Abstract
Background
Panax ginseng Meyer is cultivated because of its medicinal effects on the immune system, blood pressure, and cancer. Major ginsenosides in fresh ginseng are converted to minor ginsenosides by structural changes such as hydrolysis and dehydration. The transformed ginsenosides are generally more bioavailable and bioactive than the primary ginsenosides. Therefore, in this study, hydrothermal processing was applied to ginseng preparation to increase the yields of the transformed ginsenosides, such as 20(S)-Rg3, Rk1, and Rg5, and enhance antioxidant activities in an effective way.
Methods
Ginseng extract was hydrothermally processed using batch reactors at 100–160°C with differing reaction times. Quantitative analysis of the ginsenoside yields was performed using HPLC, and the antioxidant activity was qualitatively analyzed by evaluating 2,2'-azino-bis radical cation scavenging, 2,2-diphenyl-1-picrylhydrazyl radical scavenging, and phenolic antioxidants. Red ginseng and sun ginseng were prepared by conventional steaming as the control group.
Results
Unlike steaming, the hydrothermal process was performed under homogeneous conditions. Chemical reaction, heat transfer, and mass transfer are generally more efficient in homogeneous reactions. Therefore, maximum yields for the hydrothermal process were 2.5–25 times higher than those for steaming, and the antioxidant activities showed 1.6–4-fold increases for the hydrothermal process. Moreover, the reaction time was decreased from 3 h to 15–35 min using hydrothermal processing.
Conclusion
Therefore, hydrothermal processing offers significant improvements over the conventional steaming process. In particular, at temperatures over 140°C, high yields of the transformed ginsenosides and increased antioxidant activities were obtained in tens of minutes.
Keywords: antioxidant activity, ginseng, ginsenosides, hydrothermal processing, yield
1. Introduction
Ginseng has been widely used as a medicinal plant because it has many bioactivities, such as immune system modulation, antistress activity, and anticancer effects [1]. These bioactivities are generally attributed to a group of compounds called ginsenosides, which are the main ingredients of ginseng [2]. Ginsenosides are triterpene glycosides [3], and they can be classified into three types according to the location at which sugar moieties are attached to the triterpene skeleton: protopanaxadiol (PPD)-type, protopanaxatriol (PPT)-type, and oleanolic acid-type [4]. Among these ginsenosides, PPD-type and PPT-type are the most common [1]. In unprocessed ginseng, Rg1, Re, and Rf are PPT-type primary ginsenosides, and Rb1, Rc, Rb2, and Rd are PPD-type primary ginsenosides. These primary ginsenosides can be converted into transformed ginsenosides through deglycosylation and/or dehydration. In particular, PPD-type primary ginsenosides can be transformed into 20(S)-Rg3, Rk1, and Rg5 (Supplementary Fig. 1) [5].
Because the transformed ginsenosides contain fewer sugar moieties than the primary ginsenosides, the transformed ginsenosides are more bioavailable [6]. In addition, the transformed ginsenosides have various bioactivities that the primary ginsenosides do not have [7]. Therefore, many researchers have tried to increase the yield of the transformed ginsenosides, with three processes commonly utilized. The most typical process is steaming, and red ginseng and sun ginseng are prepared using this method [8], [9]. Other processes include the utilization of acidic catalysts [10], [11] and microorganisms or enzymes [12], [13]. However, these conventional methods are usually time-consuming and complex. The reaction time for the steaming process is over 3 h, whereas that for the biotransformation process, which requires incubation, is longer. Shorter reaction times can be achieved for the acid catalyst process, but corrosion and additional acid separation steps are inevitable. In this study, to overcome the disadvantages of these conventional processes, a hydrothermal process was introduced.
Hydrothermal reactions are chemical reactions performed in aqueous solution above 100°C and atmospheric pressure in a closed system [14], [15]. When a hydrothermal reaction is performed, as the reaction temperature increases to the critical temperature, the ion product of water increases [16], whereas the dielectric constant decreases [17]. With the increase in ion product, the concentrations of H+ and OH– increase; therefore, water with a higher ion product is a good medium for acid- or base-catalyzed reactions. Moreover, the decreased dielectric constant allows water to dissolve organic materials, similar to other organic solvents [18]. Although organic solvents and catalysts are not used, the effects achieved using a hydrothermal reaction in water are similar. Because of these properties, the hydrothermal reaction has been applied to biomass and biowaste transformation processes that conventionally need acid catalysts or organic solvents [19], [20]. Agricultural biomass and food biowaste have been hydrothermally converted to more valuable chemicals through hydrolysis and dehydration [21], [22]. As the transformation of primary ginsenosides to more valuable ginsenosides occurs through similar reaction mechanisms, we introduced a hydrothermal process for ginseng preparation.
In this study, hydrothermal reactions were performed for the first time for ginseng preparation under various operating conditions with differing temperatures and times to increase the yields of transformed ginsenosides and enhance the antioxidant activities. The hydrothermal process performed in this study was simpler, more time efficient, and more environmentally friendly than the typical transformation processes. The yields of the transformed ginsenosides, 20(S)-Rg3, Rk1, and Rg5, were calculated, and the antioxidant activities of the hydrothermally-reacted samples were measured using three methods. The obtained results were compared with those for conventionally prepared red ginseng and sun ginseng.
2. Materials and methods
2.1. Chemicals and reagents
HPLC-grade water (J.T. Baker, Center Valley, PA, USA) and acetonitrile (Samchun, Seoul, South Korea) were used as HPLC eluents. Milli-Q water from Millipore (Darmstadt, Germany) was also used as a solvent, except in HPLC measurements. Ethanol, used as an extraction solvent, was special grade (Samchun, Seoul, South Korea), whereas ethanol used for antioxidant activity measurements was HPLC grade (Fischer Scientific, Pittsburgh, PA, USA). HPLC-grade methanol (J.T. Baker, Center Valley, PA, USA) was used to check the antioxidant activity. Standard ginsenosides, Rg1, Re, Rf, Rb1, Rc, Rb2, Rd, and 20(S)-Rg3 were purchased from ChromaDex (Irvine, CA, USA). Rk1 and Rg5 were obtained from Ambo Institute (Daejoen, South Korea). 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzothiazo-line-6-sulfonic acid; ABTS), Folin–Ciocalteu reagent, 6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid (Trolox), gallic acid, and acrylamide were purchased from Sigma–Aldrich (St. Louis, MO, USA).
2.2. Plant material preparation
Fresh ginseng (4 yr old) was purchased from Kyung-dong market in Seoul, South Korea. The ginseng was washed, cut into pieces, and dried in an oven at 50°C for 3 d. Dried ginseng fragments were ground to pass through a 40-mesh sieve. The powder (10 g) was extracted for 8 h using a Soxhlet extractor with 300 mL of 50% aqueous ethanol as the extraction solvent. After extraction, the extract was evaporated to less than 30% of its initial volume using a rotary evaporator to remove ethanol. Three 0.3-mL aliquots of the concentrate were dried in a vacuum oven to determine the percent solids by weight in the extract. The concentrate was diluted to 4 weight% (wt%) solids for use as a reactant in the hydrothermal reaction.
2.3. Hydrothermal reaction
The hydrothermal reactions were performed using an oil bath (COB-22, Chosun Instruments, Seoul, South Korea) with 23-mL batch reactors. The reaction temperatures were 100°C, 120°C, 140°C, and 160°C, with corresponding reaction times of 6–72 h, 1–12 h, 15–135 min, and 5–35 min. The reaction pressures were the saturated vapor pressures (101 kPa, 199 kPa, 362 kPa, and 618 kPa) at each reaction temperature, and all experiments were repeated three times.
2.4. Control group preparation
As a control group, red ginseng and sun ginseng were prepared according to the published procedure with a slight modification [23]. Freshly cut ginseng was dried in an oven at 50°C for 3 d. The dried ginseng was then steamed in an autoclave (WiseClave WACS-1045, Witeg, Wertheim, Germany) at 100°C for 3 h to prepare red ginseng or at 120°C for 3 h to prepare sun ginseng. The steamed ginseng was dried in the oven at 50°C for 3 d. After drying, the procedures for Soxhlet extraction, evaporation, measurement of weight percent solids, and dilution to 4 wt% solids were performed as described in section 2.2.
2.5. HPLC analysis method
An Agilent 1100 series HPLC (Agilent, Santa Clara, CA) equipped with an Agilent Zorbax SB-Aq column (150 × 4.6 mm, 50 μm) was used for quantitative analysis. The flow rate was 1.0 mL/min, and the injection volume was 20 μL. The eluent system used was a slight modification of a published method [24]. Eluent A was water and eluent B was acetonitrile: 0–6 min (18–23% B), 6–48.5 min (23–40% B), 48.5–58 min (40% B), 58–60 min (40–100% B), 60–70 min (100% B), 70–71 min (100–18% B), and 71–75 min (18% B); the post time was 5 min (18% B). Detection was carried out at 203 nm and 25°C using a variable wavelength detector. Every sample was filtered with a 0.2-μm syringe filter from Advantec (Tokyo, Japan) prior to HPLC analysis.
2.6. Antioxidant activity measurements
2.6.1. DPPH radical scavenging ability
A 6 × 10–5M DPPH solution in methanol was prepared. Each sample (50 μL) was mixed with 2 mL of the DPPH solution and kept at room temperature for 16 min. The absorbance of this mixture at 517 nm was measured using an Evolution 201 UV-Vis spectrophotometer (Thermo Scientific, Waltham, MA, USA) [25]. This procedure was performed in triplicate for each sample. The DPPH radical scavenging ability is defined by the following formula:
| (1) |
where A0 is the absorbance of the DPPH solution at t = 0, A1 is the absorbance of the DPPH solution with added sample at t = 16 min, and A2 is the absorbance of 50 μL of the sample in methanol.
The DPPH radical scavenging ability was quantified according to a Trolox standard curve, and the results were converted to Trolox equivalent antioxidant capacity (TEAC) values.
2.6.2. ABTS radical cation scavenging ability
ABTS was dissolved in water (7mM), and potassium persulfate (2.45mM, final concentration) was added to the solution. The solution was kept in a dark room for 12–16 h before use and was subsequently diluted with ethanol to obtain an absorbance of 0.700 ± 0.02 at 734 nm. Each sample (20 μL) was mixed with 2 mL of the ABTS solution and then kept in a water bath at 30°C for 6 min. The absorbance at 734 nm was measured by UV-visible spectroscopy [25]. This procedure was performed in triplicate for each sample.
| (2) |
where A0 is the absorbance of the ABTS solution at t = 0, A1 is the absorbance of the ABTS solution with added sample at t = 6 min, and A2 is the absorbance of 20 μL of the sample in ethanol.
The ABTS radical cation scavenging ability was quantified using a Trolox standard curve, and the results were converted to TEAC values.
2.6.3. Total phenol content
Folin–Ciocalteu reagent was diluted with water (1:10 volume ratio), and a 20% sodium carbonate solution in water was prepared. Each sample (100 μL) was mixed with 2.2 mL of the Folin–Ciocalteu solution, and a sodium carbonate solution (1 mL) was added after 3 min. After 1 h, the absorbance at 765 nm was measured [26]. This procedure was performed in triplicate for each sample. The total phenol content was quantified using a gallic acid standard curve, and the results were converted to gallic acid equivalents.
2.7. Elemental analysis
The ginseng extract and ginseng powder were freeze-dried before elemental analysis. Elemental analysis for carbon, nitrogen, and oxygen was performed in triplicate at the National Center for Inter-University Research Facilities at Seoul National University.
2.8. Statistical analysis
Every experiment and analysis was performed in triplicate. The results were expressed as mean ± standard deviation. A one-way analysis of variance using Microsoft excel 2010 version (Microsoft Corporation, Redmond, WA, USA) was performed. Statistically significant differences were determined at p < 0.05.
3. Results and discussion
3.1. Ginsenoside yields
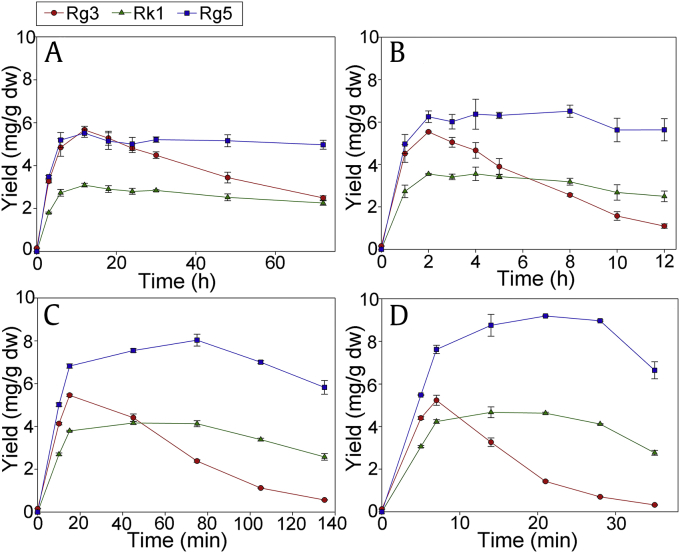
A comparison of the HPLC chromatograms of unprocessed ginseng extract (Supplementary Fig. 2A) and hydrothermally processed ginseng extract (Supplementary Fig. 2B) illustrates that the hydrothermal process transformed primary ginsenosides, such as Rb1, Rc, Rb2, and Rd, into deglycosylated and/or dehydrated ginsenosides, such as 20(S)-Rg3, Rk1, and Rg5. Hydrothermal reactions at 100°C were performed for 3 h, 6 h, 12 h, 18 h, 24 h, 30 h, 48 h, and 72 h. Among these reaction times, the product that reacted for 12 h showed the highest yields of 20(S)-Rg3 [5.67 mg/g dry weight (dw)], Rk1 (3.01 mg/g dw), and Rg5 (5.45 mg/g dw; Fig. 1A). At 120°C, the reactions were performed for 1 h, 2 h, 3 h, 4 h, 5 h, 8 h, 10 h, and 12 h. In this case, 20(S)-Rg3 (5.54 mg/g dw) was maximized at 2 h, Rk1 (3.56 mg/g dw) at 4 h, and Rg5 (6.51 mg/g dw) at 8 h (Fig. 1B). The reaction times at 140°C were 10 min, 15 min, 45 min, 75 min, 105 min, and 135 min. The yield of 20(S)-Rg3 (5.46 mg/g dw) was the highest at 15 min, Rk1 (4.16 mg/g dw) at 45 min, and Rg5 (8.03 mg/g dw) at 75 min (Fig. 1C). At 160°C, the hydrothermal reactions were performed for 5 min, 7 min, 14 min, 21 min, 28 min, and 35 min, and 20(S)-Rg3 (5.28 mg/g dw) was most abundant at 7 min, Rk1 (4.67 mg/g dw) at 14 min, and Rg5 (9.19 mg/g dw) at 21 min (Fig. 1D).
Fig. 1.
Yields of 20(S)-Rg3, Rk1, and Rg5 from hydrothermal reactions at (A) 100°C, (B) 120°C, (C) 140°C, and (D) 160°C. dw, dry weight.
For every 20°C increase in the reaction temperature, the maximum yield of 20(S)-Rg3 decreased by about 0.1 mg/g dw (5.67 mg/g dw, 5.54 mg/g dw, 5.46 mg/g dw, and 5.28 mg/g dw at 100°C, 120°C, 140°C, and 160°C, respectively). However, the corresponding maximum yields of Rk1 and Rg5 increased by approximately 0.5 mg/g dw (3.01 mg/g dw, 3.56 mg/g dw, 4.16 mg/g dw, and 4.67 mg/g dw) and 1 mg/g dw (5.45 mg/g dw, 6.51 mg/g dw, 8.03 mg/g dw, and 9.19 mg/g dw), respectively. The corresponding generation and decomposition rates determine the maximum yield of each ginsenoside. For 20(S)-Rg3, the increase in the decomposition rate with increasing reaction temperature was estimated to be greater than the corresponding increase in the generation rate. Therefore, the maximum yield of 20(S)-Rg3 gradually decreased with increasing temperature. However, the maximum yields of Rk1 and Rg5 increased because the increases in the generation rates of Rk1 and Rg5 with temperature were greater than the increases in the corresponding decomposition rates. Consequently, to maximize the yield of 20(S)-Rg3 using the hydrothermal process, a lower reaction temperature is preferred, whereas a higher reaction temperature is suitable to maximize the yields of Rk1 and Rg5.
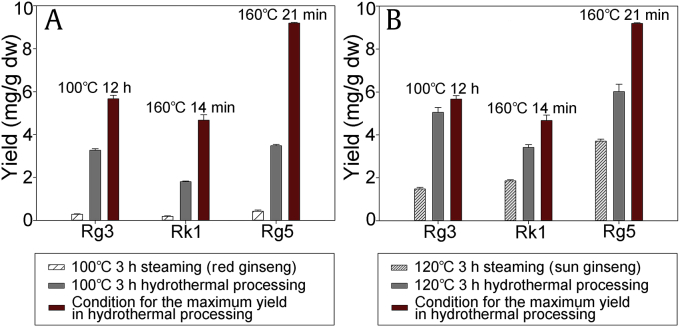
To compare the efficiencies of traditional treatment methods and the hydrothermal process, red ginseng and sun ginseng were prepared, and the yields of 20(S)-Rg3, Rk1, and Rg5 were determined (Supplementary Table 1). Because red ginseng and sun ginseng were steamed at 100°C and 120°C, respectively, for 3 h, the samples that hydrothermally reacted for 3 h at 100°C and 120°C were used for comparison (Figs. 2A and 2B). At 100°C, the yields of 20(S)-Rg3, Rk1, and Rg5 obtained by the hydrothermal reaction were about 11.3, 9.47, and 8.09 times higher, respectively, than those in red ginseng. When the hydrothermal reaction was performed at 120°C, the yields of 20(S)-Rg3, Rk1, and Rg5 were about 3.41, 1.83, and 1.62 times higher, respectively, than those in sun ginseng. In both cases, the hydrothermal reaction was more efficient than the steaming process. Moreover, the maximum yield of each transformed ginsenoside obtained by the hydrothermal reaction was increased considerably (Fig. 2).
Fig. 2.
Comparison of yields for hydrothermally reacted samples and (A) red ginseng or (B) sun ginseng. dw, dry weight.
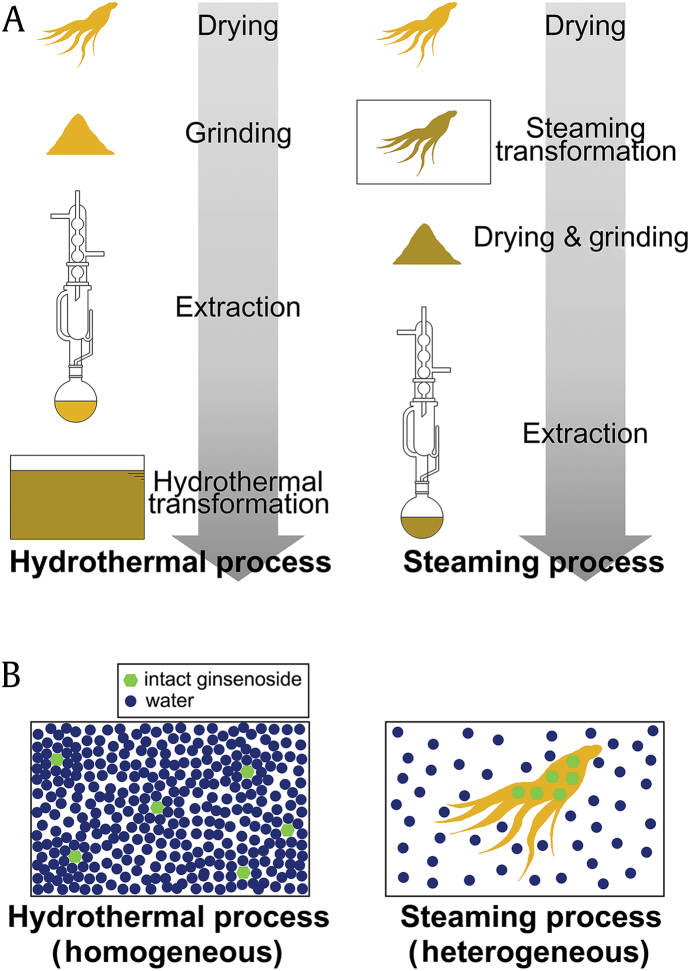
Although the reaction temperature and the heating time were the same, the yields of 20(S)-Rg3, Rk1, and Rg5 obtained using the hydrothermal reaction were higher than those obtained by steaming. This is probably due to the differences between homogeneous and heterogeneous reactions. 20(S)-Rg3, Rk1, and Rg5, which are minor ginsenosides in unprocessed ginseng, can be obtained by deglycosylation and/or dehydration of major ginsenosides, such as Rb1, Rc, and Rb2, which are abundant in fresh ginseng. The reactants for the generation of these minor ginsenosides are the major ginsenosides, which are primary ginsenosides, and water because deglycosylation of the primary ginsenosides is a type of hydrolysis reaction. In the hydrothermal process, the raw material for the transformation step was ginseng extract (Fig. 3A) and the primary ginsenosides were in water itself resulting in a homogeneous reaction (Fig. 3B). However, in the steaming process, the raw material for the transformation step was dried ginseng (Fig. 3A), and the primary ginsenosides were in the ginseng, while water existed in the form of vapor, thus resulting in a heterogeneous reaction (Fig. 3B). Because the reactants coexist in the same phase in a homogeneous reaction, the chemical reaction, heat transfer, and mass transfer are considered more efficient than those in a heterogeneous reaction. Therefore, the hydrothermal process was able to increase the yields of 20(S)-Rg3, Rk1, and Rg5 in comparison with those obtained by the steaming process.
Fig. 3.
(A) Steps in the hydrothermal process and steaming process. (B) Reaction type comparison for the hydrothermal process and steaming process.
The operating conditions that maximized the total yields of 20(S)-Rg3, Rk1, and Rg5 at 100°C, 120°C, 140°C, and 160°C were 720 min, 120 min, 45 min, and 7 min, respectively, and maximized total yields were 14.3 ± 0.43 mg/g dw, 15.3 ± 0.30 mg/g dw, 16.1 ± 0.30 mg/g dw, and 17.1 ± 0.50 mg/g dw, respectively. The natural logarithm of the reaction time was found to have a linear relation with the reaction temperature (R2 = 0.987; Supplementary Fig. 3). This result showed that the reaction time rapidly decreased with increasing reaction temperature in the hydrothermal process. Furthermore, using this graph, the operating conditions that maximize the total yield of 20(S)-Rg3, Rk1, and Rg5 can be roughly estimated, which is very useful when changing the operating conditions. For instance, although experiments were not performed at 150°C, the reaction time to maximize the total yield of the three transformed ginsenosides can be estimated to be around 16 min.
3.2. Antioxidant activities
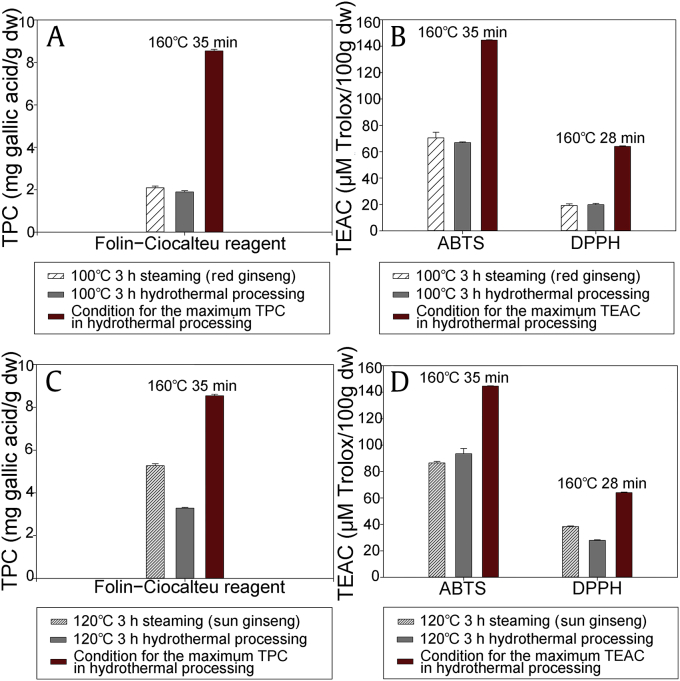
The antioxidant activities of the hydrothermally processed samples and the control group (Supplementary Table 2) were measured using three different methods. At each reaction temperature, the antioxidant activities increased as the reaction time increased, with markedly increased gradients at higher reaction temperatures. The maximum total phenol content (Supplementary Fig. 4A) at each reaction temperature was 3.13, 3.32, 3.74, and 4.74 times higher than that of raw ginseng extract. The maximum TEAC values for the hydrothermally processed product measured using ABTS (Supplementary Fig. 4B) and DPPH (Supplementary Fig. 4C) were 2.13–2.50 and 3.11–3.45 times higher, respectively, than those of the raw reactant.
The antioxidant activities of the samples that were hydrothermally reacted at 100°C and 120°C for 3 h were compared with those of red ginseng and sun ginseng, respectively (Fig. 4). Although the yields of 20(S)-Rg3, Rk1, and Rg5 in red ginseng were about 10 times lower than those in the sample hydrothermally reacted at 100°C, the antioxidant activities were similar (Figs. 4A and 4B). In the case of sun ginseng, the yields of the three ginsenosides were lower than those in the sample hydrothermally reacted at 120°C, but the antioxidant activities were higher (Figs. 4C and 4D). Although the hydrothermally reacted samples produced higher yields of the three ginsenosides, they showed lower antioxidant activities, which suggests that the three ginsenosides may not significantly influence antioxidant activity. In addition to ginsenosides, phenolic compounds and Maillard reaction products (MRPs) can influence antioxidant activities [27], [28]. It is difficult to determine which compounds enhanced the antioxidant activity more than the three ginsenosides because the antioxidant activities of each ginsenoside, phenolic compound, and MRP in red ginseng, sun ginseng, and the hydrothermally reacted samples could not be measured separately. However, these results suggest that the compounds with the greatest influence on the antioxidant activities are not 20(S)-Rg3, Rk1, and Rg5.
Fig. 4.
Comparisons of the antioxidant activities of the hydrothermally reacted samples and red ginseng [(A) total phenol content and (B) TEAC] or sun ginseng [(C) total phenol content and (D) TEAC]. ABTS, 2,2′-azino-bis (3-ethylbenzothiazo-line-6-sulfonic acid); DPPH, 2,2-diphenyl-1-picrylhydrazyl; dw, dry weight; TEAC = trolox equivalent antioxidant capacity; TPC, total phenolic content.
At the same reaction temperature and heating time, red ginseng and sun ginseng showed similar or higher antioxidant activities than the hydrothermally reacted samples. However, at 100°C over 72 h, 120°C over 8 h, 140°C over 75 min, and 160°C over 21 min, the antioxidant activities determined by all three methods for the hydrothermally reacted samples were higher than those determined for the control samples. The maximum antioxidant activities obtained using the hydrothermal process were 1.6–4 times higher than those in red ginseng and sun ginseng (Fig. 4).
MRPs are generated when amino acids and reducing sugars react at high temperatures [29]. From the yields of the three ginsenosides, it can be concluded that reducing sugars were generated in both the hydrothermal process and the steaming process because reducing sugars are obtained when sugars detach from major ginsenosides. Although sufficient amino acids exist in the ginseng powder used for the steaming process, it was necessary to determine whether amino acids were present in the ginseng extract used for the hydrothermal process. Thus, elemental analyses for carbon, nitrogen, and oxygen were performed for the ginseng extract and ginseng powder (Supplementary Table 3). While the ginseng powder had 1.89 wt% nitrogen, the ginseng extract only had 0.81 wt% nitrogen. This result indicates that amino acids were not extracted well into the ginseng extract. In the case of red ginseng and sun ginseng, the reaction media was ginseng; therefore, reducing sugars could react with amino acids present in the ginseng. However, the ginseng extract used in the hydrothermal process is deficient in amino acids; therefore, the reducing sugars may not fully react with amino acids to form MRPs. The amount of MRPs generated by the hydrothermal process seems to be lower than that generated by the steaming process, and this might be one of the factors that influence the antioxidant activities.
4. Conclusion
Hydrothermal processing of ginseng extract was carried out, and red ginseng and sun ginseng were prepared using the conventional steaming process for comparison. The hydrothermal process significantly increased the yields of 20(S)-Rg3, Rk1, and Rg5 in comparison with those of the control in every operating condition. This increase resulted from differences between the hydrothermal process as a homogeneous reaction and the steaming process as a heterogeneous reaction. As a homogeneous reaction, the hydrothermal processing of ginseng extract has the potential for commercialization in the form of a semicontinuous process to produce large amounts of the three transformed ginsenosides. Therefore, we plan to carry out hydrothermal processing of ginseng extract in a continuous system. Moreover, in the hydrothermal process, the observed linear relation between the natural logarithm of the reaction time and temperature can be used to determine optimized operating conditions. Meanwhile, it was found that 20(S)-Rg3, Rk1, and Rg5 are not the main factors that influence antioxidant activity. Although the yields of these three ginsenosides from the hydrothermal process were significantly higher than those from the steaming process, the antioxidant activities were similar or lower. The lower nitrogen content in ginseng extract indicated that fewer MRPs were generated in the hydrothermally processed samples, which might affect the antioxidant activities. However, at temperatures higher than 140°C, the antioxidant activities were successfully increased at short reaction times. Overall, the hydrothermal processing of ginseng extract can be used to greatly increase the yields of 20(S)-Rg3, Rk1, and Rg5, as well as the antioxidant activities of the samples in a short reaction time.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jgr.2016.12.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Christensen L.P. Chapter 1 Ginsenosides; Chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2008;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 2.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 3.Kim S.J., Murthy H.N., Hahn E.J., Lee H.L., Paek K.Y. Effect of processing methods on the concentrations of bioactive components of ginseng (Panax ginseng C.A. Meyer) adventitious roots. LWT–Food Sci Technol. 2008;41:959–964. [Google Scholar]
- 4.Mi J., Zhang M., Ren G., Zhang H., Wang Y., Hu P. Enriched separation of protopanaxatriol ginsenosides, malonyl ginsenosides and protopanaxadiol ginsenosides from Panax ginseng using macroporous resins. J Food Eng. 2012;113:577–588. [Google Scholar]
- 5.Qi L.W., Wang C.Z., Yuan C.S. American ginseng: Potential structure–function relationship in cancer chemoprevention. Biochem Pharmacol. 2010;80:947–954. doi: 10.1016/j.bcp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Wang J.R., Yau L.F., Zhang R., Xia Y., Ma J., Ho H.M., Hu P., Hu M., Liu L., Jiang Z.H. Transformation of ginsenosides from notoginseng by artificial gastric juice can increase cytotoxicity toward cancer cells. J Agric Food Chem. 2014;62:2558–2573. doi: 10.1021/jf405482s. [DOI] [PubMed] [Google Scholar]
- 7.Park J.Y., Choi P., Kim T., Ko H., Kim H.K., Kang K.S., Ham J. Protective effects of processed ginseng and its active ginsenosides on cisplatin-induced nephrotoxicity: In vitro and in vivo studies. J Agric Food Chem. 2015;63:5964–5969. doi: 10.1021/acs.jafc.5b00782. [DOI] [PubMed] [Google Scholar]
- 8.Chen J., Du B., Cai W., Xu B. Ginsenosides and amino acids in flavored ginseng chips as affected by food formulation and processing technology. LWT–Food Sci Technol. 2015;62:517–524. [Google Scholar]
- 9.Lee S.M., Bae B.S., Park H.W., Ahn N.G., Cho B.G., Cho Y.L., Kwak Y.S. Characterization of Korean Red Ginseng (Panax ginseng Meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39:384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim M.H., Hong H.D., Kim Y.C., Rhee Y.K., Kim K.T., Rho J. Ginsenoside changes in red ginseng manufactured by acid impregnation treatment. J Ginseng Res. 2010;34:93–97. [Google Scholar]
- 11.Quan K., Liu Q., Wan J.Y., Zhao Y.J., Guo R.Z., Alolga R.N., Li P., Qi L.W. Rapid preparation of rare ginsenosides by acid transformation and their structure–activity relationships against cancer cells. Sci Rep. 2015;5:8598. doi: 10.1038/srep08598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi H.S., Kim S.Y., Park Y., Jung E.Y., Suh H.J. Enzymatic transformation of ginsenosides in Korean Red Ginseng (Panax ginseng Meyer) extract prepared by Spezyme and Optidex. J Ginseng Res. 2014;38:264–269. doi: 10.1016/j.jgr.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu B.Y., Lu T.J., Chen C.H., Wang S.J., Hwang L.S. Biotransformation of ginsenoside Rd in the ginseng extraction residue by fermentation with lingzhi (Ganoderma lucidum) Food Chem. 2013;141:4186–4193. doi: 10.1016/j.foodchem.2013.06.134. [DOI] [PubMed] [Google Scholar]
- 14.Feng S., Xu R. New materials in hydrothermal synthesis. Acc Chem Res. 2001;34:239–247. doi: 10.1021/ar0000105. [DOI] [PubMed] [Google Scholar]
- 15.Rabenau A. The role of hydrothermal synthesis in preparative chemistry. Angew Chem Int Ed Engl. 1985;24:1026–1040. [Google Scholar]
- 16.Bandura A.V., Lvov S.N. The ionization constant of water over wide ranges of temperature and density. J Phys Chem Ref Data. 2006;35:15–30. [Google Scholar]
- 17.Fernández D.P., Mulev Y., Goodwin A.R.H., Sengers J.M.H.L. A database for the static dielectric constant of water and steam. J Phys Chem Ref Data. 1995;24:33–70. [Google Scholar]
- 18.Akiya N., Savage P.E. Roles of water for chemical reactions in high-temperature water. Chem Rev. 2002;102:2725–2750. doi: 10.1021/cr000668w. [DOI] [PubMed] [Google Scholar]
- 19.Brunner G. Near critical and supercritical water. Part I. Hydrolytic and hydrothermal processes. J Supercrit Fluids. 2009;47:373–381. [Google Scholar]
- 20.Pavlovič I., Knez Ž., Škerget M. Hydrothermal reactions of agricultural and food processing wastes in sub- and supercritical water: a review of fundamentals, mechanisms, and state of research. J Agric Food Chem. 2013;61:8003–8025. doi: 10.1021/jf401008a. [DOI] [PubMed] [Google Scholar]
- 21.Ingram T., Wörmeyer K., Lima J.C.I., Bockemühl V., Antranikian G., Brunner G., Smirnova I. Comparison of different pretreatment methods for lignocellulosic materials. Part I: conversion of rye straw to valuable products. Bioresour Technol. 2011;102:5221–5228. doi: 10.1016/j.biortech.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Ruen-ngam D., Quitain A.T., Sasaki M., Goto M. Hydrothermal hydrolysis of hesperidin into more valuable compounds under supercritical carbon dioxide condition. Ind Eng Chem Res. 2012;51:13545–13551. [Google Scholar]
- 23.Kang K.S., Kim H.Y., Baek S.H., Yoo H.H., Park J.H., Yokozawa T. Study on the hydroxyl radical scavenging activity changes of ginseng and ginsenoside-Rb2 by heat processing. Biol Pharm Bull. 2007;30:724–728. doi: 10.1248/bpb.30.724. [DOI] [PubMed] [Google Scholar]
- 24.Shehzad O., Kim H.P., Kim Y.S. State-of-the-art separation of ginsenosides from Korean white and red ginseng by countercurrent chromatography. Anal Bioanal Chem. 2013;405:4523–4530. doi: 10.1007/s00216-012-6609-z. [DOI] [PubMed] [Google Scholar]
- 25.Katalinic V., Milos M., Kulisic T., Jukic M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006;94:550–557. [Google Scholar]
- 26.Wojdyło A., Oszmiański J., Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2006;105:940–949. [Google Scholar]
- 27.Jung M.Y., Jeon B.S., Bock J.Y. Free, esterified, and insoluble-bound phenolic acids in white and red Korean ginsengs (Panax ginseng C.A. Meyer) Food Chem. 2002;79:105–111. [Google Scholar]
- 28.Kim J.H., Han I.H., Yamabe N., Kim Y.J., Lee W., Eom D.W., Choi P., Cheon G.J., Jang H.J., Kim S.N. Renoprotective effects of Maillard reaction products generated during heat treatment of ginsenoside Re with leucine. Food Chem. 2014;143:114–121. doi: 10.1016/j.foodchem.2013.07.075. [DOI] [PubMed] [Google Scholar]
- 29.Hodge J.E. Dehydrated foods: Chemistry of browning reactions in model systems. J Agric Food Chem. 1953;1:928–943. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.