Abstract
Aim
The role of clopidogrel in treating patients with acute ischaemic stroke is unclear. We have conducted the clinical trial in order to evaluate the efficacy and safety of clopidogrel with a loading dose in treating patients with non-cardiogenic acute ischaemic stroke.
Method
Clopidogrel loading dose versus maintenance dose to treat patients with acute ischaemic stroke in China (CLASS-China) was a prospective, randomised, double-blind and placebo-controlled clinical trial in China. Patients with acute ischaemic stroke of non-cardiogenic origin within 48 hours of onset were enrolled and those received thrombolysis were excluded. Enrolled patients were divided into two treatment groups: loading dose and routine dose. The primary outcome was the incidence of stroke recurrence or progression within 7 days. Primary safety outcome was measured by life-threatening haemorrhage. An intent-to-treat analysis was used for the statistical analysis.
Results
From March 2008 to March 2010, a total of 303 patients from 16 centres were recruited into this study; six were excluded because of lack of basic information. Since the enrolment was slow and the study drug expired in March 2010, this clinical trial was stopped earlier than planned. No significant baseline and demographic differences were seen between the two groups. There was no difference in primary outcome between the loading dosage group 16.1% (24/149) and control group 14.9% (22/148), respectively (p=0.782). The mortality and disability rate within 90 days in loading dose group (19.6%) was slightly lower than that in controlled group (23.4%), p=0.444. Loading dose group had two (1.3%) cases of fatal haemorrhage and control group had four (2.7%) within 90 days, p=0.674. No significant difference was detected in other adverse events between the groups.
Conclusion
In our study stopped early due to slow enrolment, loading dose of clopidogrel does not reduce the risk of recurrent stroke. Future trials with sufficient number of patients enrolled are needed to re-examine this hypothesis.
Keywords: Acute ischaemic stroke, non-cardiogenic stroke, clopidogrel, loading dose
Background
Previous studies have showed that the rate of recurrent stroke within 7 days can be as high as 10% after transient ischaemic attack (TIA) or minor stroke,1 and the rate of stroke in progression (SIP) was up or >30%.2 Although it is difficult to differentiate recurrent ischaemic stroke from SIP within 7 days of onset of the original stroke, effectively reducing stroke recurrence or SIP within 7 days can improve the prognosis and outcome of patients. The mechanism of early recurrent stroke or SIP could be caused by progress of thrombus or poststroke cerebral oedema, poor perfusion, haemorrhagic transformation and other metabolic disorders.3 To halt the progression of thrombus, the use of aspirin was controversial.4–6 However, clopidogrel has not been well studied. As an ADP receptor antagonist, clopidogrel showed better efficacy and safety in secondary prevention of stroke and myocardial infarction than 325 mg of aspirin.3 However, pharmacokinetic studies of clopidogrel showed that maximum inhibition of platelets with its regular dose (75 mg/day) required 7 days, and a loading dose of 300 mg could achieve maximum inhibition of platelets within 3 hours. Based on this phenomenon, loading dose of clopidogrel had been used in trials of treating coronary heart disease but in patients with acute ischaemic stroke. A preliminary study suggested that loading dose of clopidogrel was feasible and safe in patients with non-cardiogenic acute ischaemic stroke or TIA.7 We therefore conducted this multicentre prospective trial to examine the efficacy and safety of loading dose of clopidogrel versus standard dose of clopidogrel in patients with non-cardiogenic acute ischaemic stroke and TIA in China.
Method
Study design
Since the high incidence of stroke recurrence or progression within 7 days was used as the primary efficacy outcome for this study, and in order to reduce the incidence of primary efficacy outcome from 32% to 20% according to our open preliminary research, CLASS-China would require a sample size of 600 in order to detect the difference. CLASS-China is a prospectively, randomised, double-blinded, placebo-controlled clinical trial conducted at 17 clinical centres in the mainland southern China. Double-blind and randomising scheme was performed by CLASS-China randomisation and drug blind business operations manual developed by the data monitoring committee. Patients meeting the enrolment criteria were randomly assigned to one of the two treatment groups with the use of a double-blind, double-dummy design. The site investigator called into an automated system that randomly assigned a number corresponding to a medication kit stored at the study site, and the medication in the kit was administered to the patient.
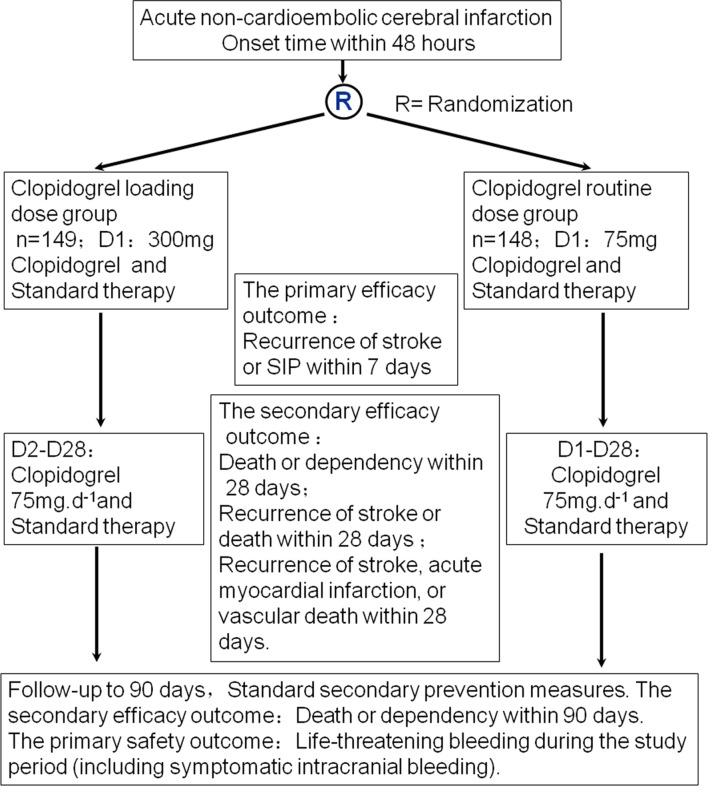
Both groups received study drug or placebo immediately after they arrived to the hospital. After enrolment, patient took the first dose of three pills of clopidogrel or placebo (225 mg) and one pill of clopidogrel (75 mg) provided by Sanofi-Aventis. All patients took 75 mg/day from the second day to 28 days. All patients had dysphagia screening prior to the initial dose immediately after they arrived to the hospital. Patients with dysphagia need not be excluded from the study. They need nasal feeding after indwelling gastric tube and study drug or placebo administered via the gastric tube. The protocol was approved by the ethics committees of The First Affiliated Hospital of Sun Yat-Sen University (figure 1). Registration number (ISRCTN07057952).
Figure 1.
CLASS-China study protocol. SIP, stroke in progression.
Study population
The main inclusion criteria had been designed as partial anterior circulation infarcts presumed to be caused by atherothrombosis. Just after the beginning of recruitment, however, such criteria were found not to be feasible for a multicentre trial. Therefore, the final inclusion criteria were: onset within 48 hours of acute non-cardioembolic cerebral infarction, 18–80 years of age, signed informed consent, and CT or MRI confirmation of cerebral infarction.
The exclusion criteria were: have received thrombolysis, cardiogenic embolism, clopidogrel allergy, intolerance or contraindication, needing oral anticoagulants and antiplatelet drugs within 1 week prior to randomisation, or use of drugs affecting platelet function; serious liver and kidney dysfunction or heart and lung diseases, malignant tumours, gastrointestinal disorders seriously affect drug absorption, coagulopathy or systemic bleeding illness or history and thrombocytopenia or neutropenia or history, pregnancy or breastfeeding women, recent surgery, trauma, or any plan for major surgery.
Combined treatment
All patients received standard medical treatment except clopidogrel loading dose or maintenance dose. Standard treatments include management of hypertension, diabetes, brain oedema, rehabilitation therapy, antibiotics when indicated and nutritional support. Traditional Chinese medicine and other antiplatelet drugs were prohibited within 7 days after enrolment.
Treatment options after 28 days were determined by the treating physician according to the indications. Clopidogrel 75 mg/day was recommended up to 90 days. Aspirin 100 mg/day was recommended if patient could not take clopidogrel. Other antiplatelet drugs were prohibited.
Study outcomes
The primary efficacy outcome was the recurrence of stroke or SIP within 7 days. Because the SIP was generally defined as neurological deterioration within 7 days, it was clinically difficult to differentiate. In this study, an increase of ≥2 points on the National Institutes of Health Stroke Scale (NIHSS) than that at the baseline within 7 days was considered as SIP.8 Patients had daily NIHSS score determination to monitor for the primary efficacy outcome.
The secondary efficacy outcomes included the following: (1) Death or dependency ratios within 28 and 90 days (modified Rankin Scale, mRS ≥3).9 Because mRS and activity of daily living measurement such as Barthel index (BI) have high level of consistency, we evaluated and recorded both mRS and BI at the same time. A score of <75 points on BI was considered dependent.10 (2) Recurrence of stroke or death within 28 days. (3) Recurrence of stroke, acute myocardial infarction or vascular death within 28 days.
To classify haemorrhagic complications, the criteria used by the MATCH study were implemented in this research.11 The primary safety outcome was life-threatening bleeding events or any fatal bleeding events, such as symptomatic intracranial bleeding (sICH, symptomatic intracerebral haemorrhage), haemoglobin decreased ≥5 g/dL, haemorrhagic shock, transfusing ≥4 units of packed red blood cells. The secondary safety outcomes include: (1) moderate bleeding events such as continuous bleeding complications, intraocular haemorrhages cause serious vision loss, transfusing >2 to 3 units of packed red blood cells; (2) small haemorrhages such as other bleeding events not matching the above standard, excluding ICH; (3) other side effects, including liver and kidney impairment, leucopenia, thrombocytopenia through the test of routine blood, blood biochemical and urine routine, and allergies, skin rash, and so on; (4) microbleeding: MRI gradient echo (MR-GRE) showing microhaemorrhage.
Statistical analysis
This was an intention-to-treat trial. All tests were one tailed, and a p value of 0.05 was considered statistically significant. χ2 test was used to examine the incidence of endpoint events. Quantitative analysis was used to test and compare the quantity of microbleeding on MR-GRE in 28 days or discharge. Descriptive statistics, frequency table and data list were used for summary. Continuous variables were described by descriptive statistics, including sample size, mean, SD, interval and median. Paired t-test and two-sample t-tests were used to analyse the change from baseline in groups. Non-parametric test was used if the data distribution was abnormal. Two-sided test with 95% CI was used if the data conformed to t distribution. Classification variables were described by descriptive statistics, including sample size, frequency counts and percentages. Non-parametric test was used for analysis of change from baseline and comparison between the two groups.
Results
From March 2008 to March 2010, a total of 17 centres were recruited but only 16 enrolled patients. A total of 303 patients were finally enrolled into this study but six cases were excluded because of lack of basic information. It was unanticipated that the enrolment was very slow. Along with the expiration of the study drug in March 2010, the trial had to stop. The demographic characteristics and baseline data between the two groups showed no significant differences (table 1).
Table 1.
Demographic and baseline characteristics of the patients
| Groups | Loading dose group | Standard dose group | p |
| n | 149 | 148 | – |
| Age (year) | 64.0 (10.7) | 63.5 (11.0) | 0.706 |
| Male | 105 (70.5%) | 92 (62.2%) | 0.130 |
| Onset to admission time (hour)* | 17.3 (11.9) | 17.7 (12.9) | 0.771 |
| BMI (kg/m2)* | 23.9 (3.2) | 23.3 (3.5) | 0.143 |
| Systolic pressure (mm Hg)* | 151 (25.1) | 150 (22.6) | 0.900 |
| Fasting blood sugar (mmol/L)* | 7.0 (4.3) | 6.3 (2.8) | 0.157 |
| Hypertension history | 87 (58.4%) | 87 (58.8%) | 0.945 |
| Diabetes history | 27 (18.1%) | 27 (18.2%) | 0.978 |
| Coronary heart disease history | 8 (5.4%) | 7 (4.7%) | 0.801 |
| TIA history | 2 (1.3%) | 1 (0.7%) | >0.999 |
| Stroke history | 20 (13.4%) | 21 (14.2%) | 0.848 |
| Current or ex-smoker | 51 (73.9%) | 46 (75.4%) | 0.845 |
| Alcoholic | 42 (28.2%) | 35 (23.6%) | 0.372 |
| Baseline mRS (3–6)† | 101 (67.8%) | 97 (65.5%) | 0.682 |
| Baseline BI (0 to <75)† | 96 (64.4%) | 97 (65.5%) | 0.841 |
| Baseline NIHSS | 5.7 (3.3) | 6.2 (3.6) | 0.222 |
*Units.
†Scores.
BI, Barthel index; BMI, body mass index; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischaemic attack.
For the primary efficacy outcome, the total stroke recurrence or SIP within 7 days was 15.5%, which was lower than our expected rate of 32%, and there was no difference between the two groups: loading dosage group 16.1% (24/149) and control group 14.9% (22/148), respectively, p=0.782. For the secondary efficacy outcome, death or dependency within 28 days was 32.2% (46/149) in the loading dose group and 30.9% (43/148) in the controlled group (p=0.808). Death or dependency within 90 days was 19.6% (27/149) in loading dose group and 23.4% (32/148) in the controlled group (p=0.444). There was no difference between the two groups on recurrent stroke, acute myocardial infarction or death within 28 days: loading dosage group 18.1% (27/149) and control group 16.2% (24/148), respectively (p=0.663, table 2).
Table 2.
Comparison of efficacy between the treatment groups of loading and standard dosage of clopidogrel
| Groups | Loading dose group | Standard dose | RRR (95% CI) | p |
| n | 149 | 148 | – | – |
| Stroke within 7 days*, n (%) | 24 (16.1%) | 22 (14.9%) | 7.7 (−64.7, 48.2) | 0.7823 |
| Death or dependency† within 28 days, n (%) | 46 (32.2%) | 43 (30.9%) | – | 0.808 |
| Death or dependency† within 90 days, n (%) | 27 (19.6%) | 32 (23.4%) | – | 0.444 |
| Recurrent stroke or death within 28 days | 27 (18.1%) | 24 (16.2%) | – | 0.663 |
| Recurrent stroke, acute myocardial infarction or death within 28 days | 27 (18.1%) | 24 (16.2%) | – | 0.663 |
*Primary outcome.
†Dependency: modified Rankin Scale ≥3.
RRR, relative risk reduce.
For the safety outcome, fatal bleeding (sICH) was found in two cases (1.3%) in loading dose group and four cases (2.7%) in control group within 90 days, p=0.674. There was no difference in adverse events or severe adverse events between the two groups (table 3).
Table 3.
The life-threatening bleeding events between loading and standard dosages of clopidogrel treating groups for all patients
| Groups | n | Life-threatening bleeding events | ||
| 7 days | 28 days | 90 days | ||
| Loading dose group | 149 | 2 (1.3%) | 2 (1.3%) | 2 (1.3%) |
| Standard dose group | 148 | 2 (1.4%) | 2 (1.4%) | 4 (2.7%) |
| p | – | 1.0 | 1.0 | 0.674 |
Discussion
Of all antiplatelet agents used during the acute period to treat ischaemic stroke, aspirin showed marginal benefit when given within 48 hours in IST/CAST (International Stroke Trial/Chinese Acute Stroke Trial) trials.12 LOAD trial using 325 mg of aspirin plus 375 mg of clopidogrel within 36 hours appeared to be safe and significantly less likely to experience neurological deterioration (p<0.002).13 Several other small trials using either a loading dose of 600 mg of clopidogrel within 24 hours, or combination of aspirin and dipyridamole in acute ischaemic stroke showed such uses were safe but efficacy was unclear. EARLY trial compared 100 mg of aspirin to 25 mg of aspirin plus 200 mg of extended-release dipyridamole within 24 hours of stroke or TIA or after 7 days of aspirin monotherapy.14 There was a very marginal benefit. The FASTER (Fast Assessment of Stroke and Transient Ischemic Attack to Prevent Early Recurrence) trial used clopidogrel to treat patients with minor strokes (NIHSS score <4) within 24 hours of qualifying event. The risk of recurrent stroke was 7.1% compared with 10.8% in the placebo arm (p=0.019).15 A trial using clopidogrel loading dose at least 300 mg (with or without aspirin) for moderate and severe ischaemic strokes (NIHSS score >3) showed such loading dose was safe and effective.16 However, this trial was a retrospective study and the maintenance dose was not combined with aspirin, as 91.5% of the group received clopidogrel loading dose combined with aspirin. A multicentre double-blinded randomised controlled trial study in Sweden showed that aspirin (325 mg/day, 5 days) given within 48 hours of onset failed to reduce the early stroke progression compared with placebo.6 CAIST (Cilostazol in Acute Ischemic Stroke Treatment) study showed cilostazol was safe and effective for acute cerebral infarction compared with aspirin, but without statistical differences between them.17 SOCRATES study suggested that there was no difference in the primary outcome (the composite outcome events, including stroke, myocardial infarction and death for the first time after enrolment) between ticagrelor and aspirin. But ticagrelor was better than aspirin of the composite outcome events on the seventh day, and haemorrhagic events were the same with aspirin.18
The original purpose of our study was to provide evidence that loading dose of clopidogrel was more effective on SIP caused by atherosclerosis. The reason for modifying the inclusion criteria during the trial was that the subtype of ischaemic stroke was difficult to be determined within 48 hours of onset in a multicentre trial. Therefore, we enrolled patients with acute non-cardioembolic cerebral infarction origin within 48 hours and who met the other inclusion criteria after research council symposium.
Our trial tested clopidogrel in a different way. Since we did not reach the prespecified 600 patients and only 297 patients were enrolled and followed up, the sample was too small to detect any meaningful difference in the primary and secondary outcomes. However, our study seemed to show a trend of lower rate of disability and haemorrhagic event in patients who received loading dose of clopidogrel. Such trend requires a large prospective trial to confirm.
The limitation of our trial was the low number of enrolment. Several reasons could explain this phenomenon. First, the current doctor–patient relationship in China is critical, which makes it difficult to enrol patients in clinical trials. Second, patient and family were sceptical in participating in treatment trials. These two reasons led to slow speed to enrol patients so that the study drugs expired. Since the trial could not continue, the research council decided to terminate the research and perform statistical analysis for those enrolled.
Another limitation of our trial was the lack of a study log allowing for determination of how our study population differs from our general population of patients with stroke. During the process of this study, only those patients who met the inclusion criteria and did not accord with standard of exclusion criteria were requested to be informed consent, and the screened data for those patients who did not agree to attend this study had not been recorded.
Conclusion
In our study stopped early due to slow enrolment, loading dose of clopidogrel does not reduce the risk of recurrent stroke. Future trials with sufficient number of patients enrolled are needed to re-examine this hypothesis.
Acknowledgments
We thank all the patients and the clinical and research teams of the 16 centers who participated in this trial and their relatives. The centers and the local investigators are listed as following: Dongguan People’s Hospital, Guangdong Province (Dr Weimin Xiao), Liwan Hospital, Guangzhou Medical University (Dr Feng Qi), Shanghai General Hospital(Prof Shaoshi Wang), Shenzhen People’s Hospital, Guangdong Province (Prof Xiaofan Chu), Sun Yat-Sen Memorial Hospital, SunYat-Sen University (Prof Ying Peng),The First Affiliated Hospital, Sun Yat-Sen University (Prof Jinsheng Zeng), The First Affiliated Hospital, Guangdong Medical University (Prof Bin Zhao), The First Affiliated Hospital, Guangzhou Medical University (Prof Ming Shao), The First Affiliated Hospital, Jinan University, Guangzhou (Prof Anding Xu), The First Affiliated Hospital, Kunming Medical University (Prof Wenmin Wang), The First People’s Hospital of Yunnan Province (Prof Li Ding), The Second Affiliated Hospital, Guangzhou Medical University (Prof Qingchun Gao), The Southwest Hospital, The Third Military Medical University (Prof Kangning Chen), Zhanjiang Central Hospital, Guangdong Province (Dr Zhanyin Chen), Zhaoqing People’s Hospital, Guangdong Province (Dr Yan Huang),Zhuhai People’s Hospital, Guangdong Province (Dr Wenyan Zhuo).
Footnotes
Contributors: All the listed authors have participated actively in the study, and have seen and approved the submitted manuscript. ZY was responsible for the statistical analysis of data and writing of paper. YW has contributed to conduct and reporting of the study. TZ, WW and XW were in charge of acquisition of data. ZJ and AX have contributed on the conception and design of the study, analysis and interpretation of data, and the modifications of the paper.
Funding: This study was supported by the Science and Technology Program of Guangzhou (No. 201508020004), and received funding from the Medical Science and Technology Research of Guangdong Province (No. A2015461).
Competing interests: None declared.
Patient consent: Obtained
Ethics approval: The First Affiliated Hospital of Sun Yat-Sen University.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
Correction notice: This article has been corrected since it published Online First. The author names have been corrected and an acknowledgement statement has been added.
References
- 1. Sl H, Gong XG. Economic burden of ischemic stroke in China. Health Economy of China 2003;22:18–22. [Google Scholar]
- 2. Petty GW, Brown RD, Whisnant JP, et al. Survival and recurrence after first cerebral infarction: a population-based study in Rochester, Minnesota, 1975 through 1989. Neurology 1998;50:208–16. doi:10.1212/WNL.50.1.208 [DOI] [PubMed] [Google Scholar]
- 3. Coull AJ, Lovett JK, Rothwell PM. Oxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ 2004;328:326 doi:10.1136/bmj.37991.635266.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese acute stroke trial) Collaborative Group. Lancet 1997;349:1641–9. [PubMed] [Google Scholar]
- 5. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet 1997;349:1569–81. [PubMed] [Google Scholar]
- 6. Rödén-Jüllig A, Britton M, Malmkvist K, et al. Aspirin in the prevention of progressing stroke: a randomized controlled study. J Intern Med 2003;254:584–90. doi:10.1111/j.1365-2796.2003.01233.x [DOI] [PubMed] [Google Scholar]
- 7. Yang WY, Ad X, Qi F, et al. The preliminary study of clopidogrel loading dose to treat non-cardiogenic ischemic stroke or transient ischemic stroke. Chinese journal of neural mental illness 2008;34:658–62. [Google Scholar]
- 8. Chen XZ, Hj L, Bx L, et al. Foreign medical (cerebrovascular disease. 2000:109–11.
- 9. Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005;352:777–85. doi:10.1056/NEJMoa042991 [DOI] [PubMed] [Google Scholar]
- 10. Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Md State Med J 1965;14:61–5. [PubMed] [Google Scholar]
- 11. Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 2004;364:331–7. doi:10.1016/S0140-6736(04)16721-4 [DOI] [PubMed] [Google Scholar]
- 12. Chen ZM, Sandercock P, Pan HC, et al. Indications for early aspirin use in acute ischemic stroke : a combined analysis of 40 000 randomized patients from the chinese acute stroke trial and the international stroke trial. on behalf of the CAST and IST collaborative groups. Stroke 2000;31:1240–9. doi:10.1161/01.STR.31.6.1240 [DOI] [PubMed] [Google Scholar]
- 13. Meyer DM, Albright KC, Allison TA, et al. LOAD: a pilot study of the safety of loading of aspirin and clopidogrel in acute ischemic stroke and transient ischemic attack. J Stroke Cerebrovasc Dis 2008;17:26–9. doi:10.1016/j.jstrokecerebrovasdis.2007.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dengler R, Diener HC, Schwartz A, et al. Early treatment with aspirin plus extended-release dipyridamole for transient ischaemic attack or ischaemic stroke within 24 h of symptom onset (EARLY trial): a randomised, open-label, blinded-endpoint trial. Lancet Neurol 2010;9:159–66. doi:10.1016/S1474-4422(09)70361-8 [DOI] [PubMed] [Google Scholar]
- 15. Kennedy J, Hill MD, Ryckborst KJ, et al. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol 2007;6:961–9. doi:10.1016/S1474-4422(07)70250-8 [DOI] [PubMed] [Google Scholar]
- 16. Shaban A, Monlezun DJ, Rincon N, et al. Clopidogrel load in patients with moderate and severe ischemic strokes. Stroke Res Treat 2016;2016:8915764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee YS, Bae HJ, Kang DW, et al. Cilostazol in Acute Ischemic Stroke Treatment (CAIST Trial): a randomized double-blind non-inferiority trial. Cerebrovasc Dis 2011;32:65–71. doi:10.1159/000327036 [DOI] [PubMed] [Google Scholar]
- 18. Johnston SC, Amarenco P, Albers GW, et al. SOCRATES Steering Committee and Investigators. Ticagrelor versus Aspirin in Acute Stroke or Transient Ischemic Attack. N Engl J Med 2016;375:35–43. doi:10.1056/NEJMoa1603060 [DOI] [PubMed] [Google Scholar]