Abstract
Background
Korean Red Ginseng extracts (RGE) have been suggested as effective immune modulators, and we reported that ginsenosides possess anti-inflammasome properties. However, the properties of nonsaponin components of RGE have not been well studied.
Methods
To assess the roles of nonsaponin fractions (NS) in NLRP3 inflammasome activation, we treated murine macrophages with or without first or second inflammasome activation signals with RGE, NS, or saponin fractions (SF). The first signal was nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)-mediated transcription of pro-interleukin (IL)-1β and NLRP3 while the second signal triggered assembly of inflammasome components, leading to IL-1β maturation. In addition, we examined the role of NS in IL-6 production and IL-1β maturation in mice.
Results
NS induced IL-1β and NLRP3 transcription via toll-like receptor 4 signaling, whereas SF blocked expression. During the second signal, SF attenuated NLRP3 inflammasome activation while NS did not. Further, NS-injected mice presented increased IL-1β maturation and IL-6 production.
Conclusion
SF and NS of RGE play differential roles in the NLRP3 inflammasome activation. Hence, RGE can be suggested as an NLRP3 inflammasome modulator.
Keywords: cytokine, inflammasome, interleukin-1beta, Korean Red Ginseng extracts, NLRP3
1. Introduction
Ginseng, which is the root of Panax ginseng Meyer of the family Araliaceae, is found in eastern Asia (mostly Korea, northeast China, and eastern Siberia) and is one of the most well researched medicinal herbs. Emerging literature has reported that ginseng has various pharmacological constituents, including ginsenosides, polyacetylenes, polyphenolic compounds, and acidic polysaccharides, which ameliorate cancer, diabetes mellitus, and neural disorders [1]. In addition, ginseng and its constituents possess antiaging, antithrombotic, wound healing, and immune regulatory activities [2]. Korean Red Ginseng is made by steaming and drying fresh ginseng root to enhance efficacy, and it contains several unique ginsenosides (Rh2, Rs4, and Rg3) derived from hydrolysis of saponins during heating procedures [3], [4]. Ginsenosides, which are the major active components of Korean Red Ginseng, exhibit anti-inflammatory properties by inhibiting nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation in association with reduced transcriptional levels of proinflammatory mediators [5]. In addition, Korean Red Ginseng and ginsenosides attenuate inflammasome activation, which plays a vital role in innate immunity [6].
Inflammasomes play critical roles in hosts against microbial pathogens and endogenous harmful signals [7]. Inflammasomes are multiprotein complexes composed of a nucleotide-binding oligomerization domain-like receptor (NLR, a pattern recognition platform), an apoptosis-related speck-like protein containing a caspase recruitment domain (ASC, an adaptor protein), and procaspase-1 (a cysteine protease). Inflammasome assembly facilitates self-activation of caspase-1, leading to cleavage and secretion of precursor proinflammatory cytokines such as interleukin (IL)-1β and IL-18. Activated inflammasomes also trigger caspase-1-mediated cell death known as pyroptosis [8]. Among several inflammasomes, nucleotide-binding oligomerization domain-like receptors (NOD)-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome is the most well characterized, since it recognizes not only pathogens, but also a variety of danger-associated molecular pattern molecules such as uric acid crystals linked with gout, β-amyloid plaques associated with Alzheimer's disease, islet amyloid polypeptide and free fatty acids involved in type 2 diabetes, and cholesterol crystals and oxidized low density lipoprotein connected with atherosclerosis [9]. Hence, inhibition of NLRP3 inflammasome activation and IL-1β/IL-18 production, which are final products of NLRP3 inflammasome, is currently a therapeutic strategy for multiple diseases [9]. Activation of NLRP3 inflammasome requires two signals. The first signal, also referred to as the priming step, is commonly induced by toll-like receptor (TLR) ligands such as lipopolysaccharide (LPS), which trigger NF-κB-mediated transcription of pro-IL-1β/IL-18 and NLRP3 [10]. The second signal, termed the activation step, induces assembly of inflammasome components and self-catalytic activation of caspase-1 through K+ efflux, Ca2+ mobilization, or reactive oxygen species (ROS) generation [11].
Previously, we reported attenuation of NLRP3 inflammasome-mediated IL-1β maturation by Korean Red Ginseng extracts (RGE) as well as inhibition of NLRP3 inflammasome second signaling by ginsenosides (Rh1 and Rg3) [6]. Hence, we investigated RGE as an anti-inflammasome agent that regulates inflammasome-mediated diseases. Although ginsenosides are key elements for regulation of NLRP3 inflammasome activation, the effects of other constituents of RGE, such as nonsaponin fractions (NS), on inflammasome activation have not been elucidated. In this study, we compared the effects of SF or NS of RGE on dual signaling in NLRP3 inflammasome activation using human and murine macrophages. The role of NS in cytokine production was further confirmed in animals.
2. Materials and methods
2.1. Cell culture and treatment
Unless otherwise indicated, all materials for cell culture were purchased from GenDEPOT Inc. (Barker, TX, USA). Bone marrow-derived macrophages (BMDMs) were obtained by differentiation of bone marrow progenitors from tibia and femur bones of C57BL/6 mice (6–12-wk-old; Narabio Co., Seoul, Korea) in L929 cell-conditioned medium as a source of macrophage colony-stimulating factor [12]. The progenitors were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 50% L929 cell-conditioned medium, 100 U/mL of penicillin, and 100 μg/mL of streptomycin. Cells were seeded in nontissue culture-treated Petri dishes (SPL Life Science Co., Phcheon-si, Gyeonggi-do, Korea) and incubated at 37°C in a 5% CO2 atmosphere for 7 d. THP-1 cells were obtained from Korea Cell Line Bank (KCLB Number 40202; Seoul, Korea) and maintained in RPMI 1640 medium containing 10% FBS, 100 U/mL of penicillin, and 100 μg/mL of streptomycin at 37°C in a 5% CO2 atmosphere.
2.2. Preparation of SF and NS of RGE
RGE were manufactured from roots of 6-yr-old fresh P. ginseng provided by Korea Ginseng Corp. (Daejeon, Korea) [6]. The SF and NS were prepared according to a previous study [13]. Briefly, RGE (2.0 kg) was subjected sequentially to Diaion HP20 adsorption chromatography (Mitsubishi Chemical Co., Tokyo, Japan) using H2O, 20% ethyl alcohol (EtOH), and absolute EtOH (Daegung Chemicals and Metal Co., Siheung-si, Gyeonggi-do, Korea) as eluents. No ginsenosides were detected in H2O or 20% EtOH, which combined and evaporated to dryness in vacuo (NS, 1.1 kg). EtOH yield was 135.4 g [saponin fraction (SF)]. According to the results of the component analysis, SF was a more dominant saponin (223.4 mg/g) than NS (5.5 mg/g). NS showed four-fold higher acidic polysaccharide content and six-fold higher arginine-fructose-glucose content than SF. Generally, ginsenoside content of Korean Red Ginseng powder was 18.5 mg/g. Endotoxin contaminations of RGE powder (0.10 ± 0.02 EU/mg), RGE solution (0.06 ± 0.02 EU/mg), NS (0.80 ± 0.02 EU/mg), and SF (0.11 ± 0.01 EU/mg) were elucidated by limulus amebocyte lysate (LAL) assays (QCL-1000 Test Kit, Lonza Group Ltd., Basel, Switzerland).
2.3. Cell treatment for cytokine and NLRP3 expression
BMDMs or THP-1 cells (2.0 × 106 cells per well for RNA extraction or 1.0 × 106 cells per well for protein analysis) were plated on six-well plates or 12-well plates (SPL Life Science Co.) and treated with RGE, NS, or SF with/without LPS (L4130, 10 ng/mL, Sigma-Aldrich Co., St. Louis, MO, USA). In addition, BMDMs were treated with LPS, RGE, NS, or SF with/without TAK-242 (5μM, CLI-095, InvivoGen, San Diego, CA, USA), Bay 11-7821 (Bay, 1744, Tocris Bioscience, Bristol, UK), or diphenyleneiodonium chloride (DPI, 0504, Tocris Bioscience). Total RNA and cellular lysates were prepared for further analysis.
2.4. Cell treatment for inflammasome activation and inhibition
BMDMs (1.0 × 106 cells per well) were plated on 12-well plates (SPL Life Science Co.) and primed with 1 μg/mL of LPS (Sigma-Aldrich Co.) with/without NS or SF in RPMI 1640 containing 10% FBS and antibiotics for 3 h [14]. After priming, BMDMs were subjected to the following activation steps. Culture medium was replaced with RPMI 1640 supplemented with nigericin (NG, 40μM; 4312, Tocris Bioscience) with/without NS or SF for 1 h. Cellular supernatant, lysate (Lys), and cross-linked pellets (Pellet) with suberic acid bis (Sigma–Aldrich Co.) were collected for further analysis.
2.5. Reverse transcription polymerase chain reaction (RT-PCR) and quantitative real time PCR (qPCR)
Total RNA was extracted using Trizol (InvitroGen, Grand Island, NY, USA) and reverse-transcribed to first-strand complementary DNA (cDNA) using an M-MLV cDNA synthesis kit (Enzynomics, Daejeon, Korea). For RT-PCR, transcription was amplified by a SimpliAmp Thermal Cycler (Thermo Fisher Scientific Inc. Grand Island, NY, USA) and nTaq polymerase (Enzynomics). PCR products were visualized by agarose gel electrophoresis and ethidium bromide staining. For qPCR, gene expression was quantified using an Eco Real-Time PCR system (Illumina, San Diego, CA, USA) and TOPrealTM qPCR 2X PreMIX containing SYBR Green (Enzynomics). Quantitation was normalized with β-actin (Actb) or glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Information on gene-specific primers is listed in Table 1.
Table 1.
Primer sequences
| Gene Name | Species | GeneBank ID | Direction | Sequence (5′ to 3′) | Expected band size (bp) |
|---|---|---|---|---|---|
| Pro-IL-1β | Mouse | NM_008361 | F | CAG GCA GGC AGT ATC ACT CA | 350 |
| R | AGG CCA CAG GTA TTT TGT CG | ||||
| IL-6 | Mouse | NM_031168 | F | GTT CTC TGG GAA ATC GTG GA | 339 |
| R | GGA AAT TGG GGT AGG AAG GA | ||||
| TNFα | Mouse | NM_013693 | F | ACG GCA TGG ATC TCA AAG AC | 324 |
| R | CGG ACT CCG CAA AGT CTA AG | ||||
| IL-10 | Mouse | NM_010548 | F | TGC TAT GCT GCC TGC TCT TA | 243 |
| R | TCA TTT CCG ATA AGG CTT GG | ||||
| IL-1α | Mouse | NM_010554 | F | GAA GCT CGT CAG GCA GAA GT | 253 |
| R | TGT TTC TGG CAA CTC CTT CA | ||||
| NLRP3 | Mouse | NM_145827 | F | CAG ACT GGC AAA AGG CTG TG | 333 |
| R | TCT TCC CGG TCT CCA TCT GT | ||||
| F4/80 | Mouse | NM_010130 | F | TTT TCA GAT CCT TGG CCA TC | 254 |
| R | AGG AGC CTG GTA CAT TGG TG | ||||
| CD11b | Mouse | NM_001082960 | F | CCA AGA CGA TCT CAG CAT CA | 296 |
| R | TAG CAG GAA AGA TGG GAT GG | ||||
| β-actin | Mouse | NM_007393 | F | AGC CAT GTA CGT AGC CAT CC | 228 |
| R | CTC TCA GCT GTG GTGGTG AA | ||||
| GAPDH | Mouse | NM_001289726 | F | AAC TTT GGC ATT GTG GAA GG | 223 |
| R | ACA CAT TGG GGG TAG GAA CA | ||||
| Pro-IL-1β | Human | NM_000576 | F | CTG TCC TGC GTG TTG AAA GA | 180 |
| R | TTC TGC TTG AGA GGT GCT GA | ||||
| β-actin | Human | NM_001101 | F | GGA CTT CGA GCA AGA GAT GG | 234 |
| R | AGC ACT GTG TTG GCG TAC AG |
GADPH, glyceraldehyde 3-phosphate dehydrogenase; IL, interleukin; TNF, tumor-necrosis factor.
2.6. Western blot analysis
Supernatant, Lys, and Pellet samples were separated on a sodium dodecyl sulfate polyacrylamide gel (10% or 16%), transferred into a polyvinylidene difluoride membrane (Pall Co., Port Washington, NY, USA), and blocked with 3% skim milk. The membrane was probed with primary antibodies against anti-mouse IL-1β antibody (AF-401-NA, R&D Systems, Minneapolis, MN, USA), anti-human IL-1β antibody (AF-201-NA, R&D Systems), anti-Asc antibody (sc-22514, Santa Cruz Biotechnology, Dallas, TX, ), anti-tumor necrosis factor (TNF)α antibody (sc-1351, Santa Cruz Biotechnology), anti-NLRP3 antibody (AG-20B-0014-C100, AddipoGen Co., San Diego, CA, USA) or anti-actin antibody (sc-1615, Santa Cruz Biotechnology) overnight at 4°C. The membranes were further probed with HRP-conjugated 2nd anti-sera (sc-2020 or sc-2004, Santa Cruz Biotechnology) and visualized using Power-Opti ECL solution (BioNote Co., Gyeonggi-do, Korea) and a cooled charge-coupled device camera system (AE-9150, EZ-Capture II, ATTO Technology, Tokyo, Japan). The intensity of bands was measured by CS Analyzer Version 3.00 (ATTO Technology).
2.7. Animals study
Male C57BL/6 mice (8-wk-old) were purchased from Narabio Co. All mice were maintained under a 12 h light/dark cycle at 24°C. Animals were provided standard sterile food and water ad libitum, after which they were allowed to adjust to the environment for 1 wk. For intraperitoneal injection of NS, mice (n = 3 per group) were injected with NS (1 mg/mouse or 5 mg/mouse) or LPS (100 μg/mouse). After 6 h, mice were anesthetized with ether inhalation and blood collected by decapitation. Peritoneal cavities were washed with 5 mL of PBS, and peritoneal exudate cells were analyzed by a cell counter (Moxi Z, ORFLO Techlogies, Ketchum, ID, USA). Sera and lavage fluids were collected for further analysis. For oral administration of NS, mice (n = 3 per group) were administered NS (800 μg/mouse/d) for 7 d and then intraperitoneal injection of LPS (100 μg/mouse). Lavage fluids were collected for further analysis. All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Kangwon National University (Chuncheon, Korea; IACUC; approval number KW-150313-1).
2.8. Cytokine and cytotoxicity assay
To quantify secreted IL-1β, cell culture supernatants of BMDMs were measured by a mouse IL-1beta/IL-1F2 Quantikine ELISA Kit (MTA00B, R&D Systems) or mouse IL-6 Quntikine ELISA Kit (M6000B, R&D Systems). For the cytotoxicity assay, BMDMs (10,000 cells/well) were treated with LPS for 3 h. The cells were treated with the indicated substances (RGE, NS, or SF) for 1 h and cytotoxicity was assessed by the Cell Counting Kit-8 (Dojindo Molecular Technologies, Inc., Rockville, MD, USA) as per the manufacturer's protocol. The plates were readout using an Epoch microplate spectrophotometer (BioTek, Winooski, VT, USA).
2.9. Statistical analysis
Statistical analyses were performed using a t test (Mann-Whitney U test) for the two groups or one-way analysis of variance (Tukey's multiple comparisons test) for multiple groups, and survival analysis for lethality test using GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. SF or NS differentially regulate cytokine expression in macrophages
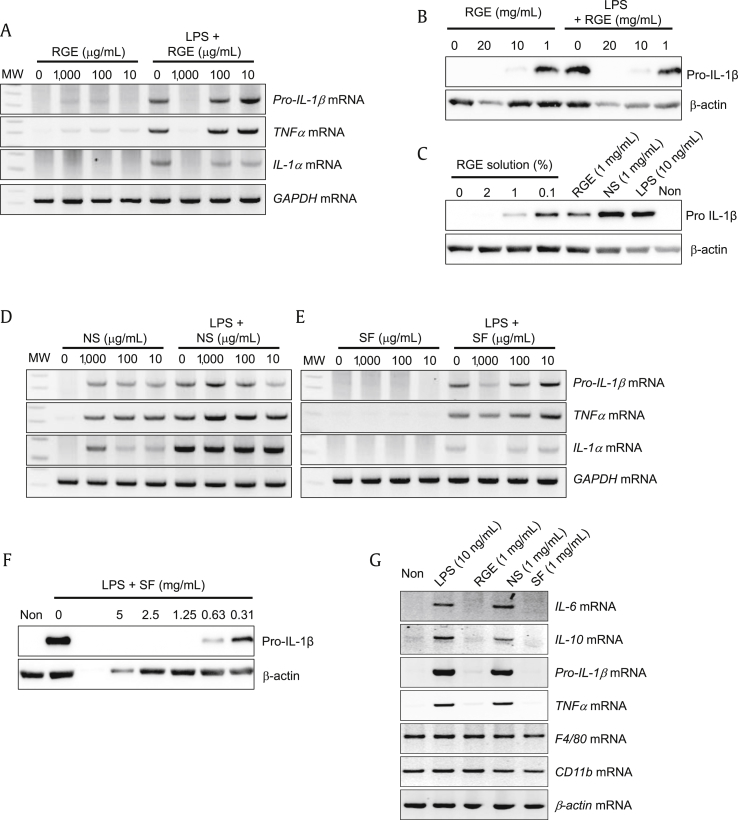
To elucidate the effects of RGE and its subfractions (SF and NS) on proinflammatory cytokine production in macrophages, we treated murine BMDMs with RGE with/without LPS. As expected, RGE blocked LPS-mediated pro-IL-1β, TNFα, and IL-1α mRNA induction (Fig. 1A) as well as pro-IL-1β protein expression (Fig. 1B). Interestingly, a lower dosage of RGE (1 mg/mL) without LPS increased pro-IL-1β and TNFα mRNA (Fig. 1A), and pro-IL-1β protein (Fig. 1B) expression, whereas a high dosage of RGE (10 mg/mL or 20 mg/mL) blocked LPS-mediated cytokine expression. In addition, RGE solution (concentrated extracts) had a similar effect on pro-IL-1β expression as RGE powder (Fig. 1C). Based on this result, we hypothesize that RGE has differential effects on cytokine production. To confirm this, we treated BMDMs with NS with/without LPS (Fig. 1D). NS treatment did not attenuate LPS-induced cytokine expression. Although SF treatment alone did not upregulate cytokine production, it did block LPS-mediated proinflammatory cytokine expression (Fig. 1E) and pro-IL-1β protein expression (Fig. 1F). In addition, we tested the effects of NS on other cytokine and macrophage markers (Fig. 1G). NS induced IL-6 and IL-10 transcription in macrophages, similar to pro-IL-1β and TNFα expression. However, NS did not affect mRNA expression of F4/80 and CD11b, which are widely used markers of murine macrophages, in BMDMs. Thus, RGE plays differential roles in cytokine production. Specifically, NS from RGE upregulates proinflammatory cytokines while SF downregulates LPS-mediated cytokine expression.
Fig. 1.
Effects of Korean Red Ginseng extracts (RGE), nonsaponin fractions (NS), and saponin fractions (SF) on cytokine expression. (A–G) Bone marrow-derived macrophages (BMDMs) were treated with the indicated concentrations of RGE, NS, or SF with/without lipopolysaccharide (LPS; 10 ng/mL) for 3 h. The indicated genes were analyzed by reverse transcription polymerase chain reaction (RT-PCR) and immunoblotting. All data shown are representative of at least two independent experiments. RGE, freeze-dried powder of Korean Red Ginseng extracts, RGE solution, concentrated extracts of Korean Red Ginseng (64 % RGE powder). IL, interleukin; TNF, tumor-necrosis factor; GADPH, glyceraldehyde 3-phosphate dehydrogenase; F4/80, EGF-like module-containing mucin-like hormone receptor-like 1; CD11b, Integrin alpha M.
3.2. NS of Korean Red Ginseng induces proinflammatory cytokines in macrophages
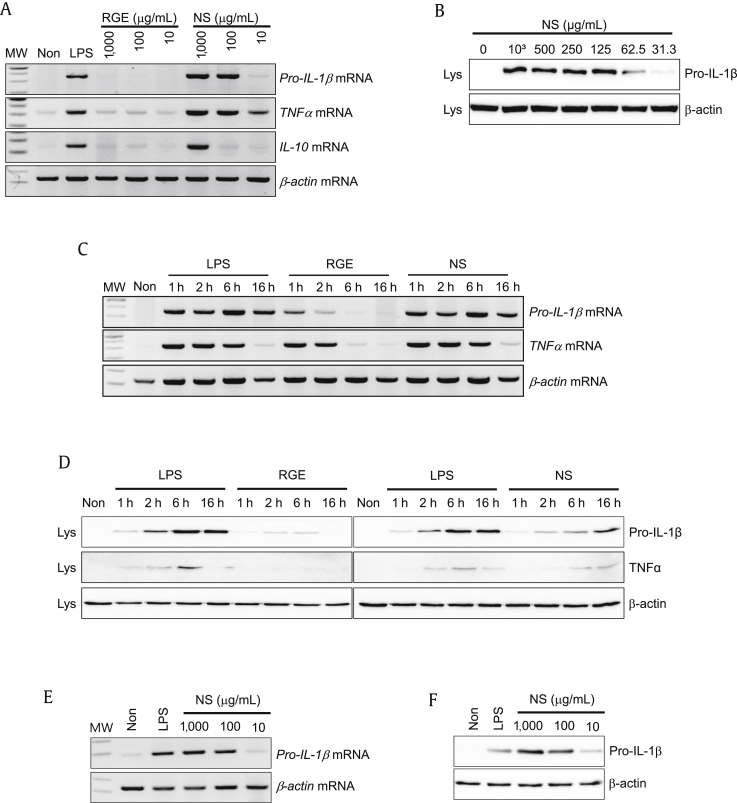
To assess the effect of NS on cytokine expression in BMDMs, increasing dosages of RGE or NS for 6 h were applied, and the expression of two proinflammatory cytokines (pro-IL-1β and TNFα) and an anti-inflammatory cytokine (IL-10) was measured. As seen in Fig. 2A, NS dose-dependently induced mRNA expression of all cytokines, similar to LPS treatment. Protein expression of pro-IL-1β was elevated by NS treatment in a dose-dependent manner (Fig. 2B). We further investigated the time-dependent expression pattern of cytokines (Fig. 2C). Pro-IL-1β and TNFα mRNAs were highly expressed at 1 h after LPS, RGE, or NS treatment, and expression levels were maintained until 16 h. The expression levels of pro-IL-1β and TNFα proteins were further confirmed by immunoblotting (Fig. 2D). The effects of NS on cytokine upregulation were measured in a human monocytic cell line (THP-1). Human pro-IL-1β mRNA (Fig. 2E) and protein (Fig. 2F) levels were elevated by NS. Taken together, NS potentially induces inflammatory cytokine expression in macrophages.
Fig. 2.
Effect of nonsaponin fractions (NS) on cytokine expression. Bone marrow-derived macrophages (BMDMs) were treated with the indicated concentrations of Korean Red Ginseng extracts (RGE) or NS for 3 h, after which gene and protein expression levels were analyzed by reverse transcription polymerase chain reaction (RT-PCR) (A) and immunoblotting (B). Bone marrow-derived macrophages (BMDMs) were treated with lipopolysaccharide (LPS; 10 ng/mL), RGE (100 μg/mL), or NS (100 μg/mL) for the indicated time, after which expression of genes (C) and proteins (D) was analyzed. A human leukemic cell line (THP-1) was treated with LPS (10 ng/mL) or the indicated concentration of NS for 6 h. Expression levels of pro-IL-1β mRNA (E) and protein (F) were measured. All data shown are representative of at least two independent experiments. IL, interleukin; TNF, tumor-necrosis factor.
3.3. NS is a ligand for TLR 4
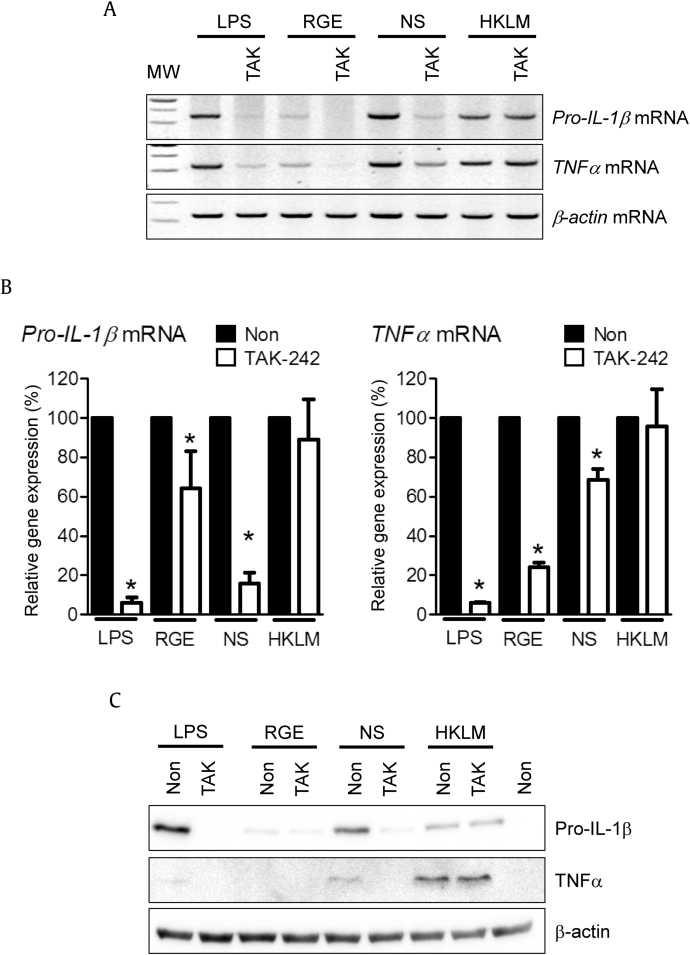
Based on the cytokine-inducing property of NS, we speculate that TLR may recognize NS. To confirm this, a TLR4 inhibitor, TAK-242 [15], was applied in the presence of NS, LPS (TLR4 agonist), or heat-killed Listeria monocytogenes (HKLM, TLR2 agonist). As shown in Fig. 3A, all TLR agonists and NS induced pro-IL-1β and TNFα mRNA expression, which was attenuated by TAK-242 cotreatment, whereas HKLM did not. Relative cytokine mRNA expression levels were further analyzed by qPCR (Fig. 3B). TAK-242 significantly inhibited RGE-, NS-, or LPS-mediated pro-IL-1β and TNFα mRNA expression, although the inhibition levels varied. The effect of TAK-242 on pro-IL-1β and TNFα protein expression was further confirmed (Fig. 3C). Thus, NS-mediated cytokine expression was blocked by the TLR4 inhibitor.
Fig. 3.
Effect of toll-like receptor 4 (TLR4) on nonsaponin fractions (NS)-mediated cytokine expression. Bone marrow-derived macrophages (BMDMs) were treated with the indicated concentrations of lipopolysaccharide (LPS) (10 ng/mL), Korean Red Ginseng extracts (RGE) (100 μg/mL), NS (100 μg/mL), or heat-killed Listeria monocytogenes (HKLM) (1%) with/without TAK (TAK-242, TLR4 inhibitor, 5μM) for 2 h. Expression levels of pro-IL-1β and TNFα were analyzed by RT-PCR (A), qPCR (B), or immunoblotting (C). All data shown are representative of at least two independent experiments. * p < 0.05 vs. non-TAK treatment. IL, interleukin; TNF, tumor-necrosis factor.
3.4. NS induces NLRP3 expression while SF attenuates expression
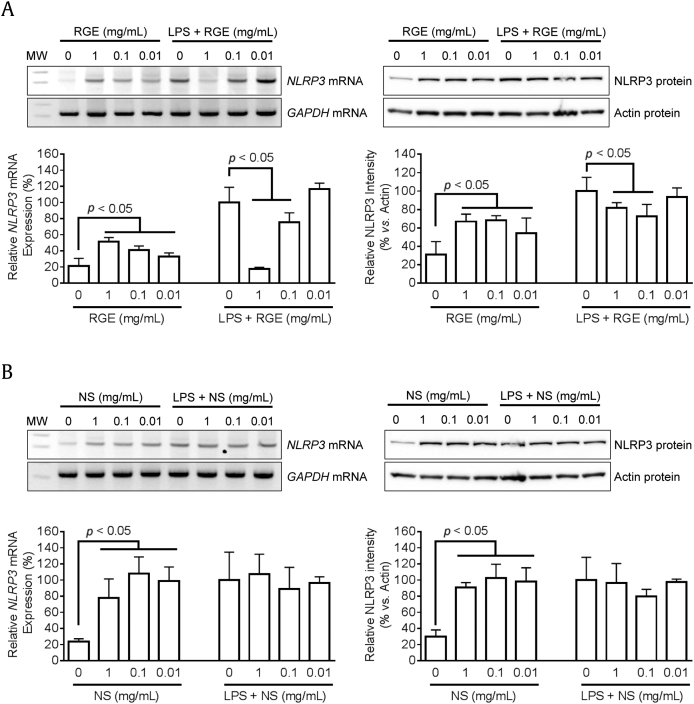
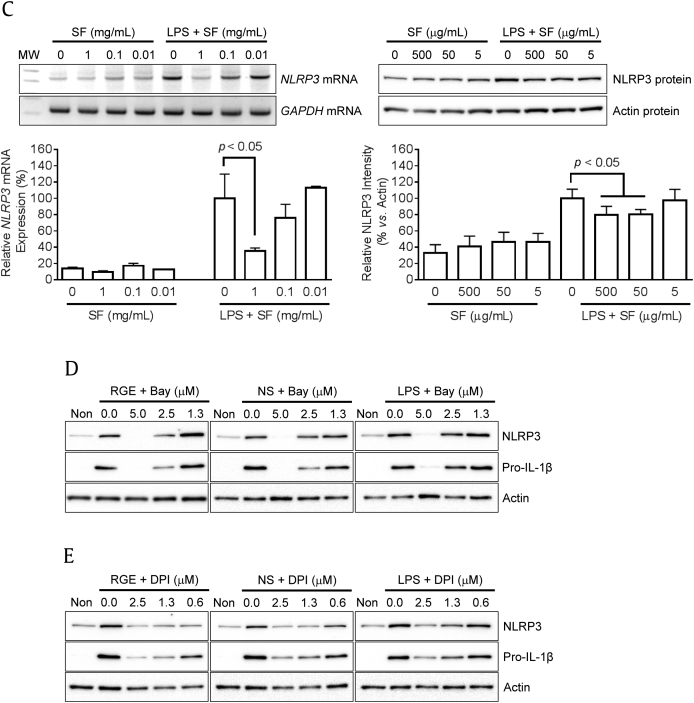
Previously, we reported that RGE attenuate NLRP3 inflammasome activation, and ginsenosides (Rg1 and Rh3) were proposed as anti-inflammasome molecules [6]. In this study, we further elucidated the effects of NS or SF on the priming (1st signal) and/or activating step (2nd signal) of NLRP3 inflammasome activation. Based on previous literature, the priming step mediated via NF-κB signaling and ROS upregulates NLRP3 expression and is a prerequisite for the activating step, which induces assembly of inflammasome components to maturation and secretes IL-1β [10], [16]. To observe the effects of RGE on the first signal, BMDMs were treated with RGE (Fig. 4A), NS (Fig. 4B), or SF (Fig. 4C) with/without LPS. Similar to the effect of RGE, NS, or SF on cytokine expression, RGE and NS without LPS induced NLRP3 mRNA and protein expressions while RGE and SF blocked LPS-mediated NLRP3 mRNA and protein expression. We further treated blockers for NF-κB signaling and ROS generation to RGE-, NS-, or LPS-mediated NLRP3 expression, as performed in the literature [10], [16]. RGE- or NS-mediated NLRP3 and pro-IL-1β upregulation was interrupted by Bay (NFκB inhibitor, Fig. 4D) and inhibited by DPI (blocker for ROS generation, Fig. 4E). Thus, NS of RGE upregulated NLRP3 and pro-IL-1β expression during the priming step through NF-κB signaling and/or ROS generation.
Fig. 4.
Effects of Korean Red Ginseng extracts (RGE), nonsaponin fractions (NS), or saponin fractions (SF) on NLRP3 expression. Bone marrow-derived macrophages (BMDMs) were treated with the indicated concentrations of RGE (A), NS (B), or SF (C) with/without lipopolysaccharide (LPS; 10 ng/mL) for 3 h. Expression of NLRP3 mRNA (left side panel) was analyzed by reverse transcription polymerase chain reaction (RT-PCR) and quantitative real time PCR (qPCR). Levels of NLRP3 protein (right side panel) were measured by immunoblotting, and the below bar graph indicates relative NLRP3 band density. (D–E) Macrophages were treated with RGE (1 mg/mL), NS (500 μg/mL), or LPS (10 ng/mL) in the presence of Bay (NF-κB signaling inhibitor) or diphenyleneiodonium chloride (DPI; blocker for ROS generation) for 3 h. The protein levels of NLRP3 and pro-IL-1β were analyzed by immunoblotting. NOD (nucleotide-binding oligomerization domain-like receptors)-like receptor family, pyrin domain containing 3; GADPH, glyceraldehyde 3-phosphate dehydrogenase
3.5. NS induces priming step of NLRP3 inflammasome activation while SF attenuates both of priming and activation
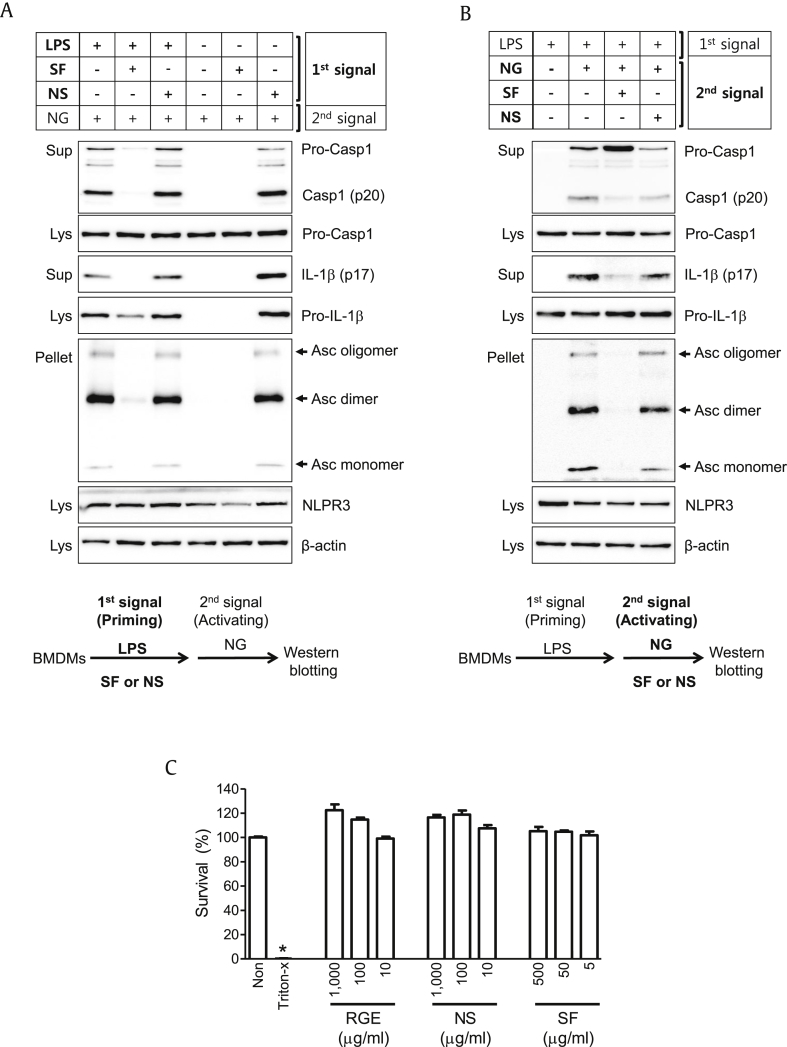
To investigate the effects of SF or NS on continuous NLRP3 inflammasome activation, we treated fractions during the priming or activation steps. As seen in Fig. 5A, BMDMs were treated with SF or NS with/without LPS (1st signal) during the priming step. Cells were further treated with NLPR3 trigger (NG) at the activation step. As expected, SF attenuated LPS-mediated pro-IL-1β and NLRP3 expression, resulting in inhibition of NG-mediated Casp1 (p20) and IL-1β (p17) secretion and Asc speck formation. By contrast, NS without LPS induced pro-IL-1β and NLRP3 expression, resulting in induction of NG-mediated NLRP3 inflammasome activation. Thus, SF and NS oppositely acted on the priming step of NLRP3 inflammasome activation. As shown in Fig. 5B, LPS-primed BMDMs were treated with NG (2nd signal) with/without SF or NS during the activation step. NS did not change NG-mediated Casp1 and IL-1β secretion or ASC pyroptosome formation, while SF attenuated NG-induced NLRP3 inflammasome activation. Thus, SF during the activation step inhibited NLRP3 inflammasome activation. The current treatment concentration of RGE, NS, or SF did not show any cytotoxicity (Fig. 5C). Taken together, NS of RGE upregulated NLRP3 expression in the priming step (1st signal) but not the activation step (2nd signal), unlike SF.
Fig. 5.
Effects of nonsaponin fractions (NS) or saponin fractions (SF) on the priming or activation steps of NLRP3 inflammasome activation. (A) Bone marrow-derived macrophages (BMDMs) were treated with lipopolysaccharide (LPS) with/without NS or SF as the first signal for 3 h, after which cells were replaced by media containing nigericin (NG, 40μM, second signal). (B) LPS-primed BMDMs were treated with NG as the second signal. Cell culture supernatants (Sup), cell lysates (Lys), and cross-linked pellets (Pellet) from whole cell lysates were analyzed by immunoblotting as indicated. (C) LPS-primed BMDMs were treated with the indicated concentration of RGE, NS, or SF, and cell viabilities were measured. All data shown are representative of at least two independent experiments. Bar graph presents the mean ± standard deviation. Casp1, caspase-1; IL, interleukin; Asc, Apoptosis-associated speck-like protein containing a C-terminal caspase-recruitment domain; NOD (nucleotide-binding oligomerization domain-like receptors) -like receptor family, pyrin domain containing 3.
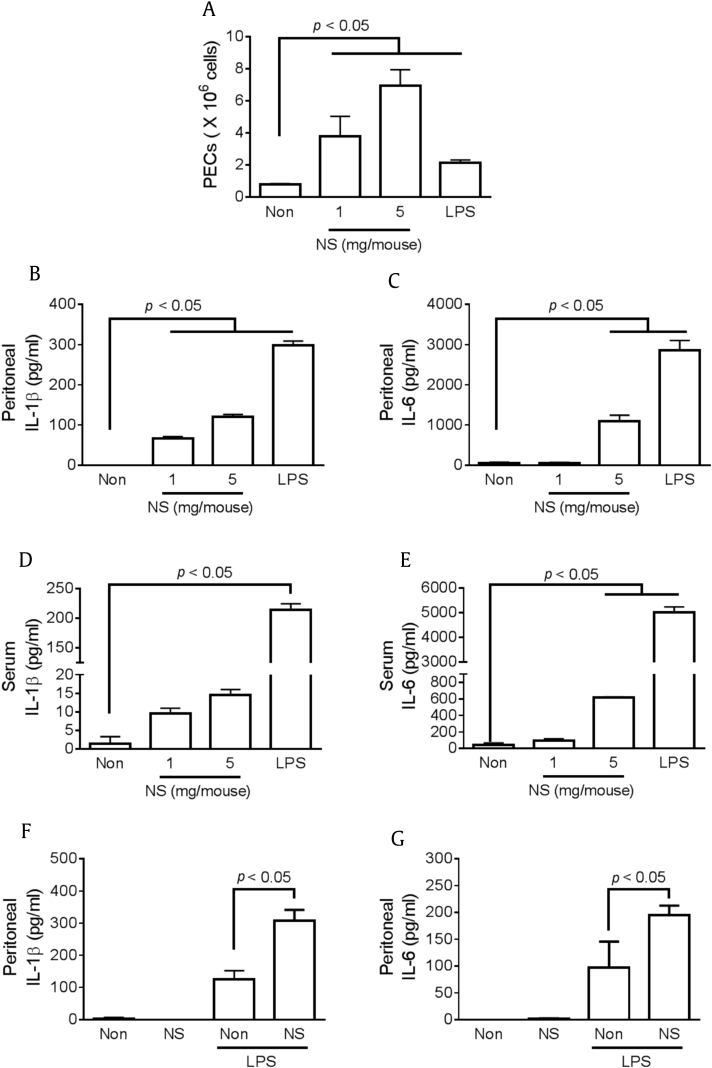
3.6. NS induces cytokine production and maturation in mice
To assess the effect of NS on in vivo cytokine production and/or maturation, mice were intraperitoneally or orally administrated NS, and cytokine levels in peritoneal lavage fluids or serum were observed. Intraperitoneal NS injection significantly increased the number of peritoneal exudate cells (Fig. 6A) as well as peritoneal IL-1β maturation (Fig. 6B) and IL-6 production (Fig. 6C), similar to LPS treatment. Serum IL-1β (Fig. 6D) and IL-6 (Fig. 6E) expression was also induced by NS injection, although the cytokine levels induced by NS treatment were lower than those induced by LPS injection. In addition, we elucidated the effect of oral administration of NS on LPS-induced peritonitis. Mice were injected with LPS or PBS after NS feeding for 7 d, after which peritoneal IL-1β maturation (Fig. 6F) and IL-6 production (Fig. 6G) were measured. Oral administration of NS did not induce any cytokine production but did increase peritoneal IL-1β and IL-6 secretion. Thus, both peritoneal injection and oral administration of NS induced production and maturation of cytokines.
Fig. 6.
Effect of nonsaponin fractions (NS) on cytokine expression in vivo. (A–E) Mice (n = 3 per group) were intraperitoneally (ip) injected with NS (1 mg/mouse or 5 mg/mouse) or lipopolysaccharide (LPS; 100 μg/mouse), and the samples (serum and peritoneal lavage fluid) were collected after 6 h. Peritoneal exudate cells (PECs) were calculated, and serum and peritoneal IL-1β and IL-6 were measured. (F–G) Mice (n = 3 per group) were orally administrated NS (800 μg/mouse/d) for 7 d and then ip injected with saline or LPS (100 μg/mouse). Peritoneal lavage fluid was collected after 6 h LPS injection, and IL-1β and IL-6 concentrations were measured. IL, interleukin.
4. Discussion
In this study, we elucidated the properties of saponin and nonsaponin subfractions of RGE on the priming and activating steps of NLRP3 inflammasome. Nonsaponin components of RGE stimulated expression of inflammatory cytokines such as pro-IL-1β via TLR4 signaling, whereas saponins of RGE attenuated TLR-mediated cytokine production. NS also upregulated NLRP3 transcription, which is an essential prerequisite for NLRP3 inflammasome activation [10], [16], whereas saponins attenuated NLRP3 transcription during the priming step. Although SF and its ginsenosides attenuated NLRP3 inflammasome-mediated IL-1β maturation [6], NS had no effect on NLRP3 inflammasome activation. The role of nonsaponins in cytokine production and maturation was further confirmed in mice. IP-injected NS increased the number of peritoneal exudate cells [17] as well as peritoneal and serum IL-1β and IL-6 secretion, similar to LPS injection. However, oral administration of nonsaponins did not induce cytokine secretion. Taken together, NS of Korean Red Ginseng are suggested to be immunostimulatory agents by acting as a first signal in NLRP3 inflammasome activation and stimulating TLR4 to produce cytokine production in macrophages.
Ginsenosides, which are biological active saponin compounds found in P. ginseng, have been reported as anti-inflammatory agents [18]. Ginsenosides significantly inhibit production of proinflammatory mediators such as TNFα, IL-1β, and monocyte chemoattractant protein-1 as well as inducible nitric oxide synthase and cyclooxygenase-2 [19]. Molecular targets of ginsenosides have been identified for regulation of inflammatory responses [18]. Ginsenosides suppress NF-κB signaling, including phosphorylation of IκBα, an inhibitor of NF-κB (p65/p50) activation, as well as activation of IκB kinase and phosphorylation of ERK (extracellular signal–regulated kinases) 1/2, JNK (c-Jun N-terminal kinases), and c-Jun [20], [21], [22]. Ginsenosides reduce cAMP, which activates protein kinase A, by inhibiting phosphodiesterase type 4, resulting in activation of NF-κB activation [23]. NF-κB signaling mediates upregulation of IL-1β precursor and NLRP3 genes in the priming step of NLRP3 inflammasome activation, and inflammasome assembly cannot be completed without NF-κB signaling [10]. SF in the priming step interrupted inflammasome activation since ginsenosides blocked NF-κB signaling. Thus, SF act as anti-inflammasome agents in the priming and activating steps.
Similar to our results, several studies revealed that nonsaponin components of ginseng upregulate expression of several cytokines in macrophages. Polysaccharides from P. ginseng, also called ginsan, upregulate proinflammatory cytokines such as TNFα, IL-1β, IL-6, and INF-γ in macrophages [24], [25]. Red ginseng acidic polysaccharide (RGAP) combined with INF-γ enhances IL-1, IL-6, TNFα, and nitric oxide (NO) production via enhanced NF-κB signaling [26]. In addition, low levels of IL-1 and IL-6 are detectable in RGAP-treated macrophages, whereas TNFα is not affected by RGAP [26]. RGAP treatment was shown to induce NO reduction in RAW264.7 cells without morphological changes, unlike LPS [27]. Induced NO production is associated with enhanced levels of inducible nitric oxide synthase and nuclear transcription factors (NF-κB, AP-1 (activator protein 1), and CREB (cAMP response element binding protein)) through ERK and JNK signaling and TLR2 as a surface receptor [27]. In addition, ginseng radix is known to induce production of TNFα and IFN-γ in spleen cells and peritoneal macrophages but had no effect in TLR4-deficient mice [28]. Ginseng radix extract was further shown to not be contaminated by LPS [28]. Thus, NS induce proinflammatory cytokines via TLR2 or TLR4 signaling in macrophages.
Inflammasomes not only recognize pathogens, but also sense abnormal metabolites, such as saturated fatty acid palmitate, lipotoxic ceramides, islet amyloid polypeptide, and minimally oxidized low density lipoprotein [29], [30]. In addition, IL-1β resulting from inflammasome activation promotes metabolic inflammation and metabolic pathologies and their complications [31]. Macrophages sense metabolites and express TLR family proteins and inflammasomes to induce inflammation by secreting proinflammatory cytokines such as IL-1β [32]. Expression of TLR2 and TLR4, the first signal for inflammasome activation, was shown to be a mediator for metabolic inflammation and insulin resistance [33]. Expression of NLRP3 is directly correlated with insulin resistance via IL-1β-mediated Th1 proinflammation in adipose tissue [29], [32]. Human clinical trials based on blockage of the IL-1 receptor significantly improved insulin sensitivity and beta-cell function [34]. Based on the present study, we speculate that saponin of RGE attenuated inflammasome activation, resulting in improved metabolic inflammation, while the nonsaponin may be present to induce inflammation.
In conclusion, we suggest that Korean Red Ginseng is not only an inflammatory modulator, but also an NLRP3 inflammasome modulator. In this study, ginsenoside-enriched SF interrupted cytokine expression while NS stimulated cytokine upregulation. During NLRP3 inflammasome activation, SF attenuated NLRP3 gene and pro-IL-1β precursor levels during the priming step and also blocked assembly of NLRP3 inflammasome. NS induced NLRP3 gene and pro-IL-1β precursor expression but did not affect inflammasome assembly. Thus, Korean Red Ginseng acts as a stimulator and inhibitor of the NLRP3 inflammasome response and activation.
Conflicts of interest
The authors have no conflicts of interest.
Acknowledgments
This research was supported by a grant (2014) from the Korean Society of Ginseng Funded by the Korean Ginseng Corporation and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2015R1A2A2A01004183).
References
- 1.Kang S., Min H. Ginseng, the ‘immunity boost’: the effects of Panax ginseng on immune system. J Ginseng Res. 2012;36:354–368. doi: 10.5142/jgr.2012.36.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ru W., Wang D., Xu Y., He X., Sun Y.E., Qian L., Zhou X., Qin Y. Chemical constituents and bioactivities of Panax ginseng (C. A. Mey.) Drug Discov Ther. 2015;9:23–32. doi: 10.5582/ddt.2015.01004. [DOI] [PubMed] [Google Scholar]
- 3.Baek S.H., Bae O.N., Park J.H. Recent methodology in ginseng analysis. J Ginseng Res. 2012;36:119–134. doi: 10.5142/jgr.2012.36.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sohn E.H., Jang S.A., Lee C.H., Jang K.H., Kang S.C., Park H.J., Pyo S. Effects of Korean Red Ginseng extract for the treatment of atopic dermatitis-like skin lesions in mice. J Ginseng Res. 2011;35:479–486. doi: 10.5142/jgr.2011.35.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu J.M., Yao Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J., Ahn H., Han B.C., Lee S.H., Cho Y.W., Kim C.H., Hong E.J., An B.S., Jeung E.B., Lee G.S. Korean Red Ginseng extracts inhibit NLRP3 and AIM2 inflammasome activation. Immunol Lett. 2014;158:143–150. doi: 10.1016/j.imlet.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 8.Lamkanfi M. Emerging inflammasome effector mechanisms. Nat Rev Immunol. 2011;11:213–220. doi: 10.1038/nri2936. [DOI] [PubMed] [Google Scholar]
- 9.Ozaki E., Campbell M., Doyle S.L. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflamm Res. 2015;8:15–27. doi: 10.2147/JIR.S51250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauernfeind F.G., Horvath G., Stutz A., Alnemri E.S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B.G., Fitzgerald K.A. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latz E., Xiao T.S., Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J., Ahn H., Woo H.M., Lee E., Lee G.S. Characterization of porcine NLRP3 inflammasome activation and its upstream mechanism. Vet Res Commun. 2014;38:193–200. doi: 10.1007/s11259-014-9602-5. [DOI] [PubMed] [Google Scholar]
- 13.Larina L., Cho B.G., Ten L., Park H. Isolation of saponin-free fraction from Ginseng (Panax ginseng C.A. Meyer) and its effects on the function of neutrophils. Korean J Chem Eng. 2001;18:986–991. [Google Scholar]
- 14.Ahn H., Kang S.G., Yoon S.I., Kim P.H., Kim D., Lee G.S. Poly-gamma-glutamic acid from Bacillus subtilis upregulates pro-inflammatory cytokines while inhibiting NLRP3, NLRC4 and AIM2 inflammasome activation. Cell Mol Immunol Forthcoming. 2016 doi: 10.1038/cmi.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawamoto T., Ii M., Kitazaki T., Iizawa Y., Kimura H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 2008;584:40–48. doi: 10.1016/j.ejphar.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 16.Bauernfeind F., Bartok E., Rieger A., Franchi L., Nunez G., Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.So A., De Smedt T., Revaz S., Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9:R28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park J., Cho J.Y. Anti-inflammatory effects of ginsenosides from Panax ginseng and their structural analogs. Afr J Biotechnol. 2009;8:3682–3690. [Google Scholar]
- 19.Cho J.Y., Yoo E.S., Baik K.U., Park M.H., Han B.H. In vitro inhibitory effect of protopanaxadiol ginsenosides on tumor necrosis factor (TNF)-alpha production and its modulation by known TNF-alpha antagonists. Planta Med. 2001;67:213–218. doi: 10.1055/s-2001-12005. [DOI] [PubMed] [Google Scholar]
- 20.Wu C.F., Bi X.L., Yang J.Y., Zhan J.Y., Dong Y.X., Wang J.H., Wang J.M., Zhang R., Li X. Differential effects of ginsenosides on NO and TNF-alpha production by LPS-activated N9 microglia. Int Immunopharmacol. 2007;7:313–320. doi: 10.1016/j.intimp.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Choo M.K., Sakurai H., Kim D.H., Saiki I. A ginseng saponin metabolite suppresses tumor necrosis factor-alpha-promoted metastasis by suppressing nuclear factor-kappaB signaling in murine colon cancer cells. Oncol Rep. 2008;19:595–600. [PubMed] [Google Scholar]
- 22.Oh G.S., Pae H.O., Choi B.M., Seo E.A., Kim D.H., Shin M.K., Kim J.D., Kim J.B., Chung H.T. 20(S)-Protopanaxatriol, one of ginsenoside metabolites, inhibits inducible nitric oxide synthase and cyclooxygenase-2 expressions through inactivation of nuclear factor-kappaB in RAW 264.7 macrophages stimulated with lipopolysaccharide. Cancer Lett. 2004;205:23–29. doi: 10.1016/j.canlet.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 23.Stancheva S.L., Alova L.G. Ginsenoside Rg1 inhibits the brain cAMP phosphodiesterase activity in young and aged rats. Gen Pharmacol. 1993;24:1459–1462. doi: 10.1016/0306-3623(93)90435-z. [DOI] [PubMed] [Google Scholar]
- 24.Lim D.S., Bae K.G., Jung I.S., Kim C.H., Yun Y.S., Song J.Y. Anti-septicaemic effect of polysaccharide from Panax ginseng by macrophage activation. J Infect. 2002;45:32–38. doi: 10.1053/jinf.2002.1007. [DOI] [PubMed] [Google Scholar]
- 25.Shin J.Y., Song J.Y., Yun Y.S., Yang H.O., Rhee D.K., Pyo S. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol Immunotoxicol. 2002;24:469–482. doi: 10.1081/iph-120014730. [DOI] [PubMed] [Google Scholar]
- 26.Choi H.S., Kim K.H., Sohn E., Park J.D., Kim B.O., Moon E.Y., Rhee D.K., Pyo S. Red ginseng acidic polysaccharide (RGAP) in combination with IFN-gamma results in enhanced macrophage function through activation of the NF-kappaB pathway. Biosci Biotechnol Biochem. 2008;72:1817–1825. doi: 10.1271/bbb.80085. [DOI] [PubMed] [Google Scholar]
- 27.Byeon S.E., Lee J., Kim J.H., Yang W.S., Kwak Y.S., Kim S.Y., Choung E.S., Rhee M.H., Cho J.Y. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean Red Ginseng. Mediators Inflamm. 2012;2012:732860. doi: 10.1155/2012/732860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakaya T.A., Kita M., Kuriyama H., Iwakura Y., Imanishi J. Panax ginseng induces production of proinflammatory cytokines via toll-like receptor. J Interferon Cytokine Res. 2004;24:93–100. doi: 10.1089/107999004322813336. [DOI] [PubMed] [Google Scholar]
- 29.Vandanmagsar B., Youm Y.H., Ravussin A., Galgani J.E., Stadler K., Mynatt R.L., Ravussin E., Stephens J.M., Dixit V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen H., Gris D., Lei Y., Jha S., Zhang L., Huang M.T., Brickey W.J., Ting J.P. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feve B., Bastard J.P. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:305–311. doi: 10.1038/nrendo.2009.62. [DOI] [PubMed] [Google Scholar]
- 32.Henao-Mejia J., Elinav E., Thaiss C.A., Flavell R.A. Inflammasomes and metabolic disease. Annu Rev Physiol. 2014;76:57–78. doi: 10.1146/annurev-physiol-021113-170324. [DOI] [PubMed] [Google Scholar]
- 33.Konner A.C., Bruning J.C. Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol Metab. 2011;22:16–23. doi: 10.1016/j.tem.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Larsen C.M., Faulenbach M., Vaag A., Volund A., Ehses J.A., Seifert B., Mandrup-Poulsen T., Donath M.Y. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]