Abstract
Action potential (AP) shape is a key determinant of cellular electrophysiological behavior. We found that in small-diameter, capsaicin-sensitive dorsal root ganglia neurons corresponding to nociceptors (from rats of either sex), stimulation at frequencies as low as 1 Hz produced progressive broadening of the APs. Stimulation at 10 Hz for 3 s resulted in an increase in AP width by an average of 76 ± 7% at 22°C and by 38 ± 3% at 35°C. AP clamp experiments showed that spike broadening results from frequency-dependent reduction of potassium current during spike repolarization. The major current responsible for frequency-dependent reduction of overall spike-repolarizing potassium current was identified as Kv3 current by its sensitivity to low concentrations of 4-aminopyridine (IC50 <100 μm) and block by the peptide inhibitor blood depressing substance I (BDS-I). There was a small component of Kv1-mediated current during AP repolarization, but this current did not show frequency-dependent reduction. In a small fraction of cells, there was a component of calcium-dependent potassium current that showed frequency-dependent reduction, but the contribution to overall potassium current reduction was almost always much smaller than that of Kv3-mediated current. These results show that Kv3 channels make a major contribution to spike repolarization in small-diameter DRG neurons and undergo frequency-dependent reduction, leading to spike broadening at moderate firing frequencies. Spike broadening from frequency-dependent reduction in Kv3 current could mitigate the frequency-dependent decreases in conduction velocity typical of C-fiber axons.
SIGNIFICANCE STATEMENT Small-diameter dorsal root ganglia (DRG) neurons mediating nociception and other sensory modalities express many types of potassium channels, but how they combine to control firing patterns and conduction is not well understood. We found that action potentials of small-diameter rat DRG neurons showed spike broadening at frequencies as low as 1 Hz and that spike broadening resulted predominantly from frequency-dependent inactivation of Kv3 channels. Spike width helps to control transmitter release, conduction velocity, and firing patterns and understanding the role of particular potassium channels can help to guide new pharmacological strategies for targeting pain-sensing neurons selectively.
Keywords: action potential, Kv3 channels, nociceptor, spike broadening
Introduction
The sensation of pain originates in primary afferent sensory neurons and some pathophysiological pain likely involves altered excitability of these neurons (Liu et al., 2000; Chung and Chung, 2002; Katz and Gold, 2006). The excitability of dorsal root ganglia (DRG) neurons corresponding to pain-sensing C-fibers is controlled by the many voltage-dependent ion channels that they express, including multiple types of sodium channels, calcium channels, and potassium channels (Gold et al., 1996; Rasband et al., 2001; Rush et al., 2007; Dib-Hajj et al., 2009; Zamponi et al., 2009). Potassium currents in DRG neurons comprise an especially complex mixture of components, including multiple inactivating and sustained voltage-gated potassium currents (Gold et al., 1996; Safronov et al., 1996; Everill et al., 1998; Rola et al., 2003), calcium-activated potassium currents (Gold et al., 1996; Scholz et al., 1998), and sodium-activated potassium current (Nuwer et al., 2010; Martinez-Espinosa et al., 2015) Reduced potassium currents likely contribute to aberrant nociceptor activity produced by inflammation (Nicol et al., 1997; Vaughn and Gold, 2010; Zhang et al., 2012) and nerve injury (Ishikawa et al., 1999; Abdulla and Smith, 2001; Rasband et al., 2001).
Action potential (AP) shape varies considerably among different types of mammalian peripheral and central neurons, reflecting different contributions of various potassium channels. For example, some neocortical GABAergic interneurons have unusually narrow APs that result from large fast-activating currents from Kv3-family channels (Rudy and McBain, 2001; Lien and Jonas, 2003), whereas repolarization of the broader APs of neocortical pyramidal neurons depends on Kv1, Kv2, and Kv4 channels and BK-calcium-activated potassium channels (Shao et al., 1999; Kim et al., 2005; Liu and Bean, 2014; Pathak et al., 2016). The APs of C-fiber nociceptors are distinctive in being unusually broad, with a pronounced “shoulder” on the falling phase (Ritter and Mendell, 1992; Djouhri et al., 1998). The AP shoulder reflects in part inward voltage-dependent calcium current (McCobb and Beam, 1991; Scroggs and Fox, 1992) and TTX-resistant Nav1.8 channel current (Renganathan et al., 2001; Blair and Bean, 2002) flowing during the falling phase and partially competing with outward potassium currents. The makeup of the potassium currents flowing during AP repolarization in small-diameter nociceptive DRG neurons is not known in detail. Recent experiments suggest that channels formed by Kv3.4 subunits are important because knock-down of Kv3.4 using siRNA results in wider APs (Ritter et al., 2012), a striking result given the previous association of Kv3 channels with cell types with narrow APs. A role of BK-calcium-activated potassium channels in spike repolarization is suggested by the ability of the BK blocker iberiotoxin to broaden APs in some small-diameter DRG neurons (Li et al., 2007; Zhang et al., 2010).
In some neurons, repetitive firing results in broadening of APs, generally appearing to reflect cumulative inactivation of potassium channels. Different potassium channel types are involved in different kinds of neurons. In various molluscan neurons, spike broadening can result from cumulative inactivation of both delayed-rectifier potassium current and A-type potassium current (Aldrich et al., 1979; Ma and Koester, 1996). In hippocampal CA1 pyramidal neurons, inactivation of both BK-calcium-activated potassium channels (Shao et al., 1999) and Kv4-mediated A-type channels (Kim et al., 2005) is involved. Spike broadening in various hypothalamic neurons reflects calcium-dependent progressive inactivation of A-type potassium current (Kirkpatrick and Bourque, 1991, Hlubek and Cobbett, 2000; Sonner et al., 2008). In hippocampal mossy fiber boutons, spike broadening reflects inactivation of a Kv1-mediated component of current, resulting in enhanced calcium entry and enhanced synaptic transmission (Geiger and Jonas, 2000).
Frequency-dependent spike broadening has been described in DRG neurons (Harper and Lawson, 1985; Park and Dunlap, 1998), but the mechanism is unknown. Studying small-diameter, capsaicin-sensitive rat DRG neurons, we found prominent spike broadening at frequencies as low as 1 Hz. Using the AP clamp method and pharmacological dissection of currents, we found that spike broadening in these neurons results primarily from frequency-dependent inactivation of Kv3 channels.
Materials and Methods
Cell preparation.
Dissociated DRG neurons were prepared as described previously (Blair and Bean, 2002). DRGs were removed from Long–Evans rats (postnatal day 14–16, either sex), cut in half, and treated for 20 min at 37°C with 20 U/ml papain (Worthington Biochemical) and 5 mm dl-cysteine in a Ca2+-free, Mg2+-free (CMF) Hank's solution containing the following (in mm): 136.9 NaCl, 5.4 KCl, 0.34 Na2HPO4, 0.44 KH2PO4, 5.55 glucose, and 5 HEPES, 0.005% phenol red, pH 7.4. Ganglia were then treated for 20 min at 37°C with 3 mg/ml collagenase (type I; Sigma-Aldrich) and 4 mg/ml dispase II (Boehringer Mannheim) in CMF Hank's solution. Cells were dispersed by trituration with a fire-polished glass Pasteur pipette in a solution of Leibovitz's L-15 medium (Invitrogen) supplemented with 10% fetal calf serum, 5 mm HEPES, 50 U/ml penicillin, 50 μg/ml streptomycin, 2 mm l-glutamine, and 100 ng/ml NGF (Invitrogen) and then plated on glass coverslips treated with 200 μg/ml poly-d-lysine. Cells were incubated in the supplemented L-15 solution at 33°C (room air) for 2–4 h, after which they were stored at 4°C and used within 48 h. Storing the neurons at 4°C inhibited the growth of neurites so that cells could be voltage clamped with fast settling of the capacity transient, enabling accurate recording of currents on the fast time scale of the AP.
Electrophysiology.
Whole-cell voltage- and current-clamp recordings were made from small-diameter DRG neurons (21–33 μm) using electrodes with resistances of 1.5–6 MΩ when filled with a potassium-aspartate internal solution containing the following (in mm): 140 K-aspartate, 13.5 NaCl, 1.6 MgCl2, 0.09 EGTA, 9 HEPES, 4 MgATP, 14 Tris-creatine PO4, and 0.3 Tris-GTP, pH 7.4 with KOH. Pipette tips were wrapped with thin strips of Parafilm to reduce capacitance. Seals were formed in Tyrode's solution containing the following (in mm): 150 NaCl, 4 KCl, 2 CaCl2, 2 MgCl2, 10 glucose, and 10 HEPES, pH 7.4 with NaOH. Series resistance was compensated by 80–95%. Solutions were applied after lifting the cell in front of an array of quartz fiber flow pipes. An initial set of experiments was done at room temperature (22 ± 1°C) and a subsequent set of experiment was done at 35 ± 1°C, with temperature controlled by heating the pipes using a feedback controller system (Warner Instruments TC-344C).
Data acquisition and analysis.
Currents and voltages were controlled and sampled using a Digidata 1321A or 1322A interface and pClamp 8 or pClamp 9 software (Molecular Devices). Current and voltage records were filtered at 5–10 kHz and digitized at 100 kHz. Analysis was performed with Igor Pro (Wavemetrics) using DataAccess (Bruxton Software) to import pClamp data. Reported membrane potentials are corrected for a liquid junction potential of −10 mV between the internal solution and the Tyrode's solution in which current was zeroed before sealing onto the cell, measured using a flowing 3 m KCl reference electrode as described by Neher (1992).
Current-clamp experiments.
APs were evoked with 0.5 ms current injections, leaving most of the AP free of the effect of injected current. For experiments examining frequency-dependent changes in APs during repetitive stimulation, current injections were set to 1.25–1.5 times threshold level to ensure reaching threshold rapidly. When neurons were stimulated repetitively multiple times, neurons were allowed to rest for at least 90 s between each train. AP widths were measured at half-maximal amplitude.
AP clamp experiments.
For most AP clamp experiments (Llinás et al., 1982; de Haas and Vogel, 1989), each cell's own AP was used as the command waveform. Potassium current was quantified by integrating net outward current during the falling phase of AP, starting at the peak of the AP and continuing to the most negative voltage in the afterhyperpolarization (or to 2 times the AP duration in cases in which there was no clear afterhyperpolarization).
Inhibitors.
Blood depressing substance I (BDS-I) was from Alomone Laboratories, TTX was from Calbiochem, and ω-conotoxin-GVIA was from Bachem; all other chemicals were from Sigma-Aldrich.
Experimental design and statistical analysis.
Summaries of data are given as mean ± SEM. For assaying frequency-dependent reduction of potassium current during spike repolarization, the experimental measurement was the difference in AP-evoked outward current from the 1st to the 15th presentation of the AP waveform delivered at 5 Hz. To better allow comparisons among cells, the change in integrated outward current was normalized to each cell's capacitance. The figures show scatter plots of all data for each experimental manipulation (replacement of external calcium by magnesium, addition of tetraethylammonium (TEA), 4-aminopyridine (4-AP), α-dendrotoxin, or BDS-I), with data points from the same cell connected by lines. Statistical significance reported in the text was assessed using the two-tailed Wilcoxon test for nonparametric paired data.
Results
AP broadening during repetitive stimulation of small DRG neurons
We recorded from small-diameter rat DRG neurons, first testing each cell for capsaicin sensitivity by applying 1 μm capsaicin. Only cells in which a current was evoked by capsaicin were studied further. In recording APs from this cell population, we found that with stimulation even at the relatively low frequency of 1 Hz, APs showed progressive spike-to-spike broadening. This effect was quite pronounced at frequencies of 5 Hz and higher. Figure 1A shows an example with stimulation at 5 Hz for 3 s. The AP width (measured at half-maximal amplitude) increased from 4.9 ms in the first AP to 6.6 ms in the 15th. Figure 1B shows the frequency dependence of AP broadening in 13 neurons that were each stimulated 30 times at 1, 5, 10, and 20 Hz. There was substantial broadening even at 1 Hz (by 12 ± 1%) and the degree of broadening increased at 5 Hz (44 ± 4%), 10 Hz (76 ± 7%), and 20 Hz (129 ± 12%). Broadening was evident by the second spike in a train and was half-maximal after three to eight spikes, taking longer to reach steady state at higher frequencies. The frequency-dependent spike broadening seen in these cells fits well with AP broadening seen previously during low-frequency stimulation in both rat DRG (Harper and Lawson, 1985) and embryonic chick DRG (Park and Dunlap, 1998) neurons.
Figure 1.
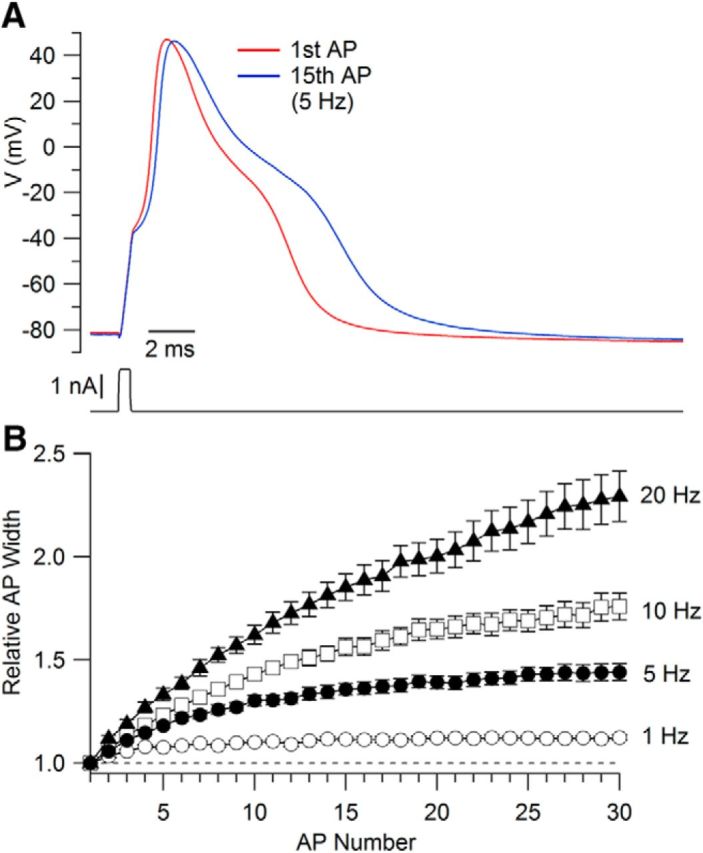
Broadening of APs during repetitive stimulation. A, First and 15th APs evoked by 5 Hz stimulation in a small-diameter, capsaicin-sensitive DRG neuron. APs were evoked by a 0.5 ms 1.7 nA current injection. B, Time course of AP broadening with stimulation at frequencies from 1 to 20 Hz. Symbols show mean ± SEM for determinations at 1, 5, 10, and 20 Hz, each determined in 13 neurons.
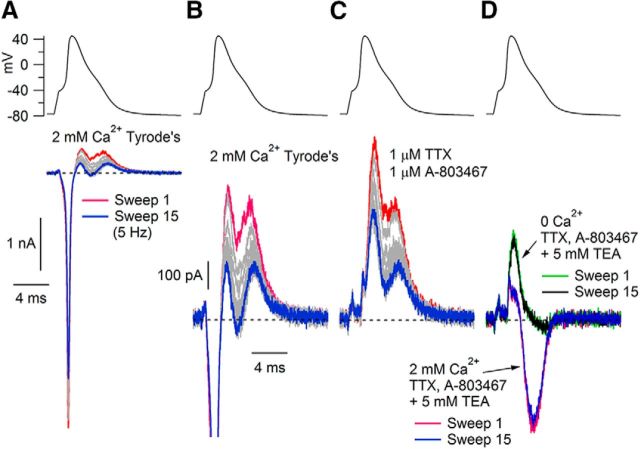
To characterize the changes in ionic currents that underlie the AP broadening, we performed AP clamp using each cell's own AP as a command waveform in voltage clamp. We used the first AP recorded during 5 Hz current-clamp stimulation (i.e., sweep 1 in Fig. 1A) to focus on changes in ionic current resulting from the repetitive application of the same voltage waveform independent of changes resulting from alterations in the shape of the AP. Figure 2A shows an example of the total ionic current recorded in external Tyrode's solution when the AP clamp was applied at 5 Hz. To isolate ionic current, capacitative current was eliminated; most capacitative current was removed electronically using the capacitative nulling circuit in the amplifier and the remaining capacitative current was corrected during analysis by performing a point-by-point subtraction using capacitative currents evoked by a 5 or 10 mV hyperpolarization from −75 mV. As expected, total ionic current was inward during the rising phase of the AP and outward during the falling phase.
Figure 2.
Reduction of outward current evoked by AP waveforms delivered at 5 Hz. The cell's own AP (evoked by a 0.5 ms, 1.1 nA current injection) was used as the command waveform in voltage clamp and applied at 5 Hz. A, Successive applications of the AP waveform using control Tyrode's solution containing 2 mm Ca evoked smaller inward currents during the upstroke and smaller outward currents during repolarization (1st sweep, red; 2nd to 14th sweeps, gray; 15th sweep, blue). B, Outward current shown at higher gain. C, Outward current after inhibiting sodium current with 1 μm TTX and 1 μm A-803467. D, Current during first (red) and 15th (blue) sweeps after switching to a solution with 5 mm TEA added to TTX and A-803467 and then after switching to a solution with TEA, TTX, and A-803467 and with equimolar Mg2+ replacing Ca2+ (first sweep, green; 15th sweep, black).
With repetitive stimulation by the same AP waveform, there were two changes in the evoked current (Fig. 2A). First, the peak inward current during the AP upstroke decreased. This frequency-dependent reduction in inward current during the upstroke results from slow inactivation of TTX-resistant sodium channels (Blair and Bean, 2003). The second change in the current evoked by 5 Hz stimulation was a decrease in net outward current during the falling phase of the AP. Addition of TTX (1 μm) and the Nav1.8 inhibitor A-803467 (1 μm) (Jarvis et al., 2007) inhibited the inward sodium current during the rising phase, helping to isolate changes in the outward potassium current. We used TTX and A-803467 in subsequent experiments to focus on changes in potassium current. In the presence of TTX and A-803467, further addition of 5 mm TEA inhibited the outward current and resulted in an inward current flowing during the falling phase of the AP, as would be expected if inhibiting potassium current reveals an inward calcium current flowing during the shoulder of the AP. This inward current was eliminated with a solution in which calcium was replaced by magnesium. There was little or no frequency-dependent change in the calcium current.
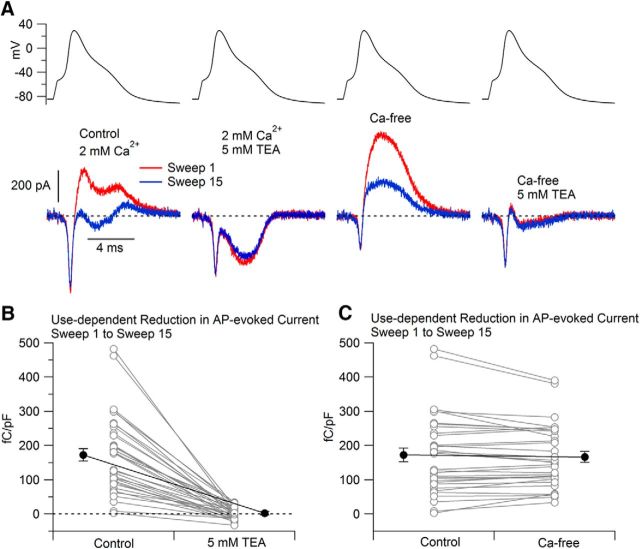
The experiment shown in Figure 2 was typical in that 5 mm TEA inhibited all or most of the outward potassium current, leaving a net inward calcium current, and there was little or no frequency dependence remaining in the presence of TEA. We quantified the frequency-dependent changes in potassium current by integrating the current during the falling phase of the AP from the time of the peak of the AP to the time of the afterhyperpolarization and calculating the difference in the AP-evoked current in the 1st and 15th stimuli delivered at 5 Hz. Figure 3A shows records in which we explored the sensitivity of the frequency-dependent component of potassium current to external TEA and to removal of calcium. TEA completely inhibited the frequency-dependent component of outward current. In collected results from 33 cells, there was a use-dependent reduction in outward current during the falling phase of the AP of 172 ± 20 fC/pF (outward current integrated during the falling phase of the AP and normalized to each cell's capacitance) and this was reduced to 2 ± 3 fC/pF in the presence of 5 mm TEA (n = 33; p < 0.0001, two-tailed Wilcoxon test).
Figure 3.
The frequency-dependent component of AP-evoked potassium current is inhibited by 5 mm TEA and is mostly calcium independent. A, Currents evoked by the cell's own AP waveform during the 1st and 15th application of the waveform delivered at 5 Hz, first in Tyrode's solution with 1 μm TTX and 1 μm A-803467 (1st, red; 15th, blue), then in a solution with added 5 mm TEA-Cl, then in a calcium-free solution (with Mg2+ replacing Ca2+), and then in the Ca-free solution with added 5 mm TEA. All solutions contained 1 μm TTX and 1 μm A-803467. B, Collected results from 33 cells showing the change in current evoked by the 1st and the 15th stimuli before and after the addition of 5 mm TEA. Current was integrated from the peak of the AP to the afterhyperpolarization and normalized to each cells' capacitance. Connected points show data for each cell and solid symbols show mean ± SEM for each condition. C, Same for currents before and after replacing Ca2+ by Mg2+ (n = 33).
Previous work has shown that BK-calcium-activated potassium channels contribute to the repolarization of APs in small-diameter DRG neurons (Gold et al., 1996; Scholz et al., 1998; Li et al., 2007; Zhang et al., 2010, 2012; Cao et al., 2012). BK channels are sensitive to external TEA and, with some combinations of accessory subunits, can undergo inactivation (Wallner et al., 1999; Xia et al., 1999, 2000). We therefore tested whether the frequency-dependent component of potassium current was from calcium-activated potassium channels by using solutions in which calcium was replaced by magnesium. In most cells, replacing calcium with magnesium had little effect on the frequency-dependent component of potassium current (Fig. 3C). On average, the frequency-dependent component of potassium current decreased from 170 ± 20 fC/pF in calcium-containing solution to 166 ± 16 fC/pF in calcium-free solution (n = 33; p = 0.64, two-tailed Wilcoxon test). In a small fraction of cells (seven of 33), eliminating calcium resulted in a clear reduction of frequency-dependent current by >10%, suggestive of a component of frequency-dependent BK current. However, this component was always much smaller than the component of frequency-dependent current remaining in calcium-free solution. We therefore focused on this calcium-independent component in further experiments.
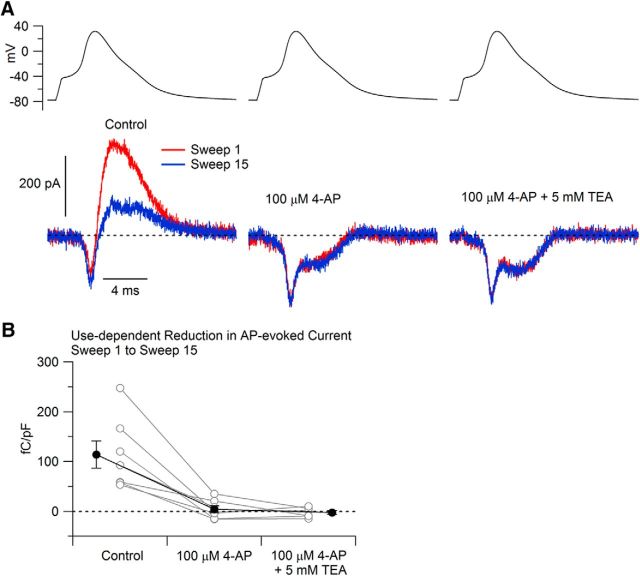
To explore what channel types underlie the calcium-independent potassium current, we next tested the effect of various inhibitors on the frequency-sensitive potassium current. We first tested its sensitivity to 4-AP. We found that 4-AP applied at 100 μm was very effective at inhibiting the frequency-dependent component of the potassium current. An example is shown in Figure 4A, in which 100 μm 4-AP completely inhibited the frequency-dependent component of potassium current and there was little effect of further addition of 5 mm TEA. In collected results, 100 μm 4-AP reduced the frequency-dependent potassium current from 114 ± 27 fC/pF to 4 ± 7 fC/pF (n = 7; p < 0.01, Wilcoxon two-tailed test).
Figure 4.
Effect of 100 μm 4-AP on frequency-dependent reduction in potassium current. A, Currents evoked by the cell's AP waveform during the 1st and 15th application of the waveform delivered at 5 Hz before (left), after (middle) applying 100 μm 4-AP, and after applying 100 μm 4-AP together with 5 mm TEA (right). Currents were recorded in a solution containing 1 μm TTX and 1 μm A-803467 to inhibit sodium current and with Mg2+ replacing Ca2+ to eliminate calcium current. B, Collected results showing the change in current evoked by the 1st and the 15th stimuli before and after 100 μm 4-AP and then 100 μm 4-AP plus 5 mm TEA. Connected points show data for each cell (n = 7 for control/4-AP, n = 6 for control/4-AP/4-AP + TEA) and solid symbols show mean ± SEM for each condition.
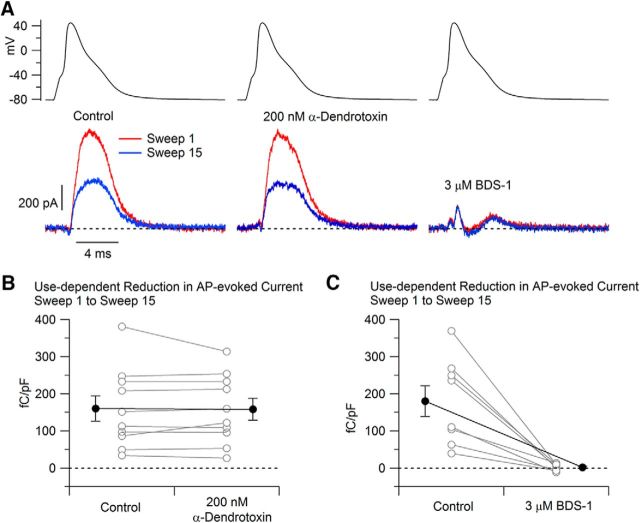
Although many types of potassium channels are inhibited by millimolar concentrations of 4-AP, only a few are effectively inhibited by submillmolar concentrations. Among the currents with high sensitivity to 4-AP are two kinds of potassium currents that can undergo inactivation, a Kv1-mediated current known as ID (Storm, 1988; Wu and Barish, 1992; Shu et al., 2007) and Kv3 channels that include the Kv3.4 subunit (Rettig et al., 1992; Diochot et al., 1998; Baranauskas et al., 2003). We therefore tested more selective inhibitors of each of these currents. ID is sensitive to the peptide inhibitor α-dendrotoxin, which inhibits the Kv1-mediated component of inactivating potassium current that underlies spike broadening in mossy fiber terminals in the hippocampus (Geiger and Jonas, 2000). However, we found that there was very little effect of α-dendrotoxin on the frequency-dependent component of current in small-diameter, capsaicin-sensitive DRG neurons (Fig. 5). On average, frequency-dependent potassium current changed from 160 ± 34 fC/pF to 158 ± 29 fC/pF with application of 200 nm α-dendrotoxin (n = 10; p = 0.67, Wilcoxon two-tailed test).
Figure 5.
Effect of α-dendrotoxin and BDS-I on frequency-dependent reduction in potassium current. A, Currents evoked by the 1st and 15th AP waveforms are shown in control (left), after the application of 200 nm α-dendrotoxin (middle), and after the application of 3 μm BDS-I (right). Currents were recorded in a solution containing 1 μm TTX and 1 μm A-803467 to inhibit sodium current and with Mg2+ replacing Ca2+ to eliminate calcium current. B, Collected results showing the change in current evoked by the 1st and 15th stimuli before and after 200 nm dendrotoxin. Connected points show data for each cell (n = 10) and solid symbols show mean ± SEM for each condition. C, Same for currents before and after application of 3 μm BDS-I to the external solution (n = 9).
We next tested the Kv3 inhibitor BDS-I (Diochot et al., 1998; Shevchenko et al., 2004; Yeung et al., 2005; Martina et al., 2007). BDS-I almost completely inhibited the frequency-dependent potassium current, reducing it from 174 ± 37 fC/pF to 1 ± 3 fC/pF (Fig. 5; n = 9, p < 0.05, Wilcoxon two-tailed test). The combination of sensitivity to low concentrations of 4-AP and BDS-I suggests that the frequency-dependent component of potassium current is carried by Kv3 channels.
AP broadening at 35°C
The kinetics of channel gating are strongly temperature dependent (Hille, 2001). The kinetics of potassium channels during the AP are expected to change with changes in temperature. We therefore did a series of experiments examining spike broadening at near-physiological temperature (35°C). We found that APs at 35°C showed frequency-dependent broadening (Fig. 6). As at room temperature, broadening was evident at frequencies as low as 1 Hz, but it took higher frequencies for a given degree of broadening at 35°C compared with room temperature. In collected results, the amount of broadening during 30 APs at 35°C was 12 ± 2% at 1 Hz (n = 16), 25 ± 3% at 5 Hz (n = 16), 38 ± 3% at 10 Hz (n = 13), and 67 ± 10% at 20 Hz (n = 11).
Figure 6.
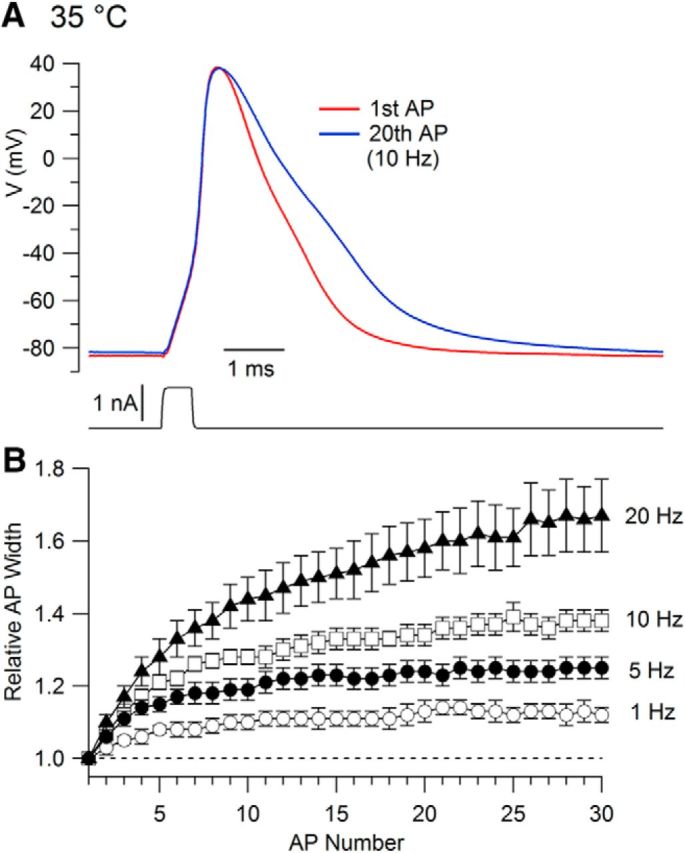
Broadening of APs during repetitive stimulation at 35°C. A, First and 15th APs evoked by 5 Hz stimulation. B, Time course of AP broadening with stimulation at different frequencies. Symbols show mean ± SEM for determinations at 1 Hz (n = 16), 5 Hz (n = 16), 10 Hz (n = 13), and 20 Hz (n = 11).
As at 22°C, the outward current evoked by APs at 35°C showed a frequency-dependent reduction. Furthermore, as at 22°C, BDS-I very effectively inhibited the frequency-dependent component of potassium current for APs at 35°C (Fig. 7), reducing it from 83 ± 18 fC/pF to 1 ± 5 fC/pF (n = 10; p = 0.0054, two-tailed Wilcoxon test). Therefore, it appears that Kv3 channels account for the inactivation of potassium channels producing frequency-dependent APs at 35°C as well as at 22°C.
Figure 7.
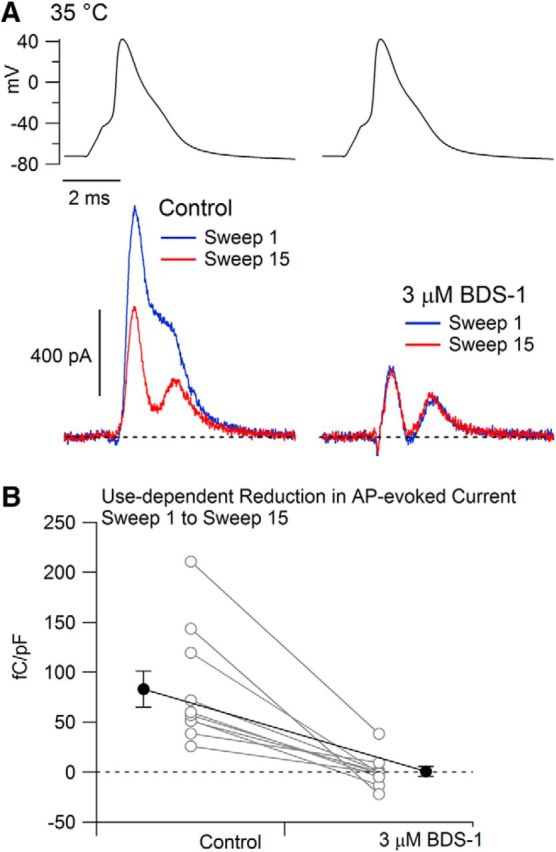
Frequency-dependent reduction in AP-evoked potassium current at 35°C is mainly from Kv3 channels. A, Current evoked by the cell's AP waveform was recorded with the waveform delivered at 5 Hz. Currents evoked by the 1st and 15th AP waveforms are shown in control (left) and after the application of 3 μm BDS-I (right) to inhibit Kv3 channels. Currents were recorded in a solution containing 1 μm TTX and 1 μm A-803467 to inhibit sodium current. B, Collected results showing the change in current evoked by the 1st and 15th stimuli before and after the addition of 3 μm BDS-I to the external solution. Connected points show data for each cell (n = 10) and solid symbols show mean ± SEM for each condition.
Discussion
These results show that, as in a number of other neuronal types, APs in small-diameter, capsaicin-sensitive DRG neurons show frequency-dependent broadening. AP clamp experiments show that the broadening results from frequency-dependent reduction of potassium current during the falling phase of the AP and that Kv3 channels account for most of the potassium current undergoing frequency-dependent reduction.
We examined AP broadening at frequencies between 1 and 20 Hz, which is within the range of firing frequencies seen in C-fiber nociceptors in response to noxious stimuli such as pinch (Chen and Levine, 2003; Djouhri et al., 2006). In fact, at the onset of mechanical stimulation, C-fiber nociceptors can fire up to 60 Hz, with firing as fast as 20 Hz sustained for several hundred milliseconds (Chen and Levine, 2003). We saw substantial broadening at 20 Hz (increase in spike width by ∼60% in the first half-second) suggesting that significant broadening likely occurs at physiological rates of firing.
Kv3 channels
The identification of Kv3 channels as the key channels underlying spike broadening fits well with recent work showing expression of both Kv3.1 subunits (Bocksteins et al., 2012) and Kv3.4 subunits Chien et al., 2007; Ritter et al., 2012; Ritter et al., 2015a) in small DRG neurons. Kv3.4 subunits confer rapid inactivation on both homomeric and heteromeric channels (Rettig et al., 1992; Diochot et al., 1998; Baranauskas et al., 2003). An inactivating component of current in small DRG neurons can be reduced using siRNA for Kv3.4 (Ritter at al., 2012) and was found to be activated early in spike repolarization (Ritter et al., 2015b), which is consistent with our results. The inactivating component of current matches the properties of a component of overall potassium current in small DRG neurons originally called IAht (Gold et al., 1996).
The key involvement of Kv3 channels in spike broadening is at first surprising. Kv3 family channels were initially associated with fast-spiking neocortical interneurons and cerebellar Purkinje neurons, both of which have narrow APs (half-amplitude width 100–500 μs) and can fire at high frequencies without reported spike broadening (Rudy et al., 1999; Rudy and McBain, 2001; Lien and Jonas, 2003; Martina et al., 2007). The reasons that inactivation of Kv3 channels produces spike broadening in DRG neurons, but apparently not in interneurons or Purkinje neurons, remain to be determined. In Purkinje neurons, with narrow APs, only a small fraction (20%) of available channels is actually activated during spike repolarization (Martina et al., 2007). With a large “buffer” of potassium current not normally activated, perhaps a small increase in AP duration can recruit a larger fraction of channels and thus limit spike broadening even if a fraction of the channels inactivate. When APs are already broad, as in small DRG neurons, available channels may be activated with a higher probability to start with (Ritter et al., 2015a) so that there is little or no buffer to compensate for reduction of available channels by inactivation. It is also possible that the kinetics of activation or inactivation of Kv3 channels are different in DRG neurons and fast-spiking neurons. Further voltage-clamp experiments will be needed to explore these possibilities.
BK channels
In most cells, the component of potassium current undergoing frequency-dependent reduction was little affected by removal of calcium. However, there was significant reduction by >10% in a minority of neurons (seven of 33). It is plausible that this represents a component of inactivating BK current. There is expression of BK current in small DRG neurons (Zhang et al., 2003; Zhang et al., 2010; Hendrich et al., 2012; cf. Cao et al., 2012) and, in some small DRG neurons, blocking BK current produces broadening of APs (Scholz et al., 1998; Zhang et al., 2003; Li et al., 2007; Zhang et al., 2010; Cao et al., 2012). BK channels can undergo inactivation when β2 and β3 subunits are expressed (Wallner et al., 1999; Xia et al., 1999, 2000) and both are found to be present in DRG neurons by single-cell PCR (Zhang et al., 2012). However, the contribution of BK channel inactivation to overall frequency-dependent reduction of potassium current is clearly small relative to that of Kv3 current and is unlikely to contribute significantly to spike broadening in most neurons.
Kv1 channels
In voltage-clamp experiments using voltage steps, the overall potassium current in small DRG neurons includes a component of α-dendrotoxin-sensitive Kv1 current in both mice (Beekwilder et al., 2003; Bocksteins et al., 2012) and rats (Yang et al., 2004; Gruss et al., 2006; Chi and Nicol, 2007; Sculptoreanu et al., 2009), However, dendrotoxin has little effect on AP duration (Chi and Nicol, 2007). Consistent with this, in our experiments, α-dendrotoxin inhibited only a very small component of overall potassium current evoked by AP waveforms and had very little effect on the potassium current undergoing frequency-dependent reduction during repetitive firing. The simplest interpretation is that, although small DRG neurons express Kv1 channels, they likely activate too slowly to be activated much during APs compared with fast-activating Kv3 channels.
Comparison with other neurons
Spike broadening from Kv3 channel inactivation has not been described before and may be unusual. In hippocampal mossy fiber boutons, Kv3 channels contribute ∼60% of the AP-evoked potassium current (Alle et al., 2011), but spike broadening is ascribed to inactivation of Kv1-mediated current (Geiger and Jonas, 2000). In hypothalamic supraoptic neurons, Kv3 channels help to mediate spike repolarization (Shevchenko et al., 2004), but spike broadening is attributed to inactivation of other potassium channels (Hlubek and Cobbett, 2000) and facilitation of calcium currents (O'Regan and Cobbett, 1993).
Spike broadening in DRG neurons was substantial at much lower frequencies than required in most other neuronal types. For example, in CA1 pyramidal neurons, frequencies >20 Hz are needed to produce spike broadening (Shao et al., 1999), similar to amygdala neurons, in which BK channels also mediate broadening (Faber and Sah, 2003). In contrast, in capsaicin-sensitive DRG neurons, spike broadening was evident at 1 Hz and very dramatic at 20 Hz (67 ± 10% at 35°C). The rapid onset of spike broadening (by the second spike) fits with the fast onset of inactivation of Kv3.4-containing channels and its occurrence at frequencies as low as 1 Hz fits with the slow recovery of inactivation, which takes several seconds at room temperature (Rettig et al., 1992; Baranauskas et al., 2003).
Functional significance of spike broadening in small DRG neurons
Immunocytochemistry shows the expression of Kv3.4 channels in axons and synaptic boutons of C-fibers and in cell bodies (Chien et al., 2007), making it plausible that AP broadening could occur in axons and boutons as well. At boutons, spike broadening would result in increased calcium entry (Jackson et al., 1991) and transmitter release (Geiger and Jonas, 2000). In axons, other things being equal, a broader AP should promote faster conduction with a larger safety factor because the longer depolarization prolongs the passive electrotonic depolarization of the downstream axon that initiates active recruitment of sodium channels. This might explain why APs of small DRG neurons associated with small-diameter, slowly conducting C-fiber axons are notable for being unusually broad to begin with (Harper and Lawson, 1985; Koerber et al., 1988; Djouhri et al., 1998). Frequency-dependent spike broadening could then help to mitigate activity-dependent slowing of conduction velocity and spike failure resulting from slow inactivation of sodium channels (De Col et al., 2008, 2012) and accumulation of intraaxonal sodium (Tigerholm et al., 2014). Currently, however, our mechanistic understanding of AP propagation in C-fiber axons is necessarily based mainly on computer modeling (Gemes et al., 2013; Petersson et al., 2014; Tigerholm et al., 2014; Sundt et al., 2015). Further data on the nature and kinetics of the potassium currents flowing during the AP should allow such modeling to be increasingly realistic and lead to new insights about how the many types of ion channels in C-fiber neurons combine to regulate excitability during natural firing patterns.
Footnotes
Author contributions: P.W.L., N.T.B., and B.P.B. designed research; P.W.L. and N.T.B. performed research; P.W.L., N.T.B., and B.P.B. analyzed data; P.W.L., N.T.B., and B.P.B. wrote the paper.
This work was supported by the National Institutes of Health (Grant NS036855).
The authors declare no competing financial interests.
References
- Abdulla FA, Smith PA (2001) Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. J Neurophysiol 85:644–658. [DOI] [PubMed] [Google Scholar]
- Aldrich RW Jr, Getting PA, Thompson SH (1979) Mechanism of frequency-dependent broadening of molluscan neurone soma spikes. J Physiol 291:531–544. 10.1113/jphysiol.1979.sp012829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alle H, Kubota H, Geiger JR (2011) Sparse but highly efficient Kv3 outpace BKCa channels in action potential repolarization at hippocampal mossy fiber boutons. J Neurosci 31:8001–8012. 10.1523/JNEUROSCI.0972-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranauskas G, Tkatch T, Nagata K, Yeh JZ, Surmeier DJ (2003) Kv3.4 subunits enhance the repolarizing efficiency of Kv3.1 channels in fast-spiking neurons. Nat Neurosci 6:258–266. 10.1038/nn1019 [DOI] [PubMed] [Google Scholar]
- Beekwilder JP, O'Leary ME, van den Broek LP, van Kempen GT, Ypey DL, van den Berg RJ (2003) Kv1.1 channels of dorsal root ganglion neurons are inhibited by n-butyl-p-aminobenzoate, a promising anesthetic for the treatment of chronic pain. J Pharmacol Exp Ther 304:531–538. 10.1124/jpet.102.042135 [DOI] [PubMed] [Google Scholar]
- Blair NT, Bean BP (2002) Roles of tetrodotoxin (TTX)-sensitive na+ current, TTX-resistant na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J Neurosci 22:10277–10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair NT, Bean BP (2003) Role of tetrodotoxin-resistant na+ current slow inactivation in adaptation of action potential firing in small-diameter dorsal root ganglion neurons. J Neurosci 23:10338–10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocksteins E, Van de Vijver G, Van Bogaert PP, Snyders DJ (2012) Kv3 channels contribute to the delayed rectifier current in small cultured mouse dorsal root ganglion neurons. Am J Physiol Cell Physiol 303:C406–C415. 10.1152/ajpcell.00343.2011 [DOI] [PubMed] [Google Scholar]
- Cao XH, Chen SR, Li L, Pan HL (2012) Nerve injury increases brain-derived neurotrophic factor levels to suppress BK channel activity in primary sensory neurons. J Neurochem 121:944–953. 10.1111/j.1471-4159.2012.07736.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Levine JD (2003) Altered temporal pattern of mechanically evoked C-fiber activity in a model of diabetic neuropathy in the rat. Neuroscience 121:1007–1015. 10.1016/S0306-4522(03)00486-X [DOI] [PubMed] [Google Scholar]
- Chi XX, Nicol GD (2007) Manipulation of the potassium channel Kv1.1 and its effect on neuronal excitability in rat sensory neurons. J Neurophysiol 98:2683–2692. 10.1152/jn.00437.2007 [DOI] [PubMed] [Google Scholar]
- Chien LY, Cheng JK, Chu D, Cheng CF, Tsaur ML (2007) Reduced expression of A-type potassium channels in primary sensory neurons induces mechanical hypersensitivity. J Neurosci 27:9855–9865. 10.1523/JNEUROSCI.0604-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JM, Chung K (2002) Importance of hyperexcitability of DRG neurons in neuropathic pain. Pain Pract 2:87–97. 10.1046/j.1533-2500.2002.02011.x [DOI] [PubMed] [Google Scholar]
- De Col R, Messlinger K, Carr RW (2008) Conduction velocity is regulated by sodium channel inactivation in unmyelinated axons innervating the rat cranial meninges. J Physiol 586:1089–1103. 10.1113/jphysiol.2007.145383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Col R, Messlinger K, Carr RW (2012) Repetitive activity slows axonal conduction velocity and concomitantly increases mechanical activation threshold in single axons of the rat cranial dura. J Physiol 590:725–736. 10.1113/jphysiol.2011.220624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haas V, Vogel W (1989) Sodium and potassium currents recorded during an action potential. Eur Biophys J 17:49–51. 10.1007/BF00257145 [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Black JA, Waxman SG (2009) Voltage-gated sodium channels: therapeutic targets for pain. Pain Med 10:1260–1269. 10.1111/j.1526-4637.2009.00719.x [DOI] [PubMed] [Google Scholar]
- Diochot S, Schweitz H, Béress L, Lazdunski M (1998) Sea anemone peptides with a specific blocking activity against the fast inactivating potassium channel Kv3.4. J Biol Chem 273:6744–6749. 10.1074/jbc.273.12.6744 [DOI] [PubMed] [Google Scholar]
- Djouhri L, Bleazard L, Lawson SN (1998) Association of somatic action potential shape with sensory receptive properties in guinea-pig dorsal root ganglion neurones. J Physiol 513:857–872. 10.1111/j.1469-7793.1998.857ba.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN (2006) Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci 26:1281–1292. 10.1523/JNEUROSCI.3388-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everill B, Rizzo MA, Kocsis JD (1998) Morphologically identified cutaneous afferent DRG neurons express three different potassium currents in varying proportions. J Neurophysiol 79:1814–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Sah P (2003) Ca2+-activated K+ (BK) channel inactivation contributes to spike broadening during repetitive firing in the rat lateral amygdala. J Physiol 552:483–497. 10.1113/jphysiol.2003.050120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JR, Jonas P (2000) Dynamic control of presynaptic ca(2+) inflow by fast-inactivating K(+) channels in hippocampal mossy fiber boutons. Neuron 28:927–939. 10.1016/S0896-6273(00)00164-1 [DOI] [PubMed] [Google Scholar]
- Gemes G, Koopmeiners A, Rigaud M, Lirk P, Sapunar D, Bangaru ML, Vilceanu D, Garrison SR, Ljubkovic M, Mueller SJ, Stucky CL, Hogan QH (2013) Failure of action potential propagation in sensory neurons: Mechanisms and loss of afferent filtering in C-type units after painful nerve injury. J Physiol 591:1111–1131. 10.1113/jphysiol.2012.242750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Shuster MJ, Levine JD (1996) Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J Neurophysiol 75:2629–2646. [DOI] [PubMed] [Google Scholar]
- Gruss M, Ettorre G, Stehr AJ, Henrich M, Hempelmann G, Scholz A (2006) Moderate hypoxia influences excitability and blocks dendrotoxin sensitive K+ currents in rat primary sensory neurones. Mol Pain 2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AA, Lawson SN (1985) Electrical properties of rat dorsal root ganglion neurones with different peripheral nerve conduction velocities. J Physiol 359:47–63. 10.1113/jphysiol.1985.sp015574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrich J, Alvarez P, Chen X, Levine JD (2012) GDNF induces mechanical hyperalgesia in muscle by reducing I(BK) in isolectin B4-positive nociceptors. Neuroscience 219:204–213. 10.1016/j.neuroscience.2012.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. (2001) Ion channels of excitable membranes, Ed 3 Sunderland, MA: Sinauer. [Google Scholar]
- Hlubek MD, Cobbett P (2000) Differential effects of K(+) channel blockers on frequency-dependent action potential broadening in supraoptic neurons. Brain Res Bull 53:203–209. 10.1016/S0361-9230(00)00335-X [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Tanaka M, Black JA, Waxman SG (1999) Changes in expression of voltage-gated potassium channels in dorsal root ganglion neurons following axotomy. Muscle Nerve 22:502–507. 10.1002/(SICI)1097-4598(199904)22:4%3C502::AID-MUS12%3E3.0.CO%3B2-K [DOI] [PubMed] [Google Scholar]
- Jackson MB, Konnerth A, Augustine GJ (1991) Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci U S A 88:380–384. 10.1073/pnas.88.2.380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, et al. (2007) A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci U S A 104:8520–8855. 10.1073/pnas.0611364104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz EJ, Gold MS (2006) Inflammatory hyperalgesia: A role for the C-fiber sensory neuron cell body? J Pain 7:170–178. [DOI] [PubMed] [Google Scholar]
- Kim J, Wei DS, Hoffman DA (2005) Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones. J Physiol 569:41–57. 10.1113/jphysiol.2005.095042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick K, Bourque CW (1991) Dual role for calcium in the control of spike duration in rat supraoptic neuroendocrine cells. Neurosci Lett 133:271–274. 10.1016/0304-3940(91)90586-I [DOI] [PubMed] [Google Scholar]
- Koerber HR, Druzinsky RE, Mendell LM (1988) Properties of somata of spinal dorsal root ganglion cells differ according to peripheral receptor innervated. J Neurophysiol 60:1584–1596. [DOI] [PubMed] [Google Scholar]
- Li W, Gao SB, Lv CX, Wu Y, Guo ZH, Ding JP, Xu T (2007) Characterization of voltage-and Ca2+-activated K+ channels in rat dorsal root ganglion neurons. J Cell Physiol 212:348–357. 10.1002/jcp.21007 [DOI] [PubMed] [Google Scholar]
- Lien CC, Jonas P (2003) Kv3 potassium conductance is necessary and kinetically optimized for high-frequency action potential generation in hippocampal interneurons. J Neurosci 23:2058–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CN, Michaelis M, Amir R, Devor M (2000) Spinal nerve injury enhances subthreshold membrane potential oscillations in DRG neurons: Relation to neuropathic pain. J Neurophysiol 84:205–215. [DOI] [PubMed] [Google Scholar]
- Liu PW, Bean BP (2014) Kv2 channel regulation of action potential repolarization and firing patterns in superior cervical ganglion neurons and hippocampal CA1 pyramidal neurons. J Neurosci 34:4991–5002. 10.1523/JNEUROSCI.1925-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Sugimori M, Simon SM (1982) Transmission by presynaptic spike-like depolarization in the squid giant synapse. Proc Natl Acad Sci U S A 79:2415–2419. 10.1073/pnas.79.7.2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Koester J (1996) The role of K+ currents in frequency-dependent spike broadening in aplysia R20 neurons: a dynamic-clamp analysis. J Neurosci 16:4089–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina M, Metz AE, Bean BP (2007) Voltage-dependent potassium currents during fast spikes of rat cerebellar purkinje neurons: Inhibition by BDS-I toxin. J Neurophysiol 97:563–571. 10.1152/jn.00269.2006 [DOI] [PubMed] [Google Scholar]
- Martinez-Espinosa PL, Wu J, Yang C, Gonzalez-Perez V, Zhou H, Liang H, Xia XM, Lingle CJ (2015) Knockout of Slo2.2 enhances itch, abolishes KNa current, and increases action potential firing frequency in DRG neurons. Elife 4: pii: e10013. 10.7554/eLife.10013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCobb DP, Beam KG (1991) Action potential waveform voltage-clamp commands reveal striking differences in calcium entry via low and high voltage-activated calcium channels. Neuron 7:119–127. 10.1016/0896-6273(91)90080-J [DOI] [PubMed] [Google Scholar]
- Neher E. (1992) Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol 207:123–131. 10.1016/0076-6879(92)07008-C [DOI] [PubMed] [Google Scholar]
- Nicol GD, Vasko MR, Evans AR (1997) Prostaglandins suppress an outward potassium current in embryonic rat sensory neurons. J Neurophysiol 77:167–176. [DOI] [PubMed] [Google Scholar]
- Nuwer MO, Picchione KE, Bhattacharjee A (2010) PKA-induced internalization of slack KNa channels produces dorsal root ganglion neuron hyperexcitability. J Neurosci 30:14165–14172. 10.1523/JNEUROSCI.3150-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Regan MH, Cobbett P (1993) Somatic currents contribute to frequency-dependent spike-broadening in supraoptic neuroendocrine cells. Neurosci Lett 161:169–173. 10.1016/0304-3940(93)90286-T [DOI] [PubMed] [Google Scholar]
- Park D, Dunlap K (1998) Dynamic regulation of calcium influx by G-proteins, action potential waveform, and neuronal firing frequency. J Neurosci 18:6757–6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak D, Guan D, Foehring RC (2016) Roles of specific Kv channel types in repolarization of the action potential in genetically identified subclasses of pyramidal neurons in mouse neocortex. J Neurophysiol 115:2317–2329. 10.1152/jn.01028.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson ME, Obreja O, Lampert A, Carr RW, Schmelz M, Fransén E (2014) Differential axonal conduction patterns of mechano-sensitive and mechano-insensitive nociceptors—a combined experimental and modelling study. PLoS One 9:e103556. 10.1371/journal.pone.0103556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS (2001) Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci U S A 98:13373–13378. 10.1073/pnas.231376298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renganathan M, Cummins TR, Waxman SG (2001) Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol 86:629–640. [DOI] [PubMed] [Google Scholar]
- Rettig J, Wunder F, Stocker M, Lichtinghagen R, Mastiaux F, Beckh S, Kues W, Pedarzani P, Schröter KH, Ruppersberg JP (1992) Characterization of a shaw-related potassium channel family in rat brain. EMBO J 11:2473–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter AM, Mendell LM (1992) Somal membrane properties of physiologically identified sensory neurons in the rat: effects of nerve growth factor. J Neurophysiol 68:2033–2041. [DOI] [PubMed] [Google Scholar]
- Ritter DM, Ho C, O'Leary ME, Covarrubias M (2012) Modulation of Kv3.4 channel N-type inactivation by protein kinase C shapes the action potential in dorsal root ganglion neurons. J Physiol 590:145–161. 10.1113/jphysiol.2011.218560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter DM, Zemel BM, Lepore AC, Covarrubias M (2015a) Kv3.4 channel function and dysfunction in nociceptors. Channels 9:209–217. 10.1080/19336950.2015.1056949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter DM, Zemel BM, Hala TJ, O'Leary ME, Lepore AC, Covarrubias M (2015b) Dysregulation of Kv3.4 channels in dorsal root ganglia following spinal cord injury. J Neurosci 35:1260–1273. 10.1523/JNEUROSCI.1594-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rola R, Witkowski G, Szulczyk PJ (2003) Voltage-dependent K+ currents in rat cardiac dorsal root ganglion neurons. Neuroscience 119:181–191. 10.1016/S0306-4522(03)00124-6 [DOI] [PubMed] [Google Scholar]
- Rudy B, McBain CJ (2001) Kv3 channels: Voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci 24:517–526. 10.1016/S0166-2236(00)01892-0 [DOI] [PubMed] [Google Scholar]
- Rudy B, Chow A, Lau D, Amarillo Y, Ozaita A, Saganich M, Moreno H, Nadal MS, Hernandez-Pineda R, Hernandez-Cruz A, Erisir A, Leonard C, Vega-Saenz de Miera E (1999) Contributions of Kv3 channels to neuronal excitability. Ann N Y Acad Sci 868:304–343. 10.1111/j.1749-6632.1999.tb11295.x [DOI] [PubMed] [Google Scholar]
- Rush AM, Cummins TR, Waxman SG (2007) Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol 579:1–14. 10.1113/jphysiol.2006.121483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronov BV, Bischoff U, Vogel W (1996) Single voltage-gated K+ channels and their functions in small dorsal root ganglion neurones of rat. J Physiol 493:393–408. 10.1113/jphysiol.1996.sp021391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz A, Gruss M, Vogel W (1998) Properties and functions of calcium-activated K+ channels in small neurones of rat dorsal root ganglion studied in a thin slice preparation. J Physiol 513:55–69. 10.1111/j.1469-7793.1998.055by.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scroggs RS, Fox AP (1992) Multiple Ca2+ currents elicited by action potential waveforms in acutely isolated adult rat dorsal root ganglion neurons. J Neurosci 12:1789–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculptoreanu A, Artim DE, de Groat WC (2009) Neurokinins inhibit low threshold inactivating K+ currents in capsaicin responsive DRG neurons. Exp Neurol 219:562–573. 10.1016/j.expneurol.2009.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao LR, Halvorsrud R, Borg-Graham L, Storm JF (1999) The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol 521:135–146. 10.1111/j.1469-7793.1999.00135.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko T, Teruyama R, Armstrong WE (2004) High-threshold, Kv3-like potassium currents in magnocellular neurosecretory neurons and their role in spike repolarization. J Neurophysiol 92:3043–3055. 10.1152/jn.00431.2004 [DOI] [PubMed] [Google Scholar]
- Shu Y, Yu Y, Yang J, McCormick DA (2007) Selective control of cortical axonal spikes by a slowly inactivating K+ current. Proc Natl Acad Sci U S A 104:11453–11458. 10.1073/pnas.0702041104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonner PM, Filosa JA, Stern JE (2008) Diminished A-type potassium current and altered firing properties in presympathetic PVN neurones in renovascular hypertensive rats. J Physiol 586:1605–1622. 10.1113/jphysiol.2007.147413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. (1988) Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature 336:379–381. 10.1038/336379a0 [DOI] [PubMed] [Google Scholar]
- Sundt D, Gamper N, Jaffe DB (2015) Spike propagation through the dorsal root ganglia in an unmyelinated sensory neuron: a modeling study. J Neurophysiol 114:3140–3153. 10.1152/jn.00226.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigerholm J, Petersson ME, Obreja O, Lampert A, Carr R, Schmelz M, Fransén E (2014) Modeling activity-dependent changes of axonal spike conduction in primary afferent C-nociceptors. J Neurophysiol 111:1721–1735. 10.1152/jn.00777.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn AH, Gold MS (2010) Ionic mechanisms underlying inflammatory mediator-induced sensitization of dural afferents. J Neurosci 30:7878–7888. 10.1523/JNEUROSCI.6053-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Meera P, Toro L (1999) Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane beta-subunit homolog. Proc Natl Acad Sci U S A 96:4137–4142. 10.1073/pnas.96.7.4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RL, Barish ME (1992) Two pharmacologically and kinetically distinct transient potassium currents in cultured embryonic mouse hippocampal neurons. J Neurosci 12:2235–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Ding JP, Lingle CJ (1999) Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J Neurosci 19:5255–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Ding JP, Zeng XH, Duan KL, Lingle CJ (2000) Rectification and rapid activation at low Ca2+ of Ca2+-activated, voltage-dependent BK currents: consequences of rapid inactivation by a novel beta subunit. J Neurosci 20:4890–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EK, Takimoto K, Hayashi Y, de Groat WC, Yoshimura N (2004) Altered expression of potassium channel subunit mRNA and alpha-dendrotoxin sensitivity of potassium currents in rat dorsal root ganglion neurons after axotomy. Neuroscience 123:867–874. 10.1016/j.neuroscience.2003.11.014 [DOI] [PubMed] [Google Scholar]
- Yeung SY, Thompson D, Wang Z, Fedida D, Robertson B (2005) Modulation of Kv3 subfamily potassium currents by the sea anemone toxin BDS: significance for CNS and biophysical studies. J Neurosci 25:8735–8745. 10.1523/JNEUROSCI.2119-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamponi GW, Lewis RJ, Todorovic SM, Arneric SP, Snutch TP (2009) Role of voltage-gated calcium channels in ascending pain pathways. Brain Res Rev 60:84–89. 10.1016/j.brainresrev.2008.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Gopalakrishnan M, Shieh CC (2003) Modulation of action potential firing by iberiotoxin and NS1619 in rat dorsal root ganglion neurons. Neuroscience 122:1003–1011. 10.1016/j.neuroscience.2003.08.035 [DOI] [PubMed] [Google Scholar]
- Zhang XL, Mok LP, Katz EJ, Gold MS (2010) BKCa currents are enriched in a subpopulation of adult rat cutaneous nociceptive dorsal root ganglion neurons. Eur J Neurosci 31:450–462. 10.1111/j.1460-9568.2009.07060.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XL, Mok LP, Lee KY, Charbonnet M, Gold MS (2012) Inflammation-induced changes in BK(ca) currents in cutaneous dorsal root ganglion neurons from the adult rat. Mol Pain 8:37-8069-8-37. 10.1186/1744-8069-8-37 [DOI] [PMC free article] [PubMed] [Google Scholar]