Abstract
Background
Panax ginseng is a traditional herb used for medicinal purposes in eastern Asia. P. ginseng contains various ginsenosides with pharmacological effects. In this study, floralginsenoside A (FGA), ginsenoside Rd (GRD), and ginsenoside Re (GRE) were purified from P. ginseng berry.
Methods
Chemical structures of FGA, GRD, and GRE were determined based on spectroscopic methods, including fast atom bombardment mass spectroscopy, ID-nuclear magnetic resonance, and infrared spectroscopy. Inhibitory activities of these compounds on melanogenesis were studied by measuring the expression of protein and melanin content in the melan-a cell line. This inhibitory activity was confirmed by observing pigmentation and tyrosinase activities of zebrafish.
Results
GRD, GRE, and FGA were not cytotoxic at concentrations less than 20μM, 80μM, and 160μM in melan-a cells, respectively. GRD, GRE, and FGA inhibited melanin biosynthesis in melan-a cells by 15.2%, 22.9%, and 23.9% at 20μM, 80μM, and 160μM, respectively. FGA was observed to display the most potent inhibitory effect. In addition, FGA decreased microphthalmia-associated transcription factor protein expression in a dose-dependent manner. Moreover, FGA induced extracellular signal-regulated kinase phosphorylation level in melan-a cells. In addition, melanin pigment content and tyrosinase activity in zebrafish treated with FGA at160μM were reduced.
Conclusion
FGA showed the most potent inhibition of melanogenesis in both in vitro and in vivo studies. This study suggests that FGA purified from P. ginseng may be an effective melanogenesis inhibitor.
Keywords: antimelanogenesis, floralginsenoside A, Panax ginseng berry
1. Introduction
Melanin pigment has several functions, including protection of the skin from ultraviolet light and removal of reactive oxygen species [1], [2]. Nevertheless, excessive production of melanin pigment and its accumulation in the skin can cause pigmentation disorders, such as solar lentigo, melasma, freckles, and postinflammatory hyperpigmentation [3], [4]. Several known depigmenting agents such as kojic acid and arbutin have already been the focus of studies and are currently being utilized as cosmetic additives [5]. However, it is necessary to find more effective and safer depigmenting agents because of the potential carcinogenic side effects of kojic acid [6]. Commercially available skin depigment cosmetics have displayed many safety concerns and low efficacy. Therefore, research into the separation of novel compounds from a natural source to prevent pigmentation is increasing.
Ginsenosides normally have the steroidal structures connected with sugar moieties. They are found in about 30 different forms of Panax ginseng [7]. Ginsenosides are reported to have various cellular activities, including immunomodulating, antitumor, antioxidative, and anti-inflammatory effects, and the ability to enhance cerebral blood flow [8], [9], [10]. Several studies have reported the discovery of inhibitors of melanin synthesis in P. ginseng [11], [12], [13]. However, these studies were performed with phenolic compounds and cinnamic acid [11], [12], [13]. We isolated floralginsenoside A (FGA), ginsenoside Rd (GRD), and ginsenoside Re (GRE) from P. ginseng berry. In other studies of GRD and GRE, various physiological activities have been reported, such as antioxidant, anti-inflammatory, antidiabetic, anticancer, and immunomodulating activities [14], [15], [16], [17]. However, there has been no research on the whitening activity of GRD and GRE. On the contrary, FGA was not reported to exhibit any physiological activity. Consequently, we studied the inhibition of melanin biosynthesis through in vitro and in vivo systems, with FGA, GRD, and GRE purified from P. ginseng berry, and estimated its potential as a new depigment substance.
2. Materials and methods
2.1. General
Kieselgel 60 and LiChroprep RP-18 resins were used for column chromatography (Merck, Darmstadt, Germany). Kieselgel 60 F254 (Merck) and RP-18 F254S (Merck, Darmstadt, Germany) were used as solid phases for the TLC experiment. Fast atom bombardment–mass spectrometry (FAB–MS) spectrum was recorded on a JMS-700 (JEOL, Tokyo, Japan). The IR spectra were obtained from a Spectrum One FT-IR spectrometer (Perkin Elmer, Buckinghamshire, England). The NMR spectra were recorded on a Varian Inova AS 400 spectrometer (400 MHz; Varian, Palo Alto, CA, USA). The compounds 12-ο-tetradecanoylphorbol-13-acetate, 1-phenyl-2-thiourea (PTU), L-3,4-dihydroxyphenylalanine (L-DOPA), and L-tyrosine were all purchased from Sigma Chemical Co. (St. Louis, MO, USA). Fetal bovine serum (FBS), antibiotics (penicillin, streptomycin) and cell culture media were obtained from Gibco BRL Life Technology (Rockville, MD, USA). Antiextracellular signal-regulated kinase (anti-ERK)1/2, antiphosphorylated ERK1/2, anti-Akt, antiphosphorylated Akt, and antimouse and antirabbit immunoglobulin G antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies against microphthalmia-associated transcription factor (MITF) were purchased from Thermo Fisher Scientific (Rockford, IL, USA). Tyrosinase was gifted by Dr V.J. Hearing, National Institutes of Health, Bethesda, MD, USA. The cell culture media and additives were purchased from Gibco (Grand Island, NY, USA).
2.2. Plant materials
Six-yr-old P. ginseng berry was preserved and cultivated at the experimental field of the National Institute of Horticultural and Herbal Science (NIHHS), Rural Development Administration, Chungbuk Province, Korea, in 2014. Voucher specimen (NIHHS141010) was deposited at the herbarium of the Department of Herbal Crop Research, National Institute of Horticultural and Herbal Science, Rural Development Administration, Eumseong, Korea.
2.3. Extraction and isolation
Fresh ginseng (5 kg) was extracted twice with 90% methanol at room temperature. Subsequently, the methanol extract was partitioned with water, ethylacetate, and n-butanol (n-BuOH) which were purchased from solvent (DAEJUNG Chemicals, Korea). Repeated silica gel and octadecyl silica gel resins used for column chromatography, and prep-liquid chromatography of BuOH fractions yielded three ginsenosides. A rare compound, FGA, along with two well-known ginsenosides, GRE and GRD, was isolated from the BuOH fraction. Identities of these compounds were confirmed by comparing their measured spectroscopic data with those reported in the literature [18], [19], [20].
2.4. Physicochemical characteristics of FGA, GRD, and GRE
FGA: white amorphous powder; Mp: 167–168°C; FAB/MS m/z: 831 [M–H]−; IR (KBr, ν): 3,469 cm−1, 1,655 cm−1, 1,462 cm−1, 1,076 cm−1; 1H- and 13C-NMR data are same as the literature value [18]. GRE: white powder; Mp 186–187°C; FAB/MS m/z: 945 [M]+; IR (KBr, ν): 3,359 cm−1, 2,929 cm−1, 1,642 cm−1, 1,072 cm−1, 1,045 cm−1; 1H- and 13C-NMR data are same as the literature value [19]. GRD: white powder; Mp 182–183°C; FAB/MS m/z: 969 [M]+; IR (KBr, ν): 3,366 cm−1, 2,943 cm−1, 1,647 cm−1, 1,077 cm−1, 1,034 cm−1; 1H- and 13C-NMR data are same as the literature value [20].
2.5. Maintenance of cells
Melan-a cells are an immortalized normal murine melanocyte cell line derived from C57BL/6 mice, with high melanin pigmentation. The melan-a cell line was donated by Dr Dorothy Bennett (St. George's Hospital, London, UK). These cells were maintained in RPMI-1640 culture medium supplemented with 10% (v/v) FBS (fetal bovine serum), 100 U/mL penicillin, 100 μg/mL streptomycin, and 200nM 12-ο-tetradecanoylphorbol-13-acetate in a humidified atmosphere of 5% CO2 at 37°C. Cell viability was measured using CCK-8 cell counting kit-8 (Dojindo, Japan).
2.6. Measurement of melanin content
Melan-a cells were treated with three ginsenosides (FGA, GRD, and GRE). The cells were then lysed in 1N NaOH (Ducsan, Korea) at 60°C for 30 min. The melanin content was then determined in the lysates at 450 nm. The data were normalized by measuring the protein content of the cell lysates with a BCA (Bicinchoninic acid) protein assay kit (Thermo Fisher Scientific, Rocford, IL, USA).
2.7. Western blot analysis
Melan-a cells were lysed in lysis buffer (Radioimmunoprecipitation assay buffer with Noniodet P-40, protease inhibitor, and phosphatase inhibitor). Cell debris was removed, and the resulting supernatant was used for Western blot analysis. Protein concentration of the cell lysate was determined using the BCA protein assay kit. Proteins in cultured cell homogenates were separated on 8% SDS (sodium dodecyl sulfate)-polyacrylamide gel, transferred to nitrocellulose membranes (Millipore, Billerica, MA, USA), and subsequently subjected to Western blot analysis using particular antibodies. The immunoreactive antigen was then visualized using the HRP (Horseradish peroxiase)-coupled secondary antibodies and the enhanced chemiluminescence detection kit from Pierce Biotechnology (Rockford, IL, USA).
2.8. Origin and maintenance of zebrafish embryo
Zebrafish embryos were obtained from a zebrafish organogenesis mutant bank in Gyeongbuk National University. Zebrafishes were kept in a 3.5-L acrylic tank. The acrylic tank was maintained at a constant temperature (28 ± 1°C) with 14/10-h cycle of light and dark. Zebrafishes were fed live brine shrimps (Artemia salina). The embryos were obtained from natural spawning that was induced in the morning by turning on the light. Embryo collection was completed in 30 min.
2.9. Phenotype-based assessment
Synchronized embryos were collected and arrayed using a pipette (10 embryos per well in 24-well plates containing 1 mL of egg water). FGA was dissolved in dimethyl sulfoxide (Sigma Chemical Co., St Louis, MO, USA) and then added to the egg water 9–72 h postfertilization. Effects on the pigment spots of zebrafish were observed under a stereomicroscope. In all experiments, 0.1mM PTU (Sigma Chemical Co., St Louis, MO, USA) was used to generate transparent zebrafish without interfering in the developmental process [21] and considered as a positive control. Phenotype-based assessment of pigmentation was carried out by dechorionation using forceps, anesthetization in tricaine methanesulfonate solution (Sigma Chemical Co., St Louis, MO, USA), mounting in glycerol on a 35-mm dish, and observation under a stereoscopic microscope to take pictures [22].
2.10. Tyrosinase activity and melanin content
Tyrosinase activity was spectrometrically determined. About 100 zebrafish embryos were treated with FGA 9–48 h postfertilization and sonicated in Pro-Prep protein extraction solution (Intron, Daejeon, Korea). The lysate was clarified by centrifuging at 10,000g for 5 min. After quantification, 250 μg of total protein was added, followed by 100 μL of 5mM L-DOPA. After incubation for 60 min at 37°C, absorbance was measured at 475 nm using a spectrophotometer. For the determination of melanin content, about 100 zebrafish embryos were treated with FGA 9–48 h postfertilization and sonicated in protein extraction solution. After the centrifugation, the pellet was lysed in 1N NaOH at 100°C for 10 min. Optical density of the supernatant was measured at 490 nm [22].
2.11. Statistical analysis
Statistical analysis was performed using SPSS 18 software (IBM, USA). All experiments described were performed three or more times. The data are expressed as the mean ± standard deviation. Significant differences between results were determined by Student t test. The statistical differences were considered significant at p < 0.05.
3. Results and discussion
3.1. Inhibitory activity of three ginsenosides on melanogenesis in melan-a cells
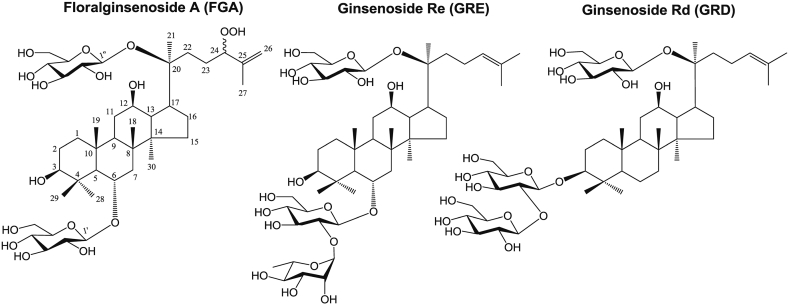
Melanin biosynthesis activity was studied by isolating FGA, GRD, and GRE from P. ginseng berry (Fig. 1).
Fig. 1.
Chemical structures of FGA, GRD, and GRE from P. ginseng. FGA, floralginsenoside A; GRD, ginsenoside Rd; GRE, ginsenoside Re.
Cell viability was determined using the CCK-8 cell viability assay kit after treating melan-a cells with the three ginsenosides (0–160μM) for 3 d. FGA did not show cytotoxicity toward melan-a cells in all tested concentrations. However, GRE and GRD were cytotoxic at concentrations 40μM and 160μM, respectively (Table 1).
Table 1.
Melan-a cell viability after treatment with three ginsenosides isolated from P. ginseng
| Ginsenosides | Concentration (μM) |
|||||
|---|---|---|---|---|---|---|
| Control | 10 | 20 | 40 | 80 | 160 | |
| FGA | 100 ± 2.1 | 99.7 ± 5.4 | 99.6 ± 2.6 | 103.4 ± 10.9 | 98.9 ± 2.9 | 98.7 ± 12.8 |
| GRD | 100 ± 2.1 | 94.7 ± 1.2 | 92.4 ± 0.7 | 71.3 ± 1.8 | 54.3 ± 1.1 | 46.3 ± 3.6 |
| GRE | 100 ± 2.1 | 95.3 ± 1.1 | 91.6 ± 2.0 | 90.7 ± 0.5 | 89.3 ± 2.7 | 69.6 ± 11.1 |
Data are presented by mean ± SD (n = 3)
The cells were cultured with 0–160μM of three ginsenosides for 3 d, and cell viability was determined by CCK-8 cell-counting kit
FGA, floralginsenoside A; GRD, ginsenoside Rd; GRE, ginsenoside Re; SD, standard deviation
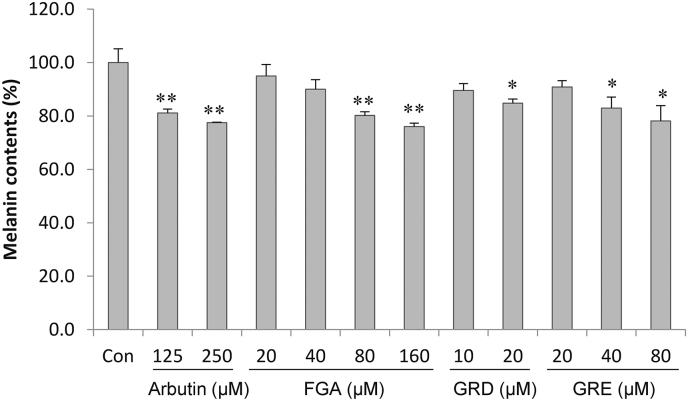
To examine the activity of three ginsenosides on melanogenesis, we measured the melanin contents of melan-a cells up to the previously measured cytotoxic concentrations. As shown in Fig. 2, three ginsenosides reduced the melanin content in a dose-dependent manner. FGA indicated a significantly high melanin inhibitory activity (23.9%) at 160μM concentration without inducing any cytotoxicity in the cells.
Fig. 2.
Effects of three ginsenosides isolated from P. ginseng on melanogenesis in melan-a cells. The cell was cultured with 125–250μM arbutin and 0–160μM of three ginsenosides (FGA, GRD, and GRE) for 3 d. The results are averages of triplicate experiments, and the data are expressed as mean ± SD. * p < 0.05 versus control group. ** p < 0.01 versus control group. Con, control; FGA, floralginsenoside A; GRD, ginsenoside Rd; GRE, ginsenoside Re; SD, standard deviation.
Ginseng ginsenoside is the main biologically active compound that is widely studied. In recent years, it not only is used for its effect on the skin, but is also clearly a scientific approach for the treatment of various diseases [23], [24]. It has been reported that several ginsenosides and cinnamic acid are inhibitory agents of melanogenesis, and are found mainly in P. ginseng [11], [25], [26]. Therefore, we studied the expression of a protein found in melanin synthesis by nontoxic FGA (it has not been studied with respect to the physiological activities, as mentioned in the introduction) in melan-a cells.
3.2. Effect of FGA on the melanogenesis-related signaling pathway
Tyrosinase is involved in the biosynthesis of melanin by catalyzing the first two steps of melanin as a rate-limiting enzyme [27]. Tyrosinase hydroxylates tyrosine to DOPA and then oxidizes DOPA into dopaquinone [28]. Gene expression of tyrosinase is reported to be regulated by the MITF, which binds to the M-box (GTCATGTGCT) within the tyrosinase promoter [29]. Downregulation of MITF expression through tyrosinase is the most significant mechanism underlying the antimelanogenesis of inhibitory agents [30]. In addition, intracellular signaling kinase family ERK and Akt have been reported to have an influential function in melanin biosynthesis [31]. The action of ERK causes phosphorylation of MITF at serine-73, which leads to ubiquitination and subsequent MITF degradation, finally decreasing tyrosinase synthesis and melanogenesis [32]. Further, melanin production is also reduced by the phosphorylation of MITF at serine-29, which activates the signaling pathways of Akt [33].
Accordingly, it was hypothesized that FGA might inhibit melanogenesis-related signaling pathways, including the inhibition of MITF–tyrosinase signaling and/or activation of ERK–Akt signaling. Therefore, we studied the effect of FGA on the expression of tyrosinase and MITF, as well as ERK and Akt, in an attempt to further elucidate the molecular mechanisms. As shown in Fig. 3, tyrosinase and MITF expression were reduced in a dose-dependent manner. Furthermore, phospho-ERK and Akt signaling pathways were significantly enhanced in a dose-dependent manner by FGA treatment until 160μM concentration.
Fig. 3.
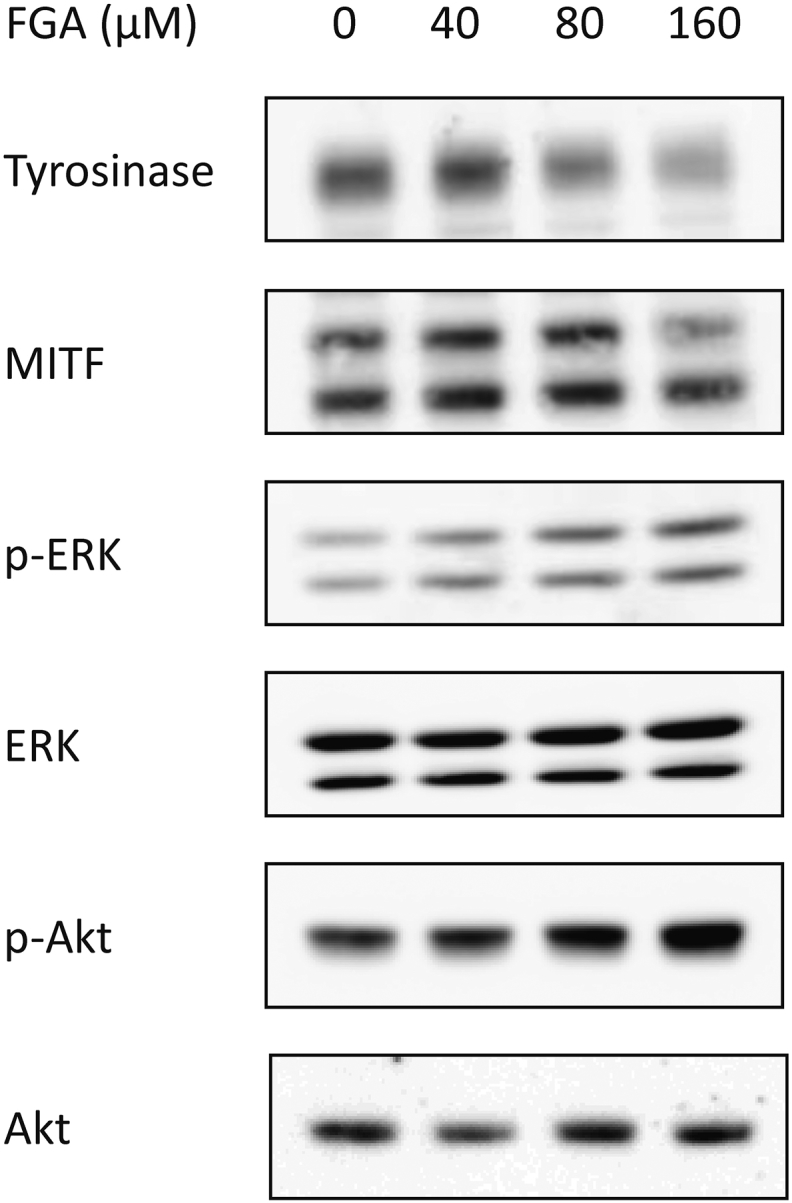
Effect of FGA on tyrosinase, MITF, Akt, and ERK protein expression in melan-a cells. The cells were cultured with 0–160μM of FGA for 24 h. Whole-cell lysate was subjected to Western blot analysis using antibodies against p-Akt, Akt, p-ERK, ERK, and MITF. Equal protein loading was confirmed using β-actin antibody. ERK, extracellular signal-regulated kinase; FGA, floralginsenoside A; MITF, microphthalmia-associated transcription factor; p-Akt, phosphorylated Akt; p-ERK, phosphorylated extracellular signal-regulated kinase.
Our findings support that Akt and ERK pathway activation may be responsible for the discovered inhibition of melanin biosynthesis by FGA (Fig. 3). In order to confirm that these signaling pathways are responsible for FGA-induced inhibitory effects on melanogenesis, we activated both Akt and ERK signaling in the presence of FGA in melan-a cells.
3.3. Effect of FGA on melanogenesis in zebrafish
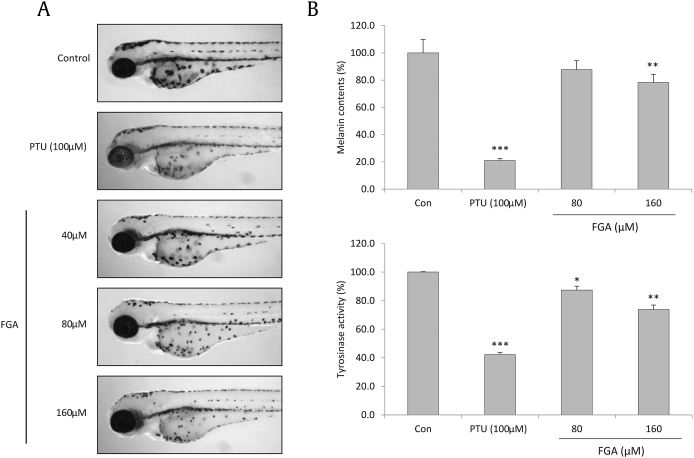
The zebrafish has become a favored model for biochemical studies because it is an efficient and robust replacement for animal experiments [34]. Observation of the pigment on the surface of zebrafish is a simple method to study the pigmentation process without complex laboratory procedures [22]. PTU (sulfur-containing tyrosinase inhibitor) is a widely used positive control in zebrafish investigations, which was included in this study [35]. The number of melanin pigmentations on the zebrafish embryo treated with FGA gradually decreased in a dose-dependent manner (Fig. 4A). Moreover, the total content of melanin and tyrosinase activity was measured using the whole zebrafish extract. FGA showed a potential reduction in the total melanin content and tyrosinase activity after treatment (Fig. 4B). More than 95% of the zebrafish survived treatments with PTU and treatment concentration of FGA (data not shown).
Fig. 4.
Effect of FGA on melanogenesis in zebrafish. Synchronized embryos were treated with melanogenic inhibitors at the indicated concentrations. FGA was dissolved in DMSO and then added to the embryo medium. (A) Effects on the pigmentation of zebrafish were observed under a stereomicroscope. (B) Total melanin content was determined using a spectrometer. To measure tyrosinase activity, 250 μg of total protein was incubated with L-DOPA (final 0.5mM) and then determined using a spectrometer. Results shown are the mean of three independent experiments ± SD. * p < 0.05 versus control group. ** p < 0.01 versus control group. *** p < 0.001 versus control group. Con, control; DMSO, dimethyl sulfoxide; FGA, floralginsenoside A; L-DOPA, L-3,4-dihydroxyphenylalanine; PTU, 1-phenyl-2-thiourea; SD, standard deviation.
A recent study reported that the melanogenesis inhibitory effect of ginsenoside Rb1 from the P. ginseng root on the B16 melanoma cells is stimulated by α-melanocyte-stimulating hormone in a dose-dependent manner [36]. Lee et al [25] reported that minor ginsenosides purified from the leaves showed inhibitory activity on body pigmentation in zebrafish. There has been no study on the biological activity of FGA after isolation by Yoshikawa et al [18]. Floralginsenosides Ka, Kc, and J were the only ginsenosides reported to have antioxidant and anti-inflammatory actions [37], [38].
In conclusion, we have found inhibitory activities of FGA, GRD, and GRE isolated from P. ginseng berry on melanin biosynthesis. All three ginsenosides were found to have an inhibitory activity on melanin biosynthesis in melan-a cells. Notably, FGA showed the strongest inhibitory activity without any cell toxicity in the in vitro and in vivo studies. Inhibition of melanin content and tyrosinase activity may be related to potential whitening. Therefore, FGA of P. ginseng berry shows how you determine whether to regulate melanin production, and this can be protection against hyperpigmentation.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
This research was supported by Export Promotion Technology Development Program (No. 314026-03-2-HD020), Ministry of Agriculture, Food and Rural Affairs.
References
- 1.Kadekaro A.L., Kavanagh R., Kanto H., Terzieva S., Hauser J., Kobayashi N., Schwemberger S., Cornelius J., Babcock G., Shertzer H.G. Alpha-melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005;65:4292–4299. doi: 10.1158/0008-5472.CAN-04-4535. [DOI] [PubMed] [Google Scholar]
- 2.Chiang H.M., Chien Y.C., Wu C.H., Kuo Y.H., Wu W.C., Pan Y.Y., Su Y.H., Wen K.C. Hydroalcoholic extract of Rhodiola rosea L. (Crassulaceae) and its hydrolysate inhibit melanogenesis in B16F0 cells by regulating the CREB/MITF/tyrosinase pathway. Food Chem Toxicol. 2014;65:129–139. doi: 10.1016/j.fct.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 3.Hearing V.J., Jr. Mammalian monophenol monooxygenase (tyrosinase): purification, properties, and reactions catalyzed. Methods Enzymol. 1987;142:154–165. doi: 10.1016/s0076-6879(87)42024-7. [DOI] [PubMed] [Google Scholar]
- 4.Briganti S., Camera E., Picardo M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003;16:101–110. doi: 10.1034/j.1600-0749.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 5.Fujimoto N., Watanabe H., Nakatani T., Roy G., Ito A. Induction of thyroid tumours in (C57BL/6N X C3H/N)F1 mice by oral administration of kojic acid. Food Chem Toxicol. 1998;36:697–703. doi: 10.1016/s0278-6915(98)00030-1. [DOI] [PubMed] [Google Scholar]
- 6.Kim S., Jung S.H., Cho C.W. Physicochemical studies of a newly synthesized molecule, 6-methyl-3-phenethyl-3,4-dihydro-1h-quinazoline-2-thione (jsh18) for topical formulations. Arch Pharm Res. 2008;31:1363–1368. doi: 10.1007/s12272-001-2118-x. [DOI] [PubMed] [Google Scholar]
- 7.Choi S., Lee J.H., Kim Y.I., Kang M.J., Rhim H., Lee S.M., Nah S.Y. Effects of ginsenoside on g protein-coupled inwardly rectifying k+ channel activity expressed in xenopus oocytes. Eur J Pharmacol. 2003;468:83–92. doi: 10.1016/s0014-2999(03)01666-2. [DOI] [PubMed] [Google Scholar]
- 8.Zhang C., Liu L., Yu Y., Chen B., Tang C., Li X. Antitumor effects of ginsenoside rg3 on human hepatocellular carcinoma cells. Mol Med Rep. 2012;5:1295–1298. doi: 10.3892/mmr.2012.808. [DOI] [PubMed] [Google Scholar]
- 9.Park D., Bae D.K., Jeon J.H., Lee J., Oh N., Yang G., Yang Y.H., Kim T.K., Song J., Lee S.H. Immunopotentiation and antitumor effects of a ginsenoside Rg(3)-fortified red ginseng preparation in mice bearing H460 lung cancer cells. Environ Toxicol Pharmacol. 2011;31:397–405. doi: 10.1016/j.etap.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Shin Y.M., Jung H.J., Choi W.Y., Lim C.J. Antioxidative, anti-inflammatory, and matrix metalloproteinase inhibitory activities of 20(s)-ginsenoside rg3 in cultured mammalian cell lines. Mol Bio Rep. 2013;40:269–279. doi: 10.1007/s11033-012-2058-1. [DOI] [PubMed] [Google Scholar]
- 11.Kong Y.H., Jo Y.O., Cho C.W., Son D., Park S., Rho J., Choi S.Y. Inhibitory effects of cinnamic acid on melanin biosynthesis in skin. Biol Pharm Bull. 2008;31:946–948. doi: 10.1248/bpb.31.946. [DOI] [PubMed] [Google Scholar]
- 12.Im S.J., Kim K.N., Yun Y.G., Lee J.C., Mun Y.J., Kim J.H., Woo W.H. Effect of radix ginseng and radix trichosanthis on the melanogenesis. Biol Pharm Bull. 2003;26:849–853. doi: 10.1248/bpb.26.849. [DOI] [PubMed] [Google Scholar]
- 13.Lee Y., Kim K.T., Kim S.S., Hur J., Ha S.K., Cho C.W., Choi S.Y. Inhibitory effects of ginseng seed on melanin biosynthesis. Pharmacogn Mag. 2014;10:S272–S275. doi: 10.4103/0973-1296.133271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie J.T., Shao Z.H., Vanden Hoek T.L., Chang W.T., Li J., Mehendale S., Wang C.Z., Hsu C.W., Becker L.B., Yin J.J. Antioxidant effects of ginsenoside re in cardiomyocytes. Eur J Pharmacol. 2006;532:201–207. doi: 10.1016/j.ejphar.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y.X., Wang L., Xiao E.L., Li S.J., Chen J.J., Gao B., Min G.N., Wang Z.P., Wu Y.J. Ginsenoside-Rd exhibits anti-inflammatory activities through elevation of antioxidant enzyme activities and inhibition of JNK and ERK activation in vivo. Int Immunopharmacol. 2013;17:1094–1100. doi: 10.1016/j.intimp.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Yang C.Y., Wang J., Zhao Y., Shen L., Jiang X., Xie Z.G., Liang N., Zhang L., Chen Z.H. Anti-diabetic effects of Panax notoginseng saponins and its major anti-hyperglycemic components. J Ethnopharmacol. 2010;130:231–236. doi: 10.1016/j.jep.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 17.Mita A., Shida R., Kasai N., Shoji J. Enhancement and suppression in production of IgM-antibody in mice treated with purified saponins. Biomedicine. 1979;31:223–227. [PubMed] [Google Scholar]
- 18.Yoshikawa M., Sugimoto S., Nakamura S., Matsuda H. Medicinal flowers. Xi. Structures of new dammarane-type triterpene diglycosides with hydroperoxide group from flower buds of Panax ginseng. Chem Pharm Bull. 2007;55:571–576. doi: 10.1248/cpb.55.571. [DOI] [PubMed] [Google Scholar]
- 19.Teng R., Li H., Chen J., Wang D., He Y., Yang C. Complete assignment of 1h and 13c NMR data for nine protopanaxatriol glycosides. Magn Reson Chem. 2002;40:483–488. [Google Scholar]
- 20.Sanada S., Kondo N., Shoji J., Tanaka O., Shibata S. Studies on the saponins of ginseng. I. Structures of ginsenoside-Ro, -Rb1, -Rb2, -Rc and -Rd. Chem Pharm Bull. 1974;22:421–428. [Google Scholar]
- 21.Karlsson J., von Hofsten J., Olsson P.E. Generating transparent zebrafish: a refined method to improve detection of gene expression during embryonic development. Mar Biotechnol. 2001;3:522–527. doi: 10.1007/s1012601-0053-4. [DOI] [PubMed] [Google Scholar]
- 22.Choi T.Y., Kim J.H., Ko D.H., Kim C.H., Hwang J.S., Ahn S., Kim S.Y., Kim C.D., Lee J.H., Yoon T.J. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment Cell Res. 2007;20:120–127. doi: 10.1111/j.1600-0749.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 23.Gillis C.N. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 24.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 25.Lee D.Y., Cha B.J., Lee Y.S., Kim G.S., Noh H.J., Kim S.Y., Kang H.C., Kim J.H., Baek N.I. The potential of minor ginsenosides isolated from the leaves of Panax ginseng as inhibitors of melanogenesis. Int J Mol Sci. 2015;16:1677–1690. doi: 10.3390/ijms16011677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee D.Y., Jeong S.C., Jeong Y.T., Lee M.K., Seo K.H., Lee J.W., Kim G.S., Lee S.E., Baek N.I., Kim J.H. Antimelanogenic effects of picrionoside a isolated from the leaves of Korean ginseng. Biol Pharm Bull. 2015;38:1663–1667. doi: 10.1248/bpb.b15-00410. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y.J., Uyama H. Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. Cell Mol Life Sci. 2005;62:1707–1723. doi: 10.1007/s00018-005-5054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costin G.E., Hearing V.J. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–994. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 29.Bentley N.J., Eisen T., Goding C.R. Melanocyte-specific expression of the human tyrosinase promoter: activation by the microphthalmia gene product and role of the initiator. Mol Cell Biol. 1994;14:7996–8006. doi: 10.1128/mcb.14.12.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shibahara S., Yasumoto K.I., Amae S., Udono T., Watanabe K.I., Saito H., Takeda K. Regulation of pigment cell-specific gene expression by MITF. Pigment Cell Melanoma Res. 2000;13:98–102. doi: 10.1034/j.1600-0749.13.s8.18.x. [DOI] [PubMed] [Google Scholar]
- 31.Singh S.K., Sarkar C., Mallick S., Saha B., Bera R., Bhadra R. Human placental lipid induces melanogenesis through p38 MAPK in B16F10 mouse melanoma. Pigment Cell Res. 2005;18:113–121. doi: 10.1111/j.1600-0749.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 32.Solano F., Briganti S., Picardo M., Ghanem G. Hypopigmenting agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006;19:550–571. doi: 10.1111/j.1600-0749.2006.00334.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee J., Jung K., Kim Y.S., Park D. Diosgenin inhibits melanogenesis through the activation of phosphatidylinositol-3-kinase pathway (pi3k) signaling. Life Sci. 2007;81:249–254. doi: 10.1016/j.lfs.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Strahle U., Scholz S., Geisler R., Greiner P., Hollert H., Rastegar S., Schumacher A., Selderslaghs I., Weiss C., Witters H. Zebrafish embryos as an alternative to animal experiments—a commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod Toxicol. 2012;33:128–132. doi: 10.1016/j.reprotox.2011.06.121. [DOI] [PubMed] [Google Scholar]
- 35.Elsalini O.A., Rohr K.B. Phenylthiourea disrupts thyroid function in developing zebrafish. Dev Genes Evol. 2003;212:593–598. doi: 10.1007/s00427-002-0279-3. [DOI] [PubMed] [Google Scholar]
- 36.Wang L., Lu A.P., Yu Z.L., Wong R.N., Bian Z.X., Kwok H.H., Yue P.Y., Zhou L.M., Chen H., Xu M. The melanogenesis-inhibitory effect and the percutaneous formulation of ginsenoside Rb1. AAPS Pharm Sci Technol. 2014;15:1252–1262. doi: 10.1208/s12249-014-0138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tung N.H., Song G.Y., Nhiem N.X., Ding Y., Tai B.H., Jin L.G., Lim C.M., Hyun J.W., Park C.J., Kang H.K. Dammarane-type saponins from the flower buds of Panax ginseng and their intracellular radical scavenging capacity. J Agric Food Chem. 2010;58:868–874. doi: 10.1021/jf903334g. [DOI] [PubMed] [Google Scholar]
- 38.Tung N.H., Quang T.H., Son J.H., Koo J.E., Hong H.J., Koh Y.S., Song G.Y., Kim Y.H. Inhibitory effect of ginsenosides from steamed ginseng-leaves and flowers on the LPS-stimulated IL-12 production in bone marrow-derived dendritic cells. Arch Pharm Res. 2011;34:681–685. doi: 10.1007/s12272-011-0419-2. [DOI] [PubMed] [Google Scholar]