Abstract
Background
Ginsenoside Rg1 belongs to protopanaxatriol-type ginsenosides and has diverse pharmacological activities. In this report, we investigated whether Rg1 could upregulate muscular stem cell differentiation and muscle growth.
Methods
C2C12 myoblasts, MyoD-transfected 10T1/2 embryonic fibroblasts, and HEK293T cells were treated with Rg1 and differentiated for 2 d, subjected to immunoblotting, immunocytochemistry, or immunoprecipitation.
Results
Rg1 activated promyogenic kinases, p38MAPK (mitogen-activated protein kinase) and Akt signaling, that in turn promote the heterodimerization with MyoD and E proteins, resulting in enhancing myogenic differentiation. Through the activation of Akt/mammalian target of rapamycin pathway, Rg1 induced myotube growth and prevented dexamethasone-induced myotube atrophy. Furthermore, Rg1 increased MyoD-dependent myogenic conversion of fibroblast.
Conclusion
Rg1 upregulates promyogenic kinases, especially Akt, resulting in improvement of myoblast differentiation and myotube growth.
Keywords: ginsenoside Rg1, myoblast differentiation, myogenic conversion, myotube hypertrophy, promyogenic signaling
1. Introduction
The maintenance of muscle mass is important for staving off the risk of metabolic syndrome and age-related muscular dystrophy, and this significantly contributes to quality of life [1]. Degenerative disorders of skeletal muscles are associated with declined regenerative abilities accompanied by deregulation of satellite cells that function as muscle stem cells [2]. Differentiation of skeletal myoblasts is a multistage process and includes self-renewal of myogenic cells, exit of cell cycle, expression in the muscle-specific transcriptional genes, and fusion into multinucleated myofibers [3], [4]. MyoD plays a role as a critical transcriptional regulator in skeletal muscle to induce myogenesis and can convert fibroblasts, non-muscular cell types, into differentiated myoblasts [5]. It has been well documented that promyogenic kinases, p38 MAPK (mitogen-activated protein kinase), and Akt play critical roles in myoblast differentiation, such as enhancement of myoblast differentiation, upregulation of heterodimerization with MyoD and E proteins, activation of Mef2, alteration in chromatin remodeling complex to myogenic loci, and cell survival [6], [7], [8], [9], [10], [11]. Both p38MAPK and Akt pathways are required for activating and reinforcing each other's activity to lead to efficient myogenesis [12]. Especially, Akt/mammalian target of rapamycin (mTOR) pathway acts as a crucial regulator of muscle protein synthesis and hypertrophy [13], [14]. In contrast, muscular atrophy arises from the decrease in protein synthesis and the decline in the amount of muscle proteins through down-regulating Akt/mTOR signaling [1], [15]. The importance of skeletal muscle function for the improvement of metabolic health and life quality has attracted much attention to understanding molecular regulatory mechanisms of controlling muscular stem cell differentiation and muscle growth as well as identifying compound(s) to improve muscle function and mass.
Panax ginseng Meyer has been used as a conventional medicinal plant for millennia in Japan, China, and Korea. In Korean medicine, ginseng has been traditionally used to revitalize the mind and body, prevent aging, and increase physical strength and vigor [16]. Ginsenosides are the main pharmacologically active compounds of P. ginseng, which are classified in three types based on aglycone moieties, namely protopanaxadiol-, protopanaxatriol- and oleanolic acid-type ginsenosides [17]. It has been reported that all ginsenosides have broad biological and physiological effects, such as anti-amnestic, anti-inflammatory, anti-oxidant, anti-diabetic, and anti-tumor activity [18], [19], [20], [21]. However, there are no reports to explore the regulatory effect of P. ginseng on myoblast differentiation and myotube growth in C2C12 myoblasts. Ginsenoside Rg1, identified as a protopanaxatriol-type, has pharmacological actions such as neuroprotective and anti-tumor effects on various cancer types [22]. Recently, it was reported that Rg1 enhances glucose uptake in insulin-resistant myoblasts, resulting from the activation of the AMPK-activated protein kinase (AMPK) signaling pathway [23]. However, the effects of Rg1 on myogenesis and myotube formation have not been examined.
In this study, we demonstrate that Rg1 from P. ginseng enhances myoblast differentiation and myotube growth. Rg1 protects myotubes from dexamethasone (DEX)-triggered atrophy through the Akt/mTOR pathway. Rg1 treatment enhances myoblast differentiation through upregulating of key promyogenic signalings, Akt, and p38MAPK, which in turn elevates the heterodimerization with MyoD and E proteins. Furthermore, Rg1 increases the MyoD-dependent trans-differentiation of mouse embryonic fibroblasts into myoblasts. Collectively, our findings suggest that Rg1 improves myoblast differentiation and myotube hypertrophy via activation of key promyogenic signalings, especially Akt and MyoD-mediated gene expression. Thus, Rg1 might be an effective therapeutic or neutraceutical remedy to intervene muscle weakness and atrophy.
2. Materials and methods
2.1. Materials
Rg1 (purity > 99%) was kindly provided by Professor Kim, Si-Kwan (Konkuk University, Chungju, Korea). Fetal bovine serum (FBS), horse serum (HS), and Dulbecco modified Eagle's medium (DMEM) were obtained from Gibco (Grand Island, NY, USA). Antibodies were as following: MHC (myosin heavy chain; the Developmental Studies Hybridoma Bank, Iowa, IA, USA), Akt, phospho-Akt (pAkt), p70S6K, phospho-p70S6K (pp70S6K), mTOR, phospho-mTOR (pmTOR), phospho-p38MAPK (pp38) (Cell Signaling Technology, Beverly, MA, USA), pan-Cadherin (Sigma, St. Louis, MO, USA), p38MAPK, MyoD, Myogenin, E2A, and α-Tubulin (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
2.2. Cell cultures
Myoblast C2C12 cells, embryonic fibroblast 10T1/2 cells, and HEK293T cells were cultured, as described previously [9], [24]. For the induction of myogenic differentiation, C2C12 cells were exchanged from growth medium (GM) containing 15% FBS to differentiation medium (DM) containing 2% HS. The efficiency of the myoblast differentiation was quantified, as previously described [25]. To induce hypertrophic myoblast differentiation, C2C12 cells were differentiated for 2 d and then treated with Rg1 for additional 2 d in DM. For the DEX-induced atrophy study, C2C12 cells were triggered to differentiate for 24 hrs and treated with 25μM DEX and 10nM Rg1, followed by incubation in DM for 2 d [15].
For our experiments that involved pharmacological inhibitors (CalBiochem, La Jolla, CA, USA), C2C12 cells were pre-incubated with 1μM SB203580 or 2.5μM LY294003 for 30 min, respectively, and then treated with Rg1 for 2 d. For the myogenic conversion study, MyoD-transfected 10T1/2 embryonic fibroblasts were treated with Rg1 and differentiated for 2 d.
2.3. Immunoblotting and immunoprecipitation analyses
Immunoblotting analysis was performed, as previously described [10]. Briefly, cells were lysed in cell lysis buffer [pH 7.2, 1mM ethylenediaminetetraacetic acid (EDTA), 10mM Tris-HCl, 1% Triton X-100, 150mM sodium chloride (NaCl)] containing complete proteinase inhibitor cocktail (Roche Diagnostics, Basel, Switzerland). Cell lysates were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis. The primary antibodies used were pp38MAPK, p38MAPK, pAkt, Akt, mTOR, pmTOR, p70S6K, pp70S6K, p4E-BP1, 4E-BP1, MHC, MyoD, Myogenin, E2A, α-Tubulin and pan-Cadherin.
For immunoprecipitation assay, C2C12 or MyoD-transfected 293T cells were treated with Rg1 and lysed in cell lysis buffer, followed by immunoprecipitation with anti-E2A antibodies overnight at 4°C. The immunoprecipitants were mixed with protein G agarose beads (Roche Diagnostics) for 2 hrs at 4°C and analyzed by immunoblotting with antibodies against MyoD and E2A.
2.4. Immunocytochemistry
Immunostaining for MHC expression was performed, as described previously [9]. Briefly, C2C12 myoblasts or 10T1/2 embryonic fibroblasts were fixed, permeabilized, blocked, and incubated with anti-MHC, followed by an Alexa Fluor 568-conjugated secondary antibody (Molecular Probes, Eugene, OR, USA). Images were captured and processed with a Nikon ECLIPSE TE-2000U microscope and NIS-Elements F software (Nikon, Tokyo, Japan).
2.5. Statistical analysis
The experiments were performed independently three times. The participants' t test was used to access the significance of the difference between two mean values. *p < 0.05 and **p < 0.01 were considered to be statistically significant.
3. Results and discussion
3.1. Rg1 enhances myogenic differentiation through activation of p38MAPK and Akt
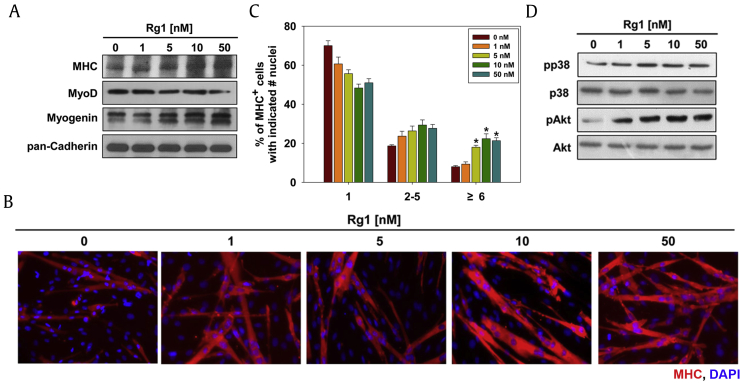
To identify potential regulators of MyoD-mediated myoblast differentiation, we had screened the MHC expression of C2C12 myoblasts. Rg1 was selected from 18 ginsenosides including crude saponin through this screening experiment. First, we investigated whether Rg1 can promote myogenic differentiation and the myotube formation. C2C12 cells were induced to differentiate for 2 d (D2) in dimethyl sulfoxide (DMSO) or the indicated concentration of Rg1 and assessed on myogenic differentiation by Western blotting with muscle-specific proteins, including MHC, MyoD, and Myogenin. C2C12 myoblasts treated with Rg1 displayed the incremental expression of Myogenin and MHC dose-dependently without change in MyoD expression (Fig. 1A). As assessed by MHC immunostaining, Rg1 treatment in C2C12 myoblasts for 2 d formed bigger MHC-positive multinucleated myotubes compared to the control cells (Fig. 1B). To quantify the myotube formation, MHC-positive myocytes were counted as mononucleate, containing two to five nuclei, or containing six or more nuclei per myotube and plotted as percentile (Fig. 1C). The treatment with Rg1 decreased the proportion of mononucleate myocytes, while it substantially elevated the proportion of bigger myotubes containing six or more nuclei dose-dependently (Fig. 1C).
Fig. 1.
Rg1 promotes myogenic differentiation through promyogenic kinases in C2C12 myoblasts. (A) Rg1-treated C2C12 cells were differentiated in DM for 2 d. Using prepared cell lysates, MHC, MyoD, and Myogenin were analyzed by immunoblotting. Pan-Cadherin was used as a loading control. (B) To observe myotube formation, the expression of MHC with cells from panel A was analyzed by immunostaining. DAPI was used to visualize nuclei. (C) The MHC-positive myocytes shown in panel B were quantified as a number of nuclei per myotube. Values are presented as means ± standard deviation (SD) of three independent experiments. (D) The total and phosphorylated forms of p38MAPK and Akt of cell lysates from panel A were analyzed by immunoblotting. **p < 0.01 compared with control group. DAPI, 4',6-Diamidine-2'-phenylindole dihydrochloride; DM, differentiation medium; MHC, myosin heavy chain.
It has been reported that p38MAPK and Akt, key promyogenic kinases, play an important role in myoblast differentiation [9], [10], [11]. To assess the molecular regulatory pathways of Rg1-mediated myoblast differentiation, C2C12 myoblasts were treated with various concentrations of Rg1 and assessed for the activation status of Akt and p38 by using antibodies recognizing pAkt or pp38 by immunoblotting. The treatment of Rg1 dramatically elevated pp38 and pAkt levels in a dose-dependent manner without changes in total protein levels (Fig. 1D). These data indicate that Rg1 enhanced C2C12 myoblast differentiation at a biochemical as well as morphological level, suggesting that the promyogenic effect of Rg1 is dependent on the activation of Akt and p38MAPK pathways to enhance MyoD activities and myoblast differentiation.
3.2. Rg1 increases the heterodimerization of MyoD with E proteins
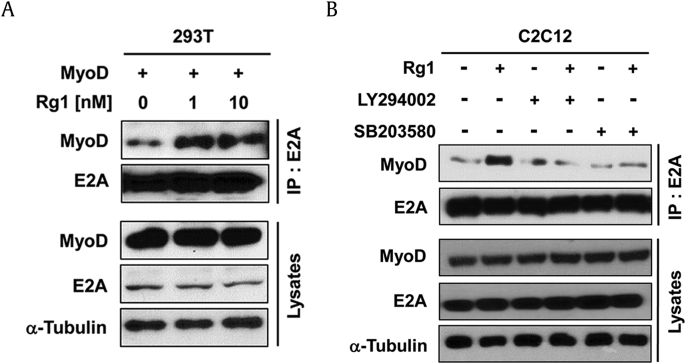
Previous studies have shown that p38MAPK and Akt enhance the heterodimer formation of MyoD and its partner E proteins leading to the MyoD activation [7], [9], [26]. Therefore, we examined whether the heterodimerization of E proteins with MyoD is enhanced by Rg1 treatment. For this, 293T cells expressing MyoD were treated with DMSO or Rg1 for 36 hrs, followed by immunoprecipitation with antibodies to E2A and immunoblotting with MyoD antibodies. E2A and MyoD protein levels were constant in total lysates regardless of the Rg1 treatment (Fig. 2A). Furthermore, treatment with Rg1 dramatically elevated the amount of MyoD in the E2A precipitates in MyoD-transfected C2C12 myoblasts compared with those of DMSO-treated cells (Fig. 2A).
Fig. 2.
Rg1 enhances the heterodimerization of E protein with MyoD. (A) 293T cells transfected with MyoD were treated with Rg1 for 36 hrs, followed by immunoprecipitation with anti-E2A antibodies and immunoblotting using anti-MyoD antibodies. Cell lysates are used as an input control for each protein. (B) C2C12 myoblasts were pretreated with 1μM Akt inhibitor (LY294002) or 2.5μM p38MAPK inhibitor (SB203580). After 30 min, C2C12 myoblasts were treated with Rg1 and then differentiated for 48 hrs. Total lysates were followed by immunoprecipitation with anti-E2A antibodies and immunoblotting analysis using anti-MyoD antibodies. Total lysates are used as the input control.
To further confirm Rg1 effects on the complex formation of MyoD with E2A, the C2C12 myoblasts treated with Rg1 for 2 d were subjected to co-immunoprecipitation analysis. The levels of E2A and MyoD protein remained constant in lysates in Rg1-treated C2C12 myoblasts relative to the DMSO-treated cells (Fig. 2B). In agreement with the exogenous interaction, Rg1 treatment increased the amount of MyoD proteins in the precipitates with E2A antibodies compared with control cells. Next, we examined whether the activation of p38MAPK and Akt is required for the Rg1-mediated heterodimerization. In Fig. 2B, both pharmacological inhibitors, LY294002 for Akt and SB203580 for p38MAPK, decreased the amount of MyoD in the E2A precipitates similar to vehicle-treated cells. Rg1 treatment failed to rescue the heterodimerization with MyoD and E2A in SB203580- or LY294002-pretreated C2C12 myoblasts. p38MAPK promotes the phosphorylation of E47 protein and MyoD/E47 association, resulting in stimulating MyoD-mediated muscle-specific gene transcription [27]. Lack of Akt impairs transcriptional functions of MyoD, and Akt signaling plays an important role in the induction of muscle-specific gene expression in early myogenesis [26]. These data suggest that Rg1 upregulates MyoD activity by augmenting its heterodimer formation with E proteins, likely through activation of promyogenic kinases, such as p38MAPK and Akt.
3.3. Rg1 promotes myotube growth through the activation of Akt/mTOR signalings
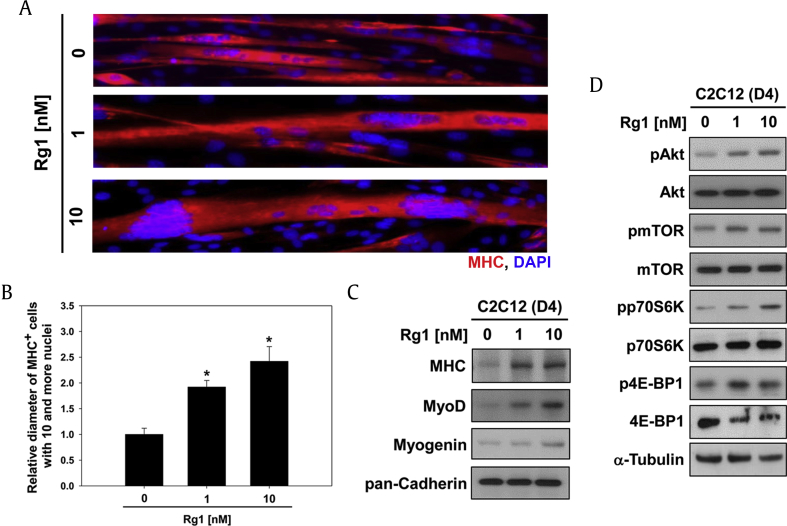
Skeletal muscle mass is tightly regulated by Akt/mTOR signalings, which act as promoters of muscular protein synthesis and hypertrophy [13], [14]. To explore whether Rg1 can promote Akt-mediated muscle hypertrophy, C2C12 myoblasts were induced to differentiate for 2 d (D2), followed by treatment of Rg1 for additional 2 d (D4). These cells were then subjected to immunostaining for MHC to assess myotube formation. Both DMSO- or Rg1-treated C2C12 cells formed large multinucleated myotubes; however, Rg1-treated C2C12 cells formed larger myotubes dose-dependently than DMSO-treated cells (Fig. 3A). Measurement of myotube diameters revealed that Rg1 treatment enhanced the myotube thickness, especially about 2.45-fold in 10nM Rg1 (Fig. 3B). To further examine whether Rg1 upregulates the expression of muscle-specific proteins and involved signaling pathways, C2C12 cells were incubated under the same experimental conditions as mentioned above, subjected to immunoblotting analysis. Similarly to early differentiation, myotubes treated with Rg1 showed higher levels of MHC, MyoD, and Myogenin expression than the control myotubes (Fig. 3C). The treatment with Rg1 increased the activation of Akt and Akt downstream targets as evident by greatly enhanced levels of the phosphorylated forms of mTOR, p70S6K, and 4E-BP1 compared with the vehicle-treated cells (Fig. 3D). Panaxatriol derived from P. ginseng has been reported to enhance resistance exercise-triggered protein synthesis through mTOR complex 1 signaling in rat skeletal muscle [28]. S6K is required for maintaining protein quality during high rates of muscle growth, and S6K and 4E-BP1 independently mediate rapamycin-sensitive muscle hypertrophy [29]. These data indicate that Rg1 elicits activation of Akt/mTOR signalings to induce myotube hypertrophy.
Fig. 3.
Rg1 promotes Akt-dependent myotube hypertrophy. (A) C2C12 myoblasts were induced to differentiate in DM for 2 d and then treated with Rg1 for additional 2 d. The myotube formation was analyzed by MHC immunostaining. DAPI was used to visualize nuclei. (B) Average myotube diameter shown in panel (A) was measured by NIS-Elements F software (Nikon, Tokyo, Japan). Data are presented as means determination of six fields ± 1 SD. ** p < 0.01 compared with control group. (C) Muscle-specific proteins from panel A were analyzed by immunoblotting. Pan-Cadherin was used as a loading control. (D) Total and phosphorylated forms of Akt, mTOR, p70S6K, and 4E-BP1 from panel A were analyzed by immunoblotting. α-Tubulin was used as the loading control. DAPI, 4',6-Diamidine-2'-phenylindole dihydrochloride; DM, differentiation medium; MHC, myosin heavy chain; SD, standard deviation.
3.4. Rg1 prevents DEX-induced myotube atrophy via Akt activation
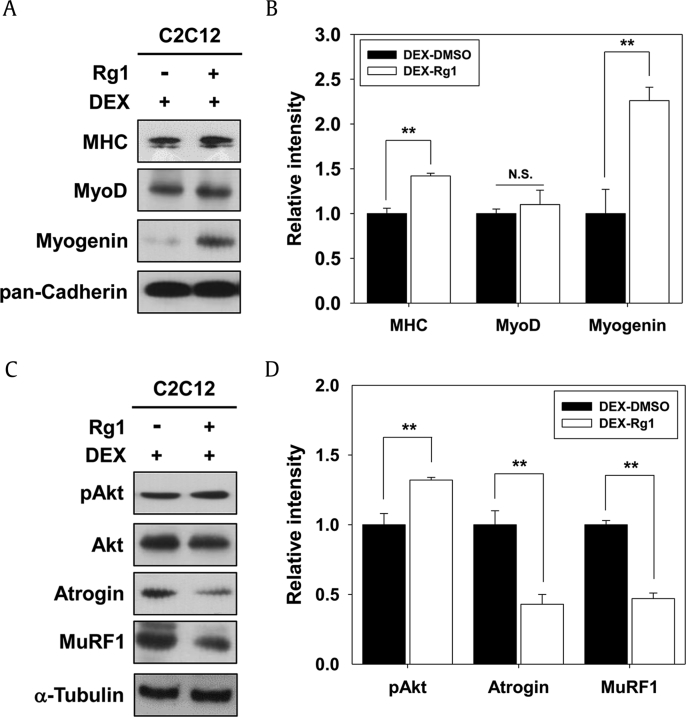
It has been reported that Akt is can suppress atrophic processes, allowing it to inhibit glucocorticoid-mediated muscular atrophy [15]. The synthetic glucocorticoid DEX triggers myotube atrophy in vivo and in vitro [15], [30]. We investigated whether Rg1 can protect myotubes from DEX-induced muscle atrophy. C2C12 myoblasts were induced to differentiate in DM for 24 hrs, followed by treatment with 25μM DEX along with vehicle or Rg1 for additional 2 d (D3). These cells were then subjected to immunoblot with MHC, MyoD, and Myogenin. As shown in Figs. 4A and 4B, the rescue effect of Rg1 treatment on DEX-induced atrophic myotubes was reflected by the restored expression of MHC and Myogenin, similar to the level of vehicle control.
Fig. 4.
Rg1 prevented DEX-induced myotube atrophy through activation of Akt signaling. (A) C2C12 cells were differentiated in DM for 1 d and then treated with 25μM DEX along with vehicle or 10nM Rg1 in fresh DM for additional 2 d. The cell lysates were followed by immunoblotting against antibodies of muscle-specific proteins. Pan-Cadherin was used as the loading control. (B) The signal intensity of indicated muscle-specific proteins was quantified in three independent experiments and normalized to the loading control pan-Cadherin. The values from DMSO-treated DEX-induced cells were set to 1.0. Values are presented as means ± SD. ** p < 0.01 compared to control. (C) Cell lysates were subjected to immunoblotting against anti-pAkt, anti-Akt, anti-Atrogin, and anti-MuRF1. α-Tubulin was used as the loading control. (D) Quantification of three experiments was performed as shown in panel C. The signal intensity of phosphorylated Akt was calculated and normalized to the loading control of total Akt. The signaling intensities of Atrogin and MuRF1 proteins were calculated and normalized to the loading control α-Tubulin. The values from DMSO-treated DEX-induced cells were set to 1.0. Values are presented as means ± SD. ** p < 0.01 compared to control group. DEX, dexamethasone; DM, differentiation medium; DMSO, dimethyl sulfoxide; MHC, myosin heavy chain; NS, not significant; SD, standard deviation.
The IGF-1/Akt pathway that has been shown to enhance hypertrophy inhibits the induction of atrophy mediators, MAFbx/Atrogin and MuRF1, which are known as the skeletal muscle-specific ubiquitin ligases [1], [15]. To investigate whether Rg1 can modulate Akt activation and these ubiquitin ligases in the above mentioned experimental conditions, the expression levels of Atrogin and MuRF1 were determined. Rg1 treatment in DEX-induced atrophic myotubes increased the levels of pAkt and starkly decreased the expression levels of Atrogin and MuRF1 compared with those of DEX-treated cells (Figs. 4C and 4D). The inhibitory mechanism for the skeletal muscle-specific ubiquitin ligases is associated with Akt-mediated inhibition of the FoxO family of transcriptional factors, thereby inhibiting Akt-mediated inhibition of the upregulation of MuRF1 and Atrogin [31], [32]. Taken together, these data suggest that Rg1 prevents DEX-induced myotube atrophy through the inhibition of muscle-specific ubiquitin ligases mediated by Akt activation.
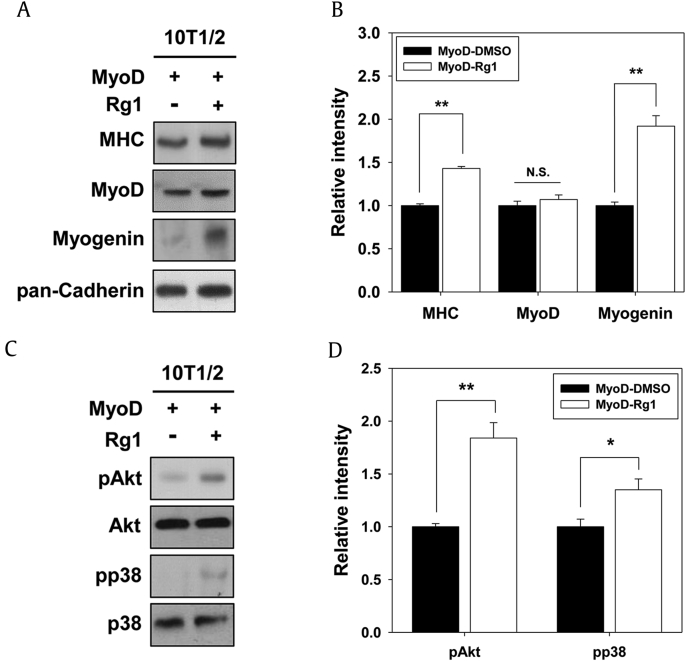
3.5. Rg1 promotes the MyoD-mediated myogenic conversion of fibroblasts
MyoD reportedly possesses the ability to convert fibroblasts into myogenic lineage [5]. Thus, we investigated whether Rg1 induces MyoD-mediated myogenic conversion of embryonic fibroblasts, one of the non-muscular cell types into myoblasts. 10T1/2 mouse embryonic fibroblast cells expressing with pBp/MyoD or control pBp (pBabe-puro) and treated with vehicle or Rg1 and induced to differentiate for 2 d, followed by immunoblotting with muscle-specific proteins. The MyoD overexpression in the vehicle-treated 10T1/2 fibroblasts triggered the expression of MHC, MyoD, and Myogenin, which was further elevated by the Rg1 treatment (Figs. 5A and 5B). We further examined the activation status of Akt and p38MAPK in 10T1/2 cells. Rg1 treatment in MyoD-transfected 10T1/2 cells slightly elevated the phosphorylated levels of p38MAPK and Akt compared with control 10T1/2 cells (Figs. 5C and 5D). MyoD can change non-muscular cell types, such as adipocytes, fibroblasts, osteosarcoma, and melanoma, into skeletal muscular cells [5]. The activation of p38MAPK is required for MyoD-dependent myogenic conversion of fibroblasts [33]. The therapy of cell replacement is paid attention to a trustworthy strategy for diverse degenerative diseases in skeletal muscle including sarcopenia, cachexia, and muscular atrophy [34]. Collectively, these data indicate that Rg1 could improve the MyoD-mediated myogenic conversion of fibroblasts into myoblasts through p38MAPK and Akt activation, indicating that Rg1 has a potentiality for the induction of differentiation capacity to change various non-muscle cell types into myogenic cells.
Fig. 5.
Rg1 augments myogenic differentiation of 10T1/2 embryonic fibroblasts transfected with MyoD. (A) MyoD-transfected 10T1/2 fibroblasts were treated with either vehicle or 10nM Rg1 and then differentiated in DM for 2 d. Muscle-specific proteins were analyzed by immunoblotting. Pan-Cadherin was used as the loading control. (B) The signal intensity of indicated muscle-specific proteins was quantified in three independent experiments and normalized to the loading control pan-Cadherin. The values from DMSO-treated MyoD-transfected cells were set to 1.0. Values are presented as means ± SD. (C) The total and phosphorylated forms of p38MAPK and Akt of cell lysates from panel (A) were analyzed by immunoblotting. (D) The signal intensity of total and phosphorylated forms of p38MAP and Akt was quantified in three independent experiments, and the relative values for the phosphorylated forms to total p38MAPK and Akt proteins were determined, respectively. The values from DMSO-treated MyoD-transfected cells were set to 1.0. Values are presented as means ± SD. * p < 0.05 and ** p < 0.01 compared to control group. DMSO, dimethyl sulfoxide; MHC, myosin heavy chain; NS, not significant; SD, standard deviation.
4. Conclusions
Our study provides a mechanistic framework for understanding how Rg1 enhances muscle growth and prevents muscle atrophy by activating Akt/mTOR signaling pathway and how Rg1 enhances myoblast differentiation, accompanied by MyoD activation and the active heterodimerization with MyoD and E proteins. Furthermore, Rg1 also augments myogenic conversion of embryonic fibroblasts into myoblasts, suggesting that Rg1 represents an excellent candidate for the treatment or prevention of muscle atrophy or related chronic diseases.
Conflicts of interest
All authors have no conflicts of interest.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2011-0030074 and 2016R1A2B4014868 to GUB) and funded by Korea Institute of Science and Technology (No. 2Z04950 to SNK).
Contributor Information
Yong Kee Kim, Email: yksnbk@sookmyung.ac.kr.
Gyu-Un Bae, Email: gbae@sookmyung.ac.kr.
References
- 1.Sanchez A.M., Csibi A., Raibon A., Docquier A., Lagirand-Cantaloube J., Leibovitch M.P., Leibovitch S.A., Bernardi H. eIF3f: a central regulator of the antagonism atrophy/hypertrophy in skeletal muscle. Int J Biochem Cell Biol. 2013;45:2158–2162. doi: 10.1016/j.biocel.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Maltzahn J., Chang N.C., Bentzinger C.F., Rudnicki M.A. Wnt signaling in myogenesis. Trends Cell Biol. 2012;22:602–609. doi: 10.1016/j.tcb.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horsley V., Pavlath G.K. Forming a multinucleated cell: molecules that regulate myoblast fusion. Cells Tissues Organs. 2004;176:67–78. doi: 10.1159/000075028. [DOI] [PubMed] [Google Scholar]
- 4.Krauss R.S. Regulation of promyogenic signal transduction by cell-cell contact and adhesion. Exp Cell Res. 2010;316:3042–3049. doi: 10.1016/j.yexcr.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tapscott S.J., Davis R.L., Thayer M.J., Cheng P.F., Weintraub H., Lassar A.B. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988;242:405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- 6.Simone C., Forcales S.V., Hill D.A., Imbalzano A.N., Latella L., Puri P.L. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat Genet. 2004;36:738–743. doi: 10.1038/ng1378. [DOI] [PubMed] [Google Scholar]
- 7.Serra C., Palacios D., Mozzetta C., Forcales S.V., Morantte I., Ripani M., Jones D.R., Du K., Jhala U.S., Simone C. Functional interdependence at the chromatin level between the MKK6/p38 and IGF1/PI3K/AKT pathways during muscle differentiation. Mol Cell. 2007;28:200–213. doi: 10.1016/j.molcel.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawlor M.A., Rotwein P. Insulin-like growth factor-mediated muscle cell survival: central roles for Akt and cyclin-dependent kinase inhibitor p21. Mol Cell Biol. 2000;20:8983–8995. doi: 10.1128/mcb.20.23.8983-8995.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae G.U., Kim B.G., Lee H.J., Oh J.E., Lee S.J., Zhang W., Krauss R.S., Kang J.S. Cdo binds Abl to promote p38alpha/beta mitogen-activated protein kinase activity and myogenic differentiation. Mol Cell Biol. 2009;29:4130–4143. doi: 10.1128/MCB.00199-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae G.U., Lee J.R., Kim B.G., Han J.W., Leem Y.E., Lee H.J., Ho S.M., Hahn M.J., Kang J.S. Cdo interacts with APPL1 and activates Akt in myoblast differentiation. Mol Biol Cell. 2010;21:2399–2411. doi: 10.1091/mbc.E09-12-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takaesu G., Kang J.S., Bae G.U., Yi M.J., Lee C.M., Reddy E.P., Krauss R.S. Activation of p38alpha/beta MAPK in myogenesis via binding of the scaffold protein JLP to the cell surface protein Cdo. J Cell Biol. 2006;175:383–388. doi: 10.1083/jcb.200608031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez I., Tripathi G., Carter E.J., Cobb L.J., Salih D.A., Lovett F.A., Holding C., Pell J.M. Akt2, a novel functional link between p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways in myogenesis. Mol Cell Biol. 2004;24:3607–3622. doi: 10.1128/MCB.24.9.3607-3622.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodine S.C., Stitt T.N., Gonzalez M., Kline W.O., Stover G.L., Bauerlein R., Zlotchenko E., Scrimgeour A., Lawrence J.C., Glass D.J. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 14.Rommel C., Bodine S.C., Clarke B.A., Rossman R., Nunez L., Stitt T.N., Yancopoulos G.D., Glass D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 15.Stitt T.N., Drujan D., Clarke B.A., Panaro F., Timofeyva Y., Kline W.O., Gonzalez M., Yancopoulos G.D., Glass D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 16.Kim J.H. Cardiovascular diseases and Panax ginseng: a review on molecular mechanisms and medical applications. J Ginseng Res. 2012;36:16–26. doi: 10.5142/jgr.2012.36.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim T.H., Lee S.M. The effects of ginseng total saponin, panaxadiol and panaxatriol on ischemia/reperfusion injury in isolated rat heart. Food Chem Toxicol. 2010;48:1516–1520. doi: 10.1016/j.fct.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Yu T., Yang Y., Kwak Y.S., Song G.G., Kim M.Y., Rhee M.H., Cho J.Y. Ginsenoside Rc from Panax ginseng exerts anti-inflammatory activity by targeting TANK-binding kinase 1/interferon regulatory factor-3 and p38/ATF-2. J Ginseng Res. 2017;41:127–133. doi: 10.1016/j.jgr.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang C.R., Lee S.H., Jang G.Y., Hwang I.G., Kim H.Y., Woo K.S., Lee J., Jeong J.S. Changes in ginsenoside compositions and antioxidant activities of hydroponic-cultured ginseng roots and leaves with heating temperature. J Ginseng Res. 2014;38:180–186. doi: 10.1016/j.jgr.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gui Q.F., Xu Z.R., Xu K.Y., Yang Y.M. The efficacy of ginseng-related therapies in type 2 diabetes mellitus: an updated systematic review and meta-analysis. Medicine. 2016;95 doi: 10.1097/MD.0000000000002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin J.Y., Lee J.M., Shin H.S., Park S.Y., Yang J.E., Cho S.K., Yi T.H. Anti-cancer effect of ginsenoside F2 against glioblastoma multiforme in xenograft model in SD rats. J Ginseng Res. 2012;36:86–92. doi: 10.5142/jgr.2012.36.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang B., Xiong Z., Yang J., Wang W., Wang Y., Hu Z.L., Wang F., Chen J.G. Antidepressant-like effects of ginsenoside Rg1 are due to activation of the BDNF signalling pathway and neurogenesis in the hippocampus. Br J Pharmacol. 2012;166:1872–1887. doi: 10.1111/j.1476-5381.2012.01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee H.M., Lee O.H., Kim K.J., Lee B.Y. Ginsenoside Rg1 promotes glucose uptake through activated AMPK pathway in insulin-resistant muscle cells. Phytother Res. 2012;26:1017–1022. doi: 10.1002/ptr.3686. [DOI] [PubMed] [Google Scholar]
- 24.Lee S.J., Leem Y.E., Go G.Y., Choi Y., Song Y.J., Kim I., Kim Y.K., Seo D.W., Kang J.S., Bae G.U. Epicatechin elicits MyoD-dependent myoblast differentiation and myogenic conversion of fibroblasts. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S.J., Hwang J., Jeong H.J., Yoo M., Go G.Y., Lee J.R., Leem Y.E., Park J.W., Seo D.W., Kim Y.K. PKN2 and Cdo interact to activate AKT and promote myoblast differentiation. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2016.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson E.M., Rotwein P. Selective control of skeletal muscle differentiation by Akt1. J Biol Chem. 2007;282:5106–5110. doi: 10.1074/jbc.C600315200. [DOI] [PubMed] [Google Scholar]
- 27.Lluis F., Ballestar E., Suelves M., Esteller M., Munoz-Canoves P. E47 phosphorylation by p38 MAPK promotes MyoD/E47 association and muscle-specific gene transcription. EMBO J. 2005;24:974–984. doi: 10.1038/sj.emboj.7600528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takamura Y., Makanae Y., Ato S., Yoshii N., Kido K., Nomura M., Uchiyama A., Shiozawa N., Fujita S. Panaxatriol derived from ginseng augments resistance exercised-induced protein synthesis via mTORC1 signaling in rat skeletal muscle. Nutr Res. 2016;36:1193–1201. doi: 10.1016/j.nutres.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Marabita M., Baraldo M., Solagna F., Ceelen J.J., Sartori R., Nolte H., Nemazanyy I., Pyronnet S., Kruger M., Pende M. S6K1 Is required for increasing skeletal muscle force during hypertrophy. Cell Rep. 2016;17:501–513. doi: 10.1016/j.celrep.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 30.Hasselgren P.O. Glucocorticoids and muscle catabolism. Curr Opin Clin Nutr Metab Care. 1999;2:201–205. doi: 10.1097/00075197-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Kimura K., Cheng X.W., Inoue A., Hu L., Koike T., Kuzuya M. Beta-hydroxy-beta-methylbutyrate facilitates PI3K/Akt-dependent mammalian target of rapamycin and FoxO1/3a phosphorylations and alleviates tumor necrosis factor alpha/interferon gamma-induced MuRF-1 expression in C2C12 cells. Nutr Res. 2014;34:368–374. doi: 10.1016/j.nutres.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Sandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Picard A., Walsh K., Schiaffino S., Lecker S.H., Goldberg A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zetser A., Gredinger E., Bengal E. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J Biol Chem. 1999;274:5193–5200. doi: 10.1074/jbc.274.8.5193. [DOI] [PubMed] [Google Scholar]
- 34.Meregalli M., Farini A., Colleoni F., Cassinelli L., Torrente Y. The role of stem cells in muscular dystrophies. Curr Gene Ther. 2012;12:192–205. doi: 10.2174/156652312800840559. [DOI] [PubMed] [Google Scholar]