Abstract
Pregnancy is a hypercoagulable state which carries an excess risk of maternal venous thrombosis. Endothelial injury, alterations in blood flow and activation of the coagulation pathway are proposed to contribute to the hypercoagulability. The risk for thrombosis may be accentuated by certain drugs and device implants that directly or indirectly affect the coagulation pathway. To help ensure that these interventions do not result in adverse maternal or fetal outcomes during pregnancy, gravid experimental animals can be exposed to such treatments at various stages of gestation and over a dosage range that would identify hazards and inform risk assessment. Circulating soluble biomarkers can also be evaluated for enhancing the assessment of any increased risk of venous thrombosis during pregnancy. In addition to traditional in vivo animal testing, efforts are under way to incorporate reliable non-animal methods in the assessment of embryofetal toxicity and thrombogenic effects. This review summarizes hemostatic balance during pregnancy in animal species, embryofetal development, biomarkers of venous thrombosis, and alterations caused by drug-induced venous thrombosis.
Keywords: pregnancy, venous thrombosis, embryofetal toxicity assessment, drugs/biologics, biomarkers, nonclinical evaluation
Introduction
Human pregnancy is a hypercoagulable state in which the homeostatic equilibrium is shifted toward coagulation, elevating the risk of thrombosis and other maternal complications. These alterations are driven by physiological changes in coagulation pathways, and may be accentuated by mechanical constriction in the main abdominal veins and arterial structures by the growing uterus as pregnancy progresses (Brenner, 2004; Lockwood; 2006, Szecsi et al., 2010; Liu et al., 2012). These and other lifestyle and predisposing factors culminate in pregnant women having increased risk for thrombosis, especially during the peripartum and puerperium. Coagulopathy and hemodynamic changes related to the metabolic syndrome have also been proposed to play a role in pregnancy-associated hypertension and pre-eclampsia (Ness and Sibai, 2006; Tchaikovski et al., 2011; Ebina et al., 2015). The prothrombotic state of pregnancy may be further escalated by certain drugs or device implants which could alter the coagulation parameters or blood flow. Moreover, treating thrombosis during pregnancy may carry risk of bleeding and fetal toxicity. Thus, evaluating effects of drugs and biologics on coagulation parameters is prudent, and helps to assure the safe use of these products during pregnancy and gestation.
Several documents have been adopted to provide guidance on the design and analysis of mammalian animal studies intended to evaluate the safety of drugs and biologics used during pregnancy and gestation. However, the risk of venous thrombosis during pregnancy is not fully addressed by the traditional studies. To address this issue, research efforts are underway to develop non-animal methods to assess for enhanced risk of thrombotic events or embryofetal developmental toxicity.
Mammalian Pregnancy and Embryofetal Development
Unlike other vertebrates, females of most mammalian species not only produce the egg, but also support the fertilized egg, the embryo, and later the fetus throughout pregnancy via the placenta. Embryofetal development is a period of exponential growth that starts with the one-cell zygote, followed by its rapid cell division to a blastocyst stage, where the embryo starts differentiating into two distinct cell populations- the surrounding outer trophoblast cells and the inner cell mass cells. In the blastocyst stage, the embryo hatches from its protective zona pellucida layer and is now able to begin implantation into the maternal endometrium. The embryo undergoes rapid cell proliferation and differentiation during morphogenesis (ending around week 8 post-fertilization in humans, when it is referred to as a “fetus”) and organogenesis (which continues for 39 weeks in humans), and culminates with the delivery of the mammalian pup. Besides the difference in size and the sexual maturity, the mammalian newborn resembles its parents in most aspects of anatomy, morphology, and physiology. Species differences in the extent of organ maturity at birth notwithstanding, musculoskeletal, cardiovascular, neuronal, digestive, and pulmonary development in the newborn mammal is almost complete at term. Developmental milestones for the main organ systems for humans and rodents exhibit species differences (Table 1). Drug-induced morphological or functional impairment that occurs during the proliferation and differentiation of the embryo before specific organs reach their maturity could potentially be severe and irreversible. For example, if a given toxicity occurs prior to or immediately after implantation, it may result in spontaneous loss of the embryo. If the exposure to a drug happens during morphogenesis and organogenesis (i.e., before the developmental milestones are reached), cardiovascular, skeletal, or other anatomical malformations can occur (Table 1) (Menger et al., 1997, van Driel et al., 2002). Functional deficit is often associated with physical malformations such as renal failure and fetal hypotension, in the case of administration of angiotensin converting enzyme inhibitors during the second and third trimesters of pregnancy, or central nervous system and heart and lung dysfunction associated with fetal exposure to warfarin (van Driel et al., 2002).
TABLE 1.
Developmental Milestones for Major System Organs in Rodents and Humans
| Humans, gestation week (GW) | Rodents, gestation day (GD) | |
|---|---|---|
| Nervous System | GW 2–38a | GD 6–22a |
| Heart | GW 3–11 | GD 9.5–15.5 |
| Respiratory | GW 3–38b | GD 9-postnatal |
| Renal | GW 3–35 | GD 10.5-postnatal |
| Reproductive | GW 6–38 | GD ~11-termc |
| Sensory (eyes, ears) | GW 4–38 | GD 11-GD18 |
| Limbs | GW 3–8 | GD 9.5–13.5 |
| Spleen | GW 2–26 | GD 8-postnatal |
Neurogenesis continues postnatally.
Alveolar phase of development continues postnatally.
Development of prostate occurs postnatally.
The safety assessment of drugs and biologics intended to be used during the embryofetal developmental stages has been traditionally assessed in experimental animals. These studies are designed to predict potential adverse effects, if any, such as fetal structural or functional abnormalities following exposure of the mother and the embryo during the period of organogenesis. The testing for embryofetal toxicity is generally conducted in a rodent (rat, mouse) and a non-rodent (rabbit) species. Two species are traditionally used because the inter-species differences in susceptibility to toxicants can increase the likelihood of capturing different aspects of embryofetal toxicity (van der Laan et al., 2012). If either the rodent or non-rodent species are unresponsive to biologics, non-human primates are considered as potential animal models.
Depending on the target of the embryofetal toxicant, temporal windows of susceptibility for morphological or functional impairment are species-specific. For example, nephrogenesis in humans is completed in utero, whereas in mice and rats it occurs postnatally (Table 1) (Cappon and Hurtt, 2010). Thus, for certain drugs that act on a specific organ, studies that include only in utero exposures in mice and rats, but not postnatal administration, may not capture all the effects that could potentially occur in humans. Species differences in the system- or organ-specific developmental milestones form the basis for choosing the appropriate species and duration of studies for assessing the safety of drugs and biologics during embryofetal development. Susceptibility to toxicological fetal injury in these species depends on the timing of exposure in utero because organs have different critical periods when they are most susceptible during gestation.
The placenta derives from the fertilized egg, and in addition to being the supporting organ for the fetus, it has immune and endocrine functions that ensure the viability of the embryo and continuation of pregnancy. There are significant differences in the morphology and physiology of the rodent, rabbit, monkey, and human placenta (Table 2). Of those, the placenta of the macaque monkey is the most similar to that of the human in structure, shape, and morphology. Regardless of placental morphology, it is generally accepted that most mammalian embryos are exposed to drugs present in the maternal circulation (Carney et al., 2004; Atkinson et al., 2006). Relevant species, optimal dosages, timing, length of period of fetal exposure, and stages of development are critical variables in the design of reproductive toxicology studies. Considerations on timing and levels of transplacental transfer in animal species versus human, especially for biotechnology-derived pharmaceuticals, such as antibody therapies, are also factors that influence the selection of species and study design in animal testing (DeSesso et al., 2012).
TABLE 2.
Placenta Morphology and Physiology in Common Animal Species
| Shape | Structure | Yolk sac/yolk sac placenta | |
|---|---|---|---|
| Rats, mice, rabbits | Discoid | Hemochorial Labyrinthine |
Develop inverted yolk sac placenta |
| Macaques | Bi-discoid | Hemochorial Villous |
Small yolk sac No inverted yolk sac placenta |
| Humans | Discoid | Hemochorial Villous |
Small yolk sac No inverted yolk sac placenta |
Alternative Methods for Assessing Drug Safety During Embryofetal Development
In addition to the traditional in vivo animal models for assessing reproductive toxicology, alternative approaches also continue to be explored. Although to date most of the alternative approaches are still in the stage of development and validation, many are being used in the early phases of drug discovery to search for the least toxic molecule during lead compound optimization and to streamline potential chemicals with severe toxicity signals. Among the alternative approaches being explored to assess embryofetal toxicity of drugs are the whole embryo culture, the Zebrafish model, and embryonic stem cells (Piersma, 2004; Lee et al., 2012). In silico computational modeling based on the similarity of physicochemical properties and activity of drugs, can also be utilized to predict embryofetal toxicity, in an effort to minimize the latter (Piparo and Worth, 2010).
The whole embryo culture system (WEC) is a well-developed in vitro approach that has been used extensively to evaluate the potential for embryo-toxicity of drugs that may be used during the vulnerable period of organogenesis. Both the rat and rabbit embryo models have been developed and tested as a rapid screen for potentially teratogenic compounds or to study the mechanisms of normal development. The advantage of this method is that a broad spectrum of normal and impaired developmental events can be monitored using the WEC system. However, the drawback of this testing method is that, at present, it can only be conducted for a limited time during embryofetal development (typically between gestational days 8 and 12 in rat and rabbit models) and, due to technical limitations with the in vitro culture system, cannot be carried out to the end of gestation (Harris and Hansen, 2006).
The Zebrafish embryo has also been used as a research animal model to study the effects and mechanisms of teratogens. The use of the Zebrafish model in developmental toxicity studies has many advantages, including the potential for high output, because of the short life span of this species and the relatively low cost of the assay. The Zebrafish assay has proven to be a rapid and relatively accurate tool for the evaluation of compounds with a teratogenic potential. When used in conjunction with other safety assessment tools, this model is a valuable assay to be utilized for the purposes of hazard identification and benefit-risk analysis for pharmaceuticals (Brannen et al., 2010).
Embryonic stem cell assays are a group of alternative tests being developed for assessing potential toxicity of pharmacologic agents on the proliferation and differentiation of pluripotent cells (Tandon and Jyoti, 2012). In these studies, both unspecialized stem cells and those triggered to undergo differentiation into various organ systems (e.g., heart, nerve, or skin cells) can be subjected to the potential toxicant. Growth inhibition (or cytotoxicity) and the inhibition of differentiation are often assessed as a readout on the effect the compound may have on embryofetal growth and development. Both mouse and human embryonic stem cell lines have been tested for feasibility of the assay. However, the embryonic stem cell assays still need to be further refined as at the present time they are still limited by their low sensitivity and specificity.
Hemostatic Balance During Pregnancy in Animal Species
The coagulation pathway is an ancient homeostatic adaptation occurring early in the evolution of vertebrates (Ribeiro et al., 2015). The principal proteins of this pathway are present in all eutherian (i.e., placental) mammalians. The activity and physiologic levels of these proteins vary markedly across species, strains, and genders; there are additional differences related to the age and the physiologic state (e.g., before and during estrus) of the tested animal (Gentry, 2004; Lemini et al., 2007; Siller-Matula et al., 2008; Doolittle, 2009; Lemini et al., 2015). However, little is known about the conservation of physiologic changes in the coagulation propensity in pregnancy and in hormonally altered states in common laboratory animals.
Numerous studies in animals have sought to recapitulate the changes in coagulation and propensity for thrombosis that occur during pregnancy and after hormonal treatment in women (Table 3). These studies indicate that animals and humans can respond differently to such states. The main alterations observed in rats and mice receiving estrogen were not thrombo-embolic but hyperplasia of some tissues (Pasquale, 1989). Changes in coagulation factors have not been thoroughly examined in animals. In studies where coagulation molecules were measured, the extent of the changes, both in terms of the number of coagulation factors affected and the magnitude of the change has not been analogous to humans (Cleuren et al., 2010, Cleuren et al., 2012).
TABLE 3.
Changes in Coagulation Cascade During Pregnancy
Abbreviations: APC, activated protein C; PT, prothrombin time; TFPI, tissue factor pathway inhibitor.
↑, increase; ↓, decrease; =no change in the coagulation parameters during pregnancy.
Reference ranges of coagulation pathway proteins during pregnancy are not widely available, and existing data indicate a complex scenario. In New Zealand white rabbits, prothrombotic changes in coagulation parameters, such as increased levels of fibrinogen and decreased prothrombin time, have been reported at the end of gestation (Mizoguchi et al., 2010). Differences in prothrombin time, as well as adaptations counteracting the prothrombotic changes (such as higher levels of antithrombin which is not seen in pregnant women), were also observed in various rabbit strains (Haneda et al., 2010; Mizoguchi et al., 2010; Szecsi et al., 2010).
Increased transcription of fibrinogen and thrombin genes was reported during pregnancy and during early post partum in Sprague Dawley rats (Urasoko et al., 2009; Urasoko et al., 2012). Significantly lower prothrombin time measurements were observed immediately following delivery in rats (Urasoko et al., 2009; Urasoko et al., 2012). Like rabbits, rats had increased antithrombin levels at the end of pregnancy and during very early lactation (Urasoko et al., 2009; Urasoko et al., 2012). It is presumed that similar variations of reference ranges may also occur in different species of non-human primates during pregnancy given the age and species-specific differences reported (Ekser et al., 2012).
The absence of data on the levels or physiologic activity of most proteins indicative of the prothrombotic state during human pregnancy is a major limitation of various studies in gravid animals. For example, most studies that have been performed in animals did not evaluate possible changes in the activated protein C-mediated thrombolytic pathway. Lower activities of protein C, protein S, and tissue factor pathway inhibitor (TFPI) are the hallmarks of activated protein C resistance both in pregnant women and those who receive oral contraceptives (Hellgren, 2003; Tchaikovski and Rosing, 2010). In a few animal studies where these parameters were measured, such as those performed in C57BL/6 mice, decreased activated protein C resistance, due to increased protein S and TFPI levels, was seen during pregnancy (Table 3) (Tchaikovski et al., 2009). Some of the most common laboratory strains, such as C57BL/6 mice, may have distinct regulatory mechanisms that offer protection against estrogen-mediated thrombosis in pregnancy. Thus, the species commonly used to assess safety during pregnancy, namely rodents, rabbits, and non-human primates, exhibit significant inter- and intra-species differences in pregnancy-related coagulation changes.
Venous Thrombosis During Pregnancy
Pregnancy itself carries the risk for hypercoagulability, which is characterized by an imbalance between procoagulant and anticoagulant factors that are essential to maintain vascular homeostasis. It is generally believed that such a shift toward hypercoagulability in pregnant women represents an evolutionary adaptation conferring survival benefit to pregnant women facing the trauma of childbirth. The anticoagulant effects are maintained by various trans-membrane proteins expressed in endothelial cells, such as thrombomodulin, tissue-type plasminogen activator (t-PA), heparin-like proteoglycans, and soluble proteins, such as antithrombin. The procoagulant effects are mediated by increased expression/activity of thrombin, plasminogen-activator inhibitor-1 (PAI-1), von Willebrand factor (vWF), tissue factor, and reactive oxygen species.
Several factors have been proposed that trigger clotting in veins during pregnancy, such as activated endothelium, altered blood flow, and hypercoagulability (Fig. 1). Hypoxia or inflammatory stimuli that may occur early during pregnancy can trigger endothelial cell activation and adhesion receptors expression, and lead to the activation of circulating leukocytes (Blann, 2003; Patil et al., 2013). Subsequent activation of leukocytes induces expression of tissue factor that switches the intimal surface to a prothrombotic state. Under normal conditions, the endothelium releases nitric oxide (NO) and prostacyclin, and expresses various anticoagulants, such as TFPI, endothelial protein C receptor, and heparin-like proteoglycans, to prevent thrombosis (Esmon and Esmon, 2011). Thrombomodulin participates in the inhibition of coagulation by binding and deactivating thrombin, and by activation of protein C, which degrades factor Va and factor VIIIa in the presence of protein S. Reduced expression of protein S in pregnancy leads to unopposed activation of these factors, and thereby promotes thrombosis. Among the prothrombotic molecules that are expressed on the surface of activated endothelial cells, tissue factor acts as a cofactor for factor VIIa, leading to activation of factors IX and X, and thrombin, resulting in initiation of blood coagulation (Fig. 2). During pregnancy, endothelial cells are activated, which leads to the expression of procoagulant tissue factor and downregulation of the anticoagulant protein S, both of which could intensify thrombotic risk (Esmon and Esmon, 2011; Patil et al., 2013).
FIGURE 1.
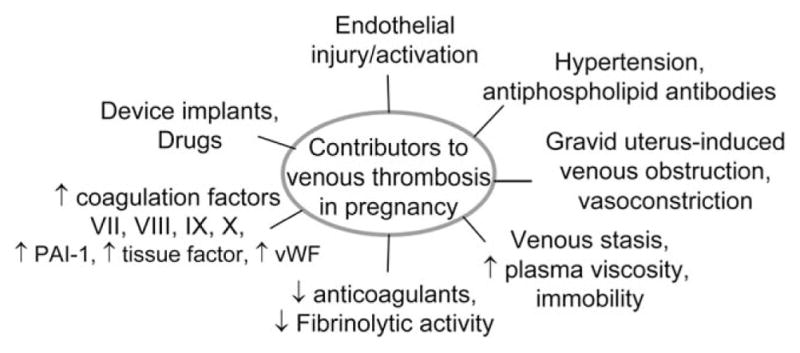
Factors influencing venous thrombosis during pregnancy: These include venous stasis, prolonged immobility, hyperviscosity, and vasoconstriction. In addition, endothelial injury may arise from high shear stress, hypertension, circulating microparticles, inflammatory cells, drugs, biomaterials, or medical device implants. Hypercoagulablity may also occur because of alterations in blood constituents, increase in fibrinogen, PAI-1, clotting factors, vWF, and decrease in antithrombin III, protein C, S, and fibrinolytic activity. PAI-1, plasminogen activator inhibitor-1; vWF, von Willebrand factor
FIGURE 2.
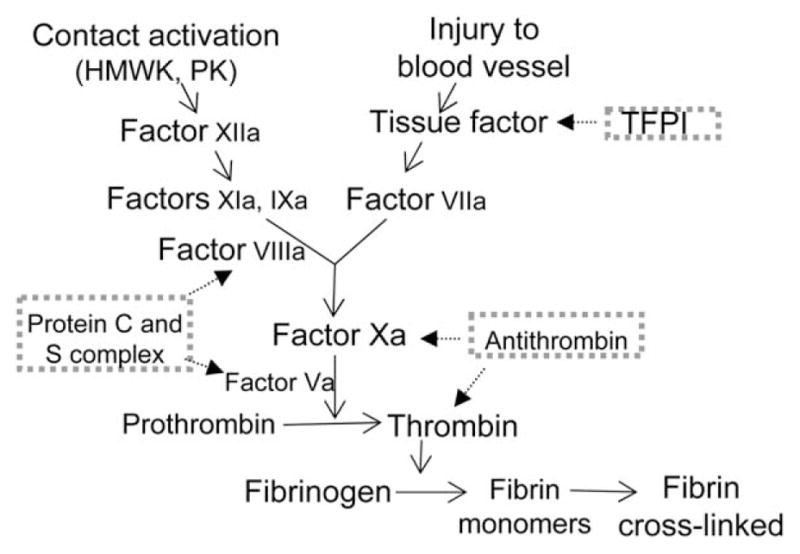
Simplified scheme of the coagulation cascade: Extrinsic (initimal injury) and intrinsic (contact activation) pathways involved in the formation of crosslinked fibrin. TFPI inhibits factor VIIa-related activation of factor Xa. Antithrombin degrades thrombin and factor Xa. Protein C and its co-enzyme protein S degrade the cofactors Va and VIIIa. HMWK, high molecular weight kininogen; PK, prekallikrein; TFPI, tissue factor pathway inhibitor
Activation of endothelial cells during pregnancy leads to increased expression of various molecules (such as P-selectin, E-selectin, and vWF) that capture leukocytes, platelets, and microparticles on the surface of the endothelium (Blann, 2003). vWF binds to hemostatic proteins, particularly factor VIII, and participates in clot formation and platelet adhesion to the endothelium. In addition, hypoxia that may occur during pregnancy could promote the release of vWF from Weibel-Palade bodies in endothelial cells to enhance prothrombotic events (Blann, 2003; Patil et al., 2013). Elevated plasma levels of clotting factors (such as fibrinogen and thrombin), and reduced anticoagulants (such as protein S, activated protein C, and antithrombin III), are reported to occur during pregnancy (James, 2009; Patil et al., 2013). In addition, fibrinolysis is impaired by an increase in PAI-1 and PAI-2, the latter synthesized from the placenta, with a net result tilting toward thrombotic state (Hellgren, 2003; Patil et al., 2013).
In venous thrombosis, thrombi tend to occur at sites where blood flow and shear stress are low, resulting in red-cell-rich thrombi. During pregnancy, venous stasis may be intensified because of enhanced responsiveness of the vessel wall to hormonal changes that switch toward thrombotic risk (Peverill, 2003; James, 2009). Moreover, reduced blood flow and stasis, because of mechanical pressure by the gravid uterus, could potentially lead to the accumulation of procoagulant proteases, such as thrombin. Altered blood flow could augment thrombotic processes by creating turbulence, which enhances the cellular and enzymatic reactions, and induces injury to the intimal surface to result in a prothrombogenic intimal surface (Brenner, 2004).
Normal laminar flow regulates endothelial-cell-related vascular reactivity by inducing the release of NO, prostacyclin, and t-PA, which are antithrombotic mediators. But under high shear stress of turbulent flow that can occur during pregnancy, the endothelium releases prothrombotic and proinflammatory mediators, such as tissue factor, intercellular adhesion molecule-1 (ICAM-1, CD54), and vascular cell adhesion molecule-1 (VCAM-1, CD106). In addition, leukocytes and platelets may be displaced from their central flow streamlines and make a closer contact with an activated endothelium. Moreover, anatomical perturbation of the compression of the left common iliac vein by the gravid uterus leads to vasoconstriction, which could increase the risk of thrombosis during pregnancy (DeStephano et al., 2014).
Changes in hormones during pregnancy create a precondition for a hypercoagulable state by affecting the venous blood flow, which is accompanied by elevated levels of coagulation factors VII, VIII, and X, fibrinogen, vWF, and PAI-1 that lead to clot formation (Brenner, 2004; James, 2009; Patil et al., 2013). Similarly, high levels of estrogen with the use of oral hormone contraceptives or hormone therapy augment the increase in the levels of factor VII, VIII, and X, prothrombin, and fibrinogen (Hellgren, 2003). Multiple mechanisms that activate thrombotic processes and mediators in the endothelium are considered to be amplified during pregnancy. Thus, increased incidence of venous thrombosis during pregnancy and the early postpartum period is the result of venous stasis, vascular endothelial injury/activation, and alterations in the constitution of the blood that lead to hypercoagulability.
Drug-Induced Thrombosis
Drugs can induce a pathological hypercoagulable state by a variety of mechanisms, including damage to the intimal surface, changes in blood coagulation constituents, and alterations in blood flow. Drugs may also attenuate the secretion of antithrombotic mediators and facilitate platelet adhesion and aggregation. Pregnancy is a hypercoagulable state, and when drugs that have thrombogenic potential are administered during pregnancy, the risk of thrombosis or embolism may become substantial because of synergistic activation of the coagulation pathways. Drugs that activate or cause injury to the endothelium could be accompanied by loss of protective molecules and disruption of hemostatic balance, leading to the initiation of thrombosis (Patil et al., 2013). The intimal injury along with platelet hyper-reactivity and circulating coagulation factors interact in a series of interlacing reactions resulting in vasoconstriction and formation of thrombus consisting of platelets and fibrin. Certain drugs that facilitate thrombosis may be problematic to administer during pregnancy because they could aggravate the incidence of venous thrombosis, as discussed below.
DRUG-INDUCED ENDOTHELIAL INJURY
Endothelial injury leads to thrombotic processes by mechanisms that involve adherence of platelets, white blood cells, and fibrin to the endothelium, leading to expression or secretion of prothrombotic mediators, such as vWF, and loss of anticoagulant molecules, such as thrombomodulin (Blann, 2003). Decrease in endothelial mediators, such as NO and prostacyclin, that act as vasodilators and inhibitors of platelet and leukocytes adhesion, lead to a prothrombogenic intimal surface. Drug-induced injury to the endothelium could be a consequence of vasoconstriction, increase in blood pressure, high shear stress, circulating microparticles, and/or biomaterial device implants (Rectenwald et al., 2005).
Examples of drugs that cause direct endothelial damage include the antineoplastic anthracyclines, such as 5-fluorouracil (5-FU) and doxorubicin, which induce free radical generation that leads to lipid peroxidation and endothelial cell membrane damage (Kinhult et al., 2003). 5-FU impairs the antioxidant defense capacity of the endothelium, and excess free radicals impair the ability of endothelial cells to activate protein C. Since pregnancy itself confers a risk of thrombosis, the risk could even be higher with 5-FU treatment. However, the use of anthracyclines during pregnancy is restricted because they were shown to be embryotoxic and teratogenic, when administered during the period of organogenesis in rats and rabbits (Kumar et al., 2006).
Rapamycin analogs are immunosuppressants that upregulate the expression of tissue factor, a transmembrane protein that serves as a high-affinity receptor for factor VIIa, and initiates the extrinsic blood coagulation pathway (Guba et al., 2005). Rapamycin analogs are also coated onto stent devices to treat restenosis, and have been implicated in stent thrombosis by increasing endothelial tissue factor (Guba et al., 2005; Camici et al., 2010). Thus, the use of rapamycin analogs during pregnancy may enhance the incidence of venous thrombosis.
Drugs that influence levels of serotonin in the blood could potentially alter the tendency toward thrombosis. Selective serotonin reuptake inhibitors may increase the risk of thrombosis because of the immediate serotonin increase adjacent to specific serotonin receptors, which leads to platelet activation and endothelium-derived contracting factors (Maurer-Spurej et al., 2004; Kurne et al., 2004). Antipsychotics cause an increase in platelet aggregation and vasoconstriction, and hence their use during pregnancy may intensify the risk of venous thrombosis (Hagg and Spigset, 2002).
Selective cyclooxygenase-2 inhibitors have been reported to increase thrombotic incidence because of inhibition of prostacyclin, in addition to thromboxane A2, in endothelial cells, and thereby tipping the hemostatic balance towards platelet aggregation and vasoconstriction (Kasliwal et al., 2005). Under certain conditions, COX-2 inhibitors may accentuate the prothrombotic state.
Dexamethasone is reported to cause an increase in plasma levels of prothrombotic factors, such as PAI-1, and decrease t-PA activity, and thus sustained exposure to glucocorticoids may contribute to increased risk of thrombosis (Brotman et al., 2006).
Phosphodiesterase type 5 (PDE-5) inhibitors induce vasodilatory effects mediated by the endothelial NO-guanosine monophosphate (GMP) pathway, which leads to blood stasis, increased endothelial permeability, and venous thrombosis. PDE-5 inhibitors are associated with reduced antithrombin III and free protein S, indicating that prolonged treatment may aggravate incidence of thrombotic or embolic events (Rufa et al., 2007).
In heparin-induced thrombocytopenia, the antibodies formed activate platelets and endothelial cells via the constant fragment (Fc) and the antigen-binding fragment (Fab’2). The latter induces expression of surface adhesion molecules, such as E-selectin and VCAM-1, which predispose to venous thrombosis (Blank et al., 2002). However, the incidence of low molecular weight heparin-induced thrombocytopenia is reported to be rare during pregnancy.
DRUG-INDUCED CHANGES IN COAGULATION FACTORS
Blood coagulation involves a series of proteolytic reactions resulting in the formation of a fibrin clot (Figure 2). Most factors are enzymes or coenzymes that are generally present in the plasma in an inactive form, but when activated, they become biologically functional in the clotting mechanism. Hormonal changes during pregnancy, in a similar fashion to estrogenic oral contraceptives alter the constitution of blood, result in hyperviscosity, and reduce antithrombin III, protein C, or S, which leads to a hypercoagulable state (Peverill, 2003). Hormone therapies have been reported to increase plasma levels of coagulation factors, decrease antithrombin levels, impair coagulation inhibitor pathways, and increase the relative risk of thrombosis (Peverill, 2003). Tamoxifen is an anti-estrogen agent, but it has also been shown to exhibit estrogenic effects, which may reduce antithrombin and protein C levels, leading to hypercoagulability (Onitilo et al., 2009). Erythropoietin causes an increase in thrombin-antithrombin III complexes, a specific indicator of thrombin generation in blood, and induces PAI-1 release, which may aggravate the risk of thrombosis (Smith et al., 2003). Prednisone treatment decreases plasma levels of fibrinogen and plasminogen, and increases levels of prothrombin, antithrombin, and vWF, any of which may enhance incidence of thrombosis (Jilma et al., 2005).
Polyclonal immunoglobulin (IG) products have been linked to increased risk for thromboembolic events, likely because of multifactorial reasons, but presence of coagulation factors that copurify with the active ingredient, for example activated factor XI, may play a potential role (Menis et al., 2013).
DRUGS THAT ALTER BLOOD FLOW
Disturbances in laminar blood flow augment the thrombotic processes because turbulence causes injury to the intimal surface and enhance thrombotic events (Wakefield et al., 2008). The increase in shear stress induces release of prothrombotic and proinflammatory endothelial mediators, such as tissue factor, vWF, endothelin, ICAM-1, and VCAM-1. Alterations in blood flow, such as slowing of blood flow, venous stasis, and/or increase in blood viscosity, promote activation of coagulation pathways. Thus, drugs that alter blood flow could activate tissue factor to bind to factor VIIa and trigger the initiation of coagulation (Stassen et al., 2004; Wakefield et al., 2008).
Antipsychotics bind to α-adrenergic receptors and cause vasodilation and hypotension by blocking adrenergic neurotransmission, which could lead to venous stasis and slowing of blood flow, and contribute to increased thrombogenicity (Zornberg and Jick, 2000). Selective serotonin reuptake inhibitors increase serotonin blood levels and cause vasoconstriction resulting in altered blood flow, and may facilitate thrombosis (Kurne et al., 2004; Maurer-Spurej et al., 2004). Although not intended for pregnancy use, ethinyl estradiol carries a greater risk of venous thrombosis because it may increase hematocrit and blood viscosity, which could lead to a decrease in blood flow and lower shear rates (Cushman et al., 2004). Glucocorticoids act at various sites in the NO synthesis pathway and down-regulate endothelial NO synthase expression through genomic responses mediated by the intracellular glucocorticoid receptors that lead to vasoconstriction and decrease blood flow (Yang and Zhang, 2004). Recombinant human erythropoietin administration results in increased erythrocyte mass with augmented blood viscosity, by increasing erythrocyte flexibility that could increase the risk of thrombosis (Vogel et al., 2003). Inhibitors of vascular endothelial growth factors could potentially lead to overproduction of erythropoietin and increase hematocrit, which may increase the risk of thrombosis via elevation of blood viscosity.
Biomarkers for Assessment of Venous Thrombosis
In the in vivo embryofetal toxicity assessment of drugs, inclusion of biomarkers may provide weight-of-evidence in the evaluation of the risk for venous thrombosis. Measurements of circulating levels of coagulation molecules from pregnant animals may enable to characterize drugs for prothrombotic signals during pregnancy. A number of biomarkers have been proposed to predict venous thrombosis (Table 4).
TABLE 4.
Potential Soluble Biomarkers of Venous Thrombosis
| Biomarker | Source | Role |
|---|---|---|
| D-dimer | Fibrin degradation product formed after blood clot fibrinolysis | Reflects global activation of blood coagulation state |
| Fibrin monomer | Thrombin-mediated breakdown product of fibrinogen | Involved in intrinsic and extrinsic coagulation pathway |
| Factor VIII | Glycoprotein cofactor released by leukocytes, endothelial cells | Essential in intrinsic coagulation pathway, activates factor IXa |
| P-selectin (CD62P) | Glycoprotein secreted by activated endothelial cells, platelets | Cell adhesion molecule, interacts with PSGL-1 to mediate binding of platelets and endothelial cells with leukocytes |
| Thrombin | Breakdown product of prothrombin mediated by prothrombinase | Activates platelets, coagulation factors V and VII, and converts fibrinogen to fibrin |
| Microparticles | Released by platelets, endothelial cells | Carriers of tissue factor, participate in coagulation, inflammation |
| CD40 ligand | Glycoprotein secreted by leukocytes, platelets | Endothelial cell activation, up-regulation of tissue factor activity |
| β-thromboglobulin, Platelet factor 4 | Secreted from activated platelet α-granules | Neutralization of heparin-like molecules, platelet activation |
Abbreviations: PSGL-1, P-selectin glycoprotein ligand-1.
A potential biomarker is D-Dimer, for which elevated levels are considered to reflect a hypercoagulable state. D-Dimer is a degradation product of cross-linked fibrin that is formed immediately after thrombin-generated fibrin clots are degraded by plasmin. D-Dimer levels rise during an acute event of venous thrombosis, and reflect a global activation of blood coagulation and fibrinolysis (Righini et al., 2008; Hansen et al., 2011). Elevated D-Dimer indicates abnormal fibrin degradation products indicating clot formation.
Fibrin monomer is the product of thrombin-induced proteolysis of fibrinogen, and is considered to be a predictor of venous thrombosis (Tanaka et al., 2009) (Fig. 2). Both D-dimer and fibrin monomer are fibrin-related markers, but D-dimer is a product of post-thrombotic state of cross-linked fibrin, whereas fibrin monomer is produced by thrombin-mediated cleavage of fibrinogen in a hyper-coagulable state (Tanaka et al., 2009). Therefore, D-dimer serves as a post-thrombotic marker, while fibrin monomer serves as a pre-thrombotic marker, and their simultaneous measurements may be more predictive of venous thrombosis (Cosmi et al., 2008; Hansen et al., 2011).
Another biomarker associated with venous thrombosis is elevated levels of factor VIII, which is considered to be a risk predictor for venous thrombosis (Cosmi et al., 2008). Factor VIII is synthesized and released from the liver and endothelium, and it participates in the contact pathway of coagulation. High levels of factor VIII are considered to be the cause rather than a consequence of venous thrombosis (Kyrle et al., 2000). When factor VIII levels are considered for assessment of venous thrombosis, it is important to evaluate whether acute phase reactants are present, as these also induce factor VIII.
Elevated soluble P-selectin (CD62P) has been implicated as a risk factor for venous thrombosis (Rectenwald et al., 2005; Wakefield et al., 2008). P-selectin, a member of the selectin family of cell adhesion molecules, is primarily stored in the α granules of platelets and the Weibel-Palade bodies of endothelial cells. After activation of platelets and endothelial cells, P-selectin is translocated to the cell surface and in part released into the plasma in soluble form. P-selectin is expressed on platelets and mediates platelet-endothelium interaction and supports fibrin formation and thrombus growth, by binding to its main counter-receptor, the P-selectin glycoprotein ligand-1 (Jilma et al., 2005; Rectenwald et al., 2005). Thus, P-selectin is considered to be another potential predictor of prothrombotic state.
Another biomarker is the thrombin generation assay, which is considered to be a valuable measure of the cumulative effects of prothrombotic tendencies, and may be a potential predictor of venous thrombosis. The measurement of thrombin is considered to be important because it is a key enzyme in the coagulation process that leads to the conversion of fibrinogen to fibrin, resulting in clot formation. The generation of thrombin is measured in plasma using fluorogenic or chromogenic substrates to detect coagulation potential.
Microparticles are small membranous vesicles released from plasma membranes of activated platelets and endothelial cells, and are the main carriers of tissue factor, which plays a major role in coagulation (Rectenwald et al., 2005). Microparticles are negatively charged membrane vesicles that are commonly defined by their procoagulant phosphatidylserine-rich surface. Drugs that increase circulating levels of microparticles are associated with increased incidence of procoagulant state (Patil et al., 2013). Tissue factor measurement in plasma or tissue factor measurement on microparticles is considered to be a predictor of prothrombotic state, however, assay standardization of microparticle isolation and measurement remains an issue.
CD40 ligand (CD40L) concentration, which is increased following orthopedic surgery and device implants, causes injury to endothelial cells, upregulates tissue factor activity, enhances coagulability, and serves as a marker for venous thromboembolism (Ahn et al., 2004; Kageyama et al., 2007). The release of soluble CD40L from activated platelets reacts with CD40 receptor on endothelial cells to induce inflammatory responses by triggering expression of cytokines and chemokines. A number of acute-phase proteins, such as C-reactive protein (CRP) and inflammatory cytokines (such as interleukins 1β, 6, 8, 10, and tumor necrosis factor-α), are associated with an increased incidence of venous thrombosis. The inflammatory cytokines upregulate prothrombotic factors, such as tissue factor and adhesion molecules, in the endothelium to switch the balance in favor of thrombosis (Cirillo et al., 2005).
β-Thromboglobulin and platelet factor 4 levels are elevated in plasma of subjects with thromboembolic disorders, including venous thrombosis (Kageyama et al., 2007). β-Thromboglobulin and platelet factor 4 are proteins stored in α-granules of platelets and are released in large amounts after platelet activation to promote blood coagulation by modulating the effects of heparin-like molecules.
The activated clotting time test is used to monitor anticoagulation effects, such as high-dose heparin procedures that require intense anticoagulant administration. Prolongation of the activated clotting time may indicate thrombocytopenia, platelet dysfunction, or a deficiency in coagulation factors, whereas shorter duration may reflect procoagulant status. However, measurements of clotting time can be directly affected by drugs, such as warfarin, aprotinin, and glycoprotein IIb/IIIa inhibitors, in addition to the physical perturbations to the body caused by pregnancy.
Conclusions
Experimental animals are used to evaluate the safety profile of drugs and biologics to reveal any adverse effects of the test substance on embryofetal development and postnatal development of the offspring. Data on coagulation changes in animals during pregnancy indicate variability in many parameters of thrombosis, and have limitations in predicting venous thrombosis in humans. Certain drugs can induce a prothrombotic state by a variety of mechanisms, including those affecting the vascular intimal surface, blood flow, and various blood constituents. Drugs that contribute to venous stasis or slow blood circulation may act in concert to increase the risk of thrombosis. The risk of developing a thrombotic state could be additive if such drugs are administered during pregnancy. The evaluation of drugs for embryofetal developmental toxicity could be enhanced by including multiple soluble biomarkers of venous thrombosis to assess the potential risk for drug-induced venous thrombosis during pregnancy. Efforts are underway to search for non-animal in vitro alternative methods of assessment of embryofetal toxicity as well as assays that can be used to predict the occurrence of venous thrombosis. Development of validated and reliable in vitro assays to assess the effect of pharmaceuticals on embryofetal development would provide an opportunity to evaluate biologically relevant and species-specific toxicity endpoints as well reduce the use of animals.
Footnotes
Disclaimer: This article reflects the views of the authors and should not be construed to represent FDA’s views or policies.
References
- Ahn ER, Lander G, Jy W, et al. Differences of soluble CD40L in sera and plasma: implications on CD40L assay as a marker of thrombotic risk. Thromb Res. 2004;114:143–148. doi: 10.1016/j.thromres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Atkinson DE, Boyd RD, Sibley CP. Placental transport. In: Neill JD, editor. Knobil and Neill’s physiology of reproduction. Elsevier; Boston, MA: 2006. pp. 2787–2846. [Google Scholar]
- Blank M, Shoenfeld Y, Tavor S, et al. Anti-platelet factor 4/heparin antibodies from patients with heparin-induced thrombocytopenia provoke direct activation of microvascular endothelial cells. Int Immunol. 2002;14:121–129. doi: 10.1093/intimm/14.2.121. [DOI] [PubMed] [Google Scholar]
- Blann AD. How a damaged blood vessel wall contibutes to thrombosis and hypertenasion. Pathophysiol Haemost Thromb. 2003;33:445–448. doi: 10.1159/000083843. [DOI] [PubMed] [Google Scholar]
- Brannen KC, Panzica-Kelly JM, Danberry TL, et al. Development of a zebrafish embryo teratogenicity assay and quantitative prediction model. Birth Defect Res B Dev Reprod Toxicol. 2010;89:66–77. doi: 10.1002/bdrb.20223. [DOI] [PubMed] [Google Scholar]
- Brenner B. Haemostatic changes in pregnancy. Thromb Res. 2004;114:409–414. doi: 10.1016/j.thromres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Brotman DJ, Girod JP, Posch A, et al. Effects of short-term glucocorticoids on hemostatic factors in healthy volunteers. Thromb Res. 2006;118:247–252. doi: 10.1016/j.thromres.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Camici GG, Steffel J, Amanovic I, et al. Rapamycin promotes arterial thrombosis in vivo: implications for everolimus and zotarolimus eluting stents. Eur Heart J. 2010;31:236–242. doi: 10.1093/eurheartj/ehp259. [DOI] [PubMed] [Google Scholar]
- Cappon G, Hurtt M. Developmental toxicity of the kidney. In: Kapp RJ, Tyl R, editors. Reproductive Toxicology. Informa Healthcare; New York, NY: 2010. pp. 193–204. [Google Scholar]
- Carney EW, Scialli AR, Watson RE, DeSesso JM. Mechanisms regulating toxicant disposition to the embryo during early pregnancy: an interspecies comparison. Birth Defect Res C Embryo Today. 2004;72:345–360. doi: 10.1002/bdrc.20027. [DOI] [PubMed] [Google Scholar]
- Cirillo P, Golino P, Calabro P, et al. C-reactive protein induces tissue factor expression and promotes smooth muscle and endothelial cell proliferation. Cardiovasc Res. 2005;68:47–55. doi: 10.1016/j.cardiores.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Cleuren AC, Van der Linden IK, De Visser YP, et al. 17alpha-Ethinylestradiol rapidly alters transcript levels of murine coagulation genes via estrogen receptor alpha. J Thromb Haemost. 2010;8:1838–1846. doi: 10.1111/j.1538-7836.2010.03930.x. [DOI] [PubMed] [Google Scholar]
- Cleuren AC, Van OR, Reitsma PH, et al. Long-term estrogen treatment of mice with a prothrombotic phenotype induces sustained increases in thrombin generation without affecting tissue fibrin deposition. J Thromb Haemost. 2012;10:2392–2394. doi: 10.1111/j.1538-7836.2012.04916.x. [DOI] [PubMed] [Google Scholar]
- Cosmi B, Legnani C, Cini M, et al. D-dimer and factor VIII are independent risk factors for recurrence after anticoagulation withdrawal for a first idiopathic deep vein thrombosis. Thromb Res. 2008;122:610–617. doi: 10.1016/j.thromres.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Cushman M, Kuller LH, Prentice R, Rodabough, et al. Women’s Health Initiative Investigators. Estrogen plus progestin and risk of venous thrombosis. Jama. 2004;292:1573–1580. doi: 10.1001/jama.292.13.1573. [DOI] [PubMed] [Google Scholar]
- DeSesso JM, Williams AL, Ahuja A, et al. The placenta, transfer of immunoglobulins, and safety assessment of biopharmaceuticals in pregnancy. Crit Rev Toxicol. 2012;42:185–210. doi: 10.3109/10408444.2011.653487. [DOI] [PubMed] [Google Scholar]
- DeStephano CC, Werner EF, Holly BP, Lessne ML. Diagnosis and management of iliac vein thrombosis in pregnancy resulting from May-Thurner Syndrome. J Perinatol. 2014;34:566–568. doi: 10.1038/jp.2014.38. [DOI] [PubMed] [Google Scholar]
- Doolittle RF. Step-by-step evolution of vertebrate blood coagulation. Cold Spring Harb Symp Quant Biol. 2009;74:35–40. doi: 10.1101/sqb.2009.74.001. [DOI] [PubMed] [Google Scholar]
- Ebina Y, Ieko M, Naito S, et al. Low levels of plasma protein S, protein C and coagulation factor XII during early pregnancy and adverse pregnancy outcome. Thromb Haemost. 2015;114:65–69. doi: 10.1160/TH14-11-0928. [DOI] [PubMed] [Google Scholar]
- Ekser B, Bianchi J, Ball S, et al. Comparison of hematologic, biochemical, and coagulation parameters in alpha1,3-galactosyl-transferase gene-knockout pigs, wild-type pigs, and four primate species. Xenotransplantation. 2012;19:342–354. doi: 10.1111/xen.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon CT, Esmon NL. The link between vascular features and thrombosis. Annu Rev Physiol. 2011;73:503–514. doi: 10.1146/annurev-physiol-012110-142300. [DOI] [PubMed] [Google Scholar]
- Gentry PA. Comparative aspects of blood coagulation. Vet J. 2004;168:238–251. doi: 10.1016/j.tvjl.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Guba M, Yezhelyev M, Eichhorn ME, et al. Rapamycin induces tumor-specific thrombosis via tissue factor in the presence of VEGF. Blood. 2005;105:4463–4469. doi: 10.1182/blood-2004-09-3540. [DOI] [PubMed] [Google Scholar]
- Hagg S, Spigset O. Antipsychotic-induced venous thromboembolism: a review of the evidence. CNS Drugs. 2002;16:765–776. doi: 10.2165/00023210-200216110-00005. [DOI] [PubMed] [Google Scholar]
- Haneda R, Mizoguchi Y, Matsuoka T, et al. Changes in blood parameters in pregnant Japanese White rabbits. J Toxicol Sci. 2010;35:773–778. doi: 10.2131/jts.35.773. [DOI] [PubMed] [Google Scholar]
- Hansen AT, Andreasen BH, Salvig JD, Hvas AM. Changes in fibrin D-dimer, fibrinogen, and protein S during pregnancy. Scand J Clin Lab Invest. 2011;71:173–176. doi: 10.3109/00365513.2010.545432. [DOI] [PubMed] [Google Scholar]
- Harris C, Hansen JM. In vitro Methods for the study of mechanisms of developmental toxicity. In: Hood R, editor. Developmental and reproductive toxicology. Taylor & Francis; Boca Raton, FL: 2006. pp. 647–695. [Google Scholar]
- Hellgren M. Hemostasis during normal pregnancy and puerperium. Semin Thromb Hemost. 2003;29:125–130. doi: 10.1055/s-2003-38897. [DOI] [PubMed] [Google Scholar]
- James AH. Venous thromboembolism in pregnancy. Arterioscler Thromb Vasc Biol. 2009;29:326–331. doi: 10.1161/ATVBAHA.109.184127. [DOI] [PubMed] [Google Scholar]
- Jilma B, Cvitko T, Winter-Fabry A, et al. High dose dexamethasone increases circulating P-selectin and von Willebrand factor levels in healthy men. Thromb Haemost. 2005;94:797–801. doi: 10.1160/TH04-10-0652. [DOI] [PubMed] [Google Scholar]
- Kageyama K, Nakajima Y, Shibasaki M, et al. Increased platelet, leukocyte, and endothelial cell activity are associated with increased coagulability in patients after total knee arthroplasty. J Thromb Haemost. 2007;5:738–745. doi: 10.1111/j.1538-7836.2007.02443.x. [DOI] [PubMed] [Google Scholar]
- Kasliwal R, Layton D, Harris S, et al. A comparison of reported gastrointestinal and thromboembolic events between rofecoxib and celecoxib using observational data. Drug Saf. 2005;28:803–816. doi: 10.2165/00002018-200528090-00005. [DOI] [PubMed] [Google Scholar]
- Kinhult S, Albertsson M, Eskilsson J, Cwikiel M. Effects of probucol on endothelial damage by 5-fluorouracil. Acta Oncol. 2003;42:304–308. doi: 10.1080/02841860310004409. [DOI] [PubMed] [Google Scholar]
- Kumar S, Lobo SW, Dubey AK, Pandey SK. Teratogenic effects of 5-fluorouracil on rat brain. Nepal Med Coll J. 2006;8:7–8. [PubMed] [Google Scholar]
- Kurne A, Ertugrul A, Anil Yagcioglu AE, Yazici KM. Venous thromboembolism and escitalopram. Gen Hosp Psychiatry. 2004;26:481–483. doi: 10.1016/j.genhosppsych.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Kyrle PA, Minar E, Hirschl M, et al. High plasma levels of factor VIII and the risk of recurrent venous thromboembolism. N Engl J Med. 2000;343:457–462. doi: 10.1056/NEJM200008173430702. [DOI] [PubMed] [Google Scholar]
- Lee HY, Inselman AL, Kanungo J, Hansen DK. Alternative models in developmental toxicology. Syst Biol Reprod Med. 2012;58:10–22. doi: 10.3109/19396368.2011.648302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemini C, Jaimez R, Figueroa A, et al. Ovariectomy differential influence on some hemostatic markers of mice and rats. Exp Anim. 2015;64:81–89. doi: 10.1538/expanim.14-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemini C, Jaimez R, Franco Y. Gender and inter-species influence on coagulation tests of rats and mice. Thromb Res. 2007;120:415–419. doi: 10.1016/j.thromres.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Liu J, Yuan E, Lee L. Gestational age-specific reference intervals for routine haemostatic assays during normal pregnancy. Clin Chim Acta. 2012;413:258–261. doi: 10.1016/j.cca.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Lockwood CJ. Pregnancy-associated changes in the hemostatic system. Clin Obstet Gynecol. 2006;49:836–843. doi: 10.1097/01.grf.0000211952.82206.16. [DOI] [PubMed] [Google Scholar]
- Maurer-Spurej E, Pittendreigh C, Solomons K. The influence of selective serotonin reuptake inhibitors on human platelet serotonin. Thromb Haemost. 2004;91:119–128. doi: 10.1160/TH03-05-0330. [DOI] [PubMed] [Google Scholar]
- Menger H, Lin AE, Toriello HV, et al. Vitamin K deficiency embryopathy: a phenocopy of the warfarin embryopathy due to a disorder of embryonic vitamin K metabolism. Am J Med Genet. 1997;72:129–134. [PubMed] [Google Scholar]
- Menis M, Sridhar G, Selvam N, et al. Hyperimmune globulins and same-day thrombotic adverse events as recorded in a large healthcare database during 2008–2011. American Journal of Hematology. 2013;88:1035–1040. doi: 10.1002/ajh.23559. [DOI] [PubMed] [Google Scholar]
- Mizoguchi Y, Matsuoka T, Mizuguchi H, et al. Changes in blood parameters in New Zealand White rabbits during pregnancy. Lab Anim. 2010;44:33–39. doi: 10.1258/la.2009.008002. [DOI] [PubMed] [Google Scholar]
- Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195:40–49. doi: 10.1016/j.ajog.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Onitilo AA, McCarty CA, Wilke RA, et al. Estrogen receptor genotype is associated with risk of venous thromboembolism during tamoxifen therapy. Breast Cancer Res Treat. 2009;115:643–650. doi: 10.1007/s10549-008-0264-2. [DOI] [PubMed] [Google Scholar]
- Pasquale SA. Oral contraceptives: significance of their effects in man and relationship to findings in animal models. Toxicol Pathol. 1989;17:396–400. doi: 10.1177/019262338901700217. [DOI] [PubMed] [Google Scholar]
- Patil R, Ghosh K, Satoskar P, Shetty S. Elevated procoagulant endothelial and tissue factor expressing microparticles in women with recurrent pregnancy loss. PLoS One. 2013;8:e81407. doi: 10.1371/journal.pone.0081407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peverill RE. Hormone therapy and venous thromboembolism. Best Pract Res Clin Endocrinol Metab. 2003;17:149–164. doi: 10.1016/s1521-690x(02)00079-9. [DOI] [PubMed] [Google Scholar]
- Piersma AH. Validation of alternative methods for developmental toxicity testing. Toxicol Lett. 2004;149:147–153. doi: 10.1016/j.toxlet.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Piparo EL, Worth A. EUR – Scientific and Technical Research series. 2010. Review of QSAR models and software tools for predicting developmental and reproductive toxicity. [Google Scholar]
- Rectenwald JE, Myers DD, Jr, Hawley AE, et al. D-dimer, P-selectin, and microparticles: novel markers to predict deep venous thrombosis. A pilot study. Thromb Haemost. 2005;94:1312–1317. doi: 10.1160/TH05-06-0426. [DOI] [PubMed] [Google Scholar]
- Ribeiro AM, Zepeda-Mendoza ML, Bertelsen MF, et al. A refined model of the genomic basis for phenotypic variation in vertebrate hemostasis. BMC Evol Biol. 2015;15:124. doi: 10.1186/s12862-015-0409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righini M, Perrier A, De MP, Bounameaux H. D-Dimer for venous thromboembolism diagnosis: 20 years later. J Thromb Haemost. 2008;6:1059–1071. doi: 10.1111/j.1538-7836.2008.02981.x. [DOI] [PubMed] [Google Scholar]
- Rufa A, Cerase A, Monti L, et al. Recurrent venous thrombosis including cerebral venous sinus thrombosis in a patient taking sildenafil for erectile dysfunction. J Neurol Sci. 2007;260:293–295. doi: 10.1016/j.jns.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Siller-Matula JM, Plasenzotti R, Spiel A, et al. Interspecies differences in coagulation profile. Thromb Haemost. 2008;100:397–404. [PubMed] [Google Scholar]
- Smith KJ, Bleyer AJ, Little WC, Sane DC. The cardiovascular effects of erythropoietin. Cardiovasc Res. 2003;59:538–548. doi: 10.1016/s0008-6363(03)00468-1. [DOI] [PubMed] [Google Scholar]
- Stassen JM, Arnout J, Deckmyn H. The hemostatic system. Curr Med Chem. 2004;11:2245–2260. doi: 10.2174/0929867043364603. [DOI] [PubMed] [Google Scholar]
- Szecsi PB, Jorgensen M, Klajnbard A, et al. Haemostatic reference intervals in pregnancy. Thromb Haemost. 2010;103:718–727. doi: 10.1160/TH09-10-0704. [DOI] [PubMed] [Google Scholar]
- Tanaka KA, Key NS, Levy JH. Blood coagulation: hemostasis and thrombin regulation. Anesth Analg. 2009;108:1433–1446. doi: 10.1213/ane.0b013e31819bcc9c. [DOI] [PubMed] [Google Scholar]
- Tandon S, Jyoti S. Embryonic stem cells: an alternative approach to developmental toxicity testing. J Pharm Bioallied Sci. 2012;4:96–100. doi: 10.4103/0975-7406.94808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchaikovski SN, Rosing J. Mechanisms of estrogen-induced venous thromboembolism. Thromb Res. 2010;126:5–11. doi: 10.1016/j.thromres.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Tchaikovski SN, Thomassen MC, Costa SD, et al. Role of protein S and tissue factor pathway inhibitor in the development of activated protein C resistance early in pregnancy in women with a history of preeclampsia. Thromb Haemost. 2011;106:914–921. doi: 10.1160/TH11-04-0244. [DOI] [PubMed] [Google Scholar]
- Tchaikovski SN, Van Vlijmen BJ, Cleuren AC, et al. Pregnancy-associated changes in the hemostatic system in wild-type and factor V Leiden mice. J Thromb Haemost. 2009;7:312–318. doi: 10.1111/j.1538-7836.2008.03240.x. [DOI] [PubMed] [Google Scholar]
- Urasoko Y, He XJ, Ebata T, et al. Changes in blood parameters and coagulation-related gene expression in pregnant rats. J Am Assoc Lab Anim Sci. 2009;48:272–278. [PMC free article] [PubMed] [Google Scholar]
- Urasoko Y, He XJ, Masao T, et al. Changes in blood parameters and the expression of coagulation-related genes in lactating Sprague-Dawley rats. J Am Assoc Lab Anim Sci. 2012;51:144–149. [PMC free article] [PubMed] [Google Scholar]
- van der Laan JW, Chapin RE, Haenen B, et al. Testing strategies for embryo-fetal toxicity of human pharmaceuticals. Animal models vs. in vitro approaches: a workshop report. Regul Toxicol Pharmacol. 2012;63:115–123. doi: 10.1016/j.yrtph.2012.03.009. [DOI] [PubMed] [Google Scholar]
- van Driel D, Wesseling J, Sauer PJ, et al. Teratogen update: fetal effects after in utero exposure to coumarins overview of cases, follow-up findings, and pathogenesis. Teratology. 2002;66:127–140. doi: 10.1002/tera.10054. [DOI] [PubMed] [Google Scholar]
- Vogel J, Kiessling I, Heinicke K, et al. Transgenic mice over-expressing erythropoietin adapt to excessive erythrocytosis by regulating blood viscosity. Blood. 2003;102:2278–2284. doi: 10.1182/blood-2003-01-0283. [DOI] [PubMed] [Google Scholar]
- Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol. 2008;28:387–391. doi: 10.1161/ATVBAHA.108.162289. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhang L. Glucocorticoids and vascular reactivity. Curr Vasc Pharmacol. 2004;2:1–12. doi: 10.2174/1570161043476483. [DOI] [PubMed] [Google Scholar]
- Zornberg GL, Jick H. Antipsychotic drug use and risk of first-time idiopathic venous thromboembolism: a case-control study. Lancet. 2000;356:1219–1223. doi: 10.1016/S0140-6736(00)02784-7. [DOI] [PubMed] [Google Scholar]


