Abstract
Background
Breast, colorectal, ovarian, and endometrial cancers constitute approximately 30% of newly diagnosed cancer cases in Switzerland, affecting more than 12,000 individuals annually. Hundreds of these patients are likely to carry germline pathogenic variants associated with hereditary breast ovarian cancer (HBOC) or Lynch syndrome (LS). Genetic services (counseling and testing) for hereditary susceptibility to cancer can prevent many cancer diagnoses and deaths through early identification and risk management.
Objective
Cascade screening is the systematic identification and testing of relatives of a known mutation carrier. It determines whether asymptomatic relatives also carry the known variant, needing management options to reduce future harmful outcomes. Specific aims of the CASCADE study are to (1) survey index cases with HBOC or LS from clinic-based genetic testing records and determine their current cancer status and surveillance practices, needs for coordination of medical care, psychosocial needs, patient-provider and patient-family communication, quality of life, and willingness to serve as advocates for cancer genetic services to blood relatives, (2) survey first- and second-degree relatives and first-cousins identified from pedigrees or family history records of HBOC and LS index cases and determine their current cancer and mutation status, cancer surveillance practices, needs for coordination of medical care, barriers and facilitators to using cancer genetic services, psychosocial needs, patient-provider and patient-family communication, quality of life, and willingness to participate in a study designed to increase use of cancer genetic services, and (3) explore the influence of patient-provider communication about genetic cancer risk on patient-family communication and the acceptability of a family-based communication, coping, and decision support intervention with focus group(s) of mutation carriers and relatives.
Methods
CASCADE is a longitudinal study using surveys (online or paper/pencil) and focus groups, designed to elicit factors that enhance cascade genetic testing for HBOC and LS in Switzerland. Repeated observations are the optimal way for assessing these outcomes. Focus groups will examine barriers in patient-provider and patient-family communication, and the acceptability of a family-based communication, coping, and decision-support intervention. The survey will be developed in English, translated into three languages (German, French, and Italian), and back-translated into English, except for scales with validated versions in these languages.
Results
Descriptive analyses will include calculating means, standard deviations, frequencies, and percentages of variables and participant descriptors. Bivariate analyses (Pearson correlations, chi-square test for differences in proportions, and t test for differences in means) will assess associations between demographics and clinical characteristics. Regression analyses will incorporate generalized estimating equations for pairing index cases with their relatives and explore whether predictors are in direct, mediating, or moderating relationship to an outcome. Focus group data will be transcribed verbatim and analyzed for common themes.
Conclusions
Robust evidence from basic science and descriptive population-based studies in Switzerland support the necessity of cascade screening for genetic predisposition to HBOC and LS. CASCADE is designed to address translation of this knowledge into public health interventions.
Trial Registration
ClinicalTrials.gov NCT03124212; https://clinicaltrials.gov/ct2/show/NCT03124212 (Archived by WebCite at http://www.webcitation.org/6tKZnNDBt)
Keywords: public health genetics, public health interventions, family communication, cancer surveillance, patient-provider communication, quality of life, psychosocial support, family-based interventions
Introduction
Breast, colorectal, ovarian, and endometrial cancers constitute approximately 30% of newly diagnosed cancer cases in Switzerland, affecting more than 12,000 individuals annually [1]. About 2%-15% of incident cases are associated with known hereditary cancer syndromes. Several hundred Swiss patients diagnosed with any of these cancers are likely to carry known pathogenic germline variants [2]. Approximately 5-10% of breast cancer cases and 10%-15% of epithelial ovarian cancer cases develop due to single gene mutations that are passed down in the family, such as the breast cancer 1 (BRCA1) and breast cancer 2 (BRCA2) genes [3,4]. Germline BRCA mutations are associated with most hereditary breast and ovarian cancer (HBOC) cases. Women with BRCA mutations have a 55%-70% risk of breast cancer and 17%-59% risk of ovarian cancer by age 70, while the corresponding lifetime risks in the general population are 12% and 1.3%, respectively [5-7]. HBOC cases have an increased risk of cancer at a younger age, often before recommendations for routine screening apply [8,9]. The prevalence of BRCA mutations varies considerably among ethnic groups and geographical areas. In Caucasian populations, the prevalence of BRCA pathogenic variants is estimated at 1:400 to 1:500, whereas the frequency of three founder mutations in the Ashkenazi Jewish population is 1:40 [10-12]. About 21% of Swiss breast cancer patients are diagnosed younger than 50 years old, which may indicate genetic susceptibility [13,14].
Lynch syndrome (LS), previously known as hereditary nonpolyposis colorectal cancer, is an inherited disorder, associated with 22%-74% lifetime risk for colorectal cancer, 14%-71% risk for endometrial cancer, 3%-22% risk for ovarian cancer, up to 13% risk for gastric cancer, and up to 25% risk for urothelial cancer [15]. LS accounts for about 2%-5% of colorectal cancer and endometrial cancer burden, as well as increased risk for several other malignancies including gastric, ovarian, small bowel, urinary and biliary tract, pancreatic, and sebaceous gland tumors [16]. Individuals with LS have a 10%-74% risk of colorectal cancer, and a 14%-71% risk of endometrial cancer by age 70, while the corresponding rates in the general population are 5.5% and 2.7%, respectively [17,18]. A hallmark of LS is early age of onset, usually before the age of 50 at which recommendations for routine screening apply [15,19]. Most LS-related tumors are characterized by a high level of microsatellite instability (MSI-H), which is distinctive of cancers with a defective DNA mismatch repair (MMR) mechanism [20]. Diagnosis of LS involves a sequential process including prescreening with MSI testing and immunohistochemistry analysis to determine expression of the main MMR proteins (MLH1, MSH2, MSH6, PMS2) in tumor tissues. Additional MLH1 promoter methylation testing eliminates the possibility of loss of MLH1 expression due to epigenetic mechanisms or identification of a somatic BRAF pathogenic variant (c.1799T>A/p.V600E). In the case of pathological prescreening results, germline analyses of two or more MMR genes (MLH1/PMS2 and/or MSH2/MSH6) and search for EPCAM deletions confirm the diagnosis. Germline mutations in the MLH1 and MSH2 genes account for up to 90% of LS cases, whereas MSH6 and PMS2 mutations account for most of the remaining cases [21]. The Amsterdam Criteria II and Revised Bethesda Guidelines are used in clinical practice for identifying individuals concerned about LS [22]. These guidelines are not sensitive enough and may miss up to 30% of LS cases [23]. Even if the population prevalence of LS is estimated at 1:440 [24], LS is vastly underdiagnosed compared to HBOC.
Germline mutations connected to HBOC and LS are inherited in an autosomal dominant manner. De novo mutations are rare in these syndromes. For every identified mutation carrier, there are multiple family members who may carry the same mutation. First- and second-degree relatives and first cousins of known carriers have 50%, 25%, and 12.5% probability for inheriting the respective cancer predisposition. The availability of cancer genetic services (counseling and testing) for HBOC and LS is a significant milestone for effective cancer prevention and control [25]. When a pathogenic variant is identified, relatives can be tested with 100% accuracy [26]. Genetic counseling can educate patients and cancer-free individuals about cancer risk and management options according to mutation status. Physicians’ attitudes [27] and coverage of cost of tests and gene panels by health insurance influence whether genetic testing is performed or not [28].
A Swiss study reported that about 11% of all breast cancer patients and 25% of those with a strong family history used genetic services [29]. These figures are lower for LS-related colorectal and endometrial cancer patients, suggesting that many Swiss mutation carriers and their family members may not benefit from advances in health care technology and medical diagnostics. HBOC and LS patients are at an increased risk of secondary cancers and can benefit from intensive surveillance, pharmacoprevention, or prophylactic surgery. Prophylactic surgery such as mastectomy, bilateral salpingo-oophorectomy, and hysterectomy should be discussed with women affected with HBOC or LS [30]. Subtotal colectomy can be considered for LS patients with colorectal cancer [18]. Family members who test positive benefit from high-risk management care starting at age 25-30, or 10 years before the earliest age of breast cancer onset in the family. This care can include annual breast magnetic resonance imaging, mammograms, pelvic ultrasound for women (HBOC) [31], and annual colonoscopy starting at age 20-25, or 2-5 years before the earliest age of colorectal cancer onset in the family, whichever comes first (LS) [15,18]. Implementing clinical recommendations and providing high-quality surveillance to patients during survivorship requires excellent coordination of health care services provided in high-risk clinics [32-35].
Mutation carriers identified through complete genetic analyses are asked to communicate test results to relatives and encourage them to use genetic services. This process is highly variable from family to family, with less than 40% of high-risk relatives using genetic services, suggesting a lack of effective communication [36,37]. Lack of understanding of genetic information combined with family conflicts most likely inhibits disclosure of test results to relatives [38,39]. In Switzerland, the Federal Act on Human Genetic Testing (HGTA) is the legal regulation that directly applies to the clinical practice of genetic analysis. HGTA states that a physician is not allowed to disclose genetic test results to anyone except the tested individual or their legal representative. Results can be disclosed to family members, spouses, or partners only with the explicit consent of the tested individual. If the tested individual refuses to disclose this information, if they are deceased, have disappeared, or are unable to consent in the absence of an authorized delegate, the physician can seek help from the expert commission on professional confidentiality. The physician may apply to the appropriate cantonal authority to be released from the duty of professional secrecy if protecting the overriding interests of the family members, spouse, or partner requires that they receive this information. Cantonal authorities may also request an opinion from the Expert Commission for Human Genetic Testing [40]. Interventions designed to facilitate patient-provider and patient-family communication can enhance understanding of genetic information and facilitate the disclosure of test results from carriers to relatives and can contribute to more effective management of hereditary cancer. Several such interventions have been developed and tested in the United States [41-51] but should be adapted before they can be implemented in Switzerland, due to cultural and possibly legal differences.
Cascade screening is the sequential process of identifying and testing blood relatives of a known mutation carrier to determine if additional individuals carry the pathogenic variant, and proposing preventive and other clinical management options to reduce morbidity and mortality [52]. Cascade screening also reassures non-carrier relatives and excludes them from intensive surveillance, making it cost-effective and contributing to personalized medicine [53]. The Centers for Disease Control and Prevention, Office for Public Health Genomics issued evidence-based recommendations justifying genetic testing in affected individuals and relatives when there is a known family history of HBOC or other BRCA-related cancers, LS-related colorectal cancer, or familial hypercholesterolemia (FH). These are Tier 1 genetic conditions suitable to promoting translation of scientific breakthroughs in genetics to public health [54]. There are currently no systematic efforts to apply cascade screening for Tier 1 genetic conditions among the general population in Europe apart from the Netherlands, which successfully implemented a cascade screening program for FH. The implementation of this pioneering public health program helped identify more than 28,000 asymptomatic cases [55] and provides proof-of-concept that cascade screening can be applied in other settings [56].
Robust evidence from basic science and descriptive population-based studies in Switzerland support the necessity of cascade screening for HBOC and LS [57-67]. However, there are currently no interventions to translate this knowledge into public health. Researchers know little about the cancer status and surveillance behaviors of mutation carriers and their relatives, and their needs for psychosocial, patient-provider, and family communication support. This is especially important over time, as little is known about decisional regret associated with genetic testing, communication, and support after the pathogenic variant has been identified in some family members but not in others, as well as impact on quality of life. A better understanding is needed of the overall response of the Swiss health care system to mutation carriers’ needs for long-term coordination of cancer surveillance and prevention. Finally, there are no interventions culturally tailored for Swiss families and designed to enhance patient-provider and patient-family communication, coping, and provide decisional support.
Establishing a registry with families harboring germline pathogenic variants associated with HBOC and LS and the collection of cancer surveillance and psychosocial data over time will greatly assist in finding sustainable solutions and developing cutting-edge interventions that optimize the health care system. However, establishing cascade screening for HBOC and LS and promoting interventions for communicating hereditary cancer risks pose several challenges at the medical and social level, requiring interprofessional collaboration with stakeholders from basic research, the health care system, and social science. In response to this challenge, the Swiss Cancer Genetic Predisposition Cascade Screening Consortium was assembled in 2015 with stakeholders from various disciplines (ie, basic science, epidemiology, medicine, nursing, psychology, public health, and sociology) to conduct the CASCADE study and examine the feasibility of establishing a family-based registry and a cohort with HBOC and LS mutation-harboring families.
The specific aims of the CASCADE study are to (1) survey index cases with HBOC or LS from clinic-based genetic testing records and determine their current cancer status and surveillance practices, needs for coordination of medical care, psychosocial needs, patient-provider and patient-family communication, quality of life, and willingness to serve as advocates for cancer genetic services to blood relatives, (2) survey blood relatives identified from pedigrees or family history records of HBOC and LS index cases and determine their current cancer and mutation status, cancer surveillance practices, needs for coordination of medical care, barriers and facilitators to using cancer genetic services, psychosocial needs, patient-provider and patient-family communication, quality of life, and willingness to participate in a study designed to increase use of cancer genetic services, and (3) explore the influence of patient-provider communication about genetic cancer risk on patient-family communication and the acceptability of a family-based communication, coping, and decision support intervention with focus group(s) of mutation carriers and relatives.
Methods
Design
CASCADE is a longitudinal study using surveys and focus groups, designed to elicit factors that enhance cascade genetic testing for HBOC and LS in Switzerland. The CASCADE study will contact known mutation carriers for HBOC and LS and systematically identify and contact their relatives to determine if they have had genetic testing, if they also carry the pathogenic variant, and how they manage their risk for hereditary cancer. Repeated observations are the optimal way for assessing these outcomes. The study will also use focus groups to examine the acceptability of a family communication, coping, and decision support intervention (Phase I). Table 1 presents a detailed description of assessments conducted for the study. The study protocol has been approved by the local ethics committee, while approval from ethics committees in other cantons is underway. The study will be carried out according to principles described in the Declaration of Helsinki and applicable Swiss laws and Swiss regulatory authority requirements.
Table 1.
Flow of assessments for the CASCADE study.
| Phase and steps | Tasks/Procedures | Data | |
| Selection of eligible index cases | |||
| Random selection of families | Each clinical site provides the principal investigator (PI) with a list of the family identifications (IDs) determined by the clinical site as harboring a pathogenic germline variant. The PI randomly selects 35% of family IDs from the list with computer-generated numbers. The number of selected family IDs at each site is based on total number of family IDs at the clinical site and stratification for representative sampling. | No identifiable data for index cases are shared with the PI. | |
| Identification of eligible index cases | Through pedigrees and family history records, each site coordinator identifies index cases (1st family member to be identified as a carrier of a germline pathogenic variant) and determines whether they can be contacted (ie, alive and living in Switzerland). If an index case cannot be contacted, site coordinators identify 1st degree relatives who carry the familial pathogenic variant, randomly select one of them (computer-generated numbers), and determine whether they can be contacted. The process is repeated until an eligible mutation carrier is identified that can initiate cascade screening in the family. | Clinical sites collect minimal data (except identifiable data) for all index cases, regardless of whether they can be reached or not. Minimum data include gender, age, mutation, cancer type, age at diagnosis, stage, age tested, alive, place of residence, preferred language. | |
| Recruitment of eligible index cases | |||
| Recruitment package to index cases | The medical director of each clinical site (co-PI or site co-investigator) and the site coordinator mail recruitment packages to index cases. If the index case did not receive genetic counseling at the testing site, then the recruitment package is sent to the referring physician who is asked to pass it on. Three attempts will be made to contact index cases. The medical director will inform treating oncologists about the participation of index cases. | Unique identification coding scheme enabling identifying index cases, site they were recruited from, and type of hereditary cancer syndrome (HBOC or LS). Dates recruitment packages were sent to physicians, dates the response from Index cases was received, and recruitment attempts made. |
|
| Engagement of index cases in the CASCADE study | The site coordinator receives the informed consent or participation refusal form from index cases. Index cases accepting participation receive the CASCADE survey in their preferred language and format (paper/pencil or online) from the PI. The PI creates a coding key for identifying participants and the Clinical Trials Unit creates a coding key for variables assessed in the CASCADE survey. | Identifiable information for index cases accepting participation is passed on from site coordinators to the PI. Response rate from index cases, acceptance to participate in various stages of the CASCADE study, reasons for nonparticipation and preferred language and format for survey. |
|
| Survey from index cases | The PI and the data management team receive the completed survey from index cases either in paper/pencil or online. | Assessment of data quality in each format (eg, percent missing data, outliers). Assessment of instrument reliability (Cronbach alpha and principal component analysis). Number of eligible relatives. Number of eligible relatives the index case is willing to invite. Characteristics of relatives reported by the index case. CASCADE study outcomes. | |
| Recruitment of eligible blood relatives | |||
| Identification of eligible relatives | Based on index cases’ response to the CASCADE survey, the PI identifies eligible blood relatives the index case is willing to invite. Information about relatives is cross-referenced with pedigrees and family history information from clinical sites. | Number of relatives and degree of relationship to the index case (1st or 2nd degree relative, or 1st cousin). | |
| Recruitment package to eligible relatives | The PI prepares recruitment packages for relatives and a personalized letter for each index case, explaining the recruitment process and asking them to pass on recruitment packages to relatives. | Unique identification coding scheme enabling matching members of the same family. | |
| Engagement of eligible relatives in the CASCADE study | The PI receives informed consents or participation refusal forms from relatives. Relatives accepting participation receive the CASCADE survey in their preferred language and format (paper/pencil or online). | Response rate from relatives, acceptance to participate in various arms of the CASCADE study, reasons for nonparticipation and preferred language and format for survey completion. | |
| Survey from relatives | The PI and the data manager receive the completed survey from relatives either in paper/pencil or online form. | Assessment of data quality in each format (eg, percent missing data, outliers). Assessment of instrument reliability (Cronbach alpha and principal component analysis). Number of eligible relatives willing to invite. CASCADE study outcomes. |
|
| Focus groups | |||
| Selection of index cases and relatives | A purposeful sample of index cases and relatives accepting participation in focus groups will be selected by the qualitative methodologist and the PI. | Characteristics of index cases invited in the focus groups (preferred language, gender, mutation, type of cancer). Characteristics of families invited in the focus groups (level of support and communication). |
|
| Invitation letters for focus groups | The PI in collaboration with the qualitative methodologist will send invitation letters initially to index cases and then to families selected for the focus groups. | Acceptance rate. | |
| Focus groups | Focus groups are organized and completed under the auspices of the qualitative methodologist. | Narrative data from focus groups. | |
Setting
This multicenter study involves contributions from oncology and genetic testing centers from three linguistic regions of Switzerland (German-, French-, and Italian-speaking). Medical directors of clinical sites are either co-principal investigators (co-PIs) or site co-investigators and will oversee recruitment procedures according to the study protocol. The PI will oversee the scientific integrity of the study, including recruitment, data collection, and data analyses. These findings will be compiled and communicated to clinical sites.
Sample and Sample Size
The CASCADE study targets individuals who have been identified through genetic testing as carrying a pathogenic germline variant associated either with HBOC or LS and their relatives (first- and second-degree, and first cousins). Textbox 1 describes applicable inclusion and exclusion criteria. Index cases include male and female cancer patients and cancer-free individuals. Cancer risk associated with HBOC and LS does not apply to children, thus, the study will include only adults (≥18 years old). Decisions to undergo genetic testing for these conditions are made by adults deemed competent to provide informed consent and should be undertaken after individuals participate in consultation regarding the benefits and drawbacks of genetic testing. Vulnerable participants (eg, those living in nursing homes) will be excluded because they may not be able to consent to genetic testing or follow recommended cancer surveillance or preventive measures. Critically ill patients will be excluded from recruiting relatives and from focus groups to avoid increasing subject burden.
Characteristics of the target populations.
Inclusion criteria
Living carriers of germline pathogenic variants associated with HBOC and LS, and their relatives (1st and 2nd degree, and 1st cousins)
Have at least one living blood relative
Both genders
Age ≥18 years old
Mentally/physically able to provide informed consent
Cancer patients and cancer-free individuals
Can read/speak German or French or Italian or English
Currently living in Switzerland
Exclusion criteria
Carriers of unclassified genetic variants in BRCA1, BRCA2 or MLH1, MSH2, MSH6, PMS2, EPCAM genes
Currently not living in Switzerland
Critically ill patients not able to complete the survey
Not able to provide an informed consent
Institutionalized (eg, nursing homes) or incarcerated
Estimates of sample size are based on the PI’s experience, consultations with medical directors of clinical sites, and assuming average prevalence rates of 5% for hereditary breast cancer and 2-5% for hereditary colorectal cancer (the two most common manifestations of HBOC and LS), respectively. Assuming that is feasible to recruit 10% of mutation carriers from each participating clinic, 300 index cases will be targeted for inclusion within 12 months. It is expected that around 70% of approached index cases will accept participation, meaning that 495 index cases need to be approached to reach 305. It is estimated that from each index case we will identify 2.5 relatives. Assuming a response rate of 50% among relatives, we expect to recruit approximately 381 relatives. Figure 1 presents the CONSORT [68] diagram for recruitment of expected index cases and relatives.
Figure 1.
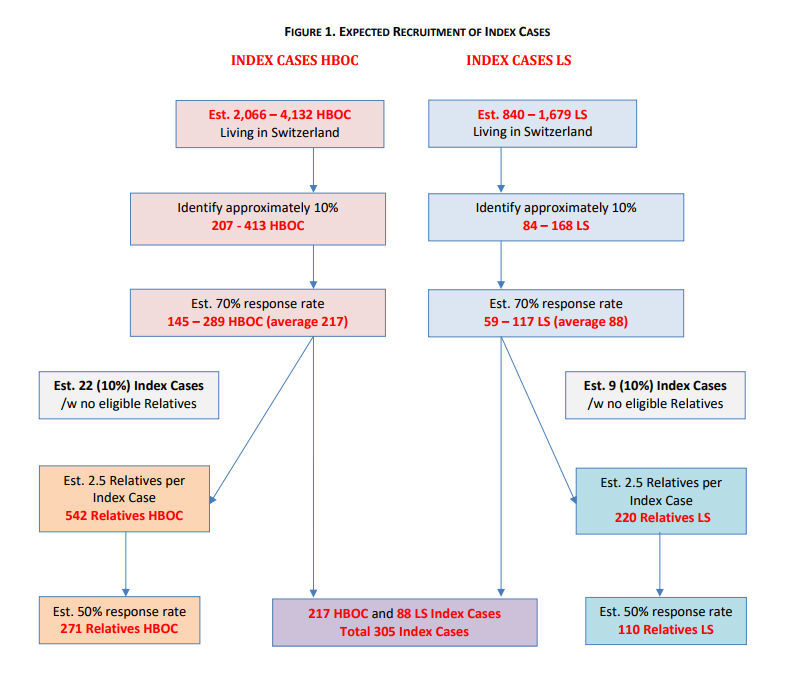
Expected recruitment of index cases.
Recruitment Procedures for Index Cases
Participating Swiss clinical sites will record the total number of mutation-harboring families for HBOC or LS. A dedicated staff person at each clinical site, the site coordinator, will identify eligible index cases (ie, first person in the family identified as carrying a germline pathogenic variant associated with HBOC or LS), determine whether they can be contacted or not, and initiate and monitor the recruitment process. Selection of mutation-harboring families from each clinical site will involve the following steps:
Each site creates a list with IDs (eg, 001…, 350) corresponding to each index case and a family with a pathogenic variant.
The ID list is sent to the PI, who selects approximately 35% of cases with the assistance of a computer-generated random list. Identifiable information is not released until the index case accepts participation through signing an informed consent form.
Site coordinators retrieve the medical charts of selected index cases and decide whether the cases can be contacted by determining living status and residence.
If the index case is not available, then site coordinators identify first-degree relatives, who have also been identified through genetic testing as carriers of the familial pathogenic variant, and randomly (computer-generated list) select one of them.
Steps 3 and 4 are repeated until an index case who can be the initial contact person for the family is identified. If this process yields no results, the next family is selected.
All information obtained from each step is recorded, including minimum information for index cases. Minimum information includes demographics (age, gender), clinical history (tumor type, age at diagnosis, stage), and genetic testing results (including MSI and IHC tumor testing for LS patients) and is obtained from medical records. Index cases are recruited to the CASCADE study by the medical director of the respective site.
Index cases will be mailed an information letter, two copies of the informed consent form, two copies of a participation refusal form, and a stamped self-addressed envelope to return their response to the clinical site. Index cases will be informed about the objectives of the CASCADE study, participation requirements, the study plan, confidentiality, and associated risk and benefits through the informed consent form, which explicitly requires their agreement to (1) complete the CASCADE survey, (2) contact one or more of their blood relatives for the study, (3) be contacted once a year for 5 years and provide updated information about their health, and (4) participate alone or with a blood relative in a focus group. Index cases can participate in all or in some of the above study steps. The information letter explains that the minimum requirement for taking part in the CASCADE study is to complete the self-administered survey once. The refusal form asks nonparticipating index cases the reason for their refusal; this information is necessary for the validity of the study.
Site coordinators will determine whether the identified index case can be contacted or not by investigating whether they are alive and whether they live in Switzerland through hospital and civil records. If the recruitment package is returned undelivered, additional address verification methods will be used to locate a new residence. If the index case cannot be contacted a priori, coordinators will determine whether a first-degree relative can be the new index case for the family. Three attempts will be made to contact index cases for each family. If the study receives no response 6 weeks after the third attempt, a new family will be selected to preserve required sample size. Index cases will be recruited to the CASCADE study on a consecutive ongoing basis. Site coordinators will review pedigrees and family history of index cases who accept participation to extract demographic and medical information and to record all blood relatives (first- and second-degree relatives and first cousins). Index cases will be asked to complete a self-administered survey.
When an index case has not received genetic counseling at the participating center, the invitation package for the CASCADE study will be sent by the referring physician. This is necessary because some clinical sites perform only genetic testing and the referring physician is considered the medical person who has direct knowledge of index case’s genetic testing results. Site coordinators and the PI will keep track of the recruitment process. Referring physicians will make three recruitment attempts by mailing a new invitation package every 6 weeks if the index case does not respond to the invitation (either positively or negatively). Contact information (address, telephone, email) of the PI and the medical director will be provided in the information letter, so that index cases can request further information about the study at any point. A signed informed consent will be requested prior to index case’s enrollment as a prerequisite for engagement in the CASCADE study.
Recruitment Procedures for Relatives
In order to alleviate ethical concerns associated with contacting blood relatives (ie, first- and second-degree relatives, and first cousins) without their explicit consent, the CASCADE study will approach them through index cases and will approach only relatives the index case is willing to contact. This recruitment method has been used in previous family-based studies with very good recruitment outcomes [69,70]. Index cases will be mailed recruitment packages to pass on to their relatives, including an information letter, two copies of the informed consent form, two copies of the participation refusal form, and a stamped self-addressed envelope for relatives to return their response to the PI. Relatives’ identifiable information will not be released to the PI. By returning a signed informed consent, the relative indicates willingness to participate and releases their identifiable information to the PI. Once this information is available, a recruiter will contact them to ascertain eligibility. If relatives do not respond after 6 weeks, the PI will contact the index case asking them to pass on a reminder letter to the nonresponding relative. If this effort yields no response, there will be no further attempts to contact the relative. Relatives agreeing to participate will receive a similar survey as the index case, asking if they are willing to (1) invite additional relatives to the CASCADE study, (2) be contacted once a year for 5 years and provide updated information about their health, and (3) participate alone or with a blood relative in a focus group. Relatives can also participate in all or some of the above study steps.
Recruitment Procedures for Focus Groups
Two series of focus groups will be organized to explore the (1) difficulties associated with patient-provider communication regarding genetic cancer risk, (2) difficulties associated with patient-family communication regarding the pathogenic mutation, (3) mutual influence of patient-provider and patient-family communication, and (4) acceptability of a family-based intervention designed to enhance communication, coping, and decision making for genetic testing. A purposeful sample of index cases and relatives will be selected from individuals who agreed to participate in focus groups. The sampling method will be based on the expertise of the qualitative methodologist from interviews with Swiss BRCA carriers [32,33,71] and the PI’s experience conducting focus groups with US BRCA families. Segmentation strategy will guide sampling methods and the composition of the focus groups. Each focus group will be relatively homogeneous, while the full set will include several potentially distinct perspectives [72]. Focus groups will include 5-10 participants. Male and female cancer patients and cancer-free individuals will be selected to represent HBOC and LS.
It is expected that data saturation will be reached with 6-10 focus groups including about 30-60 carriers and 30-60 relatives. The first series of focus groups will include only mutation carriers stratified according to level of family communication (high, intermediate, low). These focus groups will explore the difficulties in patient-provider and patient-family communication, and the interrelatedness of these two types of communication. The second series of focus groups will include carriers and relatives and will explore the acceptability of an intervention designed to facilitate communication of test results among family members, helpful coping mechanisms, and decision making for genetic testing. Two sampling methods are envisioned. One method involves several members of the same family who can be invited together; the other involves 3-4 family pairs consisting of one carrier and one relative, which will be homogeneous in terms of gender, health status, etc. The sampling method of the second series of focus groups will be informed by responses to the CASCADE survey and findings from the first series of focus groups.
Data Collection and Data Management
The CASCADE survey will be developed in English, translated into three languages (German, French, and Italian), and back-translated into English by professional translators, except for scales with validated versions in these languages (eg, 12-Item Short Form Health Survey [SF-12]). Discrepancies will be resolved by the PI with the collaboration of the translators and the co-investigators. Index cases and relatives will be given the choice to complete the CASCADE survey either as paper/pencil or using an online platform. The content of the paper/pencil and online survey are identical. Participants who choose to complete the survey online will receive an access code and will be instructed how to log into a secure Web platform. If a survey is missing important information (eg, number of relatives the index case is willing to contact), research personnel will contact participants to ascertain it.
No identifying information, such as name and address, is collected with paper/pencil or online surveys. Each index case is given a code; for example, G001-IC stands for an index case selected from the Geneva clinic with the family study code 001. Relatives recruited from this index case will be coded G001-R1, G001-R2, etc, to establish the link between family members. This code will be used for surveys, consent forms, refusal forms, and correspondence letters to match participants to the correct family unit and maintain the study’s internal validity. The PI and coordinators will keep logs with these codes. The coding key will be kept in a password-protected computer file and will be available to the PI, members of the Swiss Cancer Genetic Predisposition Cascade Screening Consortium, and key personnel. The code will be broken only to avert an immediate risk to the health of the person, in cases of withdrawal from the study, or when there is a legal basis.
All study data will be collected and stored in a secure database and handled by the data management team from the Clinical Trials Unit, University Hospital, Basel (CTU Basel). The online survey will be implemented using LimeSurvey, installed on a separate server, and exclusively used for the study. Lime Survey is an established app to perform online surveys. The system (server and data) is integrated in a regular backup process. Data transfer from and to the Web-based survey system are encrypted using secure sockets layer/transport layer security (SSL/TLS). The secure database will be used for data collection and to track returned surveys. Data entered for paper/pencil surveys will be double-checked for accuracy. The usability of the paper/pencil or online survey will be assessed based on number of individuals who choose either mode, percent of missing data, etc. Many items are parts of multi-item scales and are anticipated to correlate with each other. The reliability of these scales will be tested using principal component analyses and Cronbach alpha coefficients. Scales with alpha≥.71 will be used. On completion of approximately 30 surveys, scale psychometrics will be examined. For any given scale that shows less than required psychometric properties (ie, Cronbach alpha<.71 and factor analysis indicates item loadings <10% compared to item loadings in the original scale), a revision of the translated scale will be undertaken. This will allow comparisons of scale reliability based on delivery mode and will establish whether the survey can be administered interchangeably.
Health-related and personal data collected for the CASCADE study are confidential; coding will safeguard participants’ confidentiality. All study documents will be archived in the PI’s office. Site-related documents will be archived at the office of each medical director. Administrative data are accessible only by authorized personnel and data managers from CTU Basel. Direct access to documents will be permitted for monitoring, audits, or inspections. Ethics committee members, members of the Swiss Cancer Genetic Predisposition Cascade Screening Consortium, the statistician, and key personnel will have access to project plan, dataset, statistical code, etc, during and after the study (publication, dissemination). Paper/pencil surveys will be stored in a separate research office in the PI’s building for 5 years and then destroyed by shredding. Once all data have been collected, the complete dataset and survey setup will be exported by CTU Basel and transferred to the PI and the statistician via a secure channel. The survey system (including database) will be purged after the end of the study. The PI will archive the electronic data for a minimum of 10 years.
Outcomes
Table 2 [73-89] describes primary outcomes for index cases and relatives and the scales used to assess them. The feasibility of establishing a family-based registry will be assessed using the number of mutation-harboring families associated with HBOC and LS from each clinical site, the number of relatives identified from pedigrees and family history, index cases’ response rate to the CASCADE survey, the number of relatives each index case is willing to invite, relatives’ response rate to the CASCADE survey, and the willingness of index cases and relatives to be contacted once a year for 5 years. Additional outcomes include assessing acceptance rates of paper/pencil and online platform and quality of data (eg, percent missing values).
Table 2.
Scales used in the CASCADE survey.
| Concepts | Scale | Index case | Relatives | |
| Demographics | Age, gender, education, employment status (previously used) [73] | √ | √ | |
| Health history | ||||
| Comorbidities | Chronic conditions associated with mobility, cardiovascular disease, diabetes, anxiety, depression Self-reported list (yes/no) (previously used) [73] |
√ | √ | |
| Reproductive history (females) | Risk factors associated with the Gail model [74,75] Self-reported |
√ | √ | |
| Alcohol, tobacco, physical activity | Self-reported (previously used) [76] | √ | √ | |
| Cancer-related | ||||
| Cancer diagnoses | Type of cancer, age of onset Self-reported list (previously used) [73] |
√ | √ | |
| Surgery | Surgeries associated with HBOC & LS Prophylactic surgeries Self-reported (previously used) [29] |
√ | √ | |
| Surveillance behaviors | Surveillance for cancers associated with HBOC & LS Surveillance for common cancers Investigating tool developed per the American Society of Clinical Oncology guidelines [77] (previously used) [73] |
√ | √ | |
| Barriers & facilitators (previously used) [73] Coordination of medical care (multiple choices) High out-of-pocket costs (yes/no) |
√ | √ | ||
| Family history | Family history in 1st and 2nd degree relatives & 1st cousins – type of cancer, age of onset (previously used) [73] | √ | √ | |
| Psychosocial needs | ||||
| Fear of cancer recurrence | Concerns About Recurrence Scale [78] 4 items, 7-point Likert scale |
√ | √ | |
| Perceived cancer risk | Perceived Risk for Developing Cancer [79] 1 item, 10 points with verbal anchors |
√ | √ | |
| Decisional conflict | Decisional Conflict associated with genetic testing [80] 16 items, 7-point Likert scale |
√ | ||
| Decisional regret | Decisional Regret associated with genetic testing [81] 5 items, 7-point Likert scale |
√ | ||
| Coping with stressful events | Brief Cope [82] 25 items, 7-point Likert scale |
√ | √ | |
| Self-efficacy | Self-efficacy dealing with cancer [83] 14 items, 7-point Likert scale |
√ | √ | |
| Self-efficacy – use genetic services (counseling & testing) [83] 1 item, 7-point Likert scale |
√ | |||
| Knowledge | Breast & Ovarian Cancer Risk Factor Knowledge Index [84,85] 17 items (True, False, Don’t Know) |
√ | √ | |
| Knowledge of Breast Cancer Genetics Scale [70] 12 items (True, False, Don’t Know) |
√ | √ | ||
| LS Risk Factors & Inheritance Investigator developed 19 items (True, False, Don’t Know) |
√ | √ | ||
| Communication | ||||
| Physician | Need for physician communication about mutation Investigator developed 10 items, 7-point Likert scale |
√ | ||
| Family | Mutuality & Interpersonal Sensitivity [86] 15 items, 7-point Likert scale |
√ | √ | |
| Family Support in Illness [73,87] 10 items, 7-point Likert scale |
√ | √ | ||
| Communication with children & relatives about mutation (previously used) [29] 17 items (multiple choice) |
√ | |||
| Genetic services | ||||
| Genetic services | Barriers & facilitators (previously used) [29,88] 11 items, 7-point Likert scale & 22 items (multiple choice) |
√ | √ | |
| Genetic testing | Had genetic testing (yes/no) Self-reported |
√ | √ | |
| Referral | Source & involvement (previously used) [29] 16 items (multiple choice) |
√ | ||
| Quality of Life | SF-12 [89] Physical component & Mental component |
√ | √ | |
Data Analyses
Selection bias will be minimized by random selection of mutation-harboring families in each clinical site from three linguistic regions of Switzerland. Stratification will ensure selection of an equal proportion of index cases from clinical sites that offer genetic services for both syndromes. The study will try to recruit all index cases from clinical sites including fewer than 100 mutation-harboring HBOC/LS families to ensure a representative sample.
All statistical analyses will be conducted in licensed software packages, including Microsoft Excel, SPSS (IBM), and R. For all statistical tests, significance will be set at two-sided alpha=.05. Data values will be examined for legality (within appropriate range) using histograms and box plots and corrected when possible. Descriptive analyses will include calculating means, standard deviations, frequencies, and percentages of variables and participant descriptors. Bivariate analyses (Pearson correlations, chi-square test for differences in proportions, and t test for differences in means) will assess associations between demographics and clinical characteristics. Regression analyses will incorporate generalized estimating equations for pairing index cases with their relatives and explore to what extent predictors are in direct, mediating, or moderating relationship to an outcome.
The following comparisons will take place: between index cases and relatives, between HBOC and LS, between men and women, cancer patients versus cancer-free individuals, participants with children versus those with no children, between different age groups and different cancer diagnoses. Data from participants who withdraw will be kept in the study to ensure the internal validity of the study. Missing data from multi-item scales will be addressed with multiple imputations using R software if they exceed 5% of observations and if they are less than 25% for each specific scale. Scale reliability will be assessed with Cronbach alpha and principle component analyses. Deviations from the planned analyses are not foreseen. The study statistician will review and approve any deviations from the original statistical plan if necessary.
Narrative data from focus groups will be recorded and transcribed verbatim to allow data management and content examination. Thematic analyses to inductively classify data in concepts and categories, as these emerge through an interpretive process, will be carried out under the guidance of the qualitative methodologist [90]. Focus group participants will be shown a prototype of a family-based intervention as a PowerPoint presentation. Then they will be asked if they like the intervention, if they find it useful, and how it can be improved. Acceptability of the intervention will be assessed with a short survey using 7-point Likert-type items (1=Low to 7=High) asking overall satisfaction with the content, format and appearance of the program, and whether it can help with family communication, coping, and decision making. The survey assesses six acceptability items: ease of use, clarity, appropriate length, appropriate level of detail, able to hold interest, and satisfaction.
Results
This study is currently recruiting participants.
Discussion
Principal Considerations
Cancer predisposition cascade genetic screening combines personalized medicine and public health. Once a mutation carrier for HBOC or Lynch syndrome is identified, evidence-based interventions are available that can reduce the risk of adverse health outcomes in entire cohorts of relatives [91]. This approach is cost-effective for Tier 1 genetic conditions, leading to reduced medical and insurance coverage costs (eg, treatment and hospitalization expenses) [92-94]. Cascade screening for FH applied in the Netherlands identified thousands of mutation carriers for the disorder and has been subsidized by the Dutch government since 2015 [95,96]. Similar programs for FH have also been implemented in Scotland and Wales [97,98].
Availability of genetic testing created an increasing demand for coordination of health care services and risk communication among index cases and relatives. Knowledge of hereditary risk can serve as an information tool to reduce cancer morbidity and mortality. This necessitates the establishment of family-based registries that systematically record genetic information. Currently, this information is fragmented and dispersed across Swiss clinical sites. The establishment of high-risk clinics would allow synergistic approaches in cancer surveillance and medical care offered to these families. Effective data sharing and dissemination across disciplines is mandatory for increasing the impact of genetic screening, ensure resource allocation, and facilitate health care policy and decision making.
Conclusion
CASCADE study will promote multidisciplinary research in public health genetics at the cutting edge of medicine with strong translational application. This has significant potential to enhance the development of high-quality comprehensive support systems to improve use of cancer genetic services and facilitate patient involvement in health care decisions. The long-term outcome of this program is the development and implementation of new models for systematic surveillance and detection of individuals at risk for hereditary cancer in Switzerland. Immediate outcomes are the assessment of current use of cancer genetic services and evaluation of the public health impact of HBOC and LS. The CASCADE study will document the needs of mutation-harboring families, including barriers and facilitators to accessing cancer genetic services, and will promote use of family history for genetic risk assessment. The study will also provide information for the acceptability of an intervention that will potentially increase genetic literacy, expand understanding of health care technologies, and reduce HBOC- and LS-related morbidity and mortality in Switzerland.
Acknowledgments
Funding for this study was provided by the University of Basel, Forschungsfonds in March 2016 to MK.
Abbreviations
- BRCA1
breast cancer 1
- BRCA2
breast cancer 2
- CTU
Clinical Trial Unit
- FH
familial hypercholesterolemia
- HBOC
hereditary breast/ovarian cancer syndrome
- HFTA
Federal Act on Human Genetic Testing
- LS
Lynch syndrome
- MMR
mismatch repair
- MSI-H
microsatellite instability
Footnotes
Conflicts of Interest: None declared.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015 Mar 01;136(5):E359–386. doi: 10.1002/ijc.29210. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang X, Bannon F, Ahn JV, Johnson CJ, Bonaventure A, Marcos-Gragera R, Stiller C, Azevedo ESG, Chen W, Ogunbiyi OJ, Rachet B, Soeberg MJ, You H, Matsuda T, Bielska-Lasota M, Storm H, Tucker TC, Coleman MP. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015 Mar 14;385(9972):977–1010. doi: 10.1016/S0140-6736(14)62038-9. http://linkinghub.elsevier.com/retrieve/pii/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antoniou A, Pharoah PDP, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles DM, Tang N, Olah E, Anton-Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tulinius H, Thorlacius S, Eerola H, Nevanlinna H, Syrjäkoski K, Kallioniemi O, Thompson D, Evans C, Peto J, Lalloo F, Evans DG, Easton DF. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003 May;72(5):1117–1130. doi: 10.1086/375033. https://linkinghub.elsevier.com/retrieve/pii/S0002-9297(07)60640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, Roeb W, Agnew KJ, Stray SM, Wickramanayake A, Norquist B, Pennington KP, Garcia RL, King M, Swisher EM. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A. 2011 Nov 01;108(44):18032–18037. doi: 10.1073/pnas.1115052108. http://www.pnas.org/cgi/pmidlookup?view=long&pmid=22006311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couch FJ, Nathanson KL, Offit K. Two decades after BRCA: setting paradigms in personalized cancer care and prevention. Science. 2014 Mar 28;343(6178):1466–1470. doi: 10.1126/science.1251827. http://europepmc.org/abstract/MED/24675953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E, Evans DG, Izatt L, Eeles RA, Adlard J, Davidson R, Eccles D, Cole T, Cook J, Brewer C, Tischkowitz M, Douglas F, Hodgson S, Walker L, Porteous ME, Morrison PJ, Side LE, Kennedy MJ, Houghton C, Donaldson A, Rogers MT, Dorkins H, Miedzybrodzka Z, Gregory H, Eason J, Barwell J, McCann E, Murray A, Antoniou AC, Easton DF, EMBRACE Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013 Jun 05;105(11):812–822. doi: 10.1093/jnci/djt095. [DOI] [PubMed] [Google Scholar]
- 7.NCI SEER Data, 1973-2014, 1973-2014. 2017. May 17, [2017-05-17]. https://seer.cancer.gov/data/
- 8.Balmaña J, Díez O, Rubio IT, Cardoso F, ESMO Guidelines Working Group BRCA in breast cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2011 Sep;22 Suppl 6:vi31–34. doi: 10.1093/annonc/mdr373. [DOI] [PubMed] [Google Scholar]
- 9.Christinat A, Pagani O. Practical aspects of genetic counseling in breast cancer: lights and shadows. Breast. 2013 Aug;22(4):375–382. doi: 10.1016/j.breast.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Anglian Breast Cancer Study Group Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Br J Cancer. 2000 Nov;83(10):1301–1308. doi: 10.1054/bjoc.2000.1407. doi: 10.1054/bjoc.2000.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King M, Marks JH, Mandell JB, New York Breast Cancer Study Group Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003 Oct 24;302(5645):643–646. doi: 10.1126/science.1088759. http://www.sciencemag.org/cgi/pmidlookup?view=long&pmid=14576434. [DOI] [PubMed] [Google Scholar]
- 12.Whittemore AS, Gong G, John EM, McGuire V, Li FP, Ostrow KL, Dicioccio R, Felberg A, West DW. Prevalence of BRCA1 mutation carriers among U.S. non-Hispanic Whites. Cancer Epidemiol Biomarkers Prev. 2004 Dec;13(12):2078–2083. http://cebp.aacrjournals.org/cgi/pmidlookup?view=long&pmid=15598764. [PubMed] [Google Scholar]
- 13.Bouchardy C, Lorez M, Arndt V. Schweizer Krebsbulletin; 35 (Auflage 4200) 2015. Jun 15, [2017-08-31]. Effects of age and stage on breast cancer survival in Switzerland http://www.zora.uzh.ch/id/eprint/117137/
- 14.Schoumacher F, Glaus A, Mueller H, Eppenberger U, Bolliger B, Senn HJ. BRCA1/2 mutations in Swiss patients with familial or early-onset breast and ovarian cancer. Swiss Med Wkly. 2001 Apr 21;131(15-16):223–226. doi: 10.4414/smw.2001.09677. [DOI] [PubMed] [Google Scholar]
- 15.Giardiello FM, Allen JI, Axilbund JE, Boland CR, Burke CA, Burt RW, Church JM, Dominitz JA, Johnson DA, Kaltenbach T, Levin TR, Lieberman DA, Robertson DJ, Syngal S, Rex DK. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-society Task Force on colorectal cancer. Am J Gastroenterol. 2014 Aug;109(8):1159–1179. doi: 10.1038/ajg.2014.186. [DOI] [PubMed] [Google Scholar]
- 16.Bonis P, Trikalinos T, Chung M, Chew P, Ip S, DeVine D, Lau J. Hereditary nonpolyposis colorectal cancer: diagnostic strategies and their implications. Evid Rep Technol Assess (Full Rep) 2007 May;(150):1–180. [PMC free article] [PubMed] [Google Scholar]
- 17.Bellcross CA, Bedrosian SR, Daniels E, Duquette D, Hampel H, Jasperson K, Joseph DA, Kaye C, Lubin I, Meyer LJ, Reyes M, Scheuner MT, Schully SD, Senter L, Stewart SL, St PJ, Westman J, Wise P, Yang VW, Khoury MJ. Implementing screening for Lynch syndrome among patients with newly diagnosed colorectal cancer: summary of a public health/clinical collaborative meeting. Genet Med. 2012 Jan;14(1):152–162. doi: 10.1038/gim.0b013e31823375ea. http://europepmc.org/abstract/MED/22237445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW, American COG. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015 Feb;110(2):223–262; quiz 263. doi: 10.1038/ajg.2014.435. http://europepmc.org/abstract/MED/25645574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Preventive Services Task Force. Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, García FAR, Gillman MW, Harper DM, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Owens DK, Phillips WR, Phipps MG, Pignone MP, Siu AL. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016 Jun 21;315(23):2564–2575. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 20.Kladny J, Lubinski J. Lynch syndrome (HNPCC) Hered Cancer Clin Pract. 2008 Jun 15;6(2):99–102. doi: 10.1186/1897-4287-6-2-99. http://europepmc.org/abstract/MED/19804605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch HT, Snyder CL, Shaw TG, Heinen CD, Hitchins MP. Milestones of Lynch syndrome: 1895-2015. Nat Rev Cancer. 2015 Dec;15(3):181–194. doi: 10.1038/nrc3878. [DOI] [PubMed] [Google Scholar]
- 22.Umar A, Boland CR, Terdiman JP, Syngal S, de LCA, Rüschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R, Hamilton SR, Hiatt RA, Jass J, Lindblom A, Lynch HT, Peltomaki P, Ramsey SD, Rodriguez-Bigas MA, Vasen HFA, Hawk ET, Barrett JC, Freedman AN, Srivastava S. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004 Feb 18;96(4):261–268. doi: 10.1093/jnci/djh034. http://europepmc.org/abstract/MED/14970275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med. 2009 Jan;11(1):42–65. doi: 10.1097/GIM.0b013e31818fa2db. http://europepmc.org/abstract/MED/19125127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S, Wang W, Lee S, Nafa K, Lee J, Romans K, Watson P, Gruber SB, Euhus D, Kinzler KW, Jass J, Gallinger S, Lindor NM, Casey G, Ellis N, Giardiello FM, Offit K, Parmigiani G, Colon Cancer Family Registry Prediction of germline mutations and cancer risk in the Lynch syndrome. JAMA. 2006 Sep 27;296(12):1479–1487. doi: 10.1001/jama.296.12.1479. http://europepmc.org/abstract/MED/17003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalady MF, Heald B. Diagnostic Approach to Hereditary Colorectal Cancer Syndromes. Clin Colon Rectal Surg. 2015 Dec;28(4):205–214. doi: 10.1055/s-0035-1564432. http://europepmc.org/abstract/MED/26664327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynch JA, Venne V, Berse B. Genetic tests to identify risk for breast cancer. Semin Oncol Nurs. 2015 May;31(2):100–107. doi: 10.1016/j.soncn.2015.02.007. http://europepmc.org/abstract/MED/25951739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douma KFL, Meiser B, Kirk J, Mitchell G, Saunders C, Rahman B, Sousa MS, Barlow-Stewart K, Gleeson M, Tucker K. Health professionals' evaluation of delivering treatment-focused genetic testing to women newly diagnosed with breast cancer. Fam Cancer. 2015 Jun;14(2):265–272. doi: 10.1007/s10689-014-9770-z. [DOI] [PubMed] [Google Scholar]
- 28.Clain E, Trosman JR, Douglas MP, Weldon CB, Phillips KA. Availability and payer coverage of BRCA1/2 tests and gene panels. Nat Biotechnol. 2015 Sep;33(9):900–902. doi: 10.1038/nbt.3322. http://europepmc.org/abstract/MED/26348951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayme A, Viassolo V, Rapiti E, Fioretta G, Schubert H, Bouchardy C, Chappuis PO, Benhamou S. Determinants of genetic counseling uptake and its impact on breast cancer outcome: a population-based study. Breast Cancer Res Treat. 2014 Apr;144(2):379–389. doi: 10.1007/s10549-014-2864-3. [DOI] [PubMed] [Google Scholar]
- 30.Schmeler KM, Lynch HT, Chen L, Munsell MF, Soliman PT, Clark MB, Daniels MS, White KG, Boyd-Rogers SG, Conrad PG, Yang KY, Rubin MM, Sun CC, Slomovitz BM, Gershenson DM, Lu KH. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006 Jan 19;354(3):261–269. doi: 10.1056/NEJMoa052627. [DOI] [PubMed] [Google Scholar]
- 31.Le-Petross HT, Whitman GJ, Atchley DP, Yuan Y, Gutierrez-Barrera A, Hortobagyi GN, Litton JK, Arun BK. Effectiveness of alternating mammography and magnetic resonance imaging for screening women with deleterious BRCA mutations at high risk of breast cancer. Cancer. 2011 Sep 01;117(17):3900–7. doi: 10.1002/cncr.25971. doi: 10.1002/cncr.25971. [DOI] [PubMed] [Google Scholar]
- 32.Caiata-Zufferey M. Genetically at-risk status and individual agency. A qualitative study on asymptomatic women living with genetic risk of breast/ovarian cancer. Soc Sci Med. 2015 May;132:141–148. doi: 10.1016/j.socscimed.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 33.Caiata-Zufferey M, Pagani O, Cina V, Membrez V, Taborelli M, Unger S, Murphy A, Monnerat C, Chappuis PO. Challenges in managing genetic cancer risk: a long-term qualitative study of unaffected women carrying BRCA1/BRCA2 mutations. Genet Med. 2015 Sep;17(9):726–732. doi: 10.1038/gim.2014.183. [DOI] [PubMed] [Google Scholar]
- 34.Howell D, Hack TF, Oliver TK, Chulak T, Mayo S, Aubin M, Chasen M, Earle CC, Friedman AJ, Green E, Jones GW, Jones JM, Parkinson M, Payeur N, Sabiston CM, Sinclair S. Models of care for post-treatment follow-up of adult cancer survivors: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2012 Dec;6(4):359–371. doi: 10.1007/s11764-012-0232-z. [DOI] [PubMed] [Google Scholar]
- 35.IOM . Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: National Academies Press; 2013. [2017-09-07]. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2013/Quality-Cancer-Care/qualitycancercare_rb.pdf . [PubMed] [Google Scholar]
- 36.Blandy C, Chabal F, Stoppa-Lyonnet D, Julian-Reynier C. Testing participation in BRCA1/2-positive families: initiator role of index cases. Genet Test. 2003;7(3):225–233. doi: 10.1089/109065703322537241. [DOI] [PubMed] [Google Scholar]
- 37.Suthers GK, Armstrong J, McCormack J, Trott D. Letting the family know: balancing ethics and effectiveness when notifying relatives about genetic testing for a familial disorder. J Med Genet. 2006 Aug;43(8):665–670. doi: 10.1136/jmg.2005.039172. http://europepmc.org/abstract/MED/16371501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurmankin AD, Domchek S, Stopfer J, Fels C, Armstrong K. Patients' resistance to risk information in genetic counseling for BRCA1/2. Arch Intern Med. 2005 Mar 14;165(5):523–529. doi: 10.1001/archinte.165.5.523. [DOI] [PubMed] [Google Scholar]
- 39.Tercyak KP, Demarco TA, Mars BD, Peshkin BN. Women's satisfaction with genetic counseling for hereditary breast-ovarian cancer: psychological aspects. Am J Med Genet A. 2004 Nov 15;131(1):36–41. doi: 10.1002/ajmg.a.30317. http://europepmc.org/abstract/MED/15389697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Federal Act on Human Genetic Testing. [2017-09-08]. https://www.admin.ch/opc/en/classified-compilation/20011087/201401010000/810.12.pdf .
- 41.Dekker N, Hermens RPMG, Elwyn G, van DWT, Nagengast FM, van DP, Salemink S, Adang E, van KJHJM, Ligtenberg MJL, Hoogerbrugge N. Improving calculation, interpretation and communication of familial colorectal cancer risk: protocol for a randomized controlled trial. Implement Sci. 2010 Jan 28;5:6. doi: 10.1186/1748-5908-5-6. https://implementationscience.biomedcentral.com/articles/10.1186/1748-5908-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graves K, Wenzel L, Schwartz M, Luta G, Wileyto P, Narod S, Peshkin B, Marcus A, Cella D, Emsbo S, Barnes D, Halbert C. Randomized controlled trial of a psychosocial telephone counseling intervention in BRCA1 and BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev. 2010 Mar;19(3):648–654. doi: 10.1158/1055-9965.EPI-09-0548. http://cebp.aacrjournals.org/cgi/pmidlookup?view=long&pmid=20200423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green MJ, Peterson SK, Baker MW, Harper GR, Friedman LC, Rubinstein WS, Mauger DT. Effect of a computer-based decision aid on knowledge, perceptions, and intentions about genetic testing for breast cancer susceptibility: a randomized controlled trial. JAMA. 2004 Jul 28;292(4):442–452. doi: 10.1001/jama.292.4.442. http://europepmc.org/abstract/MED/15280342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hooker GW, Leventhal K, DeMarco T, Peshkin BN, Finch C, Wahl E, Joines JR, Brown K, Valdimarsdottir H, Schwartz MD. Longitudinal changes in patient distress following interactive decision aid use among BRCA1/2 carriers: a randomized trial. Med Decis Making. 2011;31(3):412–421. doi: 10.1177/0272989X10381283. http://europepmc.org/abstract/MED/20876346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joseph G, Beattie MS, Lee R, Braithwaite D, Wilcox C, Metrikin M, Lamvik K, Luce J. Pre-counseling Education for Low Literacy Women at Risk of Hereditary Breast and Ovarian Cancer (HBOC): Patient Experiences Using the Cancer Risk Education Intervention Tool (CREdIT) J Genet Counsel. 2010 May 19;19(5):447–462. doi: 10.1007/s10897-010-9303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaphingst KA, Persky S, McCall C, Lachance C, Loewenstein J, Beall AC, Blascovich J. Testing the effects of educational strategies on comprehension of a genomic concept using virtual reality technology. Patient Educ Couns. 2009 Nov;77(2):224–230. doi: 10.1016/j.pec.2009.03.029. http://europepmc.org/abstract/MED/19409749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kardashian A, Fehniger J, Creasman J, Cheung E, Beattie MS. A Pilot study of the Sharing Risk Information Tool (ShaRIT) for Families with Hereditary Breast and Ovarian Cancer Syndrome. Hered Cancer Clin Pract. 2012 Apr 12;10(1):4. doi: 10.1186/1897-4287-10-4. https://hccpjournal.biomedcentral.com/articles/10.1186/1897-4287-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mancini J, Noguès C, Adenis C, Berthet P, Bonadona V, Chompret A, Coupier I, Eisinger F, Fricker J, Gauthier-Villars M, Lasset C, Lortholary A, N'Guyen TD, Vennin P, Sobol H, Stoppa-Lyonnet D, Julian-Reynier C. Impact of an information booklet on satisfaction and decision-making about BRCA genetic testing. Eur J Cancer. 2006 May;42(7):871–881. doi: 10.1016/j.ejca.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 49.Tiller K, Meiser B, Gaff C, Kirk J, Dudding T, Phillips K, Friedlander M, Tucker K. A randomized controlled trial of a decision aid for women at increased risk of ovarian cancer. Med Decis Making. 2006;26(4):360–372. doi: 10.1177/0272989X06290486. [DOI] [PubMed] [Google Scholar]
- 50.Wakefield CE, Meiser B, Homewood J, Peate M, Taylor A, Lobb E, Kirk J, Young M, Williams R, Dudding T, Tucker K, AGenDA Collaborative Group A randomized controlled trial of a decision aid for women considering genetic testing for breast and ovarian cancer risk. Breast Cancer Res Treat. 2008 Jan;107(2):289–301. doi: 10.1007/s10549-007-9539-2. [DOI] [PubMed] [Google Scholar]
- 51.Wang C, Gonzalez R, Milliron KJ, Strecher VJ, Merajver SD. Genetic counseling for BRCA1/2: a randomized controlled trial of two strategies to facilitate the education and counseling process. Am J Med Genet A. 2005 Apr 01;134A(1):66–73. doi: 10.1002/ajmg.a.30577. [DOI] [PubMed] [Google Scholar]
- 52.Grosse SD, Rogowski WH, Ross LF, Cornel MC, Dondorp WJ, Khoury MJ. Population screening for genetic disorders in the 21st century: evidence, economics, and ethics. Public Health Genomics. 2010;13(2):106–115. doi: 10.1159/000226594. [DOI] [PubMed] [Google Scholar]
- 53.Hampel H. Genetic counseling and cascade genetic testing in Lynch syndrome. Fam Cancer. 2016 Jul;15(3):423–427. doi: 10.1007/s10689-016-9893-5. [DOI] [PubMed] [Google Scholar]
- 54.Khoury MJ, Evans JP. A public health perspective on a national precision medicine cohort: balancing long-term knowledge generation with early health benefit. JAMA. 2015 Jun 02;313(21):2117–2118. doi: 10.1001/jama.2015.3382. http://europepmc.org/abstract/MED/26034952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galema-Boers JMH, Versmissen J, Roeters VLHWO, Dusault-Wijkstra JE, Williams M, Roeters VLJE. Cascade screening of familial hypercholesterolemia must go on. Atherosclerosis. 2015 Oct;242(2):415–417. doi: 10.1016/j.atherosclerosis.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 56.Samimi G, Bernardini MQ, Brody LC, Caga-Anan CF, Campbell IG, Chenevix-Trench G, Couch FJ, Dean M, de HJA, Domchek SM, Drapkin R, Spencer FH, Friedlander M, Gaudet MM, Harmsen MG, Hurley K, James PA, Kwon JS, Lacbawan F, Lheureux S, Mai PL, Mechanic LE, Minasian LM, Myers ER, Robson ME, Ramus SJ, Rezende LF, Shaw PA, Slavin TP, Swisher EM, Takenaka M, Bowtell DD, Sherman ME. Traceback: A Proposed Framework to Increase Identification and Genetic Counseling of BRCA1 and BRCA2 Mutation Carriers Through Family-Based Outreach. J Clin Oncol. 2017 Jul 10;35(20):2329–2337. doi: 10.1200/JCO.2016.70.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bouchardy C, Benhamou S, Fioretta G, Verkooijen HM, Chappuis PO, Neyroud-Caspar I, Castiglione M, Vinh-Hung V, Vlastos G, Rapiti E. Risk of second breast cancer according to estrogen receptor status and family history. Breast Cancer Res Treat. 2011 May;127(1):233–241. doi: 10.1007/s10549-010-1137-z. [DOI] [PubMed] [Google Scholar]
- 58.Buehler Michelle, Tse Brian, Leboucq Alix, Jacob Francis, Caduff Rosmarie, Fink Daniel, Goldstein Darlene R, Heinzelmann-Schwarz Viola. Meta-analysis of microarray data identifies GAS6 expression as an independent predictor of poor survival in ovarian cancer. Biomed Res Int. 2013;2013:238284. doi: 10.1155/2013/238284. doi: 10.1155/2013/238284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Decollogne S, Joshi S, Chung SA, Luk PP, Yeo RX, Nixdorf S, Fedier A, Heinzelmann-Schwarz V, Hogg PJ, Dilda PJ. Alterations in the mitochondrial responses to PENAO as a mechanism of resistance in ovarian cancer cells. Gynecol Oncol. 2015 Aug;138(2):363–371. doi: 10.1016/j.ygyno.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 60.Genevay M, Benusiglio PR, Hutter P, Chappuis PO. [Lynch syndrome: when pathologist and clinician have the opportunity to reduce the risk of developing cancer] Rev Med Suisse. 2011 Jul 27;7(303):1502–1506. [PubMed] [Google Scholar]
- 61.Heinimann K. Toward a molecular classification of colorectal cancer: the role of microsatellite instability status. Front Oncol. 2013 Oct 31;3:272. doi: 10.3389/fonc.2013.00272. doi: 10.3389/fonc.2013.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henry C, Llamosas E, Knipprath-Meszaros A, Schoetzau A, Obermann E, Fuenfschilling M, Caduff R, Fink D, Hacker N, Ward R, Heinzelmann-Schwarz V, Ford C. Targeting the ROR1 and ROR2 receptors in epithelial ovarian cancer inhibits cell migration and invasion. Oncotarget. 2015 Nov 24;6(37):40310–40326. doi: 10.18632/oncotarget.5643. http://www.impactjournals.com/oncotarget/misc/linkedout.php?pii=5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kempers MJE, Kuiper RP, Ockeloen CW, Chappuis PO, Hutter P, Rahner N, Schackert HK, Steinke V, Holinski-Feder E, Morak M, Kloor M, Büttner R, Verwiel ETP, van KJH, Nagtegaal ID, Goossens M, van DPRS, Niessen RC, Sijmons RH, Kluijt I, Hogervorst FBL, Leter EM, Gille JJP, Aalfs CM, Redeker EJW, Hes FJ, Tops CMJ, van NBPM, van GME, Gómez GEB, Eccles DM, Bunyan DJ, Syngal S, Stoffel EM, Culver JO, Palomares MR, Graham T, Velsher L, Papp J, Oláh E, Chan TL, Leung SY, van KAG, Kiemeney LALM, Hoogerbrugge N, Ligtenberg MJL. Risk of colorectal and endometrial cancers in EPCAM deletion-positive Lynch syndrome: a cohort study. Lancet Oncol. 2011 Jan;12(1):49–55. doi: 10.1016/S1470-2045(10)70265-5. http://europepmc.org/abstract/MED/21145788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manegold-Brauer G, Bellin AK, Tercanli S, Lapaire O, Heinzelmann-Schwarz V. The special role of ultrasound for screening, staging and surveillance of malignant ovarian tumors: distinction from other methods of diagnostic imaging. Arch Gynecol Obstet. 2014 Mar;289(3):491–498. doi: 10.1007/s00404-013-3081-8. [DOI] [PubMed] [Google Scholar]
- 65.Schwab FD, Bürki N, Huang DJ, Heinzelmann-Schwarz V, Schmid SM, Vetter M, Schötzau A, Güth U. Impact of breast cancer family history on tumor detection and tumor size in women newly-diagnosed with invasive breast cancer. Fam Cancer. 2014 Mar;13(1):99–107. doi: 10.1007/s10689-013-9682-3. [DOI] [PubMed] [Google Scholar]
- 66.Vasen HFA, Blanco I, Aktan-Collan K, Gopie JP, Alonso A, Aretz S, Bernstein I, Bertario L, Burn J, Capella G, Colas C, Engel C, Frayling IM, Genuardi M, Heinimann K, Hes FJ, Hodgson SV, Karagiannis JA, Lalloo F, Lindblom A, Mecklin J, Møller Pal, Myrhoj T, Nagengast FM, Parc Y, Ponz DLM, Renkonen-Sinisalo L, Sampson JR, Stormorken A, Sijmons RH, Tejpar S, Thomas HJW, Rahner N, Wijnen JT, Järvinen HJ, Möslein G, Mallorca Group Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut. 2013 Jun;62(6):812–823. doi: 10.1136/gutjnl-2012-304356. http://gut.bmj.com/cgi/pmidlookup?view=long&pmid=23408351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verkooijen HM, Rapiti E, Fioretta G, Vinh-Hung V, Keller J, Benhamou S, Vlastos G, Chappuis PO, Bouchardy C. Impact of a positive family history on diagnosis, management, and survival of breast cancer: different effects across socio-economic groups. Cancer Causes Control. 2009 Nov;20(9):1689–1696. doi: 10.1007/s10552-009-9420-1. [DOI] [PubMed] [Google Scholar]
- 68.Eysenbach G, CONSORT-EHEALTH Group CONSORT-EHEALTH: improving and standardizing evaluation reports of Web-based and mobile health interventions. J Med Internet Res. 2011 Dec 31;13(4):e126. doi: 10.2196/jmir.1923. http://www.jmir.org/2011/4/e126/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katapodi MC, Duquette D, Yang JJ, Mendelsohn-Victor K, Anderson B, Nikolaidis C, Mancewicz E, Northouse LL, Duffy S, Ronis D, Milliron KJ, Probst-Herbst N, Merajver SD, Janz NK, Copeland G, Roberts S. Recruiting families at risk for hereditary breast and ovarian cancer from a statewide cancer registry: a methodological study. Cancer Causes Control. 2017 Mar;28(3):191–201. doi: 10.1007/s10552-017-0858-2. [DOI] [PubMed] [Google Scholar]
- 70.Katapodi MC, Northouse LL, Milliron KJ, Liu G, Merajver SD. Individual and family characteristics associated with BRCA1/2 genetic testing in high-risk families. Psychooncology. 2013 Jun;22(6):1336–1343. doi: 10.1002/pon.3139. [DOI] [PubMed] [Google Scholar]
- 71.Caiata-Zufferey M, Schulz PJ. Physicians' communicative strategies in interacting with Internet-informed patients: results from a qualitative study. Health Commun. 2012;27(8):738–749. doi: 10.1080/10410236.2011.636478. [DOI] [PubMed] [Google Scholar]
- 72.Morgan D, Krueger R, King J. Focus Group Kit. Thousand Oaks, CA: SAGE Publications; 1998. [Google Scholar]
- 73.Katapodi Mc, Northouse Ll, Schafenacker Am, Duquette D, Duffy Sa, Ronis Dl, Anderson B, Janz Nk, McLosky J, Milliron Kj, Merajver Sd, Duong Lm, Copeland G. Using a state cancer registry to recruit young breast cancer survivors and high-risk relatives: protocol of a randomized trial testing the efficacy of a targeted versus a tailored intervention to increase breast cancer screening. BMC Cancer. 2013 Mar 1;13(1):e1001940. doi: 10.1186/1471-2407-13-97. http://dx.plos.org/10.1371/journal.pmed.1001940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989 Dec 20;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 75.Rockhill B, Spiegelman D, Byrne C, Hunter DJ, Colditz GA. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001 Mar 07;93(5):358–366. doi: 10.1093/jnci/93.5.358. [DOI] [PubMed] [Google Scholar]
- 76.Michigan Department of Health and Human Services BRFS annual questionnaires. 2017. [2017-08-31]. http://www.michigan.gov/mdhhs/0,5885,7-339-71550_5104_5279_39424_39425-263911--,00.html .
- 77.Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, Cannady RS, Pratt-Chapman ML, Edge SB, Jacobs LA, Hurria A, Marks LB, LaMonte SJ, Warner E, Lyman GH, Ganz PA. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 2016 Feb 20;34(6):611–635. doi: 10.1200/JCO.2015.64.3809. [DOI] [PubMed] [Google Scholar]
- 78.Vickberg SMJ. The Concerns About Recurrence Scale (CARS): a systematic measure of women's fears about the possibility of breast cancer recurrence. Ann Behav Med. 2003;25(1):16–24. doi: 10.1207/S15324796ABM2501_03. [DOI] [PubMed] [Google Scholar]
- 79.Katapodi M, Jung M, Milliron K, Merajver S, Northouse L. Psychological distress of developing hereditary breast/ovarian cancer among female relatives who did not pursue BRCA1 or BRCA2 genetic testing. 11th International Conference on Psychosocial Aspects of Genetic Testing for Hereditary Cancer; April 5, 2009; Toronto, ON. 2009. pp. 23–2. [Google Scholar]
- 80.Katapodi MC, Munro ML, Pierce PF, Williams RA. Psychometric testing of the decisional conflict scale: genetic testing hereditary breast and ovarian cancer. Nurs Res. 2011;60(6):368–377. doi: 10.1097/NNR.0b013e3182337dad. http://europepmc.org/abstract/MED/22048556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brehaut JC, O'Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, Feldman-Stewart D. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281–292. doi: 10.1177/0272989X03256005. [DOI] [PubMed] [Google Scholar]
- 82.Carver CS. You want to measure coping but your protocol's too long: consider the brief COPE. Int J Behav Med. 1997;4(1):92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- 83.Bandura A. Human agency in social cognitive theory. Am Psychol. 1989 Sep;44(9):1175–1184. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]
- 84.Katapodi MC, Aouizerat BE. Do women in the community recognize hereditary and sporadic breast cancer risk factors? Oncol Nurs Forum. 2005 May 10;32(3):617–623. doi: 10.1188/05.ONF.617-623. [DOI] [PubMed] [Google Scholar]
- 85.Katapodi MC, DeFlon SL, Milliron KJ, Northouse LL, Merajver S. Knowledge of risk factors and modes of gene transmission among women at risk for hereditary breast and ovarian cancer syndrome. JCO. 2011 May 20;29(15_suppl):e12017. doi: 10.1200/jco.2011.29.15_suppl.e12017. [DOI] [Google Scholar]
- 86.Lewis FM, Hammond MA, Woods NF. The family's functioning with newly diagnosed breast cancer in the mother: the development of an explanatory model. J Behav Med. 1993 Aug;16(4):351–370. doi: 10.1007/BF00844777. [DOI] [PubMed] [Google Scholar]
- 87.Katapodi MC, Facione NC, Miaskowski C, Dodd MJ, Waters C. The influence of social support on breast cancer screening in a multicultural community sample. Oncol Nurs Forum. 2002 Jun;29(5):845–852. doi: 10.1188/02.ONF.845-852. [DOI] [PubMed] [Google Scholar]
- 88.Anderson B, McLosky J, Wasilevich E, Lyon-Callo S, Duquette D, Copeland G. Barriers and facilitators for utilization of genetic counseling and risk assessment services in young female breast cancer survivors. J Cancer Epidemiol. 2012;2012:298745. doi: 10.1155/2012/298745. doi: 10.1155/2012/298745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996 Mar;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 90.Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology. 2006 Jan;3(2):77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 91.Khoury MJ, Iademarco MF, Riley WT. Precision Public Health for the Era of Precision Medicine. Am J Prev Med. 2016 Mar;50(3):398–401. doi: 10.1016/j.amepre.2015.08.031. http://europepmc.org/abstract/MED/26547538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ademi Z, Watts GF, Pang J, Sijbrands EJG, van Bockxmeer FM, O'Leary P, Geelhoed E, Liew D. Cascade screening based on genetic testing is cost-effective: evidence for the implementation of models of care for familial hypercholesterolemia. J Clin Lipidol. 2014;8(4):390–400. doi: 10.1016/j.jacl.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 93.Sie AS, Mensenkamp AR, Adang EMM, Ligtenberg MJL, Hoogerbrugge N. Fourfold increased detection of Lynch syndrome by raising age limit for tumour genetic testing from 50 to 70 years is cost-effective. Ann Oncol. 2014 Oct;25(10):2001–2007. doi: 10.1093/annonc/mdu361. [DOI] [PubMed] [Google Scholar]
- 94.Snowsill T, Huxley N, Hoyle M, Jones-Hughes T, Coelho H, Cooper C, Frayling I, Hyde C. A systematic review and economic evaluation of diagnostic strategies for Lynch syndrome. Health Technol Assess. 2014 Sep;18(58):1–406. doi: 10.3310/hta18580. doi: 10.3310/hta18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ned RM, Sijbrands EJG. Cascade Screening for Familial Hypercholesterolemia (FH) PLoS Curr. 2011 May 23;3:RRN1238. doi: 10.1371/currents.RRN1238. doi: 10.1371/currents.RRN1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Watts GF, Gidding S, Wierzbicki AS, Toth PP, Alonso R, Brown WV, Bruckert E, Defesche J, Lin KK, Livingston M, Mata P, Parhofer KG, Raal FJ, Santos RD, Sijbrands EJG, Simpson WG, Sullivan DR, Susekov AV, Tomlinson B, Wiegman A, Yamashita S, Kastelein JJP, International FH Foundation Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation: executive summary. J Atheroscler Thromb. 2014;21(4):368–374. [PubMed] [Google Scholar]
- 97.Datta BN, McDowell IFW, Rees A. Integrating provision of specialist lipid services with cascade testing for familial hypercholesterolaemia. Curr Opin Lipidol. 2010 Aug;21(4):366–371. doi: 10.1097/MOL.0b013e32833c14e2. [DOI] [PubMed] [Google Scholar]
- 98.Finnie Rm. Cascade screening for familial hypercholesterolaemia in Scotland. The British Journal of Diabetes & Vascular Disease. 2010 Jun 21;10(3):123–125. doi: 10.1177/1474651409343245. [DOI] [Google Scholar]


