Abstract
The red-tailed Amazon parrot (Amazona brasiliensis) is a threatened species of psittacine bird that inhabit coastal regions of Brazil. In view of the threat of this species, the aim of this study was to perform a health evaluation in wild nestlings in Rasa Island, determining the prevalence of enterobacteria and infectious agents according to type of nest. Blood samples were collected from 64 birds and evaluated for antibodies of Chlamydia psittaci by commercial dot-blot ELISA. Cloacal and oropharyngeal swabs samples were collected from 23 birds from artificial wooden nests, 15 birds from PVC nests and 2 birds from natural nests for microbiological analysis. Swab samples were collected from 58 parrots for C. psittaci detection by PCR and from 50 nestlings for Avian Influenza, Newcastle Disease and West Nile viruses’ detection analysis by real-time RT-PCR. Ten bacterial genera and 17 species were identified, and the most prevalent were Escherichia coli and Klebsiella oxytoca. There was no influence of the type of nest in the nestlings’ microbiota. All samples tested by ELISA and PCR were negative. There is currently insufficient information available about the health of A. brasiliensis and data of this study provide a reference point for future evaluations and aid in conservation plans.
Keywords: Avian influenza virus, Chlamydia psittaci, Microbiology, Newcastle disease, West Nile virus
Introduction
There is a wider need for greater effort in research on disease in wild bird populations, especially in threatened species.1 Health monitoring of these species should be encouraged being that, at local scales, infectious disease is a common driver of population declines.2 Moreover, wild birds may carry or be infected with microorganisms that can affect their health and the health of livestock, pet animals and human beings.3
The red-tailed Amazon parrot (Amazona brasiliensis) is listed as vulnerable according to the International Union for the Conservation of Nature Red List of Threatened Species.4 This species is endemic to the Atlantic Forest, inhabiting southern coastal regions of São Paulo, Paraná and northern Santa Catarina in a narrow coastal strip.5 Breeding areas are mostly located in small estuarine islands, and these facts make the parrot especially vulnerable to environmental disturbance.6
Since 1997, the Society for Wildlife Research and Environmental Education (SPVS) has developed the red-tailed Amazon Conservation Project in an attempt to minimize population decline.7 Environmental modification by human activities in Rasa Island destroyed many natural nests and the lack of tree cavities can limit the population growth of parrots. One of the efforts of SPVS is to build artificial nests made from wood (Fig. 1A) and polyvinyl chloride (PVC) (Fig. 1B), and install them next to the lost natural nests in large native trees that have the potential to become future natural shelters along the coast of Paraná. Since 2003, the artificial nests have been widely used by the parrots for breeding.8
Fig. 1.
Red-tailed Amazon parrot in a wooden (A) and polyvinyl chloride (B) nest in Rasa Island, Paraná, Brazil.
In the case of the threatened red-tailed Amazon population, a health study is essential to address disease risk and to establish normal parameters previously unavailable, assisting the conservation of this species.9 It would be also important to differentiate the parrots’ microbiota in the different types of nests, since the avian gastrointestinal microbiota can be affected by environment.10 No microbiological studies comparing wild birds from different nests under same conditions are available in the literature for parrot species.
A few studies reporting health status of wild psittacine species in southern Brazil have been described in the literature.9, 11 Chlamydiosis is one of the main infectious diseases for the order Psittaciformes because this group has the highest positivity rate (45%) among birds.12 Avian influenza virus (AIV), Newcastle disease virus (NDV), and West Nile virus (WNV) are three of the most important viral diseases in industrial aviculture and have a significant burden to public health.13, 14, 15 Besides holding several bird migratory routes, Brazil is considered free from high pathogenic viruses of these viral diseases, and monitoring wild birds is essential to analyze virus circulation, because the risk of introduction is always present. Until now, wild red-tailed Amazon parrot populations in Brazil had never been evaluated for such viruses.
In view of the limited information available on the species, the aim of this study was to perform a health evaluation in wild A. brasiliensis nestlings in Rasa Island, Paraná, Brazil, by assessing the cloacal and oropharyngeal microbiota according to type of nest; and by evaluating the presence of Chlamydia psittaci, AIV, NDV-1 and -2 and WNV.
Material and methods
Study area
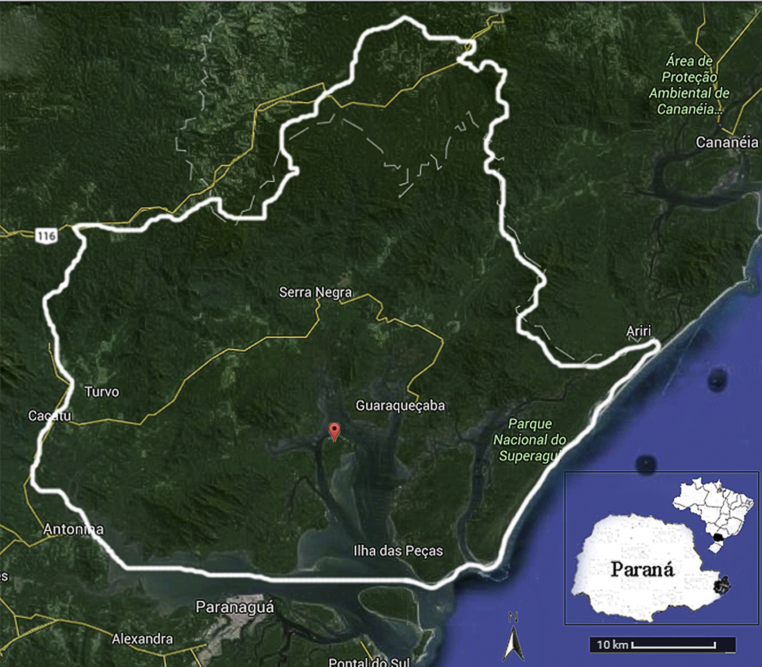
This study was approved by the Animal Use Ethics Committee of the Agricultural Sciences Campus of the Federal University of the State of Paraná, Southern Brazil (protocol number 050/2013) and by the SISBIO (number 41035-1). Sample collection was performed in Rasa Island, located in the Environmental Protection Area of Guaraqueçaba, Paraná, Brazil (Fig. 2). It is a protected area with an extensive area of Atlantic forest, consisting of estuaries, islands, mangrove forests, plains, mountains and plateaus where the parrot breeds.
Fig. 2.
Map of the Environmental Protection Area of Guaraqueçaba (white border) in the state of Paraná, Brazil. Rasa Island in the red dot. Figure adapted.37
Sampling
Sample collection of A. brasiliensis was carried out concomitantly with the monitoring of nestlings during the 2013/2014 breeding season in five field expeditions (December to February). The nests on the trees were accessed using climbing equipment and ladders. The birds were taken from the nest and carefully sampled. The age was estimated, the nestlings were weighed, and immediately put back into their nests.
Blood samples were collected from nestlings from the superficial ulnar vein using 1 mL sterile syringes pretreated with 1000 IU sodium heparin for antibody test. The samples were placed in tubes and refrigerated for up to 24 h. The tubes were centrifuged for 5 min to obtain plasma, which was frozen at −40 °C until analysis for C. psittaci.
Cloacal and oropharyngeal swab samples were collected from nestlings for microbiological analysis and kept refrigerated in Stuart transport medium up to 48 h until analysis in the laboratory. Cloacal and oropharyngeal swab samples were collected for C. psittaci detection by PCR in microtubes containing 1.0 mL of PBS pH 7.4, and for AIV, NDV and WNV detection by real time RT-PCR (rRT-PCR) in 2 mL microtubes containing antifungals, antibiotics and glycerol. All samples were kept frozen at −40 °C until analysis in the laboratory.
Microbiological analyses
Samples were plated on brain heart infusion and incubated for 24 h at 37 °C. Subsequently, they were stored in 30% glycerol solution at −20 °C. When thawed, the samples were inoculated by streaking method on blood and MacConkey's agar media and incubated at 37 °C for 24 h for the bacteria grow. Later, the cultured bacteria were picked up for the preparation of smear, stained with Gram's stain and examined under microscope for staining and morphological characterization of the isolates. Catalase test were also performed in the cultured bacteria. Each pure colony was inoculated in triple sugar iron agar by stabbing through the center of the medium to the bottom of the tube and then streaking the surface of the agar slant and incubated at 37 °C for 24 h, for detecting carbohydrate fermentation and production of H2S and gas. Finally, the fermenting colonies were inoculated in an enterobacteria kit (Newprov – Pinhais, Paraná, Brazil) for biochemical tests at 37 °C, examined at 24 and 48 h of incubation.
The kit provides the following biochemical tests: tryptophan deaminase reaction, hydrogen sulfide production, glucose fermentation, gas production from glucose, l-Lysine decarboxylation, indole production, decarboxylation of ornithine, motility, citrate as the sole source of carbon, and rhamnose fermentation. Identification of bacteria was performed according to manufacturer's instructions and the literature.16
Antibody tests
The presence of antibodies for C. psittaci was performed using a modified ELISA test according to manufacturer's instructions (ImmunoComb Avian Chlamydophila psittaci Antibody Test Kit – Biogal Galed Labs., Israel).
Conventional PCR
C. psittaci DNA analysis by conventional PCR was performed. Swab samples were vortexed for 2 min and centrifuged at 20,000 g for 30 min at 8 °C. The supernatant was discarded and DNA extraction was performed using a DNA purification kit (NucleoSpin Tissue – Macherey-Nagel, Germany) according to manufacturer's instruction.
The cycling was performed with a program of 5 min at 94 °C; 40 cycles at 94 °C for 1 min, 50 °C for 1 min and 72 °C for 2 min; and a final extension at 72 °C for 10 min, producing a 300-bp fragment.17 Positive and negative controls were included and the PCR products were analyzed by electrophoresis on 1.5% agarose gels stained with GelRed (Biotium).
Real-time RT-PCR
One step rRT-PCR was carried out for detection of AIV, NDV and WNV. RNA extraction was performed using 150 μL of swab samples, in which were added 700 μL of NucliSENS easyMAG Lysis buffer (NucliSENS Iso kit – Biomerieux). After 10 min of reaction at room temperature, 30 μL of magnetic silica was added in the solution, and the whole extraction process was carried out in automated equipment (NucliSENS Iso kit – Biomerieux) according to manufacturer's instructions. Amplification reactions for AIV detection and detection of class I and II NDV using a multiplex rRT-PCR assay were performed.13, 15 Specific primers and probes were used for WNV detection.14
The cycling was performed in an ABI 7300 PCR System (Applied Biosystems) with a program of 45 °C for 20 min (reverse transcription); 95 °C for 10 min; 40 cycles of 95 °C for 15 s and 60 °C for 45 s.
Statistical analysis
An ANOVA analysis (at P < 0.05) was carried out to determine the influence of the type of nest on prevalence of bacteria in the nestlings by using Portal Action software. All data were verified for normal distribution by the Shapiro–Wilk test.
Results
A total of 74 nestlings were sampled from 38 artificial nests (21 wooden and 17 PVC) and two natural nests (representing 5% of the total). The 74 birds had estimated age between 25 and 56 days (averaging 42 days) and weighed between 275 and 540 g (averaging 420 g).
Cloacal and oropharyngeal swab samples were collected from 40 nestlings for microbiological analysis: 23 individuals from 12 artificial wooden nests, 15 parrots from 10 PVC nests and two parrots from two natural nests. An average of two nestlings per nest was observed. In the same nest, three birds were sampled in three artificial wooden and one PVC nest; two birds were sampled in the same nest in five artificial wooden and three PVC nests; in all other nests just one nestling was sampled per nest.
Cloacal and oropharyngeal swab samples were collected from 58 nestlings for C. psittaci detection and from 50 nestlings for AIV, NDV and WNV detection.
One hundred eighteen bacterial colonies were isolated from 73 samples of 40 nestlings, totaling 91.25% (73/80) of positive samples for enterobacteria. One species of gram-positive bacteria, Staphylococcus sp., was isolated from two parrots in artificial wooden nests. Colonies were not isolated from four cloacal samples of parrots in PVC nests and from two oropharyngeal samples and one cloacal sample of parrots in artificial wooden nests.
The prevalence of bacterial species obtained from cloacal and oropharyngeal samples according to the nest can be observed in Table 1. There was no influence of the type of artificial nest in the birds’ microbiota (P < 0.05). The nestlings in the natural nests were not compared because of the small number of samples.
Table 1.
Prevalence of Enterobacteriaceae isolated from cloacal and oropharyngeal swab samples of wild red-tailed Amazon parrot (Amazona brasiliensis) nestlings in Rasa Island, Paraná, Brazil, according to the nest.
| Bacterial species | Prevalence in nestlings from artificial wooden nest |
Prevalence in nestlings from PVC nest |
Prevalence in nestlings from natural nest |
|||
|---|---|---|---|---|---|---|
| Cloacal samples | Oropharyngeal samples | Cloacal samples | Oropharyngeal samples | Cloacal samples | Oropharyngeal samples | |
| Escherichia coli | 72.7% (16/22) | 19.0% (4/21) | 72.7% (8/11) | 46.7% (7/15) | 100.0% (2/2) | 100.0% (2/2) |
| Escherichia fergusonii | 0 | 14.3% (3/21) | 0 | 20.0% (3/15) | 0 | 0 |
| Klebsiella oxytoca | 22.7% (5/22) | 23.8% (5/21) | 18.2% (2/11) | 20.0% (3/15) | 50.0% (1/2) | 100.0% (2/2) |
| Klebsiella pneumoniae | 9.1% (2/22) | 23.8% (5/21) | 0 | 20.0% (3/15) | 50.0% (1/2) | 0 |
| Proteus mirabilis | 13.6% (3/22) | 14.3% (3/21) | 0 | 13.3% (2/15) | 0 | 0 |
| Proteus vulgaris | 0 | 0 | 9.1% (1/11) | 0 | 0 | 0 |
| Citrobacter diversus (koseri) | 0 | 0 | 9.1% (1/11) | 0 | 0 | 0 |
| Citrobacter werkmanii | 0 | 4.8% (1/21) | 0 | 0 | 0 | 0 |
| Citrobacter freundii | 0 | 0 | 0 | 6.7% (1/15) | 0 | 0 |
| Enterobacter aerogenes | 22.7% (5/22) | 19.0% (4/21) | 9.1% (1/11) | 13.3% (2/15) | 0 | 0 |
| Enterobacter cloacae | 4.5% (1/22) | 0 | 18.2% (2/11) | 0 | 0 | 50.0% (1/2) |
| Enterobacter gergoviae | 4.5%(1/22) | 0 | 0 | 0 | 0 | 0 |
| Enterobacter sakazakii | 4.5% (1/22) | 4.8% (1/21) | 0 | 0 | 0 | 0 |
| Serratia liquefaciens (group) | 13.6% (3/22) | 0 | 0 | 13.3% (2/15) | 50.0% (1/2) | 0 |
| Serratia rubidaea | 0 | 4.8% (1/21) | 0 | 0 | 0 | 0 |
| Morganella morgani | 0 | 4.8% (1/21) | 9.1% (1/11) | 0 | 0 | 0 |
| Hafnia alvei | 0 | 4.8% (1/21) | 0 | 6.7% (1/15) | 0 | 0 |
| Staphylococcus sp.* | 0 | 9.5% (2/21) | 0 | 0 | 0 | 0 |
| Kluyvera spp. | 0 | 0 | 0 | 6.7% (1/15) | 0 | 0 |
Family Staphylococcaceae.
All samples tested for antibody detection and by PCR for C. psittaci and rRT-PCR for AIV, NDV and WNV were negative.
Discussion
This study provides health baseline parameters for the red-tailed Amazon parrot in the state of Paraná, Brazil. The results indicate that the microbiota of wild A. brasiliensis nestlings typically have gram-negative bacteria in its composition. Some literature states that normal bacterial flora of parrots is composed predominantly by gram-positive bacilli,18 but the normality and significance of the presence of gram-negative bacteria is divergent, since some studies indicate that their presence does not imply disease.10, 11
The avian gastrointestinal microbiota can be affected by many factors such as diet, age, environment, antibiotic administration and infection with pathogenic organisms.10 Many microbiological studies include captive adult psittacine birds in the sample,18 and high percentage of gram-positive and low percentage of gram-negative bacteria can be found.10 In the present study, only wild parrot nestlings up to 56 days were sampled, showing high prevalence of gram-negative bacteria (91.25%). Young birds are still establishing their bacterial flora and the diversity of bacterial community increases when some avian species grow older.19 Immediately after hatching, the digestive system of birds is presumed to be sterile and subsequently either passively or actively will acquire microbiota from the environment, such as nesting environment and food.19, 20 In the case of parrots, the parents regurgitate food to their young, thus permitting a mode of vertical microbial transmission.20 The influence of age (adult and young) and environment (captive and wild) could explain the difference in the diversity of bacterial community between the wild nestlings sampled in the present study and others researches.
In Brazil, the detection of gram-negative bacteria has been reported in asymptomatic wild A. brasiliensis nestlings in Paraná and São Paulo coast.11, 21 In São Paulo coast, swab samples of 14 A. brasiliensis showed predominance of E. coli and Proteus mirabilis in both cloacal and oropharyngeal samples.21 Microbiological analyses of cloacal swab samples with high positivity (74.46%) for Enterobacteriaceae were observed in a previous study with 19 A. brasiliensis from four islands of the Environmental Protection Area of Guaraqueçaba, including Rasa Island.11 High prevalence of E. coli (84.2%) was obtained in this study,11 and Pseudomonas spp. (31.5%), Enterobacter spp. (26.3%), Proteus vulgaris (26.3%), Citrobacter spp (21.0%) and Staphylococcus spp. (5.2%) were also found. Similar species of bacterial colonies observed in the present study are consistent with the findings of these investigations, showing the normality of gram-negative bacteria in the microbiota of psittacine species.
All bacteria genera showed in Table 1 were observed in previous studies with psittacine species.11, 21, 22, 23, 24 These microorganisms probably compose the normal microflora of this avian family, and the microbiological profile of the nestlings sampled was within normality. Concerning the different bacteria species, to our knowledge, this is the first isolation of Escherichia fergusonii and Citrobacter werkmanii in a psittacine bird.
Generally, the Enterobacteriaceae are considered secondary pathogens, but can function as the primary pathogen in certain circumstances, depending on the virulence of the bacteria and the host response to infections. Among Citrobacter species, C. freunddi appears to be the most pathogenic of the group, and the other species are less commonly encountered in birds and do not appear to have great health relevance, as well as E. fergusonii, already isolated from asymptomatic Brazilian birds.25, 26 The genera Enterobacter, Hafnia, Serratia and Proteus are of low pathogenicity, and Morganella morganii and Kluyvera spp. are infrequent opportunistic pathogens in birds.25 Differently, the species E. coli, Klebsiella oxytoca, K. pneumonia and Staphylococcus spp. can cause primary or secondary disease in avian species and have been associated with disease in Amazon parrots.25 Pathogenicity and antimicrobial resistance tests could be used to assess the real risks of these bacteria to the red-tailed Amazon parrots’ health in future evaluations.
Concerning the nests, the excretions of the birds can accumulate over time, increasing exposure levels of nestlings to fecal bacteria. Different materials used to build nests probably provide different environments for wild nestlings, increasing or decreasing the presence of microorganisms, but no researches have yet tested this hypothesis.3 However, in the present study, there was no influence of the type of nest in the nestlings’ microbiota, and more analyses using nest samples should be encouraged to elucidate the influence of the material in bacterial diversity. This would help to choose the best nest and to guide management measures. Unfortunately, artificial nests are still needed in the Rasa Island, since it is a small breeding area (10.5 km2) for the parrots, and deforestation leads to a lack of sufficient natural cavities.
Analyses for antibody detection by ELISA and for DNA detection by PCR for C. psittaci were negative, as well as the analysis for RNA detection by rRT-PCR for AIV, NDV and WNV. The absence of pathogens such as C. psittaci can likely be justified by the isolation of the population studied, which mostly use islands for breeding, feeding and night rest.6 On the other hand, this isolation and limited distribution of the parrots could be concerning, since island endemic birds probably have been exposed to few pathogens,27 and emergence and introduction of diseases can affect the population dynamics and regulate its abundance.1 Pathogenic organisms have been involved in numerous declines of endemic species on islands.27 These facts reinforce the importance of establishing monitoring programs to allow early detection and prevent further spread of avian diseases.
Opportunely, the Environmental Protection Area of Guaraqueçaba is located within the most preserved area of continuous Atlantic forest in Brazil and is the largest conservation unit of the region. Much of the population of parrots is found in protected areas in Paraná, but the lack of supervision in the region enables the cutting of important tree species for reproduction, shelter and feeding of the parrot.28 Besides this, these birds probably have less anthropogenic interference, less contact with human beings and domestic animals, and suffer less stress by environmental disorders, when compared to birds from other areas such as Pantanal, where modifications in the habitat are largely due to the planting of pasture for cattle. In this region, C. psittaci was detected in 6.3% (2/32) of wild Amazona aestiva by semi-nested PCR.29 Environmental conditions are often a basic cause associated with disease emergence, persistence, and spread, and must be addressed to avoid disease.1
Unlike conditions in the Guaraqueçaba area, the red-tailed Amazon parrot still faces great adversity in the state of São Paulo because of urban growth, deforestation and removal of nestlings by humans for illegal trade, and because of the lack of protected areas and lack of supervision.5 These factors endanger the future of the species in this region and can affect their health. Sera examined from this species in the state of São Paulo using dotblot ELISA demonstrated 42.85% of 14 birds older than 10 days positive for antibodies to C. psittaci.21 But this high percentage of positive animals can also be due to maternal immunity, which can last up to 2–4 weeks after hatch.30 These results are not similar to the present study, in which all 64 nestlings serologically evaluated were negative for C. psittaci. This agent is considered endemic in Brazil and has been reported in captive parrots on outbreaks and wild prevalence studies.29, 31 In the state of Paraná, C. psittaci's DNA was detected in 1.2% of the samples from 117 free-living A. brasiliensis nestlings analyzed by semi-nested PCR,9 a low prevalence, which is in accordance with the present study.
The presence of WNV, AIV and NDV in parrots has not been detected in Brazil and is also consistent with the results of the present study. Psittacine birds vary in susceptibility for NDV, and some of these species have shed NDV intermittently for over 1 year and have been associated with introducing the virus into the poultry industry.32 The detection of AIV from psittacine species is rare, occurring in captive animals, which do not appear to play a major role in the epidemiology of the disease.33 Negative results of the present study may reinforce the little importance of these agents for psittacine species.
Concerning other countries, similar results to this study were observed in wild psittacine birds. Antibodies to AIV, NDV and C. psittaci has not been found in blue-fronted Amazon parrots (A. aestiva) in Bolivia.34 Serologic testing in wild parakeets (Aratinga weddellii and Brotogeris sanctithomae) in Peru were negative to antibodies for C. psittaci and NDV,35 and four species of wild Mexican parrots showed negative serologic tests for AIV and NDV.36
The results of this study indicate that the red-tailed Amazon population located in Rasa Island is not infected by C. psittaci, AIV, WNV or NDV, and the data obtained is supported by previous studies involving wild psittacine species. Concerning the bacteria observed more information and studies are necessary for establishing their potential as disease agents for the parrots. The microbiota of wild A. brasiliensis nestlings is predominantly composed of gram-negative bacteria and was not influenced by the type of artificial nest. Unfortunately, few natural nests were found in the study area to compare the microbiota of nestlings between artificial and natural nests. Data not previously described for the species was reported here, providing a reference point for future evaluations and interpretations of laboratory findings in conservation plans. Health monitoring in threatened free-living species is an important tool when it comes to avian conservation and should be increasingly encouraged.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
We gratefully acknowledge the Society for Wildlife Research and Environmental Education (SPVS) for logistics and technical support in Guaraqueçaba area and for the efforts to conserve the red-tailed Amazon parrot. We would like to thank J.V.B. Agottani for the ELISA test provided. Funding for this project was in part provided by the São Paulo Research Foundation – FAPESP (2011/13821-7), (2013/05485-2) and (2009/05994-9).
Associate Editor: João Araujo Jr.
References
- 1.Friend M., Mclean R.G., Dein F.J. Disease emergence in birds: challenges for the twenty-first century. Auk. 2001;118:290–303. [Google Scholar]
- 2.Heard M.J., Smith K.F., Ripp K.J. The threat of disease increases as species move toward extinction. Conserv Biol. 2013;27:1378–1388. doi: 10.1111/cobi.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benskin C.M.H., Wilson K., Jones K., Hartley I.R. Bacterial pathogens in wild birds: a review of the frequency and effects on infection. Biol Rev. 2009;84:349–373. doi: 10.1111/j.1469-185X.2008.00076.x. [DOI] [PubMed] [Google Scholar]
- 4.IUCN – International Union for Conservation of Nature . 2015. Red List of Threatened Species. Version 2015-4. Available from http://www.iucnredlist.org [accessed 03.04.2016] [Google Scholar]
- 5.Galetti M., Schunck F., Ribeiro M., Paiva A.A., Toledo R., Fonseca L. Distribuição e tamanho populacional do papagaio-de-cara-roxa Amazona brasiliensis no estado de São Paulo. Rev Bras Ornitol. 2006;14:239–247. [Google Scholar]
- 6.Sipinski E.A.B. Master Dissertation, Forest Sciences, Federal University of Paraná; Curitiba, Brazil: 2003. O papagaio-de-cara-roxa (Amazona brasiliensis) na ilha Rasa, PR – aspectos ecológicos e reprodutivos e relação com o ambiente. 74 pp. [Google Scholar]
- 7.Sipinski E.A.B., Abbud M.C., Sezerban R.M. Tendência populacional do papagaio-de-cara-roxa (Amazona brasiliensis) no litoral do estado do Paraná. Ornithol. 2014;6:136–143. [Google Scholar]
- 8.Abbud M.C. Master Dissertation, Ecology and Conservation, Federal University of Paraná; Curitiba, Brazil: 2013. Reprodução e conservação do papagaio-de-cara-roxa Amazona brasiliensis (Linnaeus 1758) (Aves: Psittacidae) no litoral norte do estado do Paraná. 63 pp. [Google Scholar]
- 9.Ribas J.M., Sipinski E.A.B., Serafini P.P., Ferreira V.L., Raso T.F., Pinto A.A. Chlamydophila psittaci assessment in threatened Red-tailed Amazon (Amazona brasiliensis) parrots in Paraná, Brazil. Ornithology. 2014;6:144–147. [Google Scholar]
- 10.Flammer K., Drewes L.A. Species-related differences in the incidence of gram-negative bacteria isolated from the cloaca of clinically normal psittacine birds. Av Dis. 1988;32:79–83. [PubMed] [Google Scholar]
- 11.Serafini P.P., Meurer R., Biesdorf S.M., Sipinski E.A.B. O uso da microbiologia como ferramenta para a conservação de aves ameaçadas: dados preliminares para o papagaio-de-cara-roxa, Amazona brasiliensis (Aves: Psittacidae) no Paraná. Arq Ciênc Vet Zool. 2015;18:65–69. [Google Scholar]
- 12.Kaleta E.F., Taday E.M.A. Avian host range of Chlamydophila spp. based on isolation, antigen detection and serology. Av Pathol. 2003;32:435–461. doi: 10.1080/03079450310001593613. [DOI] [PubMed] [Google Scholar]
- 13.Kim L.M., Suarez D.L., Afonso C.L. Detection of a broad range of class I and II Newcastle disease viruses using a multiplex real-time reverse transcription polymerase chain reaction assay. J Vet Diagn Invest. 2008;20:414–425. doi: 10.1177/104063870802000402. [DOI] [PubMed] [Google Scholar]
- 14.Ometto T., Durigon E.L., Araujo J. West Nile virus surveillance Brazil, 2008–2010. Trans Royal Soc Trop Med Hyg. 2013;107:723–730. doi: 10.1093/trstmh/trt081. [DOI] [PubMed] [Google Scholar]
- 15.Araujo J., Junior S.M.A., Gaidet N. Avian Influenza Virus (H11N9) in migratory shorebirds wintering in the Amazon region, Brazil. PLoS ONE. 2014;9:1–10. doi: 10.1371/journal.pone.0110141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winn W.C., Allen S.D., Janda W.M. 6th ed. Guanabara Koogan; Rio de Janeiro: 2008. Koneman diagnóstico microbiológico: texto e atlas colorido. [Google Scholar]
- 17.Laroucau K., Souriau A., Rodolakis A. Improved sensitivity of PCR for Chlamydophila using pmp genes. Vet Microbiol. 2001;82:155–164. doi: 10.1016/s0378-1135(01)00381-9. [DOI] [PubMed] [Google Scholar]
- 18.Doneley R.J.T. Bacterial and parasitic diseases of parrots. Vet Clin: Ex An Pract. 2009;12:417–432. doi: 10.1016/j.cvex.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 19.van der Wielen P.W.J.V.D., Keuzenkamp D.A., Lipman L.J.A., Knapen F.V., Biesterveld S. Spatial and temporal variation of the intestinal bacterial community in commercially raised broiler chickens during growth. Microb Ecol. 2002;44(286):293. doi: 10.1007/s00248-002-2015-y. [DOI] [PubMed] [Google Scholar]
- 20.van Dongen W.F.D., White J., Brandl H.B. Age-related differences in the cloacal microbiota of a wild bird species. BMC Ecol. 2013;13:1–12. doi: 10.1186/1472-6785-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavalheiro M.L. Master Dissertation, Forest Science, Federal University of Paraná; Curitiba, Brazil: 1999. Qualidade do ambiente e características fisiológicas do papagaio-de-cara-roxa (Amazona brasiliensis) na Ilha Comprida – São Paul. 105 pp. [Google Scholar]
- 22.Loiko M.R., Abilheira F.A., Guedes N.R. Identificação da microbiota da orofaringe e cloaca em filhotes de arara-azul-grande (Anodorhyncus hyacinthinus) de vida livre do Pantanal – MS. Rev Inic Cient ULBRA. 2007;1:29–35. [Google Scholar]
- 23.Efstathion C.A. Master Dissertation, Biological Sciences, Charles E. Schmidt College of Science; 2011. Prevalence of antibiotic resistant commensal bacteria in endangered avian species. 48 pp. [Google Scholar]
- 24.Hidasi H.W., Neto J.H., Moraes D.M.C., Linhares G.F.C., Jayme V.S., Andrade M.A. Enterobacterial detection and Escherichia coli antimicrobial resistance in parrots seized from the illegal wildlife trade. J Zoo Wildl Med. 2013;44:1–7. doi: 10.1638/1042-7260-44.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Gerlach H. Bacteria. In: Ritchie B.W., Harrison G.J., Harrison L.R., editors. Avian Medicine: Principles and Application. Wingers Publishing; Vancouver: 1994. pp. 949–983. [Google Scholar]
- 26.Santos H.F., Flôres M.L., Lara V.M., Silva M.S., Battisti L., Lovato L.T. Microbiota cloacal aeróbia de cracídeos cativos no Rio Grande do Sul e sua susceptibilidade a antimicrobianos. Pesq Vet Bra. 2010;30:1077–1082. [Google Scholar]
- 27.Wikelski M., Foufopoulos J., Vargas H., Snell H. Galápagos birds and diseases: invasive pathogens as threats for island species. Ecol Soc. 2004;9:1–10. [Google Scholar]
- 28.Scherer-Neto P., Straube F.C. Amazona brasiliensis (Linnaeus, 1758) In: Machado A.B., Drummond G.M., Paglia A.P., editors. Livro vermelho da fauna brasileira ameaçada de extinção. 1st ed. Ministério do Meio Ambiente; Brasília: 2008. pp. 456–458. [Google Scholar]
- 29.Raso T.F., Seixas G.H.F., Guedes N.M.R., Pinto A.A. Chlamydophila psittaci in free-living Blue-fronted Amazon parrots (Amazona aestiva) and Hyacinth macaws (Anodorhynchus hyacinthinus) in the Pantanal of Mato Grosso do Sul, Brazil. Vet Microbiol. 2006;117:235–241. doi: 10.1016/j.vetmic.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 30.Bermudez A.J., Stewart-Brown B. Disease Prevention and Diagnosis. In: Saif Y.M., Fadly A.M., Glisson J.R., McDougald L.R., Nolan L.K., Swayne D.E., editors. 12th ed. Blackwell Publishing; Iowa: 2008. pp. 5–42. (Diseases of Poultry). [Google Scholar]
- 31.Raso T.F., Godoy S.N., Milanelo L. An outbreak of chlamydiosis in captive Blue-fronted Amazon parrots (Amazona aestiva) in Brazil. J Zoo Wildl Med. 2004;35:94–96. doi: 10.1638/02-090. [DOI] [PubMed] [Google Scholar]
- 32.OIE – World Organization for Animal Health . 2009. Technical Disease Cards – Newcastle Disease. Available from http://www.oie.int/animal-health-in-the-world/technical-disease-cards/ [accessed 3.4.16] [Google Scholar]
- 33.Perkins L.E.L., Swayne D.E. Varied pathogenicity of a Hong Kong – origin H5N1 Avian Influenza virus in four passerine species and budgerigars. Vet Pathol. 2003;40:14–24. doi: 10.1354/vp.40-1-14. [DOI] [PubMed] [Google Scholar]
- 34.Deem S.L., Noss A.J., Cuellar R.L., Karesh W.B. Health evaluation of free-ranging and captive blue-fronted amazon parrots (Amazona aestiva) in the Gran Chaco, Bolivia. J Zoo Wildl Med. 2005;36:598–605. doi: 10.1638/04094.1. [DOI] [PubMed] [Google Scholar]
- 35.Gilardi K.V.K., Lowentime L.J., Gilardi J.D., Munn C.A.A. A survey for selected viral, chlamydial, and parasitic diseases in wild dusky-headed parakeets (Aratinga weddelli) and tui parakeets (Brotogeris sanctithomae) in Peru. J Wildl Dis. 1995;31:523–528. doi: 10.7589/0090-3558-31.4.523. [DOI] [PubMed] [Google Scholar]
- 36.Stone E.G., Montiel-Parra G., Perez T.M. A survey of selected parasitic and viral pathogens in four species of Mexican parrots, Amazona autumnalis, Amazona oratrix, Amazona viridigenalis, and Rhynchopsitta pachyrhyncha. J Zoo Wildl Med. 2005;36:245–249. doi: 10.1638/04-026.1. [DOI] [PubMed] [Google Scholar]
- 37.IBAMA – Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis . 1995. Área de Proteção Ambiental de Guaraqueçaba – Plano de Gestão Ambiental, Curitiba, Brazil. Available from http://www.icmbio.gov.br/portal/images/stories/docs-planos-de-manejo/pm_apa_guaraquecaba.pdf [accessed 3.4.16] [Google Scholar]