Abstract
Objectives:
Anti-tumor necrosis factor (TNF)-α agents like Infliximab (IFX) are effective in the treatment of inflammatory bowel diseases (IBDs) and are widely used. However, a considerable number of patients do not respond or lose response to this therapy. Preliminary evidence suggests that transmembrane TNF-α (tmTNF-α) might be linked to response to IFX by promoting reverse signaling-induced apoptosis in inflammatory cells. The main aim of this study was the evaluation of this hypothesis in primary IFX non-responders.
Methods:
A total of 47 IFX naive IBD patients were included in the study. Blood samples were taken before the start of IFX therapy (at week 0) and after induction therapy (at week 14). Endoscopic disease activity and markers of inflammation at baseline and at week 14 were used to evaluate response. Baseline soluble TNF-α (sTNF-α), percentage of circulating TNF-α positive cells, mean fluorescence intensity (MFI) of tmTNF-α, and apoptosis rate at week 14 in the peripheral blood mononuclear cells (PBMCs) were evaluated in IFX responders and non-responders.
Results:
Mean sTNF-α was not significantly different in responders compared to non-responders (P=0.13). Mean percentage of tmTNF-α bearing lymphocytes and monocytes was higher in the PBMCs of responders (P=0.05 and P=0.014, respectively). Mean MFI of tmTNF-α in circulating lymphocytes and monocytes was greater in responders (P=0.002 and P<0.001, respectively). Moreover, the mean percentage of apoptosis in PBMCs was significantly greater in responders compared to non-responders (P=0.002).
Conclusions:
The percentage of tmTNF-α bearing lymphocytes and monocytes and the intensity of tmTNF-α in the circulating leukocyte population of IBD patients was directly related to primary response to IFX. This was likely due—as assessed by the apoptosis rate—to promotion of inflammatory cell death. Thus, our data suggest that peripheral leukocytes could in principle be used for predicting primary response to IFX in IBD patients.
Introduction
Inflammatory bowel disease (IBD)—primarily, Crohn’s disease (CD) and ulcerative colitis (UC)1—are quickly becoming a global healthcare problem2 as patients are becoming refractory to the most effective medications, the anti-tumor necrosis factor-α (anti-TNF-α) agents.3 Elevated levels of pro-inflammatory cytokines have been demonstrated in serum and intestinal mucosa of IBD patients. Among them, TNF-α appears to be a key player.4 Inhibition of TNF-α by monoclonal antibodies—such as Infliximab (IFX) and Adalimumab—has been shown for more than a decade to be the most effective treatment for IBD patients; however, despite their proven efficacy, up to 30% of biological naive patients do not respond to anti-TNF agents after the induction phase—so-called primary non response (PNR)—and up to 13% per year of those who initially respond will eventually lose response over time (secondary loss of response (SLR)).3, 5, 6 The mechanisms at the basis of PNR and SLR might be multiple and are still not fully understood. The literature suggests that PNR may be associated with stricturing disease and lack of response to any medical therapy.5, 6 In primary non-responders the disease could also be driven by a biological process (partially) independent of TNF-α. Alternatively, the drug dose might simply not be enough for the patient’s effective disease burden.7, 8 Finally, the lack of response could be due to other less clear factors (genetics, drug metabolism and disposition).5 Unfortunately, in clinical practice it is often difficult to identify the mechanism at the basis of PNR and SLR, and to accurately predict therapeutic response to anti-TNF agents from the outset. Clearly, the identification of biomarkers capable of predicting such response remains an important goal in clinical IBD research today.
Transmembrane TNF-α (tmTNF-α), a precursor of the soluble form of TNF-alpha (sTNF-α), is expressed on activated macrophages and lymphocytes as well as other cell types. After processing by TNF-α converting enzyme, the sTNF-α is cleaved from tmTNF-α and mediates its biological activities through binding to TNF receptors of remote tissues. Accumulating evidence suggests that not only sTNF-α but also tmTNF-α is involved in the inflammatory response. tmTNF-α acts as a bipolar molecule that transmits signals both as a ligand and as a receptor. On TNF-α-producing cells it binds to TNF receptors, and transmits signals to the target cell as a ligand, while also acting as a receptor transmitting outside-to-inside signals back to the cell.9, 10, 11 When playing the receptor role, tmTNF-α also acts as a receptor for IFX. Binding of IFX to tmTNF-α activates several anti-inflammatory mechanisms including 'tmTNF-α reverse signaling-induced apoptosis', which is considered one of the most important.11, 12, 13 In this process, tmTNF-α acts as a cell surface receptor binding the circulating IFX and thereby activating apoptosis of the tmTNF-α bearing inflammatory cells. Apoptosis with depletion of the inflammatory cells will then result in attenuation of inflammation.
Thus, the concentration of tmTNF-α and the number of tmTNF-α bearing cells might be related to IFX response in IBD. A greater tmTNF-α density could lead to more effective induction of IFX-induced apoptosis in inflammatory cells, and to a better clinical response to IFX. Were that the case, the density of tmTNF-α and the number of tmTNF-α bearing cells could be leveraged as biomarkers of IFX response. In this study we have tested such hypotheses in patients with PNR to IFX.
Methods
Patients
IFX naive IBD patients, who failed corticosteroids and/or immunomodulators, were included in the study. The inclusion criteria were as follows: verifiable diagnosis of CD or UC based on standard endoscopic, radiologic, and histologic criteria;14, 15, 16 age 16 years or older; testing negative for hepatitis B and C; human immunodeficiency virus; latent or current tuberculosis (by chest X-ray and tuberculin skin test); serum cytomegalovirus PCR; and negative stool Clostridium difficile and stool culture. Exclusion criteria were as follows: previous IBD-related intestinal resection, perianal disease, history of severe heart disease, neurodegenerative diseases, pregnancy, and previous history of colon cancer. In addition, patients were excluded in the presence of intestinal strictures, abscess, fistulas, or sepsis—conditions that might impact on medical response to anti-TNF-α agents or any other medication.5, 6 Hence, all the CD patients had a non-stricturing, non-penetrating phenotype (Montreal B1).17 We also stipulated that patients demonstrating intolerance to IFX had to be excluded from the study.
Written informed consent was obtained from all subjects and the protocol was approved by the local Ethical Committee.
Study protocol
The treatment protocol included a standard IFX dose of 5 mg/kg at weeks 0, 2, and 6 as induction, and every 8 weeks thereafter as maintenance. The first blood sample was drawn at baseline, before the first IFX infusion. Peripheral blood mononuclear cells (PBMCs) were extracted and subjected to flow cytometry. The percentage of TNF-α-positive lymphocytes and monocytes, along with the intensity of TNF-α expression in each cell population was also analyzed. Serum samples were analyzed for sTNF-α level.
At week 14, a blood sample was collected immediately before the 4th IFX infusion. PBMCs were extracted and subjected to flow cytometric analysis for the evaluation of apoptosis and additional tests (see below).
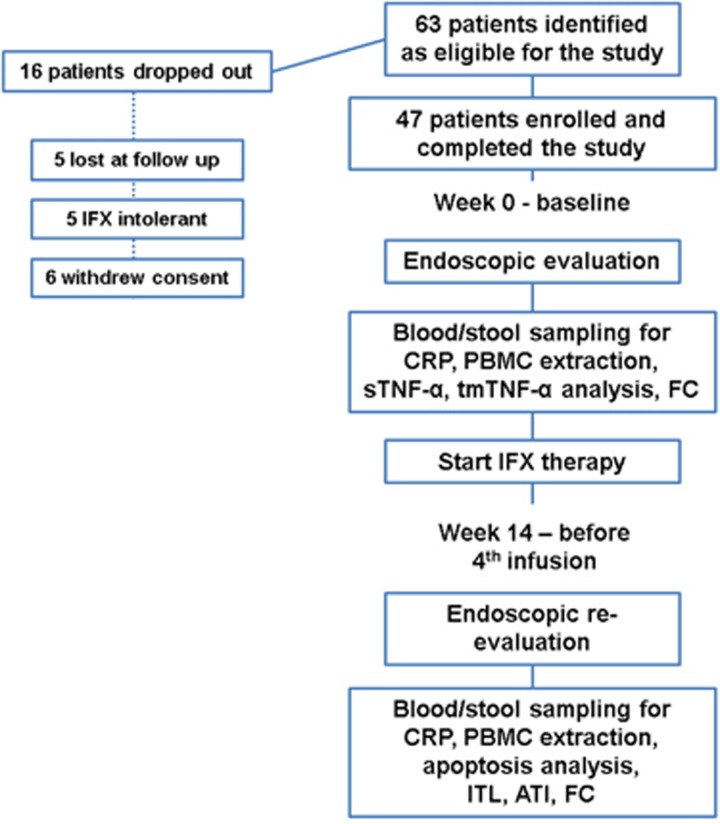
Endoscopic assessment was conducted at weeks 0 and 14 (see next paragraph). Figure 1 shows the flowchart of the study protocol in detail.
Figure 1.
Flowchart of the study. ATI, antibodies to infliximab; FC, fecal calprotectin; IFX, Infliximab; ITL, Infliximab trough level; PBMC, peripheral blood mononuclear cells; sTNF-α, serum TNF-α tmTNF-α, transmembrane TNF-α.
Evaluation of response to IFX
The common clinical definition of PNR to IFX is the lack of improvement of clinical signs and symptoms with induction therapy.3, 5 However, in our study we chose more objective and reliable parameters of therapeutic response namely endoscopic disease activity and markers of inflammation before and after treatment. For the purpose, colonoscopy was performed at week 0 (baseline) and 14, immediately before the first maintenance infusion. Endoscopic response in UC was defined as an improvement of the endoscopic Mayo score of at least one point from baseline.18 Endoscopic response in CD was defined as a decrease of ≥5 in the CDEIS score.19 In addition, C-reactive protein (CRP), and fecal calprotectin (FC) (FCal ELISA, Bühlmann, Schönenbuch, Switzerland) were measured at weeks 0 and 14, using an enzyme-linked immunosorbent assay (ELISA). A CRP level of (up to) 5 mg/l and FC level of (up to)100 μg/g were considered normal values.
Finally, to gain additional knowledge on the mechanism at the basis of PNR, IFX trough levels (ITL) and antibodies to IFX (ATI) (IDKmonitor-infliximab drug level ELISA and IDKmonitor-infliximab total ADA ELISA, Immundiagnostik AG, Bensheim, Germany) were measured immediately before the 4th IFX infusion at week 14 by ELISA. An ITL of 0.8 μg/ml and an ATI value of 10 U/ml were considered as cutoff detection levels. IDKmonitor-infliximab total ADA ELISA detects total ADA (bound and free ADA) and is categorized as a drug-tolerant assay.
Flow cytometric analysis of PBMCs
PBMCs were isolated by gradient centrifugation on a layer of Ficoll-Paque Plus (Amersham, Uppsala, Sweden). For the evaluation of tmTNF-α mean fluorescence intensity (MFI), extracted leukocytes were subjected to the acid wash process to remove receptor-bound TNF (cells were reconstituted for 5 min in RPMI 1640 medium, pH 2.5 containing 10% FBS). Cells were then washed twice with PBS before flow cytometric evaluation. The cells were stained for tmTNF-α by phycoerythrin conjugated anti-TNF-α monoclonal antibody (PE-Mouse Anti-Human TNF, Biolegend, San Diego, CA) in PBS with 1% bovine serum albumin and 0.1% sodium azide for 30 min at 4 °C. Before staining, cells were Fc-blocked by 1 μg of human IgG for 15 min at room temperature. Using the gating strategy, based on forward scatter (FSC) and side scatter (SSC) two separate gates were identified for the lymphocyte and monocytes populations (Supplementary Figure 1S).
For the assessment of PBMCs’ apoptotic activity, cells were stained by FITC Annexin V Apoptosis Detection Kit (BioLegend) with Propidium Iodide. Briefly, the cells were washed twice with cold BioLegend's Cell Staining Buffer, and then were re-suspended in Annexin V Binding Buffer. Subsequently, FITC Annexin V and Propidium Iodide Solution were added. Following 15 min incubation at room temperature in the dark, annexin V Binding Buffer was added. The cells were then subjected to flow cytometry.
Staining with the relevant isotype-matched control monoclonal antibody (BioLegend) was performed in all flow cytometric evaluations.
Immediately after staining, flow cytometric analysis was performed on a FACScalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ). At least 10 000 PBMCs per sample were analyzed. The data were analyzed using Flow Jo software (Tree star, Ashland, OR). An example of an individual run of apoptotic rates in responders and non-responders is illustrated in Supplementary Figure 2S.
sTNF-α analysis
Collected serum samples were stored at −80 °C until analysis. Serum levels of TNF-α were measured by ELISA, according to the protocol provided by the manufacturer (Human TNF-alpha Quantikine HS ELISA, R&D Systems-Minneapolis, MN). Human TNF-α controls (QuantiGlo Immunoassay Control Set 732 for Human TNF-alpha) were also used to increase the test accuracy.
Statistical analysis
Statistical analysis was performed using IBM SPSS for Windows, version 21 (IBM Armonk, NY). Central tendency and variability for numeric variables were measured using the mean and s.d., respectively. Normality test was performed for dependent variables. For normally distributed variables the parametric independent t-test and the paired t-test were used. For variables which were not normally distributed, the non-parametric Mann–Whitney U-test and Wilcoxon test were used. The Pearson/Spearman’s correlation coefficient was used to test potential correlation of variables. The analysis of covariance test was used to analyze mean differences among two groups with treatment as factor and baseline as covariate. The prognostic value of candidate variables was evaluated by plotting receiver operating characteristic curves and calculation of the area under the curve. Maximum point of the Youden's index (Youden's J) was identified as the cutoff value in the separation of responders and non-responders. The Youden's index is the likelihood of a positive test result in subjects with the condition vs. those without the condition. This index combines sensitivity and specificity into a single measure and has a value between 0 and 1. In a perfect test the Youden's value equals 1. A P value ≤0.05 was considered statistically significant.
Results
Table 1 shows the baseline characteristics of the enrolled patients. In total, 63 IFX naive IBD patients were identified as eligible for the study. Of the 63 eligible patients, 16 patients did not complete the treatment protocol and were later excluded. In total, 47 IFX naive IBD patients were evaluated in this study (Figure 1).
Table 1. Baseline characteristics of IBD patients.
| Variable |
Results |
|
|---|---|---|
| UC (n=13) | CD (n=34) | |
| Mean Age (mean±s.d.) years | 38.7±14.6 | 35±13.8 |
| Gender (Males, %) | 53.8 | 52.9 |
| Positive smoking history (%) | 15.4 | 6 |
| Positive family history of IBD (%) | 7.7 | 20 |
| Mean disease duration (mean±s.d.) years | 6.7±1.7 | 6.6±2.1 |
| Classification: | ||
| L1—ileal (%) | — | 10 (29.4) |
| L2—colonic (%) | 11 (32.4) | |
| L3—ileocolonic (%) | 13 (38.2) | |
| Classification: | ||
| E1—rectum (%) | 0 | — |
| E2—left sided (%) | 5 (38.5) | |
| E3—extensive (%) | 8 (61.5) | |
The patient population was composed by 22 females and 25 males, with 34 CD and 13 UC patients. The mean age was 36 years, ranging from 17 to 60. The mean duration of disease was 6.6 years, ranging from 3 to 12. Only 8.5% of patients were smokers at time of enrollment and 17% had a family history of IBD. Of the CD patients, equal proportions had ileal, colonic and ilecolonic disease. Of the UC patients 62% had pancolitis and 38% left sided colitis.
Based on the endoscopic criteria described in Methods, a total of 30 responders and 17 non-responders were identified in our cohort. The clinical and laboratory characteristics of responders and non-responders are compared in Table 2.
Table 2. Comparison of clinical and laboratory characteristics of IFX responders and nonresponders.
| Variable |
Responders |
Non-responders |
P value | ||
|---|---|---|---|---|---|
| UC (%) | CD (%) | UC (%) | CD (%) | ||
| Disease type | 8 (26.7) | 22 (73.3) | 5 (29.4) | 12 (70.6) | – |
| 30 (63.8) | 17 (36.2) | – | |||
| CDEIS week 0 | – | 17.6±4.7 | – | 18.9±4.3 | 0.43 |
| CDEIS week 14 | – | 9.3±3.4 | – | 17.4±4.7 | <0.001 |
| Mayo endoscopic subscore week 0 | 2.6±0.5 | – | 2.2±0.4 | – | 0.15 |
| Mayo endoscopic subscore week 14 | 1.3±0.5 | – | 2.4±0.5 | – | 0.002 |
| ITL (μg ml−1) week 14 | 5.6±3.6 | 3.5±1.6 | 0.9±1.1 | 1.2±1.1 | |
| 4.1±2.4 | 1.1±1.1 | <0.001 | |||
| ITL within therapeutic window (3–7 μg ml−1) at week 14 | 5 (63) | 12 (55) | 0 (0) | 2 (16) | |
| 17 (57) | 2 (12) | <0.001 | |||
| ITL week 14, ≥0.8 μg/ ml−1 | 8 (100) | 20(91) | 3 (60) | 6 (50) | |
| 28 (93.3) | 9 (52.9) | 0.002 | |||
| Negative ATI week 14 | 8 (100) | 19 (86.4) | 3 (60) | 8 (66.7) | |
| 27 (90) | 11 (64.7) | 0.004 | |||
| CRP (mg/L) week 0 | 34.5±47.3 | 45±43 | 35.1±29 | 42±37 | |
| 42±44 | 40±36 | 0.75 | |||
| CRP (mg/L) week 14 | 5.1±2.5 | 6.6±4.9 | 25.5±33 | 36.6±33 | |
| 6±4.5 | 33±31 | 0.005 | |||
| Mean CRP week 14, <5 mg /l−1 | 7 (87.5) | 16 (72) | 4 (80) | 4 (33) | |
| 23 (76.7) | 8 (47.1) | 0.04 | |||
| FC μg/ g−1 week 0 | 541.1±444 | 612±441 | 477.4±411 | 594.6±392 | |
| 593±435 | 560±406 | 0.86 | |||
| FC μg/ g−1 week 14 | 120.1±91.2 | 146.1±110 | 491.8±555 | 689±378 | |
| 139±107 | 631±429 | <0.001 | |||
| Mean FC week 14, <100 μg/ g−1 | 7 (87.5) | 14 (63.6) | 1 (20) | 0 (0) | |
| 21 (70) | 1 (5.9) | <0.001 | |||
Abbreviations: ATI, antibodies to infliximab; CD, Crohn’s disease; CDEIS, Crohn's disease Endoscopic Index of Severity; FC, fecal calprotectin; IFX, infliximab; ITL, IFX trough level; UC, ulcerative colitis.
Data are shown as N (%) or mean±s.d.
Almost 2/3 of UC and CD patients were primary responders. At baseline (week 0, before therapy), the CDEIS and Mayo subscore were similar in the two groups. After induction therapy (week 14), the mean CDEIS score in CD patients decreased by 8.3 points in responders compared to 1.45 points in non-responders (P<0.001). In UC patients, the mean Mayo endoscopic subscore decreased by 1.37 points in responders, while it increased by 0.2 points in non-responders (P<0.001).
ITL were 4.1±2.4 in responders compared to 1.1±1.1 μg/ml (P<0.001) in non-responders. In 57% of responders and 12% of non-responders ITL was within the therapeutic window (3–7 μg/ml).20 ATI were present in 35.3% of non-responders and in 10% of responders. Mean CRP and FC were similar at baseline in the two groups. Mean CRP decreased by 17.5% in non-responders and by 86% in responders after induction therapy (P<0.001). Mean FC decreased by 75% in responders after induction therapy, while it increased by 12% in non-responders (P<0.001).
Next, sTNFa and tm-TNF-α were measured at baseline, and apoptosis at week 14.
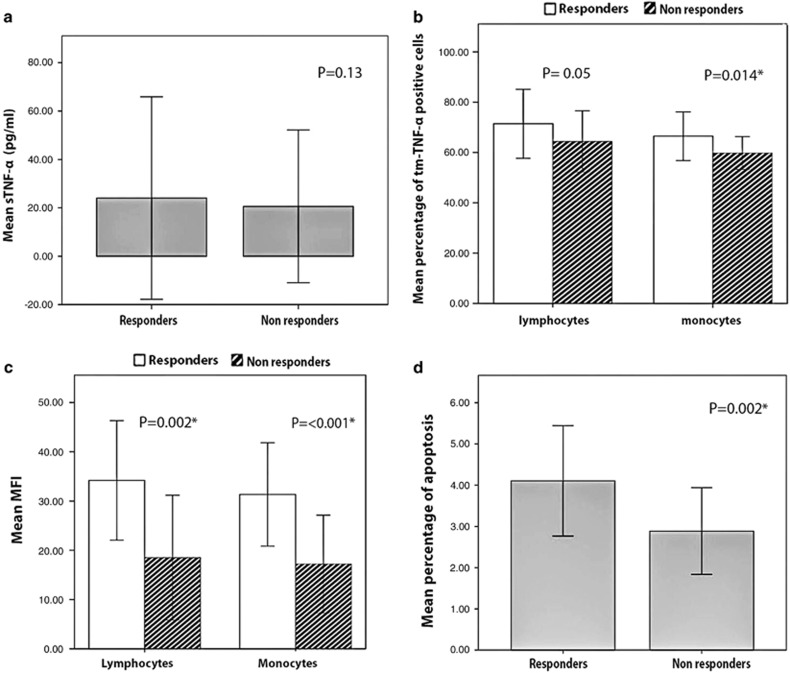
Mean sTNF-α was 24.1±41.8 pg/ml vs. 20.6±31.6 pg/ml, in responders vs. non-responders, respectively (P=0.13) (Figure 2a).
Figure 2.
sTNF-α, tmTNF-α bearing lymphocytes and monocytes, lymphocyte and monocyte tmTNF-α MFI (measured at baseline), and apoptosis in IFX responders and IFX non-responders (measured at 14 weeks). (a) Mean sTNF-α level; (b) percentage of tmTNF-α bearing lymphocytes and monocytes; (c) MFI of tmTNF-α in lymphocytes and monocytes; (d) percentage of apoptosis in PBMCs. IFX, Infliximab; MFI, mean fluorescence intensity; PBMC; sTNF-α, soluble TNF-α tmTNF-α, transmembrane TNF-α. The asterisk indicates a statistically significant difference.
The mean percentage of tmTNF-α bearing lymphocytes was borderline statistically different between responders and non-responders (71.4±13.6 vs. 64.3±12.1, respectively, P=0.05) (Figure 2b).The mean percentage of tmTNF-α bearing monocytes was significantly greater in responders compared to non-responders (66.5±9.6 vs. 59.8±6.5, respectively, P=0.014) (Figure 2b).
Mean lymphocyte MFI of tmTNF-α was greater in responders compared to non-responders (34.1±12.1 vs. 18.5±12.6, P=0.002) (Figure 2c). No significant correlation was observed between the lymphocyte MFI and sTNF-α values (r=−0.043, P=0.77). Mean monocyte MFI of tmTNF-α was also significantly greater in responders compared to non-responders (31.3±10.4 vs. 17.1±9.9, respectively, P<0.001) (Figure 2c). No significant correlation was observed between the monocyte MFI and sTNF-α values (r=−0.101, P=0.49).
The mean percentage of apoptosis in PBMCs was significantly greater in responders compared to non-responders, (4.1±1.3% vs. 2.8±1%, respectively, P=0.002) (Figure 2d). A significant positive correlation was observed between apoptosis and tmTNF-α MFI values of both lymphocytes and monocytes with correlation coefficients of 0.783 and 0.689, respectively (P<0.001). There was no significant correlation between apoptosis and sTNF-α levels (r=−0.100, P=0.5).
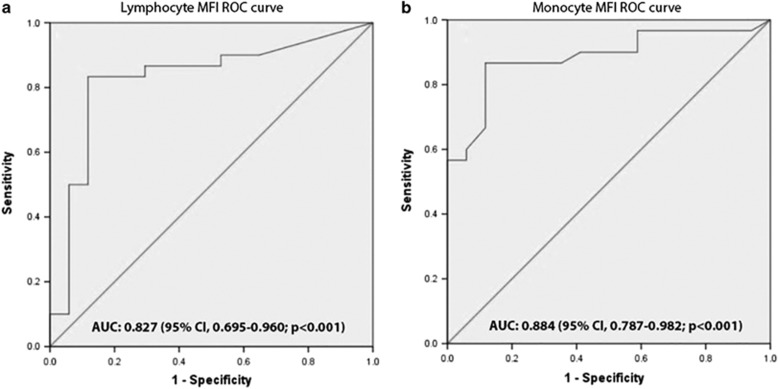
Construction of receiver operating characteristic curves and calculation of area under the curve was used to evaluate the prognostic power of MFI in tmTNF-α bearing lymphocytes and monocytes to predict response to IFX.
For the lymphocyte MFI, area under the curve was 0.827 (95% confidence interval, 0.695–0.960; P<0.001) with the optimal cutoff value of 30.5, with a sensitivity and specificity of 83.3 and 88.2, respectively (Figure 3a).
Figure 3.
ROC curves for lymphocyte (a) and monocyte (b) MFI. MFI, mean fluorescence intensity; ROC, receiver operating characteristic.
For the monocytes MFI, area under the curve was 0.884 (95% confidence interval, 0.787–0.982; P<0.001) with an optimal cutoff value of 24.5, with sensitivity and specificity of 86.7 and 88.2, respectively (Figure 3b).
Discussion
Anti-TNF agents, including IFX, are widely used in moderate to severe IBD. However, a sizeable proportion of biological naive patients fail to respond (PNR) or lose response over time (SLR). Given the potentially serious adverse effects of IFX and the financial burden it imposes to both health systems and patients, the identification of biomarkers which could help predict response to IFX might reduce the risks and costs for those patients who will fail to respond.21, 22
Several potential biomarkers have been proposed to predict PNR to IFX in IBD patients.22 Among them, the role of serum TNF-α level in IFX response has been widely investigated. However, there is no consensus as to its utility.
Martinez-Borra et al. reported that a high serum TNF-α level is associated with lack of response to IFX in CD patients.23 By contrast, Magnusson et al. showed that baseline levels of TNF-α tended to be higher in responders than in non-responders.24 Others have shown that baseline serum TNF-α in rheumatoid arthritis patients might provide a good estimation of the global disease burden and—as such—should be targeted in IFX dose titration studies.25
In addition to the neutralization of sTNF-α, IFX appears to attenuate inflammation by the so-called “tmTNF-α reverse signaling-induced apoptosis” whereby tmTNF-α appears to act as a cell surface receptor binding circulating IFX, binding which in turn triggers reverse signaling and other events, including apoptosis of the tmTNF-α bearing cells, a de facto cellular suicide.11, 12, 13 This mechanism of action of IFX seems to be clinically very important. In fact, IBD patients do not respond to Etanercept, (another anti-TNF-α agent) which—by contrast with IFX—only appears to inhibit sTNF-α, but does not induce reverse signaling through tmTNF-α binding.4, 13, 26
In this regard, Atreya et al. showed that CD patients with a high number of tmTNF-α positive cells (as tested in inflamed intestinal tissue) had a better response to anti-TNF-α therapy when compared to patients with a low number of tmTNF-α positive cells.27
In this study, we tested the hypothesis that a high density of TNF-α on the surface of TNF-α bearing circulating cells might lead to more efficient induction of reverse signaling associated apoptosis, and might predict response to IFX in IBD patients. For the purpose, we enrolled CD and UC patients naive to IFX and tested sTNF-α, percentage of circulating TNF-α positive monocytes and lymphocytes, MFI of tmTNF-α in circulating lymphocytes and monocytes, and apoptosis rate in PBMCs of IFX responders and non-responders. It is important to stress that patients were identified as responders or non-responders based on a solid objective clinical outcome—endoscopic activity—after full induction therapy. In addition, we measured blood and stool markers of inflammation before and after therapy. We believe that these are more reliable indicators of response than symptomatic improvement after the IFX induction phase.3, 5 Enrolled patients had no strictures or other disease complications which would have made them potentially resistant to any medical therapy. We also measured ITL and ATI—based on the currently held opinion that resistance to IFX might be due to low ITL or to the presence of ATI, albeit less likely in PNR than in SLR.5, 6
A total of 47 patients completed the study. More than 2/3 responded to IFX, showing a significant drop in endoscopic scores, CRP and FC levels. IFX responders had higher ITL when compared to non-responders. In addition, 35% of non-responders (and 10% of responders) had detectable ATI levels. It should be noted that the use of a drug-tolerant assay allowed us to detect ATI in the presence of an adequate IFX concentration.28 These data (as well as the very restrictive inclusion criteria) further suggest overall that in our patient population the lack of response—when present—could be driven by factors other than immune response to IFX such as a specific biologic mechanism. Indeed, the MFI value of tmTNF-α was significantly lower in IFX non-responders compared to IFX responders, in both monocytes and lymphocytes. In addition, the apoptosis rate in PBMCs was also lower in IFX non-responders compared to IFX responders. By contrast, sTNF-α did not significantly differ in the two populations and did not correlate with MFI values and apoptotic rates. Taken together these findings might suggest that in non-responders less circulating IFX binds to tmTNF-α which in turn might lead to faster clearance (leading to lower ITL) and decreased efficacy.
Other data suggest that PNR might be due to a disease burden exceeding the neutralizing capacity of the standard IFX dose.7, 8, 29 However, our findings are not inconsistent with such conclusion. They rather suggest that in PNR IFX efficacy seems to be hampered by a defective target—which could in principle be overcome by larger medication doses, a scenario functionally and clinically indistinguishable from a greater disease burden. Clearly, many other mechanisms might also be at play.
Similar observations have been made in other diseases. For example, different sensitivity to apoptosis induced by anti-TNF-α therapy has been previously shown in PBMCs of rheumatoid arthritis patients.30
Hence, our results show that in principle a simple blood test in PBMCs could be used to predict response to IFX in IBD patients. This conclusion is also supported by the good prognostic value, sensitivity and specificity of tmTNF-α MFI of both lymphocytes and monocytes as assessed by receiver operating characteristic and area under the curve.
Potential limitations of our study are the relatively small sample size and the relatively short follow up. In addition, we have only studied patients with PNR to IFX—and it is not clear whether our findings would also apply to SLR.
In conclusion, our data show that the intensity of tmTNF-α on circulating leukocytes in IBD patients is related to IFX response in both CD and UC. This observation has the potential to be used clinically given its relatively low cost and lack of invasiveness. Further studies to confirm these findings are warranted.
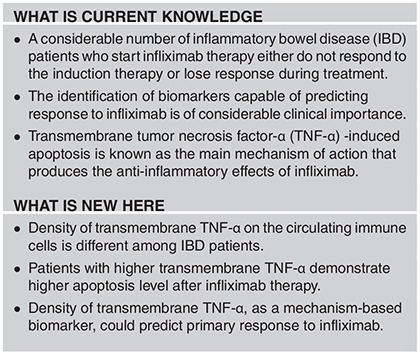
Study Highlights
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Guarantor of the article: Dario Sorrentino, MD, FRACP.
Specific author contributions: Study concept and design: Hamid Asadzadeh Aghdaei, Azade Amini Kadijani, Dario Sorrentino. Acquisition of data: Azade Amini Kadijani, Hedieh Balaii. Analysis and interpretation of data: Azade Amini Kadijani, Dario Sorrentino. Drafting of the manuscript: Azade Amini Kadijani, Alireza Mirzaei, Vu Nguyen. Critical revision of the manuscript for important intellectual content: Dario Sorrentino, Vu Nguyen. Statistical analysis: Alireza Mirzaei. Administrative, technical, and material support: Mohammad Reza Zali, Shabnam Shahrokh, Jessica L.Mays. Study supervision: Hamid Asadzadeh Aghdaei, Dario Sorrentino.
Financial support: V.N. has received grant support from AbbVie Inc. D.S. has received consulting fees from Abbott/AbbVie, Schering-Plough, MSD, Janssen Research & Development, LLC., Centocor Inc., TechLab, Hoffmann-LaRoche, Giuliani, Schering-Plough, and Ferring; research grants from AbbVie, Janssen Research & Development, LLC, Schering-Plough, TechLab, Centocor and serves in the Speakers Bureau of AbbVie and the National Faculty of Janssen. This study has been supported by Research Institute for Gastroenterology and Liver Disease Shaheed Beheshti University of Medical Sciences.
Potential competing interests: None.
Supplementary Material
References
- Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 2006; 3: 390–407. [DOI] [PubMed] [Google Scholar]
- Molodecky NA, Soon S, Rabi DM et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142: 46–54.e42. [DOI] [PubMed] [Google Scholar]
- Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn's disease: a review. Am J Gastroenterol 2009; 104: 760–767. [DOI] [PubMed] [Google Scholar]
- Van Deventer S. Review article: targeting TNFα as a key cytokine in the inflammatory processes of Crohn’s disease—the mechanisms of action of infliximab. Aliment Pharmacol Ther 1999; 13: 3–8. [DOI] [PubMed] [Google Scholar]
- Danese S, Fiorino G, Reinisch W. Review article: causative factors and the clinical management of patients with Crohn’s disease who lose response to anti-TNF-α therapy. Aliment Pharmacol Ther 2011; 34: 1–10. [DOI] [PubMed] [Google Scholar]
- Sorrentino D, Nguyen V, Henderson C et al. Therapeutic drug monitoring and clinical outcomes in immune mediated diseases: the missing link. Inflamm Bowel Dis 2016; 22: 2527–2537. [DOI] [PubMed] [Google Scholar]
- Verstockt B, Arijs I, de Bruyn M et al. Increased baseline TNF-driven pathways observed in patients with Crohn's disease not responding to infliximab. Gastroenterology 2017; 152: S767. [Google Scholar]
- Yarur AJ, Jain A, Sussman DA et al. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: the ATLAS study. Gut 2015; 65: 249–255. [DOI] [PubMed] [Google Scholar]
- Meusch U, Rossol M, Baerwald C et al. Outside-to-inside signaling through transmembrane tumor necrosis factor reverses pathologic interleukin-1β production and deficient apoptosis of rheumatoid arthritis monocytes. Arthritis Rheum 2009; 60: 2612–2621. [DOI] [PubMed] [Google Scholar]
- Mitoma H, Horiuchi T, Hatta N et al. Infliximab induces potent anti-inflammatory responses by outside-to-inside signals through transmembrane TNF-α. Gastroenterology 2005; 128: 376–392. [DOI] [PubMed] [Google Scholar]
- Horiuchi T, Mitoma H, Harashima S-I et al. Transmembrane TNF-α: structure, function and interaction with anti-TNF agents. Rheumatology 2010; 49: 1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallon BJ, Moore MA, Trinh H et al. Chimeric anti-TNF-α monoclonal antibody cA2 binds recombinant transmembrane TNF-α and activates immune effector functions. Cytokine 1995; 7: 251–259. [DOI] [PubMed] [Google Scholar]
- Mitoma H, Horiuchi T, Tsukamoto H et al. Mechanisms for cytotoxic effects of anti-tumor necrosis factor agents on transmembrane tumor necrosis factor α-expressing cells: comparison among infliximab, etanercept, and adalimumab. Arthritis Rheum 2008; 58: 1248–1257. [DOI] [PubMed] [Google Scholar]
- Magro F, Gionchetti P, Eliakim R et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017; 11: 649–670. [DOI] [PubMed] [Google Scholar]
- Gomollón F, Dignass A, Annese V et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis 2016; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- Benevento G, Avellini C, Terrosu G et al. Diagnosis and assessment of Crohn’s disease: the present and the future. Expert Rev Gastroenterol Hepatol 2010; 4: 757–766. [DOI] [PubMed] [Google Scholar]
- Satsangi J, Silverberg M, Vermeire S et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006; 55: 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Haens G, Sandborn WJ, Feagan BG et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology 2007; 132: 763–786. [DOI] [PubMed] [Google Scholar]
- Khanna R, Bouguen G, Feagan BG et al. A systematic review of measurement of endoscopic disease activity and mucosal healing in Crohn's disease: recommendations for clinical trial design. Inflamm Bowel Dis 2014; 20: 1850–1861. [DOI] [PubMed] [Google Scholar]
- Casteele NV, Ferrante M, Van Assche G et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015; 148: 1320–1329.e3. [DOI] [PubMed] [Google Scholar]
- Zampeli E, Gizis M, Siakavellas SI et al. Predictors of response to anti-tumor necrosis factor therapy in ulcerative colitis. World J Gastrointest Pathophysiol 2014; 5: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology 2011; 140: 1817–1826.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Borra J, López-Larrea C, González S et al. High serum tumor necrosis factor-α levels are associated with lack of response to infliximab in fistulizing Crohn's disease. Am J Gastroenterol 2002; 97: 2350–2356. [DOI] [PubMed] [Google Scholar]
- Magnusson MK, Dahlén R, Strid H et al. CD25 and TNF receptor II reflect early primary response to infliximab therapy in patients with ulcerative colitis. United European Gastroenterol J 2013; 1: 467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Miyasaka N, Tatsuki Y et al. Baseline tumour necrosis factor alpha levels predict the necessity for dose escalation of infliximab therapy in patients with rheumatoid arthritis. Ann Rheum Dis 2011; 70: 1208–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Hove T, Van Montfrans C, Peppelenbosch M et al. Infliximab treatment induces apoptosis of lamina propria T lymphocytes in Crohn's disease. Gut 2002; 50: 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atreya R, Neumann H, Neufert C et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn's disease. Nat Med 2014; 20: 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteele NV, Khanna R, Levesque BG et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn's disease. Gut 2015; 64: 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiet T, Cleynen I, Ballet V et al. 286 drug concentrations and antibodies to infliximab are inferior to the impact of disease burden in primary non-response to infliximab in crohn's disease patients. Gastroenterology 2015; 148: S-62. [Google Scholar]
- Coury F, Ferraro-Peyret C, Le Cam S et al. Peripheral blood lymphocytes from patients with rheumatoid arthritis are differentially sensitive to apoptosis induced by anti-tumour necrosis factor-alpha therapy. Clin Exp Rheumatol 2008; 26: 234. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.