Abstract
The development of molecular markers is one of the most useful methods for molecular breeding and marker-based molecular associated selections. Even though there is less information on the reference genome, molecular markers are indispensable tools for determination of genetic variation and identification of species with high levels of accuracy and reproducibility. The demand for molecular approaches for marker-based breeding and genetic discriminations in Panax species has greatly increased in recent times and has been successfully applied for various purposes. However, owing to the existence of diverse molecular techniques and differences in their principles and applications, there should be careful consideration while selecting appropriate marker types. In this review, we outline the recent status of different molecular marker applications in ginseng research and industrial fields. In addition, we discuss the basic principles, requirements, and advantages and disadvantages of the most widely used molecular markers, including restriction fragment length polymorphism, random amplified polymorphic DNA, sequence tag sites, simple sequence repeats, and single nucleotide polymorphisms.
Keywords: high-resolution melting, Panax ginseng, molecular marker, single-nucleotide polymorphism
1. Introduction
Korean ginseng (Panax ginseng) is one of the most favored herbal medicinal plants valued by local Korean consumers as well as those based overseas. The growth condition of Korean ginseng cultivation requires careful monitoring system of the ideal cultivar conditions in its natural habitat; owing to this as well as the stringent cultivation and processing techniques such as those required in the manufacture of red ginseng (hongsam). Furthermore, it is considered superior to the cultivars of competing ginseng producing countries [1], [2], [3]. However, recently, foreign ginseng, which is cheaper than Korean ginseng and is of questionable safety, has been illegally distributed as a Korean domestic produce, thereby hindering the development of the ginseng industry [4], [5]. In addition, relatively inexpensive local varieties with less uniform seeds are being misleadingly packaged as new varieties, putting cultivators of genuine new varieties at a disadvantage [6]. Consequently, it is becoming difficult for domestic cultivators to obtain guaranteed stable profits. As consumer trust in domestic produce declines, domestic ginseng producers face considerable losses.
An effort to ensure distinctness and seed uniformity during the seed production stage is necessary to counter this problem. International institutions such as the World Trade Organization, Trade Related Intellectual Property Rights, Organization for Economic Cooperation and Development, and the European Union have made the protection of intellectual property rights for domestically produced varieties compulsory. Moreover, with the establishment of the International Union for the Protection of New Varieties of Plants (UPOV), various institutional devices are being developed to protect rights related to plant varieties [7]. In its endeavor to protect domestic and foreign intellectual property rights for domestic varieties, Korea has joined the UPOV, to ensure the international profits and rights of breeders of newly developed varieties. Furthermore, the Korea Seed and Variety Service has put forward clear criteria for establishing varieties. In order to protect the new domestic varieties, this institution assesses for the cultivars distinctness, uniformity, and stability [8].
A comparative assessment of external morphology was performed to test the distinctness of new varieties. However, the differences that determine distinctness are often qualitative, which makes it difficult to accurately distinguish varieties based on morphology alone [9]. Accordingly, biochemical and molecular biological techniques have recently been applied. Molecular markers are especially advantageous because they are not affected by the cultivation environment, such as the soil conditions, and because large samples can be analyzed quickly and accurately in the laboratory [10], [11], [12]. Molecular markers are now being used frequently to distinguish cultivars of ginseng. Therefore, in this review article, we summarize the current knowledge regarding the development of molecular markers, and provide a focused review of their recent application for genetic diversity analysis of Panax species and Korean ginseng cultivars.
2. Molecular markers for discrimination in Panax species
2.1. Polymerase chain reaction-restriction fragment length polymorphism
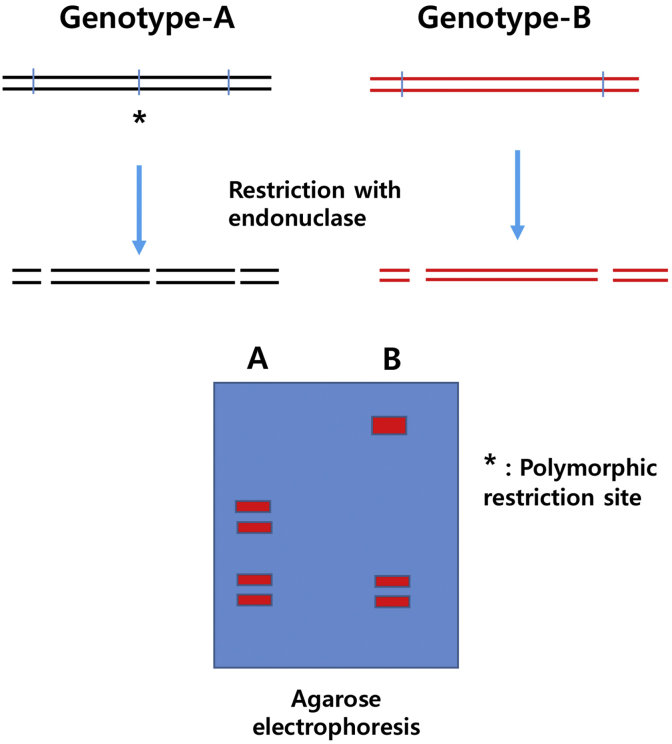
Restriction fragment length polymorphism (RFLP) is a method that is based on differences in the length of DNA fragments obtained when DNA from different sources is digested with restriction enzymes that recognize specific sequences (Fig. 1). Although RFLP has been suggested as an important tool for genome mapping and genetic fingerprinting, it requires a large amount of sample DNA and probe, which is a labeled DNA sequence that hybridizes with one or more fragments. Therefore, in order to overcome the disadvantages associated with RFLP, polymerase chain reaction (PCR)-RFLP based on endonuclease digestion of PCR-amplified DNA has been introduced as an extremely valuable tool for genotyping of species-specific variations.
Fig. 1.
Schematic explanation of restriction fragment length polymorphism.
Because of the simplicity and speed of procedures and the requirement of small amounts of DNA, this technique has been widely utilized for species identification and taxonomic research. Based on the differences in the 18S rRNA gene sequence, three species in the Panax genus can be differentiated at the DNA level by PCR-RFLP and mutant allele specific amplification [13]. Ngan et al [14] conducted an RFLP analysis of ribosomal ITS1-5.8S-ITS2 sequence among six species belonging to the Panax genus and a ginseng mixture; specifically, these researchers differentiated P. ginseng and Panax quinquefolius. This indicates that the sequence homology within the ITS1 and ITS2 regions is low in these two species. In addition, Um et al [15] carried out random amplified polymorphic DNA (RAPD) and PCR-RFLP and obtained DNA fingerprints that are different among individual ginseng plants. Yang and Kim [16] cleaved ginseng chlorophyll psbA and rbcL genes by restriction enzymes for differentiation and analyzed interspecies and intraspecies mutational variation. Furthermore, distinguishing Korean ginseng from various mixtures of medicinal plants could be possible by gradient PCR and RFLP applications [17].
Genetic analyses of Korean and foreign ginseng cultivars by Kim et al [18] were performed and successfully distinguished the cultivars Gopoong and Gumpoong (Korean ginseng cultivars). In addition, Diao et al [19] used PCR-RFLP and an amplification refractory mutation system based on the 5S rDNA sequence from commercially available complex drug mixtures and proved the existence of P. ginseng. All of the studies reviewed were able to differentiate the plants only at the species level and analyzed only a limited number of plant samples; collectively, these studies were unable to distinguish all cultivars. Thus, PCR-RFLP shows good reproducibility in the discrimination of species in the ginseng genus but the discriminatory ability of PCR-RFLP for cultivars differentiation is low.
2.2. RAPD
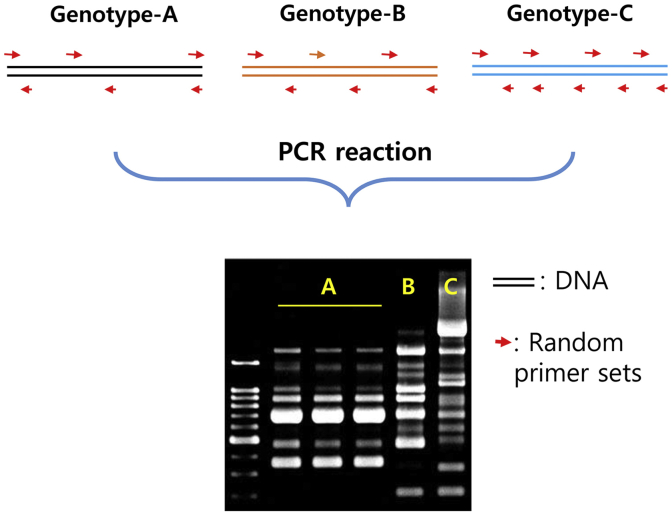
The RAPD method is relatively simpler than RFLP and can easily identify polymorphic DNA fragments (Fig. 2). Because the method requires only small amounts of DNA, RAPD can also be used for testing of genetic characteristics of many individuals, thus making it a highly economical method [20], [21]. However, RAPD is a dominant marker and cannot be used for segregation of the F2 population. This method uses a short primer, approximately a 10mer, resulting in relatively low reproducibility. Initially, Um et al [15] reported the possibility of classification of ginseng species by comparing interspecies and intraspecies mutational relations by means of polymorphic bands of RAPD. Based on the genetic diversity in American ginseng cultivated in Ontario, two more species could be identified by using RAPD analysis [22], and their results suggested that it is possible to improve the genetic improvement in ginseng cultivars. Shao et al [23] produced 18 random primers for RAPD analysis of ginseng, studied the genetic diversity of the P. quinquefolius population in North America, and identified population specific RAPD markers [24]. Moreover, 25 primers with high reproducibility, from 130 random primers, were developed by Bang et al. [25], and used these primers to study the genetic diversity among Panax species and cultivars of Korean ginseng [25].
Fig. 2.
Schematic explanation of random amplified polymorphic DNA.
At present, RAPD analysis of ginseng is broadly used for elucidating species-level genetic differences or for genetic diversity analysis of cultivars due to lower efficiency in discrimination of cultivars [26], [27], [28]. Moreover, RAPD generates nonspecific amplicons depending on experimental and ambient conditions; therefore, it is difficult to ensure reproducibility. To overcome this, an analysis of a sequence-characterized amplified region (SCAR) marker as the most effective method in terms of convenience and reproducibility among the various molecular biological methods was examined for identification of ginseng species and discrimination of cultivars [29], suggesting that a SCAR marker can be used to distinguish three species: P. ginseng, P. quinquefolius, and Panax notoginseng, and the Korean ginseng cultivar Sunone.
2.3. Sequence-tagged site
The sequence-tagged site (STS) method was developed by Olson et al [30] and involves analysis of a sequence for specific clones followed by construction of diverse primers for genetic analysis. A DNA library needs to be established for the production of specific STS primer sets [31]. Recently, the methylation filtering (MF) method, which removes methylated repetitive DNA from a library, has become a popular method that can increase cloning frequency of genomic regions [32]. The MF method enables removal of repetitive DNA and is thus useful for creating a library of genetic regions [33], [34]. Bang et al [31] built a genomic DNA library from the Yunpoong cultivar of the Korean ginseng by using the MF method; furthermore, on the basis of this information, they identified and reported eight STS markers that can differentiate six cultivars of Korean ginseng by using 208 pairs of STS primers. Several novel STS markers by establishing an MF processed genomic DNA library were developed by Jo et al [9], and were able to apply to this approach to 11 cultivars of Korean ginseng to identify a marker capable of distinguishing the new cultivar Cheonryang.
RAPD and PCR-RFLP markers show insufficient numbers of suitable loci within the genome and have poor sensitivity, reliability, discriminatory ability, and reproducibility, posing difficulties in terms of discrimination of strains [35]. In contrast, an STS marker with the simplicity of its analytical method allows for easy confirmation of results with high reproducibility and, therefore, can be applied to the analysis of massive genetic resources and to cultivar discrimination [36], [37].
2.4. Simple sequence repeats
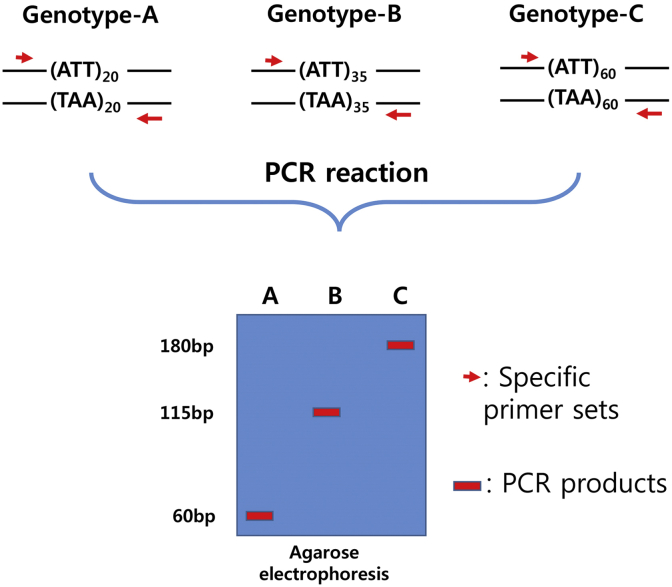
Simple sequence repeats (SSR) analysis involves the fabrication of primers for repeated monomeric, dimeric, trimeric, and tetrameric DNA sequences (Fig. 3). This method is relatively expensive and time consuming for creation of a genomic library and for primer fabrication; however, it involves a codominant marker and has very high reproducibility [38], [39]. SSR markers are reported to be effective for strain discrimination because they cover multiple alleles and have genomic region specificity, high polymorphism, and reproducibility [40], [41].
Fig. 3.
Schematic explanation of simple sequence repeat marker.
The ginseng SSR marker, although developed relatively late in comparison with other markers, has been actively used in recent years. Kim et al [42] and Ma et al [43] produced a ginseng microsatellite-enriched genomic library and identified a microsatellite sequence through screening of the library. Useful microsatellite sequences during end-sequence analysis of a Bacterial artificial chromosome (BAC) genomic library were also isolated and applied [44]. In addition, the developed SSR markers were successfully applied to American ginseng (P. quinquefolius) discrimination. However, Jo et al [45] reported no significant difference in the allele distribution of microsatellite sequences between ginseng cultivars and wild ginseng. Research performed by Hon et al [46] revealed that Korean ginseng (P. ginseng) and American ginseng (P. quinquefolius) could be distinguishable from each by analyzing the microsatellite sequences identified in the latter.
Recently, an alternative strategy has been developed that uses chloroplast genome, Expressed sequence tag (EST) and transcriptome information to identify SSR markers. Kim and Lee [47] reported 18 SSR markers that show species-specific mutations in the ginseng chlorophyll genes. Moreover, 68 sets of SSR markers from 7,055 expressed sequence tags of P. ginseng developed as powerful markers that effectively distinguish between the Chinese cultivars of P. ginseng, ‘Ji’anchangbo’ and ‘Fusong’ermaya’ [48]. Interestingly, Xu et al [49] used sequence-related amplified polymorphism analysis to study the genetic diversity of P. ginseng cultivated in China. Also, 19 EST-SSR markers to distinguish nine cultivars of Korean ginseng was proposed as an effective method for cultivar discrimination [50]. As described above, the SSR markers have been widely used in studies of genetic diversity of ginseng, analysis of genetic relations, and cultivar discrimination because of high polymorphism and reproducibility. However, this method has its limitations in that the analysis is time-consuming and because of the use of polyacrylamide gels requires skilled operators.
2.5. Single nucleotide polymorphisms
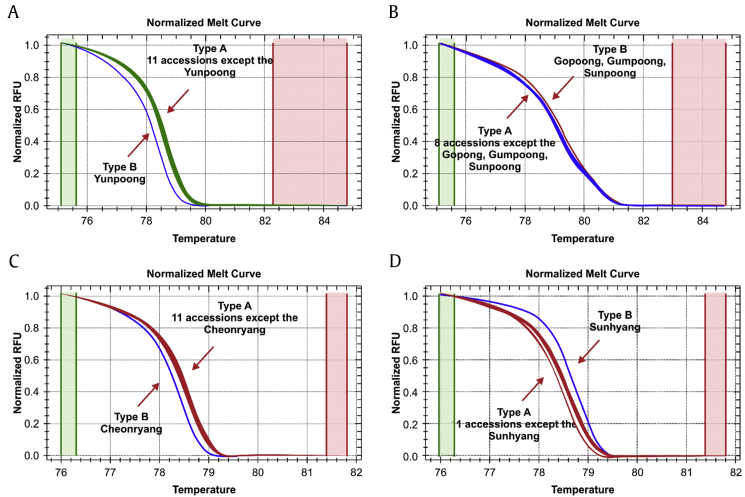
A single nucleotide polymorphism (SNP) is a single-base mutation in DNA and most frequently affects the genome of all somatic cells and has the advantage of being a stable analytical parameter [51]. Wang et al [52] examined the SNPs of mitochondrial cytochrome oxidase subunit 2, regions of introns 1 and 2, and could successfully develop a specific marker for Chunpoong, a popular cultivar in Korea. Yunpoong-specific SNP in the exon of the glyceraldehyde 3-phosphate dehydrogenase gene was identified and this polymorphism was able to distinguish this cultivar from the major ginseng cultivars in Korea [53]. Jo et al [6] produced three pairs of TaqMan-MGB probes for an SNP that is present in genomic DNA to perform genotyping; thus, they developed a method for discrimination of Korean ginseng (Fig. 4). Recent developments in next-generation sequencing technology (NGS) have accelerated the growth of sequencing information. It is now possible to elucidate a large number of SNPs using transcript information. Consistent with this trend, Jo et al [54] identified a large number of SNPs in the transcript information on ginseng and developed high-resolution melting (HRM) analysis that can distinguish cultivars (Fig. 4). Various research groups have successfully attempted species and cultivar discrimination by SNP genotyping by using HRM analysis. For example, the use of SNP genotyping with HRM analysis was usefully applied for discrimination among almond cultivars or citrus species [55], [56].
Fig. 4.
Examples of rapid discrimination of Panax ginseng cultivars and Panax quinquefolius by use of high-resolution melt analysis with (A) GHP 01095, (B) GHP 11494, (C) GHP 13830, and (D) GHP 15673 markers. Temperature unit is °C (Jo et al [54], 2015).
In the HRM method, an intercalating fluorescent dye that is added during PCR binds to amplicons, and is analyzed for changes in characteristics of the fluorescent signals dependent on a temperature increase and the sequence of the target regions [57], [58]. Therefore, HRM analysis allows for more rapid and convenient discrimination of PCR target regions based on SNP variation, thus making it possible to distinguish cultivars by genotyping of DNA samples.
3. Conclusion and future perspectives
In order to prepare for opening of the market by free-trade agreements affecting the ginseng industry, competing countries, including China, the United States, and Canada, intend to deal with the problem of illegal outflow of Korean ginseng species. It is necessary, therefore, to select DNA markers that can distinguish cultivar and to establish a database using the selected markers. In Korea, currently, research and development of ginseng cultivar is conducted in a highly competitive industry, which has also to compete with produce from other countries such as China, the United States, and Canada. For this, scientific certification technology for the development of excellent cultivars and systematic species management needs to be implemented. The lack of this certification would be a big setback to the progress of the Korean ginseng industry. The problems the ginseng industry will be faced with can be listed as follows: (1) a case where domestic cultivars are illegally shipped overseas and are imported back to Korea and sold as domestic product; (2) a case where native species are used to create new cultivars for the relevant domestic markets and these cultivars are sold at higher prices; and (3) a case when poor management of species during cultivation leads to a species of lowered genetic purity.
Conventional methods of DNA analysis are inconvenient, expensive, and time-consuming with respect to strain discrimination. These methods are also limited by the low frequency of polymorphisms in Panax species. In recent years, identification of standard genomic information and acceleration of massive production of sequence data by means of NGS has allowed for easy and rapid identification of mutations such as SNP, SSR, and Insertion/Deletion (IN/DEL) in full-length genomes. As such, the NGS method offers big benefits at low costs and is rapidly facilitating identification of genetic markers in ginseng plants. Diverse DNA markers like RFLP, RAPD, STS, SSR, and SNP analyses are being developed for discrimination of ginseng cultivar.
In conclusion, as described in this review, the development of useful efficient markers must be established. This information would be beneficial for basic ginseng breeding methods. The markers once identified and established could offer analysis of consistent results and highlight the importance of preserving genetic distinctness, uniformity, discrimination of promising line during ginseng breeding, and identification of the parent plants
Conflicts of interest
The authors have no conflicts of interest with any parties or individuals.
Acknowledgments
This work was carried out with the support of the Basic Science Research Program through the National Research Foundation of Korea (2015R1A4A1041869) Korean Ministry of Science, ICT and Future Planning and Cooperative Research Program for Agriculture Science and Technology Development (PJ01047301), Rural Development Administration, Korea.
Contributor Information
Hojin Ryu, Email: hjryu96@chungbuk.ac.kr.
Kyong Hwan Bang, Email: bang31@korea.kr.
References
- 1.Lee B.Y. Status of Korean ginseng industry and development of new ginseng products. Food Ind Nutr. 2003;8:1–9. [Google Scholar]
- 2.Yun T. Brief introduction of Panax ginseng CA Meyer. J Korean Med Sci. 2001;16(Suppl):3–5. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bang K.H., Jo I.H., Kim Y.C., Kim J.U., Park H.W., Shin M.R., Kim Y.B., Kim O.T., Hyun D.Y., Kim D.H. Molecular identification of Korean ginseng cultivars (Panax ginseng C. A. Mey.) using peptide nucleic acid (PNA) microarray. Korean J Med Crop Sci. 2012;20:387–392. [Google Scholar]
- 5.Park M.J., Kim M.K., In J.G., Yang D.C. Molecular identification of Korean ginseng by amplification refractory mutation system-PCR. Food Res Int. 2006;39:568–574. [Google Scholar]
- 6.Jo I.H., Bang K.H., Kim Y.C., Lee J.W., Seo A.Y., Seong B.J., Kim H.H., Kim D.H., Cha S.W., Cho Y.G. Rapid identification of ginseng cultivars (Panax ginseng Meyer) using novel SNP-based probes. J Ginseng Res. 2011;35:504–513. doi: 10.5142/jgr.2011.35.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutfield G. Food, biological diversity and intellectual property: The role of the international union for the protection of new varieties of plants (UPOV) Intellectual Property Issue Paper. 2011;9:1–19. [Google Scholar]
- 8.Kwon Y.S., Lee J.M., Yi G.B., Yi S.I., Kim K.M., Soh E.H., Bae K.M., Park E.K., Song I.H., Kim B.D. Use of SSR markers to complement tests of distinctiveness, uniformity, and stability (DUS) of pepper (Capsicum annuum L.) varieties. Mol Cells. 2005;19:428–435. [PubMed] [Google Scholar]
- 9.Jo I.H., Kim Y.C., Kim J.U., Lee S.H., Lim J.Y., Moon J.Y., Noh B.S., Hyun D.Y., Kim D.H., Kim K.H. A rapid identification of Korean ginseng cultivar, Cheonryang, using specific DNA markers. Korean J Med Crop Sci. 2014;22:429–434. [Google Scholar]
- 10.In D.S., Kim Y.C., Bang K.H., Chung J.W., Kim O.T., Hyun D.Y., Cha S.W., Kim T.S., Seong N.S. Genetic relationships of Panax species by RAPD and ISSR analyses. Korean J Med Crop Sci. 2005;13:249–253. [Google Scholar]
- 11.Chowdhury M.A., Vandenberg B., Warkentin T. Cultivar identification and genetic relationship among selected breeding lines and chickpea (Cicer arietinum L.) Euphytica. 2002;127:317–325. [Google Scholar]
- 12.Gostimskiĭ S.A., Kokaeva Z.G., Konovalov F.A. Studying plant genome variation using molecular markers. Genetika. 2005;41:480–492. [PubMed] [Google Scholar]
- 13.Fushimi H., Komatsu K., Isobe M., Namba T. Application of PCR-RFLP and MASA analyses on 18S ribosomal RNA gene sequence for the identification of three ginseng drugs. Biol Pharm Bull. 1997;20:765–769. doi: 10.1248/bpb.20.765. [DOI] [PubMed] [Google Scholar]
- 14.Ngan F., Shaw P., But P.P.H., Wang J. Molecular authentication of Panax species. Phytochemistry. 1999;50:787–791. doi: 10.1016/s0031-9422(98)00606-2. [DOI] [PubMed] [Google Scholar]
- 15.Um J.Y., Chung H.S., Kim M.S., Na H.J., Kwon H.J., Kim J.J., Lee K.M., Lee S.J., Lim J.P., Do K.R. Molecular authentication of Panax ginseng species by RAPD analysis and PCR-RFLP. Biol Pharm Bull. 2001;24:872–875. doi: 10.1248/bpb.24.872. [DOI] [PubMed] [Google Scholar]
- 16.Yang D.C., Kim M.S. DNA analysis of ginseng using PCR-aided RFLP technology. J Ginseng Res. 2003;27:146–150. [Google Scholar]
- 17.Shim Y.H., Park C.D., Kim D.H., Cho J.H., Cho M.H., Kim H.J. Identification of Panax species in the herbal medicine preparations using gradient PCR method. Biol Pharm Bulln. 2005;28:671–676. doi: 10.1248/bpb.28.671. [DOI] [PubMed] [Google Scholar]
- 18.Kim O.T., Bang K.H., In D.S., Lee J.W., Kim Y.C., Shin Y.S., Hyun D.Y., Lee S.S., Cha S.W., Seong N.S. Molecular authentication of ginseng cultivars by comparison of internal transcribed spacer and 5.8S rDNA sequences. Plant Biotechnol Rep. 2007;1:163–167. [Google Scholar]
- 19.Diao Y., Lin X.M., Liao C.L., Tang C.Z., Chen Z.J., Hu Z.L. Authentication of Panax ginseng from its adulterants by PCR-RFLP and ARMS. Planta medica. 2009;75:557–560. doi: 10.1055/s-0029-1185321. [DOI] [PubMed] [Google Scholar]
- 20.Jain A., Bhatia S., Banga S.S., Prakash S., Lakshmikumaran M. Potential use of random amplified polymorphic DNA (RAPD) technique to study the genetic diversity in Indian mustard (Brassica juncea) and its relationship to heterosis. Theor Appl Genet. 1994;8:116–122. doi: 10.1007/BF00222403. [DOI] [PubMed] [Google Scholar]
- 21.Paran I., Michelmore R.W. Development of reliable PCR-based markers linked to downy mildew resistance genes in lettuce. Theor Appl Genet. 1993;85:985–993. doi: 10.1007/BF00215038. [DOI] [PubMed] [Google Scholar]
- 22.Bai D., Brandle J., Reeleder R. Genetic diversity in North American ginseng (Panax quinquefolius L.) grown in Ontario detected by RAPD analysis. Genome. 1997;40:111–115. doi: 10.1139/g97-015. [DOI] [PubMed] [Google Scholar]
- 23.Shao A.J., Li X., Huang L.Q., Wei J.H., Lin S.F. Genetic analysis of cultivated ginseng population with the assistance of RAPD technology. Zhongguo Zhongyao Zazhi. 2004;29:1033–1036. [PubMed] [Google Scholar]
- 24.Lim W., Mudge K.W., Weston L.A. Utilization of RAPD markers to assess genetic diversity of wild populations of North American ginseng (Panax quinquefolium) Planta Med. 2007;73:71–76. doi: 10.1055/s-2006-951768. [DOI] [PubMed] [Google Scholar]
- 25.Bang K.H., Chung J.W., Kim Y.C., Jo I.H., Kim J.U., Shin M.R., Hyun D.Y., Kim D.H., Cha S.W., Kim K.H. Analysis of genetic polymorphism of Korean ginseng cultivars and breeding lines using RAPD markers. Korean J Intl Agri. 2013;25:184–193. [Google Scholar]
- 26.Ardiel G.S., Grewal T.S., Deberdt P., Rossnagel B.G., Scoles G.J. Inheritance of resistance to covered smut in barley and development of a tightly linked SCAR marker. Theor Appl Genet. 2002;104:457–464. doi: 10.1007/s001220100696. [DOI] [PubMed] [Google Scholar]
- 27.Deng Z., Huang S., Xiao S., Gmitter F.G. Development and characterization of SCAR markers linked to the citrus tristeza virus resistance gene form Poncorus trifoliate. Genome. 1997;40:697–704. doi: 10.1139/g97-792. [DOI] [PubMed] [Google Scholar]
- 28.Ripley V.L., Roslinsky V. Identification of an ISSR marker for 2-propenyl glucosinolate content in Brassica juncea L. and conversion to a SCAR marker. Mol Breed. 2005;16:57–66. [Google Scholar]
- 29.Lee J.W., Kim Y.C., Jo I.H., Seo A.Y., Lee J.H., Kim O.T., Hyun D.Y., Cha S.W., Bang K.H., Cho J.H. Development of an ISSR-derived SCAR marker in Korean ginseng cultivars. (Panax ginseng C. A. Meyer) J Ginseng Res. 2011;35:52–59. [Google Scholar]
- 30.Olson M., Hood L., Cantor C., Botstein D. A common language for physical mapping of the human genome. Science. 1989;245:1434–1435. doi: 10.1126/science.2781285. [DOI] [PubMed] [Google Scholar]
- 31.Bang K.H., Lee J.W., Kim Y.C., Kim D.H., Lee E.H., Jeung J.U. Construction of genomic DNA library of Korean ginseng(Panax ginseng C. A Meyer) and development of sequence-tagged sites. Biol Pharm Bull. 2010;33:1579–1588. doi: 10.1248/bpb.33.1579. [DOI] [PubMed] [Google Scholar]
- 32.Rabinowicz P.D., McCombie W.R., Martienssen R.A. Gene enrichment in plant genomic shotgun libraries. Curr Opin Plant Biol. 2003;6:150–156. doi: 10.1016/s1369-5266(03)00008-6. [DOI] [PubMed] [Google Scholar]
- 33.Gruenbaum Y., NavehMany T., Cedar H., Razin A. Sequence specificity of methylation in higher plant DNA. Nature. 1981;292:860–862. doi: 10.1038/292860a0. [DOI] [PubMed] [Google Scholar]
- 34.Bennetzen J.L., Schrick K., Springer P.S., Brown W.E., SanMiguel P. Active maize genes are unmodified and flanked by diverse classes of modified, highly repetitive DNA. Genome. 1994;37:565–576. doi: 10.1139/g94-081. [DOI] [PubMed] [Google Scholar]
- 35.Jo I.H., Bang K.H., Kim Y.C., Kim J.U., Shin M.R., Moon J.Y., Noh B.S., Hyun D.Y., Kim D.H., Cha S.W. Analysis of mitochondrial DNA sequence and molecular marker development for identification of Panax species. Korean J Med Crop Sci. 2013;21:91–96. [Google Scholar]
- 36.Jo I.H., Shin M.R., Kim Y.C., Lee S.H., Kim J.U., Moon J.Y., Noh B.S., Kang S.T., Lee D.J., Hyun D.Y. Discrimination of Korean ginseng cultivars by sequence tagged sites (STS) markers. Korean J Med Crop Sci. 2013;21:353–360. [Google Scholar]
- 37.Hwang T.Y., Jung S.M., Lee S.K., Park H.M., Jeong K.H., Lee Y.Y., Kim S.L., Yun H.T., Lee J.E., Kim D.W. Discrimination of 110 Korean soybean cultivars by sequence tagged sites(STS)-CAPS markers. Korean J Breeding Sci. 2012;44:258–272. [Google Scholar]
- 38.Hamada S.R., Petrino M.G., Kakunaga T. A novel repeated element with Z-DNA-forming potential is widely found in evolutionarily diverse eukaryotic genomes. Proc Natl Acad Sci U S A. 1982;79:6465–6469. doi: 10.1073/pnas.79.21.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tautz D., Renz M. Simple sequence are ubiquitous repetitive components of eukaryotic genomes. Nucleic Acids Res. 1984;12:4127–4138. doi: 10.1093/nar/12.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aggarwal R.K., Lalremruata A., Velavan T.P., Sowjanya A.P., Singh L. Development and characterization of ten novel microsatellite markers from olive Ridley(Lepidochelys olivacea) Conserv Genet. 2008;9:981–984. [Google Scholar]
- 41.Parida S.K., Kalia S.K., Sunita K., Dalal V., Hemaprabha G., Selvi A., Pandit A., Singh A., Gaikwad K., Sharma T.R. Informative genomic microsatellite markers for efficient genotyping applications in sugarcane. Theor Appl Genet. 2009;118:327–338. doi: 10.1007/s00122-008-0902-4. [DOI] [PubMed] [Google Scholar]
- 42.Kim J.K., Jo B.H., Lee K.L., Yoon E.S., Ryu G.H., Chung K.H. Identification of new microsatellite markers in Panax ginseng. Mol Cells. 2007;24:60–68. [PubMed] [Google Scholar]
- 43.Ma K.H., Dixit A., Kim Y.C., Lee D.Y., Kim T.S., Cho E.G., Park Y.J. Development and characterization of new microsatellite markers for ginseng (Panax ginseng CA Meyer) Conserv Genet. 2007;8:1507–1509. [Google Scholar]
- 44.Van Dan N., Ramchiary N., Choi S., Uhm T., Yang T.J., Ahn I.O., Lim Y. Development and characterization of new microsatellite markers in Panax ginseng (C.A. Meyer) from BAC end sequences. Conserv Genet. 2010;11:1223–1225. [Google Scholar]
- 45.Jo B.H., Suh D.S., Cho E.M., Kim J.K., Ryu G.H., Chung K.W. Characterization of polymorphic microsatellite loci in cultivated and wild Panax ginseng. Genes Genomics. 2009;31:119–127. [Google Scholar]
- 46.Hon C.C., Chow Y.C., Zeng F.Y., Leung F.C. Genetic authentication of ginseng and other traditional Chinese medicine. Acta Pharmacol Sin. 2003;24:841–846. [PubMed] [Google Scholar]
- 47.Kim K.J., Lee H.L. Complete chloroplast genome sequence from Korean Ginseng (Panax schiseng Nees) and comparative analysis of sequence evolution among 17 vascular plants. DNA Res. 2004;11:247–261. doi: 10.1093/dnares/11.4.247. [DOI] [PubMed] [Google Scholar]
- 48.Yang C.J., Wang J., Mu L.Q., Li S.C., Liu G.J., Hu C.Q. Development of an EST-SSR marker in Panax ginseng. Chin J Agric Biotechnol. 2008;5:175–181. [Google Scholar]
- 49.Xu Y.H., Jin H., Kim Y.C., Bang K.H., Cha S.W., Zhang L.X. Genetic diversity and genetic structures in ginseng landraces (cultivars) by SRAP Analysis. Korean J Med Crop Sci. 2010;18:180–185. [Google Scholar]
- 50.Kim N.H., Choi H.I., Ahn I.O., Yang T.J. EST-SSR marker sets for practical authentication of all nine registered ginseng cultivars in Korea. J Ginseng Res. 2012;36:298–307. doi: 10.5142/jgr.2012.36.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta P.K., Roy J.K., Prasad M. single nucleotide polymorphism: A new paradigm for molecular marker technology and DNA polymorphism detection with emphasis on their use in plants. Curr Sci. 2001;80:524–535. [Google Scholar]
- 52.Wang H., Sun H., Kwon W.S., Jin H., Yang D.C. Molecular identification of the Korean ginseng cultivar “Chunpoong” using the mitochondrial nad7 intron 4 region. Mitochondrial DNA. 2009;20:41–45. doi: 10.1080/19401730902856738. [DOI] [PubMed] [Google Scholar]
- 53.Sun H., Wang H., Kwon W.S., Kim Y.J., In J.G., Yang D.C. A simple and rapid technique for the authentication of the ginseng cultivar, Yunpoong, using an SNP marker in a large sample of ginseng leaves. Gene. 2011;487:75–79. doi: 10.1016/j.gene.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 54.Jo I.H., Lee S.H., Kim Y.C., Kim D.H., Kim H.S., Kim K.H., Chung J.W., Bang K.H. De novo transcriptome assembly and the identification of gene-associated single-nucleotide polymorphism markers in Asian and American ginseng roots. Mol Genet Genomics. 2015;290:1055–1065. doi: 10.1007/s00438-014-0974-6. [DOI] [PubMed] [Google Scholar]
- 55.Wu S.B., Wirthensohn M.G., Hunt P., Gibson J.P., Sedgley M. High resolution melting analysis of almond SNPs derived from ESTs. Theor Appl Genet. 2008;118:1–14. doi: 10.1007/s00122-008-0870-8. [DOI] [PubMed] [Google Scholar]
- 56.Distefano G., Caruso M., La Malfa S., Gentile A., Wu S.B. High resolution melting analysis is a more sensitive and effective alternative to gel-based platforms in analysis of SSR-An example in citrus. PLoS ONE. 2012;7:e44202. doi: 10.1371/journal.pone.0044202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wittwer C.T., Reed G.H., Gundry C.N., Vandersteen J.G., Pryor R.J. High-resolution genotyping by amplicon melting analysis using LCGreen. Clin Chem. 2003;49:853–860. doi: 10.1373/49.6.853. [DOI] [PubMed] [Google Scholar]
- 58.Jaakola L., Suokas M., Haggman Novel approaches based on DNA barcoding and high-resolution melting of amplicons for authenticity analyses of berry species. Food Chem. 2010;123:492–500. [Google Scholar]