Abstract
Background
Black ginseng has a more potent biological activity than non-steamed ginseng. We investigated the effects of long-term intake of dietary black ginseng extract (BG) on antioxidant activity in aged mice. We also compared the effects of BG on cognitive deficits with those of white ginseng extract (WG) and red ginseng extract (RG).
Methods
Ten-month-old mice were fed an AIN-93G-based diet containing 10 g/kg (low dose, L) or 30 g/kg (high dose, H) WG powder, RG powder, or BG powder for 24 wk. We measured serum lipids, the activities of antioxidant enzymes, and malondialdehyde levels. Additionally, the protein expression levels of choline acetyltransferase and vesicular acetylcholine transporter, which are presynaptic cholinergic markers in the cortex and hippocampus of the brain, were measured by western blotting.
Results
Triglyceride levels were reduced in all the extract-treated mice, except those in the LBG group. High-density lipoprotein cholesterol levels in the HBG group were higher than those in the control group. Total cholesterol levels were reduced in the LBG group. Additionally, glucose levels in the HBG group were significantly reduced by 41.2%. There were lower levels of malondialdehyde in the LBG group than in the control group. Furthermore, glutathione reductase activity increased in the HWG group and the HRG group. The protein expression levels of choline acetyltransferase and vesicular acetylcholine transporter significantly increased in all the ginseng-treated groups.
Conclusion
The results suggest that supplementation with the tested ginseng extracts may suppress the cognitive decline associated with aging, via regulation of the cholinergic and antioxidant defense systems.
Keywords: acetylcholinesterase, aging, choline acetyltransferase, ginseng, vesicular acetylcholine transporter
1. Introduction
Aging is a natural process associated with gradual decline in biological function and increased incidence of degenerative diseases and mortality [1]. According to the free radical theory of aging, imbalance between the production of reactive oxygen species and antioxidant activity causes age-related functional deterioration [2], [3]. A major concern in research on aging and aging-related disorders has been the impact of oxidative stress and the damage it induces [4], [5]. In addition, age-related neurodegenerative diseases cause severe damage to the cholinergic system, resulting in cognitive and memory impairments [6]. Cortical cholinergic innervation has been widely studied because of its role in memory and learning [7]. A significant decrease in cholinergic markers such as choline acetyltransferase (ChAT) and acetylcholinesterase has been observed in Alzheimer's disease [8]. Therefore, it is very important to find materials that can inhibit or delay age-associated memory loss, and to investigate defense mechanisms that enhance healthy aging [9].
Panax ginseng Meyer, a traditional herbal medicine, is a tonic used to improve physiological functions and is a key ingredient in several herbal products [10], [11]. P. ginseng is classified as white, red, or black based on its color, which is caused by repetitive steaming. It has been reported that ginsenoside content in P. ginseng is affected by the steaming process [12]. Black ginseng has been reported to have more potent pharmacological activities than white ginseng and red ginseng do. These activities include radical scavenging, anticancer, cholinesterase inhibition, and anti-inflammatory activities, and are due to the new ginsenosides that are formed during the steaming process [13], [14], [15], [16]. Recently, many studies have indicated that treatment with ginseng or individual ginsenosides has a protective effect on oxidative damage and improves learning and memory impairment caused by aging [17], [18], [19]. Although various pharmacological effects of black ginseng have been studied extensively, studies on its effects on antioxidant activity and cholinergic markers in the brains of naturally aging mice have not been conducted. Therefore, this study was performed to compare serum lipid levels, antioxidant activity, and cholinergic markers in the brains of aged mice fed an experimental diet based on an AIN-93G formula containing 1% or 3% of white ginseng extract (WG), red ginseng extract (RG), or black ginseng extract (BG) for 24 wk.
2. Materials and methods
2.1. Sample preparation
White ginseng, red ginseng, and black ginseng were obtained from Daeduck Bio Corp. (Daejeon, Korea). To prepare the ginseng extracts, each type of ginseng was ground and then subjected to extraction in 80% ethanol for 1 h in a sonicator. This extraction step was repeated three times. The solution thus obtained was filtered, evaporated, and freeze-dried. The compositions of the ginsenosides Rg1, Re, Rf, Rb1, Rc, Rb2, Rd, and Rg3 in each ginseng extract were determined via HPLC as previously described [20]. Rg3 was not detected in WG, and Rg1 and Re were not detected in BG.
2.2. Animals and diet
Twelve-week-old male Imprinting Control Region mice were purchased from Dahan BioLink (Eumseong, Korea). The animals were housed (5 mice per cage) under controlled temperature (22 ± 2°C), humidity (50–55%), and a 12/12 h light/dark cycle (light on at 08:00 AM). They were also allowed free access to food and water for 6 mo. Ten-month-old mice were divided into seven groups (5 mice per group) and fed an experimental diet based on the AIN-93G formula, containing 10 g/kg (low dose, L) or 30 g/kg (high dose, H) of WG powder, RG powder, or BG powder for 24 wk (Table 1). Food intake and body weight were measured once weekly. The dosages of the extracts used in this study were based on those used in previous studies on the efficacy of chronic intake of dietary ginseng extract on obesity in rats. In these studies, no effect was observed with the use of RG at 2,000 mg/kg/d [21], [22]. All the experimental procedures used were approved by the Institutional Animal Care and Use Committee of Chungnam National University (CNU-00245; Daejeon, Korea).
Table 1.
Experimental diet compositions (g/kg)
| Ingredient | Control | Ginseng extract |
|
|---|---|---|---|
| 1% | 3% | ||
| Casein | 200 | 200 | 200 |
| Corn starch | 457 | 447 | 427 |
| Sucrose | 200 | 200 | 200 |
| Soybean oil | 43 | 43 | 43 |
| Cellulose | 50 | 50 | 50 |
| Choline bitartrate | 2 | 2 | 2 |
| L-Cysteine | 3 | 3 | 3 |
| t-Butylhydroquinone | 0.014 | 0.014 | 0.014 |
| Mineral mix1) | 35 | 35 | 35 |
| Vitamin mix2) | 10 | 10 | 10 |
| Ginseng extract powder3) | – | 10 | 30 |
| Total | 1000 | 1000 | 1000 |
AIN-93G mineral mixture
AIN-93G vitamin mixture
Ginseng extract powder: white, red, or black ginseng extract powder
2.3. Preparation of serum and brain tissue samples
Blood samples were collected by cardiac puncture and centrifuged to obtain serum. Whole brains were removed and the hippocampus and cortex were dissected from each left hemisphere. The right hemispheres were homogenized in a 10-fold buffer (12.5mM sodium phosphate buffer (pH 7.0) and 400mM NaCl) and centrifuged to obtain the supernatant for enzyme assay.
2.4. Biochemical measurements
Serum levels of glucose, triglycerides (TG), total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-C) were determined using commercial kits (Young-Dong Pharmaceutical, Seoul, Korea). Malondialdehyde (MDA) levels were measured using the thiobarbituric acid-reactive substances method described by Mihara and Uchiyama [23]. Superoxide dismutase and glutathione reductase (GR) levels in brain homogenates were determined using commercial kits (Nianjing Jiancheng Bioengineering Institute, Nanjing, China).
2.5. Western blot analysis
Proteins in the hippocampus and cortex were extracted using a lysis buffer (PRO-PREPTM; iNtRON Biotechnology, Seongnam, Korea). The extracted proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. The membrane was blocked with 5% skim milk for 1 h and immunoblotted with primary antibodies against ChAT, vesicular acetylcholine transporter (VAChT), and β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Immunoblotting was performed by incubating the membrane with horseradish peroxidase-conjugated secondary antibodies. Blots were developed using an enhanced chemiluminescence detection kit (WEST-ONE, iNtRON Biotechnology). ImageJ software (version 1.44p, National Institutes of Health, Bethesda, MD, USA) was used for densitometric analysis of the bands.
2.6. Statistical analysis
All data were analyzed using the SPSS statistical software package (version 20; IBM Corporation, Armonk, NY, USA). Differences between groups were analyzed using analysis of variance and Dunnett's multiple comparison test. A p value < 0.05 was considered statistically significant.
3. Results and discussion
3.1. Clinical observations
No death, significant abnormal behavior, or distinct clinical symptoms were observed in any of the groups during the experiment. The feed intake, body weight, and organ weights measured in the groups are presented in Table 2. The body weights of mice supplemented with dietary ginseng extract, as well as those of the control mice, were similarly maintained from the beginning to the end of the experiment. This indicates that chronic intake of dietary ginseng extract does not change appetite or cause malnutrition.
Table 2.
Body weight, food intake, and organ weights in aged mice fed white ginseng, red ginseng, and black ginseng for 24 wk
| Control | LWG | LRG | LBG | HWG | HRG | HBG | |
|---|---|---|---|---|---|---|---|
| Initial weight (g) | 44.90 ± 1.98 | 45.80 ± 1.51 | 47.10 ± 3.15 | 45.40 ± 3.52 | 45.90 ± 1.71 | 44.40 ± 2.77 | 46.80 ± 3.11 |
| Final weight (g) | 55.00 ± 5.00 | 54.00±0.56 | 55.40 ± 4.45 | 52.90 ± 4.45 | 58.20 ± 1.68 | 50.50 ± 5.24 | 56.0 ± 3.81 |
| Food intake (g/d) | 5.21 ± 1.03 | 6.04 ± 0.92 | 5.08 ± 0.41 | 5.81 ± 1.49 | 5.96 ± 1.21 | 6.06 ± 0.64 | 6.14 ± 0.82 |
| (mg/g b.w) | |||||||
| Liver | 32.96 ± 1.36 | 29.01 ± 2.23 | 30.67 ± 2.16 | 31.96 ± 1.69 | 31.29 ± 3.39 | 32.98 ± 1.21 | 29.58 ± 0.70 |
| Kidney | 12.70 ± 0.67 | 10.34 ± 0.73 | 10.22 ± 1.04 | 11.38 ± 1.65 | 10.64 ± 0.64 | 13.32 ± 1.13 | 11.02 ± 0.43 |
| Spleen | 2.54 ± 0.48 | 2.13 ± 0.20 | 2.13 ± 0.20 | 2.08 ± 0.23 | 2.20 ± 0.23 | 2.47 ± 0.22 | 2.32 ± 0.44 |
| Heart | 4.00 ± 0.14 | 3.96 ± 0.56 | 3.77 ± 0.20 | 3.92 ± 0.29 | 3.64 ± 0.35 | 4.49 ± 0.71 | 3.81 ± 0.22 |
| Brain | 9.30 ± 0.79 | 9.36 ± 0.82 | 9.10 ± 1.03 | 9.27 ± 1.04 | 8.64 ± 0.58 | 9.03 ± 0.98 | 8.93 ± 0.87 |
Values are expressed as means ± standard deviation (n = 5)
LWG, LRG, and LBG: 1% white ginseng extract-, red ginseng extract-, and black ginseng extract-treated group, respectively
HWG, HRG, and HBG: 3% white ginseng extract-, red ginseng extract-, and black ginseng extract-treated group, respectively
b.w, body weight
3.2. Serum parameters
Serum parameters, including TC, HDL-C, TG, and glucose levels are shown in Table 3. TC levels reduced by 26.5% in the LBG-treated group (95.00 ± 21.40 mg/dL) and were lower than those in the control group (129.33 ± 39.26 mg/dL) (p < 0.05). Furthermore, HDL-C levels were about 30.9% higher in the HBG group (63.45 ± 11.21 mg/dL) than they were in the control group (48.48 ± 3.75 mg/dL) (p < 0.01). All the ginseng-treated mice, except the LBG-treated mice, showed significant reductions in TG levels. The HBG group showed reduction in glucose levels by 41.2% (92.44 ± 9.88 mg/dL) as compared with the control group (157.25 ± 22.62 mg/dL) (p < 0.001). The antihypercholesterolemic properties of BG have been reported with regard to the regulation of markers of cholesterol metabolism, such as acetyl-CoA acetyltransferase 2, sterol regulatory element-binding protein 2, and 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, in rats fed a high-cholesterol diet [24]. In addition, long-term intake of P. ginseng extract in humans for over 8 wk showed the hypolipidemic potential of the extract, which was evidenced by decreases in TC, TG, low-density lipoprotein, and MDA levels, but an increase in HDL-C level [25]. It has also been reported that supplementation with RG improves two types of diabetes in animals and humans [26], [27]. Based on the above-mentioned studies, we assumed that the components of P. ginseng might be involved in lipid and glucose metabolism.
Table 3.
Changes in serum parameters of aged mice fed white ginseng, red ginseng, and black ginseng for 24 wk
| Control | LWG | LRG | LBG | HWG | HRG | HBG | |
|---|---|---|---|---|---|---|---|
| TC (mg/dL) | 129.33 ± 39.26 | 125.1 ± 8.80 | 101.8 ± 14.60 | 95.0 ± 21.40* | 116.9 ± 10.90 | 106.80 ± 6.80 | 127.92 ± 8.04 |
| HDL-C (mg/dL) | 48.48 ± 3.75 | 56.17 ± 6.15 | 52.21 ± 6.06 | 48.34 ± 5.95 | 56.21 ± 3.67 | 52.91 ± 5.15 | 63.45 ± 11.21** |
| TG (mg/dL) | 116.00 ± 21.95 | 87.73 ± 10.51* | 84.52 ± 8.25* | 107.45 ± 17.24 | 89.00 ± 16.30* | 75.43 ± 11.78** | 82.41 ± 14.60** |
| Glucose (mg/dL) | 157.25 ± 22.62 | 173.63 ± 32.99 | 128.70 ± 15.17 | 165.33 ± 19.09 | 182.53 ± 8.44 | 132.56 ± 20.65 | 92.44 ± 9.88*** |
Values are expressed as means ± standard deviation (n = 5)
LWG, LRG, and LBG: 1% white ginseng extract-, red ginseng extract-, and black ginseng extract-treated group, respectively
HWG, HRG, and HBG: 3% white ginseng extract-, red ginseng extract-, and black ginseng extract-treated group, respectively
*p < 0.05 versus the aged control group
**p < 0.01 versus the aged control group
***p < 0.001 versus the aged control group
HDL-C, high-density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride
3.3. Antioxidant enzyme activities
Oxidative-stress-induced changes in cellular organelles and macromolecules in many pathological conditions, including neurodegenerative diseases, are related with the aging process. The brain is especially susceptible to oxidative stress because of its relatively low level of antioxidants, high levels of polyunsaturated fatty acids, and increased need for oxygen [28]. In the present study, we determined the activities of antioxidant enzymes and MDA levels in the brains of aged mice. Several studies have shown that a significant increase in MDA levels occurs in the major organs of aged rats [29], [30]. As shown in Table 4, the mean MDA level in the LBG-treated group (2.70 ± 0.29 nmol/mg protein) was significantly lower than that in the control group (3.33 ± 0.23 nmol/mg protein). There were no significant differences in superoxide dismutase levels between the control and ginseng-treated groups. A significant increase in GR activity was observed in the HWG and HRG diet groups. Reduced glutathione (GSH) is capable of scavenging reactive oxygen and nitrogen species, thereby contributing to the control of redox homoeostasis [31]. GR is an essential enzyme that recycles oxidized GSH back to its reduced form and maintains a high intracellular GSH/glutathione disulfide ratio [32]. The increased GR activity in the brains of the mice was presumably due to the active ingredients in the ginseng extracts, which include ginsenosides, acidic polysaccharides, phenolic compounds, and polyacetylenes [33], [34].
Table 4.
Antioxidant enzyme activities in the brain of aged mice fed WG, RG, and BG for 24 wk
| Control | LWG | LRG | LBG | HWG | HRG | HBG | |
|---|---|---|---|---|---|---|---|
| MDA (nmol/mg protein) | 3.33 ± 0.23 | 2.93 ± 0.26 | 3.07 ± 0.29 | 2.70 ± 0.29* | 3.21 ± 0.36 | 3.06 ± 0.46 | 3.11 ± 0.32 |
| SOD (Unit/mg protein) | 45.43 ± 10.14 | 44.80 ± 2.99 | 47.92 ± 5.52 | 50.37 ± 10.96 | 56.06 ± 4.36 | 47.71 ± 5.45 | 55.04 ± 5.60 |
| GR (Unit/mg protein) | 11.51 ± 2.56 | 13.25 ± 2.04 | 16.10 ± 3.69 | 13.67 ± 1.54 | 19.60 ± 4.19* | 18.44 ± 7.10* | 15.25 ± 2.34 |
Values are expressed as means ± standard deviation (n = 5)
LWG, LRG, and LBG: 1% white ginseng extract-, red ginseng extract-, and black ginseng extract-treated group, respectively
HWG, HRG, and HBG: 3% white ginseng extract-, red ginseng extract-, and black ginseng extract diet-treated group, respectively
*p < 0.05 versus the aged control group
GR, glutathione reductase; MDA, malondialdehyde; SOD, superoxide dismutase
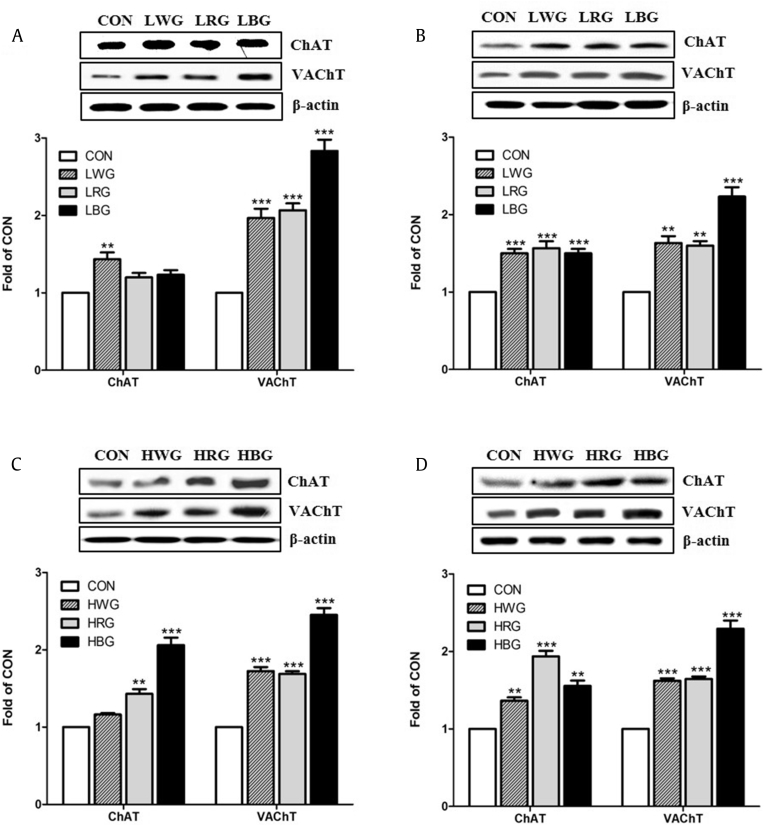
3.4. Protein expression levels of ChAT and VAChT
The expression levels of the cholinergic markers ChAT and VAChT in the brains of mice supplemented with ginseng extract for 24 wk are shown in Fig. 1. Western blot analysis showed that ChAT and VAChT protein levels were significantly increased in all the ginseng-treated groups. In addition, a significant increase of approximately two-fold in ChAT protein levels was observed in the cerebral cortex of the HBG-treated mice and in the hippocampus of the HRG-treated mice. At all dose levels, the expression of VAChT in the BG-treated groups increased by more than two-fold in the cortex and hippocampus. We observed that ChAT and VAChT protein expression in the ginseng-treated groups increased in a pattern consistent with that reported in a previous study [35]. ChAT catalyzes the synthesis of acetylcholine in the cytoplasm, whereas VAChT plays a role in the translocation of acetylcholine from the cytoplasm into synaptic vesicles [36]. Therefore, both proteins are specifically required for cholinergic neurotransmission. Kim et al. [37] demonstrated that treatment with ginsenosides Re and Rd significantly increased the expression of ChAT/VAChT genes in Neuro-2a cells. Ginsenosides Rg1 and Rb1 have been reported to have beneficial effects on learning and memory through their cholinergic activities by increasing ChAT expression and activity [38], [39]. In addition, ginsenosides Rg3 and Rg5 have been shown to reverse scopolamine-induced memory dysfunction, which is characterized by reduced central cholinergic activity and impaired learning and memory [40], [41]. Our results suggest that dietary ginseng extract might have a cognitive-enhancing effect in aged mice via regulation of the cholinergic system.
Fig. 1.
Western blot analysis of the cholinergic marker proteins, ChAT and VAChT, in the (A, C) cerebral cortex and (B, D) hippocampus of aged mice fed 1% or 3% ginseng extract for 24 wk. LWG, LRG, and LBG represent low-dose (1%) white ginseng extract, red ginseng extract, and black ginseng extract, respectively. HWG, HRG, and HBG represent high-dose (3%) white ginseng extract, red ginseng extract, and black ginseng extract, respectively.*indicates p < 0.05.**indicates p < 0.01.***indicates p < 0.001 when compared with the control group. ChAT, choline acetyltransferase; CON, control; VAChT, vesicular acetylcholine transporter.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgments
This work was supported by the grant (K16281) awarded to the Korea Institute of Oriental Medicine (KIOM) from the Ministry of Education, Science and Technology, Korea.
References
- 1.Osiewacz H.D., Hamann A. DNA reorganization and biological aging. A review. Biochemistry (Mosc) 1997;62:1275–1284. [PubMed] [Google Scholar]
- 2.Seo A.Y., Joseph A.M., Dutta D., Hwang J.C., Aris J.P., Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci. 2010;123:2533–2542. doi: 10.1242/jcs.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 4.Berry A., Greco A., Giorgio M., Pelicci P.G., de Kloet R., Alleva E., Minghetti L., Cirulli F. Deletion of the lifespan determinant p66(Shc) improves performance in a spatial memory task, decreases levels of oxidative stress markers in the hippocampus and increases levels of the neurotrophin BDNF in adult mice. Exp Gerontol. 2008;43:200–208. doi: 10.1016/j.exger.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Duffy K.B., Spangler E.L., Devan B.D., Guo Z., Bowker J.L., Janas A.M., Hagepanos A., Minor R.K., DeCabo R., Mouton P.R. A blueberry-enriched diet provides cellular protection against oxidative stress and reduces a kainate-induced learning impairment in rats. Neurobiol Aging. 2008;29:1680–1689. doi: 10.1016/j.neurobiolaging.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Bourin M., Ripoll N., Dailly E. Nicotinic receptors and Alzheimer's disease. Curr Med Res Opin. 2003;19:169–177. doi: 10.1185/030079903125001631. [DOI] [PubMed] [Google Scholar]
- 7.Voytko M.L., Olton D.S., Richardson R.T., Gorman L.K., Tobin J.R., Price D.L. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J Neurosci. 1994;14:167–186. doi: 10.1523/JNEUROSCI.14-01-00167.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeKosky S.T., Harbaugh R.E., Schmitt F.A., Bakay R.A., Chui H.C., Knopman D.S., Reeder T.M., Shetter A.G., Senter H.J., Markesbery W.R. Cortical biopsy in Alzheimer's disease: diagnostic accuracy and neurochemical, neuropathological, and cognitive correlations. Intraventricular bethanecol study group. Ann Neurol. 1992;32:625–632. doi: 10.1002/ana.410320505. [DOI] [PubMed] [Google Scholar]
- 9.Shukitt-Hale B., Lau F.C., Joseph J.A. Berry fruit supplementation and the aging brain. J Agric Food Chem. 2008;56:636–641. doi: 10.1021/jf072505f. [DOI] [PubMed] [Google Scholar]
- 10.Gillis C.N. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 11.O'Hara M., Kiefer D., Farrell K., Kemper K. A review of 12 commonly used medicinal herbs. Arch Fam Med. 1998;7:523–536. doi: 10.1001/archfami.7.6.523. [DOI] [PubMed] [Google Scholar]
- 12.Sun B.S., Gu L.J., Fang Z.M., Wang C.Y., Wang Z., Lee M.R., Li Z., Li J.J., Sung C.K. Simultaneous quantification of 19 ginsenosides in black ginseng developed from Panax ginseng by HPLC-ELSD. J Pharm Biomed Anal. 2009;15:15–22. doi: 10.1016/j.jpba.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Wan J.Y., Fan Y., Yu Q.T., Ge Y.Z., Yan C.P., Alolga R.N., Li P., Ma Z.H., Qi L.W. Integrated evaluation of malonyl ginsenosides, amino acids and polysaccharides in fresh and processed ginseng. J Pharm Biomed Anal. 2015;107:89–97. doi: 10.1016/j.jpba.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Kang K.S., Kim H.Y., Pyo J.S., Yokozawa T. Increase in the free radical scavenging activity of ginseng by heat-processing. Biol Pharm Bull. 2006;29:750–754. doi: 10.1248/bpb.29.750. [DOI] [PubMed] [Google Scholar]
- 15.Kim S.J., Kim A.K. Anti-breast cancer activity of fine black ginseng (Panax ginseng Meyer) and ginsenoside Rg5. J Ginseng Res. 2015;39:125–134. doi: 10.1016/j.jgr.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee M.R., Yun B.S., In O.H., Sung C.K. Comparative study of Korean white, red, and black ginseng extract on cholinesterase inhibitory activity and cholinergic function. J Ginseng Res. 2011;35:421–428. doi: 10.5142/jgr.2011.35.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y., Oh S. Administration of red ginseng ameliorates memory decline in aged mice. J Ginseng Res. 2015;39:250–256. doi: 10.1016/j.jgr.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H.F., Li Q., Li Y. Long-term ginsenoside administration prevents memory loss in aged female C57BL/6J mice by modulating the redox status and up-regulating the plasticity-related proteins in hippocampus. Neuroscience. 2011;183:189–202. doi: 10.1016/j.neuroscience.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 19.Heo J.H., Lee S.T., Oh M.J., Park H.J., Shim J.Y., Chu K., Kim M. Improvement of cognitive deficit in Alzheimer's disease patients by long term treatment with Korean red ginseng. J Ginseng Res. 2011;35:457–461. doi: 10.5142/jgr.2011.35.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee M.R., Yun B.S., Liu L., Zhang D.L., Wang Z., Wang C.L., Gu L.J., Wang C.Y., Mo E.K., Sung C.K. Effect of black ginseng on memory improvement in the amnesic mice induced by scopolamine. J Ginseng Res. 2010;34:51–58. [Google Scholar]
- 21.Jung S., Lee M.S., Shin Y., Kim C.T., Kim I.H., Kim Y. High hydrostatic pressure extract of red ginseng attenuates inflammation in rats with high-fat diet induced obesity. Prev Nutr Food Sci. 2015;20:253–259. doi: 10.3746/pnf.2015.20.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park S.J., Lim K.H., Noh J.H., Jeong E.J., Kim Y.S., Han B.C., Lee S.H., Moon K.S. Subacute oral toxicity study of Korean red ginseng extract in Sprague-Dawley rats. Toxicol Res. 2013;29:285–292. doi: 10.5487/TR.2013.29.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mihara M., Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 24.Saba E., Jeon B.R., Jeong D.H., Lee K., Goo Y.K., Kim S.H., Sung C.K., Roh S.S., Kim S.D., Kim H.K. Black ginseng extract ameliorates hypercholesterolemia in rats. J Ginseng Res. 2016;40:160–168. doi: 10.1016/j.jgr.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S.H., Park K.S. Effects of Panax ginseng extract on lipid metabolism in humans. Pharmacol Res. 2003;48:511–513. doi: 10.1016/s1043-6618(03)00189-0. [DOI] [PubMed] [Google Scholar]
- 26.Hong B.N., Ji M.G., Kang T.H. The efficacy of red ginseng in type 1 and type 2 diabetes in animals. Evid Based Complement Alternat Med. 2013;2013:593181. doi: 10.1155/2013/593181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vuksan V., Sung M.K., Sievenpiper J.L., Stavro P.M., Jenkins A.L., Di Buono M., Lee K.S., Leiter L.A., Nam K.Y., Arnason J.T. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis. 2008;18:46–56. doi: 10.1016/j.numecd.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Floyd R.A., Carney J.M. Free radical damage to protein and DNA: Mechanism involved and relevant observations on brain undergoing oxidative stress. Ann Neurol. 1992;32:S22–S27. doi: 10.1002/ana.410320706. [DOI] [PubMed] [Google Scholar]
- 29.Arivazhagan P., Thilakavathy T., Panneerselvam C. Antioxidant lipoate and tissue antioxidants in aged rats. J Nutr Biochem. 2000;13:122–127. doi: 10.1016/s0955-2863(99)00079-0. [DOI] [PubMed] [Google Scholar]
- 30.Ramesh T., Kim S.W., Sung J.H., Hwang S.Y., Sohn S.H., Yoo S.K., Kim S.K. Effect of fermented Panax ginseng extract (GINST) on oxidative stress and antioxidant activities in major organs of aged rats. Exp Gerontol. 2012;47:77–84. doi: 10.1016/j.exger.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Couto N., Wood J., Barber J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic Biol Med. 2016;95:27–42. doi: 10.1016/j.freeradbiomed.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 32.Foyer C.H., Theodoulou F.L., Delrot S. The functions of inter- and intracellular glutathione transport systems in plants. Trends Plant Sci. 2001;6:486–492. doi: 10.1016/s1360-1385(01)02086-6. [DOI] [PubMed] [Google Scholar]
- 33.Kim W.Y., Kim J.M., Han S.B., Lee S.K., Kim N.D., Park M.K., Kim C.K., Park J.H. Steaming of ginseng at high temperature enhances biological activity. J Nat Prod. 2000;63:1702–1704. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- 34.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 35.Gilmor M.L., Counts S.E., Wiley R.G., Levey A.I. Coordinate expression of the vesicular acetylcholine transporter and choline acetyltransferase following septohippocampal pathway lesions. J Neurochem. 1998;71:2411–2420. doi: 10.1046/j.1471-4159.1998.71062411.x. [DOI] [PubMed] [Google Scholar]
- 36.Oda Y. Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathol Int. 1999;49:921–937. doi: 10.1046/j.1440-1827.1999.00977.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim M.S., Yu J.M., Kim H.J., Kim H.B., Kim S.T., Jang S.K., Choi Y.W., Lee D.I., Joo S.S. Ginsenoside Re and Rd enhance the expression of cholinergic markers and neuronal differentiation in Neuro-2a cells. Biol Pharm Bull. 2014;37:826–833. doi: 10.1248/bpb.b14-00011. [DOI] [PubMed] [Google Scholar]
- 38.Salim K.N., McEwen B.S., Chao H.M. Ginsenoside Rb1 regulates ChAT, NGF and trkA mRNA expression in the rat brain. Brain Res Mol Brain Res. 1997;47:177–182. doi: 10.1016/s0169-328x(97)00042-9. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J.T., Liu Y., Qu Z.W., Zhang X.L., Xiao H.L. Influence of ginsenoside Rb1 and Rg1 on some central neurotransmitter receptors and protein biosynthesis in the mouse brain. Yao Xue Xue Bao. 1988;23:12–16. [PubMed] [Google Scholar]
- 40.Kim E.J., Jung I.H., Le Van T.K., Jeong J.J., Kim N.J., Kim D.H. Ginsenosides Rg5 and Rh3 protect scopolamine-induced memory deficits in mice. J Ethnopharmacol. 2013;146:294–299. doi: 10.1016/j.jep.2012.12.047. [DOI] [PubMed] [Google Scholar]
- 41.Bao H.Y., Zhang J., Yeo S.J., Myung C.S., Kim H.M., Kim J.M., Park J.H., Cho J.S., Kang J.S. Memory enhancing and neuroprotective effects of selected ginsenosides. Arch Pharm Res. 2005;28:335–342. doi: 10.1007/BF02977802. [DOI] [PubMed] [Google Scholar]