Abstract
Background
Although flowers of Panax ginseng Meyer (FPG), Panax quinquefolius L. (FPQ), and Panax notoginseng Burk. (FPN) have been historically used as both medicine and food, each is used differently in practice.
Methods
To investigate the connection between components and enhancing immunity activity of FPG, FPQ, and FPN, a method based on a rapid LC coupled with quadrupole time-of-flight MS and immunomodulatory activity study evaluated by a carbon clearance test were combined.
Results
According to quantitative results, the ratio of the total content of protopanaxatiol-type ginsenosides to protopanaxadiol-type ginsenosides in FPN was 0, but ranged from 1.10 to 1.32 and from 0.23 to 0.35 in FPG and FPQ, respectively. The ratio of the total content of neutral ginsenosides to the corresponding malonyl-ginsenosides in FPN (5.52 ± 1.33%) was higher than FPG (3.2 ± 0.64%) and FPQ (2.39 ± 0.57%). The colorimetric analysis showed the content of total ginsenosides in FPQ, FPG, and FPN to be 13.75 ± 0.60%, 17.45 ± 0.42%, and 12.45 ± 1.77%, respectively. The carbon clearance assay indicated that the phagocytic activity of FPG and FPQ was higher than that of FPN. A clear discrimination among FPG, FPQ, and FPN was observed in the principal component analysis score plots. Seven compounds were confirmed to contribute strongly by loading plots, which may be the cause of differences in efficacy.
Conclusion
This study provides basic information about the chemical and bioactive comparison of FPG, FPQ, and FPN, indicating that protopanaxtriol-type ginsenosides and malonyl-ginsenosides may play a key role in their enhancing immunity properties.
Keywords: flowers, Panax ginseng Meyer, Panax quinquefolius L., Panax notoginseng Burk., UPLC-MS/MS
1. Introduction
Panax ginseng Meyer, Panax quinquefolius L. and Panax notoginseng Burk. are three major perennial herbal plants in genus Panax (Araliaceae) [1], [2], [3]. These three herbs have been used for millenia for prevention or treatment of ailments and diseases [4] and have been sharing a large market as drugs, dietary supplements, and foods. For example, in the Compendium of Material Medica, a Chinese materia medica work written in the Ming Dynasty, P. ginseng and P. quinquefolius are often used as a tonic, a stimulant, and possess fatigue-resisting effects; P. notoginseng is considered to be a remedy for preventing bleeding and recovering from injury. For a long time, flowers of these three herbal plants have also been used extensively in the form of drugs or foods. They all, generally, were used in traditional Chinese medicine formulae. Furthermore, all the three kinds of flowers were used as an exhilarant and tonic in the form of health tea in China [5]. With the depth development, they were also exploited as drinks, and were even added into shampoo.
Recent investigation showed that the flowers of Panax ginseng Meyer (FPG), P. quinquefolius L. (FPQ), and P. notoginseng Burk. (FPN) also contain ginsenosides [6], [7], [8], [9]. Moreover, the amount of total ginsenosides in the FPG, FPQ, and FPN is higher than in any other parts [10]. These ginsenosides are defined as protopanaxatiol-type ginsenosides (PPT), protopanaxadiol-type ginsenosides (PPD), and oleanolic acid-type ginsenosides according to the dammarane skeleton. However, research into ginsenosides has always ignored malonyl-ginsenosides, which can constitute a substantial proportion of the total ginsenoside content. Malonyl-ginsenosides are thermally unstable and can degrade into corresponding neutral ginsenosides.
Since ginsenosides were reported from the flowers of these plants, pharmacological efficacies of these flowers were also studied. For example, Yoshikawa et al. [11] reported that the ginsenosides fraction from FPN can protect against liver injury induced by D-galactosamine and lipopolysaccharide in mice. Another example [6] is that, in addition to reducing pathogenic fire, brain refreshing, and invigorating the blood, ginsenosides from steamed FPG exert anti-inflammatory activity. Another study [12] showed that extract of FPG possessed antioxidant activity. However, pharmacological studies on the FPQ were yet left uncharacterized. It had been used as a tonic in the form of health protection tea with the same medicinal purposes as FPG [5].
Ginsenosides, the major components in these three flowers, are well believed by the theory of traditional Chinese medicine and local folk to stimulate immune systems leading to benefit for health. Results from basic researches support this point. Yu et al. [13] had demonstrated ginsenoside Rg1 can increase the weight of immune organs in mice and phagocytic activity of macrophages, improve serum IL-2 and complement C3 and C4, resulting in the stimulation of immune of the body. Another study [14] showed that ginsenoside Re activates microphage function to kill tumor cells. Cho et al. [15] and Rhule et al. [16] reported that ginsenosides Rb1, Rb2, and Rg1 strongly suppress the production of tumor necrosis factor-α in macrophages treated with LPS. Notoginsenosides-D, -H, -K, and -G increased serum level of IgG in mice sensitized with ovalbumin [17]. Therefore, different type of ginsenoside possesses different effects on the immune system.
Morphologically, the flowers of these three titled plants are similar. However, whether the components in these flowers are similar or not has not been determined. Since different ginsenosides possess different bioactivity, it is highly important to establish a method to characterize the relationship between the components in these three flowers and their bioactivity. In this work, we first simultaneously identify and quantify the major components in the three flowers of the titled plants by ultra-performance liquid chromatography coupled to electrospray ionization quadropole-time-of- fight mass spectrometry (UPLC-ESI-Q-TOF/MSn) and ultra-performance liquid chromatography with diode array detection (UPLC-DAD), and further discuss the relationship between the immune stimulation and the components.
2. Materials and methods
2.1. Chemicals
Ginsenosides Rg1, Re, Rb1, and Rd were purchased from the National Institutes for Food and Drug control (Beijing, China). Ginsenosides Rb2, Rc and Rb3 were purchased from Chengdu Pufeide Biological Technology Co. Ltd. (Chengdu, China). All reference standards were at least 98% purity as confirmed by HPLC. Acetonitrile (HPLC grade) was purchased from Sigma (St Louis, MO, USA); Ultrapure water (18.2 MΩ) was prepared with a Milli-Q water purification system (Millipore, Saint Quentin Fallavier, France). Other reagents were analytical grade.
2.2. Herbal materials
FPG, FPQ, and FPN were collected from 3-y-old ginseng plants from Jilin, Liaoning, and Yunnan provinces of China. All samples were morphologically authenticated as the flowers of P. ginseng Meyer, P. quinquefolius L. and P. notoginseng Burk. by Dr. Jin-cai Lu (Shenyang Pharmaceutical University, China). The pictures are shown in Fig. S1. The voucher specimens were deposited in the Pharmacognosy Laboratory, Shenyang Pharmaceutical University.
2.3. Preparation of samples and stock solution
A 0.5-g sample of powdered flower material was weight accurately. Then 20.0 mL 70% (v/v) methanol aqueous solution was added and the material was extracted by ultrasound (40 kHz, 200 W) for 0.5 h at room temperature; 70% methanol aqueous solution was added to compensate for the lost weight. The extractive was centrifuged and the supernatant was obtained as sample solution. All of the samples were passed through a 0.22-μm PTFE syringe filter prior to injection.
A mixed standard solution containing 0.445 mg/mL of Rg1, 1.790 mg/mL of Re, 0.645 mg/mL of Rb1, 0.500 mg/mL of Rc, 0.540 mg/mL of Rb2, 1.520 mg/mL of Rb3, and 0.900 mg/mL of Rd was prepared by adding each standard into a volumetric flask and dissolving with methanol. A series of standard working solutions with five different concentrations was prepared by appropriately diluting the mixed stock solution with methanol for determination of the standard curves.
2.4. Estimation of total ginsenosides
Total ginsenoside in FPG, FPQ, and FPN was determined by a colorimetric assay using UV spectrophotometry with ginsenoside Re as standard according to the method in the Appendix IV A in Chinese Pharmacopoeia (edition 2010) [18]. The wave length was at 560 nm. This assay was repeated in triplicate for each sample. The results were expressed in terms of ginsenoside Re equivalent, according to the equation of the line for the ginsenoside Re standard curve.
2.5. UPLC-Q-TOF-MS analysis
The UPLC conditions were: system, Waters Acquity; column, Acquity UPLC BEH C18 column (100 × 2.1 mm inner diameter, 1.7 μm, Waters, Milford, MA, USA); mobile phase A, 100% acetonitrile; mobile phase B, 0.1% formic acid; gradient, 0–1.5 min, 10–14% A; 1.5–3.5 min, 14–21% A; 3.5–10.3 min, 21–23% A; 10.3–10.6 min, 23–28% A; 10.6– 26.5 min, 28–31% A ; 26.5–27.5 min, 31–90% A; 27.5–30 min, 90% A; and the flow rate was 0.5 mL/min. The detection wavelength was 203 nm, column temperature was 30°C.
MS was performed on a Waters Q-TOF/MS (Waters) equipped with electrospray ionization (ESI) source operating in negative mode. The conditions of MS analysis were as follows: capillary voltage, 3 kV; cone voltage, 3 kV; cone gas (N2) flow rate, 50 L/h; nebulizing gas (N2) temperature, 450°C; nebulizing gas (N2) flow rate, 800 L/h; source temperature, 130°C; mass range, m/z 100–1,300. The energies for collision-induced dissociation were set at 20–55 V for the precursor ion. All the operations, acquisition, and analysis of data were controlled by Masslynx NT 4.1 software with QuanLynx program (Waters).
2.6. Method validation
To verify the linearity, solutions containing seven reference substances at five different concentrations were injected. The calibration curve of each standard was constructed by plotting the peak area versus the corresponding concentration. The limits of detection and quantitation were determined at signal-to-noise ratio of 3 and 10, respectively. The accuracy was explored by the percentage difference between amounts determined and spiked. The low, medium and high concentrations of the seven reference substances were spiked into samples and then prepared according to Section 2.3. The determination was performed in triplicate. The average recoveries and relative standard deviation (RSD) were calculated. The intraday and interday precision were determined on the same day or on 3 different days. The sample solutions were analyzed at 0 h, 4 h, 8 h, 12 h, and 24 h in the stability test. The reproducibility was examined in 5 times/d.
2.7. Carbon clearance test
Approximately 75 g fine powder of FPG, FPQ, and FPN were extracted according to the method described in Section 2.3, which was dried under vacuum for the carbon clearance test.
Male and female Kunming mice (18–22 g) were purchased from Liaoning Chang Sheng Biotechnology Co., Ltd [License number (Liao) SCXK2012-0004]. Before the experiments, the mice were allowed a 1-wk acclimation period at room temperature (20–25°C) and constant humidity (40–70%) under a 12-h light–dark cycle in a specific pathogen-free grade laboratory, fed with standard rodent chow and tap water freely. The procedures in this study were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Ethics Committee of the Institution.
The carbon clearance test was carried out according to references [19] and [20]. Briefly, 80 Kunming mice were randomly classified into eight groups with half male and half female, Mannatide at dose of 4.0 mg/kg/d was positive control group [21], while animals of treatment group were administrated the extracts of FPG, FPQ, and FPN at dose of 0.5 g/kg/d by or 1.0 g/kg/d by gavage for 10 d.
On Day 10 of drug treatment, all animals were received an intravenous injection (10 mL/kg) of carbon suspension (Indian ink: normal saline = 1:4) through tail vein. Blood samples (20 μL) were taken from the orbital sinus at 2–12-min intervals after the injection, and were mixed with 2 mL 0.1% (w/v) Na2CO3 solution to lyse the erythrocytes. Optical density (OD) of the samples was measured at 620 nm using a Multiskan Spectrum. At the same time, mice were sacrificed by cervical dislocation, liver and spleen weights of the mice were measured. The clearance index (k) and the calibrate index (α) were calculated as follows:
where, OD1 and OD2 are the optical densities at times t1 and t2.
3. Results and discussion
3.1. Optimization of sample preparation and chromatographic conditions
To optimize the conditions of sample preparation, the following parameters were tested by the FPG-1 extracted by an ultrasonic instrument with variables such as extraction solvent (40%, 70%, and 100% methanol or ethanol), extraction time (30 min, 40 min, 50 min, and 70 min) and solvent multiples (1:20, 1:30, 1:50, and 1:60), the results are shown in Tables S1–S4, The optimized conditions are described in Section 2.3.
Prior to the real sample analysis, the UPLC and MS parameters were fully evaluated and optimized. Column, mobile phase, and elution profile were UPLC parameters affecting separation efficiency. Different columns were tested and Acquity UPLC BEH C18 column (100 mm × 2.1 mm inner diameter, 1.7 μm) can obtain more peaks. Acetonitrile was used as mobile phrase for its perfect separation. Formic acid–water in a concentration of 0.1% (v/v) can improve the peak shape. To obtain the optimal sensitivity, ESI modes (positive and negative) and collision-induced dissociation energy were also studied. ESI negative mode with the collision energy of 20–55 V was found to provide not only optimal molecular ions but also clear information on the quasimolecular ions and fragment ions. The optimal UPLC-Q-TOF/MS conditions were described in Section 2.5.
3.2. Identification of the major components in FPG, FPQ, and FPN
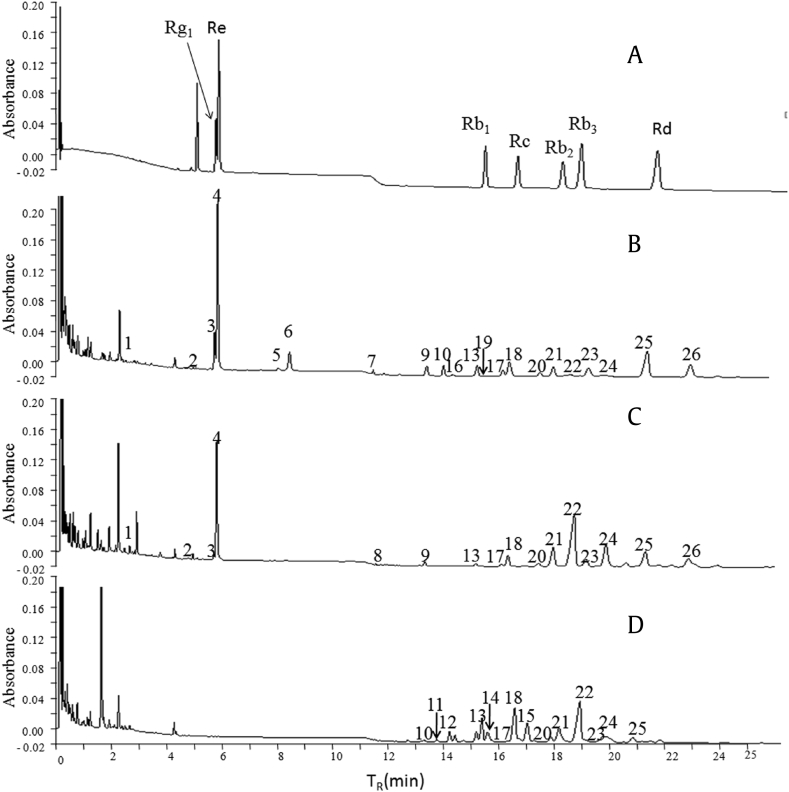
The major components in the flowers were identified by UPLC-Q-TOF/MS. The total ion chromatograms of FPG, FPQ, and FPN are shown in Fig. S2. Ginsenosides Rg1, Re, Rb1, Rc, Rb2, Rb3, and Rd were unambiguously identified by comparing their retention times and MS data with the reference standards. Other components were identified by comparing the MS/MS data with those of references [22]. The details of identified ginsenosides are summarized in Table 1. The mass error for molecular ions of all identified compounds was within ± 10 ppm, indicating that the empirical molecular formula well matched the putative molecular ions, quasimolecular ion and fragment ions.
Table 1.
Characterization of compounds in different Panax flower products by UPLC-Q-TOF/MS
| Identification | TR (min) | formula for M | [M-H]− |
Fragment ions | Sample items | |||
|---|---|---|---|---|---|---|---|---|
| Measured mass | Calculated mass | Accuracy (ppm) | ||||||
| 1 | 20-glc-Rf | 2.427 | C48H82O19 | 961.5419 | 961.5372 | 4.89 | 1,007.5474[M+HCOO]− 799.4831 [M-H-Glc]− 781.4760 [M-H-Glc-H2O]− |
FPG, FPQ |
| 2 | Noto- ginsenoside R1 |
4.363 | C47H80O18 | 931.5261 | 931.5266 | −0.54 | 977.5313[M+HCOO]− 799.4895 [M-H-Xyl]− 637.4288 [M-H-Xyl-Glc]− 475.3760 [M-H-Xyl-2Glc]− |
FPG, FPQ |
| 3 | Rg1 | 5.191 | C42H72O14 | 799.4950 | 799.4944 | 0.75 | 845.4980 [M+HCOO]− 637.4353 [M-H-Glc]− |
FPG, FPQ |
| 4 | Re | 5.911 | C48H82O18 | 945.5502 | 945.5423 | 8.35 | 991.5539 [M+HCOO]− 783.4907 [M-H-Glc]− 637.3346 [M-H-Glc-Rha]− 475.3819 [M-H-2Glc-Rha]− |
FPG, FPQ |
| 5 | Malonyl-Rg1 | 5.923 | C45H74O17 | 885.4899 | 885.4848 | 5.80 | 841.5217 [M-H-CO2]− 781.4969 [M-H-Mal-H2O]− 637.4317 [M-H-Mal-Glc]− 619.4258 [M-H-Ma-Glc-H2O]− |
FPG, FPQ |
| 6 | Malonyl- Re | 7.027 | C51H84O21 | 1,031.5496 | 1,031.5427 | 6.69 | 987.5598 [M-H-CO2]− 927.5385 [M-H-Mal-H2O]− 783.4634 [M-H-Mal-Glc]− 637.4251 [M-H-Mal-2Glc]− 475.3643[M-H-Mal-2Glc-Rha]− |
FPG, FPQ |
| 7 | Rf | 11.322 | C42H72O14 | 799.4850 | 799.4844 | 0.75 | 845.4896 [M+HCOO]− | FPG |
| 8 | P-F11 | 11.322 | C42H72O14 | 799.4850 | 799.4844 | 0.75 | 845.4896 [M+HCOO]− | FPQ |
| 9 | 20(R/S)-Rg2 | 12.475 | C42H72O13 | 783.4863 | 783.4895 | −4.08 | 829.4919[M+HCOO]− 637.4288 [M- H-Rha]− 475.3748 [M-H-Rha-Glc]− |
FPG, FPQ |
| 10 | RF3 | 13.178 | C41H70O13 | 769.4735 | 769.4738 | −3.90 | 815.4805 [M+HCOO]− | FPG |
| 11 | Ra3 | 13.158 | C59H100O27 | 1,239.6443 | 1,239.6374 | 5.57 | 1,285.6537[M+HCOO]− | FPN |
| 12 | Ra1/ Ra2/isomer | 14.020 | C58H98O26 | 1,209.6318 | 1,209.6268 | 4.13 | 1,255.6390 [M+HCOO]− | FPN |
| 13 | Rb1 | 14.170 | C54H92O23 | 1,107.5970 | 1,107.5951 | 1.72 | 1,153.6044[M+HCOO]− 945.5425 [M-H-(Glc-H2O)]− 783.4903 [M-H-2(Glc-H2O)]− |
FPG, FPQ, FPN |
| 14 | Ra1/ Ra2/isomer | 14.399 | C58H98O26 | 1,209.6318 | 1,209.6268 | 4.13 | 1,255.6390 [M+HCOO]− | FPN |
| 15 | Malonyl-Ra1/ Ra2 | 17.424 | C61H100O29 | 1,295.6252 | 1,295.6272 | −1.54 | 1077.5836[M+HCOO]− | FPN |
| 16 | RO | 14.590 | C49H80O18 | 955.4922 | 955.5001 | −8.2 | 793.4364 [M-H-Glc]− | FPG, FPQ |
| 17 | Malonyl-Rb1 | 14.774 | C57H94O26 | 1,193.5964 | 1,193.5955 | 0.75 | 1,149.6030 [M-H-CO2]− 1,107.5902 [M-H-Mal]− 1,089.5814[M-H-Mal-H2O]− 945.5450 [M-H-Mal-Glc]− |
FPG, FPQ, FPN |
| 18 | Rc | 15.251 | C53H90O22 | 1,077.5846 | 1,077.5845 | 0.09 | 1,123.5867 [M+HCOO]− 945.5430 [M-H-Ara(f)-H2O]− 783.4861 [M-H -Ara(f)-H2O- (Glc-H2O)]− 621.4187 [M –H-Ara(f)-H2O- 2(Glc-H2O)]− |
FPG, FPQ, FPN |
| 19 | Rh1 | C36H62O9 | 637.4308 | 637.4316 | −1.26 | 683.4371 [M+HCOO]− 1,475.3795 [M-H-Glc]− |
FPG | |
| 20 | Malonyl-Rc | 15.935 | C56H92O25 | 1,163.5906 | 1,163.5849 | 4.80 | 1,119.5992 [M-H-CO2]− 1,077.5874 [M-H-Mal]− 1,059.5758[M-H-Mal-H2O]− 987.5598 [M-H-CO2-Ara(f)]− 945.5584 [M-H-Mal-Ara(f)]− 915.5268 [M-H-Mal-Glc]− 783.4879 [M-H-Mal- Ara(f)-Glc]− |
FPG, FPQ, FPN |
| 21 | Rb2 | 16.654 | C53H90O22 | 1,077.5846 | 1,077.5845 | 0.09 | 1,123.5958 [M+HCOO]− 945.5456 [M-H-Ara(p)-H2O]− 783.4951 [M-H -Ara(p)-H2O- (Glc-H2O)]− 621.4277 [M-H-Ara(p)-H2O- 2(Glc-H2O)]− |
FPG, FPQ, FPN |
| 22 | Rb3 | 17.238 | C53H90O22 | 1,077.5846 | 1,077.5845 | 0.09 | 1,123.5958 [M+HCOO]− 945.5456 [M-H-Xyl-H2O]− 783.4951 [M-H-Xyl-H2O-(Glc-H2O)]− 621.4277 [M-H-Xyl-H2O- (Glc-H2O)]− |
FPG, FPQ, FPN |
| 23 | Malonyl-Rb2 | 18.020 | C56H92O25 | 1,163.5906 | 1,163.5849 | 4.90 | 1,119.5980 [M-H-CO2]− 1,077.5872 [M-H-Mal]− 1,059.5758[M-H-Mal-H2O]− 945.5480 [M-H-Mal-Ara(p)]− 915.5343 [M-H-Mal-Glc]− 783.4828[M-H-Mal-Ara(p)-Glc]− |
FPG, FPQ, FPN |
| 24 | Malonyl-Rb3 | 18.790 | C56H92O25 | 1,163.5906 | 1,163.5849 | 4.90 | 1,119.5995 [M-H-CO2]− 1,077.5841 [M-H-Mal]− 1,059.5868[M-H-Mal-H2O]− 987.5516 [M-H-CO2-Xyl]− 945.5317 [M-H-Mal-Xyl]− 915.5179 [M-H-Mal-Glc]− 783.4799 [M-H-Mal-Xyl-Glc]− |
FPG, FPQ, FPN |
| 25 | Rd | 19.836 | C48H82O18 | 945.5482 | 945.5423 | 6.24 | 991.5521 [M+HCOO]− 783.4912 [M-H-Glc]− 621.4448 [M-H-2Glc]− |
FPG, FPQ, FPN |
| 26 | Malonyl-Rd | 20.894 | C51H84O21 | 1,031.5466 | 1,031.5427 | 3.78 | 987.5532 [M-H-CO2]− 945.5439[M-H-Mal]− 927.5316 [M-H-Mal-H2O]− 783.4934 [M-H-Mal-Glc]− 621.4370[M-H-Mal-Glc-Rha]− |
FPG, FPQ |
FPG, flowers of Panax ginseng Meyer; FPN, flowers of Panax notoginseng Burk.; FPQ, flowers of Panax quinquefolius L.; UPLC-Q-TOF/MS, ultra-performance liquid chromatography coupled with quadropole-time-of- fight mass spectrometry
All ginsenosides are liable to form deprotonated ions [M-H]− and/or formic acid adduct ions [M+HCOO]− in the negative-ion mode. It is noteworthy to find that malonyl-ginsenosides could not produce adduct ions [M+HCOO]−. Due to the thermically unstable of malonyl-ginsenosides, when subjected to MS/MS analysis, the deprotonated molecules of malonyl-ginsenosides are easy to loss CO2 or demalonylation from the malonyl group, which results in detection of the peaks in the quasi-molecular ions of [M-H-CO2]− and [M-H-Mal]− [23]. According to the MS, MS/MS spectra of ginsenosides, the common fragmentation pattern was the successive or simultaneous losses of sugar unit at the site of C-20, C-3, or C-6. The characteristic ions at m/z 475 and m/z 459 were observed in the PPT and PPD types.
Fingerprint analysis showed that the major components detected in these three materials are highly different. In FPG, there were 21 major peaks identified, while only 19 and 15 peaks were identified in FPQ and FPN, respectively. Only ginsenosides Rh1, RF3, and 20-glc-Rf were detected in FPG. Pseudoginsenoside F11 is the characteristic constituent of FPQ as well as the root of P. quinquefolius L. Ginsenosides Ra1, Ra2, and Ra3 and malonyl-ginsenosides Ra1 and Ra2 were unique for FPN. Notoginsenoside R1, Ginsenosides Rg1, Re, and Ro and malonyl-ginsenosides Rg1 and Re were detected in both FPG and FPQ, but could not be detected in FPN. Apart from the aforementioned compounds, the other 10 ginsenosides were all detected in the three species. In addition, it was interesting to note that the ginsenosides detected in both FPG and FPQ, but could not be detected in FPN, were all PPTs. The 10 ginsenosides detected in all three species were all PPDs. Moreover, ginsenosides unique for FPN also were PPTs, meaning there were no PPTs in FPN. In other words, the main ginsenosides of FPN were PPTs, which can distinguish it from FPG and FPQ. By contrast, this finding would also provide foundations for the further study of the connection of bioactive constituents and curative effect.
3.3. Estimation of the major components in FPG, FPQ, and FPN
The contents of the major 14 ginsenosides were quantified by UPLC including two PPTs (Rg1, Re), five PPDs (Rb1, Rc, Rb2, Rb3 and Rd) and seven malonyl-ginsenosides (m-Rg1, m-Re, m-Rb1, m-Rc, m-Rb2, m-Rb3 and m-Rd). The chromatograms obtained with reference substances and samples solutions are shown in Fig. 1. The concentrations of ginsenosides Rg1, Re, Rb1, Rc, Rb2, Rb3, and Rd were calculated precisely according to the respective calibration curves. Malonyl-ginsenosides (m-Rg1, m-Re, m-Rb1, m-Rc, m-Rb2, m-Rb3, and m-Rd) were relatively determined by quantitative analysis of multicomponents by a single-marker approach according to references [24], [25], [26]. In theory, if the structures of compounds were similar, the relative correction factors of components were around 1, so the relative correction factors of seven malonyl-ginsenosides without reference substances to corresponding neutral ginsenosides were determined approximately as 1. Therefore, the concentrations of m-Rg1, m-Re, m-Rb1, m-Rc, m-Rb2, m-Rb3, and m-Rd were calculated relatively on the basis of the calibration curves of Rg1, Re, Rb1, Rc, Rb2, Rb3, and Rd, respectively.
Fig. 1.
UPLC chromatograms of the reference substances and samples at 203 nm. (A) Chromatogram of ginsenoside Re, Rg1, Rb1, Rb2, Rc, Rb3, and Rd. (B) Chromatogram of FPG-1. (C) Chromatogram of FPQ-1. (D) Chromatogram of FPN-1. 1–26 = the compounds in Table 1. FPG, flowers of Panax ginseng Meyer; FPN, flowers of Panax notoginseng Burk.; FPQ, flowers of Panax quinquefolius L.; UPLC, ultraperformance liquid chromatography.
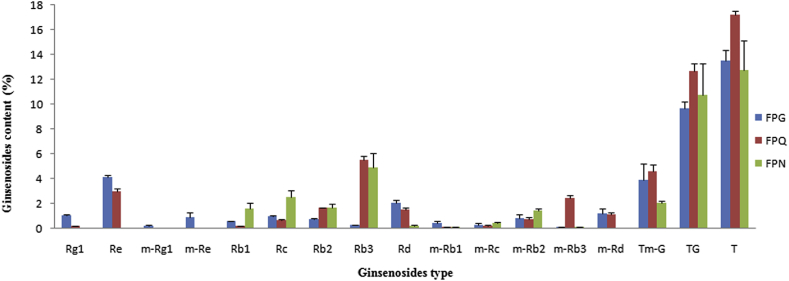
The contents of the 14 main ginsenosides and the total ginsenosides in FPG, FPQ and FPN are listed in Fig. 2 and Table 2. The results of total ginsenosides in FPG, FPQ and FPN were 13.75 ± 0.60%, 17.45 ± 0.42%, and 12.76 ± 1.77% ginsenoside Re equivalent using a colorimetric method respectively. Ginsenoside Re was found to be a major ginsenoside in FPG (4.14 ± 0.11%) and FPQ (3.00 ± 0.15%) and ginsenosides Re and Rg1 were not detected in FPN. However, Rb3 was the most abundant ginsenoside of FPQ (5.51 ± 0.20%) and FPN (5.00 ± 0.89%), which was higher than the content of the FPG (0.23 ± 0.03%). Because of the lack of ginsenoside Re and Rg1 in the FPN, the ratio of the total content of PPTs to PPDs (TPPT/TPPD) was 0. The TPPT/TPPD ratio was 1.18 ± 0.08 in FPG, 3.93 times higher than of those of in the FPQ (0.30 ± 0.06). This study clearly showed that the ratio of the total content of the neutral ginsenosides to the corresponding malonyl-ginsenosides (TG/Tm-G) in FPN (5.22 ± 1.33) was higher than in FPG (2.39 ± 0.57) and FPQ (3.20 ± 0.64). All these results suggest that the TPPT/TPPD and TG/Tm-G in FPG, FPQ, and FPN may become important assessment criteria for quality control of these flower products.
Fig. 2.
Comparison of contents of 14 ginsenosides in flowers of Panax ginseng Meyer (FPG), Panax quinquefolius L. (FPQ), and Panax notoginseng Burk. (FPN). T, TG and Tm-G represent the sum quantities of 14 ginsenosides, ginsenosides and malonyl-ginsenosides, respectively. FPG, flowers of Panax ginseng Meyer; FPN, flowers of Panax notoginseng Burk.; FPQ, flowers of Panax quinquefolius L.
Table 2.
Comparison of ginsenosides content (%) in flowers of Panax ginseng Meyer (FPG), Panax quinquefolius L. (FPQ), and Panax notoginseng Burk. (FPN)
| Batch | Collection region/date | Protopanaxtriol-type |
Protopanaxdiol-type ginsenoside |
Tm-G | TG | TPPT/ TPPD |
TG/ Tm-G |
T | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rg1 | Re | m-Rg1 | m-Re | Rb1 | Rc | Rb2 | Rb3 | Rd | m-Rb1 | m-Rc | m-Rb2 | m-Rb3 | m-Rd | |||||||
| FPG-1 | Huanren/2013.7 | 1.13 | 4.18 | 0.17 | 0.82 | 0.58 | 0.97 | 0.67 | 0.24 | 2.14 | 0.41 | 0.24 | 0.79 | 0.15 | 1.10 | 3.68 | 9.91 | 1.15 | 2.69 | 13.59 |
| FPG-2 | Changbaishan/2013.5 | 1.03 | 4.23 | 0.12 | 0.65 | 0.58 | 1.03 | 0.77 | 0.25 | 2.16 | 0.29 | 0.19 | 0.52 | 0.07 | 0.77 | 2.61 | 10.05 | 1.10 | 3.85 | 12.66 |
| FPG-3 | Shenyang/2014.5 | 1.03 | 4.04 | 0.24 | 1.27 | 0.50 | 0.93 | 0.64 | 0.21 | 1.74 | 0.61 | 0.40 | 1.09 | 0.07 | 1.58 | 5.26 | 9.09 | 1.26 | 1.73 | 14.35 |
| FPG-4 | Huanren/2013.6 | 1.13 | 4.24 | 0.22 | 0.72 | 0.6 | 0.98 | 0.69 | 0.21 | 1.99 | 0.54 | 0.32 | 0.78 | 0.06 | 1.5 | 4.14 | 9.84 | 1.20 | 2.38 | 13.78 |
| FPG-5 | Changbaishan/2014.6 | 1.1 | 4.12 | 0.19 | 1.02 | 0.48 | 1.04 | 0.75 | 0.28 | 2.01 | 0.35 | 0.42 | 0.98 | 0.11 | 1.23 | 4.3 | 9.78 | 1.14 | 2.27 | 12.88 |
| FPG-6 | Changbaishan/2014.5 | 1.03 | 3.99 | 0.18 | 1.03 | 0.51 | 0.93 | 0.77 | 0.2 | 2.16 | 0.45 | 0.43 | 0.74 | 0.08 | 1.08 | 3.99 | 9.59 | 1.10 | 2.40 | 14.23 |
| FPG-7 | Shenyang /2013.5 | 1.33 | 4.31 | 0.24 | 1.17 | 0.56 | 0.95 | 0.64 | 0.24 | 1.89 | 0.43 | 0.36 | 0.81 | 0.13 | 1.34 | 4.48 | 9.92 | 1.32 | 2.21 | 14.03 |
| FPG-8 | Shenyang /2013.5 | 1.21 | 4.21 | 0.2 | 1.23 | 0.46 | 0.99 | 0.67 | 0.21 | 1.9 | 0.61 | 0.38 | 1.09 | 0.07 | 1.28 | 4.86 | 9.65 | 1.28 | 1.99 | 14.55 |
| FPG-9 | Tonghua/2014.6 | 1 | 4.04 | 0.19 | 0.94 | 0.54 | 1.06 | 0.63 | 0.2 | 2.03 | 0.65 | 0.33 | 0.93 | 0.09 | 1.12 | 4.25 | 9.5 | 1.13 | 2.24 | 13.78 |
| FPG-10 | Tonghua/2014.7 | 1.03 | 4.05 | 0.18 | 0.89 | 0.5 | 0.93 | 0.78 | 0.25 | 2 | 0.61 | 0.4 | 1.03 | 0.07 | 1.23 | 4.41 | 9.54 | 1.14 | 2.16 | 13.65 |
| Mean | 1.10 | 4.14 | 0.10 | 0.97 | 0.53 | 0.98 | 0.70 | 0.23 | 2.00 | 0.50 | 0.35 | 0.87 | 0.09 | 1.22 | 4.20 | 9.69 | 1.18 | 2.39 | 13.75 | |
| SD (n = 3) |
0.10 | 0.11 | 0.04 | 0.21 | 0.05 | 0.05 | 0.06 | 0.03 | 0.13 | 0.13 | 0.08 | 0.18 | 0.03 | 0.22 | 0.71 | 0.28 | 0.08 | 0.57 | 0.60 | |
| FPQ-1 | Changbaishan/2013.5 | 0.20 | 3.11 | — | 0.02 | 0.18 | 0.69 | 1.66 | 5.42 | 1.57 | 0.13 | 0.25 | 0.76 | 2.34 | 1.22 | 4.72 | 12.83 | 0.35 | 2.72 | 17.55 |
| FPQ-2 | Huanren/2013.7 | 0.22 | 3.10 | — | 0.01 | 0.19 | 0.60 | 1.61 | 5.84 | 1.58 | 0.10 | 0.17 | 0.65 | 2.10 | 0.93 | 3.96 | 13.14 | 0.34 | 3.32 | 17.1 |
| FPQ-3 | Shenyang/2014.5 | 0.19 | 2.74 | — | 0.01 | 0.16 | 0.66 | 1.58 | 5.33 | 1.27 | 0.12 | 0.24 | 0.84 | 2.70 | 1.10 | 5.01 | 11.93 | 0.33 | 2.38 | 16.94 |
| FPQ-4 | Tonghua/2014.6 | 0.2 | 2.98 | — | 0.01 | 0.15 | 0.59 | 1.63 | 5.4 | 1.44 | 0.11 | 0.25 | 0.76 | 2.23 | 0.98 | 4.34 | 16.72 | 0.23 | 3.85 | 17.67 |
| FPQ-5 | Tonghua/2014.7 | 0.18 | 3.05 | — | 0.01 | 0.2 | 0.61 | 1.59 | 5.58 | 1.39 | 0.12 | 0.22 | 0.7 | 2.55 | 1.02 | 4.62 | 17.21 | 0.23 | 3.73 | 17.98 |
| Mean | 0.20 | 3.00 | — | 0.01 | 0.18 | 0.63 | 1.61 | 5.51 | 1.45 | 0.12 | 0.23 | 0.74 | 2.38 | 1.05 | 4.53 | 14.37 | 0.30 | 3.20 | 17.45 | |
| SD (n = 3) |
0.01 | 0.15 | — | 0.01 | 0.02 | 0.04 | 0.03 | 0.20 | 0.13 | 0.01 | 0.03 | 0.07 | 0.24 | 0.11 | 0.40 | 2.42 | 0.06 | 0.64 | 0.42 | |
| FPN-1 | Tongrentang/2013.9 | — | — | — | — | 1.53 | 2.56 | 1.54 | 4.45 | 0.14 | 0.06 | 0.41 | 1.40 | 0.09 | — | 2.05 | 10.22 | — | 4.99 | 12.27 |
| FPN-2 | Yunnan/2013.3 | — | — | — | — | 1.17 | 1.87 | 1.41 | 3.92 | 0.09 | 0.07 | 0.47 | 1.46 | 0.10 | — | 2.2 | 8.46 | — | 3.85 | 10.66 |
| FPN-3 | Liaoning/20145 | — | — | — | — | 2.1 | 3.02 | 1.97 | 6.19 | 0.24 | 0.07 | 0.41 | 1.14 | 0.10 | — | 1.82 | 13.52 | — | 7.43 | 15.34 |
| FPN-4 | Yunnan/2014.6 | — | — | — | — | 1.97 | 2.77 | 1.76 | 5.53 | 0.15 | 0.06 | 0.44 | 1.43 | 0.09 | — | 2.02 | 12.18 | — | 6.03 | 14.02 |
| FPN-5 | Yunnan/2014.7 | — | — | — | — | 1.89 | 2.03 | 1.84 | 4.89 | 0.21 | 0.06 | 0.4 | 1.51 | 0.09 | — | 2.06 | 10.86 | — | 5.27 | 13.08 |
| Mean | — | — | — | — | 1.73 | 2.45 | 1.70 | 5.00 | 0.17 | 0.06 | 0.43 | 1.45 | 0.09 | — | 2.03 | 11.05 | — | 5.52 | 12.76 | |
| SD (n = 3) |
— | — | — | — | 0.38 | 0.49 | 0.23 | 0.89 | 0.06 | 0.01 | 0.03 | 0.12 | 0.01 | — | 014 | 1.92 | — | 1.33 | 1.77 | |
T represents the content of the total ginsenosides using a colorimetric method. TG and Tm-G represent the content of ginsenosides and malonyl-ginsenosides, respectively. TPPD/TPPT represent ratio of the total content of protopanaxdiol-type ginsenosides to protopanaxtriol-type ginsenosides; TG/Tm-G represents the total content of determined malonyl-ginsenosides to the corresponding neutral ginsenosides
—, not detected; FPG, flowers of Panax ginseng Meyer; FPN, flowers of Panax notoginseng Burk.; FPQ, flowers of Panax quinquefolius L.; SD, standard deviation
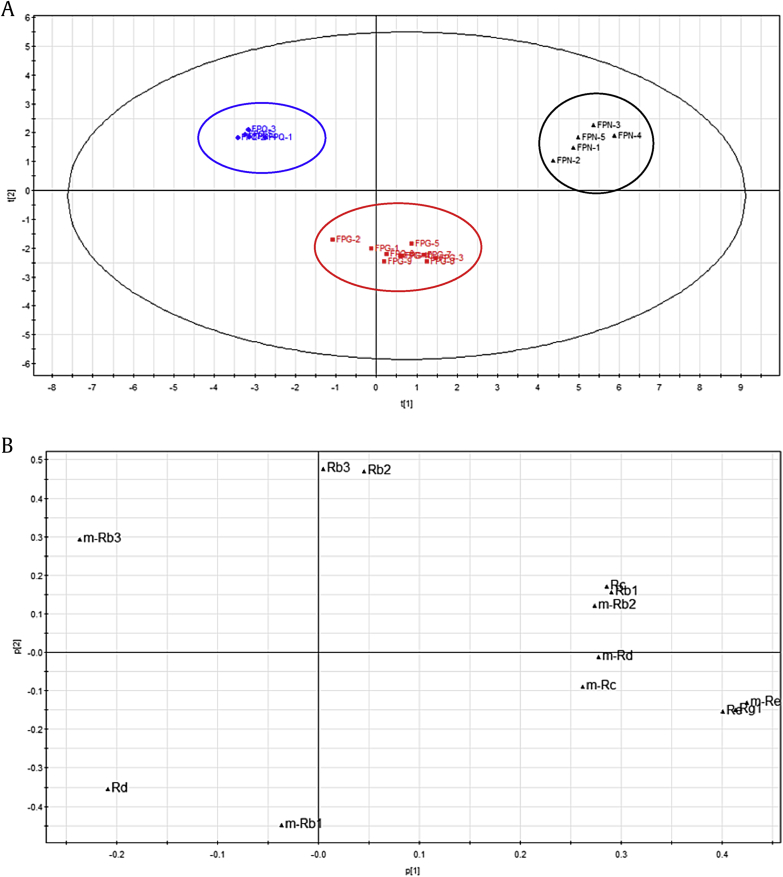
Principal component analysis (PCA) is a mathematical, multivariate, nonparametric method for extracting relevant information from confusing data sets. The idea behind the PCA was to identify markers that distinguish FPG, FPQ, and FPN from each other based on fingerprints. A clear discrimination among FPG, FPQ, and FPN was observed in the PCA score plots (Fig. 3A). All the samples were classified into three major groups, which indicate that the constituents of the samples were significantly different. Loading plots were applied to indicate the contribution of each principal component in our experiment. From Fig. 3B, seven compounds—m-Re, m-Rb1, m-Rb3, Rd, Rb2, Rb3, and Rg1—were confirmed to contribute strongly to the clusters. Of course, these compounds' profiles may be the cause of differences in efficacy.
Fig. 3.
Principal component analysis of flowers of Panax ginseng Meyer (FPG), Panax quinquefolius L. (FPQ), and Panax notoginseng Burk. (FPN). (A) Score plots, (B) loading plots.
3.4. Validation of quantitative analytical method
The quantification method was validated by defining the linearity, precision, stability, recovery, repeatability, and limits of quantification and detection (Table 3, Table 4). The calibration curves for all seven reference substances showed good linearity (r2 > 0.999) at six different concentrations, respectively. The precision of the UPLC method was determined for intraday and interday variations. The validation studies showed overall intraday and interday variations (RSD) < 9.27% and 8.51%, respectively. RSD values of the peak area of seven compounds were < 5.0% in 24 h. The recoveries for all the seven reference substances determined were in the range 82.42–106.8% with RSD < 6.46%. The repeatability of experiment method was very good (RSD < 5.0%). These results demonstrate that the established UPLC method was accurate and precise and therefore appropriate for the quantitative analyses of ginsenosides Rg1, Re, Rb1, Rc, Rb2, Rb3, and Rd.
Table 3.
Regression equation, linear range, precision, repeatability, stability, and limit of detection and quantitation
| Ginsenoside | Regression equation | Correlation factor (r2) | Linearity range (μg) | Precision (RSD%) |
Repeatability (RSD%) |
Stability (RSD%) |
Limit of detection (μg/mL) |
Limit of quantitation (μg/mL) |
|
|---|---|---|---|---|---|---|---|---|---|
| Intraday | Interday | ||||||||
| Rg1 | Y = 47404X + 1035 | 0.9993 | 8.9–445 | 4.25 | 2.70 | 1.99 | 4.86 | 0.22 | 0.67 |
| Re | Y = 64465X + 2272 | 0.9998 | 17.9–1,790 | 2.10 | 2.88 | 2.85 | 2.03 | 0.35 | 1.06 |
| Rb1 | Y = 49094X + 467.5 | 0.9991 | 12.9–645 | 2.77 | 6.13 | 2.68 | 3.92 | 0.23 | 0.68 |
| Rc | Y = 57382X + 668.6 | 0.9993 | 10–500 | 1.86 | 5.56 | 0.61 | 2.40 | 0.27 | 0.82 |
| Rb2 | Y = 53085X + 1326 | 0.9994 | 10.8–540 | 2.92 | 5.37 | 1.61 | 3.55 | 0.23 | 0.69 |
| Rb3 | Y = 53575X + 1956 | 0.9998 | 19–1,520 | 9.27 | 8.51 | 4.05 | 4.90 | 0.37 | 1.08 |
| Rd | Y = 60916X + 1605 | 0.9993 | 19–900 | 2.29 | 6.94 | 0.71 | 3.47 | 0.38 | 1.14 |
Y and X are, respectively, the peak areas and concentrations (μg/mL) of the analytes
RSD = relative standard deviation
Table 4.
Accuracy of UPLC method for the determination of reference compounds
| Analyte | Original (mg) |
Spiked (mg) |
Found (mg) |
Recovery (%) |
RSD (%) |
|---|---|---|---|---|---|
| Rg1 | 1.09 | 0.88 | 1.96 | 98.99 | 2.80 |
| 1.1 | 2.24 | 105.37 | 0.36 | ||
| 1.32 | 2.34 | 94.80 | 2.94 | ||
| Re | 4.19 | 1.2 | 5.48 | 106.83 | 2.85 |
| 1.5 | 5.69 | 99.53 | 0.85 | ||
| 1.8 | 6.01 | 101.05 | 2.62 | ||
| Rb1 | 0.58 | 0.4 | 0.98 | 98.94 | 4.52 |
| 0.5 | 1.04 | 92.82 | 1.24 | ||
| 0.6 | 1.19 | 101.99 | 0.42 | ||
| Rc | 1.02 | 0.64 | 1.68 | 104.44 | 0.96 |
| 0.8 | 1.82 | 100.13 | 0.33 | ||
| 1.06 | 1.98 | 90.64 | 1.74 | ||
| Rb2 | 0.70 | 0.44 | 1.15 | 100.80 | 0.90 |
| 0.55 | 1.24 | 97.98 | 0.57 | ||
| 0.66 | 1.34 | 97.27 | 1.24 | ||
| Rb3 | 0.20 | 0.16 | 0.33 | 84.17 | 6.46 |
| 0.20 | 0.36 | 82.42 | 1.26 | ||
| 0.24 | 0.42 | 91.73 | 2.84 | ||
| Rd | 2 | 1.60 | 3.66 | 104.05 | 1.15 |
| 2.00 | 3.95 | 97.18 | 0.62 | ||
| 2.40 | 4.32 | 96.84 | 1.49 |
RSD, relative standard deviation; UPLC, . ultraperformance liquid chromatography
3.5. Carbon clearance test
Macrophage phagocytosis targeting of xenobiotics plays a key role for host defense against a foreign invasion [27]. Clearance of carbon particles from the blood circulation is related to phagocytic activity [28]. The phagocytic activity of extracts from FPG, FPQ, and FPN was expressed by the phagocytic index and phagocytic modulus (Table 5). All data are expressed as the mean ± standard deviation. Statistical analysis was performed by a one-way analysis of variance followed by Tukey's honestly significant difference test using IBM SPSS statistics 17.0 (International Business Machines Corporation, Armonk, New York , USA). A p value < 0.05 or < 0.01 was considered statistically significant or extremely statistically significant. Table 5 shows all flowers (0.5–1.0 g/kg/d) dose dependently augmented the rate of carbon clearance from the circulatory system of rats. The FPG and FPQ presented an increased tendency of phagocytic activity when compared with the control group (p < 0.01); however, no significant difference was observed among them. Under the same conditions, FPN at different doses showed weaker phagocytic activity (p < 0.05).
Table 5.
Immunomodulatory activity [asphagocytic index (K) and phagocytic modulus (α)] of the extractives of flowers of Panax ginseng Meyer (FPG), Panax quinquefolius L. (FPQ), and Panax notoginseng Burk. (FPN)
| Batch | Dose | K | α |
|---|---|---|---|
| Control | —— | 0.011 ± 0.008 | 3.65 ± 1.13 |
| Mannatide | 4.0 mg/kg | 0.018 ± 0.007** | 4.62 ± 0.66* |
| FPG | 0.5 g/kg | 0.020 ± 0.008** | 4.90 ± 1.21** |
| 1.0 g/kg | 0.024 ± 0.007** | 5.18 ± 0.73** | |
| FPQ | 0.5 g/kg | 0.020 ± 0.006** | 4.87 ± 0.87** |
| 1.0 g/kg | 0.023 ± 0.006** | 5.03 ± 0.79** | |
| FPN | 0.5 g/kg | 0.017 ± 0.006* | 4.39 ± 0.47* |
| 1.0 g/kg | 0.018 ± 0.006* | 4.61 ± 0.43* |
Values are compared with control animals, * p < 0.05; ** p < 0.01
FPG, flowers of Panax ginseng Meyer; FPN, flowers of Panax notoginseng Burk.; FPQ, flowers of Panax quinquefolius L.
These differences may be attributed to the differentiations of components of FPG, FPQ, and FPN. Different types of ginsenosides have different pharmacology activities. On the basis of the results above, it can be clearly concluded that FPG and FPQ had stronger enhancing immunity activity than FPN in vivo, corresponding to the content of PPTs of FPN was 0, the lack of PPTs present in FPN may be responsible for its overall low enhancing immunity activity. Of course, PCA was to identify markers that distinguish FPG, FPQ, and FPN from each other based on fingerprints.
4. Conclusions
In conclusion, this study showed that the PPDs were the main ginsenosides of FPN and the TG/Tm-G in FPN was higher than FPG and FPQ; these characteristics are important assessment criteria for quality control of the flower products from the three species. Under the same conditions, FPN at different doses showed a weaker phagocytic activity than FPG and FPQ, indicating that PPTs and malonyl-ginsenosides in them may play a key role in their enhancing immunity property. There were still some limitations in this study; the developed method cannot be used to determine quantitatively the content of other ingredients, such as polysaccharide, which may be also have something to do with the enhancing immunity property. Further investigations of the relationship between the chemical profiles of different Panax flowers and their bioactivity still are indispensable. However, these results are definitely helpful for quality evaluation and the effective and safe usage of Panax flower products.
Conflicts of interest
The authors declare no competing financial interest.
Acknowledgments
The authors thank Dr Jing-hua Xu for technical assistance of the pharmacology experiment. This work was financially supported by the National Natural Science Foundation of China (No. 81373900).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jgr.2016.08.008.
Contributor Information
Jing Wang, Email: wangjingyk@126.com.
Jincai Lu, Email: jincailu@126.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Wang C.Z., McEntee E., Wicks S., Wu J.A., Yuan C.S. Phytochemical and nalytical studies of Panax notoginseng (Burk.) F. H. Chen. J Nat Med. 2006;60:97–106. [Google Scholar]
- 2.Tang W., Eisenbrand G. Springer; Berlin: 1992. Panax ginseng C. A. Mey; pp. 710–737. [Google Scholar]
- 3.Assinewe V.A., Baum B.R., Gagnon D., Thor Arnason J.T. Phytochemistry of wild populations of Panax quinquefolius L. (North American ginseng) J Agric Food Chem. 2003;51:4549–4553. doi: 10.1021/jf030042h. [DOI] [PubMed] [Google Scholar]
- 4.Zuo Y., Chen Y.Z., Kondo K., Funamoto T., Wen J., Zhou S. DNA barcoding of Panax species. Planta Med. 2011;77:182–187. doi: 10.1055/s-0030-1250166. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura S., Sugimoto S., Matsuda H., Yoshikawa M. Medicinal flowers. XVII. New dammarane-type triterpene glycosides from flower buds of American ginseng, Panax quinquefolium L. Chem Pharmaceut Bull. 2007;55:1342–1348. doi: 10.1248/cpb.55.1342. [DOI] [PubMed] [Google Scholar]
- 6.Tung N.H., Quang T.H., Son J.H., Koo J.E., Hong H.J., Koh Y.S., Song G.Y., Kim Y.H. Inhibitory effect of ginsenosides from steamed ginseng-leaves and flowers on the LPS-stimulated IL-12 production in bone marrow-derived dendritic cells. Arch Pharm Res. 2011;34:681–685. doi: 10.1007/s12272-011-0419-2. [DOI] [PubMed] [Google Scholar]
- 7.Ko S.K., Cho O.S., Bae H.M., Im B.O., Lee O.H., Lee B.Y. Quantitative analysis of ginsenosides composition in flower buds of various ginseng plants. J Korean Soc Appl Biol Chem. 2011;54:154–157. [Google Scholar]
- 8.Tung N.H., Song G.Y., Kim J.A., Hyun J.H., Kang H.K., Kim Y.H. Dammarane-type saponins from the flower buds of Panax ginseng and their effects on human leukemia cells. Bioorg Med Chem Lett. 2010;20:309–314. doi: 10.1016/j.bmcl.2009.10.110. [DOI] [PubMed] [Google Scholar]
- 9.Gao X., Dan M., Zhao A., Xie G., Jia W. Simultaneous determination of saponins in flower buds of Panax notoginseng using high performance liquid chromatography. Biomed Chromatogr. 2008;22:244–249. doi: 10.1002/bmc.915. [DOI] [PubMed] [Google Scholar]
- 10.Hong J., Hu J.Y., Liu J.H., Zhou Z., Zhao A.F. In vitro antioxidant and antimicrobial activities of flavonoids from Panax notoginseng flowers. Nat Prod Res. 2014;28:1260–1266. doi: 10.1080/14786419.2014.900768. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa M., Morikawa T., Kashima Y., Ninomiya K., Matsuda H. Structures of new dammarane-type triterpene saponins from the flower buds of Panax notoginseng and hepatoprotective effects of principal ginseng saponins 1. Nat Prod Res. 2003;66:922–927. doi: 10.1021/np030015l. [DOI] [PubMed] [Google Scholar]
- 12.Tung N.H., Song G.Y., Woo S., Hyun J.W., Koh Y.S., Kang H.K., Shoyama Y., Kim Y.H. Ginsenosides from the leaves and flower buds of panax ginseng and their pharmacological effects. Curr Bioact Comp. 2012;8:159–166. [Google Scholar]
- 13.Yu J.L., Dou D.Q., Chen X.H., Yang H.Z., Guo N., Cheng G.F. Protopanaxatriol-type ginsenosides differentially modulate type 1 and type 2 cytokines production from murine splenocytes. Planta Med. 2005;71:202–207. doi: 10.1055/s-2005-837817. [DOI] [PubMed] [Google Scholar]
- 14.Plohmann B., Bader G., Hiller K., Franz G. Immunomodulatory and antitumoral effects of triterpenoid saponins. Die Pharmazie. 1997;52:953–957. [PubMed] [Google Scholar]
- 15.Cho J.Y., Yoo E.S., Baik K.U., Park M.H., Han B.H. In vitro inhibitory effect of protopanaxadiol ginsenosides on tumor necrosis factor (TNF)-alpha production and its modulation by known TNF-alpha antagonists. Planta Med. 2001;67:213–218. doi: 10.1055/s-2001-12005. [DOI] [PubMed] [Google Scholar]
- 16.Rhule A., Navarro S., Smith J.R., Shepherd D.M. Panax notoginseng attenuates LPS-induced pro-inflammatory mediators in RAW264. 7 cells. J Ethnopharmacol. 2006;106:121–128. doi: 10.1016/j.jep.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Yoshikawa M., Morikawa T., Yashiro K., Murakami T., Matsuda H. Bioactive saponins and glycosides. XIX. Notoginseng (3): immunological adjuvant activity of notoginsenosides and related saponins: structures of notoginsenosides-L,-M, and-N from the roots of Panax notoginseng (Burk.) FH Chen. Chem Pharmaceut Bull. 2001;49:1452–1456. doi: 10.1248/cpb.49.1452. [DOI] [PubMed] [Google Scholar]
- 18.Chinese Pharmacopoeia Commission . China Medical Science and Technology Press; Beijing: 2010. Pharmacopoeia of the People’s Republic of China. [Google Scholar]
- 19.Zhao Y.L., Wu H.Z., Yang J., Zhang J., Zhou C.X. Effect of Panax ginsenoside on immunological function of immuno-suppressive mice. Chin J Biol. 2011;3:305–308. 312. [Google Scholar]
- 20.Li J., Chai J., Zhao W. Empirical study of American ginseng on anti-defatigation action and the effects of delayed hypersensitivity and the function of mononuclear phagocyte. Chin J Chin Mater Med. 2007;10:2002–2004. [Google Scholar]
- 21.Zhang Z.H., Guo Y.F., Sheng Y.C., Xie J., Mei Q.B. Systematic review of platinum antitumor drugs combined with mannatide in the treatment of malignant pleural effusion. Chin Pharm. 2011;22:1691–1695. [Google Scholar]
- 22.Fuzzati N., Gabetta B., Jayakar K., Pace R., Peterlongo F. Liquid chromatography—electrospray mass spectrometric identification of ginsenosides in Panax ginseng roots. J Chromatogr A. 1999;854:69–79. doi: 10.1016/s0021-9673(99)00463-x. [DOI] [PubMed] [Google Scholar]
- 23.Chen M., Yang W.Z., Wu W.Y., Guo D.A. Chemical analysis of xueshuantong lyophilized powder by LC-MS profiling. Chin Herb Med. 2015;7:54–61. [Google Scholar]
- 24.Xie Y.Y., Luo D., Cheng Y.J., Ma J.F., Wang Y.M., Liang Q.L., Luo G.A. Steaming-induced chemical transformations and holistic quality assessment of red ginseng derived from Panax ginseng by means of HPLC-ESI-MS/MSn-based multicomponent quantification fingerprint. J Agric Food Chem. 2012;60:8213–8224. doi: 10.1021/jf301116x. [DOI] [PubMed] [Google Scholar]
- 25.Zhu J., Wang Z., Kuang Y., Zhang Q., Gao Q., Ma N. A quantitative method using one marker for simultaneous assay of ginsenosides in Panax ginseng and P. notoginseng. Acta Pharm Sin. 2008;43:1211–1216. [PubMed] [Google Scholar]
- 26.Wang Z., Qian Z., Zhang Q., Zhu J., Gao H., Wang Z. Technical guidelines for quantitative analysis of multicomponents by single marker. Chin J Chin Mater Med. 2011;36:657–658. [Google Scholar]
- 27.Peng S., Yang Y., Li S., Wu Q., Shah N.P., Wei H., Xu F. Immunomodulatory activities of Lactobacillus rhamnosus ZDY114 and donkey milk in BALB/c mice. Int Dairy J. 2014;34:263–266. [Google Scholar]
- 28.Shukla S., Mehta A., John J., Mehta P., Vyas S.P., Shukla S. Immunomodulatory activities of the ethanolic extract of Caesalpinia bonducella seeds. J Ethnopharmacol. 2009;125:252–256. doi: 10.1016/j.jep.2009.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.