Abstract
Background
Research has revealed that manifest Alzheimer’s disease (AD) dementia is preceded by preclinical and prodromal phases during which pathology is accumulating but function remains intact. This understanding and concern that disease-modifying interventions initiated at the dementia stage may come too late in the neurodegenerative process to be successful has led to a paradigm shift in AD clinical trials. AD trials now enroll patients with mild cognitive impairment (MCI) and persons with no cognitive symptoms. Trial designs are similar to those enrolling dementia participants. We set out to test the hypothesis that attitudes towards trial design features differ among different potential AD trial populations.
Methods
We sent a survey composed of 37 items assessing specific trial elements to 246 cognitively normal, MCI, and AD dementia participants at the University of California Los Angeles (UCLA) Alzheimer’s Disease Research Center (ADRC), from whom we received 91 responses (37 cognitively normal, 32 MCI, and 22 dementia). To quantify willingness to enroll, we created three composite scenarios by summing responses and fitting proportional odds models with a binary outcome variable for whether patients were highly willing to participate in low-, moderate-, or high-risk and burden trials.
Results
MCI participants less frequently correctly self-identified their diagnoses than those with dementia or normal cognition. Compared to dementia patients, the odds of participating in a low-risk, low-burden trial were 12% lower for MCI patients (odds ratio (OR) = 0.88, 95% confidence interval (CI) 0.23–3.29) and 70% lower (OR = 0.30, 95% CI 0.08–1.09) for cognitively normal participants. With increasing risk and burden, willingness to enroll decreased and the gap in relative willingness between diagnostic groups increased. In the medium-risk, medium-burden scenario, the estimated OR was 0.64 (95% CI 0.17–2.40) for MCI and 0.21 for the cognitively normal (95% CI 0.06–0.77). In the high-risk, high-burden scenario, the estimated OR indicated reduced willingness for MCI (OR = 0.27, 95% CI 0.06–1.15) and cognitively normal respondents (OR = 0.12, 95% CI 0.03–0.54).
Conclusions
These results suggest that AD trials enrolling predementia populations, especially those requiring frequent visits and implementing biomarker testing procedures, may encounter challenges to enrollment.
Electronic supplementary material
The online version of this article (doi:10.1186/s13195-017-0311-5) contains supplementary material, which is available to authorized users.
Keywords: Alzheimer’s disease, Clinical trials, Recruitment, Mild cognitive impairment, Preclinical
Background
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder and the most common cause of dementia. A few symptomatic therapies are approved for AD but, as yet, no therapy has been successfully demonstrated to slow the cognitive and functional declines that characterize the disease [1]. Myriad challenges to developing disease-modifying therapies for AD exist. Among these, numerous studies indicate that by the time dementia is diagnosed neurobiological changes have been occurring for a decade or longer [2]. Based on this premise, research diagnostic criteria for mild cognitive impairment (MCI) due to AD [3] and prodromal AD [4, 5], as well as preclinical [6] or asymptomatic AD [4, 5], have been proposed for use in earlier disease clinical trials of potential disease-modifying therapies.
A key determinant of clinical trial success is the timely recruitment of eligible participants [7]. AD dementia clinical trials face a multitude of barriers to successful recruitment and often encounter slow or inadequate accrual [8]. While the barriers to AD dementia trial recruitment have been moderately well characterized [9–16], fewer studies explore trial decision-making in MCI [17–19] or preclinical AD [20, 21]. It will be important to understand how these populations differ in their approach to trial decisions, given that many aspects of AD trial designs, such as the outcomes used to assess efficacy, the incorporated biomarkers, and the requirement of a study partner, have been held constant as the field has evolved to include earlier and earlier disease participants. In fact, a number of candidate therapies, some with associated risks such as vasogenic edema or cerebral hemorrhage, are under simultaneous investigation in two or three AD diagnostic categories.
To begin to elucidate whether and how barriers to trial recruitment differ with disease stage, we sent a single survey instrument to cognitively normal, MCI, and AD dementia longitudinal observational research participants. We hypothesized that attitudes toward trial designs would differ and willingness to participate would decrease in less clinically affected persons.
Methods
Participants
Participants for this study were recruited from the longitudinal cohort study within the University of California Los Angeles (UCLA) Alzheimer’s Disease Research Center (ADRC). We included participants from three diagnostic groups who had consented to be contacted about additional studies: cognitively normal, MCI, and AD dementia. Patients were excluded from the study if they were determined to have dementia stemming from a non-AD etiology, did not speak English fluently, did not attend a follow-up visit at the UCLA ADRC in the 12 months preceding the survey, or had previously enrolled in a clinical trial. After applying these exclusion criteria, the survey was sent to 246 eligible participants.
Data collection
A packet was mailed to eligible participants that included the survey, a brief introduction to the goals of the study, an addressed stamped return envelope, and a consent form. To increase the response rate, we telephoned participants who had not returned completed surveys 1 month after dissemination.
The data collection instrument consisted of 37 forced choice questions. The full survey is included in Additional file 1: Appendix. The survey was created based on previous interview studies focusing on understanding barriers to participation in AD prevention clinical trials [20]. Initial survey items asked participants to self-identify as someone with AD dementia, MCI, or normal cognition and relate these diagnoses to AD trial constructs (e.g., normal participants should approach the survey by considering enrolling in an AD prevention trial, MCI participants should consider enrolling in a trial to lower the risk of dementia, and AD dementia patients should consider a clinical trial of a medication to slow the progression of the disease). To better contextualize responses, the initial survey also asked the extent to which a participant’s study partner assisted them in completing the survey (no help, help with less than half the questions, help with more than half the questions, completed in partnership, or completed on behalf of the study participant by the study partner). Remaining survey items investigated participants’ willingness to enroll in clinical trials with different attributes as well as their reasons for enrolling in such studies. Survey items examined specific trial lengths and visit frequencies, varying study procedures (e.g., magnetic resonance imaging (MRI), positron emission tomography (PET) scans), candidate therapies of different modes of administration (e.g., diet, pill, vaccines), and possible side effects (e.g., headache, bleeding in the brain or gut). Most questions elicited responses on a seven-point Likert scale in which “1” represented “Disagree very much” or “Extremely unlikely”, “4” was “Neutral”, and “7” was “Agree very much” or “Extremely Likely.”
Data analysis/statistical methods
The responses from each participant were linked to their demographic and clinical data from the UCLA ADRC database, including their research consensus diagnosis. Patient demographic data included race, gender, marital status, and living situation. The living situation of a respondent was categorized as living alone, with a spouse, with a relative or friend, or with a group. The patient sample was characterized using summary statistics, including the mean and standard deviation for quantitative variables and the count and frequency for categorical variables, stratified by clinical diagnosis.
To investigate agreement between ADRC consensus and self-reported diagnoses among those with both data points, we used unweighted and weighted kappa statistics [22, 23].
To approximate willingness to participate in trial scenarios with multiple attributes, we a priori created three composite scores by summing selected responses. The questions selected for the composite scores are based on the literature [8, 16] as well as practical experience. In the low-risk, low-burden scenario, we summed responses for 2-year clinical trials, oral experimental medications, annual visits, and trials that included MRI. For the medium-risk, medium-burden scenario, we considered responses for 5-year clinical trials, oral experimental medications, monthly follow-up visits, and trials that involved PET. Finally, the high-risk, high-burden scenario composite consisted of responses for 5-year clinical trials, infused experimental medications, weekly visits, and the use of lumbar puncture. Because the scores for each question ranged from 1 to 7, composite scores ranged from 5 to 35 for each scenario. We calculated Cronbach’s alpha for the risk and burden constructs in each scenario to investigate internal consistency. All statistics ranged from 0.73 to 0.91, indicating acceptable to excellent consistency.
To investigate differences in willingness to enroll by clinical diagnosis, we fitted a proportional odds model (ordinal logistic regression) for each scenario where the response was a discretization of the composite willingness score in each case (low risk and burden, medium risk and burden, and high risk and burden). The discretization of willingness for each of the three scenarios was derived using breakpoints at 21 and 28, which were selected a priori; > 21 represents above neutral willingness and > 28 represents high willingness. We chose to not use data-driven cutpoints in an effort to minimize potential inflation of type I errors. We a priori chose adjustment variables (gender, education, age, partner involvement in completing the survey, and marital status) in the model based upon their potential to confound the association between dementia status and trial willingness [8]. The proportional odds model assumes that the odds ratio (OR) for a “high” response that is associated with a given covariate is constant regardless of where the breakpoint that defines a “high” response lies (either above 21 or above 28 in this case). Thus, this approach avoids assumptions regarding the relative spacings of the cutpoints. The proportional odds assumption was assessed by fitting two separate logistic regression models (dichotomizing at greater than 21 and at greater than 28, respectively) and comparing the coefficients associated with each covariate across the two models. This investigation did not yield evidence that the proportional odds assumption was violated.
Missing responses were present in some completed surveys and ranged from 2% to 11% for questions in which missing data were observed. To account for missing data in our primary analysis we employed multiple imputation using 10 imputations. All reported parameter estimates accounted for the multiple imputation [24]. To assess the sensitivity of the resulting estimates to the imputation procedure, we also performed a complete-case analysis and found the estimates to be consistent with those obtained using multiple imputation.
We conducted secondary analyses to explore potential associations between the main reason for participants enrolling (personal benefit, benefit of mankind, benefit of future generations, or doctor’s suggestion) and willingness to participate. We fitted a proportional odds model to each scenario, including the reason to participate variable. Given the exploratory nature of the analyses, we only considered complete cases.
All analyses were conducted using R version 3.2.2. Imputations were performed using the Hmisc package. Kappa statistics and Cronbach’s alpha statistics were calculated using the psych package in R.
Results
Ninety-one participants completed the survey: 22 with a clinical diagnosis of dementia, 32 with MCI, and 37 with normal cognition. One respondent’s consensus diagnosis was cognitive impairment not meeting criteria for dementia or MCI. In analyses assessing attitudes towards trial participation, this patient was included as part of the MCI group. The participant was excluded from analyses of self-reported and consensus diagnosis agreement. Table 1 describes the demographic characteristics for the study respondents stratified by clinical diagnosis. The MCI group had an equal number of males and females, while the cognitively normal and dementia groups had more males. Most participants were white (91.1%). The mean age of the respondents was 73.5 years for those with dementia, 69.9 years for MCI, and 72.5 years for the cognitively normal. Dementia participants tended to be more educated. The majority of participants were married and most lived with a spouse, though the proportion living with a spouse was highest among the dementia group. As expected, most cognitively normal and MCI participants functioned independently, while the majority of dementia participants required assistance with basic or instrumental activities of daily living. Similarly, most cognitively normal and MCI participants reported completing the survey on their own (80.6% and 80.7%, respectively), while a majority of dementia participants reported receiving assistance from their study partner on more than half the questions or completing the survey together with their study partner (90.9%).
Table 1.
Characteristics of survey respondents
| Participant characteristics | Consensus diagnosis | ||
|---|---|---|---|
| CN (n = 37) | MCI (n = 32) | Dementia (n = 22) | |
| Age, mean (SD) | 72.46 (10.4) | 69.94 (9.5) | 73.55 (11.4) |
| Education, mean (SD) | 17.22 (1.9) | 16.78 (2.7) | 19.73 (17.9) |
| Female sex, n (%) | 16 (43.2%) | 16 (50.0%) | 6 (27.3%) |
| Race, n (%) | |||
| White | 35 (97.2%) | 29 (90.6%) | 18 (81.8%) |
| African-American | 0 (0.0%) | 3 (9.4%) | 1 (4.6%) |
| Asian | 1 (2.8%) | 0 (0.0%) | 2 (9.1%) |
| Other | 0 (0.0%) | 0 (0.0%) | 1 (4.6%) |
| Hispanic, n (%) | 0 (0.0%) | 1 (3.1%) | 1 (4.6%) |
| Marriage status, n (%) | |||
| Married | 25 (67.6%) | 22 (68.8%) | 17 (77.3%) |
| Widowed | 6 (16.2%) | 1 (3.1%) | 3 (13.6%) |
| Divorced | 3 (8.1%) | 6 (18.8%) | 2 (9.1%) |
| Never married | 2 (5.4%) | 3 (9.4%) | 0 (0.0%) |
| Living as married | 1 (2.7%) | 0 (0.0%) | 0 (0.0%) |
| Partner involvement, n (%) | |||
| No help | 29 (80.6%) | 25 (80.7%) | 2 (9.1%) |
| Less than half | 5 (13.9%) | 1 (3.2%) | 0 (0%) |
| More than half | 2 (5.6%) | 5 (16.1%) | 13 (59.1%) |
| Together | 0 (0.0%) | 0 (0.0%) | 7 (31.8%) |
| Living situation, n (%) | |||
| Lives alone | 7 (18.9%) | 8 (25.0%) | 3 (13.6%) |
| Lives with spouse or partner | 22 (59.5%) | 20 (62.5%) | 16 (72.7%) |
| Lives with relative or friend | 2 (5.4%) | 0 (0.0%) | 1 (4.6%) |
| Lives with group | 4 (10.8%) | 1 (3.1%) | 1 (4.6%) |
| Other | 2 (5.4%) | 3 (9.4%) | 1 (4.6%) |
| Independence, n (%) | |||
| Able to live independently | 36 (97.3%) | 31 (96.9%) | 8 (36.4%) |
| Requires some assistance with complex activities | 0 (0.0%) | 1 (3.1%) | 12 (54.6%) |
| Requires some assistance with basic activities | 1 (2.7%) | 0 (0%) | 1 (4.5%) |
| Completely dependent | 0 (0.0%) | 0 (0%) | 1 (4.5%) |
“Partner Involvement” refers to how much the study partner assisted the patient in completing the survey
“Independence” refers to how well participants can perform daily activities
CN cognitively normal, MCI mild cognitive impairment, SD standard deviation
Participants were asked to identify their diagnosis in the context of how they would complete the survey. Discrepancies between self-reported and ADRC consensus diagnoses were observed in each group (Table 2). Concordance rates were 73% for the dementia group, 48% for the MCI group, and 84% for the cognitively normal group. The weighted kappa statistic was 0.71 (95% confidence interval (CI) 0.58–0.84) indicating substantial agreement, while the unweighted kappa statistic was 0.52 (95% CI 0.36–0.67) indicating moderate agreement [25]. Table 2 provides the number of participants in each category.
Table 2.
Self-reported versus ADRC consensus diagnosisa
| Consensus diagnosis (n) | Self-reported diagnosis | ||
|---|---|---|---|
| CN (n) | MCI (n) | Dementia (n) | |
| CN | 26 | 5 | 0 |
| MCIb | 13 | 13 | 1 |
| Dementia | 2 | 4 | 16 |
aLimited to participants with both clinical and self-reported diagnoses available
bSubject with cognitive impairment not due to MCI was excluded from this analysis
Unweighted kappa statistic 0.52, 95% confidence interval (CI) 0.36–0.67
Weighted kappa statistic 0.71, 95% CI 0.58–0.84
ADRC Alzheimer’s Disease Research Center, CN cognitively normal, MCI mild cognitive impairment
Table 3 displays the group responses to questions related to the type of treatment under study and the procedures often involved in AD clinical trials. Participants within each diagnostic group were most willing to participate in trials of approved medications and trials that involved MRI. Table 4 shows the responses of the groups to questions about visit frequency and trial length; while willingness was highest for studies with annual visits, no trends relating to trial length were apparent.
Table 3.
Responses to survey questions regarding willingness to participate in clinical trials including various treatments and procedures
| Trial design elements | Diagnostic group | Likelihood of participation | ||
|---|---|---|---|---|
| Very unlikely (score = 1–2) | Neutral (score = 3–5) | Very likely (score = 6–7) | ||
| Approved | CN | 6 (16.2%) | 19 (51.4%) | 12 (32.4%) |
| MCI | 2 (6.25%) | 12 (37.5%) | 18 (56.3%) | |
| Dementia | 0 (0.00%) | 5 (22.7%) | 17 (77.3%) | |
| Experimental | CN | 11 (29.7%) | 21 (56.8%) | 5 (13.5%) |
| MCI | 4 (12.5%) | 17 (53.1%) | 11 (34.4%) | |
| Dementia | 5 (22.7%) | 4 (18.2%) | 13 (59.1%) | |
| Pill | CN | 7 (18.9%) | 21 (56.8%) | 9 (24.3%) |
| MCI | 4 (12.5%) | 14 (43.8%) | 14 (43.8%) | |
| Dementia | 4 (18.2%) | 3 (13.6%) | 15 (68.2%) | |
| Infused | CN | 12 (32.4%) | 23 (62.2%) | 2 (5.4%) |
| MCI | 9 (28.1%) | 17 (53.1%) | 6 (18.8%) | |
| Dementia | 6 (27.3%) | 9 (40.9%) | 7 (31.8%) | |
| Diet/exercise | CN | 4 (10.8%) | 18 (48.65%) | 15 (40.5%) |
| MCI | 4 (12.5%) | 10 (31.3%) | 18 (56.3%) | |
| Dementia | 3 (13.6%) | 8 (36.4%) | 11 (50.0%) | |
| Supplement | CN | 4 (10.8%) | 13 (35.1%) | 20 (54.1%) |
| MCI | 2 (6.3%) | 9 (28.1%) | 21 (65.6%) | |
| Dementia | 2 (9.1%) | 8 (36.4%) | 12 (54.6%) | |
| MRI | CN | 7 (18.9%) | 12 (32.4%) | 18 (48.7%) |
| MCI | 7 (21.9%) | 8 (25.0%) | 17 (53.1%) | |
| Dementia | 4 (18.2%) | 9 (40.9%) | 9 (40.9%) | |
| PET scan | CN | 12 (32.4%) | 11 (29.7%) | 14 (37.8%) |
| MCI | 8 (25.0%) | 9 (28.1%) | 15 (46.8%) | |
| Dementia | 4 (18.2%) | 9 (40.9%) | 9 (40.9%) | |
| Lumbar | CN | 24 (64.8%) | 12 (32.4%) | 1 (2.7%) |
| punctures | MCI | 15 (46.9%) | 9 (28.1%) | 8 (25.0%) |
| Dementia | 10 (45.4%) | 9 (40.9%) | 3 (13.6%) | |
| Bleeding in brain or gut | CN | 30 (81.1%) | 6 (16.2%) | 1 (2.7%) |
| MCI | 18 (56.3%) | 11 (34.4%) | 3 (9.4%) | |
| Dementia | 10 (45.5%) | 9 (40.9%) | 3 (13.6%) | |
| Headache or nausea | CN | 15 (40.5%) | 20 (54.1%) | 2 (5.4%) |
| MCI | 9 (28.1%) | 16 (50.0%) | 7 (21.9%) | |
| Dementia | 9 (40.9%) | 8 (36.4%) | 5 (22.7%) | |
Values are shown as n (%)
CN cognitively normal, MCI mild cognitive impairment, MRI magnetic resonance imaging, PET positron emission tomography
Table 4.
Responses to survey questions regarding willingness to participate in clinical trials of various lengths and frequency of visits
| Trial design elements | Diagnostic group | Likelihood of participation | ||
|---|---|---|---|---|
| Very unlikely (score = 1–2) | Neutral (score = 3–5) | Very likely (score = 6–7) | ||
| Annual visits | CN | 5 (13.5%) | 14 (37.8%) | 18 (48.7%) |
| MCI | 2 (6.3%) | 5 (15.6%) | 25 (78.1%) | |
| Dementia | 5 (22.7%) | 1 (4.6%) | 16 (72.7%) | |
| Monthly visits | CN | 13 (35.1%) | 16 (43.2%) | 8 (21.6%) |
| MCI | 3 (9.4%) | 14 (43.8%) | 15 (46.9%) | |
| Dementia | 6 (27.3%) | 4 (18.2%) | 12 (54.6%) | |
| Weekly visits | CN | 17 (45.9%) | 17 (45.9%) | 3 (8.1%) |
| MCI | 10 (31.3%) | 15 (46.9%) | 7 (21.9%) | |
| Dementia | 10 (45.5%) | 6 (27.3%) | 6 (27.3%) | |
| 1-year study | CN | 8 (21.6%) | 15 (40.5%) | 14 (37.8%) |
| MCI | 3 (9.4%) | 11 (34.4%) | 18 (56.3%) | |
| Dementia | 5 (22.7%) | 4 (18.2%) | 13 (59.1%) | |
| 2-year study | CN | 7 (20.0%) | 19 (54.3%) | 9 (25.7%) |
| MCI | 4 (13.8%) | 8 (27.6%) | 17 (58.6%) | |
| Dementia | 5 (25.0%) | 3 (15.0%) | 12 (60.0%) | |
| 5-year study | CN | 11 (30.6%) | 17 (47.2%) | 8 (22.2%) |
| MCI | 6 (18.8%) | 11 (34.4%) | 15 (46.9%) | |
| Dementia | 7 (31.8%) | 3 (13.6%) | 12 (54.6%) | |
Values are shown as n (%)
CN cognitively normal, MCI mild cognitive impairment
To compare the willingness of the diagnostic groups to participate, we created three composite scenarios of various risk and burden levels: low, medium, and high. Two trends were evident from the composite scenarios (Fig. 1). First, within each composite scenario, more severe diagnosis was associated with greater willingness to participate (dementia > MCI > cognitively normal). Second, with greater risk and burden, willingness to enroll was reduced for each diagnostic group. In the low-risk, low-burden scenario there was a high proportion of willing participants for each diagnostic group (63% of cognitively normal, 72% of MCI, and 80% of dementia). In the high-risk, high-burden scenario 28% of cognitively normal participants, 44% of MCI, and 59% of dementia participants were highly willing to participate.
Fig. 1.
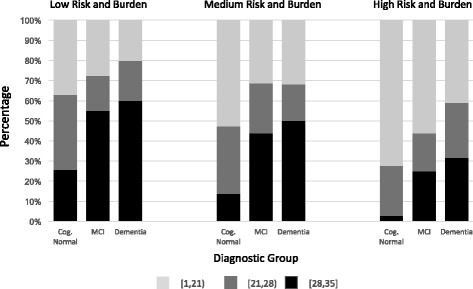
Group scores for trial composites. Diagnostic group summary scores for the composite scenarios are illustrated. Light gray = composite score of 1–21 (low willingness), dark gray = composite score 21–28 (moderate willingness), black = composite score 28–35 (high willingness). Cog. cognitively, MCI mild cognitive impairment
To more formally characterize willingness to participate among the three diagnostic groups, we fitted a proportional odds model adjusting for potential confounding factors (Table 5 and Fig. 2). The adjusted model confirmed the overall observations related to the diagnostic groups. The estimated odds of “high” versus “low” willingness comparing MCI patients to dementia patients ranged from 0.27 to 0.88, while the estimated odds of “high” versus “low” willingness comparing cognitively normal patients to dementia patients ranged from 0.12 to 0.30. Odds ratios for gender, marital status, age, and education were similar for each composite scenario (Table 5). Women were twice as likely to be willing compared to men; married participants were three times as likely as unmarried; for every 5 years of age the odds of being highly willing increased 14–30%; and higher education was associated with a roughly 20% reduction in the odds of being highly willing to participate.
Table 5.
Estimated odds ratios for each scenario based on the proportional odds model
| Covariate | Low risk, low burden | Medium risk, medium burden | High risk, high burden | |||
|---|---|---|---|---|---|---|
| Estimated OR (95% CI) | P value | Estimated OR (95% CI) | P value | Estimated OR (95% CI) | P value | |
| Diagnosis | ||||||
| Dementia | 1.0 | 1.0 | 1.0 | |||
| MCI | 0.88 (0.23–3.29) | 0.844 | 0.64 (0.17–2.40) | 0.507 | 0.27 (0.06–1.15) | 0.076 |
| CN | 0.30 (0.08–1.09) | 0.067 | 0.21 (0.06–0.77) | 0.019 | 0.12 (0.03–0.54) | 0.006 |
| Partner involvement | ||||||
| Some help | 0.65 (0.22–1.96) | 0.448 | 1.12 (0.38–3.30) | 0.834 | 0.58 (0.16–2.11) | 0.408 |
| Marital status | ||||||
| Married (living as) | 3.06 (1.12–8.35) | 0.029 | 2.26 (0.82–6.22) | 0.114 | 1.84 (0.63–5.41) | 0.266 |
| Gender | ||||||
| Female | 2.04 (0.84–4.97) | 0.117 | 1.99 (0.80–4.95) | 0.139 | 2.64 (0.97–7.17) | 0.056 |
| Age (5 years) | 1.30 (1.04–1.62) | 0.021 | 1.21 (0.96–1.51) | 0.102 | 1.14 (0.90–1.45) | 0.276 |
| Education | 0.78 (0.65–0.93) | 0.007 | 0.77 (0.64–0.93) | 0.007 | 0.80 (0.65–0.97) | 0.024 |
CI confidence interval, CN cognitively normal, MCI mild cognitive impairment, OR odds ratio
Fig. 2.

Relative willingness estimates. Proportional odds regression estimates (95% CI) for “high willingness” compared to the dementia diagnostic group are presented for the cognitively normal (Cog. Normal) and mild cognitive impairment (MCI) groups, stratified by level of trial risk and burden (low, middle, and high scenarios)
We also asked participants to state their main reason for enrolling. Figure 3 shows the proportions of patients within each diagnostic group that gave each response for a trial of an experimental medication. Thirty-eight percent of dementia patients said their main reason for enrolling was for their own benefit, 29% said it was for the good of mankind, and 29% said it was for future generations. A majority of MCI and cognitively normal participants responded that their main reason for enrolling was for the good of mankind (57% and 54%, respectively). The patterns of responses were identical when participants were asked their reason for enrolling in a trial of an approved therapy (data not shown).
Fig. 3.
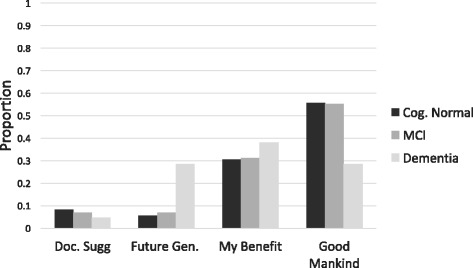
Participant reasons for enrolling. The proportion of respondents that chose each response as their main reason for enrolling in a clinical trial are presented, stratified by diagnostic group. Cog. Cognitively, Doc. doctor, Gen. generation, MCI mild cognitive impairment
In secondary analyses, we investigated whether the primary motivation for enrolling was associated with composite willingness scores in the three scenarios. We performed a likelihood ratio test to determine whether this covariate should be included in each of the models. We failed to reject the null hypothesis that the main reason for enrolling is associated with the composite score in the low- and high-risk, high-burden scenarios. The test statistics and p values were 6.10 (p = 0.11) in the low-risk, low-burden scenario, and 6.13 (p = 0.11) in the high-risk, high-burden scenario. In the medium-risk, medium-burden scenario, there was sufficient evidence to reject the null hypothesis (test statistic = 9.54, p value = 0.02), indicating an association between the primary motivation for enrolling and willingness to participate in trials.
Discussion
The results of this study indicate that the simple adoption of AD dementia trial design features in predementia trials may result in challenging trial recruitment. These recruitment challenges may be most evident in trials that involve more invasive assessment methods (such as PET or lumbar puncture) or more invasive treatments (such as infusions or vaccines). For example, the odds of high willingness to enroll in a low-risk, low-burden trial for MCI participants were estimated to be 12% less (0.88, 95% CI 0.23–3.29) than that of dementia participants. For a high-risk, high-burden trial, however, the odds of high willingness to participate were estimated to be 73% less (0.27, 95% CI 0.06–1.15). The odds of high willingness to participate for cognitively normal participants were estimated to be 70% (0.30, 95% CI 0.08–1.09), 79% (0.21, 95% CI 0.06–0.77) and 88% (0.12, 95% CI 0.03–0.54) less than those of the dementia group in low-, medium-, and high-risk and -burden composite scenarios, respectively.
The dementia group also differed from the MCI and cognitively normal groups in the reported rationale for participating in trials. Whereas altruism was the most common reason for considering enrollment in MCI and cognitively normal participants, personal benefit was the most common reason for those with dementia (Fig. 3). These results are in line with previous studies aiming to elucidate AD trial decision-making. AD is a debilitating and deadly disease; gaining access to a new therapy that may improve the health of the AD dementia patient is an important motivating factor in trial enrollment decisions, which are made in partnership between the AD patient and their caregiver [10, 14, 16, 26, 27]. In contrast, cognitively normal participants and MCI patients may emphasize altruism as a primary rationale for enrollment and may make trial decisions more unilaterally [20, 21]. In fact, in this study 80% of MCI participants reported completing the survey without input from their study partner and less than 50% of these participants correctly self-reported their diagnosis, with most incorrectly reporting as being cognitively normal (Table 2). This may suggest that these participants did not perceive a need for personal benefit of enrolling in trials. While understanding patient rationale for enrollment may provide important guidance for designing recruitment strategies, we found no relationship between the rationale for participating and willingness to enroll for two out of three composite scenarios, regardless of diagnostic category.
Some factors did predict willingness to enroll. Females were more likely than males, older age was associated with greater willingness, and married participants were 84–306% more likely to be willing to enroll compared to unmarried participants, depending on the diagnostic group. The latter finding is noteworthy given the striking pattern of overrepresentation of spousal dyads in AD dementia trials [28], suggesting that attitudes [29] rather than eligibility [30] may drive these enrollment patterns.
There are limitations to this study. Data were collected from participants in a longitudinal research study performed at an academic medical center. Most participants were Caucasian, married, and had high levels of education. This may limit generalizability to the general population, but also may recapitulate the sample biases known to occur in AD trials [28, 31]. The survey had a relatively low response rate, potentially limiting the generalizability even to the research-friendly population from which the sample is derived. Because the survey was sent to a cross-sectional selection of a time-varying cohort, we are unable to obtain demographic data on the individuals who did not respond to the survey. This limits our ability to compare the characteristics of responders and nonresponders. We excluded people who had previously participated in clinical trials; future studies should examine how previous trial participation affects attitudes toward enrollment. Our small sample size limited the power to detect differences between the three diagnosis groups. Participants are likely to have had different preferences and weightings in regards to trial characteristics and in their interpretation of the Likert scale. Future studies would benefit from incorporating these into the analysis. The paper survey was completed in the home, and the reliability of participant reporting of the involvement of the study partner is unknown. This may be particularly relevant to the MCI participants, who self-reported their own diagnosis incorrectly 50% of the time. It is possible that greater involvement of the study partner could have reduced this error rate, or could have changed the responses to other survey items. How MCI patients approach the decision whether to participate in AD trials is largely unknown. We chose to analyze the survey data based on the consensus diagnosis of the participants, rather than their self-reported diagnosis. This decision was based on the means by which these groups would be recruited to trials and the trials for which they would be eligible. We acknowledge, however, that failure of patients to recognize their own eligibility for a trial based on the diagnostic category in which it is being conducted (e.g., MCI patients who self-report as cognitively normal may ignore MCI trial invitations as not appropriate for them) could be a critical barrier to recruitment.
The study was based on hypothetical questions about trials rather than actual enrollment decisions, and failed to address some important aspects of modern AD trials, notably including the need for biomarker testing and disclosure in preclinical and prodromal trials. The biomarker disclosure process is complex and challenging to address in a paper survey [32]. This, and the desire to send identical instruments to all diagnostic groups, led us to focus on trial aspects that are more consistent across diagnostic groups.
Conclusions
Recruitment for Alzheimer’s disease clinical trials is often a difficult task. If trials are burdensome, participants and their study partners are less likely to enroll. We found that in exemplary trial scenarios, the willingness to participate was greater for dementia patients compared to MCI and cognitively normal participants. These results suggest that AD dementia clinical trial designs may encounter even more difficult recruitment when used for prodromal and preclinical AD studies. Moreover, these results indicate the need for planning studies to ensure recruitment feasibility for each diagnostic category. Major international efforts to create cohorts that are “trial ready” may address some of these challenges [33–35], but a more thorough understanding of which design elements encourage participation in each potential study population will allow researchers to carefully design trials in ways that optimize recruitment and ensure trial success.
Acknowledgements
The authors wish to acknowledge the participants and their study partners who volunteered their time to complete this survey.
Funding
This work was supported by NIA AG AG016573 and AG016570.
Availability of data and materials
The study survey is available as an appendix. To request the raw data from this study, please contact the corresponding author.
Abbreviations
- AD
Alzheimer’s disease
- ADRC
Alzheimer’s Disease Research Center
- CI
Confidence interval
- MCI
Mild cognitive impairment
- MRI
Magnetic resonance imaging
- OR
Odds ratio
- PET
Positron emission tomography
- UCLA
University of California Los Angeles
Additional file
Appendix. (DOC 59 kb)
Authors’ contributions
JDG, DE, JB, JMR, KKD, and DLG designed the study. MMN and DLG designed and performed the analytic plan. MMN drafted the manuscript. All authors contributed in an editorial capacity to the writing of the manuscript and approved the final version.
Ethics approval and consent to participate
The UCLA Institutional Review Board approved the study. Study participants signed and returned an informed consent document confirming their willingness to participate and to have their responses linked to their other data collected as part of the UCLA ADRC longitudinal study.
Consent for publication
Not applicable.
Competing interests
JDG has served as an investigator on studies sponsored by Eli Lilly & Company, Biogen Idec, and the Alzheimer’s Disease Cooperative Study. The remaining authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13195-017-0311-5) contains supplementary material, which is available to authorized users.
References
- 1.Gauthier S, Albert M, Fox N, Goedert M, Kivipelto M, Mestre-Ferrandiz J, Middleton LT. Why has therapy development for dementia failed in the last two decades? Alzheimers Dement. 2016;12:60–4. doi: 10.1016/j.jalz.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Sperling RA, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3:111–33. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–46. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 5.Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, Delacourte A, Frisoni G, Fox NC, Galasko D, Gauthier S, Hampel H, Jicha GA, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Sarazin M, de Souza LC, Stern Y, Visser PJ, Scheltens P. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9:1118–27. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 6.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kasenda B, von Elm E, You J, Blumle A, Tomonaga Y, Saccilotto R, Amstutz A, Bengough T, Meerpohl JJ, Stegert M, Tikkinen KA, Neumann I, Carrasco-Labra A, Faulhaber M, Mulla SM, Mertz D, Akl EA, Bassler D, Busse JW, Ferreira-Gonzalez I, Lamontagne F, Nordmann A, Gloy V, Raatz H, Moja L, Rosenthal R, Ebrahim S, Schandelmaier S, Xin S, Vandvik PO, Johnston BC, Walter MA, Burnand B, Schwenkglenks M, Hemkens LG, Bucher HC, Guyatt GH, Briel M. Prevalence, characteristics, and publication of discontinued randomized trials. JAMA. 2014;311:1045–51. doi: 10.1001/jama.2014.1361. [DOI] [PubMed] [Google Scholar]
- 8.Grill JD, Karlawish J. Addressing the challenges to successful recruitment and retention in Alzheimer’s disease clinical trials. Alzheimers Res Ther. 2010;2:34. doi: 10.1186/alzrt58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elad P, Treves TA, Drory M, Verchovsky R, Klimovitsky S, Ben-Laish S, Yaron S, Ginzburg K, Korczyn AD. Demented patients’ participation in a clinical trial: factors affecting the caregivers’ decision. Int J Geriatr Psychiatry. 2000;15:325–30. doi: 10.1002/(SICI)1099-1166(200004)15:4<325::AID-GPS117>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Karlawish JH, Casarett D, Klocinski J, Sankar P. How do AD patients and their caregivers decide whether to enroll in a clinical trial? Neurology. 2001;56:789–92. doi: 10.1212/WNL.56.6.789. [DOI] [PubMed] [Google Scholar]
- 11.Karlawish JH, Casarett DJ, James BD. Alzheimer’s disease patients’ and caregivers’ capacity, competency, and reasons to enroll in an early-phase Alzheimer’s disease clinical trial. J Am Geriatr Soc. 2002;50:2019–24. doi: 10.1046/j.1532-5415.2002.50615.x. [DOI] [PubMed] [Google Scholar]
- 12.Olin JT, Dagerman KS, Fox LS, Bowers B, Schneider LS. Increasing ethnic minority participation in Alzheimer disease research. Alzheimer Dis Assoc Disord. 2002;16(Suppl 2):S82–85. doi: 10.1097/00002093-200200002-00009. [DOI] [PubMed] [Google Scholar]
- 13.Gallagher-Thompson D, Solano N, Coon D, Arean P. Recruitment and retention of Latino dementia family caregivers in intervention research: issues to face, lessons to learn. Gerontologist. 2003;43:45–51. doi: 10.1093/geront/43.1.45. [DOI] [PubMed] [Google Scholar]
- 14.Karlawish J, Cary MS, Rubright J, Tenhave T. How redesigning AD clinical trials might increase study partners’ willingness to participate. Neurology. 2008;71:1883–8. doi: 10.1212/01.wnl.0000336652.05779.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelman CR. Learning from recruitment challenges: barriers to diagnosis, treatment, and research participation for Latinos with symptoms of Alzheimer’s disease. J Gerontol Soc Work. 2010;53:94–113. doi: 10.1080/01634370903361847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watson JL, Ryan L, Silverberg N, Cahan V, Bernard MA. Obstacles and opportunities in Alzheimer’s clinical trial recruitment. Health Aff (Millwood) 2014;33:574–9. doi: 10.1377/hlthaff.2013.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jefferson AL, Lambe S, Moser DJ, Byerly LK, Ozonoff A, Karlawish JH. Decisional capacity for research participation in individuals with mild cognitive impairment. J Am Geriatr Soc. 2008;56:1236–43. doi: 10.1111/j.1532-5415.2008.01752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jefferson AL, Carmona H, Gifford KA, Lambe S, Byerly LK, Cantwell NG, Tripodis Y, Karlawish J. Clinical research risk assessment among individuals with mild cognitive impairment. Am J Geriatr Psychiatry. 2012;20:878–86. doi: 10.1097/JGP.0b013e318252e5cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence V, Pickett J, Ballard C, Murray J. Patient and carer views on participating in clinical trials for prodromal Alzheimer’s disease and mild cognitive impairment. Int J Geriatr Psychiatry. 2014;29:22–31. doi: 10.1002/gps.3958. [DOI] [PubMed] [Google Scholar]
- 20.Grill JD, Karlawish J, Elashoff D, Vickrey BG. Risk disclosure and preclinical Alzheimer’s disease clinical trial enrollment. Alzheimers Dement. 2013;9:356–9. doi: 10.1016/j.jalz.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grill JD, Zhou Y, Elashoff D, Karlawish J. Disclosure of amyloid status is not a barrier to recruitment in preclinical Alzheimer’s disease clinical trials. Neurobiol Aging. 2016;39:147–53. doi: 10.1016/j.neurobiolaging.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. doi: 10.1177/001316446002000104. [DOI] [Google Scholar]
- 23.Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70:213–20. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- 24.Little RJA, Rubin DB. Statistical analysis with missing data. 2. Hoboken, NJ: Wiley-Interscience; 2002. [Google Scholar]
- 25.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 26.Bardach S, Holmes S, Jicha G. Motivators for Alzheimer’s disease clinical trial participation. Gerontologist. 2016;56:205–6. doi: 10.1007/s40520-017-0771-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leach M, Ziaian T, Francis A, Agnew T. Recruiting dementia caregivers into clinical trials lessons learnt from the Australian Transcendent Trial. Alzheimer Dis Assoc Disord. 2016;30:338–44. doi: 10.1097/WAD.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 28.Grill JD, Raman R, Ernstrom K, Aisen P, Karlawish J. Effect of study partner on the conduct of Alzheimer disease clinical trials. Neurology. 2013;80:282–8. doi: 10.1212/WNL.0b013e31827debfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cary MS, Rubright JD, Grill JD, Karlawish J. Why are spousal caregivers more prevalent than nonspousal caregivers as study partners in AD dementia clinical trials? Alzheimer Dis Assoc Disord. 2015;29:70–4. doi: 10.1097/WAD.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grill JD, Monsell S, Karlawish J. Are patients whose study partners are spouses more likely to be eligible for Alzheimer’s disease clinical trials? Dement Geriatr Cogn Disord. 2012;33:334–40. doi: 10.1159/000339361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faison WE, Schultz SK, Aerssens J, Alvidrez J, Anand R, Farrer LA, Jarvik L, Manly J, McRae T, Murphy GM, Jr, Olin JT, Regier D, Sano M, Mintzer JE. Potential ethnic modifiers in the assessment and treatment of Alzheimer’s disease: challenges for the future. Int Psychogeriatr. 2007;19:539–58. doi: 10.1017/S104161020700511X. [DOI] [PubMed] [Google Scholar]
- 32.Harkins K, Sankar P, Sperling R, Grill JD, Green RC, Johnson KA, Healy M, Karlawish J. Development of a process to disclose amyloid imaging results to cognitively normal older adult research participants. Alzheimers Res Ther. 2015;7:26. doi: 10.1186/s13195-015-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cummings J, Aisen P, Barton R, Bork J, Doody R, Dwyer J, Egan JC, Feldman H, Lappin D, Truyen L, Salloway S, Sperling R, Vradenburg G. Re-engineering Alzheimer clinical trials: Global Alzheimer’s Platform Network. J Prev Alzheimers Dis. 2016;3:114–20. doi: 10.14283/jpad.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aisen PT, Andrieu J, Boada S, Doody M, Nosheny R. Registries and cohorts to accelerate early phase Alzheimer’s trials: a report from the EU/US Clinical Trials in Alzheimer’s Disease Task Force. J Prev Alz Dis. 2016;3:68–74. doi: 10.14283/jpad.2016.97. [DOI] [PubMed] [Google Scholar]
- 35.Ritchie CW, Molinuevo JL, Truyen L, Satlin A, Van der Geyten S, Lovestone S, European Prevention of Alzheimer’s Dementia (EPAD) Consortium Development of interventions for the secondary prevention of Alzheimer’s dementia: the European Prevention of Alzheimer’s Dementia (EPAD) project. Lancet Psychiatry. 2016;3:179–86. doi: 10.1016/S2215-0366(15)00454-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study survey is available as an appendix. To request the raw data from this study, please contact the corresponding author.


