Abstract
Background
In general, after Panax ginseng is administered orally, intestinal microbes play a crucial role in its degradation and metabolization process. Studies on the metabolism of P. ginseng by microflora are important for obtaining a better understanding of their biological effects.
Methods
In vitro biotransformation of P. ginseng extract by rat intestinal microflora was investigated at 37°C for 24 h, and the simultaneous determination of the metabolites and metabolic profile of P. ginseng saponins by rat intestinal microflora was achieved using LC–MS/MS.
Results
A total of seven ginsenosides were detected in the P. ginseng extract, including ginsenosides Rg1, Re, Rf, Rb1, Rc, Rb2, and Rd. In the transformed P. ginseng samples, considerable amounts of deglycosylated metabolite compound K and Rh1 were detected. In addition, minimal amounts of deglycosylated metabolites (ginsenosides Rg2, F1, F2, Rg3, and protopanaxatriol-type ginsenosides) and untransformed ginsenosides Re, Rg1, and Rd were detected at 24 h. The results indicated that the primary metabolites are compound K and Rh1, and the protopanaxadiol-type ginsenosides were more easily metabolized than protopanaxatriol-type ginsenosides.
Conclusion
This is the first report of the identification and quantification of the metabolism and metabolic profile of P. ginseng extract in rat intestinal microflora using LC–MS/MS. The current study provided new insights for studying the metabolism and active metabolites of P. ginseng.
Keywords: ginsenoside, LC–MS/MS, metabolites, Panax ginseng, rat intestinal microflora
1. Introduction
Panax ginseng Meyer, which is a well-known plant medicine with diverse pharmacological effects, has been used as a functional food or dietary supplement in China, Korea, and Japan. As a Traditional Chinese medicine, ginseng is widely used as a single herb or in herbal mixtures to promote health and treat diseases [1]. The main active components of ginseng are ginsenosides.
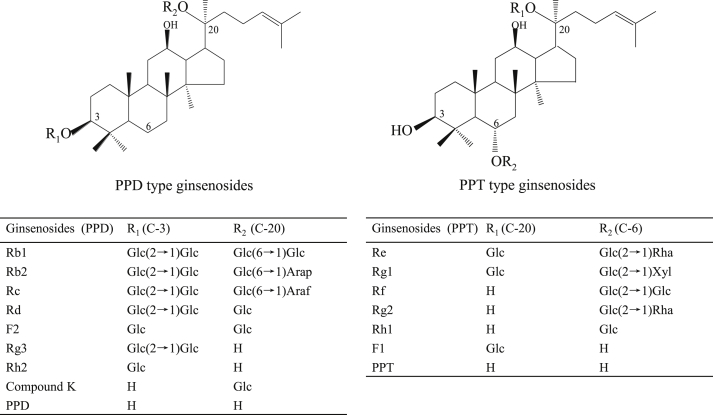
The structures of the ginsenoside monomers of more than 50 species have been isolated and identified. Ginsenosides are connected by an aglycone and a sugar and belong to the triterpenoid saponin group. Based on the structure of the aglycone, the ginsenosides can be divided into three categories as follows: oleanolic acid-type ginsenosides, protopanaxadiol (PPD)-type ginsenosides, and protopanaxatriol (PPT)-type ginsenosides. The PPD-type or PPT-type ginsenosides have different sugar moieties at positions C-3/C-6 and C-20 in the aglycon PPD or PPT (Fig. 1). The ginsenosides exhibit various biological activities including anti-inflammatory activity, anticancer activity, reduce blood pressure and cardiovascular system, immunomodulatory effects, and antioxidant activities [2], [3], [4], [5], [6]. Intestinal microflora is an important “microflora organ,” and in both human and animals, approximately 99% of the intestinal bacteria are anaerobic bacteria. In humans, the intestinal microbiota consists of more than 1014 bacteria and archaea, which include approximately 1,100 prevalent species [7], [8], [9]. It plays an important role in the metabolism of compounds that are administered orally [10].
Fig. 1.
Chemical structures of protopanaxadiol (PPD)-type ginsenosides and protopanaxatriol (PPT)-type ginsenosides Glc β-D-glucopyranosyl, Arap α-L-arabinopyranosyl, Araf α-L-arabinofuranosyl, Xyl β-D-xylopyranosyl and Rha a-L-rhamnopyranosyl.
In addition to its medicinal usage, P. ginseng is also widely consumed as a food in the form of tea, powder, drinks, liquid extracts, candy, and capsules. Therefore, the components of orally administered P. ginseng products inevitably come into contact with intestinal microbiota in the digestive tract [11], [12]. During this process, the major ginsenosides of orally administered P. ginseng are biotransformed to minor ginsenosides followed by absorption. Therefore, the characterization of the metabolism of P. ginseng saponins by intestinal microflora is important for elucidating the pharmacological actions of P. ginseng [13], [14], [15]. Many attempts have employed modern approaches for studying these important ginsenosides including the metabolism of some ginsenosides (Rb1, Rb2, Rc, Rd, Ra1, and Ra2) bioactivated by intestinal bacteria [16], [17], [18], [19], [20], [21]. However, most of these studies only investigated a single ginsenoside or one intestinal bacteria strain but the metabolism of P. ginseng extract by synergetic intestinal microflora have been ignored. Due to competitive effects and exposure to compounds with different concentrations, the administration of a single ginsenoside from a P. ginseng extract may alter the metabolic profile [1].
Studies on the metabolism of P. ginseng by microflora are important for obtaining a better understanding of their biological effects. In this study, an in vitro biotransformation study of P. ginseng extract with rat intestinal microflora was performed, and LC-MS/MS was used to identify and quantify the metabolites of P. ginseng saponins. It investigated the comprehensive metabolites and metabolic profile of P. ginseng extract.
2. Materials and methods
2.1. Chemicals and materials
The standard ginsenosides, Rg1, Re, Rf, Rb1, Rc, Rb2, Rd, Rg2, Rh1, F1, F2, Rg3, PPT, compound K, and Rh2 were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). General anaerobic medium broth was purchased from Changchun Sai wei-si Biological Technology Co., Ltd. (Changchun, China). HPLC grade methanol (MeOH) and acetonitrile were purchased from J.T. Baker (Center Valley, PA, USA).
2.2. P. ginseng extract preparation
P. ginseng roots (4 yr old) were obtained from JiAn, Jilin, China. The P. ginseng samples were pulverized into powder. One-hundred grams of the powder was extracted in an ultrasonic bath with 1 L of 80% ethanol at room temperature for 1 h. The operation was repeated twice, and the combined extracts were evaporated under vacuum and lyophilized.
2.3. Rat intestinal microflora sample preparation
Adult male Sprague-Dawley rats (Changchun) weighing 220–240 g were used in this study (n = 5). All animals were acclimated for more than 1 wk under a 12 h light and 12 h dark cycle at a room temperature of 23 ± 1°C. Animal welfare and experimental procedures were conducted in strict accordance with the guidelines for the Yanbian University Animal Care Committee.
The rat colonic contents samples (1 g) were suspended in 9 mL of cold physiological saline. The fecal suspension was centrifuged at 500g for 5 min, and then, the supernatant was centrifuged at 10,000g for 20 min. The resulting precipitates were suspended in 15 mL of general anaerobic medium broth and anaerobically (85% N2, 10% H2, 5% CO2) incubated at 37°C for 48 h.
2.4. Biotransformation of P. ginseng extract by rat intestinal microflora
In a previous study of metabolism of ginsenosides by intestinal microflora in vivo and in vitro, major ginsenosides have been reported to be mostly transformed into deglycosylated ginsenosides in 24 h [1], [22], [23]. In this study, 1 mL of the intestinal microbiota was added to 4 mg of the P. ginseng extract and anaerobically incubated at 37°C for 24 h. The reaction mixtures were extracted three times with four volumes of water-saturated n-BuOH. The n-BuOH layers were combined and dried by a speed vacuum followed by dilution to the desired volume with methanol. Each sample was filtered through a 0.2-μm filter prior to LC–MS/MS analysis.
2.5. LC–MS/MS analysis of ginsenosides
Chromatographic analysis was performed using an Agilent 1260 series LC system (Agilent Technologies, Palo Alto in California, US) equipped with a binary pump, an online degasser, an auto plate sampler, and a thermostatically-controlled column compartment. The system was operated using Mass Hunter Acquisition Software, Version B.07.00 (Agilent Technologies).
Sample separation was achieved on an Agilent Poroshell ZORBAX SB-C18 (Agilent Technologies) column (4.6 mm × 150 mm, 5 μm) with a constant flow rate of 0.5 mL/min at 25°C. The mobile phase was consisted of water (A) and acetonitrile (B), and the following gradient elution was employed: 23–30% (B) for 0–15 min, 30–44% (B) for 15–34 min, 44–68 % (B) for 34–46 min, 68–85% (B) for 46–61 min, 85–80% (B) for 61–66 min, 80–40% (B) for 66–71 min, and 40–20% (B) for 71–73 min. The injected sample volume was 2 μL.
Positive electrospray ionization–MS-MS was employed to analyze the metabolites of ginsenosides. All of the mass spectrometric experiments were performed on a triple-quadrupole tandem mass spectrometer (Agilent Technologies). The capillary voltage was set to 4,000 V for positive mode, and the gas flowed at a rate of 3 L/min with a gas temperature of 300°C. Full-scan mass spectra in a mass range of m/z 400–1,500 were acquired. The masses of the positive ion fragments [M+Na]+ or [M-2H2O+H]+ of the potential metabolites were examined to determine the ginsenoside metabolites.
2.6. Validation of quantitative ability
The quantitative analysis consisted of an external calibration method. A stock solution containing 15 reference compounds were prepared and diluted to appropriate concentrations for construction of calibration curves. The calibration curves were generated by plotting the peak area versus the concentrations of each analytes. The limits of detection that produced a signal-to-noise ratio of 3 and quantification under the present chromatographic conditions produced a signal-to-noise ratio ≥ 10. Each standard was prepared in triplicate.
The recovery of this method was achieved using the standard addition method. Three different concentration levels (100 ng/mL, 1,000 ng/mL, 2,000 ng/mL) of the references standards were added into the sample in triplicate. The average recoveries were determined by the following formula: Recovery (%) = (observed amount – original amount)/spiked amount × 100%, with relative standard deviation (%) = (standard deviation/mean) × 100%.
3. Results and discussion
3.1. Characterization of standard ginsenosides
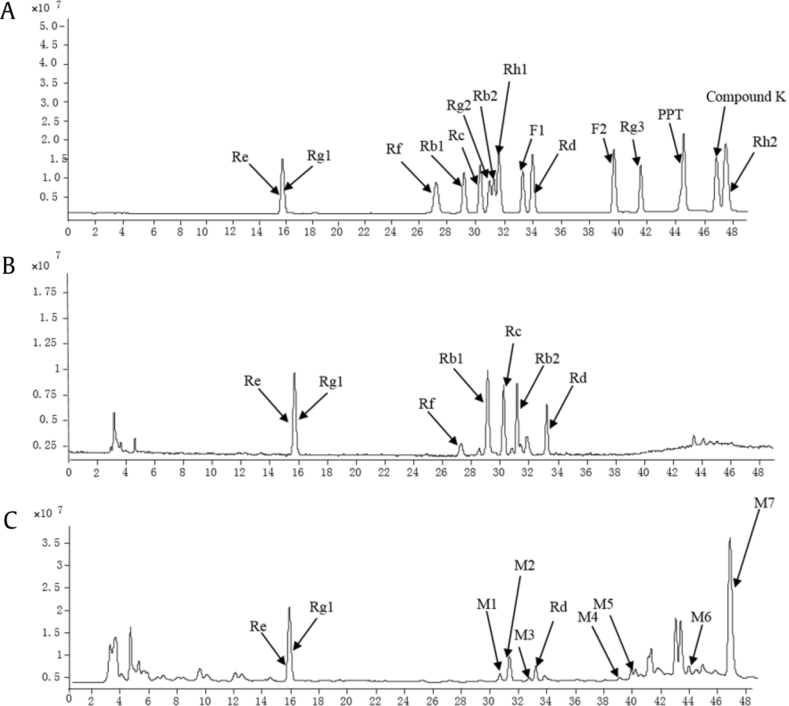
We analyzed 15 reference ginsenoside standards using LC-MS/MS. Fig. 2A shows the typical total ion chromatogram (TIC) of the extract in positive ion mode. Table 1 shows the mass spectrometric conditions and fragmentation behaviors with data in the MS/MS stage of ginsenosides and its metabolites.
Fig. 2.
The typical total ion chromatogram of ginsenosides in the positive-ion mode by LC–MS/MS. (A) mixed standards; (B) Panax ginseng extract; (C) transformed P. ginseng extract by rat intestinal microflora within 24 h.
Table 1.
LC–MS/MS data in the positive-ion mode of standard ginsenosides.
| Ginsenoside | Rt (min) | Formula | Formula (mass) |
(m/z) |
Product ions (m/z) | Collision energy | |
|---|---|---|---|---|---|---|---|
| [M+Na]+ | [M-2H2O+H]+ | ||||||
| Rg1 | 15.8 | C42H72O14 | 800.0 | 823.4 | — | 643.6 [M-glc-H2O+Na]+ 203.0 [glc+H2O+Na]+ |
40 |
| Re | 15.8 | C48H82O18 | 946.0 | 969.5 | — | 789.2 [M-glc-H2O+Na]+ 203.0 [glc+H2O+Na]+ |
50 |
| Rf | 27.0 | C42H72O14 | 800.0 | 823.3 | — | 481.0 [M-(glc+glc)-H2O+Na]+ 365.0 [(glc+glc)+H2O+Na]+ |
60 |
| Rb1 | 29.0 | C54H92O23 | 1108.0 | 1131.8 | — | 789.8 [M-(glc+glc)-H2O+Na]+ 365.3 [(glc+glc)+H2O+Na]+ |
60 |
| Rc | 30.2 | C53H90O22 | 1078.0 | 1101.7 | — | 790.7 [M-(glc+ara)-H2O+Na]+ 335.2 [(glc+ara)+H2O+Na]+ |
60 |
| Rb2 | 31.2 | C53H90O22 | 1078.0 | 1101.5 | — | 789.5 [M-(glc+ara)-H2O+Na]+ 335.2 [(glc+ara)+H2O+Na]+ |
60 |
| Rd | 33.2 | C48H82O18 | 946.0 | 969.5 | — | 789.5 [M-glc-H2O+Na]+ 365.3 [(glc+glc)+H2O+Na]+ 203.0 [glc+H2O+Na]+ |
50 |
| Rg2 | 30.8 | C42H72O13 | 784.0 | 807.5 | — | 481.0 [M-( rha+glc)-H2O+Na]+ 349.2 [(rha+glc)+H2O+Na]+ |
56 |
| Rh1 | 31.4 | C36H62O9 | 638.0 | — | 603.0 | 423.3 [M-glc-H2O+H]+ | 10 |
| F1 | 33.9 | C36H58O9 | 638.0 | 661.4 | — | 481.2 [M-glc-H2O+Na]+ 202.9 [glc+H2O+Na]+ |
34 |
| F2 | 39.8 | C42H72O13 | 784.0 | 807.3 | — | 627.2 [M-glc-H2O+Na]+ 203.0 [glc+H2O+Na]+ |
40 |
| Rg3 | 41.6 | C42H72O13 | 784.0 | 807.5 | — | 465.0 [M-(glc+glc)-H2O+Na]+ 365.0 [(glc+glc)+H2O+Na]+ |
67 |
| PPT | 44.5 | C30H52O4 | 476.0 | — | 441.0 | 423.3 [M-3H2O+H]+ | 10 |
| Compound K | 46.9 | C36H62O8 | 622.0 | 645.6 | — | 465.2 [M-glc-H2O+Na]+ 203.0 [glc+H2O+Na]+ |
30 |
| Rh2 | 47.5 | C36H62O8 | 622.0 | — | 587.0 | 407 [M-glc-H2O+H]+ | 10 |
3.2. Method validation of LC-MS/MS
The method exhibited a good linear response over concentration range using linear regression analysis. Linear calibration curves with correlation coefficients greater than 0.99 were obtained for all analytes in the concentration ranges 50–5,000 ng/mL. The results proved that this technique is capable of generating good reproducibility (data shown in Table 2). The recoveries of the investigated targets ranged from 78.5% to 123.7% and the relative standard deviation of most compounds was less than 15%.
Table 2.
Validation of the method for the LC–MS/MS determination of ginsenosides. LOD, limit of detection; LOQ, limit of quantitation; RSD, relative standard deviation
| Ginsenosides | Calibration curve equation | Correlation coefficient | Range (ng/mL) |
LOD (ng/mL) |
LOQ (ng/mL) |
Recovery % (RSD%) |
||
|---|---|---|---|---|---|---|---|---|
| 100 ng/mL | 1,000 ng/mL | 2,000 ng/mL | ||||||
| Re | y = 10.691x+ 2519.7 | R2 = 0.9973 | 50–5,000 | 7.51 | 24.99 | 104.4 (8.8) | 94.8 (6.6) | 116.5 (7.4) |
| Rg1 | y = 13.074x + 7896.7 | R2 = 0.9930 | 50–5,000 | 4.52 | 15.06 | 104.2 (11.1) | 116.8 (10.1) | 104.2 (8.9) |
| Rf | y = 9.7808x + 4535.2 | R2 = 0.9940 | 50–5,000 | 4.23 | 14.09 | 98.9 (8.9) | 98.2 (4.8) | 109.2 (9.4) |
| Rb1 | y = 4.7875x + 360.18 | R2 = 0.9964 | 50–5,000 | 12.01 | 40.03 | 97.6 (10.0) | 101.9 (0.6) | 106.2 (2.6) |
| Rc | y = 1.9243x + 520.16 | R2 = 0.9951 | 50–5,000 | 15.05 | 50.17 | 95.2 (7.3) | 95.1 (9.1) | 100.4 (4.5) |
| Rb2 | y = 1.6348x + 545.69 | R2 = 0.9955 | 50–5,000 | 12.04 | 40.12 | 90.4 (5.0) | 80.1 (8.8) | 109.1 (8.7) |
| Rd | y = 10.886x + 1745.9 | R2 = 0.9917 | 50–5,000 | 2.27 | 7.56 | 104.9 (6.9) | 101.7 (4.1) | 95.5 (3.9) |
| Rg2 | y = 0.5688x + 389.94 | R2 = 0.9902 | 50–5,000 | 20.05 | 66.83 | 115.8 (9.6) | 94.5 (10.7) | 111.7 (7.7) |
| Rh1 | y = 2.6693x + 170.07 | R2 = 0.9987 | 50–5,000 | 3.33 | 11.11 | 123.7 (9.5) | 107.7 (8.1) | 107.2 (2.2) |
| F1 | y = 4.189 x + 1495.3 | R2 = 0.9905 | 50–5,000 | 9.38 | 31.25 | 96.1 (8.1) | 100.5 (0.4) | 95.9 (4.9) |
| F2 | y = 11.384x + 4093.2 | R2 = 0.9910 | 50–5,000 | 2.64 | 8.79 | 104.9 (6.5) | 109.5 (11.5) | 107.2 (2.3) |
| Rg3 | y = 11.374x + 3971.8 | R2 = 0.9916 | 50–5,000 | 13.63 | 45.43 | 112.0 (9.2) | 99.2 (10.1) | 105.2 (7.6) |
| PPT | y = 5.7564x + 1116.6 | R2 = 0.9971 | 50–5,000 | 7.23 | 24.09 | 109.3 (4.8) | 86.5 (7.6) | 102.9 (0.9) |
| Compound K | y = 6.5315x + 1551.7 | R2 = 0.9953 | 50–5,000 | 5.35 | 17.83 | 78.5 (8.2) | 101.4 (9.6) | 82.5 (8.8) |
| Rh2 | y = 5.5618x + 1891.3 | R2 = 0.9978 | 50–5,000 | 4.68 | 13.59 | 82.0 (9.2) | 99.5 (8.7) | 113.7 (9.1) |
3.3. Characterization of ginsenosides in the P. ginseng extract
We analyzed the P. ginseng root (4 yr old) sample by LC-MS/MS. The TIC of the P. ginseng extract in positive ion mode is shown in Fig. 2B. The seven major ginsenosides were analyzed. By comparing the retention times, accurate masses, and characteristic MS/MS fragment ions of P. ginseng, the ginsenosides in the extract were identified. The seven major ginsenosides were determined to be ginsenosides Rg1, Re, Rf, Rb1, Rc, Rb2, and Rd.
3.4. Identification and quantification of metabolites of P. ginseng
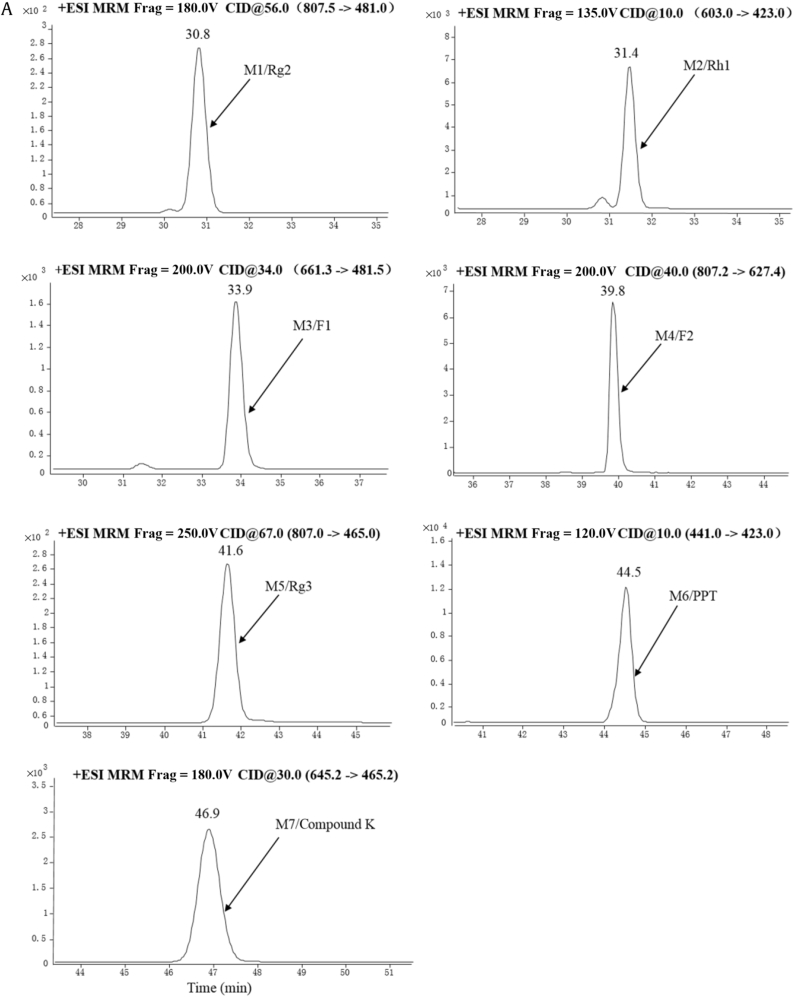
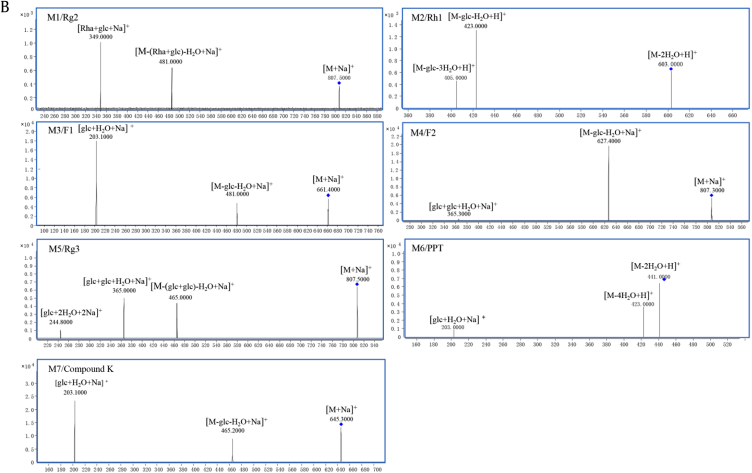
To understand the role of intestinal microflora in the metabolism of P. ginseng in the intestinal tract, rat intestinal microflora were employed to study the in vitro biotransformation of the P. ginseng extract. Fig. 2C shows the TIC of the transformed P. ginseng sample. A total of seven metabolites were identified. The representative multiple reaction monitoring chromatograms for the metabolites are shown in Fig. 3A, and the MS/MS fragmentation spectrum of the deglycosylated metabolites using electrospray ionization–MS/MS (Fig. 3B): Metabolites M1 (30.8 min) at m/z 807.5→481.0, M2 (31.4 min) at m/z 603.0→423.0, M3 (33.9 min) at m/z 661.3→481.5, M4 (39.8 min) at m/z 807.2→627.4, M5 (41.6 min) at m/z 807.0→465.0, M6 (44.5 min) at m/z 441.0→423.0 and M7 (46.9 min) at m/z 645.2→465.2 were detected in the transformed P. ginseng sample. From comparison of the retention times and molecular mass to those of standard compounds, metabolites M1, M2, M3, M4, M5, M6, and M7 were identified as ginsenosides Rg2, Rh1, F1, F2, Rg3, PPT, and compound K, respectively. Besides the metabolites determined, several peaks were also detected in the TIC. The work will be done in the future in our laboratory.
Fig. 3A.
The representative multiple reaction monitoring (MRM) chromatograms of metabolites in the transformed (A) Panax ginseng sample and the (B) MS/MS fragmentation spectrum of the deglycosylated metabolites using electrospray ionization–MS/MS (ESI–MS/MS).
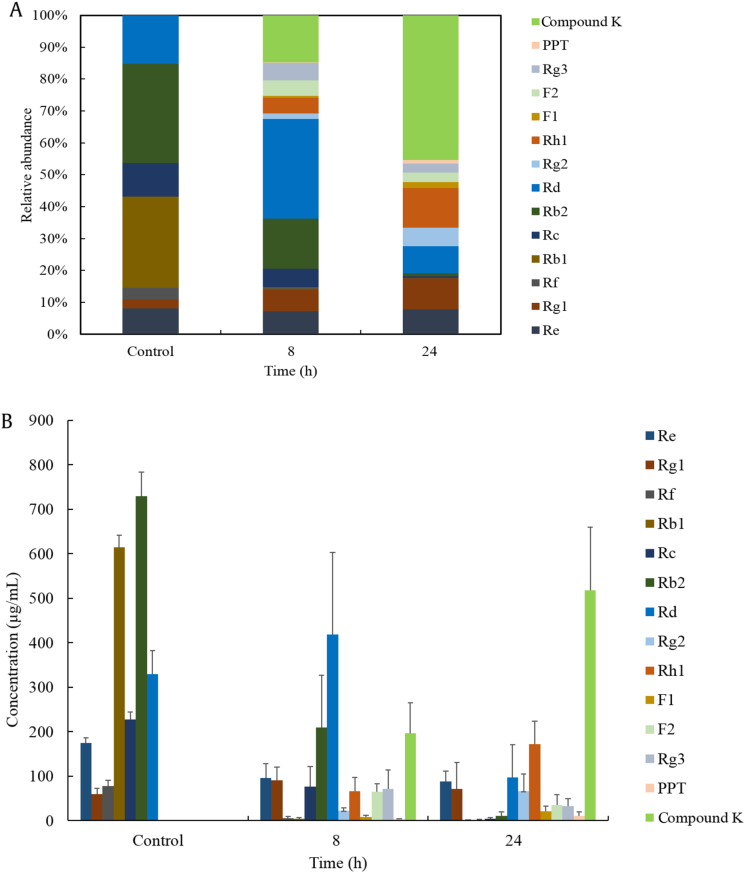
The biotransformation of P. ginseng extract by rat intestinal microflora was confirmed quantitatively by multiple reaction monitoring analysis. As shown in Fig. 4, considerable amounts of deglycosylated metabolite compound K and Rh1 were detected. In addition, minimal amounts of deglycosylated metabolites, such as ginsenosides Rg2, F1, F2, Rg3, and PPT as well as untransformed ginsenosides Re, Rg1, and Rd were detected in the transformed P. ginseng samples after 24 h. The results indicated that the primary metabolites are compound K and Rh1, and the PPD-type ginsenosides were more easily metabolized than PPT-type ginsenosides.
Fig. 4.
The (A) relative abundance and (B) concentration-time profiles of ginsenosides in the transformed Panax ginseng extract by rat intestinal microflora.
3.5. Metabolic pathways of P. ginseng saponins
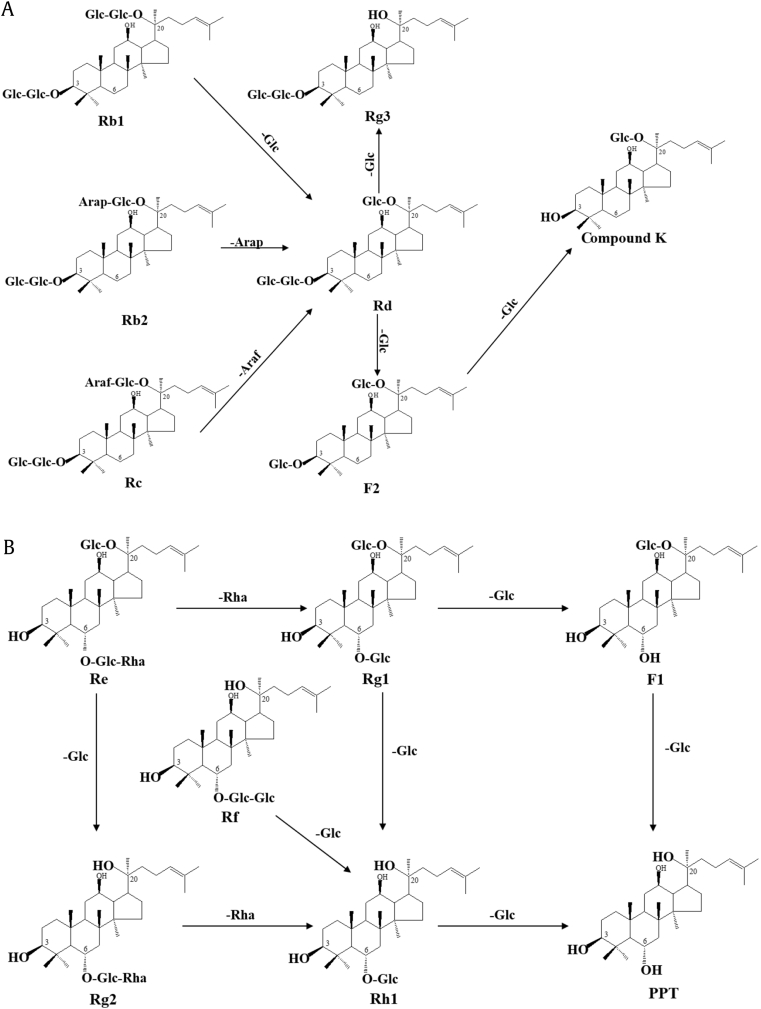
The metabolic pathways of P. ginseng saponins include deglycosylation reactions by intestinal microflora through the selective elimination of sugar moieties. According to the metabolism of ginsenosides detected in this study and previous study of metabolic pathways consulted [24], [25], we speculated the pathway of ginsenosides shown in Fig. 5. The metabolic pathways of PPD-type ginsenosides shown in Fig. 5A, illustrated the main metabolic pathways involve the selective elimination of the C-20 and C-3 outer sugar moieties to produce ginsenoside F2 from ginsenosides Rb1, Rb2, Rc and Rd, and then, C-3 sugar moieties to produce compound K. The metabolic pathways of the PPT-type ginsenosides via intestinal microflora are proposed in Fig. 5B. The metabolic pathway involves the elimination of the C-20 and C-6 sugar moieties to produce Rh1 or PPT.
Fig. 5.
Proposed deglycosylated metabolic pathway of Panax ginseng saponins by rat intestinal microflora. (A) protopanaxadiol (PPD)-type ginsenosides metabolized by rat intestinal microflora; (B) protopanaxatriol (PPT)-type ginsenosides metabolized by rat intestinal microflora.
4. Conclusion
We studied the in-vitro biotransformation of P. ginseng extract by rat intestinal microflora. A total of seven ginsenosides were detected in the P. ginseng extract including PPT-type ginsenosides Rg1, Re, and Rf, and PPD-type ginsenosides Rb1, Rc, Rb2, and Rd. In the transformed P. ginseng samples, considerable amounts of deglycosylated metabolite compound K and Rh1 were detected. In addition, minimal amounts of deglycosylated metabolite including ginsenosides Rg2, F1, F2, Rg3, and PPT as well as untransformed ginsenosides Re, Rg1, and Rd were detected after 24 h. The results indicated that the primary metabolites are compound K and Rh1, and the PPD-type ginsenosides were easily metabolized. This is the first report of the identification and quantification of the metabolism of P. ginseng extract by rat intestinal microflora. The current study provided new insights for studying the metabolism and active metabolites of P. ginseng.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This study was supported by a China Postdoctoral Science Foundation funded project 287 (Number 2015M571376) and a grant from the National Natural Science Foundation of China (Number 81660643; 81603365).
Contributor Information
Deok-Chun Yang, Email: dcyang@khu.ac.kr.
Donghao Li, Email: dhli@ybu.edu.cn.
Lin-Hu Quan, Email: lhquan@ybu.edu.cn.
References
- 1.Wan J.Y., Liu P., Wang H.Y., Qi L.W., Wang C.Z., Li P., Yuan C.S. Biotransformation and metabolic profile of American ginseng saponins with human intestinal microflora by liquid chromatography quadrupole time-of-flight mass spectrometry. J Chromatogr A. 2013;1286:83–92. doi: 10.1016/j.chroma.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 2.Kim S., Oh M.H., Kim B.S., Kim W.I., Cho H.S., Park B.Y., Park C., Shin G.W., Kwon J. Upregulation of heme oxygenase-1 by ginsenoside Ro attenuates lipopolysaccharide-induced inflammation in macrophage cells. J Ginseng Res. 2015;39:365–370. doi: 10.1016/j.jgr.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park E.H., Kim Y.J., Yamabe N., Park S.H., Kim H.K., Jang H.J., Kim J.H., Cheon G.J., Ham J., Kang K.S. Stereospecific anticancer effects of ginsenoside Rg3 epimers isolated from heat-processed American ginseng on human gastric cancer cell. J Ginseng Res. 2014;38:22–27. doi: 10.1016/j.jgr.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park J.B., Kwon S.K., Nagar H., Jung S.B., Jeon B.H., Kim C.S., Oh J.H., Song H.J., Kim C.S. Rg3-enriched Korean Red Ginseng improves vascular function in spontaneously hypertensive rats. J Ginseng Res. 2014;38:244–250. doi: 10.1016/j.jgr.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen L.P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 6.Lee M.H., Lee Y.C., Kim S.S., Hong H.D., Kim K.T. Quality and antioxidant activity of ginseng seed processed by fermentation strains. J Ginseng Res. 2015;39:178–182. doi: 10.1016/j.jgr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilg H., Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121:2126–2132. doi: 10.1172/JCI58109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon G.L., Gorbach S.L. The human intestinal microflora. Dig Dis Sci. 1986;31:147–162. doi: 10.1007/BF01295996. [DOI] [PubMed] [Google Scholar]
- 9.De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J.B., Massart S., Collini S., Pieraccini G., Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H.Y., Hua H.Y., Liu X.Y., Liu J.H., Yu B.Y. In vitro biotransformation of red ginseng extract by human intestinal microflora: metabolites identification and metabolic profile elucidation using LC–Q-TOF. J Pharm Biomed Anal. 2014;98:296–306. doi: 10.1016/j.jpba.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Crow J.M. Microbiome: that healthy gut feeling. Nature. 2011;480:88–89. doi: 10.1038/480S88a. [DOI] [PubMed] [Google Scholar]
- 12.Kim D. Chemical diversity of Panax ginseng, Panax quinquefolium, and Panax notoginseng. J Ginseng Res. 2012;36:1–15. doi: 10.5142/jgr.2012.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasegawa H., Sung J.H., Benno Y. Role of human intestinal Prevotella oris in hydrolyzing ginseng saponins. Planta Med. 1997;63:436–440. doi: 10.1055/s-2006-957729. [DOI] [PubMed] [Google Scholar]
- 14.Tawab M.A., Bahr U., Karas M., Wurglics M., Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 15.Bae E.A., Choo M.K., Park E.K., Park S.Y., Shin H.Y., Kim D.H. Metabolism of ginsenoside Rc by human intestinal bacteria and its related antiallergic activity. Biol Pharm Bull. 2002;25:743–747. doi: 10.1248/bpb.25.743. [DOI] [PubMed] [Google Scholar]
- 16.Shen H., Leung W.I., Ruan J.Q., Li S.L., Lei J.P., Wang Y.T., Yan R. Biotransformation of ginsenoside Rb1 via the gypenoside pathway by human gut bacteria. Chin Med. 2013;8:22. doi: 10.1186/1749-8546-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akao T., Kida H., Kanaoka M., Hattori M., Kobashi K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J Pharm Pharmacol. 1998;50:1155–1160. doi: 10.1111/j.2042-7158.1998.tb03327.x. [DOI] [PubMed] [Google Scholar]
- 18.Park S.Y., Bae E.A., Sung J.H., Lee S.K., Kim D.H. Purification and characterization of ginsenoside Rb1-metabolizing beta-glucosidase from Fusobacterium K-60, a human intestinal anaerobic bacterium. Biosci Biotech Bioch. 2001;65:1163–1169. doi: 10.1271/bbb.65.1163. [DOI] [PubMed] [Google Scholar]
- 19.Bae E.A., Shin J.E., Kim D.H. Metabolism of ginsenoside Re by human intestinal microflora and its estrogenic effect. Biol Pharm Bull. 2005;28:1903–1908. doi: 10.1248/bpb.28.1903. [DOI] [PubMed] [Google Scholar]
- 20.Shin H.Y., Lee J.H., Lee J.Y., Han Y.O., Han M.J., Kim D.H. Purification and characterization of ginsenoside Ra-hydrolyzing beta-D-xylosidase from Bifidobacterium breve K-110, a human intestinal anaerobic bacterium. Biol Pharm Bull. 2003;26:1170–1173. doi: 10.1248/bpb.26.1170. [DOI] [PubMed] [Google Scholar]
- 21.Shin H.Y., Park S.Y., Sung J.H., Kim D.H. Purification and characterization of alpha-L-arabinopyranosidase and alpha-L-arabinofuranosidase from Bifidobacterium breve K-110, a human intestinal anaerobic bacterium metabolizing ginsenoside Rb2 and Rc. Appl Environ Microbiol. 2003;69:7116–7123. doi: 10.1128/AEM.69.12.7116-7123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim K.A., Yoo H.H., Gu W., Yu D.H., Jin M.J., Choi H.L., Yuan K., Guerin-Deremaux L., Kim D.H. A prebiotic fiber increases the formation and subsequent absorption of compound k following oral administration of ginseng in rats. J Ginseng Res. 2015;39:183–187. doi: 10.1016/j.jgr.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang A., Zhang S., Shan J., Di L. Gut microbiota-mediated deglycosylation of ginsenoside Rb1 in rats: in vitro and in vivo insights from quantitative ultra-performance liquid chromatography-mass spectrometry analysis. Anal Methods. 2015;7:6173–6181. [Google Scholar]
- 24.Shin K.C., Choi H.Y., Seo M.J., Oh D.K. Compound k production from red ginseng extract by β-glycosidase from sulfolobus solfataricus supplemented with α-l-arabinofuranosidase from caldicellulosiruptor saccharolyticus. Plos One. 2015;10:e0145876. doi: 10.1371/journal.pone.0145876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L., Huang J., Hu X., Li K., Sun C. Simultaneous determination of ginsenoside (G-Re, G-Rg1, G-Rg2, G-F1, G-Rh1) and protopanaxatriol in human plasma and urine by LC–MS/MS and its application in a pharmacokinetics study of G-Re in volunteers. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:2011–2017. doi: 10.1016/j.jchromb.2011.05.018. [DOI] [PubMed] [Google Scholar]