Abstract
Thraustochytrids are unicellular protists belonging to the Labyrinthulomycetes class, which are characterized by the presence of a high lipid content that could replace conventional fatty acids. They show a wide geographic distribution, however their diversity in the Antarctic Region is rather scarce. The analysis based on the complete sequence of 18S rRNA gene showed that strain 34-2 belongs to the species Thraustochytrium kinnei, with 99% identity. The total lipid profile shows a wide range of saturated fatty acids with abundance of palmitic acid (16:0), showing a range of 16.1–19.7%. On the other hand, long-chain polyunsaturated fatty acids, mainly docosahexaenoic acid and eicosapentaenoic acid are present in a range of 24–48% and 6.1–9.3%, respectively. All factors analyzed in cells (biomass, carbon consumption and lipid content) changed with variations of culture temperature (10 °C and 25 °C). The growth in glucose at a temperature of 10 °C presented the most favorable conditions to produce omega-3fatty acid. This research provides the identification and characterization of a Thraustochytrids strain, with a total lipid content that presents potential applications in the production of nutritional supplements and as well biofuels.
Keywords: Thraustochytrids, Antarctic, Docosahexaenoic acid, Eicosapentaenoic acid, Temperature
Introduction
The microorganisms belonging to the Labyrinthulomycetes class and family Thraustochytriaceae are unicellular protists which are present in marine ecosystems. They have a key role on the initial stage of the microbial chain food, as organic matter degraders.1, 2 These microorganisms have been studied at the morphological, ecological and biotechnological levels.3, 4 Due to the presence of a high lipid content that could replace conventional sources of fatty acids; the Thraustochytriaceae family is of great interest.5, 6, 7, 8 Particularly, the most studied metabolites are docosahexaenoic acid (C22:6, DHA) and eicosapentaenoic acid (C20:5, EPA).9, 10 These essential biomolecules of the omega-3 family are involved in the physiological development of children and adults, cholesterol regulation, prostaglandin, thromboxane and leukotriene biosynthesis. Furthermore, they have a preventive role on different pathologies such as arteriosclerosis, asthma, thrombosis, arthritis and a wide range of tumors.11, 12, 13 Besides, a variety of biomolecules with unknown functions that could have a potential biotechnological application, such as extracellular polysaccharides, carotenoids, squalene, enzymes, osmolytes, unsaturated and saturated fatty acids could be used as biofuel.14 Taxonomic classification of the Thraustochytriaceae family comprises the genera Aplanochytrium, Ulkenia, Thraustochytrium, Japonochytrium, Aurantiochytrium (also known as Schizochytrium), Botryochytrium, Parietichytrium and Sicyoidochytrium.15, 16 Their geographic distribution includes the North Sea, India, Indonesia, Japan, Australia, South America and the Antarctic Continent.17, 18 The latter place presents poor studies of the marine microbial diversity and the number of species varies widely between taxons.19, 20, 21 Currently, in the diversity studies of the Thraustochytrids from Antarctic waters two species have been described: Thraustochytrium antarticum (Southeastern Indian Ocean) and Thraustochytrium rossii (Southwestern Pacific Ocean).23 More recently, only one microorganism has been characterized by molecular phylogeny; Aplanochytrium stocchinoi (Terra Nova Bay).23
Microorganisms have been used as alternative fatty acids source in bacteria, fungi, yeasts and microalgae.24, 25, 26 Protist species such as Crythecodiniumcohnii, Schizochytrium sp. and Ulkenia sp. have been characterized by their rapid growth, high photosynthetic activity and high production of biomass.27, 28, 29 In vitro culture of these microorganisms with high carbon/nitrogen ratio favors lipids accumulation and a decrease in cell development.29 However, this is not clearly established, since in Schizochytrium strains, the fatty acids increase is associated with biomass growth.30 Besides, in vitro studies with carbon and nitrogen sources could favor the biomass production as lipid contents, because these molecules are linked to the development of a biological model successful on fatty acids production.18, 29
This study provides a biological and biotecnological focus, using in vitro studies of a strain belonging to the Thraustochytriaceae family isolated from the Antarctic coast of King George Island. The objective of this research is based on the morphology and taxonomic classification (18S rRNA gene) studies of the culture parameters such as the carbon source and the temperature on biomass production with high content of essential fatty acids. This research offers new information about the biodiversity of Labyrinthulomycetes class in the Antarctic continent and their potential application as a biotechnological tool on LC-PUFAs or biofuels production.
Materials and methods
Isolation and culture conditions
Two samples were collected on the coast of King George Island, specifically on coordinates S 62° 12′ 34.8″ W 58° 55′ 34.3″ and the microorganisms were isolated from the water column (2.4 °C and pH 8.1). Thraustochytrids were obtained using the pine polen method.31 Then, they were incubated at different temperatures (10 °C and 25 °C) and observed using an optic microscope during 10 days to improve microorganism fixation. Inocules were cultivated in liquid medium using Honda et al. (1998)58 modified protocol (0.2% yeast extract and 0.2% sodium glutamate) in artificial seawater with 5% glucose or 5% starch, at the same temperature conditions during 8 days.32 Finally, the samples were lyophilized, centrifuged and stored at −20 °C for posterior analysis. Morphological analysis was performed with an Olympus CX21 light microscope (Tokyo, Japan).
DNA extraction and amplification of the 18S rRNA gene
DNA extraction was performed using Mo and Rinkevich33 modified protocol. Cultures were centrifuged at 14,000 rpm for 3 min, supernatant was discarded, and buffer extraction was added on pellet (0.2 M Tris–HCl pH 8.0, 1.4 M NaCl, 0.1 M EDTA and 1.5% SDS) and sonicated for 30 s. Then, the solution was centrifuged at 12,000 rpm and the supernatant was transferred to a new tube to obtain the DNA using the phenol:chloroform solution (5:1, pH 4.8). The mixture was centrifuged at 13,000 rpm for 5 min. The aquose phase was precipitated with ethanol at 4 °C overnight. Finally, the resulting pellet was suspended on nuclease free water and quantified in Infinity200 Pro NanoQuant (TECAN) and stored at −20 °C.
PCR amplification of 18S gene was carried out using comercial kit GoTaq® Green Master Mix (Reaction Buffer pH 8.5, 400 μM of dNTPs Promega and 3 mM MgCl2), and Genomic DNA (with an average ratio 260/280 of 1.6) and 1 mM of specific primers (FA1 5′-AAAGATTAAGCCATGCATGT-3′, RA1 5′-AGCTTTTTAACTGCAACAAC-3′; FA2 5′-GTCTGGTGCCAGCAGCCGCG-3′, RA2 5′-CCCGTGTTGAGTCAAATTAAG-3′; FA3 5′-CTTAAAGGAATTGACGGAAG-3′ and RA3 5′-CAATCGGTAGGTGCGACGGGCGG-3′).34 Thermocycling profile was performed with an initial denaturation step of 3 min a 95 °C, 35 cycles of amplification (1 min 94 °C, 1 min 53 °C and 1 min 72 °C), and final elongation at 72 °C of 10 min. The PCR fragments were visualized on 1.2% agarose gel using 10 μg/mL of ethidium bromide.
Sequencing and molecular phylogenetic analysis
The 18S rRNA fragments were amplified and then sequenced using automated DNA sequencer ABI-Prism (Pontifical Catholic University of Chile, Chile). For identification, a search was carried on the homolog sequences available on the data base of genes (GenBank database of the National Center for Biotechnology Information). Homologue sequences comparison was done using algorithm megablast available on NCBI server.35 Sequences alignments were performed using Clustal X2.37 Phylogenetic analysis, were done with Neighbor-joining method36 and the tree topology with MEGA 4.0.2.38 Species relations were evaluated with statistical analysis through bootstrap protocol.39 To group the phylogenetic analysis of the family Thraustochytriaceae the Prorocentrum genus was used as outgroup.
Extraction and determination of fatty acids
Fatty acids profile was done with 50 mg lyofilized biomass, using direct transesterification.40 The fatty acids were concentrate at 1000 g at 4 °C and stored at −20 °C. They were quantified by Gas Chromatography (GC) (Agilent, 7609, Santa Clara, USA) using as standard Supelco® 37 Component FAME Mix (Sigma–Aldrich) (Methyl butyrate, Methyl hexanoate, Methyl octanoate, Methyl decanoate, Methyl undecanoate, Methyl laurate, Methyl tridecanoate, Methyl tetradecanoate, Myristoleic Acid Methyl Ester, Methyl pentadecanoate, cis-10-Pentadecenoic acid methyl ester, Methyl palmitate, Methyl Palmitoleate, Methyl heptadecanoate, cis-10-Heptadecenoic acid methyl ester, Methyl octadecanoate, trans-9-Elaidic acid methyl ester, cis-9-Oleic acid methyl ester, Linolelaidic acid methyl ester, Methyl Linoleate, Methyl Arachidate, gamma-Linolenic acid methyl ester, Methyl cis-11-eicosenoate, Methyl Linolenate, Methyl heneicosanoate, cis-11,14-Eicosadienoic acid methyl, ester, Methyl docosanoate, cis-8,11,14-Eicosatrienoic acid methyl ester, Methyl Erucate, cis-11,14,17-Eicosatrienoic acid methyl ester, Methyl tricosanoate, Methyl cis-5,8,11,14-Eicosatetraenoic, cis-13-16-Docosadienoic acid methyl ester (22:2), Methyl lignocerate, Methyl cis-5,8,11,14,17-Eicosapentaenoate, Methyl Nervonate, cis-4,7,10,13,16,19-Docosahexaenoate). Biomass quantitative analysis was determined with optical density and long-chain polyunsaturated fatty acids (LC-PUFAs; DHA and EPA) content was carried out using gravimetric assay.
Neutral fatty acids (triglycerides) in 34-2 strain were visualized using Oil red O protocol modified.41 The samples were stained with 0.3% Oil Red solution for 20 min and visualized under optical microscope (Olympus CX21) using ISCapture program (http://www.tucsen.com). Images were edited with ImageJ program (http://imagej.nih.gov/ij/). Data and graphics were processed using Open Source Software IGOR Pro 6.36 (https://www.wavemetrics.com) and Canvas (http://www.canvasgfx.com).
Results
Morphologic and genetic identification
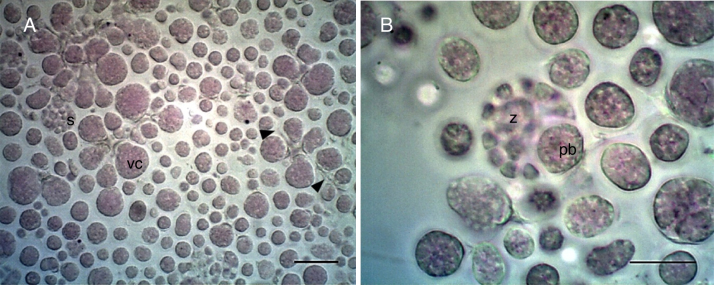
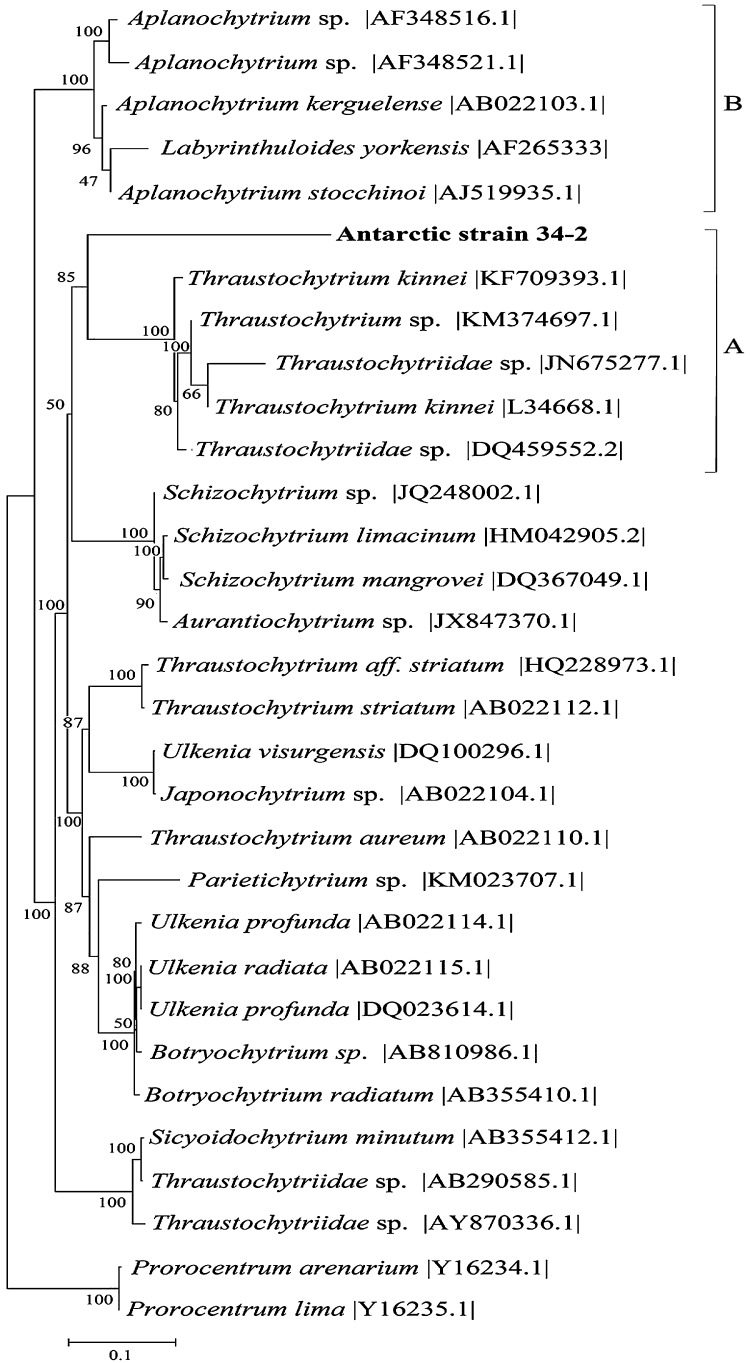
The isolated Antarctic strain had spherical cells with an average of 46 ± 5 μm in diameter (Fig. 1A). Morphological characteristics were observed such as; formation of sporangia and vegetative cells with fine filaments of ectoplasmic nets (plasma membrane extensions) (Fig. 1). The strain 34-2 has a proliferative body associated to enclosed spores within the cell wall of the sporangium (Fig. 1B). In order to determine the phylogenetic relationship the search of the homologous sequences was performed (Megablast algorithm). The comparison of 18S rRNA gene shows that the Antarctic strain exhibits high identity of 99% (over an 95% of aligned length of 1616 bp) with sequences belonging to Thraustochytrium kinnei species (GenBank accession numbers |KF709393.1| and |L34668.1|), confirming a close taxonomic clasification with Thraustochytrids microorganisms (Fig. 2). This strain shows a common ancestor, with three unclassified Thraustochytridae grouped in clade A, with a bootstrap value of 100%. In the tree topology, the sequence of A. stocchinoi, species isolated from Ross Sea, appear associated to the Antarctica clade B. The phylogenetic analysis revealed an early diversification with strain 34-2 and the rest of the genera. The 18S rRNA sequences of strain 34-2 are available in GenBank (accession numbers: LN558422; LN558423; LN558424).
Fig. 1.
Light microscopy of cells in Antarctic strain 34-2 stained with methyl violet. A Vegetative cells (vc), sporangia (s) and ectoplasmic nets (arrows). Scale bar 100 μm. B Mature sporangium with zoospores (z) and proliferative body (pb). Scale bar 50 μm.
Fig. 2.
Molecular analysis based on complete alignment of the 18S rRNA gene. The phylogenetical tree was performed with the genus belonging to the Thraustochytriaceae family, and using two species of the genus Prorocentrum as outgroup. The phylogeny was developed by the Neighbor – joining method, showing the number of GenBank accession numbers for each sequence and the bootstraps value obtained from 1000 replicates in nodes. The strain determined in this study is shown in bold type (Clade A).
Biomass and omega-3 fatty acid production
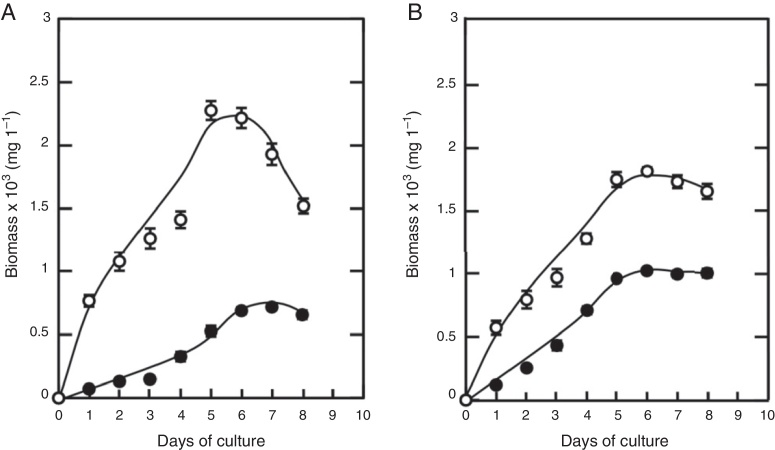
A culture of 8 days of strain 34-2 was done in order to determine kinetic biomass generation (Fig. 3). The microorganisms were cultured in four different conditions, liquid media supplemented with 0.5% glucose or starch at 10 °C and 25 °C (Fig. 3A and B). Both graphics show an exponential growth until day 5. The highest growth was registered using a supplemented medium with glucose at 25 °C, with cellular value of 2.2 × 103 mg/L. On the other hand, a low growth was registered using a glucose culture with a temperature of 10 °C, showing a maximum value of biomass at 7 days with a rate of growth of 0.7 × 103 mg/L. In starch medium, maximum values were registered at 25 °C and 10 °C at 6 days with 1.8 × 103 mg/L and 1 × 103 mg/L respectively. These results revealed that this microorganism had a high biomass produced in different carbon sources at high temperature.
Fig. 3.
The temperature and carbon source effect on biomass production. (A) Strain 34-2 supplemented with glucose 5% at 25 °C (○) and 10 °C (●). (B) supplemented with starch 5% at 25 °C (○) and 10 °C (●). This data was presented as the mean of triplicate ± SD.
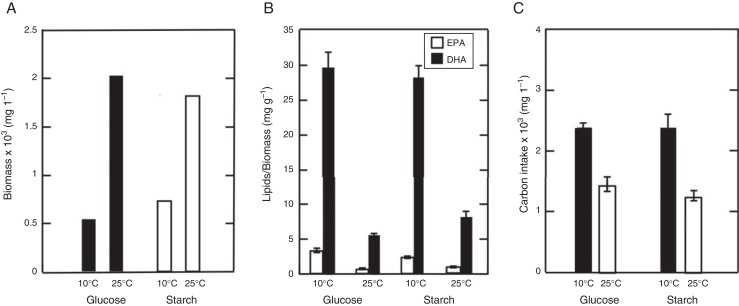
The effects of the carbon source and temperature were analyzed on biomass, carbon intake and polyunsaturated fatty acid content for 5 days of culture (Fig. 4). The results showed that there exists a major production of biomass at 25 °C, with 2 × 103 mg/L. On the other hand, low biomass was present in glucose medium at 10 °C with a value of 0.5 × 103 mg/L) (Fig. 4A). The LC-PUFAs content shows the highest values obtained with glucose as carbon source at 10 °C (Fig. 4B). The analysis revealed values of 29.8 ± 2.1 mg/g DHA and 3.3 ± 0.2 mg/g EPA. Otherwise, the lowest values were obtained at 25 °C with 5.6 ± 0.1 mg/g DHA and 0.7 ± 0.1 mg/g EPA. In carbon intake the use of glucose and starch at 10 °C presented the highest values with 2.4 × 103 mg/L and 2.4 × 103 mg/L respectively (Fig. 4C). On the other hand, the results at 25 °C were 1.4 × 103 mg/L for glucose and 1.2 × 103 mg/L for starch. The numbers of analysis already described, show standard deviation values only for variations over or equal to 0.1.
Fig. 4.
The temperature and carbon source effect on biomass, omega-3 fatty acid content (EPA y DHA) and carbon intake. (A) Quantity of biomass supplemented during 5 days of culture with glucose (black bars) and starch (white bars). (B) Relationship of DHA and EPA in relation to biomass. (C) Carbon source consumption during 5 days of culture. This data was presented as the mean of triplicate ± SD.
In general, these values show that the growth with glucose at 10 °C was the most favorable for DHA and EPA production. It was observed that with low biomass (0.5 × 103 mg/L) the higher values of DHA and EPA (29.8 ± 2.1 and 3.3 ± 0.2 mg/g respectively) were obtained, with a ratio of omega-3:6 fatty acids of 55.9% (Table 1).
Table 1.
Fatty acid profile of Antarctic strain 34-2. The values are shown as a percentage of total fatty acid content and a ratio of omega-3:omega-6 and DHA:EPA, for the temperature and carbon source conditions used in this study.
| Fatty acidsa (%) | Glucose |
Starch |
||
|---|---|---|---|---|
| 10° | 25° | 10° | 25° | |
| Saturateda | ||||
| Capric acid (C10:0) | 1.9 | 5.9 | 1 | 1 |
| Undecanoic acid (C11:0) | 0.6 | 2.8 | 0.6 | 1 |
| Lauric acid (C12:0) | 0.8 | 1.3 | 0.8 | 1.2 |
| Tridecanoic acid (C13:0) | 0.3 | 1 | 0 | 0 |
| Myristic acid (C14:0) | 2.5 | 2.7 | 2.4 | 2.5 |
| Pentadecanoic acid (15:0) | 0.8 | 3.1 | 0.6 | 2.9 |
| Palmitic acid (C16:0) | 17.9 | 16.1 | 18.6 | 19.7 |
| Stearic acid (C18:0) | 4.7 | 11.3 | 4.3 | 10.1 |
| Arachidic acid (C20:0) | 1.4 | 5.1 | 1.9 | 4.5 |
| Unsaturateda | ||||
| Elaidic acid (C18:1n9) | 1.3 | 3.4 | 1.2 | 3.2 |
| cis Oleic acid (C18:1n9c) | 1.6 | 2.8 | 2 | 3.2 |
| Linoleic acid (C18:2) | 1 | 0 | 1.1 | 1.5 |
| Eicosapentaenoic acid (C20:5) | 9.9 | 6.1 | 7.4 | 7.1 |
| Docosahexaenoic acid (C22:6) | 46.6 | 24 | 48.3 | 30.7 |
| Unknown retention time | 9 | 13.7 | 9.4 | 10.7 |
| Total (%) | 100 | 100 | 100 | 100 |
| Ratio | ||||
| DHA/EPA | 5 | 4 | 6.5 | 4.2 |
| n-3/n-6 | 55.9 | – | 50.6 | 25.2 |
Identified as fatty acids according standard retention time from standard mix.
Determination and analysis of fatty acids
The fatty acids qualitative studies were done using Oil Red O staining, a fat-soluble diazo dye. For this purpose, cultures were selected with high (glucose at 10 °C) and lower (glucose 25 °C) lipid/biomass proportion according to Fig. 4B. These results showed the presence of intracellular lipid vesicles with red color (Supplementary Material). The culture supplemented with glucose at 10 °C showed an intense staining on intracellular vesicles in 5 and 8 days of culture. On the other hand, the total composition of lipids of strain 34-2 showed 64% of saturated fatty acids (9/14) (Table 1). In this group of saturated fatty acids palmitic (C16:0) and estearic (C18:0) acids stand out. In unsaturated fatty acids, the eicosapentanoic acid (C20:59) and docohexanoic acid (C22:6) showed the highest values.
The results indicate that the production of these biomolecules changed with carbon source and temperature. Particularly, some lipids are affected according to carbon source at 25 °C. The change for glucose and starch respectively was as follows; capric acid 5.9% and 1%, linoleic acid 0% and 1.5%, undecanoic acid 2.8% and 1%, palmitic acid 16.1% and 19.7% and docosahexaenoic acid 24% and 30.7%. Other fatty acids varied with temperature changes (10 and 25 °C respectively) as follows: estearic acid 4.7–11.3%, arachidic acid 1.4–5.1%, both cultures with glucose.
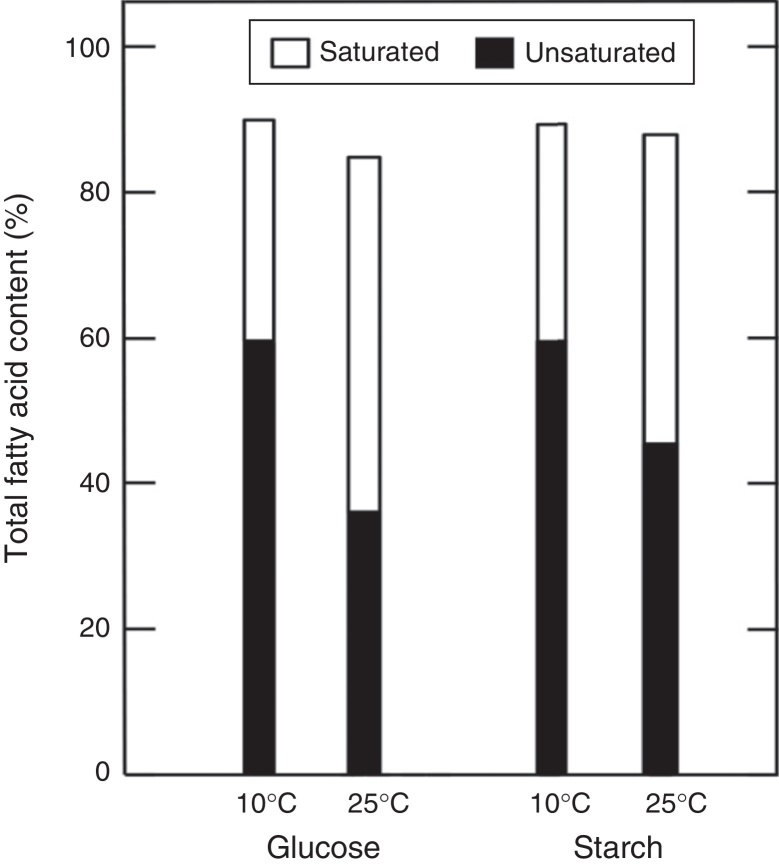
In general, the most important parameter in fatty acids profile was the effect of temperature. For example, the culture supplemented with glucose showed the highest changes (Fig. 5). The results evidenced a difference of 37% of saturated fatty acids and 40% of unsaturated fatty acids. Otherwise, microorganisms supplemented with starch presented a value of 9% and decrease 23%, of unsaturated and saturated fatty acids respectively.
Fig. 5.
Summary of saturated and unsaturated fatty acid content of Antarctic strain 34-2. The value shows the percentage of Table 1.
Discussion
The Thraustochytrids are a eukaryote group showing a wide geographic distribution, with scarce information on the diversity in the sea of the Antarctic Continent. Nowadays, reports of these microorganisms on the Antarctic Region are limited only to two researches. One of them was published by Bahnweg and Sparrow,22 and described the morphology of the strains belonging to the genus Thraustochytrium isolated from the Southeastern Indian Ocean and the Southwestern Pacific Ocean. Besides, Moro et al.23 described and characterized the species Aplanochytrium tocchinoi collected in the Terra Nova Bay (Southern Ocean, Antarctic), based on the assay of Transmission Electron Microscopy (TEM) and molecular phylogeny. The morphological studies done on the strain 34-2 revealed the presence of structures described for the genus Thraustochytrium, with piriforms sporangia to ovalated, formation of zoospores groups before liberation and the presence of a proliferation body (Fig. 1).42, 43 Besides, the molecular analysis based on the complete sequence of the 18S rRNA gene showed that strain 34-2 belongs to the species T. kinnei with identity of 99%. The phylogenetic analysis evidences a common ancestor with thraustochytrids isolated of the seashore of Chile (Valparaíso and Puerto Montt) and Australia (Southwest of Tasmania), strains located on the clade A, particularly T. kinnei |KF709393.1|, Thraustochytriidae sp. |DQ459552.2| and Thraustochytriidae sp. |JN675277.1|, respectively (Fig. 2). Therefore, this research provides basic information on the first molecular identification of this species on the Antarctic Region. Accordingly with their sites of isolation, we think that distribution of clade A is a consequence of dynamics of the Southern Hemisphere currents, because Thraustochytrids could live or be transported on hydrochory, such as sediment, senescent macroalgae and fallen mangrove leaves.10, 19 On the other side, the topology of trees show the evidence that A. stocchinoi, described above, and the strain 34-2, which are located geographically on the occidental region of the Antarctic continent, presented an early divergence, showing the first phylogenetic relation of two genera of the family Thraustochytriaceae isolated from the coasts of the Antarctic Region (Fig. 2, clade A and B).
Besides its wide distribution, these microorganisms produce metabolites with biotechnological applications such as enzymes and extracellular polysaccharides, carotenoids, polyunsaturated fatty acids (as essential fatty acids), squalene and saturated and monounsaturated fatty acids with potential on the production of biodiesel.14 The research is focused mainly on the production of essential fatty acids, being DHA28 the most studied metabolite. The synthesis of LC-PUFAs on these microorganisms is performed using two different mechanisms; one of them is the elongation/desaturation pathway which has been described on Thraustochytrium aureum.10, 44 Furthermore, Nagano et al.45 demonstrated that the content of unsaturated fatty acids precursor (C18 and C20) was consistent with the identification of Δ5-elongase and Δ4-desaturase genes on the strains of the genus Thraustochytrium. Accordingly, the results of fatty acids profile of the strain 34-2 evidence similar values of stearic acid, linoleic acid and DHA, which suggests that this microorganism could use the elongase/desaturase pathway for LC-PUFAs production (Table 1). In consistence with this it is observed that the increase in temperature produced an increase of stearic acid (C18:0) in both cultures, which decreased the total content of unsaturated fatty acids (Table 1 and Fig. 5). These results suggest that at 25 °C the enzyme Δ9-fatty acid desaturase, in charge of the transformation of stearic acid in oleic acid, decreased its activity, which could be related to the response of adaptation to the change of temperature in this microorganism. Conversely, the increase in the synthesis of DHA at low temperatures could be an adaptative response which could favor the storing of this polyunsaturated fatty acid. In consistence, Jain et al.46 described that DHA could play a role as energy source of ready energy during starvation. On the other hand, it is already known that low temperature has important consequences for the synthesis of unsaturated fatty acids, increasing availability of oxygen, needed for the activity of desaturases enzymes and diminishing the fluidity of the membrane.47, 48
In general terms, our results show that independent of the carbon source, the cultures incubated at high temperature present a major production of biomass, while the cultures incubated at lower temperature synthesize high contents of DHA and minor biomass (Fig. 3, Fig. 4A and B). However, the content of LC-PUFAs has a high concentration on all the analyzed conditions, particularly DHA with a range 24–48.3% (Table 1). These values are in agreement with researches that point that synthesis of DHA represents more than 25% of the total content of fatty acids produced by the strains of Thraustochytrids.3, 10 The high ratios of omega-3:omega-6 and DHA/EPA are important parameters in omega-3 oil production.49, 50 According to this, the culture supplemented with glucose at 10 °C evidenced the best results with a value of 55.9% and 5%, respectively (Table 1). It has been described that profiles of fatty acids are used as a methodology to establish phylogenetic homologies with other strains belonging to the family Thraustochytriaceae.16, 19 Consequently, the profile of strain 34-2 is consistent with researches carried out by Lee et al.50 that cultured 36 Thraustochytrid strains with 0.2% of glucose and separated them into eight groups based on their fatty acids and 18S rDNA gene, evidenced a mean percentage of 35.6% DHA, 9.2% EPA and 17.5% palmitic acid for the strain of the Thraustochytrium genera. On the other side, the qualitative analysis of fatty acids showed that using the methodology of Oil Red O, the presence of vesicles of red color was observed (Supplementary Material). The staining intensity evidenced a consistency with quantitative analysis between cultures of glucose at 10 °C and 25 °C (Table 1 and Fig. 4B). Because this methodology is based on triglycerides present on these microorganisms, which constitute among 70–98% of the total lipids, which 45% present DHA on their structure.51, 52 The Oil Red is used mainly on the cytological and histological analysis; however there is no evidence that point their use in Thraustochytrids.41, 50
Consequently, the results show that growth on glucose at 10 °C was the most favorable condition for the production of omega-3 fatty acid observed at the 5th day. In parallel, it was observed that the major changes were present with the variation of the temperature, affecting biomass, content of DHA and carbon intake (Fig. 3, Fig. 4, Fig. 5 and Table 1). Particularly it should be highlighted that at 10 °C there exists more consumption of the carbon source, production of DHA, EPA and minor biomass. On the contrary, the cultures at 25 °C present results opposite to those described before. This shows that at high temperatures the carbon source is not completely used up, which translate into a minor production of omega-3 fatty acids, however, the increase of the biomass suggests that the source of nitrogen is being used (Fig. 3, Fig. 4C). Studies point out that a high proportion of carbon/nitrogen in culture increases the synthesis of DHA on microorganisms that synthetize big amounts of fatty acids.10, 53 In consequence, our results suggest that the ratio carbon:nitrogen is temperature dependent; incubated at 25 °C favors the biomass formation, while at 10 °C the high consumption of the carbon source is consistent with the content of DHA and EPA, diminishing the culture biomass (Fig. 4).
Finally, is important to consider the quality of the lipids produced by strain 34-2, with the essential fatty acids as the DHA, EPA and the linoleic acid (Table 1). This confers a great interest in the clinical area, particularly on treatments where inflammation is a key physiological response on several pathologies.54, 55 On the other hand, the profile of fatty acids presents a potential for the production of biofuels, particularly on cultures supplemented with glucose at a temperature of 25 °C (Table 1 and Fig. 5). This is due to the fact that production of biofuels requires high quantities of fatty acids, mainly C16 and C18 that allow a major oxidative and thermal stability.4, 28, 56, 57 However, different areas with potential in biotechnology are still poorly understood such as; presence of viruses, extracellular polysaccharides, osmolyte systems, their role in organic matter decomposition, the biological association with marine invertebrates, potential in bioremediation, compatible solute and heavy metals resistance.
In conclusion, this research provides an identification of the diversity in Antarctic Thraustochytrids. The phylogenetic analysis allows the classification of the strain 34-2 with a 99% of identity with the species T. kinnei. The total lipid content of this strain suggests that it offers potential biotechnological applications such as production of biofuels and nutritional supplements. These results allow us to focus on molecular studies to determine the effects of the temperature on the expression of the genes that codify for enzymes present on the LC-PUFAs biosynthetic pathway and other bioactive compounds which could act at lower or room temperatures.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors wish to thank Mauricio Quiroz for his valuable assistance in microscopy work and Barbara Burmeister for proof-reading the manuscript. This work was supported by DIULA 09/14 (AG), PROJECT 79112042 from PROGRAMA PAI-CONICYT (FG), Grant Basales Usa1555 USACH-MECESUP to MT, Fund Research Support 30/2014 DPI Universidad Autónoma de Chile to (GC) and CONICYT Doctoral Fellowship N° 21130177 and Chilean Antarctic Institute Grants DG_07-16 (PP).
Associate Editor: Dra. Vania Melo
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bjm.2017.01.011.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Raghukumar S. Bacterivory: a novel dual role for Thrausochytrids in the sea. Mar Biol. 1998;113:165–169. [Google Scholar]
- 2.Raghukumar S. Ecology of the marine protists, the Labyrinthulomycetes (Thraustochytrids and Labyrinthulids) Eur J Protistol. 2002;38:127–145. [Google Scholar]
- 3.Gupta A., Barrow C.J., Puri M. Omega-3 biotechnology: Thraustochytrids as a novel source of omega-3 oils. Biotechnol Adv. 2012;30:1733–1745. doi: 10.1016/j.biotechadv.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Lee Chang K.J., Nichols C.M., Blackburn S.I., Dunstan G., Koutoulis A., Nichols P.D. Comparison of Thraustochytrids Aurantiochytrium sp., Schizochytrium sp., Thraustochytrium sp. and Ulkenia sp. for production of biodiesel, long-chain Omega-3 oils, and Exopolysaccharide. Mar Biotechnol (New York, N.Y.) 2014;16:396–411. doi: 10.1007/s10126-014-9560-5. [DOI] [PubMed] [Google Scholar]
- 5.Johnson M.B., Wen Z. Production of biodiesel fuel from the microalga Schizochytrium limacinum by direct transesterification of algal biomass. Energy Fuel. 2009;23:5179–5183. [Google Scholar]
- 6.Khanna M., Crago C.L., Black M. Can biofuels be a solution to climate change? The implications of land use change-related emissions for policy. Interface Focus. 2011;1:233–247. doi: 10.1098/rsfs.2010.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naylor R.L., Hardy R.W., Bureau D.P. Feeding aquaculture in an era of finite resources. Proc Natl Acad Sci USA. 2009;106:15103–15110. doi: 10.1073/pnas.0905235106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pauly D., Christensen V., Guenette S. Towards sustainability in world fisheries. Nature. 2002;418:689–695. doi: 10.1038/nature01017. [DOI] [PubMed] [Google Scholar]
- 9.Leander C.A., Porter D., Leander B.S. Comparative morphology and molecular phylogeny of Aplanochytrids (Labyrinthulomycota) Euro J Protistol. 2004;40:317–328. [Google Scholar]
- 10.Raghukumar S. Thraustochytrid marine protists: production of PUFAs and other emerging technologies. Mar Biotechnol. 2008;10:631–640. doi: 10.1007/s10126-008-9135-4. [DOI] [PubMed] [Google Scholar]
- 11.Damude H.G., Kinney A.J. Engineering oilseed plants for a sustainable, land-based source of long chain polyunsaturated fatty acid. Lipids. 2007;42:179–185. doi: 10.1007/s11745-007-3049-1. [DOI] [PubMed] [Google Scholar]
- 12.Funk C.D. Prostagladins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 13.Qiu X. Biosynthesis of docosahexaenoic acid (DHA, 22:6-4, 7, 10, 13, 16, 19): two distinct pathways. Prostaglandins Leukot Essent Fatty Acids. 2003;68:181–186. doi: 10.1016/s0952-3278(02)00268-5. [DOI] [PubMed] [Google Scholar]
- 14.Singh P., Liu Y., Li L., Wang G. Ecological dynamics and biotechnological implications of Thraustochytrids from marine habitats. Appl Microbiol Biotechnol. 2014;13:5789–57805. doi: 10.1007/s00253-014-5780-x. [DOI] [PubMed] [Google Scholar]
- 15.Honda D., Yokochi T., Nakahara T. Molecular phylogeny of Labyrinthulids and Thraustochytrids based on the sequencing of 18S ribosomal RNA gene. J Eukaryot Microbiol. 1999;46:637–647. doi: 10.1111/j.1550-7408.1999.tb05141.x. [DOI] [PubMed] [Google Scholar]
- 16.Yang H.L., Lu C.K., Chen S.F., Chen Y.M., Chen Y.M. Isolation and characterization of Taiwanese heterotrophic microalgae: screening of strains for docosahexaenoic acid (DHA) production. Mar Biotechnol. 2010;12:173–185. doi: 10.1007/s10126-009-9207-0. [DOI] [PubMed] [Google Scholar]
- 17.Lewis T.E., Nichols P.D., Mc Meekin T.A. The biotechnological potential of Thraustochytrids. Mar Biotechnol. 1999;1:580–587. doi: 10.1007/pl00011813. [DOI] [PubMed] [Google Scholar]
- 18.Shene C., Leyton A., Rubilar M., Pinelo M., Acevedo F., Morales E. Production of lipids and docosahexasaenoic acid (DHA) by a native Thraustochytrium strain. Eur J Lipid Sci Tech. 2013;115:890–900. [Google Scholar]
- 19.Barnes D.K.A., Hodgson D., Convey P., Allen C.S., Clarke A. Incursion and excursion of Antarctic biota: past present and future. Global Ecol Biogeogr. 2006;15:121–142. [Google Scholar]
- 20.De Wever A., Leliaert F., Verleyen E. Hidden levels of phylodiversity in Antarctic green algae: further evidence for the existence of glacial refugia. Proc Biol Sci R Soc. 2009;276:3591–3599. doi: 10.1098/rspb.2009.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffiths H.J. Antarctic marine biodiversity – what do we know about the distribution of life in the southern ocean? PLoS ONE. 2010;5:e11683. doi: 10.1371/journal.pone.0011683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bahnweg G., Sparrow F.K. Four new species of Thraustochytrium from Antarctic regions, with notes on the distribution of zoosporic fungi in the Antarctic marine ecosystems. Am J Bot. 1974;61:754–766. [Google Scholar]
- 23.Moro I., Negrisolo E., Callegaro A., Andreoli C. Aplanochytrium stocchinoi: a new Labyrinthulomycota. Protist. 2003;154:331–340. doi: 10.1078/143446103322454103. [DOI] [PubMed] [Google Scholar]
- 24.Meng X., Yang J., Xu X., Zhang L., Nie Q., Xian M. Biodiesel production from oleaginous microorganisms. Renew Energ. 2009;34:1–5. [Google Scholar]
- 25.Metz J.G., Roessler P., Facciotti D. Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science. 2001;293:290–293. doi: 10.1126/science.1059593. [DOI] [PubMed] [Google Scholar]
- 26.Miller M.R., Nichols P.D., Carter C.G. Replacement of fish oil with Thraustochytrid Schizochytrium sp. L oil in Atlantic salmon parr (Salmo salar L.) diets. Comp Biochem Physiol A: Mol Integr Physiol. 2007;148:382–392. doi: 10.1016/j.cbpa.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Gavrilescu M., Chisti Y. Biotechnology-a sustainable alternative for chemical industry. Biotech Adv. 2005;23:471–499. doi: 10.1016/j.biotechadv.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Ratledge C. Microbial oils: an introductory overview of current status. OCL. 2013;20:D602. [Google Scholar]
- 29.Ratledge C., Wynn J. The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv Appl Microbiol. 2002;51:1–51. doi: 10.1016/s0065-2164(02)51000-5. [DOI] [PubMed] [Google Scholar]
- 30.Ganuza E., Izquierdo M.S. Lipid accumulation in Schizochytrium G13/2S produced in continuous culture. Appl Microbiol Biotechnol. 2007;76:985–990. doi: 10.1007/s00253-007-1019-4. [DOI] [PubMed] [Google Scholar]
- 31.Bremen G.B. Lower marine fungi (Labyrinthulomycetes) and the decay of mangrove leaf litter. Hydrobiologia. 1995;295:89–96. [Google Scholar]
- 32.Hinzpeter I., Stead R., Trujillo L., Vidal J., Shene C. Isolation of Thraustochytrids strains in the coastal zone of Puerto Montt, Chile and evaluation of Docosahexaenoic acid (22:6n-3, DHA) production. Afinidad. 2009;66:482–487. [Google Scholar]
- 33.Mo C., Rinkevich B. A simple, reliable, and fast protocol for Thraustochytrid DNA extraction. Mar Biotechnol. 2001;3:100–102. doi: 10.1007/s101260000069. [DOI] [PubMed] [Google Scholar]
- 34.Mo C., Douek J., Rinkevich B. Development of a PCR strategy for Thraustochytrid identification based on 18S r DNA sequence. Mar Biol. 2002;140:883–889. [Google Scholar]
- 35.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 36.Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 37.Thompson J.D., Higgins D.G., Gibson T.J., Clustal W. improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position – specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamura K., Dudley J., Nei M., Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 39.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 40.Lewis T.E., Nichols P.D., Mac Meekin T.A. Evaluation of extraction methods for recovery of fatty acids from lipid producing microheterotrophs. J Microbiol Meth. 2000;43:107–116. doi: 10.1016/s0167-7012(00)00217-7. [DOI] [PubMed] [Google Scholar]
- 41.Ramírez-Zacarías J., Castro-Muñozledo F., Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochem Cell Biol. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 42.Bongiorni L., Pusceddu A., Danovaro R. Enzymatic activities of epiphytic and benthic Thraustochytrids involved in organic matter degradation. Aquat Microb Ecol. 2005;41:299–305. [Google Scholar]
- 43.Raghukumar S. Morphology, taxonomy and ecology of thraustochytrids and labyrinthulids, the marine counterparts of zoosporic fungi. In: Dayal R.M.D., editor. Advances in Zoosporic Fungi. Publications Pvt. Ltd.; New Delhi: 1996. pp. 35–60. [Google Scholar]
- 44.Matsuda T., Sakaguchi K., Hamaguchi R. Analysis of D12- fatty acid desaturase function revealed that two distinct pathways are active for the synthesis of PUFAs in T. aureum ATCC 34304. J Lipid Res. 2012;53:1210–1223. doi: 10.1194/jlr.M024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagano N., Sakaguchi K., Taoka Y. Detection of genes involved in fatty acid elongation and desaturation in Thraustochytrid marine eukaryotes. J Oleo Sci. 2011;60:475–481. doi: 10.5650/jos.60.475. [DOI] [PubMed] [Google Scholar]
- 46.Jain R., Raghukumar S., Sambaiah K., Kumon Y., Nakahara T. Docosahexaenoic acid accumulation in Thraustochytrids: search for the rationale. Mar Biol. 2007;151:1657–1664. [Google Scholar]
- 47.Jain R., Raghukumar S., Chandramohan D. Enhanced production of polyunsaturated fatty acid docosahexaenoic acid by Thraustochytrid protists. Mar Biotechnol. 2004;6 59–S65. [Google Scholar]
- 48.Singh A., Ward O.P. Microbial production of docosahexaenoic acid (DHA, C22:6) Adv Appl Microbiol. 1997;45:271–312. doi: 10.1016/s0065-2164(08)70266-1. [DOI] [PubMed] [Google Scholar]
- 49.Nichols P.D., Blackburn S.I., Green A.G. Omega-3 oils down under – an update. Inform. 2004;15:383–385. [Google Scholar]
- 50.Lee Chang K.J., Dunstan G., Abell G. Biodiscovery of new Australian Thraustochytrids for production of biodiesel and long-chain omega-3 oils. Appl Microbiol Biotechnol. 2012;93:2215–2231. doi: 10.1007/s00253-011-3856-4. [DOI] [PubMed] [Google Scholar]
- 51.Ashford A., Barclay W.R., Weaver C., Giddings T.H., Zeller S. Electron microscopy may reveal structure of docosahexaenoic acid-rich oil within Schizochytrium sp. Lipids. 2000;35:1377–1386. doi: 10.1007/s11745-000-0655-2. [DOI] [PubMed] [Google Scholar]
- 52.Nakahara T., Yokochi T., Higashihara T., Tanaka S., Yaguchi T., Honda D. Production of docosahexaenoic and docosapentaenoic acid by Schizochytrium sp. isolated from Yap islands. J Am Oil Chem Soc. 1996;73:1421–1426. [Google Scholar]
- 53.Bowles R.D., Hunt A.E., Bremer G.B., Duchars M.G., Eaton R.A. Long-chain n-3 polyunsaturated fatty acid production by members of the marine protistan group the Thraustochytrids: screening of isolates and optimization of docosahexaenoic acid production. J Biotechnol. 1999;70:193–220. [Google Scholar]
- 54.Serhan C.N., Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153:200–215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wall R., Ross R.P., Fitzgerald G.F., Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- 56.Falk O., Meyer-Pitroff R. The effect of fatty acid composition on biodiesel oxidative stability. Eur J Lipid Sci Technol. 2004;106:837–843. [Google Scholar]
- 57.Fisher L., Nicholls D., Sanderson K. Production of biodiesel. World Intellect Prop Organ. 2008:067605. [Google Scholar]
- 58.Honda D., Yokochi Y., Nakahara T., Erata M., Higashihara T. Schizochytrium limacinum sp. Nov., a new thraustochytrid from a mangrove area in the west Pacific Ocean. Mycol. Res. 1998;102(4):439–448. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.