyfunctionality was reduced in the case of HCMV reactivation.
Interestingly, there is a sequential order to the loss of T-cell functions caused by T-cell exhaustion. First, exhausted T cells lose the ability to produce IL-2 and induce cytotoxicity; then, the production capacities of TNF-α, IFN-γ and MIP-1β are progressively lost as T-cell exhaustion becomes more severe.40 In our data, the gMFIs of TNF-α and CD107a (a marker of degranulating cytotoxic activity) were decreased in patients with HCMV reactivation, while the gMFIs of IFN-γ and MIP-1β were preserved, suggesting that the HCMV-specific CD8+ T cells were in the early stage of T-cell exhaustion in the severe sepsis patients with HCMV reactivation.
PD-1 is a T cell inhibitory receptor and is considered a marker of T-cell exhaustion.40 PD-1 is upregulated in T cells after prolonged antigenic stimulation in the context of cancer and chronic viral infection and is responsible for T-cell exhaustion in these diseases.41, 42 Therefore, anti-PD-1 or anti-PD-L1 blocking antibodies have been used for cancer treatment.41, 42 Recently, PD-1 upregulation has been reported to correlate with poor outcomes in sepsis,43, 44 and the blockade of the PD-1 pathway is now considered to be an immunomodulatory therapy in sepsis-induced immunosuppression.45, 46 Therefore, it is of interest to determine whether PD-1/PD-L1 blocking therapy can reduce the reactivation of HCMV in patients with severe sepsis. Furthermore, an investigation into whether PD-1/PD-L1 blocking therapy can be guided by HCMV reactivation (which represents severe immunosuppression among sepsis patients) is necessary in patients with severe sepsis.
One of the shortcomings of the current study is the study time point. We analyzed HCMV reactivation and T-cell functions within 3 days after the patients arrived at the ED. However, T-cell functions can change over time, and HCMV can reactivate at a later time point during sepsis.9, 19, 23 Therefore, sepsis patients who suffered HCMV reactivation at a later time point might have been excluded from the HCMV reactivation group in the present study. Moreover, the stimulation of CD8+ T cells using HCMV pp65 overlapping peptide mix is not enough to measure the whole CD8+ T-cell responses against HCMV antigens because there are HCMV pp65 non-responders among HCMV-infected patients.47 Further studies with HCMV antigens other than pp65 are warranted. Further investigations with serial samples at later time points would be also required to address HCMV-specific T-cell functions in severe sepsis patients more clearly. In addition, it is hard to know whether the decreased CD8+ T-cell polyfunctionality is a cause or consequence of HCMV reactivation in this study. This question should be addressed by future studies examining proliferation markers such as Ki-67 in HCMV-specific T cells.
In conclusion, we demonstrate that HCMV reactivation in severe sepsis patients is associated with decreased polyfunctionality and increased PD-1 expression of CD8+ T cells and that HCMV reactivation can be a surrogate marker for severe immunosuppression among sepsis patients.
Acknowledgments
This work was supported by grants from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI13C1263). This work was also supported by National Research Foundation Grants (NRF-2014R1A2A1A10053662), which was funded by the Ministry of Science, Information/Communication Technology (ICT), and Future Planning of Korea.
Author contributions
YJC, SBK, MSP, JMK, SHH and E-CS designed study. YJC, SBK and JHK performed the experiments. MSP, JMK and S-HH provided clinical samples. YJC, JHK, S-HP and E-CS analyzed the data. YJC, SBK, S-HH and E-CS wrote and edited the manuscript.
Footnotes
Supplementary Information accompanies the paper on Experimental & Molecular Medicine website (http://www.nature.com/emm)
The authors declare no conflict of interest.
Supplementary Material
References
- Kalil AC, Levitsky J, Lyden E, Stoner J, Freifeld AG. Meta-analysis: the efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med 2005; 143: 870–880. [DOI] [PubMed] [Google Scholar]
- Sinclair J, Sissons P. Latency and reactivation of human cytomegalovirus. J Gen Virol 2006; 87(Pt 7): 1763–1779. [DOI] [PubMed] [Google Scholar]
- Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood 2009; 113: 5711–5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson EM, Jones CA, Webster AC, Strippoli GF, Barclay PG, Kable K et al. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: a systematic review of randomised controlled trials. Lancet 2005; 365: 2105–2115. [DOI] [PubMed] [Google Scholar]
- Strippoli GF, Hodson EM, Jones C, Craig JC. Preemptive treatment for cytomegalovirus viremia to prevent cytomegalovirus disease in solid organ transplant recipients. Transplantation 2006; 81: 139–145. [DOI] [PubMed] [Google Scholar]
- Domart Y, Trouillet JL, Fagon JY, Chastre J, Brun-Vezinet F, Gibert C. Incidence and morbidity of cytomegaloviral infection in patients with mediastinitis following cardiac surgery. Chest 1990; 97: 18–22. [DOI] [PubMed] [Google Scholar]
- Heininger A, Haeberle H, Fischer I, Beck R, Riessen R, Rohde F et al. Cytomegalovirus reactivation and associated outcome of critically ill patients with severe sepsis. Crit Care 2011; 15: R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann M, Sedemund-Adib B, Reiland P, Schmucker P, Hennig H. Increased mortality in long-term intensive care patients with active cytomegalovirus infection. Crit Care Med 2008; 36: 3145–3150. [DOI] [PubMed] [Google Scholar]
- Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA 2008; 300: 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Muller L, Klemm A, Weiss M, Schneider M, Suger-Wiedeck H, Durmus N et al. Active cytomegalovirus infection in patients with septic shock. Emerg Infect Dis 2006; 12: 1517–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton AH, Muenzer JT, Rasche D, Boomer JS, Sato B, Brownstein BH et al. Reactivation of multiple viruses in patients with sepsis. PLoS ONE 2014; 9: e98819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coisel Y, Bousbia S, Forel JM, Hraiech S, Lascola B, Roch A et al. Cytomegalovirus and herpes simplex virus effect on the prognosis of mechanically ventilated patients suspected to have ventilator-associated pneumonia. PLoS ONE 2012; 7: e51340. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jaber S, Chanques G, Borry J, Souche B, Verdier R, Perrigault PF et al. Cytomegalovirus infection in critically ill patients: associated factors and consequences. Chest 2005; 127: 233–241. [DOI] [PubMed] [Google Scholar]
- Chiche L, Forel JM, Roch A, Guervilly C, Pauly V, Allardet-Servent J et al. Active cytomegalovirus infection is common in mechanically ventilated medical intensive care unit patients. Crit Care Med 2009; 37: 1850–1857. [DOI] [PubMed] [Google Scholar]
- Guidry CA, Mansfield SA, Sawyer RG, Cook CH. Resistant pathogens, fungi, and viruses. Surg Clin North Am 2014; 94: 1195–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docke WD, Prosch S, Fietze E, Kimel V, Zuckermann H, Klug C et al. Cytomegalovirus reactivation and tumour necrosis factor. Lancet 1994; 343: 268–269. [DOI] [PubMed] [Google Scholar]
- Cook CH, Trgovcich J, Zimmerman PD, Zhang Y, Sedmak DD. Lipopolysaccharide, tumor necrosis factor alpha, or interleukin-1beta triggers reactivation of latent cytomegalovirus in immunocompetent mice. J Virol 2006; 80: 9151–9158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield S, Griessl M, Gutknecht M, Cook CH. Sepsis and cytomegalovirus: foes or conspirators? Med Microbiol Immunol 2015; 204: 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J, Trgovcich J, Kincaid M, Zimmerman PD, Klenerman P, Sims S et al. Transient CD8-memory contraction: a potential contributor to latent cytomegalovirus reactivation. J Leukoc Biol 2012; 92: 933–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CH, Zhang Y, Sedmak DD, Martin LC, Jewell S, Ferguson RM. Pulmonary cytomegalovirus reactivation causes pathology in immunocompetent mice. Crit Care Med 2006; 34: 842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PD, Shimamura M, Musgrove LC, Dennis EA, Bimczok D, Novak L et al. Cytomegalovirus enhances macrophage TLR expression and MyD88-mediated signal transduction to potentiate inducible inflammatory responses. J Immunol 2014; 193: 5604–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilet M, Aguilar G, Benet I, Belda J, Tormo N, Carbonell JA et al. Virological and immunological features of active cytomegalovirus infection in nonimmunosuppressed patients in a surgical and trauma intensive care unit. J Med Virol 2010; 82: 1384–1391. [DOI] [PubMed] [Google Scholar]
- von Muller L, Klemm A, Durmus N, Weiss M, Suger-Wiedeck H, Schneider M et al. Cellular immunity and active human cytomegalovirus infection in patients with septic shock. J Infect Dis 2007; 196: 1288–1295. [DOI] [PubMed] [Google Scholar]
- Forster MR, Trgovcich J, Zimmerman P, Chang A, Miller C, Klenerman P et al. Antiviral prevention of sepsis induced cytomegalovirus reactivation in immunocompetent mice. Antiviral Res 2010; 85: 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano C, Benet I, Clari MA, Nieto J, de la Camara R, Lopez J et al. Enumeration of cytomegalovirus-specific interferongamma CD8+ and CD4+ T cells early after allogeneic stem cell transplantation may identify patients at risk of active cytomegalovirus infection. Haematologica 2008; 93: 1434–1436. [DOI] [PubMed] [Google Scholar]
- Tormo N, Solano C, Benet I, Clari MA, Nieto J, de la Camara R et al. Lack of prompt expansion of cytomegalovirus pp65 and IE-1-specific IFNgamma CD8+ and CD4+ T cells is associated with rising levels of pp65 antigenemia and DNAemia during pre-emptive therapy in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant 2010; 45: 543–549. [DOI] [PubMed] [Google Scholar]
- Clari MA, Aguilar G, Benet I, Belda J, Gimenez E, Bravo D et al. Evaluation of cytomegalovirus (CMV)-specific T-cell immunity for the assessment of the risk of active CMV infection in non-immunosuppressed surgical and trauma intensive care unit patients. J Med Virol 2013; 85: 1802–1810. [DOI] [PubMed] [Google Scholar]
- Caston JJ, Cantisan S, Gonzalez-Gasca F, Paez-Vega A, Abdel-Hadi H, Illescas S et al. Interferon-gamma production by CMV-specific CD8+ T lymphocytes provides protection against cytomegalovirus reactivation in critically ill patients. Intensive Care Med 2016; 42: 46–53. [DOI] [PubMed] [Google Scholar]
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D et al2001SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003; 31: 1250–1256. [DOI] [PubMed] [Google Scholar]
- Durier N, Ananworanich J, Apornpong T, Ubolyam S, Kerr SJ, Mahanontharit A et al. Cytomegalovirus viremia in Thai HIV-infected patients on antiretroviral therapy: prevalence and associated mortality. Clin Infect Dis 2013; 57: 147–155. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22: 707–710. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- Needham DM, Scales DC, Laupacis A, Pronovost PJ. A systematic review of the Charlson comorbidity index using Canadian administrative databases: a perspective on risk adjustment in critical care research. J Crit Care 2005; 20: 12–19. [DOI] [PubMed] [Google Scholar]
- Aktas E, Kucuksezer UC, Bilgic S, Erten G, Deniz G. Relationship between CD107a expression and cytotoxic activity. Cell Immunol 2009; 254: 149–154. [DOI] [PubMed] [Google Scholar]
- Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011; 306: 2594–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Price DA, Casazza JP, Ferrari G, Nason M, Chattopadhyay PK et al. Surface expression patterns of negative regulatory molecules identify determinants of virus-specific CD8+ T-cell exhaustion in HIV infection. Blood 2011; 117: 4805–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa C, Krishnan A, Longmate J, Martinez J, Manchanda P, Lacey SF et al. Programmed death-1 expression in liver transplant recipients as a prognostic indicator of cytomegalovirus disease. J Infect Dis 2008; 197: 25–33. [DOI] [PubMed] [Google Scholar]
- Gallez-Hawkins GM, Thao L, Palmer J, Dagis A, Li X, Franck AE et al. Increased programmed death-1 molecule expression in cytomegalovirus disease and acute graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2009; 15: 872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks J, Tas H, Schmidt T, Kirsch S, Gartner BC, Sester U et al. PD-1 analysis on CD28(-) CD27(-) CD4 T cells allows stimulation-independent assessment of CMV viremic episodes in transplant recipients. Am J Transplant 2013; 13: 3132–3141. [DOI] [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nat Immunol 2011; 12: 492–499. [DOI] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol 2015; 36: 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan SF, Thakkar RK, Tran ML, Huang X, Cioffi WG, Ayala A et al. Programmed death 1 expression as a marker for immune and physiological dysfunction in the critically ill surgical patient. Shock 2012; 38: 117–122. [DOI] [PubMed] [Google Scholar]
- Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care 2011; 15: R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013; 13: 862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 2013; 13: 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 2005; 202: 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.