Abstract
The evaluation of physical functioning is valuable in the intensive care unit (ICU) to help inform patient recovery after critical illness, to identify patients who may require rehabilitation interventions, and to monitor responsiveness to such interventions. This viewpoint article discusses: (1) the concept of physical functioning with reference to the World Health Organization International Classification of Functioning, Disability and Health; (2) the importance of measuring physical functioning in the ICU; and (3) methods for evaluating physical functioning in the ICU. Recommendations for clinical practice and research are made, along with discussion of future directions.
Electronic supplementary material
The online version of this article (doi:10.1186/s13054-017-1827-6) contains supplementary material, which is available to authorized users.
Keywords: Critical illness, Physical function, Outcome measurement, Early mobility, Physical rehabilitation
Introduction
Improving the survivorship experience of patients is a defining challenge for modern critical care medicine due to improving mortality and increasing awareness of patient morbidity [1–3]. Intensive care unit (ICU) survivors with multi-organ failure are particularly susceptible to physical morbidity, with up to 30% muscle loss within the first 10 days of ICU admission [4, 5]. The prevalence of ICU-acquired weakness is 25–40% in patients ventilated for ≥ 48 h [6–8] and even higher in patients with sepsis or a prolonged ICU length of stay (LOS) [9–11]. Importantly, weakness and physical functioning are predictive of subsequent LOS, post-discharge survival, healthcare utilization, quality of life (QOL), and return to home [12–14]. The evaluation of physical functioning in the ICU is needed to help inform patient recovery, identify patients who may require rehabilitation interventions, and monitor intervention responsiveness.
Physical functioning in the context of the International Classification of Functioning (ICF) framework
The World Health Organization (WHO) ICF framework defines functioning as an umbrella term for the interaction between three distinct constructs: body function and structure (physiological and anatomical structure of the body systems), activities (execution of a specific task within a standardized environment), and participation (involvement in everyday life situations) [15]. The ICF framework explicitly recognizes that functioning is affected by the interplay between an individual’s health condition and contextual factors, which may include personal (e.g., education) and environmental/social (e.g., home set-up, family support) factors [15].
Using this framework, physical functioning can be evaluated across the three ICF constructs. First, functioning can be evaluated in terms of physiological impairment at the level of individual organs or body systems (i.e., the “body function” level of assessment of the ICF) [15], with a specific focus on the neurological, cardiac, respiratory, and musculoskeletal systems. Second, functioning can be evaluated in terms of performance-based measurement focused on limitations in specific activities, such as sitting, standing, or walking [15]. Third, evaluation can include assessment of participation restrictions, such as the ability to perform activities of daily living (ADLs). These perspectives evaluate distinct aspects of physical functioning and thus structure and function impairment (e.g., muscle weakness) does not necessarily strongly correlate with activity limitations (e.g., 6-min walk test) and participation restrictions (e.g., ADLs) [13, 16, 17].
Importance of measuring physical functioning in the ICU
While post-ICU impairments in physical functioning are common, our understanding of the specific subgroups of patients at highest risk for such impairments, and with the greatest potential benefit from rehabilitation interventions, is evolving. Measuring physical functioning early and longitudinally in the ICU is important to identify patients at risk of poor physical outcomes, monitor intervention efficacy, and inform recovery trajectories [12, 18, 19].
Pre-ICU factors, such as age, comorbidities, and pre-ICU trajectories for muscle mass and physical functioning, impact on the physical functioning of patients in the ICU (Fig. 1). In addition, there are many factors related to critical illness and the ICU environment that can impact on impairment in physiological body systems that are critical to the physical functioning of patients in the ICU (Fig. 1).
Fig. 1.
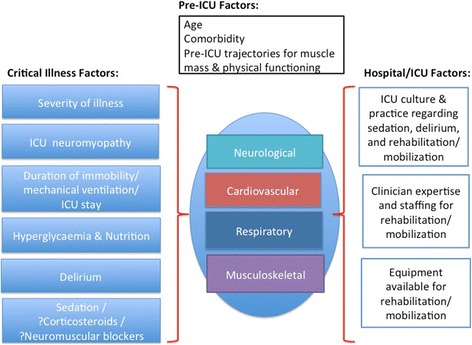
Impact of pre-ICU, critical illness and hospital/ICU factors on body systems related to physical functioning. Pre-ICU, critical illness, environmental factors, and body-system impairments, have interdependent effects on physical functioning (e.g., ICU culture regarding sedation may lead to neurological impairment resulting in immobility and musculoskeletal impairment)
Major considerations in choosing an instrument
In this next section we discuss four major considerations when selecting an instrument (Fig. 2) and synthesise current evidence (Table 1; Additional file 1: Table S1).
Fig. 2.
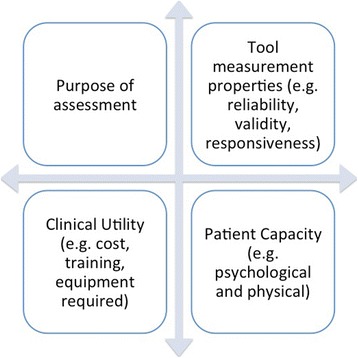
Factors to consider when selecting an outcome measure
Table 1.
Summary of measurement properties of physical functioning instruments for the ICU
| Instrument name (range for score) | Evidence of reliability? | Evidence of validity? | Evidence of predictive validity? | Evidence of responsiveness? | Evidence for MID? | Evaluation of floor and ceiling effects?# |
|---|---|---|---|---|---|---|
| ACIF (0–1) | Yes | Construct validity: Yes | Yes: for discharge to home | No | No | Low floor and ceiling in ICU |
| CPAx (0–50) | Yes | Content validity: Yes Construct validity: Yes |
Yes: for discharge to home | Yesa | Yesa | High floor at ICU admission; Low floor and ceiling at ICU and hospital dischargea |
| CcFROM (0–63) | Yes | Face/content validity: Yes | No | No | No | Low floor and ceiling in ICU |
| DEMMI (0–100) | Yes | Convergent validity: Yes Divergent validity: Yes |
No | No | No | Low floor and ceiling in ICU |
| FSS-ICU (0–35) | Yes | Construct validity: Yes Discriminant validity: Yes Known groups validity: Yes |
Yes: for discharge to home and post-ICU hospital LOSb | Yes | Yes | Low floor and ceiling at awakening and ICU discharge, high ceiling at hospital discharge |
| IMS (0–10) | Yes | Construct validity: Yes Divergent validity: Yes |
Yes: for discharge to home and 90-day survivalb | Yes | No | High floor at ICU admission; Low floor and ceiling at ICU awakening and ICU discharge |
| MMS (0–7) | Yes | Construct validity: Yes | Yes: for post-ICU hospital LOS | No | No | High floor during ICU stay |
| Perme (0–32) | Yes | Construct validity: Yes | No | No | No | High floor during ICU stay |
| PFIT-s (0–10) | Yes | Construct validity: Yes Divergent validity: Yes |
Yes: for discharge to home, post-ICU hospital LOS; Not predictive of 28-day and 12-month mortalityc | Yes | Yes | High floor at ICU admission; Low floor and ceiling at awakening and ICU discharge |
| SOMS (0–4) | Yes | Construct validity: Yes Divergent validity: Yes |
Yes: for ICU and hospital LOS, and in-hospital mortalityd | No | No | Low floor and ceiling at ICU admission |
| SPPB (0–12) | No | Construct validity: Yes Divergent validity: Yes |
Not predictive of discharge to homeb | Yes | Yes | High floor at awakening and ICU discharge |
#A low floor and ceiling effect is necessary. A low floor/ceiling effect was defined as <15%, and high floor/ceiling effect as >15% at any time point [26]
aThe MID has only been reported within the burns population for the CPAx; floor and ceiling effects have mainly been reported for the burns population. At ICU discharge the floor and ceiling effect was 13% and 0% in the burns population versus a floor and ceiling effect of 3% and 1% in a general ICU population
bPredictive validity for FSS-ICU, IMS, and SPPB were evaluated from ICU discharge physical functioning scores
cPredictive validity for PFIT-s were evaluated from ICU admission (scores evaluated a median of 6 days (range 5–9 days) after admission for all patient outcomes except discharge to home which has been evaluated across three time points: ICU admission, ICU awakening, and ICU discharge)
dPredictive validity for SOMS was evaluated from baseline ICU admission scores
ACIF Acute Care Index of Function, CPAx Chelsea Critical Care Physical Assessment Tool, CcFROM Critical Care Functional Rehabilitation Outcome Measure, DEMMI De Morton Mobility Index, FSS-ICU Functional Status Score for the ICU, ICU intensive care unit, IMS ICU mobility scale, LOS length of stay, MID minimal important difference, MMS, Perme Perme ICU Mobility Score, PFIT-s Physical Function in intensive care test scored, SOMS Surgical Optimal Mobility Scale, SPPB Short Physical Performance Battery, MMS Manchester Mobility Score
Purpose of assessment
The evaluation of physical functioning is complex and is influenced by multiple interacting factors, including strength, range of motion, proprioception, balance, cognition, and psychological issues (e.g., motivation) [20]. There are also unique patient and environmental factors (e.g., sedation, severity of illness, medical devices) specific to the ICU. Determining the specific purpose for assessing physical functioning is important when selecting an appropriate instrument. For example, if the purpose is to evaluate intervention efficacy, users should consider the specific effect of the intervention and match it with an instrument that evaluates that effect. Table 3 highlights that there are important differences when each physical function instrument is mapped to the relevant subdomains of the ICF framework. For example, the Chelsea Critical Care Physical Assessment Tool assesses both respiratory and mobility ICF subdomains; the ICU Mobility Scale only evaluates mobility subdomains; and the Physical Functional in ICU Test-scored is a composite measure of mobility, strength, and endurance. Hence, if the primary aim of an intervention is to improve patient mobility via increased muscle strength, it may be most appropriate to use a composite instrument which evaluates mobility and strength (e.g., Physical Functional in ICU Test-scored or Chelsea Critical Care Physical Assessment Tool) or separate instruments individually focused on strength and mobility (e.g., dynamometry, plus ICU Mobility Scale or Functional Status Score for the ICU). Whilst domains such as climbing and jumping, which are evaluated within the Acute Care Index of Function and the Critical Care Functional Rehabilitation Outcome Measure, are less relevant during an ICU admission, they are relevant later in the recovery process. Assessment of ‘climbing’ or stair walking ability is often a critical consideration in evaluating a patient’s safety for discharge to home. Currently there is not a single measure available that can be utilized across the entire recovery trajectory. Therefore, consideration of the elements evaluated under the subdomains of the ICF framework is important when selecting the relevant instrument based on the assessment purpose.
Table 3.
Mapping of outcome measures against ICF framework
| FSS-ICU | PFIT-s | IMS | CPAx | ACIF# | ccFROM | DEMMI | SOMS | SPPB | MMS | Perme# | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Body functions | |||||||||||
| B4. Functions of cardiovascular and respiratory systems | |||||||||||
| Respiratory functions, other specified [b4408] | X | ||||||||||
| Additional respiratory functions [b450] | X | ||||||||||
| General physical endurance [b4550] | X | X | |||||||||
| B7. Neuromuscular and movement-related functions | |||||||||||
| Mobility of joint functions [b710] | X | ||||||||||
| Power of isolated muscles and muscle groups [b7300] | X | X | X | X | X | ||||||
| Power of muscle of one limb [b7301] | |||||||||||
| Activities and participation | |||||||||||
| D4. Mobility | |||||||||||
| Lying down [d4100] | X | X | X | X | X | X | X | X | X | ||
| Sitting [d4103] | X | X | X | X | X | X | X | X | X | X | |
| Standing [d4104] | X | X | X | ||||||||
| Maintaining a lying position [d4150] | X | X | |||||||||
| Maintaining a sitting position [d4153] | X | X | X | X | X | X | X | X | |||
| Maintaining a standing position [d4154] | X | X | X | X | X | X | X | X | |||
| Transferring one-self while sitting [d4200] | X | X | X | X | X | X | |||||
| Fine hand use (picking up) [d4400] | X | ||||||||||
| Jumping [d4553] | X | ||||||||||
| Walking short distances [d4500] | X | X | X | X | X | X | X | X | X | ||
| Walking, other specified [d4508] | X1 | X2 | X3 | ||||||||
| Climbing [d4551] | X | ||||||||||
| Moving around using equipment [d465] | X | ||||||||||
In the development of this table the World Health Organization International Classification of Functioning linkages were used from http://apps.who.int/classifications/icfbrowser/, accessed May 2016. The three most relevant domains identified were: B4—Functions of cardiovascular and respiratory system; B7—Neuromuscular and Movement-Related Functions; and D4—Mobility. The final subdomain classification is identified in the first column including coding (e.g., power of isolated muscles and muscle groups is coded b7300 in the ICF browser). Subdomains under D4—Mobility of the ICF framework not considered by these functional measures include: squatting [d4101], kneeling [d4102], bending [d4106], shifting the body’s center of gravity [d4106], maintaining a squatting or kneeling position [d4151 and d4152], transferring one-self while lying [d4201], lifting and carrying objects [d430], moving objects with lower extremities [d435], hand and arm use [d445], and walking long distances, on different surfaces and around obstacles [d4501, d4502, and d4503, respectively]
#The tools ACIF and Perme assess additional subdomains not outlined in the table. For ACIF, these specific subdomains are: acquiring basic skills [d1550], communicating with receiving—spoken messages [d310], and communicating when receiving—body gestures [d3150]. For Perme, these specific subdomains are: communicating with receiving—spoken messages [d310], generalized pain [D2800], and consciousness functions [b110]. Additionally, Perme had subdomains which could not be mapped to the ICF framework, including: need for mechanical ventilation or non-invasive ventilation; lines and attachments, and presence of drips
1In the IMS this referred to the item ‘marching on the spot (at the bedside)’
2In the CPAX this referred to the item ‘stepping’
3In the ccFROM this referred to the item ‘marching on the spot’
ACIF Acute Care Index of Function, CPAx Chelsea Critical Care Physical Assessment Tool, CcFROM Critical Care Functional Rehabilitation Outcome Measure, DEMMI De Morton Mobility Index, FSS-ICU Functional Status Score for the ICU, ICF International Classification of Functioning, ICU intensive care unit, IMS ICU mobility scale, MMS, Perme Perme ICU Mobility Score, PFIT-s Physical Function in intensive care test scored, RPE rating of perceived exertion, SOMS Surgical Optimal Mobility Scale, SPPB Short Physical Performance Battery, MMS Manchester Mobility Score
Measurement properties
Relevant measurement properties to consider when selecting an instrument include the ability to measure what is intended (validity). This includes subjective interpretation (face validity), whether the instrument’s content adequately reflects the parameter of interest (content validity), comparison with other tools measuring a similar construct (construct validity), and prediction of future outcomes (predictive validity) [21, 22]. In addition, the ability to obtain accurate results within or between assessors (intra- and inter-rater reliability, respectively), or when measures are repeated longitudinally (test-retest reliability) is important. Instruments should detect change over time (responsiveness) and have a limited floor (proportion of patients scoring the lowest score possible) and ceiling (proportion of patients scoring the highest score possible) effect across the expected evaluation time points [22].
Notably, caution is needed if trying to extrapolate instruments developed for one setting or patient population to the ICU setting. This issue is particularly important given many unique issues within the ICU, including sedation, delirium, fatigability, and weakness, that can affect patient performance. For example, Acute Care Index of Function, De Morton Mobility Index, and Short Physical Performance Battery were initially developed for use in non-ICU patient populations (e.g., geriatrics and neurology), [23–25] with relatively little evaluation, at present, within the ICU setting.
Table 1 and Additional file 1 (Table S1) synthesize data on measurement properties for each instrument. An adequate level of inter-rater reliability has been demonstrated for all measurement instruments, except the Short Physical Performance Battery, although this instrument has established reliability in geriatrics. All instruments have evidence of construct validity compared to other concurrent measurements of physical function and/or strength, except the Critical Care Functional Rehabilitation Outcome Measure. This instrument only has published data on face/content validity. Seven measurement instruments have evidence of predictive validity. The most commonly evaluated predictive outcome was discharge to home, which was evaluated in six instruments. The Short Physical Performance Battery was the only instrument not predictive of discharge to home, albeit the study may have been under-powered for this assessment [26]. Accurately predicting individuals unable to be discharged to home is important to help optimize the consistency, appropriateness, and timeliness of discharge planning recommendations and rehabilitation referral. There has been limited evaluation of the predictive validity beyond hospital discharge with only three instruments (ICU Mobility scale, Surgical Optimal Mobility scale, and Physical Functional in ICU Test-scored) examining post-hospital mortality with conflicting findings (Table 1; Additional file 1: Table S1).
The ability to detect a clinically meaningful change over time (responsiveness) was examined in five instruments (Table 1; Additional file 1: Table S1): the Chelsea Critical Care Physical Assessment Tool; the Functional Status Score for the ICU; the ICU Mobility Scale; the Physical Functional in ICU Test-scored; and the Short Physical Performance Battery. All demonstrated significant change over time within the ICU, and moderate to large effect sizes (an indicator of moderate to good responsiveness) were observed for the Functional Status Score for the ICU and Physical Functional in ICU Test-scored.
The presence of floor and ceiling effects are important considerations in assessing the recovery trajectories of patients and the intervention efficacy [22]. High floor or ceiling effects indicate that the instrument is too challenging or too easy, respectively, limiting its ability to detect a change in the physical functioning of patients. The majority of instruments have low floor and ceiling effects during an ICU stay (Table 1; Additional file 1: Table S1). However, the Short Physical Performance Battery demonstrated large floor effects which limits its potential utility in the ICU (Table 1; Additional file 1: Table S1).
Based on published measurement properties alone, the most robust ICU instruments are: Physical Functional in ICU Test-scored; Chelsea Critical Care Physical Assessment Tool; Functional Status Score for the ICU; and ICU Mobility Scale (Table 1). Ongoing research is needed to further understand the measurement properties of existing instruments to ensure appropriateness and usability within the ICU setting.
Patient capacity
All instruments outlined herein (Tables 1 and 2) are dependent on patient effort. Consequently, assessing the feasibility of each instrument’s use within the ICU is critical. Feasibility should consider the requirements of the instrument, including issues related to a patient’s alertness, ability to follow instructions, motivation, weakness, and fatigability. A standardized method for determining patient mental capacity (including validated and reliable determinations of pain, sedation, and delirium status) is important to enable comparison of results across patients [27] (Fig. 3). The Perme ICU Mobility Score is unique as it includes evaluation of potential barriers to mobility that may affect patient performance (e.g., medical devices, pain, and respiratory support). Impairments in the balance of patients may also affect performance, with the De Morton Mobility Index and Short Physical Performance Battery including balance evaluation.
Table 2.
Clinical utility and practical considerations of functional measures in the ICU setting
| Outcome measure | Type of assessment# | Patient population with original development | Equipment required* | Scoring information (minimum to maximum score) | Time required to physically undertake testing | Training Rresources |
|---|---|---|---|---|---|---|
| ACIF [32] | Comprehensive | Acute neurological (including neurosurgery) [23] | Access to 5 stairs; walking marker (distance) | 20 items, 4 subcategories (0–1.00) | 12 min | No specified training package or video currently available, instructions and recording sheet available [23] |
| CPAx [33, 34–36] | Comprehensive | General and trauma ICU [31] | Handgrip dynamometera | 10 items, each scored 0–5 (0 –50) | 2–10 min | Online 40–60 min free training (requires registration) at http://cpax.ocbmedia.com |
| CcFROM [37] | Comprehensive | General, neurosurgery and trauma ICU [33] | Stopwatch | 9 items, each scored 0–7 (0–63) | 10–30 min | Instructions and recording sheet available [33], no training package or video currently available |
| DEMMI [38] | Comprehensive | General hospitalized geriatric medical patients [24] | Chair with 45 cm seat height with arm rests; stopwatch; pen (DEMMI item 13); walking marker (distance) | 15 items, 5 subcategories, each scored 0–2 (0–100) | 10–30 min | Instructions and recording sheet available in supplementary [24] (no details specific to the ICU setting) |
| FSS-ICU [26, 31, 39, 40–42] | Comprehensive | Medical ICU [37] | Walking marker (distance) | 5 items, each scored 0–7 (0–35) | 10–30 min | Detailed free instructions (registration required) at www.improvelto.com/, free training package including video available from primary author1 |
| IMS [43, 26, 44, 45] | Simple | General ICU (medical, surgical, trauma) [28] | None | 1 item, score based on highest classification level (11 options) (0–10) | <1 min | Instructions available [28] |
| MMS [46] | Simple | General ICU (medical, surgical, trauma) [39] | None | 1 item, score based on highest classification level (7 options) (0–7) | <1 min | Instructions and recording sheet available [39]—further detailed instructions available from primary author2, no training package or video currently available |
| PFIT-s [26, 30, 47, 48] | Comprehensive | General ICU (medical, surgical) [43] | Stopwatch; Borg RPE sheet (optional) | 4 items, individual items scored 0–3 (0–10) | 10–15 min | Free training package, including video, available from primary author3 |
| Perme Score [49, 50, 44] | Comprehensive | General ICU; cardiovascular ICU [46, 47] | None | 15 items, individual items scored 0–3 (0–32) | 15–60 min | No training package or video currently available. The scoring criteria and detailed instructions are available in the manuscript [46] |
| SOMS [51, 52–54] | Simple | Surgical ICU [49] | None | 1 item, score based on highest classification level (5 options) (0–4) | <1 min | No training package or video currently available, scoring criteria available in manuscript [49] |
| SPPB [26] | Comprehensive | Geriatric, non-hospitalized | Stopwatch; tape-measure (for 4-m course) | 3 items, each item scored 0–4 (0–12) | 5–10 min | Free training via: https://www.irp.nia.nih.gov/branches/leps/sppb/index.htm and https://www.youtube.com/watch?v=XgiuciJXPm4 (no details specific for ICU) |
*Additional equipment required beyond standard hospital bed, chair, and gait aids
#Type of assessment was defined into two categories: 1) “simple” involving observation of patient’s current ability (time to complete: <5 min); and 2) “Comprehensive” providing greater understanding of the impairments in physical functioning (time to complete: 10–15 min)
1Dale Needham, School of Medicine, Johns Hopkins University. Contact email: dale.needham@jhmi.edu
2David Williams, Therapy Services, University Hospitals Birmingham NHS Foundation Trust. Contact email: david.mcwilliams@uhb.nhs.uk
3Linda Denehy, Physiotherapy Department, The University of Melbourne. Contact email: l.denehy@unimelb.edu.au
a A table is able to be downloaded at the end of the eLearning module which provides the gender/age values for handgrip strength in order to work out percentage grip strength which is required to complete the CPAx
ACIF Acute Care Index of Function, CPAx Chelsea Critical Care Physical Assessment Tool, CcFROM Critical Care Functional Rehabilitation Outcome Measure, DEMMI De Morton Mobility Index, FSS-ICU Functional Status Score for the ICU, ICU intensive care unit, IMS ICU mobility scale, MMS, Perme Perme ICU Mobility Score, PFIT-s Physical Function in intensive care test scored, RPE rating of perceived exertion, SOMS Surgical Optimal Mobility Scale, SPPB Short Physical Performance Battery, MMS Manchester Mobility Score
Fig. 3.
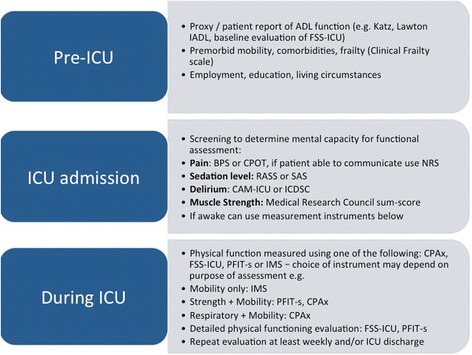
Recommendations for Clinical Practice – Measurement of Physical Functioning. Abbreviations: ADL activities of daily living; BPS Behavioural Pain Scale; CAM-ICU Confusion assessment method for the ICU; CPAx Chelsea Physical assessment Tool; CPOT Critical Care Pain Observation Tool; FSS-ICU Functional Status Score for the ICU; IADL instrumented activities of daily living; ICU intensive care unit; ICDSC Intensive Care Delirium Screening Checklist; IMS ICU Mobility Scale; NRS Numerical rating scale; PFIT-s Physical Function in ICU Test-scored; RASS Richmond Agitation and Sedation Scale; SAS, Sedation Agitation Scale
Clinical utility
The levels of expertise, training, and time required, as well as any specialized equipment, are important in assessing clinical utility. All instruments require minimal additional equipment, apart from the Chelsea Critical Care Physical Assessment Tool which requires a handgrip dynamometer, and the Acute Care Index of Function which requires a set of five steps (Table 2). Dedicated ICU training packages are available for three instruments: Chelsea Critical Care Physical Assessment Tool; Functional Status Score for the ICU; and Physical Function in ICU test-scored (Table 2). The fastest tests are the simple one-item mobility scales that indicate the patients highest level, while other more comprehensive instruments require more time to assess multiple specific activities and/or levels of assistance required (Table 2).
Recommendations for clinical practice
We propose a staged approach for assessing physical functioning in the ICU (Fig. 3). In terms of pre-ICU status, we recommend obtaining physical functioning data as part of the patient history to inform appropriate patient goals for recovery and rehabilitation [2]. The ability to obtain a validated baseline measure of physical functioning (or pre-ICU health status) is challenging due to the severity of illness, sedation, and reduced ability of patients to engage in volitional assessments. The Clinical Frailty Scale can be used to obtain a baseline assessment of frailty. Patients who are frail prior to ICU admission have worse mortality and morbidity, and require institutionalization at discharge; thus, frailty may be a useful prognostic tool [28]. Similar to the process used with the Functional Independence Measure instrument, commonly used throughout the inpatient rehabilitation setting, it is possible to conduct a baseline assessment for the Functional Status Score for the ICU measure via proxy assessment, as performed in prior research [29]; however, this baseline version of the Functional Status Score for the ICU has not been specifically validated. The ability to measure pre-ICU physical functioning is an area for future research.
Screening for mental capacity should commence from ICU admission and include assessments of pain, sedation, and delirium status [27]. We also recommend regular screening for muscle weakness using the Medical Research Council sum-score. It is likely less important to evaluate physical functioning in ICU patients who lack muscle weakness; however, strength should not be a sole guide for determining the need for physical functioning assessment because strength and function are only weakly correlated in ICU survivors [16]. At present, there is a lack of robust, validated predictive models for physical functioning impairments within the ICU. There is a predictive model for physical functioning after hospital discharge, but not whilst in hospital [12]. Therefore, identification of patients who need evaluation of physical functioning in the ICU is largely reliant on clinical judgment regarding many potential risk factors (Fig. 1). Once the patient can follow commands, we recommend, at a minimum, one of the four recommended physical functioning tools: Physical Functional in ICU Test-scored; Chelsea Critical Care Physical Assessment Tool; Functional Status Score for the ICU; and ICU Mobility scale. Summary information about these instruments (including how to access and use them) is available through a free website: www.improveLTO.com.
When selecting specific instrument(s) for a particular ICU setting, the following are important considerations: available clinician resources and expertise; and rationale for assessment (e.g., simple versus comprehensive evaluation). In settings with limited access to rehabilitation clinicians, a simple one-item scale (e.g., ICU mobility scale) can be used, which can be feasibly completed by the bedside ICU nurse. For patients with identified mobility restrictions, consultation with physiotherapists and occupational therapists may be warranted, with more comprehensive instruments used as part of their routine clinical evaluation (Fig. 3).
Areas for future investigation
There is an ongoing need to examine the measurement properties and clinical utility of ICU physical functioning instruments. In addition to primary measurement studies, valuable insights could be achieved through secondary analyses of existing studies that include relevant instruments, enabling larger sample sizes across multi-center trials [26, 30, 31]. Predictive validity is a critical consideration and needs additional evaluation for all instruments to assist with meaningful interpretation of the scores and the effects of associated interventions. The purpose of assessment should be considered when selecting an instrument. As highlighted in Table 3, there is variability in the subdomains evaluated across instruments. Future research is required to determine the most critical subdomains of physical functioning that always should be encompassed within evaluations in the ICU and across the recovery trajectory. It is currently unknown whether a single instrument, which encompasses all relevant subdomains and has robust measurement properties, is feasible; it is likely more than one instrument may be required.
There is often a delay in initiating evaluations of physical functioning in the ICU due to sedation, delirium, and illness severity impacting the volitional ability of patients. Hence, during this very early stage of critical illness, non-volitional instruments may be appropriate (e.g., screening neuromuscular electrophysiological or ultrasound tests [2]). Generally, these non-volitional assessments are not part of routine clinical practice. Further examination of their clinical utility and measurement properties is needed. Future work should also explore how psychological and cognitive capacity impact patient performance, engagement, and the timing and frequency of evaluation of physical functioning.
Conclusions
Impairment in physical functioning among ICU survivors results in significant morbidity and burden to patients, caregivers, and society. With a growing population of ICU survivors, greater utilization and standardization of physical functioning instruments is needed. This article has provided a framework and recommendations for practice. Measuring physical functioning early and longitudinally in the ICU is important to determine patients at risk of poor physical outcomes, monitor intervention efficacy, and inform recovery trajectories. These insights are important to improving the outcomes of critically ill patients.
Acknowledgements
We thank the following individuals who provided critical review of a draft version of the manuscript: Susan Berney PT, PhD; David Hager, MD, PhD; Pedro Mendez-Tellez, MD; Amy Toonstra, PT, DPT, CCS; and Jennifer Zanni, PT DScPT. We thank the following individuals who provided critical review of a draft version of Additional file 1 (Table S1) and Table 2 regarding the measurement instruments that they created and/or investigated within the ICU setting: Bernie Bissett PT, PhD; Evelyn Corner PT; Linda Denehy, PT, PhD; Carol Hodgson PT, PhD; George Kasotakis, MD; David McWilliams PT; Christiane Perme PT, CCS; Paul Twose PT; and Marike Van der Schaaf PT, PhD.
Funding
This work is supported, in part, by National Institutes of Health (R24HL111895). SMP is supported by a National Health and Medical Research Council Early Career Fellowship (1111640).
Availability of data and materials
Not applicable
Abbreviations
- ADL
Activity of daily living
- ICF
International Classification of Functioning
- ICU
Intensive care unit
- LOS
Length of stay
- QOL
Quality of life
- WHO
World Health Organization
Additional file
Detailed summary of measurement properties for the ICU setting. (DOCX 60 kb)
Authors’ contributions
Manuscript concept and design: SMP, MH, and DMN. Analysis and interpretation of data: SMP, MH, and DMN. Drafting and approval of final manuscript: SMP, MH, and DMN. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
A potential conflict of interest may exist for DMN who is a co-creator of the Functional Status Score for the ICU instrument, which is one of the measurement instruments discussed in this article. The Functional Status Score for the ICU is freely available for non-commercial use as indicated by its Creative Commons licensing. Moreover, DMN is a principal investigator on a NIH-funded, multi-centered randomized trial evaluating nutrition and exercise in acute respiratory failure and, related to this trial, is currently in receipt of an unrestricted research grant and donated amino acid product from Baxter Healthcare Corporation and an equipment loan from Reck Medical Device. DMN and MH received funding from the National Heart, Lung, and Blood Institute (NHLBI) (grant #R24HL111895). SMP reports National Health and Medical Research Council fellowship funding during conduct of this study. SMP and MH declare that they have no competing interests.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13054-017-1827-6) contains supplementary material, which is available to authorized users.
Contributor Information
Selina M. Parry, Email: Selina.parry@unimelb.edu.au
Minxuan Huang, Email: minxuan.huang@emory.edu.
Dale M. Needham, Email: dale.needham@jhmi.edu
References
- 1.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–94. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jolley S, Bunnell A, Hough C. Intensive care unit acquired weakness. Chest. 2016;S0012-3692(16):47575–6. [Google Scholar]
- 3.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–9. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 4.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–70. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 5.Parry SM, El-Ansary D, Cartwright MS, Sarwal A, Berney S, Koopman R, et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J Crit Care. 2015;30(5):1151.e9–14. doi: 10.1016/j.jcrc.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 6.De Jonghe B, Sharshar T, Lefaucheur J, Authier F, Durand-Zaleski I, Boussarsar M, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. J Am Medical Assoc. 2002;288(9):2859–67. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 7.Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez P, Sevransky JE, Shanholtz C, et al. Physical complications in acute lung injury survivors: a 2-year longitudinal prospective study. Crit Care Med. 2014;42(4):849–59. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Appleton R, Kinsella J, Quasim T. The incidence of intensive care unit-acquired weakness syndromes: a systematic review. J Intensive Care Soc. 2015;16(2):126–36. doi: 10.1177/1751143714563016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tennila A, Salmi T, Pettila V, Roine RO, Varpula T, Takkunen O. Early signs of critical illness polyneuropathy in ICU patients with systemic inflammatory response syndrome or sepsis. Intensive Care Med. 2000;26(9):1360–3. doi: 10.1007/s001340000586. [DOI] [PubMed] [Google Scholar]
- 10.Berek K, Margreiter J, Willeit J, Berek A, Schmutzhard E, Mutz NJ. Polyneuropathies in critically ill patients: a prospective evaluation. Intensive Care Med. 1996;22(9):849–55. doi: 10.1007/BF02044106. [DOI] [PubMed] [Google Scholar]
- 11.De Jonghe B, Cook D, Sharshar T, Lefaucheur J, Carlet J, Outin H. Acquired neuromuscular disorders in critically ill patients: a systematic review. Intensive Care Med. 1998;24:9. doi: 10.1007/s001340050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herridge MS, Chu LM, Matte A, Tomlinson G, Chan L, Thomas C, et al. The RECOVER Program: disability risk groups and one year outcome after ≥7 days of mechanical ventilation. Am J Respir Crit Care Med. 2016. [DOI] [PubMed]
- 13.Hermans G, Van Mechelen H, Clerckx B, Vanhullebusch T, Mesotten D, Wilmer A, et al. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2014;190(4):410–20. doi: 10.1164/rccm.201312-2257OC. [DOI] [PubMed] [Google Scholar]
- 14.Parker A, Huang M, Colantuoni E, Lord R, Dinglas V, Chong A, et al. Health care resource use and costs in long-term survivors of ARDS: a 5-year longitudinal cohort study. Crit Care Med. 2017;45(2):196–204. [DOI] [PMC free article] [PubMed]
- 15.World Health Organisation . Towards a common language for functioning, disability and health. Geneva: WHO; 2002. [Google Scholar]
- 16.Needham D, Wozniak A, Hough C, Morris P, Dinglas V, Jackson J, et al. Risk factors for physical impairments after acute lung injury in a national multicenter study. AJRCCM. 2014;189(10):1214–24. doi: 10.1164/rccm.201401-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 18.Iwashyna TJ. Trajectories of recovery and dysfunction after acute illness, with implications for clinical trial design. Am J Respir Crit Care Med. 2012;186(4):302–4. doi: 10.1164/rccm.201206-1138ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwashyna T, Hodgson C, Pilcher D, Bailey M, van Lint A, Chavan S, et al. Timing of onset and burden of persistent critical illness in Australia and New Zealand: a retrospective population based observational study. Lancet Respir Med. 2016;4(7):566–73. doi: 10.1016/S2213-2600(16)30098-4. [DOI] [PubMed] [Google Scholar]
- 20.Parry S, Granger C, Berney S, Jones J, Beach L, El-Ansary D, et al. Assessment of impairment and activity limitations in the critically ill: a systematic review of measurement instruments and their clinimetric properties. ICM. 2015;41(5):744–62. doi: 10.1007/s00134-015-3672-x. [DOI] [PubMed] [Google Scholar]
- 21.Connolly B. Describing and measuring recovery and rehabilitation after critical illness. Curr Opinion Crit Care. 2015;21:445–52. doi: 10.1097/MCC.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 22.Portney LG, Watkins MP. Foundations of clinical research, applications to practice. 3. Conneticut: Appleton and Lange; 2009. [Google Scholar]
- 23.Roach K, Van Dillen L. Development of an Acute Care Index of Functional status for patients with neurologic impairment. Phys Ther. 1988;68(7):1102–8. doi: 10.1093/ptj/68.7.1102. [DOI] [PubMed] [Google Scholar]
- 24.De Morton N, Davidson M, Keating J. The de Morton Mobility Index (DEMMI): an essential health index for an ageing world. Health Qual Life Outcomes. 2008;19(6):63. doi: 10.1186/1477-7525-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- 26.Parry S, Denehy L, Beach L, Berney S, Williamson H, Granger C. Functional outcomes in ICU—what should we be using? An observational study. Crit Care. 2015;19:127. doi: 10.1186/s13054-015-0829-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barr J, Fraser GL, Puntillo K, Ely EW, Gelinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit: executive summary. Am J Health Syst Pharm. 2013;70(1):53–8. doi: 10.1093/ajhp/70.1.53. [DOI] [PubMed] [Google Scholar]
- 28.Muscedere J, Waters B, Varambally A, Bagshaw S, Boyd J, Maslove D, et al. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med. 2017;43(8):1105–22. doi: 10.1007/s00134-017-4867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kho M, Truong A, Zanni J, Ciesla N, Brower R, Palmer J, et al. Neuromuscular electrical stimulation in mechanically ventilated patients: a randomized sham-controlled pilot trial with blinded outcome assessment. J Crit Care. 2015;30(1):32–9. doi: 10.1016/j.jcrc.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nordon-Craft A, Schenkman M, Edbrooke L, Malone D, Moss M, Denehy L. The Physical Function Intensive Care Test: implementation in survivors of critical illness. Phys Ther. 2014;94:1499–507. doi: 10.2522/ptj.20130451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang M, Chan K, Zanni J, Parry S, Neto S, da Silva V, et al. Functional status score for the intensive care unit (FSS-ICU): an international clinimetric analysis of validity, responsiveness and minimal important difference. Crit Care Med. 2016;44(120):e1155–64. [DOI] [PMC free article] [PubMed]
- 32.Bissett B, Green M, Marzano V, Byrne S, Leditschke I, Needman T, et al. Reliability and utility of the acute care index of function in intensive care patients: an observational study. Heart Lung. 2016;45:10–4. doi: 10.1016/j.hrtlng.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Corner E, Wood H, Englebretsen C, Thomas A, Grant R, Nikoletou D, et al. The Chelsea critical care physical assessment tool (CPAx): validation of an innovative new tool to measure physical morbidity in the general adult critical care population: an observational proof of concept pilot study. Physiotherapy. 2013;99:33–41. doi: 10.1016/j.physio.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Corner E, Handy J, Brett S. E-learning to facilitate the education and implementation of the Chelsea Critical Care Physical Assessment: a novel measure of function in critical illness. BMJ Open. 2016;6:e010614. doi: 10.1136/bmjopen-2015-010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corner E, Soni N, Handy J, Brett S. Construct validity of the Chelsea Critical Care Physical Assessment tool: an observational study of recovery from critical illness. Crit Care. 2014;18:R55. doi: 10.1186/cc13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corner EJ, Hichens LV, Attrill KM, Vizcaychipi MP, Brett SJ, Handy JM. The responsiveness of the Chelsea Critical Care Physical Assessment tool in measuring functional recovery in the burns critical care population: an observational study. Burns. 2015;41(2):241–7. doi: 10.1016/j.burns.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Twose P, Wise M, Enright S. Critical care functional rehabilitation outcome measure: developing a validated measure. Physiother Theory Pract. 2015;31:474–82. doi: 10.3109/09593985.2015.1025320. [DOI] [PubMed] [Google Scholar]
- 38.Sommers J, Vredeveld T, Lindeboom R, Nollet F, Engelbert R, van der Schaaf M. The De Morton Mobility Index is feasible, reliable and valid in critically ill patients. Phys Ther. 2016;96(10):1658–66. [DOI] [PubMed]
- 39.Zanni J, Korupolu R, Fan E, Pradhan P, Janjua K, Palmer J, et al. Rehabilitation therapy and outcomes in acute respiratory failure: an observational pilot project. J Crit Care. 2010;25:254–62. doi: 10.1016/j.jcrc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Ragavan V, Greenwood K, Bibi K. The Functional Status Score for the Intensive Care Unit Scale: is it reliable in the intensive care unit? Can it be used to determine discharge placement? JAcute Care Physical Ther. 2016;7(3):93–100. doi: 10.1097/JAT.0000000000000030. [DOI] [Google Scholar]
- 41.Maldaner da Silva V, Neto J, Cipriano G, Pinedo M, Needham D, Zanni J, et al. Brazilian version of the Functional Status Score for the ICU: translation and cross-cultural adaptation. Revista Brasileira de Terapia Intensiva. 2017;29(1):34–8. doi: 10.5935/0103-507X.20170006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thrush A, Rozek M, Dekerlegand J. The clinical utility of the functional status score for the intensive care unit (FSS-ICU) at a long term acute care hospital: a prospective cohort study. Phys Ther. 2012;92:1536–45. doi: 10.2522/ptj.20110412. [DOI] [PubMed] [Google Scholar]
- 43.Hodgson C, Needham D, Haines K, Bailey M, Ward A, Harrold M, et al. Feasibility and inter-rater reliability of the ICU Mobility Scale. Heart Lung. 2014;43(1):19–24. doi: 10.1016/j.hrtlng.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Kawaguchi W, Nawa R, Figueiredo T, Martins L, Pires-Neto R. Perme Intensive Care Unit Mobility Score and ICU Mobility Scale: translation into Portugese and cross-cultural adaptation for use in Brazil. Journal Brasileiro de Pneumologia. 2016;42(6):429–34. doi: 10.1590/s1806-37562015000000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tipping C, Bailey M, Bellomo R, Berney S, Buhr H, Denehy L, et al. The ICU Mobility scale has construct and predictive validity and is responsive: a multi-centre observational study. Ann Am Thoracic Soc. 2016;13(6):887–93. [DOI] [PubMed]
- 46.McWilliams D, Atkins G, Hodson J, Boyers M, Lea T, Snelson C. Feasibility and reliability of the Manchester Mobility Score as a measure of physical function within the intensive care unit. ACPRC J. 2016; in press.
- 47.Denehy L, De Morton N, Skinner E, Edbrooke L, Haines K, Warrillow S, et al. A Physical Function test for use in the intensive care unit: validity, responsiveness, and predictive utility of the physical function ICU test (scored) Phys Ther. 2013;93:1636–45. doi: 10.2522/ptj.20120310. [DOI] [PubMed] [Google Scholar]
- 48.Skinner EH, Berney S, Warrillow S, Denehy L. Development of a physical function outcome measure (PFIT) and a pilot exercise training protocol for use in intensive care. Crit Care Resusc. 2009;11(2):110–5. [PubMed] [Google Scholar]
- 49.Perme C, Nawa R, Winkelman C, Masud F. A tool to assess mobility status in critically ill patients: the Perme Mobility Score. Methodist Debakey Cardiovasc J. 2014;10(1):41–9. doi: 10.14797/mdcj-10-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nawa RK, Lettvin C, Winkelman C, Evora PRB, Perme C. Initial inter-rater reliability for a novel measure of patient mobility in a cardiovascular ICU. J Crit Care. 2014;29(3):475.e1–5. [DOI] [PubMed]
- 51.Garzon-Serrano J, Ryan C, Waak K, Hirschberg R, Tully S, Bittner E, et al. Early mobilization in critically ill patients: patients' mobilization level depends on health care provider's profession. Physical Med Rehabil. 2011;3(4):307–13. doi: 10.1016/j.pmrj.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 52.Schaller S, Stauble C, Susemasa M, Heim M, Duarte I, Mensch O, et al. The German validation study of the surgical intensive care unit optimal mobility score. J Crit Care. 2016;32:201–6. doi: 10.1016/j.jcrc.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 53.Kasotakis G, Schmidt U, Perry D, Grosse-Sundrup M, Benjamin J, Ryan C, et al. The surgical intensive care unit optimal mobility scale predicts mortality and length of stay. Crit Care. 2012;40:1122–8. doi: 10.1097/CCM.0b013e3182376e6d. [DOI] [PubMed] [Google Scholar]
- 54.Piva S, Dora G, Minelli C, Michelini M, Turla F, Mazza S, et al. The surgical optimal mobility score predicts mortality and length of stay in an Italian population of medical, surgical and neurologic intensive care unit patients. J Crit Care. 2015;30:1251–7. doi: 10.1016/j.jcrc.2015.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable


