Abstract
Background
Ginsenosides are the major components of Panax ginseng Meyer, an herbal medicine used for the treatment of various diseases. Different ginsenosides contribute to the biological properties of ginseng, such as antimicrobial, anticancer, and immunomodulatory properties. In this study, we investigated the antiviral effects of 15 ginsenosides and compound K on gammaherpesvirus.
Methods
The antiviral activity of ginsenosides was examined using the plaque-forming assay and by analyzing the expression of the lytic gene.
Results
20(R)-Ginsenoside Rh2 inhibited the replication and proliferation of murine gammaherpesvirus 68 (MHV-68), and its half-maximal inhibitory concentration (IC50) against MHV-68 was estimated to be 2.77 μM. In addition, 20(R)-ginsenoside Rh2 inhibited 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced lytic replication of human gammaherpesvirus in the Kaposi's sarcoma-associated herpesvirus (KSHV)-positive cell line BC3.
Conclusion
Our results indicate that 20(R)-ginsenoside Rh2 can inhibit the replication of mouse and human gammaherpesviruses, and thus, has the potential to treat gammaherpesvirus infection.
Keywords: antiviral activity, Kaposi's sarcoma-associated herpesvirus, murine gammaherpesvirus 68, 20(R)-ginsenoside Rh2
1. Introduction
Ginseng, the root of Panax ginseng Meyer (Araliaceae), is one of the most popular herbal medicines that has been traditionally used in East Asian countries, including Japan, China, and Korea [1]. Ginseng has pharmacological effects on immune function, cancer, neurodegenerative disorders, inflammation, and viral diseases [2], [3], [4], [5], [6], [7].
Ginseng contains various physiologically active compounds such as ginsenosides, polysaccharides, polyacetylenes, phytosterols, and essential oils [8]. Ginsenosides are triterpenoid glycosides containing dammarane and are generally divided into two groups: the protopanaxadiol ginsenosides (e.g., Rb1, Rb2, Rb3, Rc, Rd, Rg3, and Rh2) and protopanaxatriol ginsenosides (e.g., Re, Rf, Rg1, Rg2, and Rh1) [7], [9]. Ginsenosides and their enzymatic or heat-processed metabolites, such as compound K, are the major active components of ginseng that contribute to its pharmacological activities [7], [10], [11], [12].
Human gammaherpesviruses such as the Epstein–Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV) are oncoviruses, distinguished by their ability to establish a lifelong persistent infection in lymphocytes and to induce latent and lytic replication [13], [14]. This characteristic life cycle of gammaherpesviruses, especially latency and persistent infection, is highly associated with the development of various cancers [13], [15]. KSHV, also known as human herpesvirus 8, is associated with Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma [16], [17], [18]. EBV, or human herpesvirus 4, causes Burkitt's lymphoma, nasopharyngeal carcinoma, Hodgkin's disease, and some gastric cancers [19], [20]. In a few cases, lytic replication is also related to the development of malignancies, AIDS-associated epidemic Kaposi's sarcoma caused by KSHV, and hemophagocytic lymphohistiocytosis caused by EBV [21], [22]. Hence, regulation of the life cycle of a gammaherpesvirus through effective antiviral agents is very important for the treatment of viral disorders.
Owing to the absence of an appropriate experimental model for human gammaherpesviruses, studies on KSHV and EBV are limited [23]. The murine gammaherpesvirus 68 (MHV-68), a member of the subfamily of Gammaherpesvirinae from wild rodents, has been developed as an experimental model for these studies [24]. Similar to other human gammaherpesviruses, MHV-68 exhibits two distinct phases of life cycle and has genetic similarity with KSHV and EBV [24], [25]. Thus, MHV-68 is regarded as a substitute for the human gammaherpesvirus and has been widely used for in vitro and in vivo studies on gammaherpesviruses. In this study, we aimed to investigate the antiviral effects of 15 ginsenosides and compound K on gammaherpesviruses, and our results showed that 20(R)-ginsenoside Rh2 had an inhibitory effect on lytic replication and viral proliferation of the gammaherpesvirus MHV-68. Furthermore, we identified the antiviral activity of 20(R)-ginsenoside Rh2 on human gammaherpesvirus by using KSHV-positive cell lines.
2. Materials and methods
2.1. Cell lines and viruses
Vero (green monkey kidney cell) cells were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (Welgene, Seoul, Korea), 100 U/mL penicillin, and 100 μg/mL streptomycin. The mouse fibroblast cell line NIH-3T3 was cultured in DMEM with 10% bovine calf serum (Gibco, Grand Island, NY, USA). KSHV-positive B cell lymphoma (BC-3) cells were cultured in RPMI1640 supplemented with 20% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were cultured at 37°C in a humidified 5% CO2 atmosphere. MHV-68 was originally obtained from American Type Culture Collection (VR1465). Working virus stocks were grown in Vero cells in normal growth media at a multiplicity of infection (MOI) of 0.05 plaque-forming unit (PFU)/cell.
2.2. Chemicals
Ginsenosides Rb1, Rb2, Rb3, Rc, Re, Rf, Rg1, 20(R)- and 20(S)-Rg2, 20(R)- and 20(S)-Rg3, 20(S)-Rh1, 20(R)- and 20(S)-Rh2, Rp1, and compound K were purchased from Ambo Institute (Daejeon, Korea), and 12-O-tetradecanoylphorbol-13-acetate (TPA) for virus reactivation was from Sigma-Aldrich (St. Louis, MO, USA). The working stock solutions were prepared by dissolving the compounds in dimethyl sulfoxide (DMSO) and diluted to the appropriate concentrations in culture medium.
2.3. Cytotoxicity assays
Cytotoxicity was determined by measuring the mitochondrial-dependent reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) to formazan by viable cells. NIH-3T3 cells were seeded at a density of 2 × 103 cells/100 μL per well in 96-well plates, incubated for 6 h and treated with 0.5 μL of ginsenosides and compound K. After 72 h, 10 μL MTT (5 mg/mL solution; Sigma Aldrich) was added to the culture, followed by incubation for 6 h at 37°C in a CO2 incubator. Isopropanol with hydrochloric acid (0.04N, 100 μL) was added to each well to dissolve the formazan crystals. Estimation of MTT reduction to formazan by viable cells was quantified by measuring the absorbance at 540 nm, and cytotoxicity was represented as the percentage of cell viability rate, calculated as {[(sample OD – blank OD)/(DMSO OD – blank OD)] × 100}. Where OD = optical density.
2.4. Ginsenoside treatment and virus infection
For ginsenoside treatment, NIH-3T3 cells were plated onto 24-well plates at 2 × 104 cells/well and stabilized for 6 h. After 6 h stabilization, cells were treated with each ginsenoside at the appropriate concentration and incubated for 24 h. Cells were then rinsed with warm phosphate-buffered saline (PBS) twice, and inoculated with MHV-68 at an MOI of 0.1 pfu/cell or 0.05 pfu/cell for 90 min at 37°C, rocking every 15 min. After inoculation, NIH-3T3 cells were washed with warm PBS and cultured with ginsenoside-containing complete culture media. After 72 h culture, cells, and supernatants were used for further experiments.
2.5. Cell-based antiviral assays
The number of PFUs in virus stocks and virus-infected samples were titrated using the plaque-forming assay with Vero cells. Vero cells were plated onto 12-well plates at 2.5 × 104 cells/well and incubated for 24 h. Serial 10-fold dilutions of the virus stock or appropriately diluted samples were added to Vero cells at a volume of 400 μL and allowed to inoculate for 90 min at 37°C, rocking every 15 min. After inoculation, Vero cells were washed once in warm PBS and overlaid with complete DMEM containing 0.6% methylcellulose (Sigma-Aldrich). After 7 d culture, the cells were fixed and stained with 2% crystal violet in 20% ethanol for 8 h, and plaques were counted to determine the viral titers.
2.6. Real-time quantitative polymerase chain reaction
Total RNA and genomic DNA were extracted by using RNAiso plus (Takara Bio Inc., Otsu, Shiga, Japan) according to manufacturer's instructions. The expressions of the mRNA and genomic DNA of the genes of interest were analyzed by real-time quantitative polymerase chain reaction (qRT-PCR) with SYBR green (Enzynomics, Daejeon, Korea) as previously described [26]. MHV-68 replication and transcription activator (RTA)- and MHV-68 ORF56-specific primers were used to measure MHV-68 lytic gene expression and genomic DNA amplification. KSHV ORF57 locus- and RTA-specific primers were used to quantitate the KSHV viral load and lytic gene expression, as previously reported. All gene expressions were normalized using Actb (actin, beta; mouse) or GAPDH (glyceraldehyde 3-phosphate dehydrogenase; human) and the primer sequences are listed in Table 1. qRT-PCR reactions were performed using CFX Connect Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA).
Table 1.
The list of primers used for qRT-PCR
| Primer | Direction | Sequence (5′ to 3′) |
|---|---|---|
| MHV-68 RTA | Sense | GGCCGCAGACATTTAATGAC |
| Antisense | GCCTCAACTTCTCTGGATATGCC | |
| MHV-68 ORF56 | Sense | GTAACTCGAGACTGAAACCTCGCAGAGGTCC |
| Antisense | CCGAAGCTTGCACGGTGCAATGTGTCACAG | |
| KSHV RTA | Sense | CCCTGAGCCAGTTTGTCATT |
| Antisense | ATGGGTTAAAGGGGATGATG | |
| KSHV ORF57 | Sense | TGGACATTATGAAGGGCATCCTA |
| Antisense | CGGGTTCGGACAATTGCT | |
| Actb | Sense | ACCCACACTGTGCCCATCTAC |
| Antisense | GCCATCTCCTGCTCGAAGTC | |
| GAPDH | Sense | GAAGGTGAAGGTCGGAGT |
| Antisense | GASAGATGGTGATGGGATTTC |
KSHV, Kaposi's sarcoma-associated herpesvirus; MHV, murine gammaherpesvirus; qRT-PCR, quantitative real-time PCR; RTA, replication and transcription activator; Actb, actin, beta; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase.
2.7. Western blot analysis
For western blot analysis, total protein samples were extracted using the RIPA Lysis and Extraction Buffer (Thermo Fisher Scientific, Rockford, IL, USA) and further experiments were performed as previously described [27]. Rabbit polyclonal anti-ORF45 and anti-M9 antibodies were kindly provided by Dr. Moon Jung Song (Korea University, Seoul, Korea) and used at a dilution of 1:1,000. Other primary antibodies were diluted as follows: anti-K8α (Santa Cruz Biotechnology, Dallas, TX, USA) 1:200, and anti-β-tubulin (Cell Signaling, Beverly, MA, USA) 1:2,000. Horseradish peroxidase-conjugated antirabbit immunoglobulin G (Bio-Rad) and horseradish peroxidase-conjugated antimouse immunoglobulin G (Cell Signaling) were used as secondary antibodies.
2.8. Plaque reduction assays for 20(R)-ginsenoside Rh2
To determine the half-maximal inhibitory concentration (IC50) of 20(R)-ginsenoside Rh2 against MHV-68, we performed the plaque reduction assay as previously described [23]. Briefly, Vero cells were seeded at 2.5 × 104 cells/well in 12-well plates. After 24 h, the cells were incubated with MHV-68 (100 pfu/well) for 90 min and the overlay media was added upon removal of the virus inoculum. 20(R)-ginsenoside Rh2 was diluted at various concentrations into the normal growth media for pretreatment (24 h before virus inoculation) or the overlay media for posttreatment and added into the cells. After 7 days culture, the cells were fixed and stained with 2% crystal violet in 20% ethanol for 8 h. The plaques were counted, and the IC50 value was determined using the Prism 5 (GraphPad Software Inc., San Diego, CA, USA).
2.9. Statistical analysis
All data were expressed as mean ± standard deviation, and the two-tailed, unpaired Student t test was applied using Microsoft Excel 2013 (Redmond, WA, USA). A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Cytotoxic effects of ginsenosides and compound K
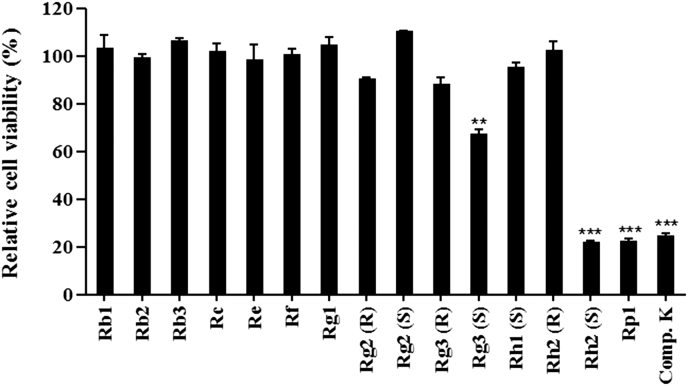
Prior to determining the antiviral activity of 15 ginsenosides and compound K, MTT assays were performed to test their cytotoxic effects at 100 μM in NIH-3T3 cells. Most ginsenosides did not affect the cell viability, while ginsenosides 20(S)-Rg3, 20(S)-Rh2, and Rp1 and compound K showed cytotoxicity at 100 μM (Fig. 1). Therefore, we did not include these cytotoxic compounds in further experiments.
Fig. 1.
Cytotoxicity of ginsenosides and compound K. Cell viability was expressed as the percent ratio of ginsenoside or compound K treatment normalized over 0.5% DMSO control value. The data are representative of three experiments with similar results. ** p < 0.01; *** p < 0.001. DMSO, dimethyl sulfoxide.
3.2. Antiviral activity of ginsenosides against MHV-68
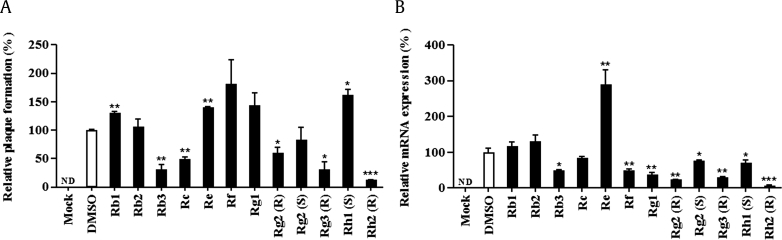
To evaluate the antiviral activity of ginsenosides, we measured the inhibition of plaque formation in NIH-3T3 cells infected with MHV-68 at an MOI of 0.1 pfu/cell by using 12 ginsenosides at 100 μM. Our results showed that ginsenosides Rb3, Rc, 20(R)-Rg3, and 20(R)-Rh2 reduced plaque formation, which indicated a decrease in viral proliferation (Fig. 2A). In addition, the level of MHV-68 RTA, an immediate-early lytic gene required for the initiation of viral replication, decreased following treatment with ginsenosides Rb3, Rf, Rg1, 20(R)-Rg2, 20(R)-Rg3, and 20(R)-Rh2 (Fig. 2B). In particular, 20(R)-ginsenoside Rh2 significantly inhibited viral proliferation and lytic gene expression, which indicated that 20(R)-ginsenoside Rh2 had a marked antiviral activity against MHV-68.
Fig. 2.
Effect of ginsenosides. (A) Effect on the levels of virus production. (B) Effect on viral transcript RTA. All ginsenosides were used at a concentration of 100 μM, and uninfected (mock) and DMSO-treated infected samples were used as control. Virus replication levels were represented as a relative value compared with that obtained using a DMSO control. The data are representative of three experiments with similar results. * p < 0.05; ** p < 0.01; *** p < 0.001. ND, not detected; RTA, replication and transcription activator.
3.3. 20(R)-ginsenoside Rh2 inhibits viral replication
To confirm the antiviral effect of 20(R)-ginsenoside Rh2 against MHV-68, we treated NIH-3T3 cells with 100 μM of 20(R)-ginsenoside Rh2 before and after infection with the MHV-68 virus at an MOI of 0.05 pfu/cell, and we examined the viral replication at 72 h after infection. The infected cells treated with DMSO were used as negative controls. Treatment with 20(R)-ginsenoside Rh2 suppressed the expression of RTA mRNA at an MOI 0.05 pfu/cell; these results were consistent with those observed with infection with the MHV-68 virus at an MOI of 0.01 pfu/cell. In addition, treatment with 20(R)-ginsenoside Rh2 inhibited the expression of the viral ORF56 gene associated with the regulation of viral DNA synthesis (Figs. 3A and 3B). Furthermore, we examined viral protein expressions. Treatment with 20(R)-ginsenoside Rh2 treatment had inhibitory effects on the expressions of late genes such as ORF45 (viral tegument protein) and M9 (also known as ORF65, small capsid protein) (Fig. 3C). These results confirmed the antiviral activity of 20(R)-ginsenoside Rh2 against MHV-68.
Fig. 3.
Effects of 20(R)-ginsenoside Rh2 (100 μM) on MHV-68 replication determined by different measurements. (A) The levels of viral transcript RTA. (B) Viral genome ORF56 amplification. (C) Viral lytic protein expression. Uninfected (mock) and DMSO-treated infected samples were used as control. Relative values were calculated against DMSO-treated infected control. The data are representative of three experiments with similar results. *** p < 0.001.
3.4. Antiviral efficacy of 20(R)-ginsenoside Rh2
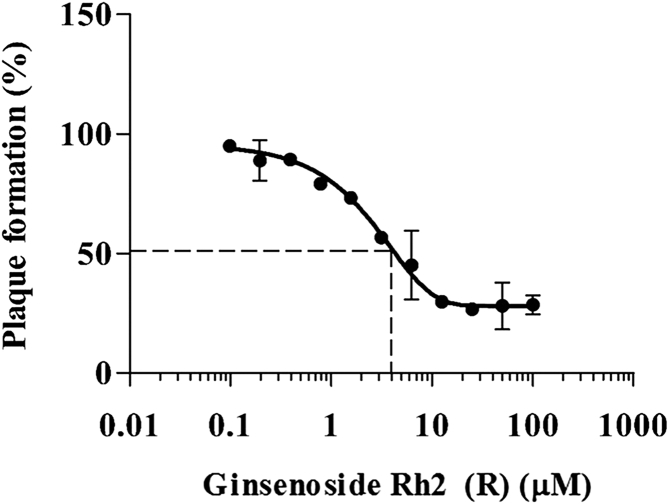
We performed plaque reduction assays using 20(R)-ginsenoside Rh2 at a concentration ranging from 0.098 μM to 100 μM, and we calculated the IC50 of 20(R)-ginsenoside Rh2. The IC50 of 20(R)-ginsenoside Rh2 against MHV-68 was estimated to be 2.77 μM (Fig. 4).
Fig. 4.
The antiviral efficacy (IC50) of 20(R)-ginsenoside Rh2. Plaque formation was described as relative data compared with those obtained using DMSO-treated infected control. IC50 represents the concentration of 20(R)-ginsenoside Rh2 required for 50% inhibition of MHV-68 replication. The data are representative of three experiments with similar results.
3.5. 20(R)-ginsenoside Rh2 inhibits virus replication following virus entry
To examine the stage of viral replication at which 20(R)-ginsenoside Rh2 exerts its activity, we treated Vero cells with 20(R)-ginsenoside Rh2 before virus infection (pretreatment group), after infection (posttreatment group), or before and after infection (pretreatment and posttreatment group) (Fig. 5A). Then, we performed plaque formation assays to measure viral replication, and the expressions of RTA and ORF56 were analyzed using qRT-PCR.
Fig. 5.
Stage of antiviral activity against MHV-68 by 20(R)-ginsenoside Rh2. (A) A schematic diagram describing the experimental scheme for measuring the stage at which 20(R)-ginsenoside Rh2 exerts its effects. (B) A representative picture of plaque reduction assays. (C) Relative plaque formations (%) compared with DMSO-treated lytic sample were calculated. (D) Changes in viral transcript RTA. (E) Changes in genome ORF56 replication. The data are representative of three experiments with similar results. * p < 0.05; *** p < 0.001.
Pretreatment with 20(R)-ginsenoside Rh2 had no effects on the inhibition of plaque formation, while posttreatment and pretreatment and posttreatment groups inhibited viral proliferation (Figs. 5B and 5C). Similar to the results of the plaque formation assay, the results of analysis of the expressions of RTA and ORF56 showed that the expressions of RTA and ORF56 were not repressed in the group pretreated with 20(R)-ginsenoside Rh2 but were repressed in the posttreatment and preretreatment and posttreatment groups (Figs. 5D and 5E). Taken together, these results suggest that 20(R)-ginsenoside Rh2 does not block the entry of the virus into the cells but inhibits the replication of MHV-68 after entry into the cells.
3.6. Antiviral activity of 20(R)-ginsenoside Rh2 against KSHV
MHV-68 is genetically related to human gammaherpesviruses such as KSHV and EBV, and its life cycle is similar to that of KSHV and EBV. Thus, we examined the antiviral effects of 20(R)-ginsenoside Rh2 against KSHV. To evaluate the effects of 20(R)-ginsenoside Rh2 on KSHV replication, we used the BC-3 tumor cell line latently infected with KSHV and analyzed lytic reactivation after TPA induction. Compared with untreated TPA-induced BC-3 cells, those treated with 20(R)-ginsenoside Rh2 induced a significant dose-dependent decrease in the protein expression of the late lytic gene KSHV K8α (Figs. 6A and 6B). In addition, KSHV RTA mRNA and viral DNA amplification levels were inhibited by 20(R)-ginsenoside Rh2 treatment (Figs. 6C and 6D). Taken together, our results indicate that 20(R)-ginsenoside Rh2 possesses effective antiviral activity against human gammaherpesviruses such as KSHV, as well as the murine gammaherpesvirus MHV-68.
Fig. 6.
Effects of 20(R)-ginsenoside Rh2 on KSHV reactivation were determined by different measurements. (A, B) The levels of a viral lytic protein K8α. (C) KSHV RTA mRNA expression. (D) Viral genome amplification. * p < 0.05; ** p < 0.01; *** p < 0.001. DMSO, dimethyl sulfoxide; KSHV, Kaposi's sarcoma-associated herpesvirus; TPA, 12-O-tetradecanoylphorbol-13-acetate.
4. Discussion
Antiviral activities of several ginsenosides have been reported in previous studies. Protopanaxatriol-type ginsenosides such as ginsenoside Re, Rf, and Rg2 protect the host from rhinovirus 3 and coxsackievirus infections [9], while ginsenoside Rb1 suppresses viral infection and proliferation of various viruses, such as hepatitis A virus, norovirus, and herpes simplex virus (HSV) [28], [29], [30]. In addition, ginsenoside Rg3 markedly inhibits the secretion of hepatitis B surface antigen, hepatitis B envelope antigen, and viral particles in hepatitis B virus-infected HepG2.2.15 cells by downregulating tumor necrosis factor receptor-associated factor 6/transforming growth factor β activated kinase-1 and the mitogen-activated protein kinase signaling pathway [31]. However, to date, no study has reported the antiviral activity of ginsenosides against gammaherpesviruses, and thus, we assessed the antiviral activities of 15 ginsenosides and compound K using MHV-68, a murine model similar to human gammaherpesviruses. We found that ginsenosides Rb3, Rc, 20(R)-Rg2, 20(S)-Rg2, 20(R)-Rg3, and 20(R)-Rh2 exerted antiviral activities by reducing plaque formation and mRNA expression of RTA. In particular, 20(R)-ginsenoside Rh2 showed the most significant antiviral effect against MHV-68.
Natural ginseng contains small amounts of ginsenoside Rh2, which is about 0.01% of the ginseng extract [32], [33], [34]. However, some naturally abundant ginsenosides such as Rg3, Rb1, Rb2, and Rc could also be metabolized into Rh2 by the intestinal bacteria after absorption, which suggests that the level of ginsenoside Rh2 after ingestion of the ginseng extract would be higher than expected [34], [35]. In addition, the antiviral activities of ginsenosides Rg3 and Rb1 might be partly derived from ginsenoside Rh2, which is intestinally converted from Rg3 and Rb1.
Recent studies have shown that ginsenoside Rh2 has two stereoisomers, 20(R)-ginsenoside and 20(S)-ginsenoside, and they exert different pharmacological effects [32], [35], [36]. Typically, the 20(S)-ginsenoside Rh2 possesses strong cytotoxic activities, and thus, it has been considered as an anticancer agent. Previous studies showed that 20(S)-ginsenoside Rh2 had cytotoxic effects on various tumor cell lines such as breast cancer, leukemia, lung cancer, and cervical carcinoma and had an IC50 as low as 22 μM against lymphocytic leukemia cells. However, treatment with 20(R)-ginsenoside Rh2 at concentrations up to 100 μM had no significant cytotoxic effects, which is consistent with the results of our cytotoxicity test using the fibroblast cell line NIH-3T3 [35], [37]. 20(R)-ginsenoside Rh2 exerts anti-inflammatory effects in lipopolysaccharide-activated macrophages and naïve keratinocytes through the suppression of reactive oxygen species and inflammatory mediators [32], [38], [39]. Relatively fewer functions of 20(R)-ginsenoside Rh2 have been identified thus far; therefore, it is important to reveal novel antiviral effects of 20(R)-ginsenoside Rh2 on MHV-68.
MHV-68 is a surrogate model for the study of human KSHV infections, and thus, we examined the antiviral effects of 20(R)-ginsenoside Rh2 in a KSHV model [24]. Similar to the results observed in the MHV-68 study, results of the treatment of KSHV-infected cells with TPA showed that 20(R)-ginsenoside Rh2 inhibited virus reactivation by inhibiting plaque formation, expression of viral transcript, and genome replication. RTA is necessary and sufficient for the reactivation of KSHV, resulting in the switch from latency to lytic replication, and nuclear factor kappa B (NF-κB) pathways negatively regulate RTA and repress lytic viral replication [40]. Qi et al [41] reported that 20(R)-ginsenoside Rh2 increased the phosphorylation of inhibitor of kappa B (IκB) and activated NF-κB; therefore it is highly likely that 20(R)-ginsenoside Rh2 might activate NF-κB via phosphorylation of IκB, repress RTA, and inhibit lytic replication in the KSHV model.
In addition to KSHV, we anticipate that 20(R)-ginsenoside Rh2 might have antiviral effects on other types of human herpesviruses such as HSV-1, HSV-2, varicella-zoster virus, and human cytomegalovirus, or other classes of viruses whose life cycles are entirely different from those of gammaherpesviruses. Furthermore, the antiviral activities of ginsenosides Rb3, Rf, Rg1, 20(R)-Rg2, and 20(R)-Rg3 need to be examined more thoroughly.
Conflicts of interest
None declared.
Acknowledgments
This work was supported by the National Research Foundation (NRF), funded by the Ministry of Science, ICT, and Future Planning [NRF-2013R1A1A3005097, 2015R1C1A2A01054457 to H.M.] and by the Chung-Ang University Excellent Student Scholarship in 2016 (G.K.).
References
- 1.Lee M.H., Lee B.H., Jung J.Y., Cheon D.S., Kim K.T., Choi C. Antiviral effect of Korean red ginseng extract and ginsenosides on murine norovirus and feline calicivirus as surrogates for human norovirus. J Ginseng Res. 2011;35:429–435. doi: 10.5142/jgr.2011.35.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin S.H., Park J.K., Nam K.Y., Park S.N., Jung N.P. Korean red ginseng saponins with low ratios of protopanaxadiol and protopanaxatriol saponin improve scopolamine-induced learning disability and spatial working memory in mice. J Ethnopharmacol. 1999;66:123–129. doi: 10.1016/s0378-8741(98)00190-1. [DOI] [PubMed] [Google Scholar]
- 3.Surh Y.J., Ferguson L.R. Dietary and medicinal antimutagens and anticarcinogens: molecular mechanisms and chemopreventive potential–highlights of a symposium. Mutat Res. 2003;523–524:1–8. doi: 10.1016/s0027-5107(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 4.Jeon C., Kang S., Park S., Lim K., Hwang K.W., Min H. T cell stimulatory effects of Korean Red Ginseng through modulation of myeloid-derived suppressor cells. J Ginseng Res. 2011;35:462–470. doi: 10.5142/jgr.2011.35.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–274. [PubMed] [Google Scholar]
- 6.Lim D.S., Bae K.G., Jung I.S., Kim C.H., Yun Y.S., Song J.Y. Anti-septicaemic effect of polysaccharide from Panax ginseng by macrophage activation. J Infect. 2002;45:32–38. doi: 10.1053/jinf.2002.1007. [DOI] [PubMed] [Google Scholar]
- 7.Kang S., Min H. Ginseng, the ‘Immunity Boost’: the effects of Panax ginseng on immune system. J Ginseng Res. 2012;36:354–368. doi: 10.5142/jgr.2012.36.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park Y., Storkson J.M., Albright K.J., Liu W., Pariza M.W. Biological activities of conjugated fatty acids: conjugated eicosadienoic (conj. 20:2delta(c11,t13/t12,c14)), eicosatrienoic (conj. 20:3delta(c8,t12,c14)), and heneicosadienoic (conj. 21:2delta(c12,t14/c13,t15)) acids and other metabolites of conjugated linoleic acid. Biochim Biophys Acta. 2005;1687:120–129. doi: 10.1016/j.bbalip.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Song J.H., Choi H.J., Song H.H., Hong E.H., Lee B.R., Oh S.R., Choi K., Yeo S.G., Lee Y.P., Cho S. Antiviral activity of ginsenosides against coxsackievirus B3, enterovirus 71, and human rhinovirus 3. J Ginseng Res. 2014;38:173–179. doi: 10.1016/j.jgr.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang K.A., Piao M.J., Kim K.C., Zheng J., Yao C.W., Cha J.W., Kim H.S., Kim D.H., Bae S.C., Hyun J.W. Compound K, a metabolite of ginseng saponin, inhibits colorectal cancer cell growth and induces apoptosis through inhibition of histone deacetylase activity. Int J Oncol. 2013;43:1907–1914. doi: 10.3892/ijo.2013.2129. [DOI] [PubMed] [Google Scholar]
- 11.Kim A.D., Kang K.A., Kim H.S., Kim D.H., Choi Y.H., Lee S.J., Hyun J.W. A ginseng metabolite, compound K, induces autophagy and apoptosis via generation of reactive oxygen species and activation of JNK in human colon cancer cells. Cell Death Dis. 2013;4:e750. doi: 10.1038/cddis.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saw C.L.L., Yang A.Y.Q., Cheng D.C., Boyanapalli S.S.S., Su Z.Y., Khor T.O., Gao S., Wang J.R., Jiang Z.H., Kong A.N.T. Pharmacodynamics of ginsenosides: antioxidant activities, activation of Nrf2, and potential synergistic effects of combinations. Chem Res Toxicol. 2012;25:1574–1580. doi: 10.1021/tx2005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rickabaugh T.M., Brown H.J., Wu T.T., Song M.J., Hwang S., Deng H., Mitsouras K., Sun R. Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 RTA reactivates murine gammaherpesvirus 68 from latency. J Virol. 2005;79:3217–3222. doi: 10.1128/JVI.79.5.3217-3222.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steed A.L., Barton E.S., Tibbetts S.A., Popkin D.L., Lutzke M.L., Rochford R., Virgin H.W., 4th Gamma interferon blocks gammaherpesvirus reactivation from latency. J Virol. 2006;80:192–200. doi: 10.1128/JVI.80.1.192-200.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du M.Q., Diss T.C., Liu H., Ye H., Hamoudi R.A., Cabecadas J., Dong H.Y., Harris N.L., Chan J.K., Rees J.W. KSHV- and EBV-associated germinotropic lymphoproliferative disorder. Blood. 2002;100:3415–3418. doi: 10.1182/blood-2002-02-0487. [DOI] [PubMed] [Google Scholar]
- 16.Cesarman E., Moore P.S., Rao P.H., Inghirami G., Knowles D.M., Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi's sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood. 1995;86:2708–2714. [PubMed] [Google Scholar]
- 17.Chang Y., Cesarman E., Pessin M.S., Lee F., Culpepper J., Knowles D.M., Moore P.S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 18.Soulier J., Grollet L., Oksenhendler E., Cacoub P., Cazals-Hatem D., Babinet P., d'Agay M.F., Clauvel J.P., Raphael M., Degos L. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 19.Brooks L., Yao Q.Y., Rickinson A.B., Young L.S. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J Virol. 1992;66:2689–2697. doi: 10.1128/jvi.66.5.2689-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iizasa H., Nanbo A., Nishikawa J., Jinushi M., Yoshiyama H. Epstein-Barr Virus (EBV)-associated gastric carcinoma. Viruses. 2012;4:3420–3439. doi: 10.3390/v4123420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan M.B., Allen C.E., Weitzman S., Filipovich A.H., McClain K.L. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118:4041–4052. doi: 10.1182/blood-2011-03-278127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F., Xue M., Qin D., Zhu X., Wang C., Zhu J., Hao T., Cheng L., Chen X., Bai Z. HIV-1 Tat promotes Kaposi's sarcoma-associated herpesvirus (KSHV) vIL-6-induced angiogenesis and tumorigenesis by regulating PI3K/PTEN/AKT/GSK-3beta signaling pathway. PLoS One. 2013;8:e53145. doi: 10.1371/journal.pone.0053145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho H.J., Jeong S.G., Park J.E., Han J.A., Kang H.R., Lee D., Song M.J. Antiviral activity of angelicin against gammaherpesviruses. Antiviral Res. 2013;100:75–83. doi: 10.1016/j.antiviral.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Simas J.P., Efstathiou S. Murine gammaherpesvirus 68: a model for the study of gammaherpesvirus pathogenesis. Trends Microbiol. 1998;6:276–282. doi: 10.1016/s0966-842x(98)01306-7. [DOI] [PubMed] [Google Scholar]
- 25.Wu T.T., Tong L.M., Rickabaugh T., Speck S., Sun R. Function of Rta is essential for lytic replication of murine gammaherpesvirus 68. J Virol. 2001;75:9262–9273. doi: 10.1128/JVI.75.19.9262-9273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Im K., Lee J.Y., Byeon H., Hwang K.W., Kang W., Whang W.K., Min H. In Vitro antioxidative and anti-inflammatory activities of the ethanol extract of eggplant (Solanum melongena) stalks in macrophage RAW 264.7 cells. Food Agric Immunol. 2016;27:758–771. [Google Scholar]
- 27.Lee K.P., Kang S., Noh M.S., Park S.J., Kim J.M., Chung H.Y., Je N.K., Lee Y.G., Choi Y.W., Im D.S. Therapeutic effects of s-petasin on disease models of asthma and peritonitis. Biomol Ther. 2015;23:45–52. doi: 10.4062/biomolther.2014.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee M.H., Lee B.H., Lee S., Choi C. Reduction of hepatitis A virus on FRhK-4 cells treated with Korean red ginseng extract and ginsenosides. J Food Sci. 2013;78:M1412–M1415. doi: 10.1111/1750-3841.12205. [DOI] [PubMed] [Google Scholar]
- 29.Lee M.H., Seo D.J., Kang J.H., Oh S.H., Choi C. Expression of antiviral cytokines in Crandell-Reese feline kidney cells pretreated with Korean red ginseng extract or ginsenosides. Food Chem Toxicol. 2014;70:19–25. doi: 10.1016/j.fct.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 30.Liang Y.Y., Wang B., Qian D.M., Li L., Wang Z.H., Hu M., Song X.X. Inhibitory effects of Ginsenoside Rb1 on apoptosis caused by HSV-1 in human glioma cells. Virol Sin. 2012;27:19–25. doi: 10.1007/s12250-012-3220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang L.J., Choi Y.J., Lee S.G. Stimulation of TRAF6/TAK1 degradation and inhibition of JNK/AP-1 signalling by ginsenoside Rg3 attenuates hepatitis B virus replication. Int J Biochem Cell Biol. 2013;45:2612–2621. doi: 10.1016/j.biocel.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Liu J., Shiono J., Shimizu K., Yu H., Zhang C., Jin F., Kondo R. 20(R)-ginsenoside Rh2, not 20(S), is a selective osteoclastgenesis inhibitor without any cytotoxicity. Bioorg Med Chem Lett. 2009;19:3320–3323. doi: 10.1016/j.bmcl.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z., Zheng Q., Liu K., Li G., Zheng R. Ginsenoside Rh(2) enhances antitumour activity and decreases genotoxic effect of cyclophosphamide. Basic Clin Pharmacol Toxicol. 2006;98:411–415. doi: 10.1111/j.1742-7843.2006.pto_348.x. [DOI] [PubMed] [Google Scholar]
- 34.Bae E.A., Han M.J., Kim E.J., Kim D.H. Transformation of ginseng saponins to ginsenoside Rh2 by acids and human intestinal bacteria and biological activities of their transformants. Arch Pharm Res. 2004;27:61–67. doi: 10.1007/BF02980048. [DOI] [PubMed] [Google Scholar]
- 35.Bae E.A., Han M.J., Choo M.K., Park S.Y., Kim D.H. Metabolism of 20(S)- and 20(R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol Pharm Bull. 2002;25:58–63. doi: 10.1248/bpb.25.58. [DOI] [PubMed] [Google Scholar]
- 36.Shibata S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J Korean Med Sci. 2001;16(Suppl.):S28–S37. doi: 10.3346/jkms.2001.16.S.S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh M., Choi Y.H., Choi S., Chung H., Kim K., Kim S.I., Kim D.K., Kim N.D. Anti-proliferating effects of ginsenoside Rh2 on MCF-7 human breast cancer cells. Int J Oncol. 1999;14:869–875. doi: 10.3892/ijo.14.5.869. [DOI] [PubMed] [Google Scholar]
- 38.Choi W.Y., Lim H.W., Lim C.J. Anti-inflammatory, antioxidative and matrix metalloproteinase inhibitory properties of 20(R)-ginsenoside Rh2 in cultured macrophages and keratinocytes. J Pharm Pharmacol. 2013;65:310–316. doi: 10.1111/j.2042-7158.2012.01598.x. [DOI] [PubMed] [Google Scholar]
- 39.Park E.K., Choo M.K., Kim E.J., Han M.J., Kim D.H. Antiallergic activity of ginsenoside Rh2. Biol Pharm Bull. 2003;26:1581–1584. doi: 10.1248/bpb.26.1581. [DOI] [PubMed] [Google Scholar]
- 40.Lingel A., Ehlers E., Wang Q., Cao M., Wood C., Lin R., Zhang L. Kaposi's sarcoma-associated herpesvirus reduces cellular myeloid differentiation primary-response gene 88 (MyD88) expression via modulation of its RNA. J Virol. 2016;90:180–188. doi: 10.1128/JVI.02342-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi X.D., Hou J.C., Yu H.T., Zhang C.J. 20 (S)-Ginsenoside-Rh2 and 20 (R)-Ginsenoside-Rh2 activate IkappaB phosphorylation expression in human lung adenocarcinoma A549 cells. Adv Mat Res. 2011;268–270:1205–1210. [Google Scholar]