Abstract
Background
Hyperkinetic dysarthria is characterized by abnormal involuntary movements affecting respiratory, phonatory, and articulatory structures impacting speech and deglutition. Speech–language pathologists (SLPs) play an important role in the evaluation and management of dysarthria and dysphagia. This review describes the standard clinical evaluation and treatment approaches by SLPs for addressing impaired speech and deglutition in specific hyperkinetic dysarthria populations.
Methods
A literature review was conducted using the data sources of PubMed, Cochrane Library, and Google Scholar. Search terms included 1) hyperkinetic dysarthria, essential voice tremor, voice tremor, vocal tremor, spasmodic dysphonia, spastic dysphonia, oromandibular dystonia, Meige syndrome, orofacial, cervical dystonia, dystonia, dyskinesia, chorea, Huntington’s Disease, myoclonus; and evaluation/treatment terms: 2) Speech–Language Pathology, Speech Pathology, Evaluation, Assessment, Dysphagia, Swallowing, Treatment, Management, and diagnosis.
Results
The standard SLP clinical speech and swallowing evaluation of chorea/Huntington’s disease, myoclonus, focal and segmental dystonia, and essential vocal tremor typically includes 1) case history; 2) examination of the tone, symmetry, and sensorimotor function of the speech structures during non-speech, speech and swallowing relevant activities (i.e., cranial nerve assessment); 3) evaluation of speech characteristics; and 4) patient self-report of the impact of their disorder on activities of daily living. SLP management of individuals with hyperkinetic dysarthria includes behavioral and compensatory strategies for addressing compromised speech and intelligibility. Swallowing disorders are managed based on individual symptoms and the underlying pathophysiology determined during evaluation.
Discussion
SLPs play an important role in contributing to the differential diagnosis and management of impaired speech and deglutition associated with hyperkinetic disorders.
Keywords: Hyperkinetic dysarthria, dystonia, dyskinesia, chorea, myoclonus, essential vocal tremor
Introduction
Speech disorders arising from abnormal activity affecting the cortico-basal ganglia-thalamocortical circuitry causing involuntary movements are broadly classified as hyperkinetic dysarthria.1–6 Hyperkinetic dysarthria is generally characterized by abnormal voice, resonance, speech sound production, and prosody that may impact intelligibility.1,2,7 The involuntary movements that are characteristic of hyperkinetic dysarthria significantly impact communication, deglutition, and quality of life.1,2,8–14 Approximately 5–7% of adult patient referrals to speech–language pathologists (SLPs) are represented by individuals with voice and motor speech disorders.15,16 Within the Speech Pathology program in the Department of Neurology at Mayo Clinic in Rochester, MN, the prevalence of individuals diagnosed with motor speech disorders (i.e., those diagnosed with dysarthria or apraxia of speech) from 1993 to 2008 was estimated at 57%.7 Within the latter group, hyperkinetic dysarthria represented approximately 20% of individuals diagnosed with dysarthria.7 The role of the SLP is to contribute to the diagnosis and management of impaired communication and deglutition of this population as part of a multidisciplinary team.16
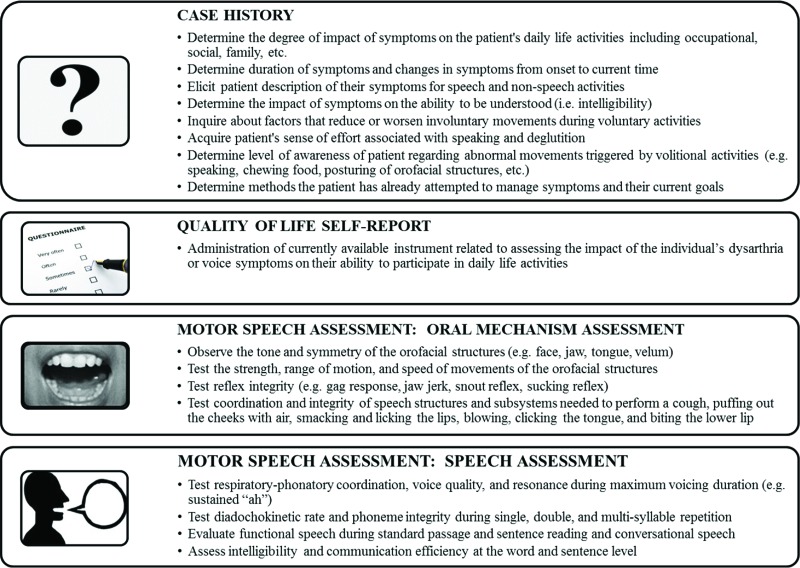
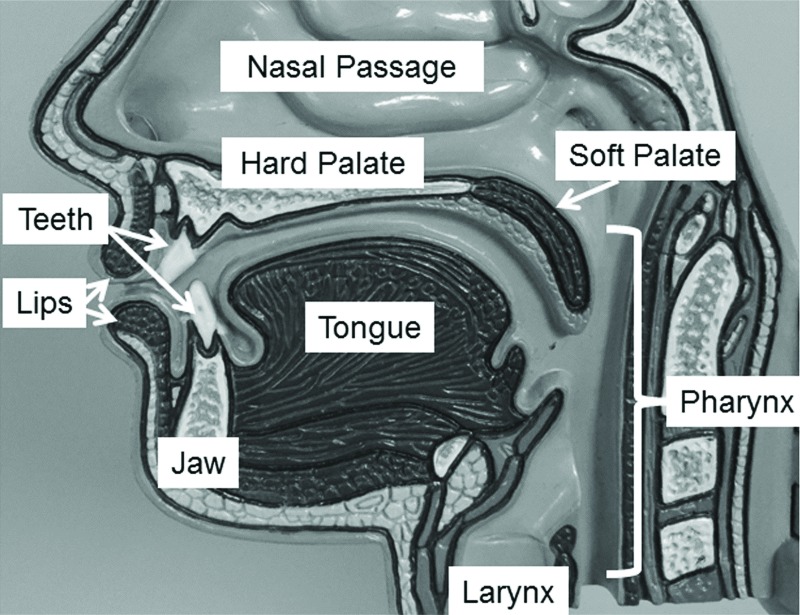
The standard SLP speech and swallowing evaluation includes 1) case history; 2) examination of the tone, symmetry, and sensorimotor function of the speech structures (see Figures 1 and 2) during non-speech, speech and deglutition activities (i.e., cranial nerve assessment); 3) evaluation of speech characteristics; and 4) patient self-report of the impact of their disorder on activities of daily living.1,2,7,17–20 Additional evaluation approaches may be incorporated to further characterize concomitant communication disorders (e.g., other dysarthria types, language and cognitive disorders) and inform the differential diagnosis.1,2,7,17,19 The SLP also provides behavioral modifications that improve communication and deglutition function.1,7,17,21,22 SLP management of dysarthria generally addresses respiratory, voice, resonance, articulation, or speaking patterns to improve intelligibility and comprehensibility.7,11,21,23,24 Augmentative and alternative communication approaches may also be used.17,25 Abnormal deglutition, or dysphagia, is managed based on the pathophysiology identified during the evaluation.7,8,26,27
Figure 1. Components of a speech motor evaluation. This figure describes the typical activities associated with the case history, quality of life self-report, and motor speech assessment (oral mechanism and speech assessment portions).
Figure 2. Mid-sagittal view of the speech structures. The speech structures shown in this figure are involved in breathing, articulation and resonance during speech and voice production. The speech structures include the tongue, jaw, lips, larynx, soft palate, and pharynx (i.e. pharyngeal wall).
The purpose of this review is to describe the standard SLP approach to evaluation and treatment of hyperkinetic dysarthria and associated dysphagia. The characteristics of hyperkinetic dysarthria populations frequently seen by SLPs will be described including patients with chorea/Huntington’s disease, myoclonus, focal and segmental dystonia, and essential vocal tremor.
Methods
A review of the literature was conducted using PubMed, EBSCO academic search, Web of Science Core Collection, Google Scholar, Library of Congress, and the American Speech-Language-Hearing Association (ASHA) online professional practice documents. Only publications considered relevant to current SLP clinical evaluation and treatment of individuals with hyperkinetic dysarthria were retrieved. A search was conducted for publications containing specific disorder-based terms including 1) hyperkinetic dysarthria, essential voice tremor, voice tremor, vocal tremor, spasmodic dysphonia, spastic dysphonia, oromandibular dystonia, Meige syndrome, orofacial, dystonia, chorea, Huntington’s disease, myoclonus, and clinical evaluation; and treatment terms including 2) speech–language pathology, speech pathology, evaluation, assessment, dysphagia, swallowing, treatment, and management. Only references written in English between November 1968 and June 2017 were included. A total of 186 references were identified of which those directly addressing speech and deglutition and speech–language pathology evaluation and treatment with relevant hyperkinetic dysarthria populations, or commonly used speech and dysphagia evaluation tools and treatment options, were selected for this review resulting in a total of 159 references.
This review is organized to describe 1) the typical SLP speech evaluation completed with individuals with hyperkinetic dysarthria; 2) clinical and instrumented approaches for evaluating dysphagia; 3) general treatment approaches for dysphagia and dysarthria; and 4) specific characteristics and unique clinical approaches for evaluating and treating each of the highlighted hyperkinetic dysarthria populations.
Speech evaluation of hyperkinetic dysarthria
Case history
The SLP evaluation is influenced by several factors acquired during the case history. SLPs frequently observe abnormal movements associated with posture, orofacial, speech patterns, or gait in those with hyperkinetic dysarthria. During the case history discussion, questions are posed to elicit the patient’s perspective regarding their involuntary abnormal movements. Of particular interest are factors that modulate the involuntary movements associated with the patient’s perception of increased or lessened effort levels during speaking.7 Individuals are often aware of difficulty speaking, chewing, or swallowing but unaware of abnormal movements triggered by volitional activities.7 Patients may report a sense of tightness and difficulty controlling the movements of specific structures or their voice. Of critical importance are specific conditions that improve or eliminate the unwanted movements or improve the quality of speech in those with hyperkinetic dysarthria. This kind of information can distinguish those with hyperkinetic dysarthria from other forms of dysarthria or functional speech disorders. For example, speakers with either oromandibular dystonia or functional speech disorders may report that their speech is normal for the first few minutes after awakening in the morning followed by deterioration throughout the day. However, individuals with functional speech disorders often exhibit worsening of abnormal movements and disrupted speech during structured assessment tasks (e.g., sentence or paragraph reading, sustained phonation as described below) compared with conversational speech. In contrast, speakers with dystonia, including spasmodic dysphonia, may demonstrate improvement in symptoms, such as reduction of laryngeal tension, during sustained phonation compared to connected speech (e.g., conversation, oral reading). In general, speakers with hyperkinetic dysarthria typically describe a sensation of “tightening” in the affected muscles or structures associated with abnormal movements that is beyond their control. Speakers with functional disorders report a similar sense of tightening, but also describe their movement disorder as associated with paralysis, numbness, or heaviness.
Quality of life self-report instruments
The case history portion of the speech evaluation is supplemented by administration of a quality of life (QOL) self-report instrument.28 QOL instruments provide an estimation of the degree to which the disorder impacts the individual’s participation in activities of daily living. Several instruments are available to determine the QOL impact of dysarthria and associated voice problems (see Table 1).29–36 Information obtained from selected instrument(s) can inform SLP evaluation tasks and management goals.
Table 1. Examples of Instruments Developed to Evaluate the Impact of Dysarthria or Voice on Quality of Life.
| Quality of Life Instruments for Dysarthria and Voice | General Description | Reference |
|---|---|---|
| Dysarthria Impact Profile | 48 statements are rated on a 5-point scale (1 = strongly agree to 5 = strongly disagree) that reflect 5 aspects of dysarthria impact: 1) The effect of dysarthria on the person, 2) Acceptance of dysarthria, 3) How the individual feels when others react, 4) Impact on communication with others, and 5) Other worries and concerns | Walshe et al.29 |
| Living with dysarthria | 50 statements divided across 10 sections of possibly impact that are rated from 1 (totally disagree) to 6 (fully agree) | Hartelius et al.30 |
| QOL for the dysarthric speaker questionnaire (QOL-DyS) | 40-item instrument in which each statement is rated from 0 (never) to 4 (always) across the domains of speech characteristics, situational difficulty, compensatory strategies, and perceived reactions of others. | Piacentini et al.31 |
| Voice Handicap Index | 30-item statements that are rated on a 5-point scale (0 = never to 4 = always) addressing 3 subscales of physical, functional and emotional impact of the voice problem on daily life activities | Jacobson et al.32 |
| Voice-Related Quality of Life (V-RQOL) | 10 statements are rated on a 5-point scale from 1 (none, not a problem) to 5 (problem is as “bad as it can be”) regarding voice function over the past 2 weeks. A standard score is then calculated across each domain of social-emotional, physical functioning, and total score | Hogikyan et al.33 |
| Voice Activity and Participation Profile (VAPP) | Uses a 10-cm visual analog scale to judge the degree to which the individual is affected as described in each of 28 statements (left side of line indicates never affected and right side represents always affected). Statements represent such aspects of voice use as Effect on the job, daily communication, social communication, and emotion | Ma and Yiu34 |
| Voice Symptom Scale (VoiSS) | 44-question items rated on a 5-point scale from 1 (never) to 5 (all the time). Items are linked to five domains including communication problems, throat infection, psychosocial distress, voice sound and variability, and phlegm | Deary et al.35 |
| Communication Participation Item Bank (CPIB) | The short form version of this instrument includes 10 question items rated on a scale from 0 (very much) to 3 (not at all). Items reflect the degree the individual experiences interference with participation in various situations due to their disorder | Baylor et al.36 |
Motor speech evaluation
The motor speech evaluation (Current Procedural Terminology (CPT) 92522) includes evaluation of the speech structures and their function to inform diagnostic impressions and management planning. The motor speech evaluation includes examination of the tone, symmetry, and function of speech structures during non-speech and speech activities.1,2,6,7,37 Formal instruments are available to SLPs to facilitate systematic evaluation and guide differential diagnosis of dysarthria types.37,38 However, SLPs with expertise in motor speech disorders can implement systematic evaluation of respiration, articulation, voicing, resonance, and prosody using clinical methods developed nearly 40 years ago.1,2,7 Table 2 provides a simplified overview of the auditory–perceptual features used to classify each type of dysarthria based on the work of Darley, et al.1,2 The motor speech evaluation may also identify impairments likely to cause dysphagia.7,14,17
Table 2. Simplified Overview of the Dysarthria Classification System Created by Darley et al.1 Based upon Clusters of Auditory–Perceptual Features.
| General Perceptual Features | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of Dysarthria | Articulation Inaccuracy | Vowel Distortions | Prosodic Abnormalities | Breathy Voice Quality | Harsh Voice Quality | Strained–Strangled Voice Quality | Hypernasality | Nasal Emission | Mono Pitch | Mono Loudness |
| Spastic | √ | √ | √ | √ | √ | √ | √ | √ | ||
| Flaccid | √ | √ | √ | √ | √ | √ | √ | |||
| Mixed | √ | √ | √ | √ | √ | √ | √ | √ | ||
| Ataxic | √ | √ | √ | √ | ||||||
| Hypokinetic | √ | √ | √ | √ | √ | |||||
| Hyperkinetic (chorea) | √ | √ | ||||||||
| Hyperkinetic (dystonia) | √ | √ | √ | √ | √ | √ | √ | √ | ||
The core components of a motor speech evaluation include examination of the oral mechanism and speech assessment. Examination of the oral mechanism (see Figure 1) entails observation of speech structure symmetry, tone, strength, range, and speed of volitional non-speech movements, including reflex testing elucidating reduced cortical pathway inhibition of primitive reflexes such as the jaw jerk, snout, sucking, and palmomental reflexes, or reduced/absent presence of the gag and cough reflexes typically present in adults.6,7,39,40 Motor speech testing evaluates speech behaviors under varied conditions to determine 1) respiratory–phonatory coordination during sustained voicing of vowels, 2) diadochokinesis testing of “speech-like” movements during rapid single, double, and multisyllabic utterance repetition (alternate motion rate, or AMR (repetition of a single syllable such as “puh-puh-puh…” or “tuh-tuh-tuh…” or “kuh-kuh-kuh…”) and sequential motion rate, or SMR (repetition of syllable sequences such as “puh-tuh-kuh…”), and 3) speech characteristics during production of single words, sentence and paragraph reading, and conversation.1,2,6,7 Auditory–perceptual impressions are formed during these tasks regarding voice quality (e.g., strained, rough, breathy), resonance (e.g., hypernasal, hyponasal), articulatory precision, prosody (e.g., speaking tempo, syllabic stress, pitch, and loudness variation), intelligibility, and comprehensibility.1,2,7,17,41 Intelligibility refers to the ability of a listener to understand a speaker based solely on acoustic signal information.23,42–44 Comprehensibility reflects the level of listener understanding of information shared by the speaker based on a combination of visible and contextual information paired with the acoustic signal.17,45,46 Another measure related to intelligibility is communication efficiency, or the rate by which an individual can successfully convey information to listeners.41 Intelligibility can be informally reported from the speech motor evaluation,44,47 or quantified using standardized tools.42,48 The observations made during the motor speech evaluation enable the SLP to determine the presence/absence and type and severity of dysarthria.
General motor speech patterns of hyperkinetic dysarthria
The characteristics of hyperkinetic dysarthria vary considerably across patients. The nature and timing of abnormal speech structure movements as well as strategies used by the speaker to control abnormal movements can be gleaned from speech production characteristics. Interestingly, affected speech structures exhibit normal strength as well as speed and range of motion, though involuntary movements may occur symmetrically, asymmetrically, or unilaterally.1,2,6,7 Commonly observed speech patterns in those with hyperkinetic dysarthria include unexpected variations in pitch or loudness, inappropriate pauses, constant or intermittent dysphonia, constant or intermittent hyper- or hyponasality, articulatory imprecision, and slow speaking rate due to frequent or extended pause durations.1,2,7,8,20,49–63
The sustained voicing task elucidates minor variations in vocal tract configuration in those with hyperkinetic dysarthria. For example, vocal unsteadiness and tremor can be perceived, as can subtle unintended articulatory movements associated with production of the vowel, “ah”.1,64–70 In addition, oscillation of the jaw, soft palate, tongue, larynx, or pharyngeal wall is reflected by involuntary rhythmic voice modulations during sustained voice production. Respiratory musculature may also produce rhythmic or sudden involuntary muscular contractions affecting loudness levels during sustained voicing and speech tasks. Abrupt or unpredictable distortions of vowel production may implicate dystonic, or choreiform contractions affecting the respiratory system or articulators.
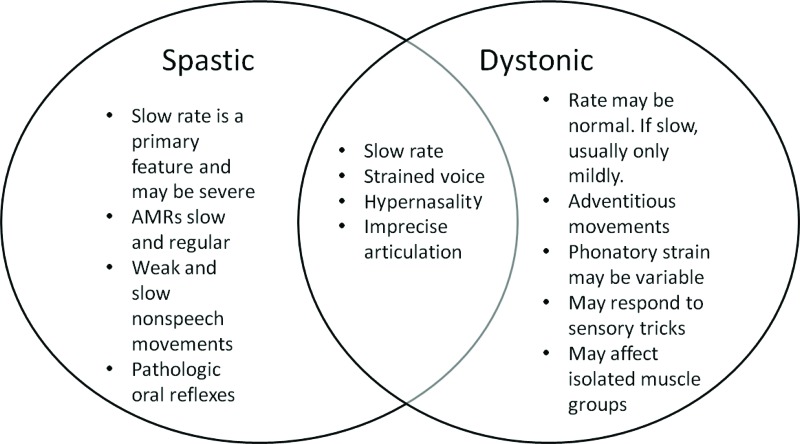
In summary, the motor speech evaluation is critical for differential diagnosis of hyperkinetic dysarthria and other dysarthria types. The hallmark feature of hyperkinetic dysarthria is the presence of involuntary movements. Table 3 provides a summary of the typical characteristics associated with each of the forms of hyperkinetic dyarthria reviewed in this paper. Although involuntary movements are not typical of other dysarthria types, speech features may overlap across more than one type of dysarthria (see Figure 3). This requires the SLP to carefully consider the entirety of case history, self-report and motor speech evaluation outcomes to differentially diagnose individuals. Further, the identification of a specific type of dysarthria, with or without concomitant language and cognitive deficits, provides a differential diagnosis indicative of specific neurologic pathology.1,2,6,7
Table 3. Motor Speech Evaluation Characteristics Associated with Specific Hyperkinetic Dysarthrias.
| Hyperkinetic Dysarthria | Chorea/Huntington’s Disease | Myoclonus | Oromandibular Dystonia | Hyoid Dystonia | Spasmodic Dysphonia | Essential Vocal Tremor |
|---|---|---|---|---|---|---|
| Physical findings | ||||||
| Quick non-rhythmic involuntary movements of speech structures at rest or during sustained postures | X | |||||
| Rapid rhythmic involuntary movements of the soft palate, pharyngeal, or laryngeal structures | X | |||||
| Sensory “tricks” | X | X | X | |||
| Involuntary contraction of anterior neck muscles associated with the hyoid bone resulting in “neck tightness” | X | |||||
| Involuntary spasms of laryngeal musculature during speech production | X | |||||
| Involuntary oscillation (tremor) of the head, tongue, jaw, lips, soft palate, pharynx, larynx, or respiratory musculature. | X | |||||
| Impaired volitional movement of the jaw (particularly opening or closing) that can sometimes involve the lips, tongue, and soft palate | X | |||||
| Speech characteristics | ||||||
| Voice stoppages | X | X | ||||
| Transient breathiness | X | |||||
| Vocal tremor | X | |||||
| Beat-like modulation of prolonged vowel | X | |||||
| Perceived clicking sound during speaking | X | |||||
| Intermittent hypernasality | X | X | ||||
| Inappropriate vocal noises | X | |||||
| Intermittent strained–strangled voice | X | |||||
| Intermittent breathy voice breaks | X | |||||
| Slow and irregular AMRs | X | |||||
| Variable speaking tempo | X | |||||
| Variable pitch and loudness patterns during speaking | X | X | X | |||
| Variable duration of sustained phonation | X | |||||
| Imprecise articulation and co-articulation | X | |||||
| Altered resonance | X | X | ||||
| Slowed speaking tempo | X | X | ||||
Figure 3. Example of shared speech features by two types of dysarthria. Speech characteristics and physical findings may be shared requiring that the entire clinical picture of individuals be considered to successfully differentially diagnose each type.
Swallowing evaluation
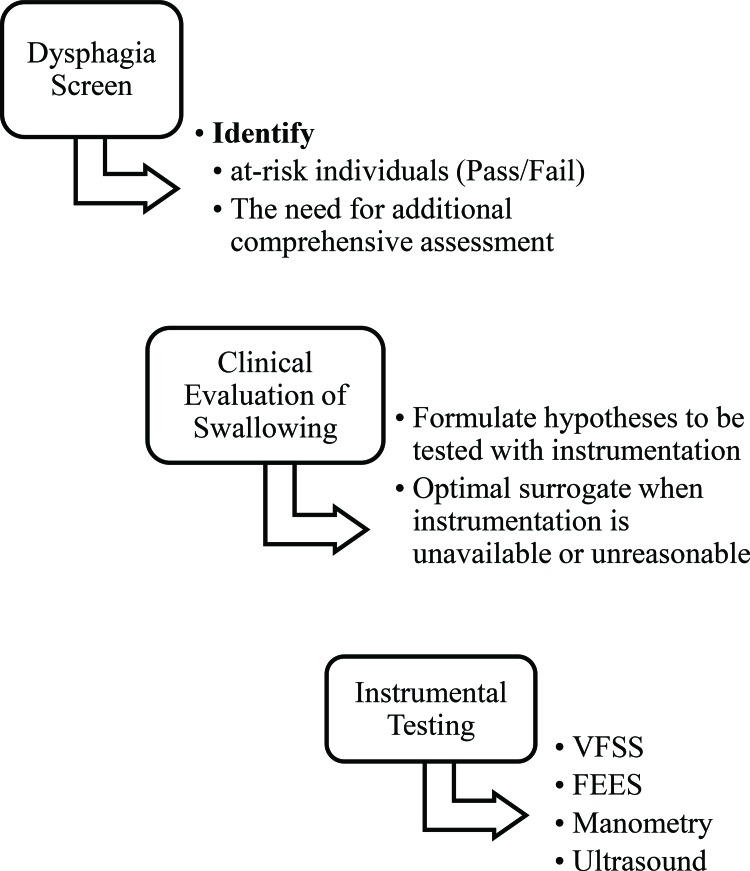
Evaluation of deglutition in those with hyperkinetic dysarthria begins during the motor speech evaluation. When dysphagia signs and symptoms are identified, additional testing methods are required to determine the pathophysiology of the individual’s dysphagia issues for optimal management. This section describes the process for evaluating dysphagia in adults recommended by ASHA (see Figure 4).26,27,71–76
Figure 4. Process for identifying and evaluating dysphagia. This figure illustrates the recommended clinical practice pattern for speech-language pathologist identification and evaluation of individuals with dysphagia.
Screening
Individuals suspected of dysphagia are typically referred to a SLP for screening, or for evaluation of dysphagia complaints.77 The screening process determines the likelihood that an individual is at risk for dysphagia and determines whether additional evaluation is needed to define the nature and severity of the problem. A dysphagia screening can be conducted by interview, questionnaire, or a brief swallowing assessment by a trained professional (e.g., SLP, Registered Nurse (RN), Licensed Practical Nurse (LPN)).27,77
Clinical evaluation of swallowing
Individuals failing the dysphagia screen undergo a clinical evaluation of swallowing by the SLP during which the following components are completed: 1) case history, 2) sensorimotor cranial nerve assessment relevant to the aerodigestive structures involved in swallowing (i.e., cranial nerves I, V, VII, IX, X, XI, XII), 3) patient self-report of function, severity, and emotional impact of dysphagia on activities of daily living, 4) evaluation of deglutition with and without food administration that is systematically varied by volume, consistency, and temperature as related to reported difficulties, and 5) evaluation of eating safety and strategies associated with modified diet, swallowing postures and maneuvers.26,27,72,78
Based on the findings of the clinical evaluation of swallowing, the SLP may be able to recommend a management plan to address signs and symptoms of dysphagia. The SLP might also recommend additional instrumental evaluation (e.g., videofluoroscopic swallow study [VFSS], flexible endoscopic evaluation of swallowing [FEES]) or referral to other specialties (e.g., gastroenterology, dietician, otolaryngology, etc.).27,71–74 The clinical evaluation of swallowing informs final conclusions regarding the nature and severity of the dysphagia. Observations from the clinical evaluation further suggest specific management strategies for improving swallowing function. The strategies can be applied during instrumental assessment to determine their effectiveness for any specific patient.27,71,73,74,79,80
Instrumental evaluation using FEES and VFSS
The most common instrumental approaches used by SLPs to evaluate dysphagia include FEES and VFSS. Manometry and ultrasound can also be utilized in some cases.81,82 The FEES approach to evaluating deglutition offers a direct observation of the pharyngeal structures during eating.27,74–76,83 This examination can be conducted at bedside in acute and inpatient hospital contexts, or in an outpatient clinic.74,83–86 The advantage of the FEES evaluation is the absence of radiation exposure and the ability to directly observe the bolus pathway as well as residue amount and location within the pharynx during a meal.27,71,84,85,87 FEES can also be used as a means of biofeedback to facilitate improved swallowing and bolus clearance.26 However, FEES does not afford views of the pharynx during the exact moment of swallow (when the pharynx is maximally constricted) unless compromised pharyngeal constriction provides a portal for viewing. In addition, views of the oral cavity are limited to the base of tongue region, so the SLP must consider information gained during the clinical exam to infer the integrity of oral bolus management. Finally, some individuals may not tolerate placement of the endoscope through the nasal passageway.
The VFSS method entails a coordinated appointment with the SLP and radiology. Optimally, the SLP executes one of the currently published standardized protocols to evaluate the oral, pharyngeal, and esophageal stages of deglutition using progressive administration of bolus volumes and consistencies.88,89 In addition, swallowing strategies based upon the clinical evaluation of swallowing findings will be evaluated for effectiveness. The most common strategies tested during the VFSS include bolus modifications (e.g., nectar or honey thick liquids, or liquid swallows following solid boluses), postural adjustments (e.g., head turn, neck flexion, side lean), and swallowing maneuvers (e.g., effortful swallow, or the Mendelsohn maneuver during which patients prolong the highest position of the hyoid bone during a swallow for ∼2 seconds to facilitate clearance of food through the throat into the esophagus).26 Upon completion of VFSS testing, a systematic analysis of the recording is recommended to elucidate the pathophysiology of the dysphagia signs and symptoms and to inform effective treatment approaches.88,89
SLP treatment of hyperkinetic dysarthria
The SLP utilizes evaluation outcomes to determine optimal behavioral or augmentative and alternative strategies (e.g., hand gestures/cues [i.e., unaided communication systems], pictures of symbols or photos, or the use of electronic devices to facilitate communication of messages [i.e., aided communication systems]) to improve the speaker’s intelligibility, comprehensibility, and communication efficiency with particular focus on conditions eliciting the greatest impairment. Several factors influence recommendations for management of hyperkinetic dysarthria including the medical etiology of the problem, prognosis for improvement, level of severity and impact on quality of life, support from family members or caregivers, environment of the patient (e.g., home, assisted living, skilled nursing facility, etc.), and the individual’s motivation level and personal goals.7 In some cases, medical/surgical management is recommended for management of specific types of hyperkinetic dysarthria.7
Common speech treatment approaches
Common SLP behavioral treatment approaches include techniques that optimize respiratory–phonatory coordination techniques for improved pitch and loudness control, phrasing, and consistency of sound production.7,24,90–92 In addition, facilitative speaking strategies may be used to improve comprehensibility by modifying speaking tempo, phrase length, and incorporate purposeful pauses during speaking.7,11,17,91,93 Augmentative and alternative communication strategies may be required to facilitate improved intelligibility and comprehensibility in more severely affected individuals.17,25,90 Potential speech–language pathology treatment approaches for addressing impaired function and coordination of the respiratory, voice or articulatory components of speech production are summarized in Table 4. These treatment approaches currently lack evidence-based outcomes for treatment of hyperkinetic dysarthria. However, these treatment approaches were selected based on their ability to address specific physiologic issues known to impact speech production in those with hyperkinetic dysarthria as well as publications mentioning their use for speech–language pathology treatment of dysarthria, in general (see Table 4).21,24,66,90,91,94–96 For example, the coordination of respiratory function with speech production is critical to speaking with adequate loudness and for speech phrasing. Thus, treatment approaches known to effectively address impaired/weakened respiratory drive and its coordination with speech production are listed as possible treatment approaches for addressing signs and symptoms associated with impaired respiratory drive, or coordination for speech production.24,66,90,93,94,96 Alternatively, speaking patterns can be directly modified using speech rhythm techniques, slowing speaking rate, delayed auditory feedback, or direct magnitude production.17,21,90,91,97 Further, in those with severely impaired speech, alternative and augmentative approaches can be used.25,98 Finally, those with impaired voice function, such as occurs with spasmodic dysphonia and essential vocal tremor, may benefit from respiratory–phonatory coordination approaches and other voice therapy treatment approaches listed, typically supplementary to medical management approaches.66,92,95,99
Table 4. Speech–Language Pathologist Treatment Approaches to Managing Impaired Respiratory, Voice, and Articulatory Functions in Those with Hyperkinetic Dysarthria.
| Hyperkinetic Dysarthria Characteristic | Sign/Symptom | Treatment Options |
|---|---|---|
| Impaired respiratory drive, or coordination for speech production | Reduced or inconsistent loudness | Expiratory muscle strength training |
| Dramatic reduction in loudness during a single breath group during speaking | Lee Silverman Speech Treatment (LSVT) | |
| Inhalation appears inadequate, prolonged, or speaking initiation occurs at unusual locations within the respiratory cycle, or utterance | Maximum inhalation/exhalation tasks, or sustained phonation tasks to improve respiratory/phonatory coordination and steadiness | |
| Few words or syllables produced per breath group, runs out of air before taking a breath | Body positioning to optimize breathing and respiratory efficiency during speaking | |
| Paradoxical movements of the rib cage and abdomen during breathing or speaking | Accent Method of Voice Therapy | |
| Abnormal posture or movements associated with volitional respiratory-phonatory coordination during speaking | Rehearse taking deeper inhalations prior to speaking and implementing increased respiratory effort during speaking | |
| Reduced maximum phonation time (may also indicate impaired voice function) | Rehearse optimal breath groups during phrasing of spoken utterances | |
| Impaired voice function | Laryngeal relaxation techniques such as easy voice onset, yawn-sign, chanting, chewing method | |
| Poor integrity, loudness, and rate of laryngeal diadochokinesis (e.g., ee-ee-ee-ee) | Laryngeal Manipulation | |
| Accent Method of Voice Therapy | ||
| Confidential Voice Technique/Flow Phonation | ||
| Hyperadduction of the vocal folds | Biofeedback during voicing/speech tasks | |
| Impaired speech function | Articulation therapy | |
| Modify speaking rate (typically encourage slower) | ||
| Impaired articulation | Speech rhythm techniques | |
| Abnormal speech pattern or rate | Delayed auditory feedback | |
| Abnormal resonance (e.g., hypernasality) | Direct magnitude production | |
| Augmentative and alternative communication intervention | ||
| Referral for prosthetic device |
Common dysphagia treatment approaches
Treatment approaches for dysphagia in those with hyperkinetic dysarthria are selected to address the underlying pathophysiology elucidated during the evaluation of deglutition. As with speech treatment options, there is a dearth of literature offering evidence-based outcomes regarding dysphagia treatment in those diagnosed with hyperkinetic dysarthria. Therefore, the most commonly used approaches for treating dysphagia are listed in Table 3 offering a comprehensive summary of currently available dysphagia treatment approaches, in general. Literature describing dysphagia in those with hyperkinetic dysarthria commonly reports the use of diet modifications to optimize bolus preparation, cohesive formation and safe clearance during eating due to oral preparation and bolus transport problems (see Table 5).8,9,26,89,100–104 Another common approach identified in this patient population is the use of compensatory strategies that modify posturing or manner of swallowing to improve airway closure and bolus clearance (see Table 5).8,9,26,89,100,101,104,105 In some cases, exercise approaches can be utilized to improve muscle strength and structural performance during mastication and swallowing (see Table 5, indirect treatment).26,27 Adaptations or compensations for improving eating and swallowing safety as well as cognitive contributions to dysphagia may also be recommended (see Table 5).26,27
Table 5. Common Speech–Language pathologist Treatment Approaches to Dysphagia.
| Diet Modification | Compensatory Strategies | Adaptations/Compensations | Indirect Treatment |
|---|---|---|---|
| Regular oral diet | Positional strategies | Assistance with feeding | Progressive resistive tongue exercises |
| PO with modification or dietary restrictions (select from the following): | Neck flexion (i.e., chin tuck) | Verbal cues | Shaker exercises |
| Water protocol between meals only (requires oral hygiene) | Head turn to left or right | Food placement on plate | Masako method |
| Liquids only (broth, nutritional supplements, milkshakes) | Lean or tilt to the right or left | Complete feeding assistance by other person | Expiratory muscle Strengthening exercises |
| Thickened liquids (specify viscosity of thin, nectar, honey, or spoon thickness) | Swallowing maneuvers | Adaptive feeding device or method | Neuromuscular electrical stimulation |
| Puree (specify runny versus thicker viscosity) | Multiple swallows per bolus | Alternate liquids with food | Tongue strengthening and ROM exercises |
| Soft and moist solids (easy to chew and easy to digest: avoid dry, dense, and stringy foods) | Breath hold prior to swallow | Reduce rate of eating | Lip strengthening exercises |
| Medication Form may require modification (e.g., pill, liquid) | Mendelsohn maneuver | Add moisture to dry foods (e.g., gravy, condiments, etc.) | Jaw strengthening and ROM exercises |
| NPO | Effortful swallow | Temperature of food (specify cold, room, hot) | Hawk exercise |
| NPO with supplemental intake | Audible exhalation after the swallow | Small and more frequent meals | Neck flexion against resistance in upright position |
| Supraglottic swallow | Oral tongue/finger sweep | Jaw depression against resistance in upright position | |
| Super supraglottic swallow | Biofeedback approaches (e.g., surface EMG, FEES, etc.) |
Abbreviations: EMG, Electromyography; FEES, Flexible Endoscopic Evaluation of Swallowing; NPO, Nil Per Oral; PO, Per Oral; ROM, Range of Motion.
Specific hyperkinetic disorder characteristics
Chorea/Huntington’s disease
Chorea is characterized by quick non-rhythmic involuntary movements at rest or during attempts to sustain a posture.7,57 The incidence of dysarthria in Huntington’s disease (HD) is estimated at 78%63 to 93%,60 although the prevalence is unknown. Speech features may fluctuate dramatically.7 For example, speech may be perceived as intermittently hypernasal, vacillate between a monotone and excessively variable pitch, or vary from a slow to rapid speech tempo.63 These opposing features reflect both the unpredictability of the abnormal movements affecting speech production as well as the strategies speakers employ to maximize communicative effectiveness.
Descriptions of hyperkinetic dysarthria accompanying HD suggest that dysphonia and disruptions in prosody are prominent and most detrimental to intelligibility.18,58,59,61 This includes weak voice, breathiness, monotone, and voice arrests.15,58–60 Further, shorter and more variable sustained phonation is often observed during the motor speech evaluation. In addition, rapid repetition of syllables (AMRs such as “puh”, “tuh”, or “kuh”) may be irregular in rhythm and variable in loudness.18 There is preliminary evidence to suggest that some of the abnormal speech features observed in HD (e.g., excessive pitch and loudness variations) are exacerbated by the use of antipsychotic medication, whereas precision of vowel articulation may slightly improve with antipsychotic therapy.60 Overall, the severity of hyperkinetic dysarthria is strongly associated with HD disease severity.18
Although dysphagia is considered a complication of HD, it has not been thoroughly studied.10 Primary dysphagia characteristics in those with HD are associated with disrupted timing, amplitude, and force of structural movements in the upper aerodigestive tract as well as from cognitive impairments.9,100–102,106,107 Hyperkinetic features of dysphagia in HD include rapid, unpredictable tongue movements, and premature loss of the bolus into the pharynx prior to swallow initiation.9,100–102,106,107 During swallowing, incoordination between breathing and swallowing may occur in addition to prolonged laryngeal elevation and frequent eructation.9 Cognitive impairments impact the patient’s ability to compensate for these difficulties and may lead to high-risk eating behaviors such as taking large bites.102,107
Myoclonus
Palatal myoclonus (PM) is characterized by rapid rhythmic movements of the velopharynx that remain constant during breathing as well as during volitional and vegetative movements and sleeping.7,108,109 If the amplitude of contractions is low, speech may be unaffected. When movements are larger, the rhythmic contractions may be perceived as beating vocal tremor during sustained phonation or as intermittent hypernasality during speaking. Some individuals perceive a clicking sound in their ear associated with palatal muscle spasms that open and close the Eustachian tube.110 The rhythmic movements are usually readily visible during instrumental assessment.10
The abnormal movements of the soft palate may extend to the pharyngeal and extrinsic laryngeal musculature. In such cases, dysphagia symptoms may reflect the disruption of airway protection timing and impaired bolus transit during swallowing.103,105,111 For example, one case example111 exhibited disruption of breathing related to rhythmic myoclonic jerks affecting the soft palate and larynx associated with difficulty swallowing solid and liquid foods. This individual’s speech and swallowing symptoms were significantly alleviated using a pharmacologic treatment, sodium valproate. Other case examples reported disruption of speech and swallowing due to rhythmic myoclonic jerks affecting the pharynx and larynx.103,105 In these cases, dysphagia was characterized as difficulty initiating swallowing and aspiration during swallowing105 as well as delayed onset of swallowing and laryngeal penetration/aspiration of liquids.103 Safe oral intake for both cases was achieved using compensatory strategies,105 or diet modifications103 for dysphagia treatment (see Table 5).
Dystonias
Speech affected by dystonia is characterized by involuntary activation of isolated muscle groups or diffuse disruption of multiple muscle groups affecting speech production.2,7 Common observations in dystonia are adventitious movements of the orofacial structures during rest, specific tasks (e.g., speaking or eating) or during volitional movements in general. Supporting the diagnosis of hyperkinetic dysarthria of dystonia are variations in performance across tasks and response to sensory tricks. Sensory tricks can include a tactile mechanism (e.g., finger touching the cheek, or chin, or a toothpick touching the lips, etc.), proprioceptive, or kinesthetic effect (e.g., tilting of the head, or bite block to limit jaw movements). The SLP can explore the use of sensory tricks that reduce symptoms and devise strategies using sensory tricks to facilitate reduced symptoms during conversations.7
The impact of dystonia on deglutition varies. Speech-induced dystonias are often suppressed during mastication and have limited impact on deglutition. When the movements interfere with deglutition, weight loss may occur.104 Alternatively, some oral dystonias are specifically triggered by eating.112 Hyperkinesias can cause abnormal movements that disrupt timing of airway protection and interfere with bolus propulsion,8,113 thereby increasing risk of aspiration, malnutrition, and dehydration.
Oromandibular dystonia
Oromandibular dystonia, by strict definition, affects jaw musculature, with rare involvement of abnormal movements of the tongue, lips, or soft palate.8,50 Focal dystonias can also affect the lips, tongue, and soft palate.2,8,50 Oromandibular dystonia (OMD) accompanied by blepharospasm may be termed Meige’s syndrome.7,114
The hyperkinetic dysarthria accompanying oromandibular dystonia may be characterized by imprecise articulation, including impaired co-articulation (i.e., accommodation of speech movements to surrounding speech sounds), hypernasality, breathiness, and disrupted fluency.8 Some speakers with oromandibular dystonia accommodate the abnormal movements such that speech disruption is imperceptible, even when abnormal movements are visible. Unfortunately, the aesthetic effects of dystonia may limit communicative effectiveness independently of speech impairment as listeners may avoid looking at the speaker and therefore miss important nonverbal cues.45 Dysphagia in those with OMD occurred in 15.6% of a small case series.100 Those with OMD and dysphagia8,104 exhibit impaired movements of the oral stage of deglutition affecting oral bolus control during chewing and oral bolus transport to the pharynx as well as difficulty swallowing solid foods when pharyngeal involvement occurs. Successful dysphagia management utilizes pharmacologic treatment approaches (e.g., Botulinum toxin A injections, antidystonic medications) as well as diet modification and compensatory swallowing approaches.8,104
Hyoid dystonia
A relatively rare form of dystonia uniquely affects the hyoid musculature.115 This variation of dystonia is characterized by speech resonance changes, anterior neck tightness and dysphagia.115 The abnormal contractions in hyoid musculature are typically visible during evaluation. Hyoid dystonia may occur as a focal dystonia or as part of the broader cranial dystonias.
Spasmodic dysphonia
Spasmodic dysphonia is a rare type of idiopathic focal dystonia estimated to affect one in 100,000 dystonia cases50 with proportionally more females than males affected.116–118 Symptoms typically begin between 40 and 50 years of age with gradual progression during the first year before stabilizing119,120 although some individuals report continued progression of symptoms over time.120 Spasmodic dysphonia is characterized by involuntary spasms isolated to the laryngeal muscles during speech production resulting in intermittent onset of voice quality changes.118,121 Two primary types of spasmodic dysphonia are described based on whether the vocal folds abduct during spasms (abductor spasmodic dysphonia, or AbSD), or adduct during spasms (adductor spasmodic dysphonia, or AdSD).49,122,123
The majority of individuals with spasmodic dysphonia exhibit AdSD type symptoms.118,120 AdSD is characterized by spasmodic over-adduction of the vocal folds associated with production of voiced speech sounds, or phonemes, resulting in an intermittently strained–strangled voice quality, or voice stoppage.49,122,123 AbSD is characterized by spasmodic over-abduction of the vocal folds associated with production of voiceless phonemes resulting in prolonged breathy breaks, or upward pitch breaks during talking.49,122,123 In rare instances, individuals exhibit symptoms of both types of spasmodic dysphonia referred to as a mixed-type spasmodic dysphonia.120 Approximately 30–50% of individuals with spasmodic dysphonia present with a co-occurring vocal tremor.116,118,120
Individuals with spasmodic dysphonia report increased sense of effort and inconsistency of their voice symptoms (i.e., intermittent normal voicing) with worsening under conditions of anxiety or stress.123–125 Patients may report or exhibit normal voice production during laughter, crying, yawning, singing, whispering, or shouting.124
During a speech evaluation, spasmodic dysphonia speech patterns exhibit distinct auditory–perceptual and acoustic patterns of intermittent phoneme-specific voice spasms or voice quality changes during production of connected speech stimuli such as sentence or paragraph reading.49,51,53,64,65,121–123,126–128 Current standard speech stimuli utilized during voice evaluations include sentences loaded with voiced phonemes (e.g., We mow our lawn all year.) compared with sentences loaded with voiceless phonemes (e.g., Peter will keep at the peak.).129 A multidisciplinary working group of experts recommended use of a specific list of sentences to distinguish between AdSD and AbSD in individuals suspected of having spasmodic dysphonia.123 The sentence lists were created from speech stimuli presented in a speech drill workbook by Fairbanks130 that include a concentration of voiceless or voiced phonemes, respectively.123 A comparison of the patient’s reported and observed difficulty reading aloud of the voiceless-loaded or voice-loaded phoneme sentence lists is compared with whispered reading of the sentences. Individuals exhibiting strained–strangled voice quality and voice stoppages on voice-loaded phoneme sentences and improved voice quality during voiceless phoneme sentences implicate AdSD. In contrast, individuals exhibiting breathy or upward pitch breaks during reading of voiceless-loaded phoneme sentences compared with voiced phoneme sentences implicate AbSD. Whispered speaking is perceived as easier for both sentence lists in those with spasmodic dysphonia. A second task that distinguishes individuals with spasmodic dysphonia is determining whether voice quality improves during shouting compared to typical speaking patterns.123 Finally, a comparison of voice quality during sustained phonation (e.g., sustained voicing of “ah” and “ee” vowels) to connected speech tasks (e.g., reading sentences, or conversation) is important for two reasons.121,123 First, sustained voicing is the optimal context for identifying a co-occurring vocal tremor.67,69,116,118,120,121 The second reason is to compare voice symptom consistency across speech contexts. Individuals showing similar degrees of strained–strangled voice quality across both sustained phonation and connected speaking tasks may have a moderate to severe muscle tension dysphonia (MTD).53,65,121,127,128 In some cases, MTD may co-occur with spasmodic dysphonia secondary to efforts by the individual to control their spasmodic dysphonia symptoms.
In addition to a thorough speech assessment, nasoendoscopic evaluation is recommended to observe pharyngeal and laryngeal speech structures directly during sustained voicing and connected speech tasks. Nasoendoscopy enables confirmation of the diagnosis of spasmodic dysphonia with or without vocal tremor.123,131,132 Uniquely, application of topical anesthesia during nasoendoscopy with individuals suspected of having spasmodic dysphonia is not advised, or should only be minimally applied within the immediate nasal passageways to improve comfort level during this procedure.123 This recommendation is due to the sensory changes topical anesthesia imposes on the throat mucosa potentially facilitating improved speech symptoms during imaging (i.e., “sensory trick”).123 In some cases, the placement of the scope alone may serve as a “sensory trick” during the examination resulting in improved voice symptoms. When this occurs, the likelihood of the individual having a speech dystonia is increased. During nasoendoscopic evaluation, observation of oscillation of speech structures during sustained voicing compared with intermittent spasm of the vocal folds in the abductory or adductory plane can be directly observed for improved diagnostic precision.123,131,132 High-speed imaging, when available, may also be used to directly observe brief spasmodic changes in the vocal fold vibratory patterns during sustained voicing that may not be as readily observed during nasoendoscopic or typical stroboscopic examination.133
In some individuals, the signs and symptoms presented during a speech evaluation may require additional differential diagnosis between spasmodic dysphonia and muscle tension dysphonia. In such cases, a short trial of speech therapy focused on reducing muscle tension is recommended to elucidate the pathophysiology of voice symptoms.92 Spasmodic dysphonia will not be cured by speech therapy in comparison to a significant reduction in symptoms of MTD with implementation of speech therapy.92,123,134–136 Although speech therapy does not cure spasmodic dysphonia, speaking strategies for lessening effortful voicing responses to vocal fold spasms may benefit the patient’s response to medical management. Thus, a trial of speech therapy may be beneficial even if symptoms are not resolved.92,95,99,137
The most effective treatment approach for managing the symptoms of spasmodic dysphonia involves medical management via injection of botulinum neurotoxin (BTN) into the affected muscles of the larynx by a laryngologist.120,132,138–144 BTN benefits individuals with spasmodic dysphonia by inducing a short-term paresis/paralysis localized to the injected laryngeal musculature by preventing the release of acetylcholine from the presynaptic terminal membrane at the neuromuscular junction.141,145,146 Reduced muscle function onset begins within 6–72 hours after injection with gradual loss of effect over 3–6 months as the BTN is broken down by the body’s enzymes. Individuals presenting with a combination of spasmodic dysphonia and co-occurring vocal tremor may experience differing responses to BTN injections requiring modified treatment approaches when speech structures outside of the larynx exhibit oscillation.131,132,147 Associated with treatment using BTN injections, individuals with spasmodic dysphonia may experience a period of side effects during which swallowing problems can arise.138,148–150 Swallowing problems associated with BTN injection typically occur due to weakened laryngeal closure/airway protection during swallowing with subsequent choking on liquids.138,148–150 These symptoms typically resolve within 2-6 weeks (depending on the injected dosage and location) following BTN treatment as the neurotoxin is broken down. In anticipation of these side effects, individuals may be counseled by the SLP to manage swallowing difficulties during the side effect phase by drinking small sips of liquid and flexing their neck (i.e., chin tuck) during swallowing of liquids for improved airway protection.138,148–150 Dysphagia associated solely with spasmodic dysphonia was reported in one case presentation in the literature.151 This case was characterized with onset of dysphagia due to pharyngolaryngeal pain and voice changes associated with difficulty breathing and intermittent aspiration symptoms.151 No other reports of dysphagia due to spasmodic dysphonia unrelated to BTN treatment were identified.
Essential vocal tremor
Speech production characterized by involuntary rhythmic modulation of pitch and loudness perceived as a shaky voice (i.e., vocal tremor) in those with essential tremor is referred to as essential vocal tremor (EVT).66,152–154 This disorder can occur in 30–40% of individuals with essential tremor,69,153–155 or may be the primary sign of essential tremor.54,154 Approximately 90% of those presenting with EVT are female.54,154 During the case history, some individuals with EVT may report improved symptoms with ingestion of alcohol similar to the effect upon limb tremor.54,154 In addition, Voice Handicap Index scores show comparable scoring across the three subscales of functional, physical, and emotional impact on activities of daily living, particularly in those with more severe voice symptoms.156 Individuals with mild EVT may not exhibit perceptible symptoms during connected speech tasks (e.g., reading sentences, or conversation).67,69 However, severe EVT is characterized by a slowed speaking tempo52 and perception of vocal tremor during both sustained phonation and connected speech tasks.67,69 Thus, evaluation of EVT across speech contexts is important for determining severity level.52,67 Further, changes in EVT severity should be evaluated across different pitch and loudness levels to determine conditions under which vocal tremor is improved or worsened.66,157
Nasoendoscopy is used to image speech structures of the pharynx and larynx and identify oscillating structures during speech tasks. This information is useful for judging the severity of EVT and anticipated responsiveness to medical management.68,131 Involvement of the larynx as well as other articulators in the upper airway is associated with poor treatment outcomes using BTN treatment.68,131 However, individuals showing mild vocal tremor or the ability to reduce their voicing duration may be candidates for speech treatment.66,153,158,159 Current speech treatment approaches with EVT are limited to case-based publications66,153 with one reporting benefit from shortening voicing duration during speaking combined with improved respiratory–phonatory coordination.66 Shortened voicing duration reduces perception of vocal tremor by disrupting the cyclic modulation of the voice.66,159 Improved respiratory–phonatory coordination aims to reduce speech structure muscle tension levels. Thus, methods found effective in reducing throat and voicing tension include the use of increased airflow and reduced effort levels during talking.66,159
Conclusion
Hyperkinetic dysarthria is characterized by abnormal involuntary movements affecting respiratory, phonatory, and articulatory structures significantly impacting communication, deglutition, and quality of life. Spasmodic dysphonia and essential voice tremor have been studied more thoroughly than other hyperkinetic speech impairments and are also the disorders for which speech therapy is most often sought by patients and requested by physicians. Speech therapy may reduce the impact of hyperkinetic dysarthria on functional communication and the effort associated with speaking.92,95,99,137 However, speech therapy does not cure hyperkinetic dysarthria and, as such, is often paired with the preferred practice of BTN injection in the management of dystonia and tremor. The risks inherent in BTN injection for oromandibular, pharyngeal, and laryngeal dystonia include the potential negative impact on breathing, speech, and deglutition. Several therapeutic strategies are described in the literature for managing the impact of speech problems and dysphagia associated with hyperkinetic dysarthria. However, few studies have identified the relevant factors predictive of successful treatment outcomes such as candidacy for specific treatment approaches, optimal treatment dosage, and cost effectiveness. One challenge to identifying such important factors is the consultative nature of, and relative rarity of hyperkinetic dysarthria on the average speech–language pathology case load. Future research addressing optimal treatment approaches and important factors predictive of outcomes as well as the incorporation of technologic advancements in imaging and physiology analysis may yield novel methods for the assessment and treatment of hyperkinetic dysarthria.
Footnotes
Funding: None.
Financial Disclosures: None.
Conflict of Interest: The authors report no conflict of interest.
Ethics Statements: Not applicable for this category of article.
References
- 1.Darley FL, Aronson AE, Brown JR. Differential diagnostic patterns of dysarthria. 1969;12:246–269. doi: 10.1044/jshr.1202.246. doi: 10.1044/jshr.1202.246. [DOI] [PubMed] [Google Scholar]
- 2.Darley FL, Aronson AE, Brown JR. Clusters of deviant speech dimensions in the dysarthrias. J Speech Hear Res. 1969;12:462–496. doi: 10.1044/jshr.1203.462. doi: 10.1044/jshr.1203.462. [DOI] [PubMed] [Google Scholar]
- 3.DeLong M, Wichmann T. Changing views of basal ganglia circuits and circuit disorders. Clin EEG Neurosci. 2010;41:61–67. doi: 10.1177/155005941004100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 5.Kent RD. Research on speech motor control and its disorders: a review and prospective. J Commun Disord. 2000;33:391–427. doi: 10.1016/s0021-9924(00)00023-x. quiz 8. doi: 10.1016/S0021-9924(00)00023-X. [DOI] [PubMed] [Google Scholar]
- 6.Kent RD, Duffy JR, Slama A, Kent JF, Clift A. Clinicoanatomic studies in dysarthria: review, critique, and directions for research. J Speech Lang Hear Res. 2001;44:535–551. doi: 10.1044/1092-4388(2001/042). doi: 10.1044/1092-4388(2001/042) [DOI] [PubMed] [Google Scholar]
- 7.Duffy J. Motor speech disorders: substrates, differential diagnosis, and management. Second edition. St. Louis: Elsevier Mosby; 2013. [Google Scholar]
- 8.Kreisler A, Verpraet AC, Veit S, Pennel-Ployart O, Behal H, Duhamel A, et al. Clinical characteristics of voice, speech, and swallowing disorders in oromandibular dystonia. J Speech Lang Hear Res. 2016;59:940–949. doi: 10.1044/2016_JSLHR-S-15-0169. doi: 10.1044/2016_JSLHR-S-15-0169. [DOI] [PubMed] [Google Scholar]
- 9.Kagel MC, Leopold NA. Dysphagia in Huntington’s disease: a 16-year retrospective. Dysphagia. 1992;7:106–114. doi: 10.1007/BF02493441. [DOI] [PubMed] [Google Scholar]
- 10.Klasner ER. Huntington disease. In: Jones HN, Rosenbek J, . Dysphagia in rare conditions. 2010. pp. 267–272. editors. [Google Scholar]
- 11.Schröter-Morasch H, Ziegler W. Rehabilitation of impaired speech function (dysarthria, dysglossia) GMS Curr Top Otorhinolaryngol Head Neck Surg. 2005;4 Doc15. [PMC free article] [PubMed] [Google Scholar]
- 12.Hu A. Reflections: the value of patient support groups. Otolaryngol Head Neck Surg. 2017;156:587–588. doi: 10.1177/0194599817697030. doi: 10.1177/0194599817697030. [DOI] [PubMed] [Google Scholar]
- 13.Kongsaengdao S, Maneeton B, Maneeton N. Quality of life in cervical dystonia after treatment with botulinum toxin A: a 24-week prospective study. Neuropsychiatr Dis Treat. 2017;13:127–132. doi: 10.2147/NDT.S116325. doi: 10.2147/NDT.S116325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin BJ, Corlew MM. The incidence of communication disorders in dysphagic patients. J Speech Hear Disord. 1990;55:28–32. doi: 10.1044/jshd.5501.28. doi: 10.1044/jshd.5501.28. [DOI] [PubMed] [Google Scholar]
- 15.Ramig LA. Acoustic analyses of phonation in patients with Huntington’s disease. Preliminary report. Ann Otol Rhinol Laryngol. 1986 doi: 10.1177/000348948609500315. [DOI] [PubMed] [Google Scholar]
- 16.ASHA . SLP Health care survey report: patient caseload characteristics trends, 2005-2011. American Speech-Language-Hearing Association; Rockville, MD: 2011. [Google Scholar]
- 17.Yorkston K, Strand E, Kennedy M. Comprehensibility of dysarthric speech: implications for assessment and treatment planning. Am J Speech Lang Pathol. 1996;5:55–66. doi: 10.1044/1058-0360.0501.55. [Google Scholar]
- 18.Hartelius L, Carlstedt A, Ytterberg M, Lillvik M, Laakso K. Speech disorders in mild and moderate Huntington Disease: Results of dysarthria assessments of 19 individuals. J M Speech Lang Pathol. 2003;11:1–14. [Google Scholar]
- 19.Bunton K, Kent RD, Duffy JR, Rosenbek JC, Kent JF. Listener agreement for auditory-perceptual ratings of dysarthria. J Speech Lang Hear Res. 2007;50:1481–95. doi: 10.1044/1092-4388(2007/102). doi: 10.1044/1092-4388(2007/102) [DOI] [PubMed] [Google Scholar]
- 20.Liss JM, White L, Mattys SL, Lansford K, Lotto AJ, Spitzer SM, et al. Quantifying speech rhythm abnormalities in the dysarthrias. J Speech Lang Hear Res. 2009;52:1334–1352. doi: 10.1044/1092-4388(2009/08-0208). doi: 10.1044/1092-4388(2009/08-0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammen VL. Managing speaking rate in dysarthria. SIG 2 Persp Neurophysiol Neurogen Speech Lang Disord. 2002;12:19–23. [Google Scholar]
- 22.Schroter-Morasch H, Ziegler W. Impaired speech – restoration processes in impaired speech functions (dysarthria, dysglossia) Laryngo Rhino Otol. 2005;84:S213–S20. doi: 10.1055/s-2005-861145. [DOI] [PubMed] [Google Scholar]
- 23.Kim Y, Kuo C. Effect of level of presentation to listeners on scaled speech intelligibility of speakers with dysarthria. Folia Phoniatr Logop. 2012;64:26–33. doi: 10.1159/000328642. doi: 10.1159/000328642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spencer KA, Yorkston KM. Evidence for the treatment of respiratory/phonatory dysfunction from dysarthria. SIG 2 Persp Neurophysiol Neurogen Speech Lang Disord. 2002;12:5–17. doi: 10.1044/nnsld12.4.4. [Google Scholar]
- 25.Mathy P. Augmentative and alternative communication intervention in neurogenic disorders with acquired dysarthria. SIG 2 Persp Neurophysiol Neurogen Speech Lang Disord. 2002;12:33–41. doi: 10.1044/nnsld12.4.28. [Google Scholar]
- 26.ASHA Adult dysphagia. (Practice Portal) 2017 June 9, 2017. www.asha.org/Practice-Portal/Clinical-Topics/Adult-Dysphagia/ Available from.
- 27.ASHA . Preferred practice patterns for the profession of speech-language pathology [Preferred Practice Patterns] Rockville, MD: ASHA; 2004. [Google Scholar]
- 28.Eadie TL, Yorkston KM, Klasner ER, Dudgeon BJ, Deitz JC, Baylor CR, et al. Measuring communicative participation: a review of self-report instruments in speech-language pathology. Am J Speech Lang Pathol. 2006;15:307–320. doi: 10.1044/1058-0360(2006/030). doi: 10.1044/1058-0360(2006/030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walshe M, Peach RK, Miller N. Dysarthria impact profile: development of a scale to measure psychosocial effects. Int J Lang Commun Disord. 2009;44:693–715. doi: 10.1080/13682820802317536. [DOI] [PubMed] [Google Scholar]
- 30.Hartelius L, Elmberg M, Holm R, Lovberg AS, Nikolaidis S. Living with dysarthria: evaluation of a self-report questionnaire. Folia Phoniatr Logop. 2008;60:11–19. doi: 10.1159/000111799. doi: 10.1159/000111799. [DOI] [PubMed] [Google Scholar]
- 31.Piacentini V, Zuin A, Cattaneo D, Schindler A. Reliability and validity of an instrument to measure quality of life in the dysarthric speaker. Folia Phoniatr Logop. 2011;63:289–295. doi: 10.1159/000322800. doi: 10.1159/000322800. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson BH JA, Grywalski C, Silbergleit A, Jacobson G, Benninger MS. The Voice Handicap Index (VHI): development and validation. Am J Speech Lang Pathol. 1997;6:66–70. doi: 10.1044/1058-0360.0603.66. [Google Scholar]
- 33.Hogikyan ND, Sethuraman G. Validation of an instrument to measure voice-related quality of life (V-RQOL) J Voice. 1999;13:557–569. doi: 10.1016/s0892-1997(99)80010-1. doi: 10.1016/S0892-1997(99)80010-1. [DOI] [PubMed] [Google Scholar]
- 34.Ma EP, Yiu EM. Voice activity and participation profile: assessing the impact of voice disorders on daily activities. J Speech Lang Hear Res. 2001;44:511–524. doi: 10.1044/1092-4388(2001/040). doi: 10.1044/1092-4388(2001/040) [DOI] [PubMed] [Google Scholar]
- 35.Deary IJ, Wilson JA, Carding PN, MacKenzie K. VoiSS: a patient-derived Voice Symptom Scale. J Psychosom Res. 2003;54:483–489. doi: 10.1016/s0022-3999(02)00469-5. doi: 10.1016/S0022-3999(02)00469-5. [DOI] [PubMed] [Google Scholar]
- 36.Baylor C, Yorkston K, Eadie T, Kim J, Chung H, Amtmann D. The Communicative Participation Item Bank (CPIB): item bank calibration and development of a disorder-generic short form. J Speech Lang Hear Res. 2013;56:1190–208. doi: 10.1044/1092-4388(2012/12-0140). doi: 10.1044/1092-4388(2012/12-0140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziegler W, Staiger A, Scholderle T, Vogel M. Gauging the auditory dimensions of dysarthric impairment: reliability and construct validity of the Bogenhausen Dysarthria Scales (BoDyS) J Speech Lang Hear Res. 2017:1–19. doi: 10.1044/2017_JSLHR-S-16-0336. doi: 10.1044/2017_JSLHR-S-16-0336. [DOI] [PubMed] [Google Scholar]
- 38.Enderby PM, Palmer R. Frenchay dysarthria assessment. Second edition. Austin, TX: PRO-ED, Inc; 2008. [Google Scholar]
- 39.Estep ME. Modulation, adaptation, and control of orofacial pathways in healthy adults. J Commun Disord. 2009;42((4)):280–5. doi: 10.1016/j.jcomdis.2009.03.005. doi: 10.1016/j.jcomdis.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solomon NP, Clark HM, Makashay MJ, Newman LA. Assessment of orofacial strength in patients with dysarthria. J Med Speech Lang Pathol. 2008;16:251–258. [PMC free article] [PubMed] [Google Scholar]
- 41.Yorkston KM, Beukelman DR. Communication efficiency of dysarthric speakers as measured by sentence intelligibility and speaking rate. J Speech Hear Disord. 1981;46((3)):296–301. doi: 10.1044/jshd.4603.296. doi: 10.1044/jshd.4603.296. [DOI] [PubMed] [Google Scholar]
- 42.Yorkston KC, Beukelman DR. Assessment of intelligibility of dysarthric speech. Austin, TX: PRO-ED; 1981. [Google Scholar]
- 43.Kent RD, Weismer G, Kent JF, Rosenbek JC. Toward phonetic intelligibility testing in dysarthria. J Speech Hear Disord. 1989;54:482–499. doi: 10.1044/jshd.5404.482. doi: 10.1044/jshd.5404.482. [DOI] [PubMed] [Google Scholar]
- 44.Walshe M, Miller N, Leahy M, Murray A. Intelligibility of dysarthric speech: perceptions of speakers and listeners. Int J Lang Commun Disord. 2008;43:633–648. doi: 10.1080/13682820801887117. [DOI] [PubMed] [Google Scholar]
- 45.Keintz CK, Bunton K, Hoit JD. Influence of visual information on the intelligibility of dysarthric speech. Am J Speech Lang Pathol. 2007;16:222–234. doi: 10.1044/1058-0360(2007/027). doi: 10.1044/1058-0360(2007/027) [DOI] [PubMed] [Google Scholar]
- 46.Barkmeier JM. Intelligibility of dysarthric speakers: audio-only and audio-visual presentations. Iowa City, IA: University of Iowa; 1988. [Google Scholar]
- 47.Wannberg P, Schalling E, Hartelius L. Perceptual assessment of dysarthria: comparison of a general and a detailed assessment protocol. Logoped Phoniatr Vocol. 2016;41:159–167. doi: 10.3109/14015439.2015.1069889. doi: 10.3109/14015439.2015.1069889. [DOI] [PubMed] [Google Scholar]
- 48.dos Santos Barreto S, Zazo Ortiz K. Protocol for the evaluation of speech intelligibility in dysarthrias: evidence of reliability and validity. Folia Phoniatr Logop. 2015;67:212–218. doi: 10.1159/000441929. doi: 10.1159/000441929. [DOI] [PubMed] [Google Scholar]
- 49.Ludlow CL, Connor NP. Dynamic aspects of phonatory control in spasmodic dysphonia. J Speech Hear Res. 1987;30:197–206. doi: 10.1044/jshr.3002.197. doi: 10.1044/jshr.3002.197. [DOI] [PubMed] [Google Scholar]
- 50.Nutt JG, Muenter MD, Aronson A, Kurland LT, Melton LJ., 3rd Epidemiology of focal and generalized dystonia in Rochester, Minnesota. Mov Disord. 1988;3:188–194. doi: 10.1002/mds.870030302. doi: 10.1002/mds.870030302. [DOI] [PubMed] [Google Scholar]
- 51.Sapienza CM, Walton S, Murry T. Acoustic variations in adductor spasmodic dysphonia as a function of speech task. J Speech Lang Hear Res. 1999;42:127–140. doi: 10.1044/jslhr.4201.127. doi: 10.1044/jslhr.4201.127. [DOI] [PubMed] [Google Scholar]
- 52.Lundy DS, Roy S, Xue JW, Casiano RR, Jassir D. Spastic/spasmodic vs. tremulous vocal quality: motor speech profile analysis. J Voice. 2004;18:146–152. doi: 10.1016/j.jvoice.2003.12.001. doi: 10.1016/j.jvoice.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Roy N, Gouse M, Mauszycki SC, Merrill RM, Smith ME. Task specificity in adductor spasmodic dysphonia versus muscle tension dysphonia. Laryngoscope. 2005;115:311–316. doi: 10.1097/01.mlg.0000154739.48314.ee. doi: 10.1097/01.mlg.0000154739.48314.ee. [DOI] [PubMed] [Google Scholar]
- 54.Sulica L, Louis ED. Clinical characteristics of essential voice tremor: a study of 34 cases. Laryngoscope. 2010;120:516–528. doi: 10.1002/lary.20702. doi: 10.1002/lary.20702. [DOI] [PubMed] [Google Scholar]
- 55.Cannito MP, Doiuchi M, Murry T, Woodson GE. Perceptual structure of adductor spasmodic dysphonia and its acoustic correlates. J Voice. 2012;26:818.e5–e13. doi: 10.1016/j.jvoice.2012.05.005. doi: 10.1016/j.jvoice.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 56.Rojas GVE, Ricz H, Tumas V, Rodrigues GR, Toscano P, Aguiar-Ricz L. Vocal parameters and self-perception in individuals with adductor spasmodic dysphonia. J Voice. 2017;31:391.e7–e18. doi: 10.1016/j.jvoice.2016.09.029. doi: 10.1016/j.jvoice.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 57.Walker FO. Huntington’s disease. Lancet. 2007;369:218–228. doi: 10.1016/S0140-6736(07)60111-1. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- 58.Velasco Garcia MJ, Cobeta I, Martin G, Alonso-Navarro H, Jimenez-Jimenez FJ. Acoustic analysis of voice in Huntington's disease patients. J Voice. 2011;25:208–217. doi: 10.1016/j.jvoice.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Rusz J, Klempir J, Baborova E, Tykalova T, Majerova V, Cmejla R, et al. Objective acoustic quantification of phonatory dysfunction in Huntington's disease. PLoS One. 2013;8:e65881. doi: 10.1371/journal.pone.0065881. doi: 10.1371/journal.pone.0065881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rusz J, Klempir J, Tykalova T, Baborova E, Cmejla R, Ruzicka E, et al. Characteristics and occurrence of speech impairment in Huntington's disease: possible influence of antipsychotic medication. J Neural Transm (Vienna) 2014;121:1529–1539. doi: 10.1007/s00702-014-1229-8. [DOI] [PubMed] [Google Scholar]
- 61.Skodda S, Schlegel U, Hoffmann R, Saft C. Impaired motor speech performance in Huntington's disease. J Neural Transm (Vienna) 2014;121:399–407. doi: 10.1007/s00702-013-1115-9. [DOI] [PubMed] [Google Scholar]
- 62.Rusz J, Hlavnicka J, Cmejla R, Ruzicka E. automatic evaluation of speech rhythm instability and acceleration in dysarthrias associated with basal ganglia dysfunction. Front Bioeng Biotechnol. 2015;3:104. doi: 10.3389/fbioe.2015.00104. doi: 10.3389/fbioe.2015.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Novotny M, Rusz J, Cmejla R, Ruzickova H, Klempir J, Ruzicka E. Hypernasality associated with basal ganglia dysfunction: evidence from Parkinson's disease and Huntington’s disease. Peer J. 2016;4:e2530. doi: 10.7717/peerj.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sapienza CM, Murry T, Brown WS., Jr Variations in adductor spasmodic dysphonia: acoustic evidence. J Voice. 1998;12:214–222. doi: 10.1016/s0892-1997(98)80041-6. doi: 10.1016/S0892-1997(98)80041-6. [DOI] [PubMed] [Google Scholar]
- 65.Sapienza CM, Walton S, Murry T. Adductor spasmodic dysphonia and muscular tension dysphonia: acoustic analysis of sustained phonation and reading. J Voice. 2000;14:502–520. doi: 10.1016/s0892-1997(00)80008-9. doi: 10.1016/S0892-1997(00)80008-9. [DOI] [PubMed] [Google Scholar]
- 66.Barkmeier-Kraemer J, Lato A, Wiley K. Development of a speech treatment program for a client with essential vocal tremor. Semin Speech Lang. 2011;32:43–57. doi: 10.1055/s-0031-1271974. [DOI] [PubMed] [Google Scholar]
- 67.Lederle A, Barkmeier-Kraemer J, Finnegan E. Perception of vocal tremor during sustained phonation compared with sentence context. J Voice. 2012;26:668.e1–e9. doi: 10.1016/j.jvoice.2011.11.001. doi: 10.1016/j.jvoice.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 68.Hemmerich AL, Finnegan EM, Hoffman HT. The distribution and severity of tremor in speech structures of persons with vocal tremor. J Voice. 2017;31(3):366–377. doi: 10.1016/j.jvoice.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 69.Brown JR, Simonson J. Organic voice tremor. A tremor of phonation. Neurology. 1963;13:520–525. doi: 10.1212/wnl.13.6.520. doi: 10.1212/WNL.13.6.520. [DOI] [PubMed] [Google Scholar]
- 70.Aronson AE, Brown JR, Litin EM, Pearson JS. Spastic dysphonia. II. Comparison with essential (voice) tremor and other neurologic and psychogenic dysphonias. J Speech Hear Disord. 1968;33:219–231. doi: 10.1044/jshd.3303.219. doi: 10.1044/jshd.3303.219. [DOI] [PubMed] [Google Scholar]
- 71.ASHA . Clinical indicators for instrumental assessment of dysphagia [Guidelines] Rockville, MD: ASHA; 2000. [Google Scholar]
- 72.ASHA . Roles of speech-language pathologists in swallowing and feeding disorders: technical report [Technical Report] Rockville, MD: ASHA; 2001. [Google Scholar]
- 73.ASHA . Guidelines for speech-language pathologists performing videofluoroscopic swallowing studies [Guidelines] Rockville, MD: ASHA; 2004. [Google Scholar]
- 74.ASHA . Role of the speech-language pathologist in the performance and interpretation of endoscopic evaluation of swallowing: guidelines [Guidelines] Rockville, MD: ASHA; 2004. [Google Scholar]
- 75.ASHA . The role of the speech-language pathologist in the performance and interpretation of endoscopic evaluation of swallowing: position statement [Position Statement]. Rockville, MD: ASHA Special Interest Division 13, Swallowing and Swallowing Disorders (Dysphagia) Committee on Endoscopic Evaluation of Swallowing. Rockville, MD: ASHA; 2005. [Google Scholar]
- 76.ASHA . Rockville, MD: ASHA; 2005. The role of the speech-language pathologist in the performance and interpretation of endoscopic evaluation of swallowing: technical report [Technical Report] [Google Scholar]
- 77.Steele CM, Molfenter SM, Bailey GL, Polacco RC, Waito AA, Zoratto DCBH, et al. Exploration of the utility of a brief swallow screening protocol with comparison to concurrent videofluoroscopy. Can J Speech Lang Pathol Audiol. 2011;35(3):228. [Google Scholar]
- 78.Coyle JL. The clinical evaluation: a necessary tool for the dysphagia sleuth. SIG 13 Persp Neurophysiol Neurogen Speech Lang Disord. 2015;24:18–25. doi: 10.1044/sasd24.1.18. [Google Scholar]
- 79.Logemann JA. Dysphagia: evaluation and treatment. Folia Phoniatr Logop. 1995;47:140–164. doi: 10.1159/000266348. doi: 10.1159/000266348. [DOI] [PubMed] [Google Scholar]
- 80.Ricci Maccarini A, Filippini A, Padovani D, Limarzi M, Loffredo M, Casolino D. Clinical non-instrumental evaluation of dysphagia. Acta Otorhinolaryngol Ital. 2007;27:299–305. [PMC free article] [PubMed] [Google Scholar]
- 81.Knigge MA, Thibeault S, McCulloch TM. Implementation of high-resolution manometry in the clinical practice of speech language pathology. Dysphagia. 2014;29:2–16. doi: 10.1007/s00455-013-9494-5. doi: 10.1007/s00455-013-9494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van Den Engel-Hoek L, Lagarde M, Van Alfen N. Ultrasound of oral and masticatory muscles: why every neuromuscular swallow team should have an ultrasound machine. Clin Anat. 2017;30:183–193. doi: 10.1002/ca.22818. doi: 10.1002/ca.22818. [DOI] [PubMed] [Google Scholar]
- 83.Giraldo-Cadavid LF, Leal-Leano LR, Leon-Basantes GA, Bastidas AR, Garcia R, Ovalle S, et al. Accuracy of endoscopic and videofluoroscopic evaluations of swallowing for oropharyngeal dysphagia. Laryngoscope. 2016 doi: 10.1002/lary.26419. doi: 10.1002/lary.26419. [DOI] [PubMed] [Google Scholar]
- 84.Park WY, Lee TH, Ham NS, Park JW, Lee YG, Cho SJ, et al. Adding endoscopist-directed flexible endoscopic evaluation of swallowing to the videofluoroscopic swallowing study increased the detection rates of penetration, aspiration, and pharyngeal residue. Gut Liver. 2015;9:623–628. doi: 10.5009/gnl14147. doi: 10.5009/gnl14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Butler SG, Stuart A, Case LD, Rees C, Vitolins M, Kritchevsky SB. Effects of liquid type, delivery method, and bolus volume on penetration-aspiration scores in healthy older adults during flexible endoscopic evaluation of swallowing. Ann Otol Rhinol Laryngol. 2011;120:288–295. doi: 10.1177/000348941112000502. doi: 10.1177/000348940911800204. [DOI] [PubMed] [Google Scholar]
- 86.O'Horo JC, Rogus-Pulia N, Garcia-Arguello L, Robbins J, Safdar N. Bedside diagnosis of dysphagia: a systematic review. J Hosp Med. 2015;10:256–265. doi: 10.1002/jhm.2313. doi: 10.1002/jhm.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Butler SG, Stuart A, Kemp S. Flexible endoscopic evaluation of swallowing in healthy young and older adults. Ann Otol Rhinol Laryngol. 2009;118:99–106. doi: 10.1177/000348940911800204. doi: 10.1177/000348941112000502. [DOI] [PubMed] [Google Scholar]
- 88.Martin-Harris B, Brodsky MB, Michel Y, Castell DO, Schleicher M, Sandidge J, et al. MBS measurement tool for swallow impairment—MBSImp: establishing a standard. Dysphagia. 2008;23:392–405. doi: 10.1007/s00455-008-9185-9. doi: 10.1007/s00455-010-9275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leonard RJ, Kendall KA. Dysphagia assessment and treatment planning: a team approach. Third edition. San Diego, CA: Plural Publishing, Inc.; 2013. [Google Scholar]
- 90.Yorkston K, Spencer K, Duffy J, Beukelman D, Golper LA, Miller R, et al. Evidence-based medicine and practice guidelines: application to the field of speech-language pathology. J Med Speech Lang Pathol. 2001;9:243–256. [Google Scholar]
- 91.Theodoros DG, Thompson-Ward EC. Treatment of dysarthria. In: Murdoch BE, editor. Dysarthria: a physiological approach to assessment and treatment. Cheltenham, UK: Stanley Thornes (Publishers) Ltd; 1998. p. 144. editor. p. [Google Scholar]
- 92.Roy N, Ford CN, Bless DM. Muscle tension dysphonia and spasmodic dysphonia: the role of manual laryngeal tension reduction in diagnosis and management. Ann Otol Rhinol Laryngol. 1996;105:851–856. doi: 10.1177/000348949610501102. doi: 10.1177/000348949610501102. [DOI] [PubMed] [Google Scholar]
- 93.Kotby MN, Shiromoto O, Hirano M. The accent method of voice therapy: effect of accentuations on FO, SPL, and airflow. J Voice. 1993;7:319–325. doi: 10.1016/s0892-1997(05)80120-1. doi: 10.1016/S0892-1997(05)80120-1. [DOI] [PubMed] [Google Scholar]
- 94.Sapienza CM. Respiratory muscle strength training applications. Curr Opin Otolaryngol Head Neck Surg. 2008;16:216–220. doi: 10.1097/MOO.0b013e3282fe96bd. doi: 10.1097/MOO.0b013e3282fe96bd. [DOI] [PubMed] [Google Scholar]
- 95.Silverman EP, Garvan C, Shrivastav R, Sapienza CM. Combined modality treatment of adductor spasmodic dysphonia. J Voice. 2012;26:77–86. doi: 10.1016/j.jvoice.2010.08.004. doi: 10.1016/j.jvoice.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 96.Laciuga H, Rosenbek JC, Davenport PW, Sapienza CM. Functional outcomes associated with expiratory muscle strength training: narrative review. J Rehabil Res Dev. 2014;51:535–546. doi: 10.1682/JRRD.2013.03.0076. doi: 10.1682/JRRD.2013.03.0076. [DOI] [PubMed] [Google Scholar]
- 97.Fox CM, Ramig LO, Ciucci MR, Sapir S, McFarland DH, Farley BG. The science and practice of LSVT/LOUD: neural plasticity-principled approach to treating individuals with Parkinson disease and other neurological disorders. Semin Speech Lang. 2006;27:283–299. doi: 10.1055/s-2006-955118. [DOI] [PubMed] [Google Scholar]
- 98.Enderby P. Disorders of communication: dysarthria. Handb Clin Neurol. 2013;110:273–81. doi: 10.1016/B978-0-444-52901-5.00022-8. [DOI] [PubMed] [Google Scholar]
- 99.Murry T, Woodson GE. Combined-modality treatment of adductor spasmodic dysphonia with botulinum toxin and voice therapy. J Voice. 1995;9:460–465. doi: 10.1016/s0892-1997(05)80211-5. doi: 10.1016/S0892-1997(05)80211-5. [DOI] [PubMed] [Google Scholar]
- 100.Leopold NA, Kagel MC. Dysphagia in Huntington’s disease. Arch Neurol. 1985;42:57–60. doi: 10.1001/archneur.1985.04060010063017. doi: 10.1001/archneur.1985.04060010063017. [DOI] [PubMed] [Google Scholar]
- 101.Hamakawa S, Koda C, Umeno H, Yoshida Y, Nakashima T, Asaoka K, et al. Oropharyngeal dysphagia in a case of Huntington's disease. Auris Nasus Larynx. 2004;31:171–176. doi: 10.1016/j.anl.2003.11.001. doi: 10.1016/j.anl.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 102.Heemskerk AW, Roos RA. Dysphagia in Huntington's disease: a review. Dysphagia. 2011;26:62–66. doi: 10.1007/s00455-010-9302-4. doi: 10.1007/s00455-010-9302-4. [DOI] [PubMed] [Google Scholar]
- 103.Juby AG, Shandro P, Emery D. Palato-pharyngo-laryngeal myoclonus … an unusual cause of dysphagia. Age Ageing. 2014;43:877–879. doi: 10.1093/ageing/afu066. doi: 10.1093/ageing/afu066. [DOI] [PubMed] [Google Scholar]
- 104.Papapetropoulos S, Singer C. Eating dysfunction associated with oromandibular dystonia: clinical characteristics and treatment considerations. Head Face Med. 2006;2:47. doi: 10.1186/1746-160X-2-47. doi: 10.1186/1746-160X-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Drysdale AJ, Ansell J, Adeley J. Palato-pharyngo-laryngeal myoclonus: an unusual cause of dysphagia and dysarthria. J Laryngol Otol. 1993;107:746–747. doi: 10.1017/s0022215100124326. doi: 10.1017/S0022215100124326. [DOI] [PubMed] [Google Scholar]
- 106.Hunt VP, Walker FO. Dysphagia in Huntington's disease. J Neurosci Nurs. 1989;21:92–95. [PubMed] [Google Scholar]
- 107.de Tommaso M, Nuzzi A, Dellomonaco AR, Sciruicchio V, Serpino C, Cormio C, et al. Dysphagia in Huntington’s disease: Correlation with Clinical Features. Eur Neurol. 2015;74:49–53. doi: 10.1159/000435833. doi: 10.1159/000435833. [DOI] [PubMed] [Google Scholar]
- 108.Wheeler Hegland K. Palatal myoclonus. In: Jones HN, Rosenbek J, editors. Dysphagia in rare conditions. San Diego: Plural; 2010. pp. 449–454. p. [Google Scholar]
- 109.Panda AK, Kushwaha S, Kaur M. Essential palatal tremor with hemifacial and vocal cord tremor. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-201327. doi: 10.1136/bcr-2013-201327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scozzafava J, Yager J. Images in clinical medicine. Essential palatal myoclonus. N Engl J Med. 2010;362:e64. doi: 10.1056/NEJMicm0806049. doi: 10.1056/NEJMicm0806049. [DOI] [PubMed] [Google Scholar]
- 111.Sumer M. Symptomatic palatal myoclonus: an unusual cause of respiratory difficulty. Acta Neurol Belg. 2001;101:113–115. [PubMed] [Google Scholar]
- 112.Bader B, Walker RH, Vogel M, Prosiegel M, McIntosh J, Danek A. Tongue protrusion and feeding dystonia: a hallmark of chorea-acanthocytosis. Mov Disord. 2010;25:127–129. doi: 10.1002/mds.22863. doi: 10.1002/mds.22863. [DOI] [PubMed] [Google Scholar]
- 113.Riski JE, Horner J, Nashold BS., Jr Swallowing function in patients with spasmodic torticollis. Neurology. 1990;40:1443–1445. doi: 10.1212/wnl.40.9.1443. doi: 10.1212/WNL.40.9.1443. [DOI] [PubMed] [Google Scholar]
- 114.LeDoux MS. Meige syndrome: what’s in a name? Parkinsonism Relat Disord. 2009;15:483–489. doi: 10.1016/j.parkreldis.2009.04.006. doi: 10.1016/j.parkreldis.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Norby E, Orbelo D, Strand E, Duffy J, Ekbom D, Bower J, et al. Hyoid muscle dystonia: A distinct focal dystonia syndrome. Parkinsonism Relat Disord. 2015;21:1210–1213. doi: 10.1016/j.parkreldis.2015.08.022. doi: 10.1016/j.parkreldis.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 116.Schweinfurth JM, Billante M, Courey MS. Risk factors and demographics in patients with spasmodic dysphonia. Laryngoscope. 2002;112:220–223. doi: 10.1097/00005537-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 117.Adler CH, Edwards BW, Bansberg SF. Female predominance in spasmodic dysphonia. J Neurol Neurosurg Psychiatry. 1997;63:688. doi: 10.1136/jnnp.63.5.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Patel AB, Bansberg SF, Adler CH, Lott DG, Crujido L. The Mayo Clinic Arizona spasmodic dysphonia experience: a demographic analysis of 718 patients. Ann Otol Rhinol Laryngol. 2015;124:859–863. doi: 10.1177/0003489415588557. [DOI] [PubMed] [Google Scholar]
- 119.Izdebski K, Dedo HH, Boles L. Spastic dysphonia: a patient profile of 200 cases. Am J Otolaryngol. 1984;5:7–14. doi: 10.1016/s0196-0709(84)80015-0. doi: 10.1016/S0196-0709(84)80015-0. [DOI] [PubMed] [Google Scholar]
- 120.Tanner K, Roy N, Merrill RM, Sauder C, Houtz DR, Smith ME. Spasmodic dysphonia: onset, course, socioemotional effects, and treatment response. Ann Otol Rhinol Laryngol. 2011;120:465–473. doi: 10.1177/000348941112000708. doi: 10.1177/000348941112000708. [DOI] [PubMed] [Google Scholar]
- 121.Barkmeier JM, Case JL, Ludlow CL. Identification of symptoms for spasmodic dysphonia and vocal tremor: a comparison of expert and nonexpert judges. J Commun Disord. 2001;34:21–37. doi: 10.1016/s0021-9924(00)00039-3. doi: 10.1016/S0021-9924(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 122.Ludlow CL. Spasmodic dysphonia: a laryngeal control disorder specific to speech. J Neurosci. 2011;31:793–797. doi: 10.1523/JNEUROSCI.2758-10.2011. doi: 10.1523/JNEUROSCI.2758-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ludlow CL, Adler CH, Berke GS, Bielamowicz SA, Blitzer A, Bressman SB, et al. Research priorities in spasmodic dysphonia. Otolaryngol Head Neck Surg. 2008;139:495–505. doi: 10.1016/j.otohns.2008.05.624. doi: 10.1016/j.otohns.2008.05.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aronson AE, Brown JR, Litin EM, Pearson JS. Spastic dysphonia. I. Voice, neurologic, and psychiatric aspects. J Speech Hear Disord. 1968;33:203–218. doi: 10.1044/jshd.3303.203. doi: 10.1044/jshd.3303.219. [DOI] [PubMed] [Google Scholar]
- 125.Murry T, Cannito MP, Woodson GE. Spasmodic dysphonia. Emotional status and botulinum toxin treatment. Arch Otolaryngol Head Neck Surg. 1994;120:310–316. doi: 10.1001/archotol.1994.01880270056010. doi: 10.1001/archotol.1994.01880270056010. [DOI] [PubMed] [Google Scholar]
- 126.Edgar JD, Sapienza CM, Bidus K, Ludlow CL. Acoustic measures of symptoms in abductor spasmodic dysphonia. J Voice. 2001;15:362–372. doi: 10.1016/S0892-1997(01)00038-8. doi: 10.1016/S0892-1997(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 127.Roy N, Mauszycki SC, Merrill RM, Gouse M, Smith ME. Toward improved differential diagnosis of adductor spasmodic dysphonia and muscle tension dysphonia. Folia Phoniatr Logop. 2007;59:83–90. doi: 10.1159/000098341. doi: 10.1159/000098341. [DOI] [PubMed] [Google Scholar]
- 128.Roy N, Whitchurch M, Merrill RM, Houtz D, Smith ME. Differential diagnosis of adductor spasmodic dysphonia and muscle tension dysphonia using phonatory break analysis. Laryngoscope. 2008;118:2245–2253. doi: 10.1097/MLG.0b013e318184577c. doi: 10.1097/MLG.0b013e318184577c. [DOI] [PubMed] [Google Scholar]
- 129.Kempster GB, Gerratt BR, Verdolini Abbott K, Barkmeier-Kraemer J, Hillman RE. Consensus auditory-perceptual evaluation of voice: development of a standardized clinical protocol. Am J Speech Lang Pathol. 2009;18:124–132. doi: 10.1044/1058-0360(2008/08-0017). doi: 10.1044/1058-0360(2008/08-0017) [DOI] [PubMed] [Google Scholar]
- 130.Fairbanks GA. Drill and articulation workjournal. Second edition. New York, NY: Harper and Row; 1960. [Google Scholar]
- 131.Bove M, Daamen N, Rosen C, Wang CC, Sulica L, Gartner-Schmidt J. Development and validation of the vocal tremor scoring system. Laryngoscope. 2006;116:1662–1667. doi: 10.1097/01.mlg.0000233255.57425.36. doi: 10.1097/01.mlg.0000233255.57425.36. [DOI] [PubMed] [Google Scholar]
- 132.Kendall KA, Leonard RJ. Interarytenoid muscle botox injection for treatment of adductor spasmodic dysphonia with vocal tremor. J Voice. 2011;25:114–119. doi: 10.1016/j.jvoice.2009.08.003. doi: 10.1016/j.jvoice.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 133.Patel RR, Liu L, Galatsanos N, Bless DM. Differential vibratory characteristics of adductor spasmodic dysphonia and muscle tension dysphonia on high-speed digital imaging. Ann Otol Rhinol Laryngol. 2011;120:21–32. doi: 10.1177/000348941112000104. doi: 10.1177/000348941112000104. [DOI] [PubMed] [Google Scholar]
- 134.Van Lierde KM, De Bodt M, Dhaeseleer E, Wuyts F, Claeys S. The treatment of muscle tension dysphonia: a comparison of two treatment techniques by means of an objective multiparameter approach. J Voice. 2010;24:294–301. doi: 10.1016/j.jvoice.2008.09.003. doi: 10.1016/j.jvoice.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 135.Van Lierde KM, De Ley S, Clement G, De Bodt M, Van Cauwenberge P. Outcome of laryngeal manual therapy in four Dutch adults with persistent moderate-to-severe vocal hyperfunction: a pilot study. J Voice. 2004;18:467–474. doi: 10.1016/j.jvoice.2004.02.003. doi: 10.1016/j.jvoice.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 136.Watts CR, Diviney SS, Hamilton A, Toles L, Childs L, Mau T. The effect of stretch-and-flow voice therapy on measures of vocal function and handicap. J Voice. 2015;29:191–199. doi: 10.1016/j.jvoice.2014.05.008. doi: 10.1016/j.jvoice.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 137.Paniello RC, Edgar JD, Perlmutter JS. Vocal exercise versus voice rest following botulinum toxin injections: a randomized crossover trial. Ann Otol Rhinol Laryngol. 2009;118:759–763. doi: 10.1177/000348940911801102. [PMC free article] [PubMed] [Google Scholar]
- 138.Ludlow CL, Naunton RF, Sedory SE, Schulz GM, Hallett M. Effects of botulinum toxin injections on speech in adductor spasmodic dysphonia. Neurology. 1988;38:1220–1225. doi: 10.1212/wnl.38.8.1220. doi: 10.1212/WNL.38.8.1220. [DOI] [PubMed] [Google Scholar]
- 139.Ludlow CL, Naunton RF, Terada S, Anderson BJ. Successful treatment of selected cases of abductor spasmodic dysphonia using botulinum toxin injection. Otolaryngol Head Neck Surg. 1991;104:849–855. doi: 10.1177/019459989110400614. doi: 10.1177/019459989110400614. [DOI] [PubMed] [Google Scholar]
- 140.Davidson BJ, Ludlow CL. Long-term effects of botulinum toxin injections in spasmodic dysphonia. Ann Otol Rhinol Laryngol. 1996;105:33–42. doi: 10.1177/000348949610500106. doi: 10.1177/000348949610500106. [DOI] [PubMed] [Google Scholar]
- 141.Bielamowicz S, Ludlow CL. Effects of botulinum toxin on pathophysiology in spasmodic dysphonia. Ann Otol Rhinol Laryngol. 2000;109:194–203. doi: 10.1177/000348940010900215. doi: 10.1177/000348940010900215. [DOI] [PubMed] [Google Scholar]
- 142.Pearson EJ, Sapienza CM. Historical approaches to the treatment of adductor-type spasmodic dysphonia (ADSD): review and tutorial. NeuroRehabilitation. 2003;18:325–338. [PubMed] [Google Scholar]
- 143.Ludlow CL. Treatment for spasmodic dysphonia: limitations of current approaches. Curr Opin Otolaryngol Head Neck Surg. 2009;17:160–165. doi: 10.1097/MOO.0b013e32832aef6f. doi: 10.1097/MOO.0b013e32832aef6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Merati AL, Heman-Ackah YD, Abaza M, Altman KW, Sulica L, Belamowicz S. Common movement disorders affecting the larynx: a report from the neurolaryngology committee of the AAO-HNS. Otolaryngol Head Neck Surg. 2005;133:654–665. doi: 10.1016/j.otohns.2005.05.003. doi: 10.1016/j.otohns.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 145.Benninger MS, Smith LJ. Noncosmetic uses of botulinum toxin in otolaryngology. Cleve Clin J Med. 2015;82:729–732. doi: 10.3949/ccjm.82a.14096. doi: 10.3949/ccjm.82a.14096. [DOI] [PubMed] [Google Scholar]
- 146.Cai BB, Francis J, Brin MF, Broide RS. Botulinum neurotoxin type A-cleaved SNAP25 is confined to primary motor neurons and localized on the plasma membrane following intramuscular toxin injection. Neuroscience. 2017;352:155–169. doi: 10.1016/j.neuroscience.2017.03.049. doi: 10.1016/j.neuroscience.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 147.Gurey LE, Sinclair CF, Blitzer A. A new paradigm for the management of essential vocal tremor with botulinum toxin. Laryngoscope. 2013;123:2497–2501. doi: 10.1002/lary.24073. doi: 10.1002/lary.24073. [DOI] [PubMed] [Google Scholar]
- 148.Holzer SE, Ludlow CL. The swallowing side effects of botulinum toxin type A injection in spasmodic dysphonia. Laryngoscope. 1996;106((Pt 1)):86–92. doi: 10.1097/00005537-199601000-00017. doi: 10.1097/00005537-199601000-00017. [DOI] [PubMed] [Google Scholar]
- 149.Buchholz DW, Neumann S. The swallowing side effects of botulinum toxin type A injection in spasmodic dysphonia. Dysphagia. 1997;12:59–60. [PubMed] [Google Scholar]
- 150.Novakovic D, Waters HH, D’Elia JB, Blitzer A. Botulinum toxin treatment of adductor spasmodic dysphonia: longitudinal functional outcomes. Laryngoscope. 2011;121:606–612. doi: 10.1002/lary.21395. doi: 10.1002/lary.21395. [DOI] [PubMed] [Google Scholar]
- 151.Yeo HG, Lee SJ, Hyun JK, Kim TU. Diagnosis of spasmodic dysphonia manifested by swallowing difficulty in videofluoroscopic swallowing study. Ann Rehabil Med. 2015;39:313–317. doi: 10.5535/arm.2015.39.2.313. doi: 10.5535/arm.2015.39.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dromey C, Smith ME. Vocal tremor and vibrato in the same person: acoustic and electromyographic differences. J Voice. 2008;22:541–545. doi: 10.1016/j.jvoice.2006.12.001. doi: 10.1016/j.jvoice.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 153.Massey EW, Paulson GW. Essential vocal tremor: clinical characteristics and response to therapy. South Med J. 1985;78:316–317. [PubMed] [Google Scholar]
- 154.Patel A, Frucht SJ. Isolated vocal tremor as a focal phenotype of essential tremor: a retrospective case review. J Clin Mov Disord. 2015;2:4. doi: 10.1186/s40734-015-0016-5. doi: 10.1186/s40734-015-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hachinski VC, Thomsen IV, Buch NH. The nature of primary vocal tremor. Can J Neurol Sci. 1975;2:195–197. doi: 10.1017/s0317167100020254. doi: 10.1017/S0317167100020254. [DOI] [PubMed] [Google Scholar]
- 156.Louis ED, Gerbin M. Voice handicap in essential tremor: a comparison with normal controls and Parkinson's disease. Tremor Other Hyperkinet Mov. 2013;3 doi: 10.7916/D8KD1WN3. doi: 10.7916/D8KD1WN3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lester RA, Barkmeier-Kraemer J, Story BH. Physiologic and acoustic patterns of essential vocal tremor. J Voice. 2013;27:422–432. doi: 10.1016/j.jvoice.2013.01.002. doi: 10.1016/j.jvoice.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 158.Barkmeier-Kraemer J. Updates on vocal tremor and its management. SIG 3 Persp Voice Voice Disord. 2012;22:97–103. doi: 10.1044/vvd22.3.97. [Google Scholar]
- 159.Barkmeier-Kraemer J, editor. Neurolaryngologie. Belsele, Belgium: Vlaamse Vereniging voor Logopedisten; 2016. Evaluation and treatment of vocal tremor. editor. StEMINARIES 2015-2016. [Google Scholar]