Abstract
Background
Chronic heavy alcohol consumption may raise the risk of developing type 2 diabetes mellitus. Saponins inhibit apoptosis of pancreatic islet cells and reduce lipid parameters. The present study was designed to investigate the effect of saponin on chronic ethanol-treated diabetic rats.
Methods
Long–Evans Tokushima Fatty (LETO) and Otsuka Long–Evans Tokushima Fatty (OLETF) rats were pair-fed a Lieber–DeCarli diet with and without 5% ethanol for 12 wks. Two weeks after starting the pair-feeding with the Lieber–DeCarli diet, intraperitoneal injection of saponin was performed for 10 wks. To perform the experiments, rats were divided as follows: LETO-Control (LC), LETO-Ethanol (LE), LETO-Ethanol-Saponin (LES), OLETF-Control (OC), OLETF-Ethanol (OE), and OLETF-Ethanol-Saponin (OES).
Results
The weights of epididymal and mesenteric fat tissue in LES and OES rats were the lightest from among the LETO and OLETF groups, respectively. The secretion of alanine aminotransferase and cholesterol in OES rats decreased significantly compared to their secretion in OC and OE rats, respectively. The islets of the pancreas in LE and OE rats showed clean, unclear, and smaller morphology compared to those of LC, LES, OC, and OES rats. In addition, the expression of insulin in the islets of the pancreas in LC, LES, OC, and OES rats was higher than in LE and OE rats.
Conclusion
Saponin may not only be helpful in alleviating the rapid progress of diabetes due to chronic alcohol consumption in diabetic patients, but may also show potential as an antidiabetic drug candidate for diabetic patients who chronically consume alcohol.
Keywords: alcohol, diabetes, insulin, pancreas, saponin
1. Introduction
Type 2 diabetes mellitus (T2DM), one of the most common metabolic disorders, is characterized by impaired glucose tolerance and insulin resistance of tissues and cells [1], [2]. As a result of the inappropriate response of tissues and cells to insulin, dysfunction of pancreatic beta cells occurs, resulting in the impairment of insulin production and secretion [2], [3]. Various risk factors, including obesity and chronic alcohol consumption, are responsible for the development of T2DM, which causes cardiovascular disease [4], [5]. In particular, chronic heavy alcohol consumption is known to raise the risk of developing T2DM [6].
Previous studies have observed that chronic heavy alcohol consumption leads to increases in blood glucose levels and insulin resistance in healthy controls as well as in patients with T2DM [6], [7]. Recently, it was reported that the impairment of insulin production in the pancreas is caused by chronic alcohol use, leading to the development of T2DM as well as the onset of T2DM [8], [9]. In the study of Lee et al [9], endoplasmic reticulum stress markers in the pancreas increased after chronic exposure of early diabetic stage rats to ethanol, suggesting that pancreatic dysfunction mediated by the activation of the endoplasmic reticulum stress proapoptotic pathway by ethanol may accelerate the development of T2DM. Kim et al [8] found that chronic ethanol treatment of diabetic-like mice induced through a high-fat diet promoted a decrease in islet cell mass and insulin expression. In addition to the induction of pancreatic dysfunction in T2DM, chronic alcohol consumption has been reported to occur in 50–70% of chronic pancreatitis patients, implying that chronic alcohol consumption could be a risk factor for chronic pancreatitis [10]. In considering these results, the damage done to the pancreas by chronic alcohol consumption may be linked to the occurrence or development of diabetes. However, although the effects of chronic alcohol consumption on T2DM have been widely studied, there are few reports on the development of medicine for treating T2DM worsened by chronic alcohol use.
Saponins are naturally occurring glycosides typically obtained from a variety of plants, including Panax ginseng and Bryonia laciniosa Linn [11], [12]. Saponins have been reported to not only induce hypoglycemia but also to lower plasma triglyceride, resulting in antidiabetic effects [2], [13]. Previous studies have observed that saponins from red ginseng (P. ginseng) inhibited apoptosis of pancreatic β cells induced by cytokines [14] and decreased cardiac injury induced by isoproterenol in porcine [15]. Patel et al [2] found that saponins from the seeds of B. laciniosa decreased the magnitude of the elevated blood glucose in diabetic rats. Furthermore, a variety of lipid parameters, including cholesterol and triglycerides, were reduced in these rats. Meanwhile, it was reported that saponins attenuate obesity through the reduction of weight gain and an increase of adiponectin in high-fat diet-induced obese rats [12]. In the light of these previous studies, saponins have potential as a candidate medicine for treating diabetes and obesity.
To identify which saponin affects the development of diabetes in chronic ethanol-exposed rats, we induced diabetes using Otsuka Long–Evans Tokushima Fatty (OLETF) rats. As OLETF rats exhibit features such as hyperglycemia, a chronic course of disease, abnormalities of the pancreas, and mild obesity, they have been commonly used as an animal model to investigate T2DM and obesity [16], [17], [18]. After chronically exposing male OLETF and nondiabetic male Long–Evans Tokushima Otsuka (LETO) rats to ethanol and saponin, we analyzed the fasting glucose levels, the degree of depression and anxiety, and factors related to lipid metabolism. Furthermore, to investigate the effect of saponin on pancreas, we analyzed the histological characteristic of the pancreas.
2. Materials and methods
2.1. Animals
Fourteen-week-old male OLETF and nondiabetic male LETO rats were purchased from Central Lab Animal Inc. (Seoul, Korea). The rats were randomly divided into two groups (LETO and OLETF groups), and three subgroups were established in each group. In the LETO group, LETO-Control (LC, n = 10 rats), LETO-Ethanol (LE, n = 10 rats), and LETO-Ethanol-Saponin (LES, n = 10 rats) were established. The weights of LC, LE, and LES rats were 408 ± 12.20 g, 414.90 ± 12.99 g, and 404 ± 15.14 g, respectively. Likewise, in the OLETF group, OLETF-Control (OC, n = 10 rats), OLETF-Ethanol (OE, n = 10 rats), and OLETF-Ethanol-Saponin (OES, n = 10 rats) were established. The weights of OC, OE, and OES rats were 509.50 ± 20.11 g, 517.55 ± 15.77 g, and 496.00 ± 25.85 g, respectively. The rats were housed on a 12-h:12-h light–dark cycle at constant room temperature (22 ± 1°C) throughout the experiments. All experimental procedures were approved by the Catholic University College of Medicine experimental animal laboratory management committee (CUMC-2012-0045-01). The animal care and experimental procedures conformed to the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised in 1996.
2.2. Diet and treatment
All experimental groups were given 100 mL/d of pair-fed Lieber–DeCarli Liquid Diet with or without 5% ethanol for 12 wks in the same manner as previously described [16]. In the LES and OES groups, after a 2-wk period to allow the animals to adjust to the liquid diet and ethanol, the rats received daily intraperitoneal injections of saponin. The dosage of saponin was determined at 200 mg/kg as was done in a previous study [19]. The other groups of rats received daily intraperitoneal injections of saline for 10 wks, as described in Fig. S1. Saponin produced from steam-treated Korean Red Ginseng (P. ginseng) was purchased from Vitallink Inc. (Gyeonggi-do, Korea).
2.3. Intraperitoneal glucose tolerance test
Intraperitoneal glucose tolerance tests (IP-GTT) were performed at 3 wks, 6 wks, and 8 wks after saponin injection (at 5 wks, 8 wks, and 10 wks, respectively, after beginning the ethanol-containing diet), as shown in Fig. S1. From this point on, we will describe the standard week based on the starting point of saponin injections as follows: experiment at Week 3 means 3 wks after saponin injection (5 wks after ethanol exposure). After all the rats have been transferred to clean cages, they were fasted for 12 h and then received an intraperitoneal injection of glucose (2 g/kg, dextrose). Blood was obtained from the tail vein at 0 min (fasting), 30 min, 60 min, 90 min, and 120 min, and glucose levels in the blood were measured with a portable glucose meter (One Touch Ultra; Johnson & Johnson Medical, Milpitas, CA, USA).
2.4. Behavioral test
For 1 wk between Wk 8 and Wk 9, rats were evaluated in a forced swim test to analyze the occurrence of depressive-like behaviors and in an elevated-plus maze (EPM) test to measure the occurrence of anxiety-like behaviors. The procedure for these behavioral tests was based on previously published studies [20], [21].
2.4.1. Forced swim test
To pretrain the animals, they were individually forced to swim inside vertical Plexiglas cylinders (height, 25 cm; diameter, 20 cm) filled with water maintained at 24 ± 1°C for 15 min. After swimming, they were placed in a heated enclosure maintained at 32°C prior to being returned to their home cages. In order to perform the forced swim test, the rats were returned to the cylinders 24 h later, and the durations of immobility and swimming were measured by a blind observer for 5 min.
2.4.2. EPM test
The EPM, which uses a black wooden platform with two open arms (30 cm × 5 cm) and two closed arms (30 cm × 5 cm × 15 cm) arranged in the shape of a plus sign, was used to measure anxiety-like behaviors. Each rat was placed on a central platform facing a closed arm and allowed to explore the maze freely for 5 min. Time spent in the open arms or closed arms was recorded manually. To avoid bias, the observer was blinded to the group classification of the rats.
2.5. Measurement of organ weights, biochemical factors, and proteins in blood serum
Ten weeks after the injection of saponin, following starvation for 12 h, the animals were anesthetized with isoflurane (1–2% with 0.8 L/min O2 administered with a facemask) and blood (3–5 mL) was drawn directly from the heart [22]. After the autopsy, the organs were measured and stored in liquid nitrogen until further experiments were performed. Blood samples were centrifuged at 800g at 25°C for 20 min, and the upper phase was transferred into a fresh tube. The biochemical parameters of serum were measured by the Green Cross Institute of Medicine (Seoul, Korea).
2.6. Histological and immunohistological examination of the pancreas
Pancreatic tissues dissociated from rats were fixed with 4% formaldehyde for 24 h, washed with distilled water, and dehydrated gradually with a series of 70–100% ethanol. The tissues were immersed in xylene, embedded in paraffin, and sectioned at 4 μm. The obtained sections were transferred onto slides and the slides were deparaffinized with xylene. The slides were stained with hematoxylin and eosin (H&E), dehydrated, mounted, and observed using a Fluorescence Attached Microscope (AX70, TR-62A02; Olympus Corporation, Tokyo, Japan).
To observe the expression of insulin, the slides prepared from the sections were deparaffinized in xylene. Antigen retrieval was accomplished by placing the slides in Dako Target Retrieval Solution, pH 6.0 (Dakocytomation, Carpinteria, CA, USA). After cooling for 20 min, the slides were quenched with 3% H2O2 for 5 min. The slides were incubated with mouse anti-insulin antibody (1:500; Catalog No. 05-1066; Merck Millipore, Darmstadt, Germany) for 30 min at room temperature. They were washed with phosphate-buffered saline, and signal was detected with the Polink-2 HRP plus mouse-NR DAB detection system (Catalog No. D58-110; GBI, Inc., Mukilteo, WA, USA) according to the manufacturer's recommendation. The slides were stained with hematoxylin, washed with phosphate-buffered saline, and mounted. They were observed using an Olympus AX-70 fluorescence microscope (Olympus Corporation), and the images were captured using an Olympus DP70 digital camera system (Olympus Corporation).
The images were digitalized using cellSens standard 1.6 (Olympus Corporation) imaging software and were evaluated on a Pannoramic Viewer 1.15.1 (3DHISTECH Ltd., Budapest, Hungary) using the positive pixel counting algorithm, which scores the stains as negative, weak-positive, medium, and strong, as reported previously [23]. This algorithm established the HScore of each case, based on the percentage and intensity of staining. The HScore takes into consideration the intensity of the staining and the percentage of positive cells per the formula:
| HScore = 1 × (% light staining) + 2 × (% moderate staining) + 3 × (% strong staining). | (1) |
HScores range from 0 to 150. Therefore, all immunoexpression results were correlated with insulin expression.
2.7. Statistical analysis
All of the data were expressed as the mean ± standard error of the mean based on seven to nine rats in each subgroup. Differences between groups were analyzed by one-way analysis of variance using SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA). Analysis of variance results followed by Tukey's honestly significant different post hoc test with p < 0.05 were considered to be statistically significant.
3. Results
3.1. Alterations in blood glucose levels
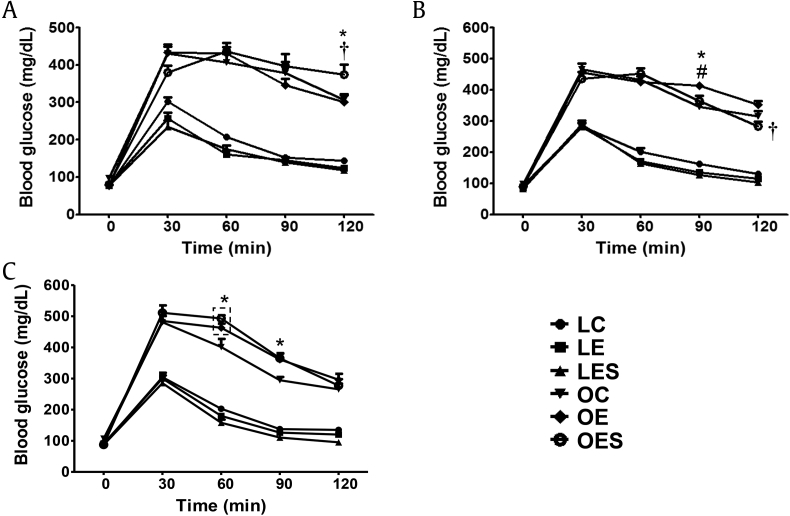
To examine alterations in glucose homeostasis due to alcohol and saponin, we performed an IP-GTT at 3 wks, 6 wks, and 8 wks. When we performed IP-GTT at 3 wks, fasting glucose levels in the LETO groups, including LC, LE, and LES, were similar (76.7 ± 2.3 mg/dL, 78 ± 2.2 mg/dL, and 77.1 ± 2.1 mg/dL, respectively), whereas fasting glucose levels in the OLETF groups, including OC, OE, and OES rats, were different (97.1 ± 2.4 mg/dL, 77.3 ± 1.5 mg/dL, and 79.7 ± 3.1 mg/dL, respectively; Fig. 1A). Among the OLETF groups, glucose levels at 30 min (379.7 ± 19.2 mg/dL) in the OES rats were lower than in the OC and OE rats (430.6 ± 23.5 mg/dL and 433.8 ± 15.1 mg/dL, respectively), whereas the glucose levels at 120 min (374.1 ± 26.6 mg/dL) in OES rats were significantly higher than in OC and OE rats (306.7 ± 15.8 mg/dL and 300.4 ± 18.8 mg/dL, respectively), resulting in a pattern with both slower increases in glucose and slower decreases in glucose relative to the other subgroups.
Fig. 1.
Comparison of IP-GTT results after exposure of LETO (LC, LE, and LES rats) and OLETF (OC, OE, and OES rats) groups to ethanol and saponin. After intraperitoneal glucose injection, blood glucose levels were measured at 0 min (fasting), 30 min, 60 min, 90 min, and 120 min. (A) IP-GTT 3 wks after saponin injection (5 wks after ethanol exposure). (B) IP-GTT 6 wk after saponin injection (8 wks after ethanol exposure). (C) IP-GTT 8 wks after saponin injection (10 wks after ethanol exposure). The data represented the mean ± SEM (n = 7–9). The data were analyzed by one-way ANOVA, followed by Tukey's HSD post hoc test. * p < 0.05 versus OC rats; †p < 0.05 versus OE rats; #p < 0.05 versus OES rats. ANOVA, analysis of variance; HSD, honestly significant different; IP-GTT, intraperitoneal glucose tolerance test; LC, LETO-Control; LE, LETO-Ethanol; LES, LETO-Ethanol-Saponin; OC, OLETF-Control; OLETF, Otsuka Long–Evans Tokushima Fatty; SEM, standard error of the mean.
As shown in Fig. 1B, fasting glucose levels were similar among all groups at 6 wks. Within the LETO group, LES rats had the fastest glucose metabolism at 120 min (103.7 ± 2 mg/dL), as well as the lowest fasting glucose level (82.4 ± 1.4 mg/dL) compared to LC and LE rats (fasting glucose levels, 92.5 ± 2.7 mg/dL and 85.8 ± 2.5 mg/dL, respectively; glucose levels at 120 min, 130.8 ± 6 mg/dL and 114.6 ± 4.8 mg/dL, respectively). Within the OLETF group, OE rats had significantly higher glucose levels not only at 90 min (414 ± 7.5 mg/dL) compared to OC and OES (345 ± 11.3 mg/dL and 357.5 ± 18.3 mg/dL, respectively) rats, but also at 120 min (352.2 ± 11.2 mg/dL) compared to OES rats (278.4 ± 15.2 mg/dL). In particular, OES rats exhibited very fast glucose metabolic activity for 120 min (from 91.4 ± 2.4 mg/dL to 278.4 ± 15.2 mg/dL) compared to the OC (from 97.4 ± 2.3 mg/dL to 314.5 ± 17.8 mg/dL) and OE (from 89 ± 2.1 mg/dL to 352.2 ± 11.2 mg/dL) rats.
Eight weeks after saponin injection, the fasting glucose levels in LC, LE, LES, and OES rats (88.6 ± 1.1 mg/dL, 90.9 ± 1.8 mg/dL, 87.1 ± 1.7 mg/dL, and 89.4 ± 2.1 mg/dL, respectively) were similar, whereas the levels in OC and OEC rats (106.9 ± 2.6 mg/dL and 96.3 ± 3.1 mg/dL, respectively) were higher than in the other rats (Fig. 1C). Within the LETO group, the glucose level of LES rats at 120 min (95.2 ± 4 mg/dL) was lowest compared to LC and LE rats (135.1 ± 4.5 mg/dL and 120 ± 2.7 mg/dL, respectively), indicating the fastest glucose metabolic activity. By contrast, the OE and OES rats (463.1 ± 13.3 mg/dL and 507.2 ± 6.7 mg/dL at 60 min, respectively; 361.1 ± 18.6 mg/dL and 351.1 ± 12.3 mg/dL at 90 min, respectively) within the OLETF group exhibited significantly higher glucose levels at 60 min and 90 min compared to OC rats (401.2 ± 26 mg/dL at 60 min; 294.5 ± 10.5 mg/dL at 90 min). Nevertheless, OES rats (262.8 ± 18 mg/dL) had lower glucose levels at 120 min compared to OC and OE rats (265.2 ± 18.6 mg/dL and 296.1 ± 19 mg/dL, respectively), indicating rapid glucose metabolic activity.
Considering that there was little difference in fasting glucose levels between the LETO and OLETF groups, the higher glucose levels of the OLETF group during the period of IP-GTT compared to the LETO group imply reduced glucose metabolism in the OLETF group. However, OES rats at 6 wks and 8 wks exhibited lower glucose levels 120 min after glucose injection compared to OC and OE rats, suggesting that saponin positively affects glucose metabolism in OLETF rats that drink ethanol as the exposure period to saponin increases.
3.2. Behavioral test
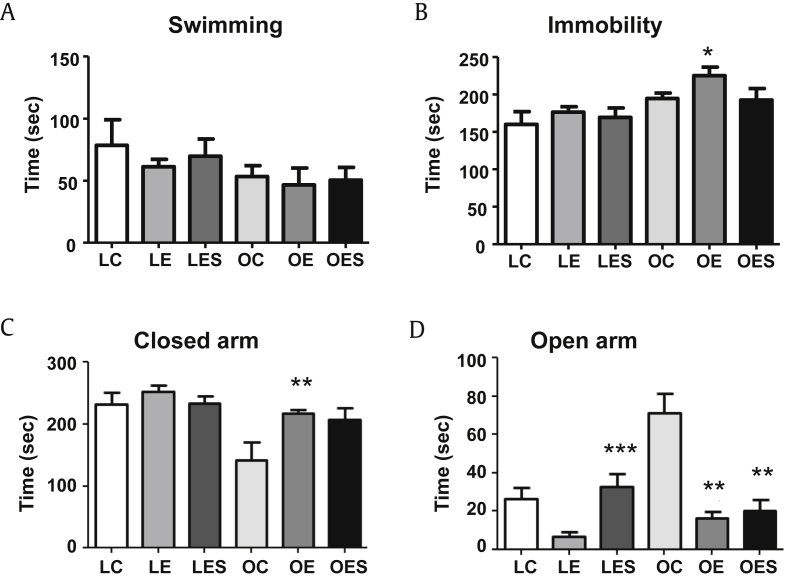
To analyze the occurrence of depressive-like behaviors, we performed forced swimming tests on the LETO and OLETF groups. The LETO group, including LC, LE, and LES rats, generally had longer swimming times than did the OLETF group, including OC, OE, and OES rats (Fig. 2A). However, LE and OE rats that received only ethanol exhibited shorter swimming times compared to control rats (LC and OC) and saponin-injected rats (LES and OES), demonstrating that saponin may decrease the depressive-like behaviors in both groups. The immobility time of both groups exhibited the opposite pattern compared to swimming times (Fig. 2B). The immobility time of OE rats was significantly longer than that of LC rats.
Fig. 2.
Examination of the occurrence of depressive- and anxiety-like behaviors. (A) Swimming time in the forced swim test. (B) Immobility in the forced swim test. (C) Time in the closed arms of the elevated plus maze. (D) Time in the open arms of the elevated plus maze. The data represent the mean ± SEM (n = 7–9). The data were analyzed by one-way ANOVA, followed by Tukey's HSD post hoc test. * p < 0.05 versus LC rats; ** p < 0.05 versus OC rats; *** p < 0.05 versus LE rats. ANOVA, analysis of variance; HSD, honestly significant different; LC, LETO-Control; LE, LETO-Ethanol; OC, OLETF-Control; SEM, standard error of the mean.
To identify the degree of anxiety in the LETO and OLETF groups, we performed the EPM test. When we measured the time spent in the closed arms of the maze, OC rats spent the shortest amount of time in the closed arms from among the tested groups (Fig. 2C). By contrast, OC rats spent the longest time in the open arms compared to LC, LE, LES, OE, and OES groups (Fig. 2D). When we compared to rats belonging to the LETO group, the amount of time LE rats spent in the open arms was significantly shorter compared to LES rats. Within the OLETF group, the amount of time OE and OES rats spent in the open arms was significantly shorter compared to OC rats. Considering the time spent in the closed and open arms, OC rats exhibited the least degree of anxiety.
3.3. Decreases in fat weight by saponin
We measured the weight of various organs as soon as rats were sacrificed. Liver weight was generally greater in the OLETF group than in the LETO group (Table 1). Within the LETO group, the liver weight of the LES rats was slightly heavier compared to the LC and LE rats, whereas within the OLETF group, the liver weight of OES rats was lighter than those of OC and OE rats. Heart weight in OES rats was significantly heavier than that in OC rats. Meanwhile, the spleen weight of the LES rats was significantly heavier than that of other rats within the LETO group. Within the OLETF group, the spleens of OES rats were heaviest. The spleen weights of LES and OES rats exposed to saponin were highest within each group. The weight of the pancreas, which produces insulin and glucagon responsible for glucose metabolism, was generally heavier in the LETO group compared to the OLETF group. Notably, within the LETO group, pancreas weight in LES rats treated with saponin was particularly heavy (Table 1). In the cases of epididymal fat tissue and mesenteric fat tissue, their weights in the LES and OES groups were lightest within each group, implying that saponin impedes the increase in fat tissue weight promoted by obesity, resulting in an improvement in metabolic function.
Table 1.
Weights of various organs in rats after saponin injection for 10 wks (after ethanol exposure for 12 wks)
| Organ | Weight (g) |
|||||
|---|---|---|---|---|---|---|
| LC | LE | LES | OC | OE | OES | |
| Liver | 2.32 ± 0.151) | 2.32 ± 0.07 | 2.41 ± 0.07 | 2.93 ± 0.25 | 2.98 ± 0.40 | 2.77 ± 0.29 |
| Heart | 0.29 ± 0.05 | 0.31 ± 0.01 | 0.33 ± 0.02 | 0.25 ± 0.02 | 0.27 ± 0.02 | 0.29 ± 0.04 *** |
| Kidney | 0.29 ± 0.02 | 0.31 ± 0.02 | 0.33 ± 0.02 * | 0.29 ± 0.03 | 0.29 ± 0.02 | 0.29 ± 0.02 |
| Spleen | 0.14 ± 0.00 | 0.14 ± 0.00 | 0.17 ± 0.01 *,** | 0.15 ± 0.04 | 0.13 ± 0.02 | 0.16 ± 0.01 **** |
| Pancreas | 0.33 ± 0.09 | 0.37 ± 0.10 | 0.46 ± 0.07 * | 0.17 ± 0.04 | 0.19 ± 0.06 | 0.19 ± 0.04 |
| Epididymal fat tissue | 2.71 ± 0.38 | 1.59 ± 0.28 * | 1.54 ± 0.26 * | 3.14 ± 0.25 | 3.13 ± 0.21 | 2.65 ± 0.09 ***,**** |
| Mesenteric fat tissue | 1.07 ± 0.28 | 0.74 ± 0.17 * | 0.64 ± 0.11 * | 2.17 ± 0.30 | 2.14 ± 0.31 | 1.62 ± 0.26***,**** |
* p < 0.05 versus LC; ** p < 0.05 versus LE; *** p < 0.05 versus OC; **** p < 0.05 versus OE (n = 7–9).
LC, LETO-Control; LE, LETO-Ethanol; LES, LETO-Ethanol-Saponin; OC, OLETF-Control; OE, OLETF-Ethanol; OES, OLETF-Ethanol-Saponin.
Organ weight per 100 g of body weight.
3.4. Expression patterns of toxicity-related enzymes in response to alcohol and saponin
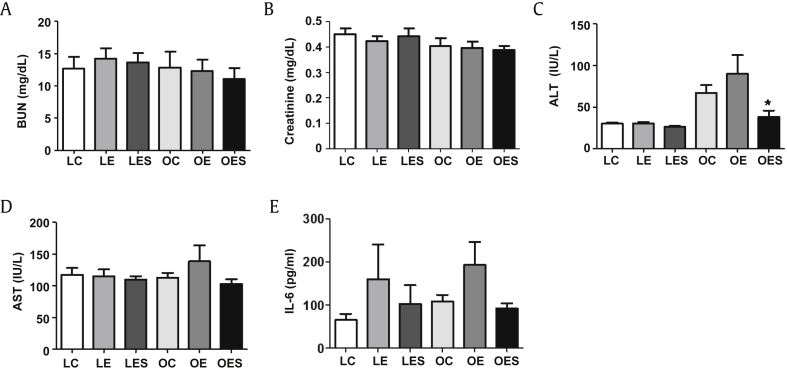
To determine the effects of alcohol and saponin on the LETO and OLETF groups, we measured the blood levels of factors related to toxicity. Among blood urea nitrogen (BUN) and creatinine, markers related to kidney toxicity, BUN did not show significant differences in both the LETO and OLETF groups (Fig. 3A). Except for a slight decrease in creatinine in the LE rats in the LETO group, the levels of creatinine in LC and LES animals were similar (Fig. 3B). The level of creatinine in the OLETF group was also similar among the OC, OE, and OES rats. Therefore, in the present study, neither ethanol nor saponin showed signs of deeply affecting the kidney. When we measured alanine aminotransferase (ALT) and aspartate aminotransferase (AST), markers for liver fat accumulation, the levels of ALT were not significantly changed in the LETO group, whereas ALT levels in the OC and OE groups were significantly higher than in the OES group (Fig. 3C). The levels of AST were not different in the LETO group, whereas OE rats exhibited the highest AST from among animals in the OLETF group (Fig. 3D). As a result, saponin may lead to a decrease in liver fat accumulation. The level of interleukin (IL)-6, known as a pleiotropic cytokine showing both protective and pathogenetic actions in diabetes, increased as a result of treatment with ethanol in both the LETO and OLETF groups, whereas this increase in IL-6 caused by ethanol was reduced by saponin (Fig. 3E).
Fig. 3.
Alterations in blood serum levels of parameters related to toxicity. Ten weeks after saponin injection (12 wks after ethanol exposure), following starvation for 12 h, animals were sacrificed and blood was drawn directly from the heart. Blood serum levels of factors related to toxicity were measured biochemically. (A, B) Factors associated with kidney toxicity. (C, D) Factors associated with liver fat accumulation. (E) IL-6 associated with inflammation. The data represent the mean ± SEM (n = 7–9). The data were analyzed by one-way ANOVA, followed by Tukey's HSD post hoc test. * p < 0.05 versus OC rats. ANOVA, analysis of variance; HSD, honestly significant different; IL, interleukin; SEM, standard error of the mean.
3.5. Decreases in some lipids by saponin
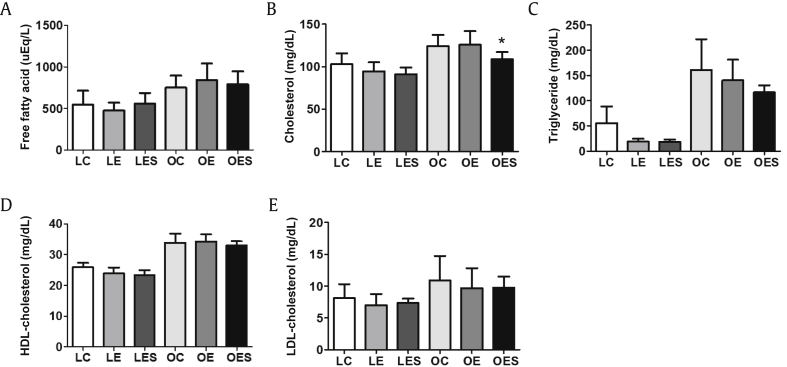
To identify changes in lipid metabolism caused by ethanol and saponin, we measured several lipids in blood. Free fatty acid was higher in the LETO group than in the OLETF group, whereas there were few differences in the expression of free fatty acid among rats within each group (Fig. 4A). As shown in Fig. 4B, the level of expression of cholesterol in the LETO group was lower than in the OLETF group. Within the OLETF group, the level of cholesterol in OES rats was significantly lower than in OE rats. The level of triglyceride in the LETO group was lower than in the OLETF group, and within the LETO group LE and LES rats had lower levels than LC rats, even though these differences were not significant (Fig. 4C). Within the OLETF group, the triglyceride level in OC rats was highest compared to animals in the OE and OES groups. However, within both the LETO and OLETF groups, the rats injected with saponin exhibited lower expression levels of triglyceride compared to the other rats. As a result of assessing the expression of high-density lipoprotein (HDL)-cholesterol and low-density lipoprotein (LDL)-cholesterol, we found that their expression was generally higher in the OLETF group compared to the LETO group (Figs. 4D and 4E). There were no significant differences in HDL-cholesterol and LDL-cholesterol among rats within each group.
Fig. 4.
Alterations in blood serum levels of parameters related to lipid metabolism. Ten weeks after saponin injection (12 wks after ethanol exposure), following starvation for 12 h, the animals were sacrificed and blood was drawn directly from the heart. Levels of factors related to lipid metabolism, including free fatty acid, cholesterol, triglyceride, HDL-cholesterol and LDL-cholesterol, in blood serum were measured biochemically. The data represent the mean ± SEM (n = 7–9). The data were analyzed by one-way ANOVA, followed by Tukey's HSD post hoc test. * p < 0.05 versus OE rats. ANOVA, analysis of variance; HDL, high-density lipoprotein; HSD, honestly significant different; LDL, low-density lipoprotein; SEM, standard error of the mean.
3.6. Expression of insulin in the pancreas
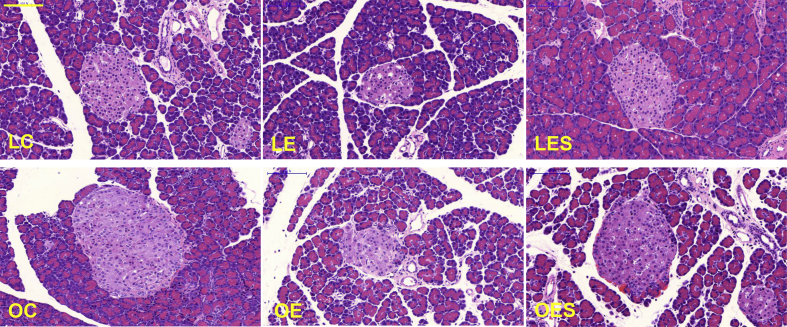
To assess the general pattern of cell and tissue morphology and distribution, we stained pancreases using the H&E staining method. The islets of Langerhans from LE and OE rats in both the LETO and OLETF groups exhibited an unclear and smaller morphology compared with those of LC, LES, OC, and OES rats, demonstrating that saponin promotes the recovery of pancreases impaired by ethanol (Fig. 5). Based on the H&E staining results, we suggest that saponin may reverse damage to the pancreas induced by ethanol.
Fig. 5.
Morphological changes in the pancreas. After exposure of rats to ethanol and saponin for 12 wks and 10 wks, respectively, the pancreases were obtained from rats in all groups (LC, LE, LES, OC, OE, and OES) and stained using an H&E staining method. The histological morphology and distribution of the islet cells and tissues in the pancreases were observed. Scale bar = 100 μm. H&E, hematoxylin and eosin; LC, LETO-Control; LE, LETO-Ethanol; OC, OLETF-Control; OLETF, Otsuka Long–Evans Tokushima Fatty; OE, OLETF-Ethanol; OES, OLETF-Ethanol-Saponin.
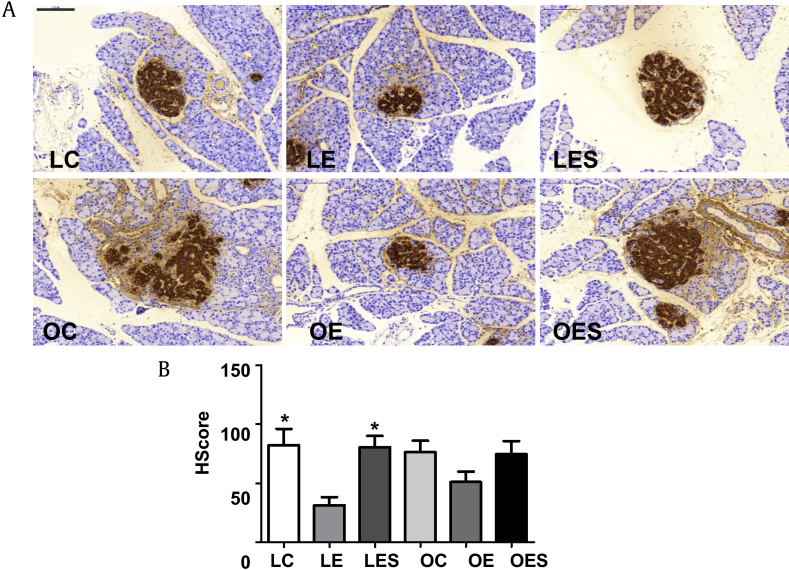
When we measured the expression of insulin in the pancreas, insulin was significantly decreased in LE rats exposed to only ethanol compared to the LC and LES rats (Figs. 6A and 6B). Furthermore, within the OLETF group, in OE rats insulin from the pancreas was also decreased compared to levels in the OC and OES rats. As a result, it is supposed that saponin inhibits not only the damage to cells and tissue in the pancreas, but also specifically the decreases in insulin expression caused by ethanol in LETO rats.
Fig. 6.
Expression of insulin in the pancreas. After exposure of rats to ethanol and saponin for 12 wks and 10 wks, respectively, pancreases were obtained from rats in all groups (LC, LE, LES, OC, OE, and OES) and immunohistochemical staining was performed. (A) The expression of insulin. (B) HScore of insulin. The intensity of the staining and the percentage of cells positive for insulin were expressed as HScore. The data represent the mean ± SEM (n = 5–6). The data were analyzed by one-way ANOVA, followed by Tukey's HSD post hoc test. Scale bar = 100 μm. * p < 0.05 versus LE rats. ANOVA, analysis of variance; HSD, honestly significant different; LC, LETO-Control; LE, LETO-Ethanol; LES, LETO-Ethanol-Saponin; OC, OLETF-Control; OLETF, Otsuka Long–Evans Tokushima Fatty; OE, OLETF-Ethanol; OES, OLETF-Ethanol-Saponin; SEM, standard error of the mean.
4. Discussion
T2DM, which is caused by metabolic disorders such as impaired glucose tolerance and insulin resistance, is worsened by chronic heavy alcohol abuse, resulting in an increase in cellular toxicity and dysfunction of the pancreas [9], [24]. Therefore, in addition to the development of diabetes-targeted medicines, it is necessary to develop medicine for diabetics chronically exposed to alcohol. Based on this, we analyzed the effect of saponin on rats with diabetes exposed to alcohol and demonstrated that saponin led to partially improved glucose tolerance, hypolipidemia, and improvements in pancreatic damage.
In the present study, the fasting glucose levels of nondiabetic rats were similar among the LC, LE, and LES rats during the period of IP-GTT measurement, whereas their postprandial glucose levels were lowest in LES rats 120 min postprandial. These findings suggest that saponin plays a certain role in glucose metabolism. However, the glucose levels on the LETO group were lowered to nearly fasting glucose levels at postprandial 120 min, indicating normal glucose metabolic activity as reported in previous studies [16], [22]. With the exception that OC rats showed higher fasting glucose levels than OE and OES rats, diabetes-induced OLETF rats exhibited fasting glucose levels similar to nondiabetic rats. However, the glucose levels of the OLETF group at postprandial 120 min were more than twice as high as those of the LETO group, demonstrating the typical phenomenon of impaired glucose metabolism in T2DM. In previous studies [9], [16], [22], postprandial glucose levels of OLETF rats fed according to the same diet method used to induce diabetes were also higher than fasting glucose levels. Interestingly, the postprandial glucose levels of the prediabetes group were lowered to nearly fasting glucose levels, and levels in the prediabetes group exposed to ethanol were also lowered quickly compared to those of the diabetes group exposed to ethanol [9]. In considering these results, it would appear that the more severe the diabetes and the longer the duration of diabetes in rats, the slower glucose metabolism is activated by ethanol. However, in the present study, postprandial glucose levels of OES rats were lower than those of OC and OE rats at 6 wks and 8 wks after saponin injection; this result was not obtained at 3 wks. Saponin has been reported to induce hypoglycemia or attenuate hyperglycemia in blood via alterations in glucose homeostasis [2], [11]. The chronic treatment with saponin underlying these results may be helpful in improving glucose metabolism in chronic alcohol-consuming diabetics.
In the present study, the depressive-like behaviors in the LETO group were not different among LC, LE, and LES rats, whereas OE rats exposed to ethanol showed significantly higher depressive-like behaviors compared to OC and OES rats. This result suggests that saponin may reduce depressive-like behaviors induced by ethanol. It has been reported in both animal and human studies that depression is associated with diabetes [25], [26], [27]. Recently, Ates et al [27] observed an increase in depressive-like behavior in diabetic rats using the Porsolt swim test. Given that the increase in depression in diabetes and the depressant effect of ethanol that may change neurotransmitter effluence followed by depression of the central nervous system [28], chronic alcohol consumption in diabetes accelerates depression. Meanwhile, it is believed that saponin shows antidepressant effects by normalizing the hypothalamus–pituitary–adrenal axis and enhancing the hippocampal inhibitory phosphorylation of GSK-3β [29], [30]. Taken together, saponin may attenuate the aggravation of depression in diabetic patients chronically consuming alcohol.
In the present study, we measured liver weights in animals from both the LETO and OLETF groups. The liver weights of the OLETF group were heavier than those of the LETO group. The liver weight of OE rats was the heaviest within the OLETF group, whereas that of OES rats was the lightest, implying that saponin inhibits the accumulation of liver fat. Recently, it has been demonstrated that liver fat substance is associated with some of the characteristics of insulin resistance in mildly overweight individuals, highlighting the possibility of developing of diabetes [31]. ALT and AST are responsible for the pathological toxicity of the liver, and ALT has been reported to be an indicator of liver fat accumulation [32]. In the present study, the ALT levels of the LETO group were not different from those of the LC, LE, and LES groups, whereas within the OELTF group the ALT levels of OC and OE rats were significantly higher than those of OES rats. In particular, the ALT levels of OES rats almost reached those of LC rats, demonstrating the strong effect of saponin against ALT secretion. As was the case with ALT, AST levels in OES rats within the OELTF group dropped into a normal ratio. It has been reported that ALT and AST are increased in the blood of streptozotocin-induced diabetic rats [33], [34]. In the study reported by Patel et al [2], saponin extracted from B. laciniosa Linn led to reductions in ALT and AST secretion into blood in streptozotocin-induced diabetic rats. Taken together, it is plausible that saponin reverses alcohol-induced toxicity and fat accumulation in liver in diabetes.
IL-6 plays a known role in inflammatory regulation and has been shown to restrain the secretion of insulin in rodent islets stimulated by glucose [35], [36]. Prystupa et al [37] observed that serum IL-6 concentrations in alcoholics were increased compared to healthy controls. However, saponin from ginseng inhibits the expression of IL-6 in cardiac tissue of guinea pigs and rat hemorrhagic shock models [38], [39]. In the present study, IL-6 levels in both the LETO and OLETF groups increased because of ethanol and were reversed by saponin. In considering these studies, saponin has an anti-inflammatory mechanism against chronic alcohol consumption-induced lL-6 in diabetics as well as in normal individuals.
We measured the weights of epididymal and mesenteric fat tissues, types of white adipose tissues, to indirectly investigate the effects of ethanol and saponin. Both the LE and LES rats within the LETO group showed significantly lighter weights of epididymal and mesenteric fat tissues than did LC rats. When people drink alcohol heavily and chronically, they rarely eat snacks or other foods, resulting in a loss of weight and a decrease in fat. Similar to this, LE rats chronically exposed to ethanol showed not only a decrease in fat tissue but also a decrease in triglyceride compared to LC rats. LES rats also exhibited the same pattern as LE rats. As a consequence, saponin treatment in normal rats may not have a great effect on lipid metabolism controlled by ethanol. However, we cannot make any conclusions regarding the role of saponin in lipid metabolism in normal rats as we did not perform treatments with only saponin in normal LETO rats. Within the OLETF group, the epididymal and mesenteric fat tissues of the OES rats were drastically lighter than those of the OC and OE rats. In addition, the cholesterol and triglyceride levels of OES rats were lower than those of OC and OE rats. Lipid abnormalities in diabetes are generally associated with hypercholesterolemia and hypertriglyceridemia [40], [41]. Saponin has been observed to contribute to a decrease in serum cholesterol and triglyceride [42], [43]. Patel et al [2] demonstrated that chronic treatment with saponin in diabetic rats led to drastic reductions in cholesterol and triglyceride compared to diabetic controls. Therefore, chronic saponin treatment in diabetic rats exposed to large quantities of ethanol may inhibit hypercholesterolemia and hypertriglyceridemia as well as the abnormal accumulation of fat.
In the present study, we observed that chronic ethanol consumption in both normal and diabetic-induced rats induced a reduction in islets of the pancreas. This finding is similar to the findings from a previous study, which reported that consumption of a 5% ethanol diet by C57BL/6L male mice for 8 wks reduced islet cell mass [8]. Other studies have observed that chronic ethanol consumption promotes not only the dysfunction of pancreatic beta cells in diabetic rats, followed by apoptosis through glucokinase nitration, but also causes oxidative stress in RINm5F cells, resulting in cell death [8], [44]. Therefore, it is plausible that chronic ethanol treatment of diabetic rats induces nitrative and oxidative stresses in the islets of the pancreas causing apoptosis and resulting in a decrease in cell mass. By contrast, chronic exposure to both ethanol and saponin suppressed the reduction of islets caused by ethanol. In the study of Kim and Kim [14], ginseng extracts containing saponin inhibited cytokine-induced apoptosis in the pancreatic beta cell line MIN6N8. Kim et al [45] reported that ginsenoside Rg3, one of the total saponins, not only improved islet cell function but also debilitated apoptosis in mouse islets. In considering these and our results, chronic treatment with saponin in chronic alcohol-exposed diabetic rats may attenuate damage to the pancreatic islet cells.
In the present study, insulin expression in the islets of the pancreas was higher in other rats relative to the rats exposed to only ethanol. In the study of Kim et al [8], exposure of 7-wk-old male C57BL/6 mice to ethanol for 8 wk led to a decrease of insulin expression as well as a significant reduction in islet cell mass. It has been reported that saponin induces insulin expression in islets via the protection of islet cells against apoptosis [45], [46]. Taken together, chronic saponin exposure in normal and diabetic rats inhibits the decrease in insulin expression induced by ethanol.
In conclusion, we demonstrated that chronic treatment with saponin enhances glucose metabolism impaired by chronic ethanol consumption and leads to a decrease in fat tissue weights and lipids, including cholesterol and triglyceride. In addition, chronic treatment with saponin inhibits the reduction in islet cell mass and the decrease in insulin expression in beta cells worsened by chronic ethanol consumption. Therefore, based on these results, saponin may be not only be helpful in alleviating the rapid progress of diabetes due to chronic alcohol consumption in diabetic patients but may also show potential as an antidiabetic drug candidate for diabetic patients who chronically consume alcohol. However, our study has several limitations. There were no control rats exposed to only saponin in either the LETO or the OLETF group. To analyze the effects of only saponin in the absence of ethanol, studies using experimental rats exposed to only saponin are needed. To obtain more significant results, it will also be necessary to perform experiments with more rats per subgroup. In addition, further studies will be needed to investigate the exact molecular mechanism regulated by saponin in the pancreas of diabetic rats chronically exposed to ethanol. However, to the best of our knowledge, this is the first report to demonstrate that saponin positively affects chronic ethanol-exposed diabetic rats.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This research was supported by the Globalization of Korean Foods R&D program, funded by the Ministry of Food, Agriculture, Forestry and Fisheries, and a grant from the Korean Healthcare Technology R&D Project, Ministry of Health and Welfare (911001-2), Republic of Korea.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jgr.2016.09.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Fig. S1. Experimental design. IHC, immunohistochemistry.
References
- 1.Hodge A.M., Dowse G.K., Collins V.R., Zimmet P.Z. Abnormal glucose tolerance and alcohol consumption in three populations at high risk of non-insulin-dependent diabetes mellitus. Am J Epidemiol. 1993;137:178–189. doi: 10.1093/oxfordjournals.aje.a116658. [DOI] [PubMed] [Google Scholar]
- 2.Patel S.B., Santani D., Patel V., Shah M. Anti-diabetic effects of ethanol extract of Bryonia laciniosa seeds and its saponins rich fraction in neonatally streptozotocin-induced diabetic rats. Pharmacognosy Res. 2015;7:92–99. doi: 10.4103/0974-8490.147217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J.Y., Park K.J., Kim G.H., Jeong E.A., Lee D.Y., Lee S.S., Kim D.J., Roh G.S., Song J., Ki S.H. In vivo activating transcription factor 3 silencing ameliorates the AMPK compensatory effects for ER stress-mediated beta-cell dysfunction during the progression of type-2 diabetes. Cell Signal. 2013;25:2348–2361. doi: 10.1016/j.cellsig.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Ozcan U., Cao Q., Yilmaz E., Lee A.H., Iwakoshi N.N., Ozdelen E., Tuncman G., Gorgun C., Glimcher L.H., Hotamisligil G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds K., Lewis B., Nolen J.D., Kinney G.L., Sathya B., He J. Alcohol consumption and risk of stroke: a meta-analysis. JAMA. 2003;289:579–588. doi: 10.1001/jama.289.5.579. [DOI] [PubMed] [Google Scholar]
- 6.Wei M., Gibbons L.W., Mitchell T.L., Kampert J.B., Blair S.N. Alcohol intake and incidence of type 2 diabetes in men. Diabetes Care. 2000;23:18–22. doi: 10.2337/diacare.23.1.18. [DOI] [PubMed] [Google Scholar]
- 7.Fueki Y., Miida T., Wardaningsih E., Ito M., Nakamura A., Takahashi A., Hanyu O., Tsuda A., Saito H., Hama H. Regular alcohol consumption improves insulin resistance in healthy Japanese men independent of obesity. Clin Chim Acta. 2007;382:71–76. doi: 10.1016/j.cca.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 8.Kim J.Y., Song E.H., Lee H.J., Oh Y.K., Park Y.S., Park J.W., Kim B.J., Kim D.J., Lee I., Song J. Chronic ethanol consumption-induced pancreatic {beta}-cell dysfunction and apoptosis through glucokinase nitration and its down-regulation. J Biol Chem. 2010;285:37251–37262. doi: 10.1074/jbc.M110.142315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S.S., Hong O.K., Ju A., Kim M.J., Kim B.J., Kim S.R., Kim W.H., Cho N.H., Kang M.I., Kang S.K. Chronic alcohol consumption results in greater damage to the pancreas than to the liver in the rats. Korean J Physiol Pharmacol. 2015;19:309–318. doi: 10.4196/kjpp.2015.19.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talamini G., Bassi C., Falconi M., Sartori N., Salvia R., Rigo L., Castagnini A., Di Francesco V., Frulloni L., Bovo P. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Dig Dis Sci. 1999;44:1303–1311. doi: 10.1023/a:1026670911955. [DOI] [PubMed] [Google Scholar]
- 11.Elekofehinti O.O. Saponins: anti-diabetic principles from medicinal plants — a review. Pathophysiology. 2015;22:95–103. doi: 10.1016/j.pathophys.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Kuate D., Kengne A.P., Biapa C.P., Azantsa B.G., Abdul Manan Bin Wan Muda W. Tetrapleura tetraptera spice attenuates high-carbohydrate, high-fat diet-induced obese and type 2 diabetic rats with metabolic syndrome features. Lipids Health Dis. 2015;14:50. doi: 10.1186/s12944-015-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elekofehinti O.O., Kamdem J.P., Kade I.J., Rocha J.B.T., Adanlawo I.G. Hypoglycemic, antiperoxidative and antihyperlipidemic effects of saponins from Solanum anguivi Lam. fruits in alloxan-induced diabetic rats. S Afr J Bot. 2013;88:56–61. [Google Scholar]
- 14.Kim H.Y., Kim K. Protective effect of ginseng on cytokine-induced apoptosis in pancreatic beta-cells. J Agric Food Chem. 2007;55:2816–2823. doi: 10.1021/jf062577r. [DOI] [PubMed] [Google Scholar]
- 15.Lim K.H., Cho J.Y., Kim B., Bae B.S., Kim J.H. Red ginseng (Panax ginseng) decreases isoproterenol-induced cardiac injury via antioxidant properties in porcine. J Med Food. 2014;17:111–118. doi: 10.1089/jmf.2013.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung K.I., Ju A., Lee H.M., Lee S.S., Song C.H., Won W.Y., Jeong J.S., Hong O.K., Kim J.H., Kim D.J. Chronic ethanol ingestion, type 2 diabetes mellitus, and brain-derived neurotrophic factor (BDNF) in rats. Neurosci Lett. 2011;487:149–152. doi: 10.1016/j.neulet.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Kawano K., Hirashima T., Mori S., Saitoh Y., Kurosumi M., Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long–Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- 18.Rector R.S., Uptergrove G.M., Borengasser S.J., Mikus C.R., Morris E.M., Naples S.P., Laye M.J., Laughlin M.H., Booth F.W., Ibdah J.A. Changes in skeletal muscle mitochondria in response to the development of type 2 diabetes or prevention by daily wheel running in hyperphagic OLETF rats. Am J Physiol Endocrinol Metab. 2010;298:E1179–E1187. doi: 10.1152/ajpendo.00703.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J.H., Hahm D.H., Yang D.C., Kim J.H., Lee H.J., Shim I. Effect of crude saponin of Korean red ginseng on high-fat diet-induced obesity in the rat. J Pharmacol Sci. 2005;97:124–131. doi: 10.1254/jphs.fp0040184. [DOI] [PubMed] [Google Scholar]
- 20.Brocardo P.S., Boehme F., Patten A., Cox A., Gil-Mohapel J., Christie B.R. Anxiety- and depression-like behaviors are accompanied by an increase in oxidative stress in a rat model of fetal alcohol spectrum disorders: protective effects of voluntary physical exercise. Neuropharmacology. 2012;62:1607–1618. doi: 10.1016/j.neuropharm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Patki G., Solanki N., Atrooz F., Allam F., Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013;1539:73–86. doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S.J., Ju A., Lim S.G., Kim D.J. Chronic alcohol consumption, type 2 diabetes mellitus, insulin-like growth factor-I (IGF-I), and growth hormone (GH) in ethanol-treated diabetic rats. Life Sci. 2013;93:778–782. doi: 10.1016/j.lfs.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Lavorato-Rocha A.M., Anjos L.G., Cunha I.W., Vassallo J., Soares F.A., Rocha R.M. Immunohistochemical assessment of PTEN in vulvar cancer: best practices for tissue staining, evaluation, and clinical association. Methods. 2015;77–78:20–24. doi: 10.1016/j.ymeth.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Risinger F.O., Cunningham C.L. Genetic differences in ethanol-induced hyperglycemia and conditioned taste aversion. Life Sci. 1992;50:Pl113–Pl118. doi: 10.1016/0024-3205(92)90463-y. [DOI] [PubMed] [Google Scholar]
- 25.Ali S., Stone M.A., Peters J.L., Davies M.J., Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23:1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 26.Anderson R.J., Freedland K.E., Clouse R.E., Lustman P.J. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 27.Ates M., Dayi A., Kiray M., Sisman A.R., Agilkaya S., Aksu I., Baykara B., Buyuk E., Cetinkaya C., Cingoz S. Anxiety- and depression-like behavior are correlated with leptin and leptin receptor expression in prefrontal cortex of streptozotocin-induced diabetic rats. Biotech Histochem. 2014;89:161–171. doi: 10.3109/10520295.2013.825319. [DOI] [PubMed] [Google Scholar]
- 28.George F.R., Collins A.C. Prostaglandin synthetase inhibitors antagonize the depressant effects of ethanol. Pharmacol Biochem Behav. 1979;10:865–869. doi: 10.1016/0091-3057(79)90059-5. [DOI] [PubMed] [Google Scholar]
- 29.Abbas G., Rauf K., Mahmood W. Saponins: the phytochemical with an emerging potential for curing clinical depression. Nat Prod Res. 2015;29:302–307. doi: 10.1080/14786419.2014.942661. [DOI] [PubMed] [Google Scholar]
- 30.Chen L., Dai J., Wang Z., Zhang H., Huang Y., Zhao Y. The antidepressant effects of ginseng total saponins in male C57BL/6N mice by enhancing hippocampal inhibitory phosphorylation of GSK-3beta. Phytother Res. 2014;28:1102–1106. doi: 10.1002/ptr.5103. [DOI] [PubMed] [Google Scholar]
- 31.Seppala-Lindroos A., Vehkavaara S., Hakkinen A.M., Goto T., Westerbacka J., Sovijarvi A., Halavaara J., Yki-Jarvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 32.Tiikkainen M., Bergholm R., Vehkavaara S., Rissanen A., Hakkinen A.M., Tamminen M., Teramo K., Yki-Jarvinen H. Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Diabetes. 2003;52:701–707. doi: 10.2337/diabetes.52.3.701. [DOI] [PubMed] [Google Scholar]
- 33.Celik S., Erdogan S., Tuzcu M. Caffeic acid phenethyl ester (CAPE) exhibits significant potential as an antidiabetic and liver-protective agent in streptozotocin-induced diabetic rats. Pharmacol Res. 2009;60:270–276. doi: 10.1016/j.phrs.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Shinde U.A., Goyal R.K. Effect of chromium picolinate on histopathological alterations in STZ and neonatal STZ diabetic rats. J Cell Mol Med. 2003;7:322–329. doi: 10.1111/j.1582-4934.2003.tb00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandler S., Bendtzen K., Eizirik D.L., Welsh M. Interleukin-6 affects insulin secretion and glucose metabolism of rat pancreatic islets in vitro. Endocrinology. 1990;126:1288–1294. doi: 10.1210/endo-126-2-1288. [DOI] [PubMed] [Google Scholar]
- 36.Southern C., Schulster D., Green I.C. Inhibition of insulin secretion from rat islets of Langerhans by interleukin-6. An effect distinct from that of interleukin-1. Biochem J. 1990;272:243–245. doi: 10.1042/bj2720243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prystupa A., Kicinski P., Sak J., Boguszewska-Czubara A., Torun-Jurkowska A., Zaluska W. Proinflammatory cytokines (IL-1alpha, IL-6) and hepatocyte growth factor in patients with alcoholic liver cirrhosis. Gastroenterol Res Pract. 2015;2015 doi: 10.1155/2015/532615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aravinthan A., Kim J.H., Antonisamy P., Kang C.W., Choi J., Kim N.S., Kim J.H. Ginseng total saponin attenuates myocardial injury via anti-oxidative and anti-inflammatory properties. J Ginseng Res. 2015;39:206–212. doi: 10.1016/j.jgr.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu H.Z., Liu Z.L., Zhao S.P., Sun C.Z., Yang M.S. Protective mechanism of Panax notoginseng saponins on rat hemorrhagic shock model in recovery stage. Cell Biochem Biophys. 2014;70:1719–1724. doi: 10.1007/s12013-014-0119-x. [DOI] [PubMed] [Google Scholar]
- 40.Abbate S.L., Brunzell J.D. Pathophysiology of hyperlipidemia in diabetes mellitus. J Cardiovasc Pharmacol. 1990;16(Suppl 9):S1–S7. [PubMed] [Google Scholar]
- 41.Oki J.C. Dyslipidemias in patients with diabetes mellitus: classification and risks and benefits of therapy. Pharmacotherapy. 1995;15:317–337. [PubMed] [Google Scholar]
- 42.Kelley D.E., Goodpaster B.H. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care. 2001;24:933–941. doi: 10.2337/diacare.24.5.933. [DOI] [PubMed] [Google Scholar]
- 43.Tammi A., Ronnemaa T., Gylling H., Rask-Nissila L., Viikari J., Tuominen J., Pulkki K., Simell O. Plant stanol ester margarine lowers serum total and low-density lipoprotein cholesterol concentrations of healthy children: the STRIP project. Special Turku Coronary Risk Factors Intervention Project. J Pediatr. 2000;136:503–510. doi: 10.1016/s0022-3476(00)90014-3. [DOI] [PubMed] [Google Scholar]
- 44.Dembele K., Nguyen K.H., Hernandez T.A., Nyomba B.L. Effects of ethanol on pancreatic beta-cell death: interaction with glucose and fatty acids. Cell Biol Toxicol. 2009;25:141–152. doi: 10.1007/s10565-008-9067-9. [DOI] [PubMed] [Google Scholar]
- 45.Kim S.S., Jang H.J., Oh M.Y., Eom D.W., Kang K.S., Kim Y.J., Lee J.H., Ham J.Y., Choi S.Y., Wee Y.M. Ginsenoside Rg3 enhances islet cell function and attenuates apoptosis in mouse islets. Transplant Proc. 2014;46:1150–1155. doi: 10.1016/j.transproceed.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 46.Luo J.Z., Luo L. American ginseng stimulates insulin production and prevents apoptosis through regulation of uncoupling protein-2 in cultured beta cells. Evid Based Complement Alternat Med. 2006;3:365–372. doi: 10.1093/ecam/nel026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Experimental design. IHC, immunohistochemistry.