Abstract
Neisseria gonorrhoeae is the agent of gonorrhea, a sexually transmitted infection with an estimate from The World Health Organization of 78 million new cases in people aged 15–49 worldwide during 2012. If left untreated, complications may include pelvic inflammatory disease and infertility. Antimicrobial treatment is usually effective; however, resistance has emerged successively through various molecular mechanisms for all the regularly used therapeutic agents throughout decades. Detection of antimicrobial susceptibility is currently the most critical aspect for N. gonorrhoeae surveillance, however poorly structured health systems pose difficulties. In this review, we compiled data from worldwide reports regarding epidemiology and antimicrobial resistance in N. gonorrhoeae, and highlight the relevance of the implementation of surveillance networks to establish policies for gonorrhea treatment.
Keywords: Neisseria gonorrhoeae, Surveillance, Resistance mechanisms
Neisseria gonorrhoeae infections: symptoms, surveillance and treatment
The gonococcal disease
N. gonorrhoeae is the etiological agent of gonorrhea, the second most frequently reported sexually transmitted infection (STI) in the world. This bacterium typically colonizes and infects the genital tract in men and women, but may be found in additional body sites such as the rectal and oropharyngeal mucosa, with or without clinically evident infection.1
Gonorrhea is usually symptomatic in men, most often as urethritis, with pain or burning sensation during urination, urethral discharge, and painful testicles. In contrast, women develop symptomatic gonococcal cervicitis less frequently, presenting a slight increase in vaginal discharge, and rare vaginal bleeding unrelated to periods, or pain and a burning sensation when urinating. The absence of symptoms in men and women can lead to sustained infections. Complicated gonorrhea may cause infertility.2
Surveillance programs around the world
A report from the World Health Organization (WHO) indicated the occurrence of 78 million new cases of gonococcal infection in people aged 15–49 worldwide during 2012.3 Despite the high rate of incidence, just a few countries, including the United States (U.S.), Canada, members of the European Union (EU), and Australia conduct broad surveillance programs on gonorrhea. Additionally, some countries from Latin-America and the Caribbean region (LAC), and countries from Western Pacific Region (WPR) and South East Asian Region (SEAR) joined the Gonococcal Antimicrobial Surveillance Program (GASP) that was conducted by WHO in the early 1990s. Since antimicrobial resistance is the main challenge associated with this microorganism, published reports emphasize the antimicrobial susceptibility profile of isolates. However, analysis of published data can be challenging, considering the breakpoints to define resistance vary across different surveillance programs. Regardless of this limitation, resistance is clearly an emerging phenomenon.
United States
In the U.S., the Centers for Disease Control and Prevention (CDC) supports the Gonococcal Isolate Surveillance Project (GISP). This program analyzes the first 25–30 N. gonorrhoeae isolates collected from men with gonococcal urethral-syndrome in sentinel laboratories located in five regions of the U.S. monthly. Surveillance includes analysis of demographic and clinical data, and antimicrobial susceptibility.4 In recent years, the southern region reported the highest rate of gonorrhea, reaching 131.4 cases per 100,000 individuals in 2014.5 The CDC estimates 820,000 new gonorrhea cases per year throughout the country.6
The STI Surveillance Network in the U.S. indicated higher incidence rates of gonorrhea in men who have sex with men (MSM) of any age, followed by men who have sex with women (MSW), and women, in 2014.5 The network also reported that the risk of gonococcal infection declines with age, with most cases occurring in individuals under the age of 24.5 In regards to antimicrobial resistance, GISP data has shown that nearly 30% of the isolates obtained from MSM, and 12% of those obtained from MSW were resistant to ciprofloxacin, with a minimum inhibitory concentration (MIC) ≥ 1 μg/mL in 2014.7 Irrespective of the sexual partners gender, in that same year, 0.7% of the isolates (n = 38) presented a decrease in cefixime susceptibility (MIC ≥ 0.25 μg/mL), and 2.5% showed azithromycin MIC alert values (MIC ≥ 2 μg/mL) (Table 1).7 Moreover, a genomic epidemiology study with 236 GISP isolates obtained in 2009–2010 has shown that an N. gonorrhoeae cluster with decreased susceptibility to extended-spectrum cephalosporins (ESC) spread predominantly among MSM during those years.8
Table 1.
Breakpoints or alert values adopted for testing susceptibility of Neisseria gonorrhoeae to key antimicrobials by CLSI, GISP, NSP, EUCAST and AGSP and selected resistance rates.
| Antimicrobial | MIC breakpoint or alert values (μg/mL)a |
Resistance or Reduced Susceptibility rates in the most recent reports of selected Gonococcal Surveillance Programs (GISP, 2014; NSP, 2014; EURO-GASP, 2013; AGSP, 2014) | ||||
|---|---|---|---|---|---|---|
| CLSI | GISPb | NSP | EUCAST/EURO-GASP | AGSP | ||
| Ciprofloxacin | ≥1.0 | ≥1.0 | ≥1.0 | >0.06 | ≥1.0 | GISP: 30% in MSM and 12% in MSW EURO-GASP: 53% AGSP: 36% |
| Ceftriaxone | S ≤ 0.25c | AV ≥ 0.125 | ≥0.125 | >0.125 | 0.06–0.125d | NSP: 3.1% AGSP: 54% (RS) |
| Cefixime | S ≤ 0.25c | AV ≥ 0.25 | ≥0.25 | >0.125 | ND | GISP: 0.7% (RS) NSP: 3.1% EURO-GASP: 4.7% |
| Azithromycin | ECV ≥ 2.0e | AV ≥ 2.0 | ≥2.0 | >0.5 | ≥1.0 | GISP: 2.5% NSP: 2.3% EURO-GASP: 5% AGSP: 2.5% |
Breakpoints define resistance, except otherwise described, adopted as reference by the Gonococcal Surveillance Programs listed.
GISP proposes minimum inhibitory concentration (MIC) alert values (AV) for ceftriaxone, cefixime and azithromycin.
CLSI does not propose resistance breakpoint for ceftriaxone and cefixime.
AGSP defines ceftriaxone decreased susceptibility for isolates with MIC = 0.06–0.125 μg/mL.
CLSI indicates that isolates with MIC ≥2.0 μg/mL for azithromycin have mutational and/or acquired resistance mechanisms, and defines as epidemiological cutoff value.
CLSI, Clinical Laboratory Standards Institution; S, susceptible; ECV, epidemiological cutoff value; ND, not determined; GISP, Gonococcal Isolate Surveillance Project; NSP, National Surveillance Program in Canada; EUCAST, European Committee on Antimicrobial Susceptibility Testing; EURO-GASP, European Gonococcal Antimicrobial Surveillance Program; AGSP, Australian Gonococcal Surveillance Program; MSM, men who have sex with men; MSW, men who have sex with women.
Canada
The National Surveillance Program (NSP) in Canada, implemented in 1985, provides data from different provinces across the country regarding N. gonorrhoeae antimicrobial susceptibility. Provincial public health laboratories (PL) send resistant isolates or isolates not submitted to antimicrobial susceptibility testing to the National Microbiology Laboratory (NML). In 2014, 2101 of 3089 N. gonorrhoeae isolates cultured in the PL were sent to NML. Interestingly, contrasting with other countries, NSP data showed a consistent diminishing trend in the ESC reduced susceptibility rates (from 7.6% in 2011 to 3.1% in 2014). However, regarding azithromycin, resistance rates rose from 0.4% in 2011 to 2.3% in 2014.9
Europe
In Europe, the extent of N. gonorrhoeae surveillance varies in different countries, according to national public health policies. However, data compiled from 21 countries composing the European GASP (Euro-GASP) conducted by the European CDC (ECDC) reported 50,001 cases in 2013.10 Euro-GASP has two surveillance modules: one decentralized, based on the communication of antimicrobial susceptibility test (AST) results to ECDC; and the other centralized. In this case, participating laboratories send isolates to the Public Health England (London) for AST.10
In the most recent Euro-GASP report, each country was required to make a contribution of 100–200 isolates (depending on the number of gonorrhoeae cases detected by the national protocols), obtained during April/May and October/November 2013. With this sampling strategy, the number of isolates included in the study, compared to the total reported cases in each country, varied from less than 1% (in consequence of high incidence levels or very efficient surveillance) to more than 100% (when under-reporting from the national protocols occurred). For instance, the United Kingdom (U.K.), with a well-established national surveillance program, reported the most cases, 32,377 in 2013, followed by the Netherlands (n = 4171), Spain (n = 3314), and Hungary (n = 1526); while Portugal reported only 116 cases in the same year, and Germany did not provide any information on this matter. Notwithstanding such differences, these six countries contributed to the Euro-GASP analysis with similar number of isolates, varying from 88 in Hungary to 240 in the U.K. Considering such a heterogeneous collection, data compiled showed in Europe, in addition to the U.S., MSM as the main group at risk of developing gonorrhea. However, in contrast to the predominant age group in the U.S., most cases reported for European citizens were with individuals over the age of 25.10
Concerning detection of resistance, the Euro-GASP adopts breakpoints set by The European Committee on Antimicrobial Susceptibility Testing (EUCAST), which are lower than the values adopted by the U.S.4, 11, 12 With this caveat in mind, the Euro-GASP reported ciprofloxacin and azithromycin resistance rates of 53% and 5%, respectively, among 1994 isolates studied in the program during 2013.10 In contrast, for cefixime, the Euro-GASP adopts the same resistance breakpoint used by the GISP. Still, cefixime resistance in Europe was higher than in the U.S., with 93 confirmed cases (4.7% of total isolates) in 2013 (Table 1).10
Australia
The Australian Gonococcal Surveillance Program (AGSP) showed a different epidemiological trend between remote regions and eastern states (Victoria, New South Wales, and Queensland) in 2012. In remote regions, notification rates were higher but stable compared to previous years, about 933 cases per 100,000 individuals, with low antimicrobial resistance rates. In contrast, in the eastern region, where an international community predominates, the incidence of gonorrhea was lower but had grown since 2009 (reaching 38.5 per 100,000 individuals), and the isolates showed higher resistance levels.13 Considering data from the whole country, and MIC breakpoint of ≥1 μg/mL for both ciprofloxacin and azithromycin,14 the AGSP reported resistance rates of 36% and 2.5% for these drugs, respectively.13 Moreover, Australia does not conduct surveillance for cefixime, but detected 5.4% (n = 258) reduced susceptibility to ceftriaxone (MIC 0.06–0.125 μg/mL) among 4804 isolates in 2014.13 As a reference for comparison, with a slight difference taken into account in breakpoints adopted, this percentage rate is more than ten times higher than that observed by GISP (0.4%) in 2014, or Euro-GASP (0.1%) in 2013 (Table 1).7, 10
Asia
Surveillance in N. gonorrhoeae resistance has been conducted in WPR and SEAR since 1992 and 2007, respectively, through a WHO-GASP initiative, under the Australian Health Department supervision. According to a GASP-WPR/GASP-SEAR report, 9744 N. gonorrhoeae isolates from 19 countries were submitted to antimicrobial susceptibility testing in 2010. Among these isolates, quinolone resistance or reduced susceptibility was widespread, reaching rates over 90% in 11 countries. Azithromycin resistance rates varied largely, from 0 to 1% in countries such as Cambodia and India to 34% in Mongolia. Different rates were also reported for decreased susceptibility to ceftriaxone: 1.3% in Singapore, 10.8% in India, 20.3% in Japan, 29.3% in Korea, and 55.8% in China.15
Africa
According to a WHO report published in 2015, a stable and efficient surveillance program for STI in Africa Region has not been implemented yet.16 In 2013, a good review about gonococcal antimicrobial resistance studies performed in Africa demonstrated that the small number of isolates tested and the lack of standardization in the sampling strategy adopted in different countries make measuring and comparisons of resistance rates difficult.17 In a general perspective, data obtained between 2004 and 2012 in the African continent demonstrated a rise in the antimicrobial resistance, especially with quinolones, and emphasizes the need for improving infrastructure and laboratory network to perform surveillance in that region.17, 18
Latin America & Caribbean
In addition to Asia and Africa, WHO-GASP has also been implemented in Latin America and the Caribbean region (GASP-LAC) since the 1990s.19 WHO-GASP-LAC published a report in 2013 that gathered information on the antimicrobial susceptibility of N. gonorrhoeae in 23 countries from 1990 to 2011. Among 12,730 isolates tested for ciprofloxacin susceptibility, a rising trend for resistance was observed during these years. Ciprofloxacin resistance rates stayed below 5% until 2004, ranged to more than 15% in 2006, finally reaching values of greater than 40% in 2010. Resistant N. gonorrhoeae isolates to azithromycin were not detected until 2000 and onwards, with the exception of Cuba, where 10 N. gonorrhoeae azithromycin resistant isolates were detected between 1995 and 1998. Azithromycin resistance rates remained above 6% from 2000 to 2009, rose to more than 25% in 2008, and reduced down to 1% in 2010. Among 5171 isolates tested for ceftriaxone between 2007 and 2011, 20 (0.4%) N. gonorrhoeae isolates obtained from Argentina, Brazil, Chile, Cuba and Uruguay exhibited reduced susceptibility to ceftriaxone (MIC ≥ 0.125 μg/mL).19
Brazil
Brazil, the largest and economically most relevant country in South America, does not have a national gonococcal surveillance program. Over the last 20 years, three attempts of establishing laboratory networks with a focus in N. gonorrhoeae resistance were conducted by the Brazilian Health Ministry (BHM). The first one occurred in 1996 as an invitation of Pan American Health Organization (PAHO)/WHO. The second one, initiated in 2007, was denominated as Sengono Project, and engaged seven previously established laboratories and research groups. Both attempts failed, since no reports of the obtained results have been published. In 2013, a new edition of the Sengono Project was initiated. The project provided preliminary results published as a BHM communication note in the BHM website in 2016.20
The BHM estimated the occurrence of 9,285,000 cases per year amongst the age group of 15–49, in 2015. Unfortunately, this number comes from compiled data obtained in small studies performed with different subpopulations in seven Brazilian states, from 2002 to 2012. The data include cohort sampling reports and asymptomatic infections, differently from cases notified in surveillance programs associated with Health Care Systems.21 According to statistics currently posted on the BHM website, WHO estimates 1,541,800 new gonorrhea infections per year in Brazil. However, no information about the data source for the estimations were provided.22
In regards to antimicrobial resistance, a report presenting GASP-LAC outcomes for 2936 isolates obtained in Brazil from 2000 to 2009 indicated that during this time ciprofloxacin resistance rates were lower than 6%. Azithromycin resistance rates varied from 22% (9/41) in 2004 to 6% (7/110) in 2007, with one additional resistant isolate detected in 2009. However, it is not clear if such data are representative for the whole country, since no information about the geographic origin of the isolates or sampling strategy is available. The same report described seven isolates obtained in Manaus in 2007 exhibiting decreased susceptibility to ceftriaxone (MIC > 0.25 μg/mL).23 Beyond this report, a small number of studies were performed sporadically throughout different regions of the country. During 2004 and 2005, five ciprofloxacin resistant isolates (8%) were detected among 65 N. gonorrhoeae isolates recovered from patients with urethritis in São Paulo.24 A few years later, ciprofloxacin resistance reached 17% among 152 isolates obtained between 2006 and 2010 from patients with gonorrhea in Rio de Janeiro.25 More recently (2011–2012), among 201 N. gonorrhoeae isolates obtained from patients with urethritis and cervicitis in a Health Service Facility in Minas Gerais, 21% were resistant to ciprofloxacin and 5% were resistant to azithromycin.26
Neisseria gonorrhoeae genotyping
Understanding clonal relationships among N. gonorrhoeae resistant isolates is an important strategy to control the spread and increase of resistance. There are two main DNA sequence-based typing methods performed in gonococcus with data stored on public website. The N. gonorrhoeae multiantigen sequence type (NG-MAST) is specific to this species and analysis two variable loci, porB and tbpB, which encode one of the two porins expressed by N. gonorrhoeae (PIB porin), and the B subunit of binding transferrin protein, respectively (http://www.ng-mast.net/). NG-MAST is a convenient tool for micro-epidemiological studies due to its discriminatory property.27 The other method is multilocus sequence typing (MLST), available for the gender Neisseria. This approach is based on the analysis of seven housekeeping genes, and is appropriate to track the spread of international clones (http://pubmlst.org/neisseria/). Both typing tools have demonstrated that infections may occur in clusters, and resistant clones may spread across continents.28 In addition to those methods, whole genome sequencing has brought new epidemiologic information in studies of transmission pathways.8, 29
Current treatment recommendations
Published guidelines usually state policies for syndromic treatment of gonorrhea, defined as symptomatic urethritis in men, and mucopurulent cervicitis in women, even while a microbiological diagnostic is not possible.6, 21 The treatment recommendations vary slightly, especially concerning antimicrobial doses, and are described in Table 2. The BHM proposes two different strategies for Brazil. For most territories, ciprofloxacin should be prescribed with a combination of azithromycin. However, studies showing ciprofloxacin resistance rates over 5% in São Paulo, Minas Gerais, and Rio de Janeiro, encourage ciprofloxacin to be replaced by ceftriaxone in those states.21 This recommendation placed Brazil in the vast group of countries (U.S., European countries, Australia, and others) that recommend a combination of ceftriaxone-azithromycin for therapy.6, 30, 31, 32, 33 The WHO Guideline for the treatment of N. gonorrhoeae based on data from high, middle- and low-income countries, published in 2016, recommends ceftriaxone or cefixime plus azithromycin primarily for treatment of genital and anorectal gonococcal infections (Table 2).3
Table 2.
Antimicrobial therapy recommended for gonococcal infection.
| Country/region/organization | Therapy | Special situations | Year guideline published | Reference |
|---|---|---|---|---|
| Europe | CRO 500 mg IM + AZM 2 g PO | If CRO is unavailable, or antimicrobial injection is impossible, or patient refuses to take the medication: CFX 400 mg PO + AZM 2 g PO If AZM is unavailable, or patient cannot take oral medication: CRO 500 mg IM In case of ESC resistance, or if patient is allergic to penicillin or cephalosporin: SPC 2 g IM + AZM 2 g PO |
2013 | 29 |
| U.S. | CRO 250 mg IM + AZM 1 g PO | If CRO is unavailable: CFX 400 mg PO + AZM 1 g PO | 2015 | 6 |
| Canada | CRO 250 mg IM + AZM 1 g PO or CFX 800 mg PO | If CRO is unavailable: SPC 2 g IM + AZM 1 g PO If patient is allergic to cephalosporin: AZM 2 g PO |
2013 | 32 |
| Brazil | CIP 500 mg PO + AZM 500 mg PO or CRO 500 mg IM + AZM 500 mg PO | If patient is allergic to cephalosporin: AZM 2 g PO If patient is < 18 year-old or pregnant: CRO 500 mg IM + AZM 500 mg PO |
2015 | 20 |
| Australia | CRO 500 mg IM + AZM 1 g PO | None | 2016 | 30 |
| WHO | CRO 250 mg IM + AZM 1 g PO or CFX 400 mg PO + AZM 1 g PO | If local recent data confirm susceptibility, one antimicrobial in single dose is a possibility: CRO 250 mg IM or CFX 400 mg PO or SPC 2 g IM | 2016 | 3 |
AZM, azithromycin; CFX, cefixime; CIP, ciprofloxacina; CRO, ceftriaxone; SPC, spectinomycin; ESC, extend spectrum cephalosporin.
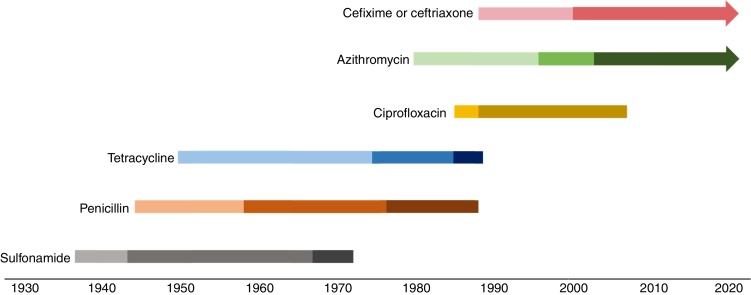
Over the last 80 years, N. gonorrhoeae has developed or acquired resistance mechanisms for sulfonamides, penicillins, tetracycline, ciprofloxacin, and more recently azithromycin and ceftriaxone, making this microorganism a candidate to cause an untreatable disease (Fig. 1).34, 35, 36 In the next section, we describe the evolution of N. gonorrhoeae resistance in a historical and molecular perspective, and provide the basis for understanding why this perception fits among the threats of the post-antibiotic era.
Fig. 1.
Evolution of Neisseria gonorrhoeae resistance to antimicrobials. Color changes indicate events that impacted the level of resistance along the time each antimicrobial was used. Sulfonamide: introduction, resistance reported, combined use with trimethoprim; penicillin and tetracycline: introduction, chromosomally-mediated resistance reported, plasmid-mediated resistance reported; ciprofloxacin: introduction, resistance reported; azithromycin: introduction, resistance reported, high level resistance reported; cefixime or ceftriaxone: introduction, reduced susceptibility reported.
Evolution of antimicrobial resistance
Sulfonamides, penicillin and tetracycline: three obsolete choices
Sulfonamides
Sulfonamides were introduced as a therapy for gonorrhea during the 1930s.37 However, by 1944, a rate of 75% treatment failure with sulphathiazole or sulphapyridine were reported amongst World War II soldiers in the Italian and Sicilian campaign.38 This class of antimicrobials acts as a competitive substrate to the dihydropteroate synthetase (DHPS) enzyme in the folic acid biosynthesis. In the gonococcus, resistance is achieved by increased production of the usual substrate, p-aminobenzoic acid, or synthesis of a mutated DHPS with low affinity to the antimicrobial.39
In the 1960s, in order to improve the efficacy of sulfonamide to treat uncomplicated gonorrhea, a combined therapy with trimethoprim was proposed as an option.40 This second compound inhibits an additional reaction in the same metabolic pathway catalyzed by the dihydrofolate reductase (DHFR) enzyme. However, N. gonorrhoeae DHRF has low affinity for trimethoprim, and may also be genetically modified, making the bacteria less sensitive to this antimicrobial.41 Still, the synergic combination of sulfonamides with trimethoprim was used to treat gonorrhea in high and multi-dose therapeutic schemes until the 1970s.42, 43
Penicillin
Penicillin, successfully used for decades, was introduced as antimicrobial treatment for gonorrhea in 1943, primarily in cases of sulfonamide treatment failure.44 However, penicillin decreased susceptibility in gonococcus starting in the 1960s.45 From then until the 1980s, the efficient penicillin dose for gonococcus infections increased 24-folds, from 200,000 U to 4.8 million U. Meanwhile, penicillin MIC of clinical isolates varied from ≤0.015 μg/mL to 2.0 μg/mL.39
As a β-lactam antimicrobial, penicillin inhibits bacterial cell wall synthesis by binding to transpeptidase enzymes called penicillin-binding proteins (PBP) in the periplasm.46 Accordingly, over the first 50 years of use, penicillin resistance mechanisms in gonococcus were related to decreased susceptibility by cumulative chromosomal mutations in different genes related to cell wall biosynthesis (penA and ponA1), or structures affecting the periplasmic drug concentration (penB, penC and mtrR).
PBP2 is an important PBP in N. gonorrhoeae. Mutations such as an aspartate insertion after the 345 position, and a variable number of substitutions in the protein c-terminal have been detected in isolates with penicillin decreased susceptibility.47, 48 Such new alleles are reported as numbered penA variants.49, 50, 51, 52, 53, 54, 55 A single base mutation in PBP1, called ponA1 (T to C in 1261 bp, resulting in the alteration L421P), decreases the acylation with the β-lactam.56
Mutations in the porB gene are known as penB mutations. Specific substitutions G120K and A121D have been characterized to affect to penicillin and tetracycline MIC in gonococcus.57 Since these positions are presumably located in a region that forms a pore restriction zone, it was first hypothesized that these substitutions affect the penetration of penicillin across the outer membrane.57, 58 However, further studies demonstrated that penB mutations affect the MIC of this drug only when MtrC-MtrD-MtrE efflux pump is over-expressed in the gonococcus, through a possible synergistic mechanism.59
The multiple transferable resistance (mtr) system of N. gonorrhoeae encodes proteins that resemble bacterial/efflux-transport molecules occurring in other bacterial species. In the gonococcus, its expression is affected by two transcriptional regulators, MtrR (repressor) and MtrA (activator), and impacts on the susceptibility of the microorganism to a variety of hydrophobic substances, including antimicrobials such as penicillin, tetracycline and macrolides, and detergent-like fatty acids.58, 60 Many different mechanisms have been described to affect the expression of mtrCDE. These include mutations leading to dramatic amino acid changes A39T or G45D in the helix-turn-helix DNA binding structure of MtrR,61 and a single base pair (bp) deletion (T:A) or a dinucleotide insertion (TT:AA) within the mtrR promoter, which may impair mtrR expression.60, 61, 62 Other changes are increased mtrCDE expression related to a mutation outside the mtrR locus,61 and a cytosine to timine mutation 120 bp upstream mtrC, which generates a second promoter to the gene insensitive to the repressive effect of mtrR.61, 63
PilQ is an important gonococcal outer membrane component, member of secretin protein family, and involved in Type IV pilus formation. PilQ and Type IV pilus form together an SDS-resistant multimeric complex, which acts as a pore for antibiotics and small molecules. The replacement G666L in the pilQ gene, known as pilQ2 mutation or penC, hinders the creation of the PilQ multimeric, disrupting the pore formed, and hence decreasing the influx of penicillin.56, 64
Through simultaneous occurences, chromosomally mediated mechanisms lead to a penicillin MIC up to 4 μg/mL in gonococcus, which is twice the MIC reported in infections only treatable with very high penicillin doses.39, 56 However, during the 1970s, N. gonorrhoeae isolates presenting MIC up to 128 μg/mL emerged,65, 66 and ended the penicillin era for gonorrhea treatment. The new resistance mechanism was a plasmid-mediated β-lactamase (bla) gene type TEM (bla-TEM), and the isolates became known as penicillinase-producing N. gonorrhoeae (PPNG).66, 67 N. gonorrhoeae blaTEM carried plasmids are genetically related, but present different sizes and insertion/deletion sites, and are named according to their epidemiological origin.68, 69 The most frequently described bla-plasmids in N. gonorrhoeae are Asia (7426 bp), Africa (5588 bp), and Rio/Toronto (5154 bp). Nevertheless, other types, such as Nimes (6798 bp), New Zealand (9309 bp), Johannesburg (4865 bp), and Australian (3269 bp) have been identified in gonococci.65, 66, 67, 69, 70, 71, 72, 73, 74
Tetracycline
Tetracycline was introduced as a treatment option for gonorrhea in patients allergic to penicillin in the 1950s.34 This antimicrobial agent affects protein synthesis by binding to the 30S ribosomal subunit.75 Similar to penicillin resistance, tetracycline resistance gradually evolved. In fact, some of the chromosomal mutations related to penicillin resistance, such as penB and mtr overexpression, impair the tetracycline activity, raising the MIC to 1 μg/mL.39, 47 Moreover, an additional single amino acid alteration V57M in the ribosomal protein 10S (rpsJ1) is enough to elevate tetracycline MIC to ≥2 μg/mL,76 the current CLSI breakpoint for resistance.12
The first gonococcus isolates showing tetracycline high-level resistance (MIC 24–32 μg/mL) were detected in the U.S. in 1985.77, 78 The TetM protein was identified as the mechanism responsible for the resistance phenotype, protecting the ribosome from tetracycline binding.39 In N. gonorrhoeae, tetM is conservatively carried by two conjugative plasmids (Dutch and American), similar in size, but probably evolutionarily unrelated. Endonuclease restriction-fragment patterns of both plasmids demonstrate large differences, and even the sequences of the respective tetM encoded are not identical.79 Given the spread of resistance, the quinolone era in the gonorrhea treatment started when tetracycline was no longer recommended by the mid-1980s.80
The quinolone era in gonorrhea treatment
Ciprofloxacin was developed in 1983 and was introduced to the U.K. and U.S. markets in the second part of the 1980s.81 Initially, gonorrhea treatment using ciprofloxacin was performed with a single dose of 250 mg. However, because of early reported cases of decreased susceptibility, the first CDC recommendation for ciprofloxacin therapy for this STI was 500 mg in a single dose.80, 82 Although isolates exhibiting reduced susceptibility (MIC ≥ 0.25 μg/mL) had been detected in London before 1989,82 and with many therapeutic failures reported during the 1990s,83, 84, 85 ciprofloxacin therapy was continued to be used at the same dosage throughout the world, for additional 10–25 years depending on the country.
Quinolones affect the activity of DNA gyrase and topoisomerase IV, two topoisomerases essential for DNA replication, transcription, recombination, and repair. This class of antimicrobial agents acts by forming a drug–enzyme–DNA complex, with further release of double-strand-DNA breaks.86 N. gonorrhoeae resistance to ciprofloxacin is mediated by mutations in the quinolone resistance-determining region (QRDR), located near the topoisomerases DNA binding site. Such mutations influence susceptibility of isolates cumulatively.87 Mutations leading to a single amino acid change in GyrA positions 91 or 95 lead to intermediary resistance level (MIC 0.1–0.5 μg/mL); however, three or more amino acid changes in GyrA (at positions 91, 95 and 102) and ParC (87 and 91) and/or Par E (439) proteins may lead to MIC ≥ 32 μg/mL.25, 88
One decade after being recommended as the first choice for gonorrhea treatment in the U.S., ciprofloxacin treatment was abandoned in the Asian Western Pacific, because of high resistance rates. In Japan, for example, the resistance percentage was 6% in 1993–1994 and rose to 24% in 1997–1998. Unfortunately, no information about the phylogeny of resistant strains was available.89 By then, ciprofloxacin-resistant gonococcus also emerged in Europe. Studies confirmed the genetic correlation among the isolates, highlighting the clonal pattern of ciprofloxacin resistant N. gonorrhoeae spread, mainly due to sexual networks.90, 91, 92 In a survey study conducted in Brazil from 2006 to 2010, 23 of the 25 resistant ciprofloxacin N. gonorrhoeae isolates were clustered into two clonal groups.25
In the U.S., the gonococcal ciprofloxacin resistance reached the west coast of the country and then spread through the rest of the territory. The first ciprofloxacin resistance isolates (MIC 1–16 μg/mL) were detected in Hawaii in 1999, affecting 9.5% of the isolates.93 Soon afterwards, the same trend was seen in California (4.9% in 2001), which made the CDC withdraw the ciprofloxacin recommendation treatment in those states.94 Ciprofloxacin was removed from guidelines in Asia, Europe, and the U.S. in the early to mid-2000s.95
Extend spectrum cephalosporins and azithromycin: the current recommended antimicrobials to treat gonorrhea
Azithromycin
Azithromycin was included as a possible therapy for gonorrhea in the beginning of the 1980s.95 This macrolide interacts with the P site of the 50S ribosomal subunit, impairing the peptidyl transferase polypeptide chain elongation.96
Different mechanisms may impact azithromycin activity in N. gonorrhoeae. One of them is the overexpression of the efflux pump mtrCDE directed by the same molecular mechanisms reported to decrease the susceptibility of N. gonorrhoeae to penicillin, increasing the azithromycin MIC to 0.5 μg/mL.62, 97 An additional mechanism of azithromycin reduced susceptibility is the occurrence of mutations in the L4 ribosomal protein.97, 98 This protein is in close contact with the peptidyl-transferase region in the V domain of the 23S rRNA, and mutations leading to dramatic amino acid changes, as the reported G70D in its extended loop, may indirectly impact the rRNA conformation.97, 99, 100, 101
When mutations occur directly in this 23S rRNA domain, resistance to azithromycin emerges. In this case, two main mechanisms are described. A single adenine methylation, promoted by plasmid mediated erm enzymes in the 2058 position of the peptidyl transferase V domain, prevents antimicrobial target binding, and rises the azithromycin MIC to 1–4 μg/mL.95, 102, 103 Moreover, the specific substitutions A2143G and/or C2599 T present in one to four rrl gene alleles, encoding the 23S RNA, result in varied azithromycin MICs. Mutations in a single allele usually do not significantly impact the MIC103, 104 however, MICs of 96 μg/mL or >256 μg/mL were reported for isolates showing C2599T or A2143G mutations in the four alleles, respectively.105, 106
N. gonorrhoeae exhibiting a high level of resistance to azithromycin has emerged in the past few years. The first case occurred in Argentina in 2001.107 Since then, the detection of high level azithromycin resistance has occurred in different countries, such as Scotland, England, Italy, U.S. and China.108, 109, 110 An outbreak with isolates exhibiting MIC ≥ 256 μg/mL affected five men and three women, all heterosexual, in England, between November 2014 and March 2015. Seven isolates were from the same NG-MAST ST9768 and presented the A2143G substitution in all four 23S encoding gene alleles.111 Nonetheless, other studies characterizing collections of isolates presenting high MIC to azithromycin demonstrated that such resistance frequently occur in non-clonally related isolates, with a variety of NG-MAST STs being detected in a same country or region.104, 106, 112 In this case it is worth mentioning that the occurrence of NG-MAST ST649 presenting similar azithromycin MIC (≥256 μg/mL) and 23S mutations in three different continents.104, 106, 109
Ceftriaxone
The ESC ceftriaxone presents high activity against gram-negative bacteria, such as N. gonorrhoeae, by high affinity binding to PBP2.113 Ceftriaxone introduction as a monotherapy for gonococcal infections was driven by the rise of ciprofloxacin resistance rates.35 Cefixime, an oral ESC, was in use before ceftriaxone; however, susceptibility to this antimicrobial decreased very quickly. For example, resistance rates reached 30% in six hospitals in the central territory of Japan in the beginning of the 2000s.49, 114 Subsequently, treatment failures with cefixime monotherapy were reported in Europe.115, 116, 117
ESC resistance might be promoted by alterations in penB, mtrR, and penC gene, although mutations in penA gene, which encodes PBP2, seem to be the main ceftriaxone resistance determinant. The altered penA gene can result from point mutations, or from genetic recombination between commensal Neisseria spp. colonizing other human sites, the last generating a mosaic-like structure.95, 118 The altered PBP2 presents diminished affinity to ceftriaxone.
Resistance to ceftriaxone is characterized by MIC > 0.5 μg/mL by CDC5 and >0.125 μg/mL by EUCAST,11 and detection of resistant isolates is still rare, with one reported case in each Japan, France and Spain.55, 119, 120 ESC resistance has been related to the presence of different patterns of PBP2. Nevertheless, studies have associated the reduced susceptibility to the clonal spread of specific lineages.55, 119, 120, 121 In Japan, the resistant isolate exhibited MIC 8 μg/mL and 4 μg/mL to cefixime and ceftriaxone, respectively, presenting PBP2 pattern highly similar to the PBP2 X, with four additional mutated amino acids residues A311V, T316P, A328T, and T484S.55 This isolate was assigned to ST7363 by MLST, the same ST of previous cefixime-resistant N. gonorrhoeae isolates circulating in Japan.54 The isolates detected in France and Spain were included in the same NG-MAST ST1407, a successful clone circulating in Europe and associated with decreased susceptibility to ESC. The isolates exhibited MIC 4 μg/mL and 1.5 μg/mL to cefixime, and 2 μg/mL and 1.5 μg/mL to ceftriaxone in France and Spain, respectively. The PBP2 XXXIV pattern with one additional amino acid substitution A501P was detected in the isolates from both countries.119, 120 The occurrence of this clone is epidemiologically related to MSM.
The emergence of ESC resistance in gonococcus and the absence of a new perspective for treatment of gonorrhea have motivated the dual therapy protocols with ceftriaxone and azithromycin. According to agencies performing surveillance programs, this strategy may decelerate the increase of resistance rates to these antimicrobials.30, 122
Concluding remarks
Currently, comparing N. gonorrhoeae resistance rates and epidemiological trends among countries is a difficult task, considering the different breakpoints and sampling strategies that have been adopted worldwide. Data obtained in different countries emphasize that surveillance is indeed essential to preserve the possibility of treatment based on syndromic diagnosis for gonorrhea.
The implementation of such surveillance programs may pose some challenges. For instance, in Brazil, the lack of interaction between research centers and the official health systems facilities face major obstacles. Moreover, continuing institutional and financial assistance is necessary to obtain, maintain and analyze N. gonorrhoeae isolates in a standard way for consecutive years. Collecting antimicrobial susceptibility testing results from diagnostic laboratories may prove useful to have an idea of current resistance rates. However, such approach is not applicable to epidemiological studies since patients’ data and even the isolates are not available for further studies
In conclusion, given the particularities of the disease, which is community based, and the difficulties of obtain and preserve N. gonorrhoeae isolates, extensive organization is necessary to implement adequate surveillance programs. In this sense, we believe that a good strategy would be to establish associations between STI medical centers and accredited laboratories that can report results to a governmental agency responsible for standardized protocols, centralize data and publish regular reports. The international agencies collaboration may prove necessary in achieving an efficient global action, especially in low income countries.
Associate Editor: Marina Baquerizo
References
- 1.Chan P.A., Robinette A., Montgomery M. Extragenital infections caused by Chlamydia trachomatis and Neisseria gonorrhoeae: a review of the literature. Infect Dis Obstet Gynecol. 2016;2016:5758387. doi: 10.1155/2016/5758387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ison C.A. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In: Gross G.E., Tyring S.K., editors. Sexually Transmitted Infections and Sexually Transmitted Diseases. Springer; Berlin Heidelberg: 2011. pp. 77–90. [Google Scholar]
- 3.World Health Organization; Geneva: 2016. WHO Guidelines for the Treatment of Neisseria gonorrhoeae.http://www.ncbi.nlm.nih.gov/books/NBK379221/ [PubMed] [Google Scholar]
- 4.CDC . CDC; A Atlanta: 2016. Gonococcal Isolates Surveillance Project Protocol 2016.http://www.cdc.gov/std/gisp/gisp-protocol-many-2016.pdf Accessed 02.06.17. [Google Scholar]
- 5.CDC . 2014. Sexually Transmitted Disease Surveillance, National Profile.https://www.cdc.gov/std/stats14/gonorrhea.htm Accessed 01.06.17. [Google Scholar]
- 6.CDC . 2015. Sexually Transmitted Diseases Treatment Guidelines.http://www.cdc.gov/mmwr/pdf/rr/rr6403.pdf Accessed 02.06.17. [DOI] [PubMed] [Google Scholar]
- 7.CDC . 2014. Gonococcal Isolates Surveillance Project Profiles.http://www.cdc.gov/std/gisp2014/gisp-2014-text-fig-tables.pdf Accessed 02.06.17. [Google Scholar]
- 8.Grad Y.H., Kirkcaldy R.D., Trees D. Genomic epidemiology of Neisseria gonorrhoeae with reduced susceptibility to cefixime in the USA: a retrospective observational study. Lancet Infect Dis. 2014;14(3):220–226. doi: 10.1016/S1473-3099(13)70693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canada PHA of, Canada PHA of . 2014. National Surveillance of Antimicrobial Susceptibilities of Neisseria gonorrhoeae – Annual Summary.https://www.canada.ca/en/public-health/services/publications/drugs-health-products/national-surveillance-antimicrobial-susceptibilities-neisseria-gonorrhoeae-annual-summary-2014.html Published 05.02.16, Accessed 01.06.17. [Google Scholar]
- 10.ECDC . ECDC; Stockholm: 2016. Gonococcal Antimicrobial Susceptibility Surveillance in Europe 2014.http://ecdc.europa.eu/en/publications/Publications/gonococcal-antimicrobial-susceptibility-surveillance-Europe-2014.pdf Accessed 02.06.17. [Google Scholar]
- 11.EUCAST . EUCAST; 2017. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 7.0.http://www.eucast.org/clinical_breakpoints/ Accessed 02.06.17. [Google Scholar]
- 12.CLSI . CLSI; Wayne: 2017. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Seventh Informational Supplement M100-S27. [Google Scholar]
- 13.Lahra M.M., Enriquez R.P. National Neisseria Network. Australian Gonococcal Surveillance Programme annual report, 2015. Commun Dis Intell Q Rep. 2017;41(1) E. [PubMed] [Google Scholar]
- 14.Lahra M.M., Enriquez R.P. Australian Gonococcal Surveillance Programme, 1 January to 31 March 2015. Commun Dis Intell Q Rep. 2015;39(2):E297–E298. [PubMed] [Google Scholar]
- 15.Lahra M.M. WHO Western Pacific and South East Asian Gonococcal Antimicrobial Surveillance Programme. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the WHO Western Pacific and South East Asian Regions, 2010. Commun Dis Intell Q Rep. 2012;36(1):95–100. [PubMed] [Google Scholar]
- 16.WHO . WHO; 2012. Baseline Report on Global Sexually Transmitted Infection Surveillance.http://www.who.int/reproductivehealth/publications/rtis/9789241505895/en/ Accessed 02.06.17. [Google Scholar]
- 17.Ndowa F.J., Francis J.M., Machiha A., Faye-Kette H., Fonkoua M.C. Gonococcal antimicrobial resistance: perspectives from the African region. Sex Transm Infect. 2013;89(suppl 4):iv11–iv15. doi: 10.1136/sextrans-2012-050907. [DOI] [PubMed] [Google Scholar]
- 18.WHO | Progress report of the implementation of the global strategy for prevention and control of sexually transmitted infections: 2006–2015. WHO. http://www.who.int/reproductivehealth/publications/rtis/progress-report-stis-strategy/en/ Accessed 02.06.17.
- 19.Dillon J.-A.R., Trecker M.A., Thakur S.D. Gonococcal Antimicrobial Surveillance Program Network in Latin America and Caribbean 1990–2011. Two decades of the gonococcal antimicrobial surveillance program in South America and the Caribbean: challenges and opportunities. Sex Transm Infect. 2013;89(suppl 4):iv36–iv41. doi: 10.1136/sextrans-2012-050905. [DOI] [PubMed] [Google Scholar]
- 20.Notícias B da S Blog, Saúde, Ministério. Pesquisa revela altas taxas de resistência aos antimicrobianos no país. Blog da Saúde. http://www.blog.saude.gov.br/gx0dy8 Accessed 01.06.17.
- 21.Protocolo Clínico e Diretrizes Terapêuticas para Atenção Integral às Pessoas com Infecções Sexualmente Transmissíveis (IST) | Departamento de Vigilância, Prevenção e Controle das IST, do HIV/Aids e das Hepatites Virais. http://www.aids.gov.br/publicacao/2015/protocolo-clinico-e-diretrizes-terapeuticas-para-atencao-integral-pessoas-com-infecc Accessed 02.06.17.
- 22.DST no Brasil | Departamento de Vigilância, Prevenção e Controle das IST, do HIV/Aids e das Hepatites Virais. http://www.aids.gov.br/pagina/dst-no-brasil Accessed 02.06.17.
- 23.Starnino S., GASP-LAC Working Group, Galarza P. Retrospective analysis of antimicrobial susceptibility trends (2000–2009) in Neisseria gonorrhoeae isolates from countries in Latin America and the Caribbean shows evolving resistance to ciprofloxacin, azithromycin and decreased susceptibility to ceftriaxone. Sex Transm Dis. 2012;39(10):813–821. doi: 10.1097/OLQ.0b013e3182631c9f. [DOI] [PubMed] [Google Scholar]
- 24.Belda Junior W., Velho P.E.N.F., Arnone M., Fagundes L.J. Emergence of fluoroquinolone-resistant Neisseria gonorrhoeae in São Paulo, Brazil. Braz J Microbiol. 2007;38(2):293–295. [Google Scholar]
- 25.Uehara A.A., Amorin E.L.T., Ferreira M. de F. Molecular characterization of quinolone-resistant Neisseria gonorrhoeae isolates from Brazil. J Clin Microbiol. 2011;49(12):4208–4212. doi: 10.1128/JCM.01175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa L.M.B., Pedroso E.R.P., Vieira Neto V., Souza V.C.P., Teixeira M.J.B. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates from patients attending a public referral center for sexually transmitted diseases in Belo Horizonte, State of Minas Gerais, Brazil. Rev Soc Bras Med Trop. 2013;46(3):304–309. doi: 10.1590/0037-8682-0009-2013. [DOI] [PubMed] [Google Scholar]
- 27.Ilina E.N., Oparina N.Y., Shitikov E.A., Borovskaya A.D., Govorun V.M. Molecular surveillance of clinical Neisseria gonorrhoeae isolates in Russia. J Clin Microbiol. 2010;48(10):3681–3689. doi: 10.1128/JCM.00565-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unemo M., Dillon J.-A.R. Review and international recommendation of methods for typing Neisseria gonorrhoeae isolates and their implications for improved knowledge of gonococcal epidemiology, treatment, and biology. Clin Microbiol Rev. 2011;24(3):447–458. doi: 10.1128/CMR.00040-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vidovic S., Caron C., Taheri A. Using crude whole-genome assemblies of Neisseria gonorrhoeae as a platform for strain analysis: clonal spread of gonorrhea infection in Saskatchewan, Canada. J Clin Microbiol. 2014;52(10):3772–3776. doi: 10.1128/JCM.01502-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bignell C., Unemo M., European STI Guidelines Editorial Board 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2013;24(2):85–92. doi: 10.1177/0956462412472837. [DOI] [PubMed] [Google Scholar]
- 31.Gonorrhoea – Australian STI Management Guidelines. http://sti.guidelines.org.au/sexually-transmissible-infections/gonorrhoea Accessed 01.06.17.
- 32.Hamasuna R., Yasuda M., Ishikawa K. The second nationwide surveillance of the antimicrobial susceptibility of Neisseria gonorrhoeae from male urethritis in Japan, 2012–2013. J Infect Chemother Off J Jpn Soc Chemother. 2015;21(5):340–345. doi: 10.1016/j.jiac.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Government of Canada PHA of C. Canadian Guidelines on Sexually Transmitted Infections – Public Health Agency of Canada. http://www.phac-aspc.gc.ca/std-mts/sti-its/cgsti-ldcits/index-eng.php Published 01.02.13, Accessed 02.06.17.
- 34.Unemo M., Shafer W.M. Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future. Ann N Y Acad Sci. 2011;1230:E19–E28. doi: 10.1111/j.1749-6632.2011.06215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unemo M., Nicholas R.A. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol. 2012;7(12):1401–1422. doi: 10.2217/fmb.12.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unemo M., Shafer W.M. Future treatment of gonorrhea – novel emerging drugs are essential and in progress? Expert Opin Emerg Drugs. 2015;20(3):357–360. doi: 10.1517/14728214.2015.1039981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kampmeier R.H. Introduction of sulfonamide therapy for gonorrhea. Sex Transm Dis. 1983;10(2):81–84. doi: 10.1097/00007435-198304000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Campbell D.J. Gonorrhoea in N. Africa and central Mediterranean. Br Med J. 1944;2(4357):44. doi: 10.1136/bmj.2.4357.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson S.R., Morse S.A. Antibiotic resistance in Neisseria gonorrhoeae: genetics and mechanisms of resistance. Sex Transm Dis. 1988;15(4):217–224. doi: 10.1097/00007435-198810000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Csonka G.W., Knight G.J. Therapeutic trial of trimethoprim as a potentiator of sulphonamides in gonorrhoea. Br J Vener Dis. 1967;43(3):161–165. doi: 10.1136/sti.43.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Averett D.R., Roth B., Burchall J.J., Baccanari D.P. Dihydrofolate reductase from Neisseria sp. Antimicrob Agents Chemother. 1979;15(3):428–435. doi: 10.1128/aac.15.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawrence A., Phillips I., Nicol C. Various regimens of trimethoprim-sulfamethoxazole used in the treatment of gonorrhea. J Infect Dis. 1973;128(suppl):673–678. doi: 10.1093/infdis/128.supplement_3.s673. [DOI] [PubMed] [Google Scholar]
- 43.Lewis D.A. The Gonococcus fights back: is this time a knock out? Sex Transm Infect. 2010;86(6):415–421. doi: 10.1136/sti.2010.042648. [DOI] [PubMed] [Google Scholar]
- 44.Van Slyke C.J., Arnold R.C., Buchholtz M. Penicillin therapy in sulfonamide-resistant gonorrhea in men. Am J Public Health Nations Health. 1943;33(12):1392–1394. doi: 10.2105/ajph.33.12.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin J.E., Lester A., Price E.V., Schmale J.D. Comparative study of gonococcal susceptibility to penicillin in the United States, 1955–1969. J Infect Dis. 1970;122(5):459–461. doi: 10.1093/infdis/122.5.459. [DOI] [PubMed] [Google Scholar]
- 46.Zapun A., Contreras-Martel C., Vernet T. Penicillin-binding proteins and beta-lactam resistance. FEMS Microbiol Rev. 2008;32(2):361–385. doi: 10.1111/j.1574-6976.2007.00095.x. [DOI] [PubMed] [Google Scholar]
- 47.Sparling P.F., Sarubbi F.A., Blackman E. Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae. J Bacteriol. 1975;124(2):740–749. doi: 10.1128/jb.124.2.740-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fedarovich A., Cook E., Tomberg J., Nicholas R.A., Davies C. Structural effect of the Asp345a insertion in penicillin-binding protein 2 from penicillin-resistant strains of Neisseria gonorrhoeae. Biochemistry (Mosc) 2014;53(48):7596–7603. doi: 10.1021/bi5011317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ito M., Deguchi T., Mizutani K.-S. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in Central Japan. Antimicrob Agents Chemother. 2005;49(1):137–143. doi: 10.1128/AAC.49.1.137-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindberg R., Fredlund H., Nicholas R., Unemo M. Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime and ceftriaxone: association with genetic polymorphisms in penA, mtrR, porB1b, and ponA. Antimicrob Agents Chemother. 2007;51(6):2117–2122. doi: 10.1128/AAC.01604-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whiley D.M., Limnios E.A., Ray S., Sloots T.P., Tapsall J.W. Diversity of penA alterations and subtypes in Neisseria gonorrhoeae strains from Sydney, Australia, that are less susceptible to ceftriaxone. Antimicrob Agents Chemother. 2007;51(9):3111–3116. doi: 10.1128/AAC.00306-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pandori M., Barry P.M., Wu A. Mosaic penicillin-binding protein 2 in Neisseria gonorrhoeae isolates collected in 2008 in San Francisco, California. Antimicrob Agents Chemother. 2009;53(9):4032–4034. doi: 10.1128/AAC.00406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee S.-G., Lee H., Jeong S.H. Various penA mutations together with mtrR, porB and ponA mutations in Neisseria gonorrhoeae isolates with reduced susceptibility to cefixime or ceftriaxone. J Antimicrob Chemother. 2010;65(4):669–675. doi: 10.1093/jac/dkp505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohnishi M., Watanabe Y., Ono E. Spread of a chromosomal cefixime-resistant penA gene among different Neisseria gonorrhoeae lineages. Antimicrob Agents Chemother. 2010;54(3):1060–1067. doi: 10.1128/AAC.01010-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohnishi M., Golparian D., Shimuta K. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea? Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother. 2011;55(7):3538–3545. doi: 10.1128/AAC.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ropp P.A., Hu M., Olesky M., Nicholas R.A. Mutations in ponA, the gene encoding penicillin-binding protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2002;46(3):769–777. doi: 10.1128/AAC.46.3.769-777.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olesky M., Hobbs M., Nicholas R.A. Identification and analysis of amino acid mutations in porin IB that mediate intermediate-level resistance to penicillin and tetracycline in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2002;46(9):2811–2820. doi: 10.1128/AAC.46.9.2811-2820.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maier T.W., Zubrzycki L., Coyle M.B., Chila M., Warner P. Genetic analysis of drug resistance in Neisseria gonorrhoeae: production of increased resistance by the combination of two antibiotic resistance loci. J Bacteriol. 1975;124(2):834–842. doi: 10.1128/jb.124.2.834-842.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olesky M., Zhao S., Rosenberg R.L., Nicholas R.A. Porin-mediated antibiotic resistance in Neisseria gonorrhoeae: ion, solute, and antibiotic permeation through PIB proteins with penB mutations. J Bacteriol. 2006;188(7):2300–2308. doi: 10.1128/JB.188.7.2300-2308.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hagman K.E., Pan W., Spratt B.G., Balthazar J.T., Judd R.C., Shafer W.M. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiol Read Engl. 1995;141(Pt 3):611–622. doi: 10.1099/13500872-141-3-611. [DOI] [PubMed] [Google Scholar]
- 61.Warner D.M., Shafer W.M., Jerse A.E. Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC-MtrD-MtrE Efflux pump system confer different levels of antimicrobial resistance and in vivo fitness. Mol Microbiol. 2008;70(2):462–478. doi: 10.1111/j.1365-2958.2008.06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zarantonelli L., Borthagaray G., Lee E.H., Veal W., Shafer W.M. Decreased susceptibility to azithromycin and erythromycin mediated by a novel mtr(R) promoter mutation in Neisseria gonorrhoeae. J Antimicrob Chemother. 2001;47(5):651–654. doi: 10.1093/jac/47.5.651. [DOI] [PubMed] [Google Scholar]
- 63.Ohneck E.A., Zalucki Y.M., Johnson P.J.T. A novel mechanism of high-level, broad-spectrum antibiotic resistance caused by a single base pair change in Neisseria gonorrhoeae. mBio. 2011;2(5) doi: 10.1128/mBio.00187-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao S., Tobiason D.M., Hu M., Seifert H.S., Nicholas R.A. The penC mutation conferring antibiotic resistance in Neisseria gonorrhoeae arises from a mutation in the PilQ secretin that interferes with multimer stability. Mol Microbiol. 2005;57(5):1238–1251. doi: 10.1111/j.1365-2958.2005.04752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ashford W.A., Golash R.G., Hemming V.G. Penicillinase-producing. Lancet Lond Engl. 1976;2(7987):657–658. doi: 10.1016/s0140-6736(76)92467-3. [DOI] [PubMed] [Google Scholar]
- 66.Phillips I. Beta-lactamase-producing, penicillin-resistant gonococcus. Lancet Lond Engl. 1976;2(7987):656–657. doi: 10.1016/s0140-6736(76)92466-1. [DOI] [PubMed] [Google Scholar]
- 67.Percival A., Rowlands J., Corkill J.E. Penicillinase-producing gonococci in Liverpool. Lancet Lond Engl. 1976;2(8000):1379–1382. doi: 10.1016/s0140-6736(76)91919-x. [DOI] [PubMed] [Google Scholar]
- 68.Pagotto F., Aman A.T., Ng L.K., Yeung K.H., Brett M., Dillon J.A. Sequence analysis of the family of penicillinase-producing plasmids of Neisseria gonorrhoeae. Plasmid. 2000;43(1):24–34. doi: 10.1006/plas.1999.1431. [DOI] [PubMed] [Google Scholar]
- 69.Müller E.E., Fayemiwo S.A., Lewis D.A. Characterization of a novel β-lactamase-producing plasmid in Neisseria gonorrhoeae: sequence analysis and molecular typing of host gonococci. J Antimicrob Chemother. 2011;66(7):1514–1517. doi: 10.1093/jac/dkr162. [DOI] [PubMed] [Google Scholar]
- 70.Yeung K.H., Dillon J.R., Pauzé M., Wallace E. A novel 4.9-kilobase plasmid associated with an outbreak of penicillinase-producing Neisseria gonorrhoeae. J Infect Dis. 1986;153(6):1162–1165. doi: 10.1093/infdis/153.6.1162. [DOI] [PubMed] [Google Scholar]
- 71.Van Embden J.D., Dessens-Kroon M., Van Klingeren B. A new beta-lactamase plasmid in Neisseria gonorrhoeae. J Antimicrob Chemother. 1985;15(2):247–250. doi: 10.1093/jac/15.2.247. [DOI] [PubMed] [Google Scholar]
- 72.Gouby A., Bourg G., Ramuz M. Previously undescribed 6.6-kilobase R plasmid in penicillinase-producing Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1986;29(6):1095–1097. doi: 10.1128/aac.29.6.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brett M. A novel gonococcal beta-lactamase plasmid. J Antimicrob Chemother. 1989;23(4):653–654. doi: 10.1093/jac/23.4.653. [DOI] [PubMed] [Google Scholar]
- 74.Trembizki E., Buckley C., Lawrence A., Lahra M., Whiley D., GRAND Study Investigators Characterization of a novel Neisseria gonorrhoeae penicillinase-producing plasmid isolated in Australia in 2012. Antimicrob Agents Chemother. 2014;58(8):4984–4985. doi: 10.1128/AAC.02993-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chopra I., Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev MMBR. 2001;65(2):232–260. doi: 10.1128/MMBR.65.2.232-260.2001. second page, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu M., Nandi S., Davies C., Nicholas R.A. High-level chromosomally mediated tetracycline resistance in Neisseria gonorrhoeae results from a point mutation in the rpsJ gene encoding ribosomal protein S10 in combination with the mtrR and penB resistance determinants. Antimicrob Agents Chemother. 2005;49(10):4327–4334. doi: 10.1128/AAC.49.10.4327-4334.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.CDC. Tetracycline-resistant Neisseria gonorrhoeae – Georgia, Pennsylvania, New Hampshire. https://www.cdc.gov/mmwr/preview/mmwrhtmL/00000611.htm Accessed 02.06.17. [PubMed]
- 78.Morse S.A., Johnson S.R., Biddle J.W., Roberts M.C. High-level tetracycline resistance in Neisseria gonorrhoeae is result of acquisition of streptococcal tetM determinant. Antimicrob Agents Chemother. 1986;30(5):664–670. doi: 10.1128/aac.30.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pachulec E., van der Does C. Conjugative plasmids of Neisseria gonorrhoeae. PLoS ONE. 2010;5(4):e9962. doi: 10.1371/journal.pone.0009962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.CDC . 1989. Sexually Transmitted Diseases Treatment Guidelines.https://www.cdc.gov/mmwr//preview/mmwrhtmL/00001459.htm Accessed 02.06.17. [DOI] [PubMed] [Google Scholar]
- 81.Anderson J.E., Hobbs M.M., Biswas G.D., Sparling P.F. Opposing selective forces for expression of the gonococcal lactoferrin receptor. Mol Microbiol. 2003;48(5):1325–1337. doi: 10.1046/j.1365-2958.2003.03496.x. [DOI] [PubMed] [Google Scholar]
- 82.Gransden W.R., Warren C.A., Phillips I., Hodges M., Barlow D. Decreased susceptibility of Neisseria gonorrhoeae to ciprofloxacin. Lancet Lond Engl. 1990;335(8680):51. doi: 10.1016/0140-6736(90)90177-7. [DOI] [PubMed] [Google Scholar]
- 83.Birley H., McDonald P., Carey P., Fletcher J. High level ciprofloxacin resistance in Neisseria gonorrhoeae. Genitourin Med. 1994;70(4):292–293. doi: 10.1136/sti.70.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tapsall J.W., Limnios E.A., Thacker C. High-level quinolone resistance in Neisseria gonorrhoeae: a report of two cases. Sex Transm Dis. 1995;22(5):310–311. doi: 10.1097/00007435-199509000-00007. [DOI] [PubMed] [Google Scholar]
- 85.Deguchi T., Saito I., Tanaka M. Fluoroquinolone treatment failure in gonorrhea. Emergence of a Neisseria gonorrhoeae strain with enhanced resistance to fluoroquinolones. Sex Transm Dis. 1997;24(5):247–250. doi: 10.1097/00007435-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 86.Jacoby G.A. Mechanisms of resistance to quinolones. Clin Infect Dis Off Publ Infect Dis Soc Am. 2005;41(suppl 2):S120–S126. doi: 10.1086/428052. [DOI] [PubMed] [Google Scholar]
- 87.Giles J.A., Falconio J., Yuenger J.D., Zenilman J.M., Dan M., Bash M.C. Quinolone resistance-determining region mutations and por type of Neisseria gonorrhoeae isolates: resistance surveillance and typing by molecular methodologies. J Infect Dis. 2004;189(11):2085–2093. doi: 10.1086/386312. [DOI] [PubMed] [Google Scholar]
- 88.Lindbäck E., Rahman M., Jalal S., Wretlind B. Mutations in gyrA, gyrB, parC, and parE in quinolone-resistant strains of Neisseria gonorrhoeae. APMIS Acta Pathol Microbiol Immunol Scand. 2002;110(9):651–657. doi: 10.1034/j.1600-0463.2002.1100909.x. [DOI] [PubMed] [Google Scholar]
- 89.Tanaka M., Nakayama H., Haraoka M., Saika T. Antimicrobial resistance of Neisseria gonorrhoeae and high prevalence of ciprofloxacin-resistant isolates in Japan, 1993 to 1998. J Clin Microbiol. 2000;38(2):521–525. doi: 10.1128/jcm.38.2.521-525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Neeling A.J., van Santen-Verheuvel M., Spaargaren J., Willems R.J. Antimicrobial resistance of Neisseria gonorrhoeae and emerging ciprofloxacin resistance in the Netherlands, 1991 to 1998. Antimicrob Agents Chemother. 2000;44(11):3184–3185. doi: 10.1128/aac.44.11.3184-3185.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mavroidi A., Tzouvelekis L.S., Tassios P.T., Flemetakis A., Daniilidou M., Tzelepi E. Characterization of Neisseria gonorrhoeae strains with decreased susceptibility to fluoroquinolones isolated in Greece from 1996 to 1999. J Clin Microbiol. 2000;38(9):3489–3491. doi: 10.1128/jcm.38.9.3489-3491.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Su X., Lind I. Molecular basis of high-level ciprofloxacin resistance in Neisseria gonorrhoeae strains isolated in Denmark from 1995 to 1998. Antimicrob Agents Chemother. 2001;45(1):117–123. doi: 10.1128/AAC.45.1.117-123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.CDC Fluoroquinolone-resistance in Neisseria gonorrhoeae, Hawaii, 1999, and decreased susceptibility to azithromycin in N. gonorrhoeae, Missouri, 1999. MMWR Morb Mortal Wkly Rep. 2000;49(37):833–837. [PubMed] [Google Scholar]
- 94.CDC Increases in fluoroquinolone-resistant Neisseria gonorrhoeae – Hawaii and California, 2001. MMWR Morb Mortal Wkly Rep. 2002;51(46):1041–1044. [PubMed] [Google Scholar]
- 95.Unemo M., Shafer W.M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev. 2014;27(3):587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Poehlsgaard J., Douthwaite S. The bacterial ribosome as a target for antibiotics. Nat Rev Microbiol. 2005;3(11):870–881. doi: 10.1038/nrmicro1265. [DOI] [PubMed] [Google Scholar]
- 97.Belkacem A., Jacquier H., Goubard A. Molecular epidemiology and mechanisms of resistance of azithromycin-resistant Neisseria gonorrhoeae isolated in France during 2013–14. J Antimicrob Chemother. 2016;71(9):2471–2478. doi: 10.1093/jac/dkw182. [DOI] [PubMed] [Google Scholar]
- 98.Allen V.G., Seah C., Martin I., Melano R.G. Azithromycin resistance is coevolving with reduced susceptibility to cephalosporins in Neisseria gonorrhoeae in Ontario, Canada. Antimicrob Agents Chemother. 2014;58(5):2528–2534. doi: 10.1128/AAC.02608-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Urlaub H., Kruft V., Bischof O., Müller E.C., Wittmann-Liebold B. Protein-rRNA binding features and their structural and functional implications in ribosomes as determined by cross-linking studies. EMBO J. 1995;14(18):4578–4588. doi: 10.1002/j.1460-2075.1995.tb00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tait-Kamradt A., Davies T., Cronan M., Jacobs M.R., Appelbaum P.C., Sutcliffe J. Mutations in 23S rRNA and ribosomal protein L4 account for resistance in pneumococcal strains selected in vitro by macrolide passage. Antimicrob Agents Chemother. 2000;44(8):2118–2125. doi: 10.1128/aac.44.8.2118-2125.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zengel J.M., Jerauld A., Walker A., Wahl M.C., Lindahl L. The extended loops of ribosomal proteins L4 and L22 are not required for ribosome assembly or L4-mediated autogenous control. RNA. 2003;9(10):1188–1197. doi: 10.1261/rna.5400703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roberts M.C., Chung W.O., Roe D. Erythromycin-resistant Neisseria gonorrhoeae and oral commensal Neisseria spp. carry known rRNA methylase genes. Antimicrob Agents Chemother. 1999;43(6):1367–1372. doi: 10.1128/aac.43.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Demczuk W., Martin I., Peterson S. Genomic epidemiology and molecular resistance mechanisms of azithromycin-resistant Neisseria gonorrhoeae in Canada from 1997 to 2014. J Clin Microbiol. 2016;54(5):1304–1313. doi: 10.1128/JCM.03195-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chisholm S.A., Dave J., Ison C.A. High-level azithromycin resistance occurs in Neisseria gonorrhoeae as a result of a single point mutation in the 23S rRNA genes. Antimicrob Agents Chemother. 2010;54(9):3812–3816. doi: 10.1128/AAC.00309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bercot B., Belkacem A., Goubard A. High-level azithromycin-resistant Neisseria gonorrhoeae clinical isolate in France, March 2014. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2014;19(44) doi: 10.2807/1560-7917.es2014.19.44.20951. [DOI] [PubMed] [Google Scholar]
- 106.Stevens K., Zaia A., Tawil S. Neisseria gonorrhoeae isolates with high-level resistance to azithromycin in Australia. J Antimicrob Chemother. 2015;70(4):1267–1268. doi: 10.1093/jac/dku490. [DOI] [PubMed] [Google Scholar]
- 107.Galarza P.G., Alcalá B., Salcedo C. Emergence of high level azithromycin-resistant Neisseria gonorrhoeae strain isolated in Argentina. Sex Transm Dis. 2009;36(12):787–788. doi: 10.1097/OLQ.0b013e3181b61bb1. [DOI] [PubMed] [Google Scholar]
- 108.Chisholm S.A., Neal T.J., Alawattegama A.B., Birley H.D.L., Howe R.A., Ison C.A. Emergence of high-level azithromycin resistance in Neisseria gonorrhoeae in England and Wales. J Antimicrob Chemother. 2009;64(2):353–358. doi: 10.1093/jac/dkp188. [DOI] [PubMed] [Google Scholar]
- 109.Katz A.R., Komeya A.Y., Soge O.O. Neisseria gonorrhoeae with high-level resistance to azithromycin: case report of the first isolate identified in the United States. Clin Infect Dis Off Publ Infect Dis Soc Am. 2012;54(6):841–843. doi: 10.1093/cid/cir929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ni C., Xue J., Zhang C., Zhou H., van der Veen S. High prevalence of Neisseria gonorrhoeae with high-level resistance to azithromycin in Hangzhou, China. J Antimicrob Chemother. 2016;71(8):2355–2357. doi: 10.1093/jac/dkw131. [DOI] [PubMed] [Google Scholar]
- 111.Chisholm S.A., Wilson J., Alexander S. An outbreak of high-level azithromycin resistant Neisseria gonorrhoeae in England. Sex Transm Infect. 2016;92(5):365–367. doi: 10.1136/sextrans-2015-052312. [DOI] [PubMed] [Google Scholar]
- 112.Jacobsson S., Golparian D., Cole M. WGS analysis and molecular resistance mechanisms of azithromycin-resistant (MIC >2 mg/L) Neisseria gonorrhoeae isolates in Europe from 2009 to 2014. J Antimicrob Chemother. 2016;71(11):3109–3116. doi: 10.1093/jac/dkw279. [DOI] [PubMed] [Google Scholar]
- 113.Barry P.M., Klausner J.D. The use of cephalosporins for gonorrhea: the impending problem of resistance. Expert Opin Pharmacother. 2009;10(4):555–577. doi: 10.1517/14656560902731993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tanaka M., Nakayama H., Tunoe H. A remarkable reduction in the susceptibility of Neisseria gonorrhoeae isolates to cephems and the selection of antibiotic regimens for the single-dose treatment of gonococcal infection in Japan. J Infect Chemother Off J Jpn Soc Chemother. 2002;8(1):81–86. doi: 10.1007/s101560200011. [DOI] [PubMed] [Google Scholar]
- 115.Unemo M., Golparian D., Syversen G., Vestrheim D.F., Moi H. Two cases of verified clinical failures using internationally recommended first-line cefixime for gonorrhoea treatment, Norway, 2010. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2010;15(47) doi: 10.2807/ese.15.47.19721-en. [DOI] [PubMed] [Google Scholar]
- 116.Unemo M., Golparian D., Stary A., Eigentler A. First Neisseria gonorrhoeae strain with resistance to cefixime causing gonorrhoea treatment failure in Austria, 2011. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2011;16(43) [PubMed] [Google Scholar]
- 117.Unemo M., Golparian D., Hestner A. Ceftriaxone treatment failure of pharyngeal gonorrhoea verified by international recommendations, Sweden, July 2010. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2011;16(6) [PubMed] [Google Scholar]
- 118.Ameyama S., Onodera S., Takahata M. Mosaic-like structure of penicillin-binding protein 2 Gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob Agents Chemother. 2002;46(12):3744–3749. doi: 10.1128/AAC.46.12.3744-3749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Unemo M., Golparian D., Nicholas R., Ohnishi M., Gallay A., Sednaoui P. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother. 2012;56(3):1273–1280. doi: 10.1128/AAC.05760-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cámara J., Serra J., Ayats J. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J Antimicrob Chemother. 2012;67(8):1858–1860. doi: 10.1093/jac/dks162. [DOI] [PubMed] [Google Scholar]
- 121.Lahra M.M., Ryder N., Whiley D.M. A new multidrug-resistant strain of Neisseria gonorrhoeae in Australia. N Engl J Med. 2014;371(19):1850–1851. doi: 10.1056/NEJMc1408109. [DOI] [PubMed] [Google Scholar]
- 122.CDC . 2010. Sexually Transmitted Diseases Treatment Guidelines.https://www.cdc.gov/mmwr/preview/mmwrhtmL/rr5912a1.htm Accessed 02.06.17. [DOI] [PubMed] [Google Scholar]