Abstract
Background
Korean Red Ginseng has been used for several decades to treat many diseases, enhancing both immunity and physical strength. Previous studies have documented the therapeutic effects of ginseng, including its anticancer, antiaging, and anti-inflammatory activities. These activities are mediated by ginsenosides present in the ginseng plant. Ginsenoside Rg3, an effective compound from red ginseng, has been shown to have antiplatelet activity in addition to its anticancer and anti-inflammatory activities. Platelets are important for both primary hemostasis and the repair of the vessels after injury; however, they also play a crucial role in the development of acute coronary diseases. We prepared ginsenoside Rg3-enriched red ginseng extract (Rg3-RGE) to examine its role in platelet physiology.
Methods
To examine the effect of Rg3-RGE on platelet activation in vitro, platelet aggregation, granule secretion, intracellular calcium ([Ca2+]i) mobilization, flow cytometry, and immunoblot analysis were carried out using rat platelets. To examine the effect of Rg3-RGE on platelet activation in vivo, a collagen plus epinephrine-induced acute pulmonary thromboembolism mouse model was used.
Results
We found that Rg3-RGE significantly inhibited collagen-induced platelet aggregation and [Ca2+]i mobilization in a dose-dependent manner in addition to reducing ATP release from collagen-stimulated platelets. Furthermore, using immunoblot analysis, we found that Rg3-RGE markedly suppressed mitogen-activated protein kinase phosphorylation (i.e., extracellular stimuli-responsive kinase, Jun N-terminal kinase, p38) as well as the PI3K (phosphatidylinositol 3 kinase)/Akt pathway. Moreover, Rg3-RGE effectively reduced collagen plus epinephrine-induced mortality in mice.
Conclusion
These data suggest that ginsenoside Rg3-RGE could be potentially be used as an antiplatelet therapeutic agent against platelet-mediated cardiovascular disorders.
Keywords: collagen, ginsenoside Rg3-RGE, Panax ginseng, platelet aggregation
1. Introduction
Cardiovascular diseases (CVDs), such as thrombosis, atherosclerosis, and myocardial infarction, are the primary causes of morbidity and mortality in Western countries. Platelets, derived from megakaryocytes as anucleate cells, circulate in the bloodstream for approximately 10 d and are crucial for primary hemostasis and prevention of posttraumatic blood loss. When blood vessels are damaged, agonists bind their cognate platelet membrane G-protein-coupled receptors (GPCR), such as GPVI (glycoprotein VI), αIIbβ3, and GPIb, leading to platelet activation and a reduction of Ca2+ mobilization. Several paracrine and autocrine mediators, such as adenosine diphosphate (ADP), thromboxane A2, epinephrine, and thrombin, initiate platelet activation. However, in thrombotic diseases, such as atherosclerosis, abnormal platelet activation can result in thrombus formation [1], [2], [3], [4]. At present, the drugs available for treatment of thrombotic diseases have serious side effects, such as bleeding [5], leading to a need for a safe and more effective antithrombotic agent.
Panax ginseng has been used for more than 2,000 yr to treat different ailments and enhance immunity. Ginseng contains numerous active compounds, including ginsenosides, peptides, polysaccharides, mineral oils, and fatty acids [6]. Recently, several studies have focused on single ginsenosides. Among them, ginsenoside Rg3 is well known for the treatment of numerous diseases, such as hypertension, diabetes mellitus, and breast cancer [7], [8], [9]. Ginsenoside Rg3's two enantiomers, ginsenosides 20(R)-Rg3 and 20(S)-Rg3, vary in the spatial arrangement of the OH¯ on the chiral center at carbon 20; the S form generally has a much higher antioxidant activity compared with the R form [10].
In our previous studies, we showed that dihydroginsenoside Rg3 mediates antiplatelet activity via ERK (extracellular stimuli-responsive kinase) signaling [11]; however, the antiplatelet activity of Rg3-enriched red ginseng extract (Rg3-RGE) remained unknown. In this study, we characterized the modulatory activity of Rg3-RGE on platelet function.
2. Materials and methods
2.1. Chemicals
Rg3-RGE was obtained from Korea Ginseng Corporation (Daejeon, Korea). In brief, red ginseng (stem/root = 75:25) was extracted with water followed by extraction with 55% ethanol. A concentrated extract was prepared by multiple extractions. Consequently, the extract was subjected to GC-MS and the resulting extract constituent profile is shown in Table 1, which confirms that the extract is Rg3-enriched red ginseng extract. Collagen was obtained from Chrono-Log Co. (Havertown, PA, USA). Fura-2/AM and dimethyl sulfoxide were purchased from Sigma Chemical Co. (St. Louis, MO, USA). ATP (adenosine triphosphate) assay kits were obtained from Biomedical Research Service Center (Buffalo, NY, USA). Fibrinogen Alexa Fluor 488 conjugate was purchased from Molecular Probes (Eugene, OR, USA). Antibodies directed against total-ERK1/2 (p44/42), phospho-ERK1/2 (p44/42), total-JNK, phospho-JNK, total-p38, phosphor-p38, total-PI3K (phosphatidylinositol 3 kinase) p85/p55, phospho-PI3K p85/p55, total-Akt (Ser473), phospho-Akt (Ser473), total-MKK4 (mitogen-activated protein kinase kinase 4), and phospho-MKK4 were purchased from Cell Signaling (Beverly, MA, USA). All chemicals used were of reagent grade.
Table 1.
Rg3-enriched red ginseng extract constituent profile
| Ginsenosides | Contents (mg/g) |
|---|---|
| Rb1 | 3.86 |
| 20(S)-Rg3 | 44.91 |
| Rc | 1.20 |
| Rb2 | 1.53 |
| Rd | 1.60 |
| Rf | 1.28 |
| Rh1 | 3.71 |
| 20(S)-Rg2 | 3.55 |
| 20(R)-Rg3 | 6.78 |
| Total | 67.41 |
2.2. Animals
Male Sprague–Dawley rats (8 wk old) and male C57BL/6J mice (8 wk old) were purchased from Orient Co. (Busan, Korea). Rats and mice were housed in an air-conditioned animal room with a light/dark cycle of 12/12 h at a temperature of 22 ± 2°C and humidity of 50 ± 10%, and were acclimated to the environment 1 wk prior to experiment initiation. All animal-related experiments were performed according to the guidelines of the National Institute of Health and were approved by the Ethics Committee of the College of Veterinary Medicine, Kyungpook National University (Daegu, Korea).
2.3. Platelet preparation
Platelet preparation was conducted at room temperature. After collection, blood was transferred to an anticoagulant citrate dextrose solution (21mM citric acid, 85mM trisodium citrate, and 83mM dextrose) and centrifuged at 170g for 7 min to isolate platelet-rich plasma (PRP). Platelets were then isolated from PRP by centrifugation at 350g for 7 min. Platelet concentration was adjusted to 3 × 108/mL with Tyrode buffer (137mM NaCl, 12mM NaHCO3, 5.5mM glucose, 2mM KCl, 1mM MgCl2, and 0.3mM NaHPO4, pH 7.4).
2.4. Platelet aggregation assay
Platelet aggregation was measured using light transmission aggregometry (Chrono-Log Co.) as previously described [12]. Washed platelets were pretreated with Rg3-RGE, or vehicle control, at 37°C for 2 min in the presence of 1mM CaCl2 followed by the induction of aggregation with collagen. The mixture was further incubated with constant stirring at 1,200 rpm for 5 min and observed for aggregation.
2.5. Scanning electron microscopy analysis
A field emission scanning electron microscope (SU8220; Hitachi, Kanagawa, Japan) was used for aggregated platelet analysis. After the platelet aggregation assay, platelets were fixed with 0.5% paraformaldehyde (1st fixation) and washed three times with phosphate-buffered sulfate, and then fixed with osmium tetroxide (2nd fixation). Samples were then dehydrated using various concentrations of ethanol, then freeze-dried and analyzed using electron microscopy.
2.6. Intracellular calcium measurement
PRP was isolated from blood by centrifugation at 170g for 7 min and loaded with 5μM Fura-2/AM for 1 h at 37°C. Fluorescently labeled platelets were then pretreated with Rg3-RGE for 2 min at 37°C and stimulated with agonist for 5 min. Fluorescence was recorded with a fluorescence spectrofluorometer (F-2500; Hitachi).
2.7. ATP and serotonin release assay
The platelet aggregate supernatant was isolated from the platelet aggregate mixture by centrifugation at 12,000 rpm for 5 min at 4°C. ATP secretion was measured with an ATP assay kit (Biomedical Research Service Center) using a luminometer (GloMax20/20; Promega, Madison, WI, USA), and serotonin release was measured with a serotonin ELISA kit (Labor Diagnostika Nord GmbH & Co., Nordhorn, Germany) according to manufacturer's instructions.
2.8. Flow cytometry
Fibrinogen binding and P-selectin expression was measured using flow cytometry as previously described [13]. Briefly, washed platelets were pretreated with Rg3-RGE and stimulated with agonist for 5 min in the presence of anti-CD62P (P-selectin) and fluorescein isothiocyanate-labeled (FITC) antifibrinogen antibody. Platelets were then fixed with 0.5% paraformaldehyde. Flow cytometric analysis was performed using the FACS Aria III flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA), and data were analyzed using CellQuest software (Becton Dickinson Immunocytometry Systems).
2.9. Immunoblotting
After terminating platelet aggregation, lysis buffer (PRO-PREP; iNtRON Biotechnology, Seoul, Korea) was added to the mixture. A BCS assay (PRO-MEASURE; iNtRON Biotechnology) was used to measure protein concentration. Total platelet protein was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes. PVDF membranes were then incubated with primary and secondary antibodies conjugated to horseradish peroxidase, and antibody binding was visualized using enhanced chemiluminescence (Advansta Inc., Menlo Park, CA, USA).
2.10. Acute pulmonary thromboembolism assay
The collagen plus epinephrine-induced acute pulmonary thromboembolism (APT) study was carried out in mice as previously described [14]. Briefly, mice were divided into three groups (n = 10), saline-treated and unchallenged, saline-treated and collagen plus epinephrine-challenged, and Rg3-RGE-treated and collagen plus epinephrine-challenged. Rg3-RGE treatment was administered by oral injection 3 d prior to collagen plus epinephrine challenge. Two hours after the final Rg3-RGE dose, mice were then challenged with 0.1 mL of a mixture containing 500 μg/mL of collagen and 60 μg/mL of epinephrine by intravenous tail vein injection. Mouse mortality was monitored for a duration of 15 min, and data represent the percentage of mice surviving in the respective treatment group. At the end of each experimental session, the surviving mice were euthanized using an overdose of anesthesia.
2.11. Lung histology
Two minutes after thrombotic challenge, mice were quickly euthanized using an overdose of anesthesia, and lungs were perfused with a fixing solution (10% formalin buffered with calcium carbonate). The trachea was ligated and harvested together with the lungs, and the organs were soaked in cold saline and fixed in 10% formalin for 24 h. The lungs were then embedded in paraffin, and sections (5–6 μm thick) were cut and stained with hematoxylin and eosin for thrombus identification. The samples were observed under a light microscope (Axio LAb A1; Carl Zeiss MicroImaging, Jena, Germany) by a blinded histopathologist, where a minimum of 10 fields were observed for every specimen. The magnification used was 100×.
2.12. Statistical analysis
Statistical significance was measured using one–way analysis of variance followed by Dunnett's post hoc test (SAS Institute Inc., Cary, NC, USA). The data are presented as mean ± standard deviation. A p value ≤ 0.05 was considered statistically significant.
3. Results
3.1. Effect of Rg3-RGE on collagen-induced platelet aggregation
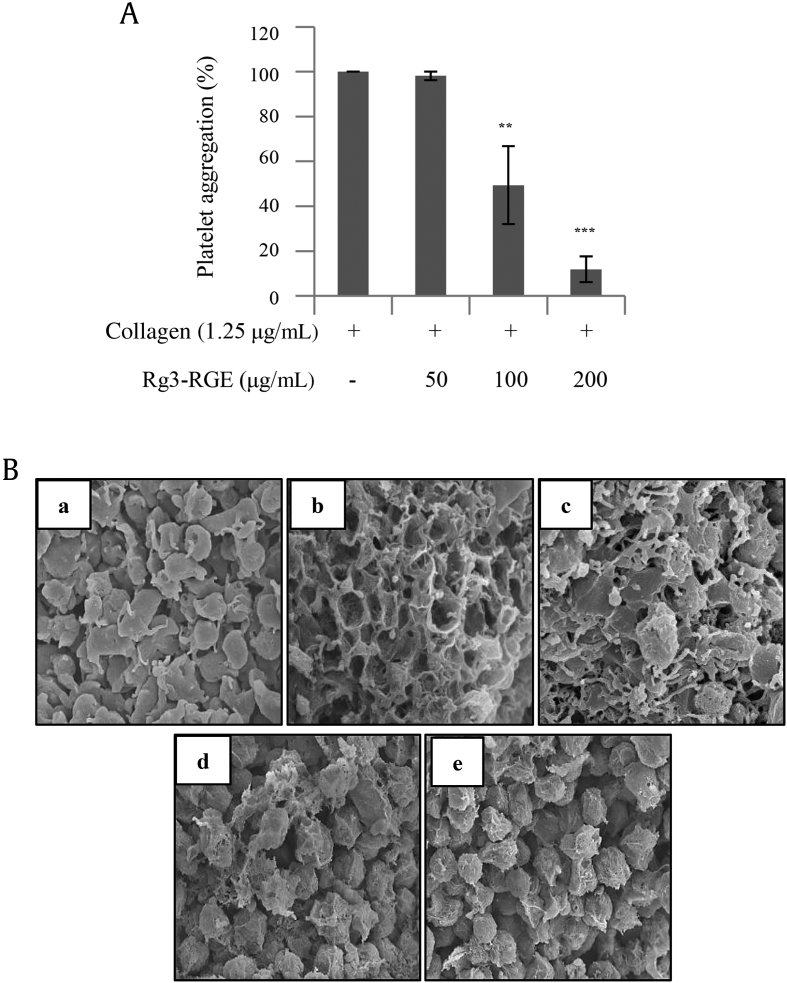
To determine whether platelet aggregation is inhibited by Rg3-RGE, we used a collagen-induced platelet aggregation assay. Using light transmission aggregometry, we found that Rg3-RGE treatment strongly inhibited collagen-induced platelet aggregation in a dose-dependent manner (Fig. 1A). Furthermore, scanning electron microscopy confirmed our findings, showing a clear dose-dependent decrease in aggregation (Fig. 1B).
Fig. 1.
Rg3-RGE inhibits collagen-induced platelet aggregation. (A) Washed platelets were pretreated with Rg3-RGE or vehicle for 2 min in the presence of 1mM CaCl2, and stimulated with collagen for 5 min. (B) After the platelet aggregation reaction, scanning electron microscopy was performed. Representative scanning electron microscopy images of platelets with (a) no treatment, (b) treated with collagen 1.25 μg/mL, (c) treated with Rg3-RGE 50 μg/mL + collagen 1.25 μg/mL, (d) treated with 100 μg/mL Rg3-RGE + collagen 1.25 μg/mL, and (e) treated with 200 μg/mL Rg3-RGE + collagen 1.25 μg/mL. Graph represents the mean ± SD of at least four independent experiments. **p < 0.01, ***p < 0.001 compared to the agonist control. Rg3-RGE, Rg3-enriched red ginseng extract; SD, standard deviation.
3.2. Effect of Rg3-RGE on intracellular calcium mobilization
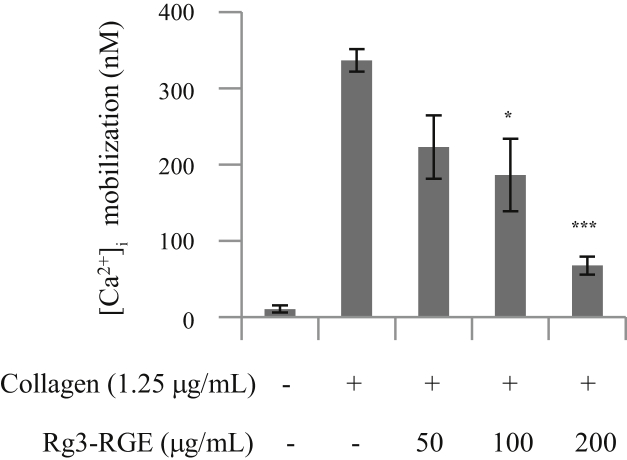
Calcium mobilization is crucial for platelet activation and the elevation of intracellular calcium ([Ca2+]i) levels triggers multiple signaling events. To determine whether Rg3-RGE reduces the collagen-induced increase of [Ca2+]i, we used a fluorescent Ca2+ indicator, Fura-2/AM. We found that Rg3-RGE treatment markedly decreased collagen-induced [Ca2+]i in a dose-dependent manner (Fig. 2), suggesting that Rg3-RGE inhibits platelet aggregation by blocking calcium mobilization.
Fig. 2.
The inhibitory effect of Rg3-RGE on collagen-induced [Ca2+]i increase. Washed platelets were loaded with a calcium fluorophore (5μM, Fura-2/AM) for 1 h. Fura 2/AM-loaded platelets were pretreated with Rg3-RGE for 2 min at 37°C and stimulated with collagen (1.25 μg/mL). The results represent the mean ± SD of at least four independent experiments. *p < 0.05 and ***p < 0.001 versus control. [Ca2+]i, intracellular calcium; Rg3-RGE, Rg3-enriched red ginseng extract; SD, standard deviation.
3.3. Effect of Rg3-RGE on granule secretion
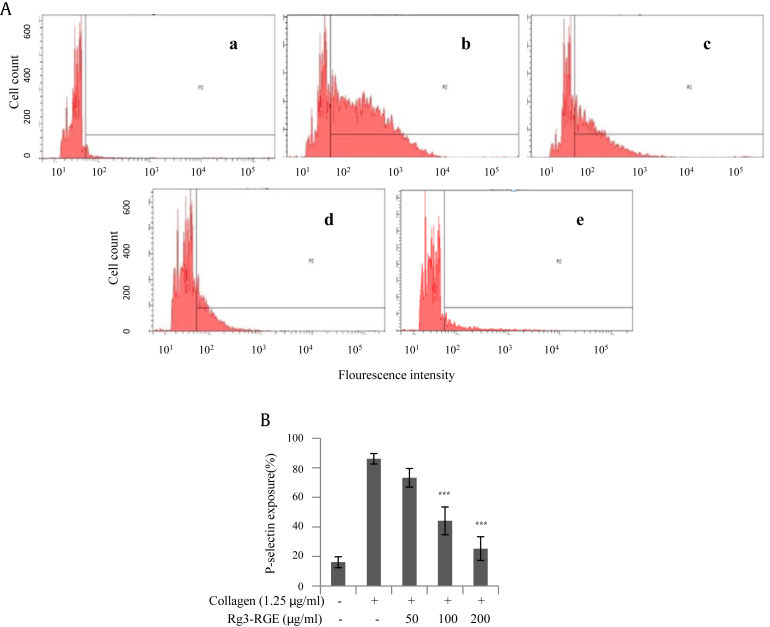
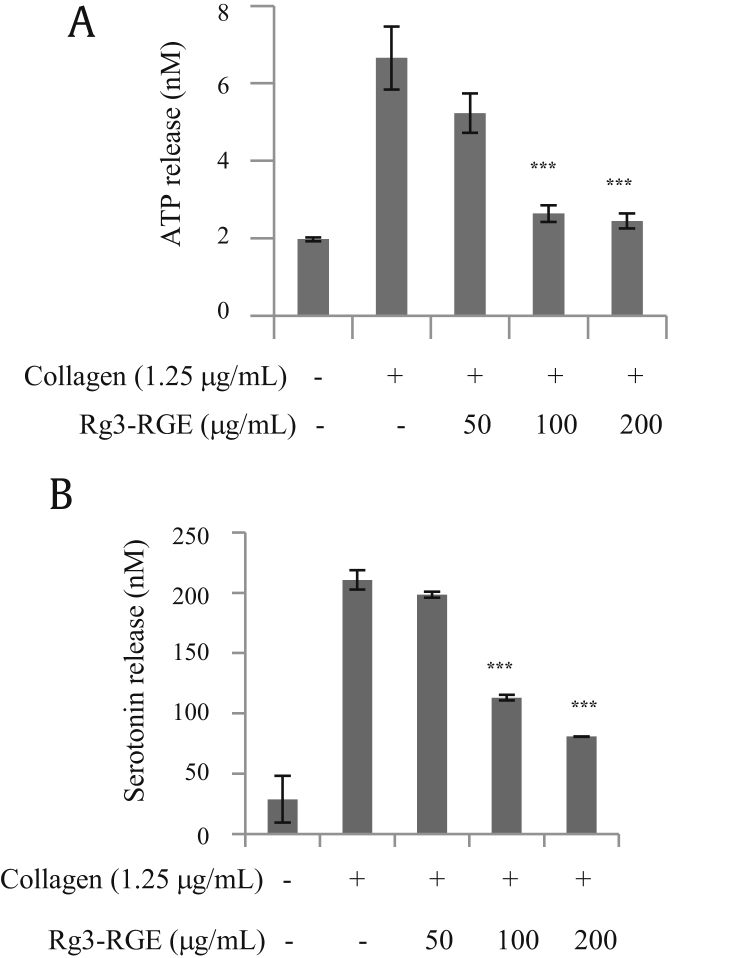
Platelets contain both α and dense granules that store factors that are important for hemostasis when released via platelet activation. To determine whether Rg3-RGE inhibits α granule secretion, we examined P-selectin expression by flow cytometry. Rg3-RGE inhibited P-selectin expression in a dose-dependent manner (Fig. 3). To determine whether Rg3-RGE also inhibited collagen-stimulated dense granules secretion, we measured ATP and serotonin release. Collagen (1.25 μg/mL) treatment alone strongly increased ATP and serotonin secretion from dense granules; however, platelets pretreated with Rg3-RGE had significantly decreased ATP and serotonin secretion in a dose-dependent manner (Fig. 4).
Fig. 3.
Rg3-RGE-mediated decrease in secretion of α granules. After platelet aggregation was completed, the platelets were incubated with anti-CD62P antibody for P-selectin and flow cytometry was performed using the FACS Aria III flow cytometer. (A) Representative flow cytometric analysis of five treatment groups (a) no treatment, (b) collagen 1.25 μg/mL, (c) Rg3-RGE 50 μg/mL + collagen 1.25 μg/mL, (d) 100 μg/mL Rg3-RGE + collagen 1.25 μg/mL, and (e) 200 μg/mL Rg3-RGE + collagen 1.25 μg/mL. (B) Bar graph summarizing the inhibitory effect of Rg3-RGE on P-selectin exposure. ***p < 0.001 versus control. Rg3-RGE, Rg3-enriched red ginseng extract.
Fig. 4.
Rg3-RGE-mediated decrease in secretion of dense granules. After platelet aggregation was terminated, the concentration of ATP and serotonin was measured in the supernatant. (A) The concentration of ATP in collagen-stimulated platelets treated with Rg3-RGE was measured using a luminometer. (B) Levels of serotonin from platelets stimulated with collagen and treated with Rg3-RGE were measured using ELISA. ***p < 0.001 versus control. ATP, adenosine triphosphate; ELISA, enzyme-linked immunosorbent assay; Rg3-RGE, Rg3-enriched red ginseng extract.
3.4. Effect of Rg3-RGE on activation of integrin αIIbβ3
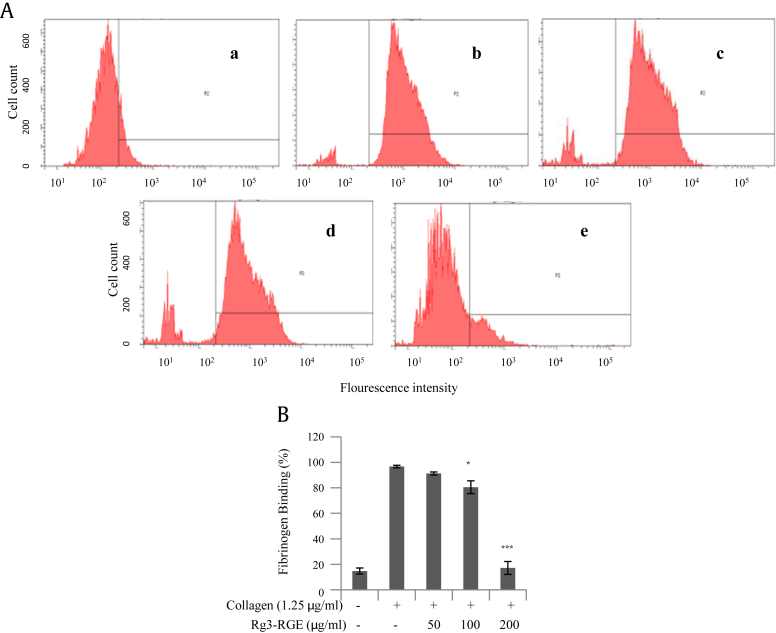
Platelet activation leads to a conformational change in integrin αIIbβ3, fibrinogen binding, and aggregation [15]. To determine whether Rg3-RGE inhibits αIIbβ3 activation and fibrinogen binding, flow cytometry was used to analyze platelets preincubated with various concentrations of Rg3-RGE and FITC-conjugated antifibrinogen antibody. Rg3-RGE inhibited fibrinogen binding in a dose-dependent manner, blocking the process of inside-out signaling (Fig. 5).
Fig. 5.
Rg3-RGE blocked fibrinogen binding to integrin αIIbβ3 in collagen-stimulated platelets. (A) Flow cytometry was used to measure fibrinogen binding to platelets after (a) no treatment, (b) treatment with collagen 1.25 μg/mL, (c) treatment with Rg3-RGE 50 μg/mL + collagen 1.25 μg/mL, (d) treatment with 100 μg/mL Rg3-RGE + collagen 1.25 μg/mL, and (e) treatment with 200 μg/mL Rg3-RGE + collagen 1.25 μg/mL. (B) Bar graph summarizing the flow cytometry data on the inhibitory effect of Rg3-RGE on fibrinogen binding. * p < 0.05 and ***p < 0.001 versus control. Rg3-RGE, Rg3-enriched red ginseng extract.
3.5. Effect of Rg3-RGE on mitogen-activated protein kinase and MKK4 phosphorylation
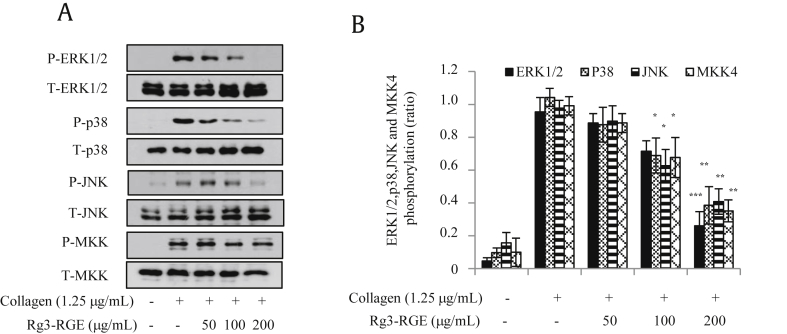
Collagen stimulated rat platelet mitogen-activated protein kinases (MAPKs) include c-Jun N-terminal kinase (JNK), extracellular stimuli-responsive kinase 1/2 (ERK1/2), and p38- [16], [17], [18]. To elucidate the underlying mechanism by which Rg3-RGE inhibits platelet aggregation, we examined the phosphorylation of downstream intracellular signaling molecules including MAPKs and mitogen-activated protein kinase kinase 4 (MKK4). We found that Rg3-RGE decreased phosphorylation of ERK1/2, JNK, and p38 (Fig. 6). In addition, Rg3-RGE also inhibited the phosphorylation of MKK4, an upstream signaling molecule of JNK (Fig. 6).
Fig. 6.
Effect of Rg3-RGE on MAPK phosphorylation in collagen-activated platelets. Washed platelets were pretreated with Rg3-RGE and stimulated with collagen. After the reaction was terminated, protein was extracted from platelets and analyzed for the phosphorylation of MAPKs by immunoblot. Immunoblot images are representative of at least three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to the agonist-treated group. ERK1/2, extracellular stimuli-responsive kinase 1/2; JNK, Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MKK4, mitogen-activated protein kinase kinase 4; RGE, Rg3-enriched red ginseng extract.
3.6. Effect of Rg3-RGE on PI3K–Akt phosphorylation
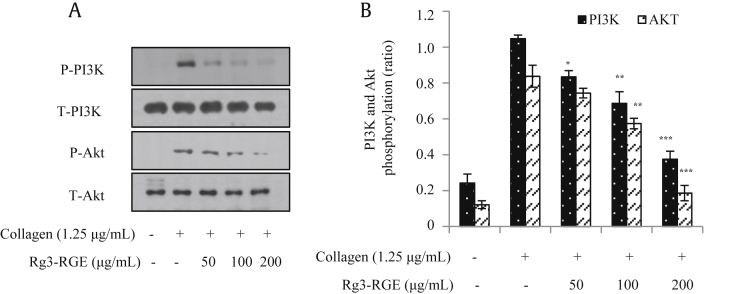
The PI3K/Akt pathway plays a crucial role in platelet function, including activation, adhesion, migration, and aggregation [19]. To examine whether Rg3-RGE affects PI3K/Akt phosphorylation in collagen-activated platelets, we used immunoblot analysis. Our data show that Rg3-RGE markedly inhibited collagen-induced PI3K–Akt signaling in a dose-dependent manner (Fig. 7).
Fig. 7.
Rg3-RGE attenuated the phosphorylation of PI3K and Akt. After the platelet aggregation reaction, platelet proteins were extracted and subjected to immunoblot analysis with specific antibodies to examine the levels of total and phosphorylated PI3K and Akt. All immunoblots represent at least three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 versus control. PI3K, phosphatidylinositol 3 kinase; RGE, Rg3-enriched red ginseng extract.
3.7. Rg3-RGE protects mice from thrombosis
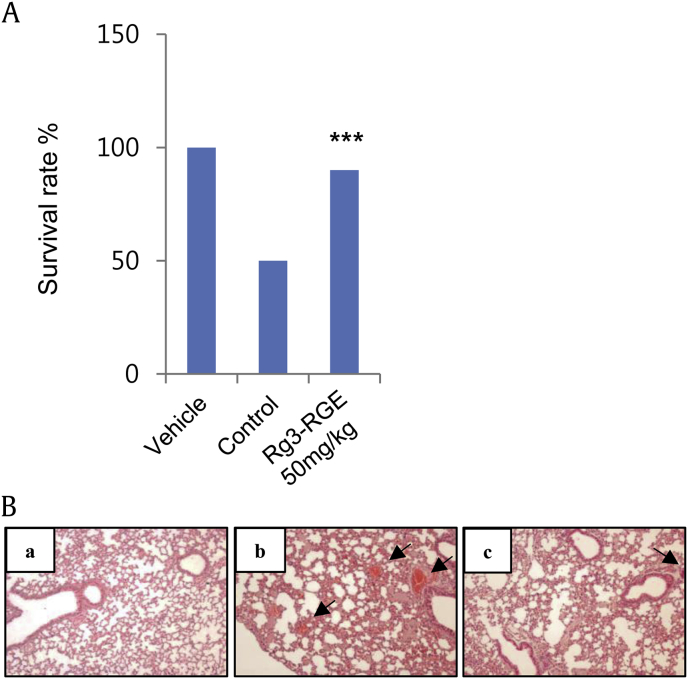
To determine whether Rg3-RGE protects mice from thrombosis, we used a collagen plus epinephrine-induced APT mouse model. After an intravenous injection of the collagen plus epinephrine, we found that the mortality of mice treated with Rg3-RGE was significantly lower compared to that of the control mice (Fig. 8A). Histologic analysis of slices of isolated lungs collected 2 min after APT induction showed that the percentage of vessels occluded by platelet thrombi was significantly lower in Rg3-RGE-treated mice than in control mice (Fig. 8B). These data show that Rg3-RGE plays a crucial role in preventing thrombus formation in vivo.
Fig. 8.
Analysis of thrombus formation. Mice were pretreated with Rg3-RGE by oral injection for 3 days. (A) Two hours after the final Rg3-RGE dose, mice were challenged with a collagen plus epinephrine mixture and checked for survival at 15 min. (B) Representative histology images (100×) of H&E-stained lungs from mice treated with (a) vehicle, (b) collagen and epinephrine mixture, and (c) with Rg3-RGE 50 mg/kg and collagen and epinephrine mixture. Arrows indicate occlusive thrombi in pulmonary vessels. ***p < 0.001 compared to the agonist-treated group. H&E, hematoxylin and eosin; Rg3-RGE, Rg3-enriched red ginseng extract.
4. Discussion
CVDs are a group of diseases that involve the blood vessels or the heart. Recently, CVDs, such as coronary artery disease, thrombosis, and acute myocardial infarction, have become the primary cause of death in developed countries. An increase in thrombus formation is likely due to the increasing prevalence of high blood pressure, diabetes, obesity, and high cholesterol. Currently, numerous studies are focusing on platelet function for the treatment and prevention of CVD. Previous studies have shown that antiplatelet therapy reduces mortality and morbidity in acute myocardial infarction [20], [21]. Platelets, small fragments of megakaryocytes that exist in the bloodstream for 7–10 d, play a crucial role in primary hemostasis and wound healing, but have also been implicated in inflammation, angiogenesis, and tumor growth and metastasis [22], [23], [24]. Platelets harbor both α granules and dense granules, which contain proteins and adhesion factors. When platelets are stimulated, the granule contents are released, binding their cognate receptors on the platelet plasma membrane leading to aggregation. However, abnormal thrombus formation can result from alterations in platelet homeostasis, leading to serious cardiovascular ailments.
P. ginseng Meyer, a Korean ginseng, is an established traditional herbal medicine for health improvement. The biological activities of ginseng include vital energy recovery, antioxidant activity, and immunity and memory enhancement [25], [26], [27], [28]. Recently, an increase in health interest has been observed in Korea, and consumer demand for red ginseng is rapidly boosting the dietary supplement market [29]. In a previous study, we showed that ginsenoside-Rg3, an active component of ginseng, has a strong antiplatelet activity mediated by the ERK pathway [11]. In this study, we hypothesized that Rg3-RGE blocks platelet-induced thrombus formation. We first tested whether Rg3-RGE inhibits collagen-induced platelet aggregation. As expected, our results showed that Rg3-RGE dramatically inhibited collagen-induced platelet aggregation in a dose-dependent manner. To elucidate the underlying inhibitory mechanism of Rg3-RGE, we analyzed downstream signaling pathways. Platelet function, including degranulation, actin cytoskeleton restructuring, and thrombus formation, is regulated by the elevation of intracellular calcium levels [30]. To determine whether Rg3-RGE regulates intracellular calcium levels, we performed a calcium assay using a fluorescence spectrophotometer. The data show that Rg3-RGE significantly decreased a rise in intracellular calcium levels in response to collagen-induced platelet activation.
Platelets contain abundant protein-rich α granules, which contain hemostatic, angiogenic, antiangiogenic, necrotic factor, growth factor, and adhesive proteins that help in the healing process. Platelets also contain less abundant dense granules, which contain small nonprotein molecules. Degranulation and release of granule contents improve platelet activation and intracellular signaling [31]. We found that Rg3-RGE significantly inhibited degranulation, hindering aggregation.
Aggregation of platelets is concomitant with the inside-out signaling event that induces a conformational change in integrin αIIbβ3 to increase its affinity to its ligand, fibrinogen. When platelets become activated, inside-out signaling induces a conformational change in integrin αIIbβ3, allowing serum fibrinogen binding, which leads to aggregation. Rg3-RGE markedly inhibited fibrinogen binding and blocked inside-out signaling, blocking aggregation. Activation of integrin αIIbβ3 and secondary mediator secretion are mediated by PI3K and PLC. Because the PI3K pathway regulates fibrinogen binding to integrin αIIbβ3 [32], we tested if Rg3-RGE altered downstream signaling events in the PI3K/Akt pathway. Previous studies have shown that PI3K inhibitors can prevent integrin αIIbβ3-mediated adhesion and thrombus formation [33]. Adam et al [34] has showed that MAPKs, such as JNK1, ERK2, and p38, are expressed in platelets and activated by many agonists. Additionally, ERK2 was found to strengthen collagen-stimulated platelet activation, and its activation was reliant on ADP secretion and TXA2 production by collagen-stimulated aggregated platelets [17]. Moreover, the phosphorylation of MAPKs trigger platelet degranulation. The function of MKK4 is linked to the JNK signaling pathway, as it is upstream of JNK [35]. Our study suggests that Rg3-RGE has the potential to suppress GPVI downstream signaling events, such as the MAPK and PI3K/Akt pathway, leading to the potential for future therapeutics to focus on GPVI antagonism. Our data show that Rg3-RGE is a potent antithrombotic agent, capable of inhibiting intracellular signaling pathways that mediate platelet activation.
Collagen and epinephrine, standard agonists for inducing platelet activation, have been used to study defects in platelet activation and their respective properties in in vivo thrombosis [36]. As expected, we found that an injection of collagen plus epinephrine rapidly induced a fatal pulmonary embolism in the majority of control mice; however, the mice treated with Rg3-RGE were protected against thromboembolism, indicating that Rg3-RGE is capable of modulating platelet function and inhibiting thrombus formation.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgments
This research was supported by the Korean Society of Ginseng (2014).
References
- 1.Ghoshal K., Bhattacharyya M. Overview of platelet physiology: its hemostatic and nonhemostatic role in disease pathogenesis. Sci World J. 2014;2014:781857. doi: 10.1155/2014/781857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davi G., Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 3.Grozovsky R., Giannini S., Falet H., Hoffmeister K.M. Regulating billions of blood platelets: glycans and beyond. Blood. 2015;126:1877–1884. doi: 10.1182/blood-2015-01-569129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bye A.P., Unsworth A.J., Gibbins J.M. Platelet signaling: a complex interplay between inhibitory and activatory networks. J Thromb Haemost. 2016;14:918–930. doi: 10.1111/jth.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett N.E., Holbrook L., Jones S., Kaiser W.J., Moraes L.A., Rana R., Sage T., Stanley R.G., Tucker K.L., Wright B. Future innovations in anti-platelet therapies. Br J Pharmacol. 2008;154:918–939. doi: 10.1038/bjp.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S.M., Bae B.S., Park H.W., Ahn N.G., Cho B.G., Cho Y.L., Kwak Y.S. Characterization of Korean Red Ginseng (Panax ginseng Meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39:384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim K.S., Jung Yang H., Lee I.S., Kim K.H., Park J., Jeong H.S., Kim Y., Ahn K.S., Na Y.C., Jang H.J. The aglycone of ginsenoside Rg3 enables glucagon-like peptide-1 secretion in enteroendocrine cells and alleviates hyperglycemia in type 2 diabetic mice. Sci Rep. 2015;5:18325. doi: 10.1038/srep18325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee K.H., Bae I.Y., Park S.I., Park J.D., Lee H.G. Antihypertensive effect of Korean Red Ginseng by enrichment of ginsenoside Rg3 and arginine–fructose. J Ginseng Res. 2016;40:237–244. doi: 10.1016/j.jgr.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Liu Q.Z., Xing S.P., Zhang J.L. Inhibiting effect of Endostar combined with ginsenoside Rg3 on breast cancer tumor growth in tumor-bearing mice. Asian Pac J Trop Med. 2016;9:180–183. doi: 10.1016/j.apjtm.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Liu T., Peng Y.F., Jia C., Yang B.H., Tao X., Li J., Fang X. Ginsenoside Rg3 improves erectile function in streptozotocin-induced diabetic rats. J Sex Med. 2015;12:611–620. doi: 10.1111/jsm.12779. [DOI] [PubMed] [Google Scholar]
- 11.Lee W.M., Kim S.D., Park M.H., Cho J.Y., Park H.J., Seo G.S., Rhee M.H. Inhibitory mechanisms of dihydroginsenoside Rg3 in platelet aggregation: critical roles of ERK2 and cAMP. J Pharm Pharmacol. 2008;60:1531–1536. doi: 10.1211/jpp/60.11.0015. [DOI] [PubMed] [Google Scholar]
- 12.Endale M., Lee W.M., Kamruzzaman S.M., Kim S.D., Park J.Y., Park M.H., Park T.Y., Park H.J., Cho J.Y., Rhee M.H. Ginsenoside-Rp1 inhibits platelet activation and thrombus formation via impaired glycoprotein VI signalling pathway, tyrosine phosphorylation and MAPK activation. Br J Pharmacol. 2012;167:109–127. doi: 10.1111/j.1476-5381.2012.01967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bye A.P., Unsworth A.J., Vaiyapuri S., Stainer A.R., Fry M.J., Gibbins J.M. Ibrutinib inhibits platelet integrin alphaIIbbeta3 outside-in signaling and thrombus stability but not adhesion to collagen. Arterioscler Thromb Vasc Biol. 2015;35:2326–2335. doi: 10.1161/ATVBAHA.115.306130. [DOI] [PubMed] [Google Scholar]
- 14.Canobbio I., Cipolla L., Consonni A., Momi S., Guidetti G., Oliviero B., Falasca M., Okigaki M., Balduini C., Gresele P., Torti M. Impaired thrombin-induced platelet activation and thrombus formation in mice lacking the Ca(2+)-dependent tyrosine kinase Pyk2. Blood. 2013;121:648–657. doi: 10.1182/blood-2012-06-438762. [DOI] [PubMed] [Google Scholar]
- 15.Shattil S.J., Newman P.J. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–1615. doi: 10.1182/blood-2004-04-1257. [DOI] [PubMed] [Google Scholar]
- 16.Oury C., Toth-Zsamboki E., Vermylen J., Hoylaerts M.F. P2X(1)-mediated activation of extracellular signal-regulated kinase 2 contributes to platelet secretion and aggregation induced by collagen. Blood. 2002;100:2499–2505. doi: 10.1182/blood-2002-03-0812. [DOI] [PubMed] [Google Scholar]
- 17.Arslan R., Bor Z., Bektas N., Mericli A.H., Ozturk Y. Antithrombotic effects of ethanol extract of Crataegus orientalis in the carrageenan-induced mice tail thrombosis model. Thromb Res. 2011;127:210–213. doi: 10.1016/j.thromres.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 18.Li Z., Delaney M.K., O'Brien K.A., Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. 2010;30:2341–2349. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuang W.Y., Kung P.H., Kuo C.Y., Wu C.C. Sulforaphane prevents human platelet aggregation through inhibiting the phosphatidylinositol 3-kinase/Akt pathway. Thromb Haemost. 2013;109:1120–1130. doi: 10.1160/TH12-09-0636. [DOI] [PubMed] [Google Scholar]
- 20.Michelson A.D. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov. 2010;9:154–169. doi: 10.1038/nrd2957. [DOI] [PubMed] [Google Scholar]
- 21.Sharma G., Berger J.S. Platelet activity and cardiovascular risk in apparently healthy individuals: a review of the data. J Thromb Thrombolysis. 2011;32:201–208. doi: 10.1007/s11239-011-0590-9. [DOI] [PubMed] [Google Scholar]
- 22.Stegner D., Dutting S., Nieswandt B. Mechanistic explanation for platelet contribution to cancer metastasis. Thromb Res. 2014;133(Suppl 2):S149–S157. doi: 10.1016/S0049-3848(14)50025-4. [DOI] [PubMed] [Google Scholar]
- 23.Kakudo N., Morimoto N., Kushida S., Ogawa T., Kusumoto K. Platelet-rich plasma releasate promotes angiogenesis in vitro and in vivo. Med Mol Morphol. 2014;47:83–89. doi: 10.1007/s00795-013-0045-9. [DOI] [PubMed] [Google Scholar]
- 24.Thomas M.R., Storey R.F. The role of platelets in inflammation. Thromb Haemost. 2015;114:449–458. doi: 10.1160/TH14-12-1067. [DOI] [PubMed] [Google Scholar]
- 25.Byeon S.E., Lee J., Kim J.H., Yang W.S., Kwak Y.S., Kim S.Y., Choung E.S., Rhee M.H., Cho J.Y. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediators Inflamm. 2012;2012:732860. doi: 10.1155/2012/732860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H., Kang T., Qi B., Kong L., Jiao Y., Cao Y., Zhang J., Yang J. Neuroprotective effects of ginseng protein on PI3K/Akt signaling pathway in the hippocampus of d-galactose/AlCl3 inducing rats model of Alzheimer's disease. J Ethnopharmacol. 2016;179:162–169. doi: 10.1016/j.jep.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Kim S., Lee Y., Cho J. Korean red ginseng extract exhibits neuroprotective effects through inhibition of apoptotic cell death. Biol Pharm Bull. 2014;37:938–946. doi: 10.1248/bpb.b13-00880. [DOI] [PubMed] [Google Scholar]
- 28.Joo S.S., Won T.J., Lee D.I. Reciprocal activity of ginsenosides in the production of proinflammatory repertoire, and their potential roles in neuroprotection in vivo. Planta Med. 2005;71:476–481. doi: 10.1055/s-2005-864145. [DOI] [PubMed] [Google Scholar]
- 29.Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Annie-Jeyachristy S., Geetha A., Surendran R. Changes in the level of cytosolic calcium, nitric oxide and nitric oxide synthase activity during platelet aggregation: an in vitro study in platelets from normal subjects and those with cirrhosis. J Biosci. 2008;33:45–53. doi: 10.1007/s12038-008-0020-0. [DOI] [PubMed] [Google Scholar]
- 31.Cimmino G., Golino P. Platelet biology and receptor pathways. J Cardiovasc Transl Res. 2013;6:299–309. doi: 10.1007/s12265-012-9445-9. [DOI] [PubMed] [Google Scholar]
- 32.Guidetti G.F., Canobbio I., Torti M. PI3K/Akt in platelet integrin signaling and implications in thrombosis. Adv Biol Regul. 2015;59:36–52. doi: 10.1016/j.jbior.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Jackson S.P., Schoenwaelder S.M., Goncalves I., Nesbitt W.S., Yap C.L., Wright C.E., Kenche V., Anderson K.E., Dopheide S.M., Yuan Y. PI 3-kinase p110beta: a new target for antithrombotic therapy. Nat Med. 2005;11:507–514. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- 34.Adam F., Kauskot A., Rosa J.P., Bryckaert M. Mitogen-activated protein kinases in hemostasis and thrombosis. J Thromb Haemost. 2008;6:2007–2016. doi: 10.1111/j.1538-7836.2008.03169.x. [DOI] [PubMed] [Google Scholar]
- 35.Haeusgen W., Herdegen T., Waetzig V. The bottleneck of JNK signaling: molecular and functional characteristics of MKK4 and MKK7. Eur J Cell Biol. 2011;90:536–544. doi: 10.1016/j.ejcb.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Angelillo-Scherrer A., de Frutos P., Aparicio C., Melis E., Savi P., Lupu F., Arnout J., Dewerchin M., Hoylaerts M., Herbert J. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat Med. 2001;7:215–221. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]